Abstract
Mouse Paneth cells respond to bacteria and bacterial cell surface antigens by discharging secretory granules into the lumen of small intestinal crypts (T. Ayabe et al., Nat. Immunol. 1:113-118, 2000). To investigate mechanisms regulating these responses, purified surface glycolipid molecules with known acyl chain modifications and attenuated properties were tested for the ability to stimulate Paneth cell secretion. The antigens included lipopolysaccharide (LPS) from wild-type and msbB-null Escherichia coli and phoP-null and phoP-constitutive Salmonella enterica serovar Typhimurium strains, as well as LPS, lipid A, and lipoteichoic acid from Pseudomonas aeruginosa and Listeria monocytogenes grown in Mg2+-limited media. Measurements of total secreted protein, secreted lysozyme, and the bactericidal peptide activities of collected secretions showed that the purified antigens elicited similar secretory responses from Paneth cells in mouse crypts ex vivo, regardless of glycolipid acyl chain modification. Despite their impaired Tlr4 pathway, Paneth cells in ex vivo C3H/HeJ mouse crypts released equivalent amounts of bactericidal peptide activity in response to purified bacterial antigens, including lipid A. Thus, mouse Paneth cells respond equivalently to purified bacterial cell envelope glycolipids, regardless of functional Tlr4, the structural properties of glycolipid acyl chains, or their association with virulence in humans.
The α-defensins are 3- to 4-kDa microbicidal peptides expressed by cells of myeloid origin and by epithelial lineages in mammals (30, 58). These cationic, β-sheet-containing peptides contain a defining tridisulfide array and amphipathicity that contributes to peptide bactericidal activity by inducing target cell membrane disruption (23, 47, 53). In cells of myeloid origin, fully processed α-defensins accumulate in azurophilic granules of phagocytic leukocytes, from which they function in microbial cell killing by nonoxidative mechanisms following phagolysosomal fusion (17). Epithelial cells on mucosal surfaces also express α- or β-defensins that may be secreted by apparent constitutive means or as granule constituents of regulated secretory pathways (39, 40, 44, 52). In mouse small intestinal epithelium, exocytotic Paneth cells located at the base of the crypts of Lieberkühn release activated α-defensins, termed cryptdins, in response to cholinergic and bacterial stimuli (2, 3, 48, 51).
Paneth cells secrete granules in a dose-dependent manner following interaction with bacterial antigens and in response to pharmacologic stimulation (2, 49-51). Live gram-negative and gram-positive bacteria and also commercially available preparations of lipopolysaccharide (LPS), lipoteichoic acid (LTA), lipid A, and muramyl dipeptide all induced rapid secretion by mouse Paneth cells. In contrast, mouse Paneth cells are unresponsive to Candida albicans, Cryptococcus neoformans, and trophozoites of Giardia lamblia (2). Both carbamyl choline and bacterial antigens stimulate Paneth cells in isolated mouse small intestinal crypts to secrete by inducing an increase in cytosolic [Ca2+] by the sequential mobilization of intracellular and extracellular Ca2+ stores (3, 49). The inhibition of Paneth cell secretion by highly selective blockers of mIKCa1, a Ca2+-activated K+ channel (3), provided evidence that bacterial antigens also induce secretion by modulating cytosolic Ca2+ dynamics.
Bacteria regulate and modify lipid A, the glycolipid anchor of their cell surface LPS, in response to the host microenvironment. For example, growth under Mg2+ limitation results in a modification of lipid A acylation in several bacterial species (13, 19-21), and the extent of lipid A acylation modulates LPS-mediated bacterial recognition when human host cells are exposed to viable bacteria. pagP, a gene activated by the phoP-phoQ regulon of salmonellae, increases lipid A acylation (22, 59), and pagP mutants show increased outer membrane permeability to α-helical peptides (22), suggesting that lipid A modification may influence sensitivity to endogenous cationic antimicrobial peptides, including α-defensins. Also, the prevailing lipid A synthesized by wild-type PAK and PAO-1 strains of Pseudomonas aeruginosa grown in high concentrations of Mg2+ is a penta-acylated form, and growth of P. aeruginosa strains in low concentrations of Mg2+ results in lipid A modification to contain aminoarabinose (4-amino-4-deoxy-l-arabinose) on the 1′ and/or 4′ phosphates and also palmitate at the 3′-3-oxo-C10:0 position (31, 32). In addition, the bacteria that colonize the human gingival crevice also produce modified cell surface glycolipids with attenuated proinflammatory and chemotactic properties (8, 9).
This study was initiated to test whether bacterial antigens with modified lipid A or lipoteichoic acid moieties would elicit differential secretory responses in Paneth cells. Paneth cells in isolated mouse small intestinal crypts were exposed to a variety of purified gram-positive and gram-negative bacterial cell surface antigens containing well-characterized glycolipid modifications. The data show that Paneth cells respond to effective doses of all structurally distinct antigens to approximately the same extent.
MATERIALS AND METHODS
Purification of bacterial antigens.
LPS and LTA samples were isolated by using the Mg2+ precipitation procedure of Darveau and Hancock or by using the phenol extraction procedure (11). In addition, LPS preparations were subjected to Folch extraction (15) to remove residual lipid contamination and the procedure of Manthey and Vogel to remove LPS contaminating proteins (24, 33). Preparations were tested for activity in 293 cells transfected with human or mouse TLR2 to exclude contaminating bacterial pathogen-associated molecular patterns.
Preparations of LPS.
LPS prepared from P. aeruginosa came from the following sources: PAK cells grown in a low concentration (8 μM) of Mg2+; PAK cells grown in a high concentration (1 mM) of Mg2+; PAK phoP-null (ΔphoP) cells grown in a low concentration (8 μM) of Mg2+; PAK ΔphoP cells grown in a high concentration (1 mM) of Mg2+; P. aeruginosa cells from clinical isolate CF1188, grown in Luria-Bertani medium (LB); and P. aeruginosa cells from clinical isolate Bronch267, grown in LB. LPS prepared from Salmonella enterica serovar Typhimurium was from the following sources: phoP-constitutive [phoP(Con)] serovar Typhimurium cells grown in LB, ΔphoP serovar Typhimurium cells grown in LB, wild-type (WT) serovar Typhimurium cells grown in LB and extracted with phenol, WT waaP-null (ΔwaaP) serovar Typhimurium cells grown in LB and extracted with phenol, phoP(Con) serovar Typhimurium cells grown in LB and extracted with phenol, and phoP(Con) ΔwaaP serovar Typhimurium cells grown in LB and extracted with phenol. LPS also was prepared from WT and ΔmsbB Escherichia coli JM83 (54), WT and ΔmsbB E. coli H16 (55), Porphyromonas gingivalis ATCC 33277, and Bacteroides forsythus ATCC 25285 as described previously (7, 10).
Preparations of lipid A.
Lipid A molecules prepared from P. aeruginosa were from the following sources: WT PAK cells grown in a low concentration (8 μM) of Mg2+, WT PAK cells grown in a high concentration (1 mM) of Mg2+, and phoP(Con) and ΔphoP serovar Typhimurium cells grown in LB.
Preparations of LTA.
Glycolipid fractions containing LTA were prepared from wild-type and petite Listeria monocytogenes “Scott A” cells grown in trypticase soy broth (TSB) supplemented with 0.6% yeast extract and 0.5% glucose (TSBYG) that were generously provided by Nancy Freitag (University of Washington). Glycolipid fractions containing “Scott A-M” LTA were prepared from WT L. monocytogenes cells grown in TSBYG, and the strain was generously provided by Thomas J. Montville (Rutgers, The University of New Jersey). Other preparations were Lm700302 LTA from L. monocytogenes 700302 cells grown in TSBYG, Lm10403S LTA from L. monocytogenes 10403S cells grown in TSBYG, Lm10403S-L LTA from L. monocytogenes 10403S cells grown in 8 μM Mg2+, and Lm10403S-H LTA from L. monocytogenes 10403S cells grown in a high concentration (1 mM) of Mg2+.
Preparation and analysis of isolated mouse crypts and Paneth cells.
The small intestine was removed from adult mice euthanized by CO2 inhalation, the lumen was rinsed with water, and crypts were eluted from segments everted and shaken in Ca2+- and Mg2+-free phosphate-buffered saline (PBS) containing 30 mM EDTA (2, 3). Villi and crypts eluted during 5-min intervals were deposited by centrifugation at 700 × g and resuspended in PBS, and the numbers of crypts in enriched fractions were estimated by hemocytometry. Crypts were resuspended in isotonic piperazine-N-N′-bis(2-ethanesulfonic acid) (PIPES) buffer consisting of 10 mM PIPES (pH 7.4) and 137 mM NaCl (iPIPES) for exposure to microbial antigens. All procedures performed on mice were performed in compliance with the policies of the Institutional Animal Care and Use Committee of the University of California, Irvine.
For preparation and isolation of single Paneth cells, crypt preparations were incubated in 2 ml of Hanks balanced salt solution with 150 U of collagenase (Sigma-Aldrich, St. Louis, Mo.)/ml at 37°C for 15 min. Cells were deposited by centrifugation at 1,500 rpm for 5 min in a Beckman GS-6R centrifuge, resuspended, and washed two times with ice-cold PBS, and the preparation was centrifuged through a 30-μm-pore-size filter Cell Strainer cap (Falcon model 352235). Intact crypts, villi, Paneth cells, and agranular crypt epithelial cells were identified under phase microscopy, drawn into capillary tubes, and transferred to PCR tubes for amplification.
For each series of antigens, preparations of crypts, numbering 2,000 to 4,000 crypts, were divided equally, resuspended in 500 μl of nominal Ca2+-free isotonic PIPES buffer, and exposed to antigens (100 ng of LPS/ml, 10 μg of LTA/ml, and 1 μg of lipid A/ml) for 30 min at 37°C. After incubation, secretions were obtained by the collection of supernatants from crypts deposited by low-speed centrifugation (2). Protein concentrations were determined by using the Bradford assay, and lysozyme activities were measured with the Micrococcus luteus turbidometric assay (18, 42). For each set of determinations, a standard curve was determined by using hen egg white lysozyme (Sigma) in sample tubes containing 175 μl of Micrococcus lysodeikticus (Sigma) suspended in PBS at 200 μg/ml, and activity was measured as the absorbance at 530 nm at 25°C. Dilutions of collected crypt secretions were assayed similarly, and the lysozyme concentrations in samples of secretions were extrapolated from the linear region of the standard curves.
RT-PCR detection of Toll-like receptor mRNAs in mouse Paneth cells.
For reverse transcriptase-PCR (RT-PCR) experiments, isolated villi, intact crypts, individual Paneth cells, or individual agranular crypt epithelial cells were transferred to individual microfuge tubes, sonicated in 1 μl of RNAguard RNase inhibitor, and stored at −70°C. The detection of Toll-like receptor (Tlr), MD-2, and CD14 mRNAs was performed by RT-PCR with nested primer sets. mRNAs from intestinal epithelial cells and structures were amplified with GeneAmp EZ rTth RNA PCR kit (Perkin-Elmer, Foster City, Calif.) using the sequence-specific primer set 1 (Table 1). Samples (500 ng) of total RNA from mouse bone marrow, stomach, small intestine, and colon were analyzed similarly. Samples consisting of 5 μl of 1:100 dilutions of the primary RT-PCR products were used as templates for nested amplification using primer set 2 (Table 2). PCR mixes lacking AmpliTaq DNA polymerase were preheated at 94°C for 2 min, and then complete reaction mixes were run for 40 cycles as described before, except for small intestinal and bone marrow amplifications, which were run for 30 cycles. Samples of the PCRs were analyzed by separation in 2% agarose gels to visualize and to size amplification products. Amplification products purified with the QIAEX II gel extraction kit (QIAGEN, Valencia, Calif.) were cloned into the pCR2.1 TOPO vector (Invitrogen, Burlingame, Calif.), and their identities were confirmed by DNA sequencing (data not shown).
TABLE 1.
Primers for primary Tlr amplificationa
| Receptor | Primer (sequence)
|
Accession no. | |
|---|---|---|---|
| Sense | Antisense | ||
| mMD2 | mMD2-S11 (5′-ATGGTCTTCCTGGCGAGTTT) | mMD2-AS456 (5′-GCAACACATCTGTAATGGCC) | B018550 |
| mCD14 | mCD14-S50 (5′-CTTGTTGCTGTTGCTTCTGG) | mCD14-AS1188 (5′-AAGCAGAGGATTCGTTGACG) | NM_009841 |
| mTLR-1 | mTLR1-S50 (5′-GTTGGTGAGAACTCAGGCG) | mTLR1-AS1198 (5′-TGTGGACCATGTGTGTTCC) | AY009154 |
| mTLR-2 | mTLR2-S330 (5′-TTGGCTCTTCTGGATCTTGG) | mTLR2-AS1585 (5′-TGTTCCTGCTGATGTCAAGG) | AF216289 |
| mTLR-3 | mTLR3-S804 (5′-TCTCTCTGGCTAACAACCAGC) | mTLR3-AS2223 (5′-TCGATGTGAATGAGCAGTACC) | AF355152 |
| mTLR-4 | mTLR4-S82 (5′-ACACCAGGAAGCTTGAATCC) | mTLR4-AS1262 (5′-GCACTCATAATGATGGCACC) | AF110133 |
| mTLR-5 | mTLR5-S255 (5′-ATGGATGGATGCTGAGTTCC) | mTLR5-AS1170 (5′-AGTTGAAGCTGAGCAGGAGC) | NM_016928 |
| mTLR-6 | mTLR6-S223 (5′-ATATCTGAGCTTCGGATGCC) | mTLR6-AS1549 (5′-AGTTATGGTCGATGACCAGC) | AF314636 |
| mTLR-7 | mTLR7-S297 (5′-TTCCTTCCGTAGGCTGAACC) | mTLR7-AS1535 (5′-CCATATATGTGGCAGTCTGCA) | AY035889 |
| mTLR-8 | mTLR8-S862 (5′-CTCATCCATCCACATACATCC) | mTLR8-AS1766 (5′-ATCCTAGACGGTGCGTTACC) | AY035890 |
| mTLR-9 | mTLR9-S999 (5′-AAGGTCTGGTCAACCTCTCG) | mTLR9-AS1850 (5′-GAATGTCATTGTGTGCCAGG) | AF314224 |
The primer pairs shown were used to amplify Tlr, MD-2, and CD14 mRNAs by RT-PCR from samples (500 ng) of total RNA from mouse bone marrow, stomach, small intestine, and colon and from isolated villi, intact crypts, individual Paneth cells, or individual agranular crypt epithelial cells (see Materials and Methods).
TABLE 2.
Primers for nested Tlr amplificationa
| Receptor | Primer (sequence)
|
Product size (bp) | |
|---|---|---|---|
| Sense | Antisense | ||
| mMD2 | mMD2-S117 (5′-AGTGGTTCTGCAACTCCTCC) | mMD2-AS338 (5′-GGCACAGAACTTCCTTACGC) | 222 |
| mCD14 | mCD14-S317 (5′-GCGGATTCCTAGTCGGATTC) | mCD14-AS951 (5′-GGTTCCTATCCAGCCTGTTG) | 635 |
| mTLR-1 | mTLR1-S722 (5′-AATCTCTTCGGCACGTTAGC) | mTLR1-AS1049 (5′-ATCCTGAAGGCAAGTTGACC) | 328 |
| mTLR-2 | mTLR2-S658 (5′-TTATCTTCCTCCTGGTTCGG) | mTLR2-AS1339 (5′-TGATTCGCTTCACCTTCTCC) | 682 |
| mTLR-3 | mTLR3-S895 (5′-CAACAACCTCCATGATGTCG) | mTLR3-AS1412 (5′-TCAGACCTCTCCATTCCTGG) | 518 |
| mTLR-4 | mTLR4-S664 (5′-GACTTCATTCAAGACCAAGCC) | mTLR4-AS968 (5′-ACACCTGCCAGAGACATTGC) | 305 |
| mTLR-5 | mTLR5-S507 (5′-TTGTCCGATCATCAGCTAAGC) | mTLR5-AS867 (5′-GGCAGATTCTTCTTGTCTTGG) | 361 |
| mTLR-6 | mTLR6-S347 (5′-TGGATGTCTCACACAATCGG) | mTLR6-AS790 (5′-TCAATAAGGTTGGACCTCTGG) | 444 |
| mTLR-7 | mTLR7-S499 (5′-CATCTTCTGAGCCTTGAGGC) | mTLR7-AS866 (5′-CACGGTGTACACGGATATGG) | 368 |
| mTLR-8 | mTLR8-S941 (5′-AGGACGATTCCTTCTACCTGG) | mTLR8-AS1485 (5′-GCCTTGCCATAAGCAGTACAC) | 545 |
| mTLR-9 | mTLR9-S1195 (5′-CATGAACGGCATCTTCTTCC) | mTLR9-AS1548 (5′-ATAGTCACCAGGTTGTTCCG) | 354 |
The primer pairs shown were used in nested amplification reactions for Tlr, MD-2, and CD14 mRNAs. Samples consisted of 5 μl of 1:100 dilutions of the primary RT-PCR products (Table 1; see Materials and Methods). PCR mixes lacking AmpliTaq DNA polymerase were preheated at 94°C for 2 min, and then complete reaction mixes were run as described in Materials and Methods.
Bactericidal peptide assays.
Two types of bactericidal assays were performed on secretions collected from crypts exposed to bacterial antigens, both using the defensin-sensitive ΔphoP strain of serovar Typhimurium as the test species (14, 36). In the first assay, 500 to 1,000 CFU of exponentially growing cells were deposited by centrifugation, resuspended in 45 μl of PIPES buffer, and combined with 5 μl of secretion collected from crypts or villi. After 60 min at 37°C, the surviving CFU, in triplicate assays, were quantitated by growth on semisolid media at 37°C overnight. Because bacterial numbers in individual experiments varied slightly, groups were normalized by expressing bacterial cell killing as a percentage (± standard deviation) relative to bacteria incubated for 1 h at 37°C in iPIPES alone. In the second assay, secretions were collected from ≥103 crypts, and the bactericidal activities of samples (0.1 to 10 μl) of secretions were assayed against 5 × 106 ΔphoP serovar Typhimurium cells. Exponential-phase bacteria grown in trypticase soy broth at 37°C were deposited by centrifugation at 1,700 × g for 10 min, washed in 10 mM PIPES (pH 7.4), and resuspended in 10 mM PIPES (pH 7.4) supplemented with 0.01 volume of TSB. Test ΔphoP serovar Typhimurium cells were incubated with samples of secretions in a total volume of 50 μl for 1 h in a shaking incubator at 37°C, 20-μl samples of incubation mixtures were diluted 1:200 with 10 mM PIPES (pH 7.4), and 50 μl of the diluted samples were plated on trypticase soy agar plates by using an Autoplate 4000 plater (Spiral Biotech Inc., Bethesda, Md.). Surviving microorganisms were quantitated as CFU per milliliter after incubation at 37°C for 12 to 18 h.
RESULTS
Paneth cell secretion in response to purified antigens.
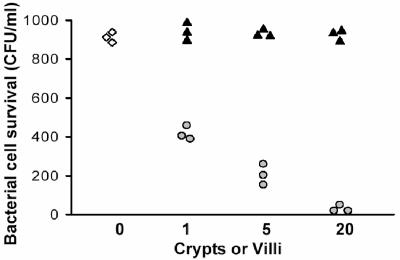
When isolated mouse small intestinal crypts are exposed to live bacteria ex vivo, Paneth cells secrete bactericidal peptides that kill bacteria in the medium (2). This fact is illustrated in Fig. 1; isolated crypts or villi were exposed to ∼1,000 CFU of serovar Typhimurium (ΔphoP) for 30 min and then tested for survival of the exposed bacteria. As reported previously (2, 3), bacterial cell survival was unaffected by incubation with villi (Fig. 1), but bacteria exposed to intact crypts were killed in a dose-dependent fashion (Fig. 1). A previous study has shown that the majority of the bactericidal peptide activity released by Paneth cells in response to bacteria is attributable to cryptdins (2). In these earlier studies (2, 3), LPS, lipid A, LTA, and muramyl dipeptide had been obtained from commercial sources, and the possibility that potential contaminants may have been influencing secretion was a concern.
FIG. 1.
Paneth cell secretion of bactericidal peptide activity in response to live bacteria. Individual crypts and villi were isolated from mouse small intestine, and numbers of each were incubated with 1,000 CFU of ΔphoP serovar Typhimurium cells at 37°C for 60 min in the presence of 1, 5, or 20 intact individual crypts or sheets of villus enterocytes (see Materials and Methods). Surviving bacteria were quantitated by plating incubation mixtures on nutrient plates, and replicates are shown. Symbols: ⋄, bacteria incubated but not exposed to crypts or villi; ▴, bacteria exposed to villus epithelium; ○, bacteria exposed to intact crypts.
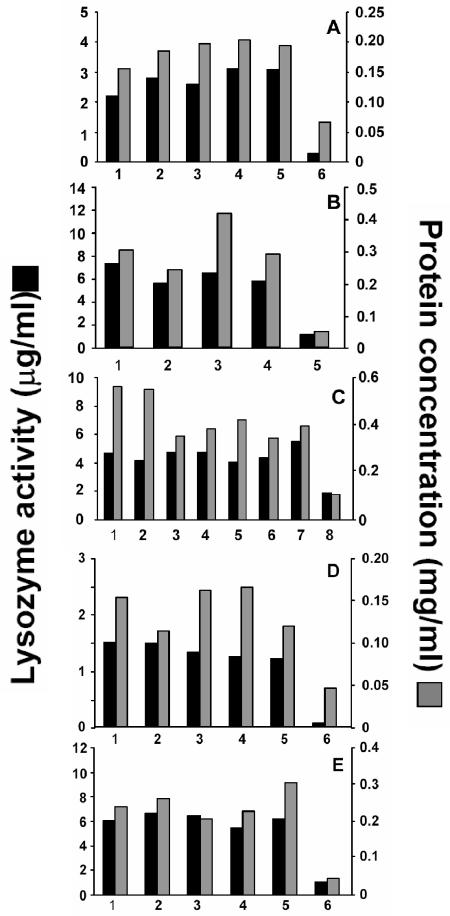
To investigate mechanisms regulating Paneth cell secretory responses, we tested whether pure bacterial surface glycolipids with known acyl chain modifications and attenuated properties would differ in the extents to which they stimulate Paneth cell secretion. Subsequent to the exposure of the crypts to purified antigens, secretions were collected from the crypts and assayed for protein, lysozyme, and bactericidal peptide activity. In the first series of experiments, the antigens consisted of LPS molecules purified (i) from WT and ΔmsbB mutants of E. coli strains (55); (ii) from WT, ΔphoP, phoP(Con), and ΔwaaP mutants of serovar Typhimurium (57); and (iii) from clinical isolates of P. aeruginosa, as well as WT and ΔphoP strains of P. aeruginosa (13). Relative to crypts incubated under identical conditions but not exposed to antigens, Paneth cells in all crypt preparations released protein and lysozyme to similar extents in response to LPS (Fig. 2A through C). Unexposed crypt preparations secreted 50 to 100 μg of total protein/ml and 0.4 to 2 μg of lysozyme/ml, approximately 2 ng of total protein and 30 pg of lysozyme on a per crypt basis. For example, with a given set of antigens, e.g., LPS from P. aeruginosa, the preparation of crypts was divided equally among the five experimental conditions so that the quantities of peptides secreted in response to antigens are comparable within a given experiment. On the other hand, because the actual numbers of crypts differed between experiments, secretions released by crypts exposed to different antigens cannot be compared directly. Whether measured by protein secretion or lysozyme activity, WT and variant LPS molecules elicited equivalent Paneth cell secretion at levels similar to the release induced by commercial LPS. Although all bacterial glycolipids tested induced similar levels of secretion, previous studies had shown that Paneth cells in intact mouse crypts did not respond to live fungi C. neoformans and C. albicans or to live trophozoites of G. lamblia. Under the conditions of these assays, secretion was independent of the species from which the LPS was isolated and the extent or quality of acyl chain modification (Fig. 2A through C), but we cannot exclude the possibility that differential secretory responses may occur at submaximal antigen concentrations.
FIG. 2.
Protein and lysozyme secretion by Paneth cells in crypts stimulated with varied bacterial cell surface antigens. For each series of antigens, preparations of crypts, numbering 2,000 to 4,000 crypts, were divided equally and exposed to antigens (100 ng of LPS/ml, 10 μg of LTA/ml, and 1 μg of lipid A/ml) for 30 min at 37°C. After incubation, secretions were obtained by the collection of supernatants from crypts deposited by low-speed centrifugation (2, 3), and protein and lysozyme activities were measured (see Materials and Methods). In each panel, the data represent the means of triplicate assays performed for one experiment per panel. Antigens used were as follows. (A) E. coli LPS (55) was from Sigma (bars 1), WT JM83 (bars 2), ΔmsbB JM83 (bars 3), WT H16 (bars 4), and ΔmsbB H16 (bars 5). Also shown are results for control crypts incubated in iPIPES without antigen (bars 6). (B) P. aeruginosa LPS (13) was from WT PAK cells (bars 1), phoPΔ PAK cells (bars 2), clinical isolate CF1188 (bars 3), and clinical isolate Bronch267 (bars 4). Also shown are results for control crypts incubated in iPIPES without antigen (bars 5). (C) LPS was from serovar Typhimurium 14028 variants (57) as follows: phoP(Con) (bars 1), ΔphoP (bars 2), WT (bars 3), WT ΔwaaP (bars 4), phoP(Con) (bars 5), and phoP(Con) ΔwaaP (bars 6). Also shown are results for E. coli LPS (bars 7) and control crypts incubated in iPIPES without antigen (bars 8). (D) Serovar Typhimurium lipid A was from PAK cells grown in a low concentrationof Mg2+ (bars 1), PAK cells grown in a high concentration of Mg2+ (bars 2), phoP(Con) PAK cells (bars 3), ΔphoP PAK cells (bars 4), and Sigma (commercial lipid A; bars 5). Also shown are results for control crypts incubated in iPIPES without antigen (bars 6). (E) Results for L. monocytogenes glycolipid fractions that contain LTA (see Materials and Methods) from Scott A (bars 1), nisin-resistant Scott A strain 700302 (34) (bars 2), clinical isolate strain 10403S (bars 3), strain 10403S grown in a low concentration of Mg2+ (bars 4), and strain 10403S grown in a high concentration of Mg2+ (bars 5) and for control crypts incubated in iPIPES without antigen (bars 6) are shown.
Paneth cell secretion also was measured in response to purified cell surface glycolipids from gram-negative and gram-positive bacteria grown under conditions of phoP activation or repression. Because activity of the phoP/phoQ regulon is associated with resistance to defensins and polymyxin (12, 22), lipid A molecules isolated from WT, phoP(Con), and ΔphoP mutants of serovar Typhimurium grown under low-Mg2+ (phoP-activated) or high-Mg2+ (phoP-repressed) conditions were tested for the ability to elicit Paneth cell secretion. At the concentrations tested, all lipid A molecules stimulated equivalent secretion regardless of acyl chain modifications associated with phoP regulation as judged by the quantity of protein and lysozyme released by Paneth cells (Fig. 2D). Similarly, analyses of LTA-containing glycolipid fractions from L. monocytogenes grown in low and high concentrations of Mg2+ also showed that the extent of Paneth cell secretion was unaffected by modifications associated with phoP activity (Fig. 2E).
Bactericidal activities of induced Paneth cell secretions.
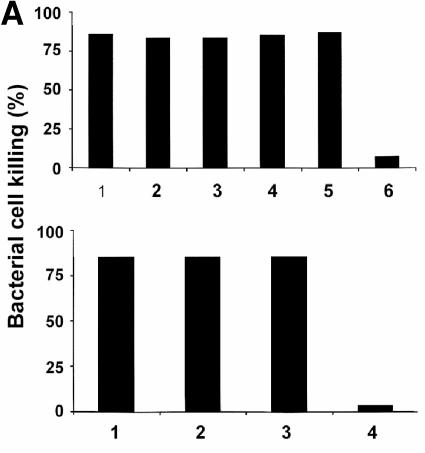
Although measurements of secreted protein and lysozyme supported the view that glycolipid modification did not affect Paneth cell secretory responses, we tested whether secretions induced by different antigens differed in their bactericidal properties. The bactericidal activities of all secretions were measured against defensin-sensitive ΔphoP serovar Typhimurium, and they were not distinguishable (Fig. 3). Samples (10 μl) of Paneth cell secretions induced by LPS from ΔmsbB strains of E. coli had the same bactericidal activities as those elicited by corresponding WT strains (Fig. 3A, upper panel). In addition, secretions elicited by LPS from P. gingivalis, a species recognized for its attenuated proinflammatory activity (4, 7, 10), were indistinguishable from secretions stimulated by LPS from B. forsythus and E. coli (Fig. 3A, lower panel). Similarly, secretions collected from crypts stimulated by serovar Typhimurium LPS had equivalent bactericidal activities, regardless of whether phoP was active or repressed (Fig. 3B).
FIG. 3.
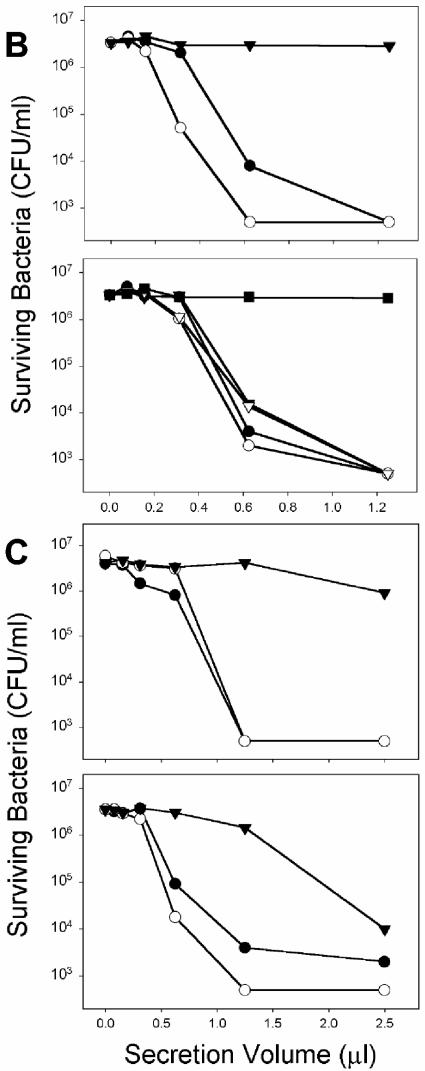
Microbicidal activity secreted from Paneth cells in response to diverse LPS, lipid A, and LTA molecules. (A) Ten-microliter samples collected from crypts exposed to WT and ΔmsbB strains of E. coli JM83 and H16 were combined with 1,000 CFU of ΔphoP serovar Typhimurium cells resuspended in 40 μl of PIPES buffer. After 60 min at 37°C, surviving CFU were quantitated by growth on semisolid media at 37°C overnight as described in the legend to Fig. 1. Groups were normalized by expressing bacterial cell killing as a percentage relative to bacteria incubated for 1 h at 37°C in iPIPES alone. Shown in the upper panel are results for E. coli LPS from Sigma (bar 1), WT JM83 (bar 2), ΔmsbB JM83 (bar 3), WT H16 (bar 4), and ΔmsbB H16 (bar 5). Also shown are results for control crypts incubated in iPIPES without antigen (bar 6). In the lower panel, results are shown for LPS from P. gingivalis (bar 1), B. forsythus (bar 2), and E. coli (Sigma) (bar 3). Also shown are results for control crypts incubated in iPIPES without antigen (bar 4). (B and C) Serial dilutions of supernatants collected from stimulated crypts exposed to purified antigens were assayed for bactericidal activity against ΔphoP serovar Typhimurium cells (see Materials and Methods). The results are representative of duplicate assays performed on separate days. (B) In the upper panel, results for secretions from crypts exposed to Mg2+-precipitated serovar Typhimurium LPS from phoP(Con) (•) and ΔphoP (○) cells and from control crypts incubated in iPIPES without antigen (▾) are shown. In the lower panel, results for secretions from crypts exposed to phenol-extracted serovar Typhimurium LPS from WT (•), WT ΔwaaP (○), phoP(Con) (▾), and phoP(Con) ΔwaaP (▿) cells and from control crypts incubated in iPIPES without antigen (▪) are shown. (C) The upper panel shows results from secretions from crypts exposed to P. aeruginosa lipid A from PAK cells grown in a low concentration of Mg2+ (•) or a high concentration of Mg2+ (○) and from control crypts incubated in iPIPES without antigen (▾). The lower panel shows results from secretions from crypts exposed to L. monocytogenes LTA from WT strain 10403S grown in a low (•) or high (○) concentration of Mg2+ and from control crypts incubated in iPIPES without antigen (▾).
To test whether purified glycolipids with known acyl chain modifications elicit differential Paneth cell secretion, crypts were exposed to lipid A from P. aeruginosa or LTA-containing fractions from L. monocytogenes to induce the release of Paneth cell bactericidal peptide activity (Fig. 3C). Regardless of the Mg2+-mediated status of phoP in the cells from which the glycolipids were isolated, the overall bactericidal activities of the collected secretions were similar to each other and to secretions induced by LPS (Fig. 3B and C). Thus, by these independent criteria, purified LPS and lipid A molecules and LTA-containing fractions induce equivalent levels of secretion from mouse Paneth cells, regardless of the covalent modifications that are associated with virulence in humans or diminished sensitivity to defensins.
Toll-like receptor mRNAs in mouse Paneth cells.
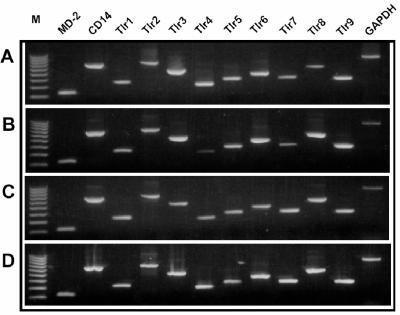
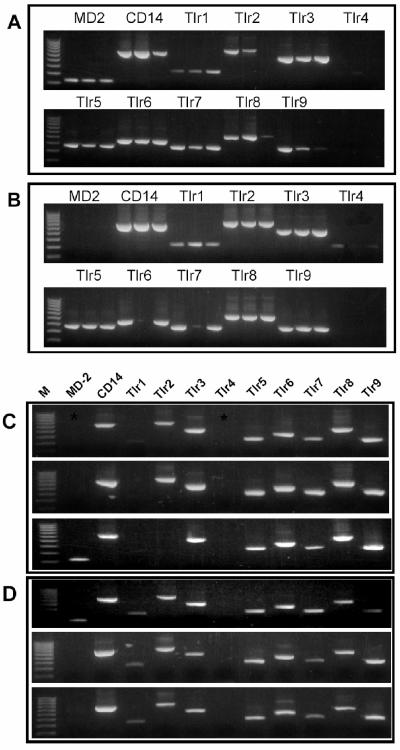
To test for potential mediators of antigen recognition by Paneth cells, the distribution of Toll-like receptor (Tlr) mRNAs was investigated in mouse intestinal epithelial cells. By a nested RT-PCR approach, mRNAs for MD-2, CD14, and Tlr1 to Tlr9 were detected in whole-organ mRNAs from mouse bone marrow, stomach, small bowel, and colon RNAs (Fig. 4), demonstrating that all primer sets supported amplification of the target sequences. Similar nested RT-PCR amplifications of isolated intact crypts and villi showed that MD-2, CD14, and all Tlr mRNAs except Tlr4 were present in isolated crypts. Individual intact villi lacked MD-2 but appeared to express Tlr4, although the cell lineages expressing the sequences remained unknown (Fig. 5A and B). Accordingly, Tlr mRNA was assayed in triplicate pools, each consisting of five individual Paneth cells or villus enterocytes (3). Tlr1 was not found in villus enterocytes, Tlr4 was not detected in either epithelial cell population, and MD-2 was absent from two of the three replicates in each case (Fig. 5C and D). The fact that Tlr mRNAs could be detected only by a nested PCR approach is an indication of their low levels of gene expression in these epithelial cell lineages. Widespread Tlr expression by small intestinal epithelia differs from the expression of the Ca2+-activated K+ channel mIKCa1, which occurs only in Paneth cells and not in villus enterocytes (3). These data support the view that mouse Paneth cells respond to bacteria by Tlr4-independent mechanisms.
FIG. 4.
Toll-like receptor mRNAs in selected adult mouse organs. CD14, MD-2, and Tlr mRNAs were amplified by nested RT-PCR (see Materials and Methods), and samples of amplified products were electrophoresed in 1.5% agarose gels and visualized by ethidium bromide staining. Products were amplified by using the primer sets noted from RNA from adult bone marrow (A), small intestine (B), stomach (C), and colon (D), with the lanes at the left containing a 100-bp DNA ladder. Note that all samples of whole-organ RNA support amplification of all sequences.
FIG. 5.
CD14, MD-2, and Tlr mRNAs in isolated mouse Paneth cells and villus enterocytes. As described in the legend to Fig. 4, MD-2, CD14, and Tlr mRNAs were amplified by nested RT-PCR (see Materials and Methods), and samples of amplified products were electrophoresed in 1.5% agarose gels and visualized by ethidium bromide staining. Products noted in the panels were amplified from single intact crypts (A), single intact villi (B), replicate pools containing five villus enterocytes (C), and replicate pools containing five Paneth cells (3) (D). (A and B) Products of replicate RT-PCR are shown in adjacent lanes; (C and D) replicate reactions are aligned vertically. Lanes at the left contain a 100-bp DNA ladder.
Tlr4 is not required for Paneth cell secretion in response to bacteria.
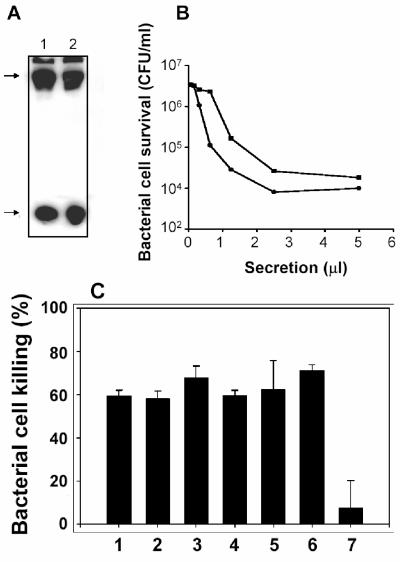
To test whether the absence of Tlr4 gene expression in Paneth cells (Fig. 5D) meant that Tlr4 was not involved in the induction of Paneth cell secretion by cell surface antigens, secretion was tested in the Tlr4-deficient C3H/HeJ mouse strain (5, 43). As Western blot analyses of small intestinal protein extracts from C3H/HeJ and outbred Swiss mice showed, the Tlr4 mutation had no effect on overall cryptdin peptide levels (Fig. 6A). As expected, if secretions were mediated by Toll-like receptors other than Tlr4, C3H/HeJ mouse crypts had normal secretory responses to challenge by exposure to 1,000 CFU of live ΔphoP serovar Typhimurium cells/crypt, as judged by the bactericidal activity of the elicited secretions (Fig. 6B). If Tlr4 were required for mouse Paneth cell secretory responses to pure LPS and lipid A molecules, Paneth cells in C3H/HeJ crypts should not be responsive to those antigens. However, C3H/HeJ mouse crypts released bactericidal peptide activity when exposed to lipid A, and the responses were similar to secretions elicited by varied LPS molecules and LTA (Fig. 6C). Although the mechanisms by which lipid A could induce secretion from Tlr4-null cells remain to be defined, Paneth cell secretion in response to bacteria is independent of Tlr4, consistent with the absence of Tlr4 mRNA in Paneth cells.
FIG. 6.
Tlr4-null Paneth cells from C3H/HeJ mice release bactericidal peptide activity in response to bacterial antigens. (A) Intestinal proteins (≤10 kDa) prepared from outbred Swiss (lane 1) and C3H/HeJ (lane 2) mice by Centricon 30 centrifugal filtration were separated by acid-urea polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane, and probed with anti-cryptdin-1 rabbit serum (2, 3). Arrows at the left denote the presence of immunoreactive procryptdin (upper arrow) and mature cryptdins 1 through 3 and 6 (lower arrow), respectively. (B) Approximately 5,000 crypts were incubated with 103 CFU of ΔphoP serovar Typhimurium cells/crypt for 30 min at 37°C in 500 μl of iPIPES. Samples of collected secretions were assayed for bactericidal activity against 5 × 106 CFU of ΔphoP serovar Typhimurium cells. The release of abundant bactericidal peptide activity by crypts from C3H/HeJ mice is evidence that Paneth cell secretory responses to bacteria are not mediated by Tlr4. (C) Solid bars denote the bactericidal activity of secretions released by C3H/HeJ mouse small intestinal crypts exposed to LPS (100 ng/ml) from P. aeruginosa PAK cells grown in 8 μM MgCl2 (bar 1), LPS (100 ng/ml) from P. aeruginosa ΔphoP PAK cells grown in 8 μM MgCl2 (bar 2), LPS (100 ng/ml) from ΔphoP serovar Typhimurium (bar 3), lipid A (1 μg/ml) from ΔphoP serovar Typhimurium (bar 4), LTA (10 μg/ml) from WT L. monocytogenes Scott A-M (see Materials and Methods) (bar 5), commercially available LPS (Sigma, 100 ng/ml) from serovar Typhimurium (bar 6), and control crypts incubated in iPIPES without antigen (bar 7).
DISCUSSION
In these studies, we tested the hypothesis that mouse Paneth cells would exhibit differential sensitivity to bacterial glycolipids associated with reduced human virulence and greater sensitivity to defensins. The findings show that Paneth cells in intact crypts respond equivalently to all forms of lipid A and LTA tested, suggesting that they recognize common components of these glycolipids rather than secrete differentially in response to particular acyl chain modifications. Because intact crypts were exposed to antigens, we cannot exclude the possibility that the antigens act on crypt epithelial cells, which release mediators that stimulate Paneth cells to release granules. Because secretion is rapid (having been detected within 5 to 15 min following exposure to antigen [2]), we speculate that the mechanism would involve components associated with vesicular fusion mechanisms at the apical cell surface. However, whether antigens interact with Paneth cells directly or at the apical or basolateral surface remains to be established.
Paneth cells release α-defensins and additional secretory granule constituents in response to pharmacologic stimulation and exposure to bacteria and bacterial antigens (2). In mouse small intestinal crypts stimulated with carbamyl choline, the cytosolic calcium dynamics change only in Paneth cells and in a biphasic pattern consistent with the mobilization of intracellular Ca2+ stores, followed by the influx of extracellular Ca2+ (49). Also, the mouse Ca2+-activated K+ channel mIKCa1 modulates mouse Paneth cell secretion, because channel blockade with highly selective mIKCa1 inhibitors diminished Paneth cell secretion in intact crypts by ∼50%. In mouse small intestines, the expression of mIKCa1 is restricted to Paneth cells, identifying the channel as a functional Paneth cell marker that appears to regulate the influx of extracellular Ca2+ essential for sustaining the secretory response. In preliminary studies of LPS-induced secretion from crypts of mice null for the phosphoinositide 3-kinase p85-α subunit (16, 56), defective crypts released bactericidal peptide activity at levels similar to those of isogenic control crypts, suggesting that secretion in response to LPS does not require phosphoinositide 3-kinase (T. Ayabe, D. Fruman, and A. J. Ouellette, unpublished data). Thus, the responses that we have characterized to pure cell surface glycolipids from several bacterial species must initiate increased cytosolic Ca2+, but, despite the exclusion of Tlr4-mediated signaling, those mechanisms remain obscure. Recently, mouse Paneth cells were shown to be immunopositive for Tlr9 (45), immunolocalizing Tlr9 to apical secretory granules. The injection of mice with CpG-containing oligonucleotides downregulated Tlr9 and appeared to stimulate Paneth cell degranulation, although the effects of CpG injection may have been an indirect action rather than a direct action on Paneth cell Tlr9. Also, the finding that C3H/HeJ mouse crypts are responsive to pure LPS and lipid A molecules was unexpected but consistent with the lack of Tlr4 mRNA in Paneth cells and also excludes Tlr4-mediated signaling in Paneth cell responses to lipid A. In mice and humans, the NOD2 cytoplasmic muramyl dipeptide receptor has been associated with susceptibility to Crohn's disease (6, 28, 35, 37), and that pattern recognition receptor is localized specifically in Paneth cells (29, 38). Thus, it is possible that Paneth cells may harbor additional, as yet uncharacterized receptors that may mediate secretory responses to glycolipids. Also, the possibility that glycolipid acyl chains may disrupt Paneth cell membranes or otherwise physically induce vesicular fusion at the apical membrane cannot be excluded.
Paneth cell α-defensin levels have been found to be generally invariant and unresponsive to the presence or absence of the resident microflora (1, 25-27, 41). Oral infection of mice with WT strains of serovar Typhimurium reduces the Paneth cell α-defensin mRNA concentration to approximately 35% of the normal level, and peptide levels also appear to decline. On the other hand, attenuated ΔphoP strains of serovar Typhimurium do not diminish cryptdin mRNA levels (46). The observed effects may be mediated by direct interactions between virulent Salmonella and Paneth cells or indirectly by inducing villus enterocytes or mesenchymal cells to release mediators via a p38-signaling pathway (46). In either case, there appears to be an association between virulence and the effects on α-defensin mRNA levels. That finding is in contrast to the absence of differential effects of glycolipids associated with attenuation or virulence in humans and Paneth cell responses. Thus, our findings appear to exclude the possibility that the selective inhibition of Paneth cell secretion by WT serovar Typhimurium cell surface antigens or phoP-regulated cell envelope modifications contributes to virulence.
Acknowledgments
This study was supported by National Institutes of Health grants AI30479 (S.I.M.), DE12768 (R.P.D.), and DK44632 (A.J.O.).
We thank Michael E. Selsted and Donald P. Satchell for useful discussions.
Editor: F. C. Fang
REFERENCES
- 1.Ayabe, T., D. P. Satchell, P. Pesendorfer, H. Tanabe, C. L. Wilson, S. J. Hagen, and A. J. Ouellette. 2002. Activation of Paneth cell α-defensins in mouse small intestine. J. Biol. Chem. 277:5219-5228. [DOI] [PubMed] [Google Scholar]
- 2.Ayabe, T., D. P. Satchell, C. L. Wilson, W. C. Parks, M. E. Selsted, and A. J. Ouellette. 2000. Secretion of microbicidal α-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 1:113-118. [DOI] [PubMed] [Google Scholar]
- 3.Ayabe, T., H. Wulff, D. Darmoul, M. D. Cahalan, K. G. Chandy, and A. J. Ouellette. 2002. Modulation of mouse Paneth cell α-defensin secretion by mIKCa1, a Ca2+-activated, intermediate conductance potassium channel. J. Biol. Chem. 277:3793-3800. [DOI] [PubMed] [Google Scholar]
- 4.Bainbridge, B. W., and R. P. Darveau. 2001. Porphyromonas gingivalis lipopolysaccharide: an unusual pattern recognition receptor ligand for the innate host defense system. Acta Odontol. Scand. 59:131-138. [DOI] [PubMed] [Google Scholar]
- 5.Beutler, B. 2000. Tlr4: central component of the sole mammalian LPS sensor. Curr. Opin. Immunol. 12:20-26. [DOI] [PubMed] [Google Scholar]
- 6.Cho, J. H. 2001. The Nod2 gene in Crohn's disease: implications for future research into the genetics and immunology of Crohn's disease. Inflamm. Bowel Dis. 7:271-275. [DOI] [PubMed] [Google Scholar]
- 7.Coats, S. R., R. A. Reife, B. W. Bainbridge, T. T. Pham, and R. P. Darveau. 2003. Porphyromonas gingivalis lipopolysaccharide antagonizes Escherichia coli lipopolysaccharide at toll-like receptor 4 in human endothelial cells. Infect. Immun. 71:6799-6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham, M. D., C. Seachord, K. Ratcliffe, B. Bainbridge, A. Aruffo, and R. P. Darveau. 1996. Helicobacter pylori and Porphyromonas gingivalis lipopolysaccharides are poorly transferred to recombinant soluble CD14. Infect. Immun. 64:3601-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darveau, R. P., S. Arbabi, I. Garcia, B. Bainbridge, and R. V. Maier. 2002. Porphyromonas gingivalis lipopolysaccharide is both agonist and antagonist for p38 mitogen-activated protein kinase activation. Infect. Immun. 70:1867-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darveau, R. P., C. M. Belton, R. A. Reife, and R. J. Lamont. 1998. Local chemokine paralysis, a novel pathogenic mechanism for Porphyromonas gingivalis. Infect. Immun. 66:1660-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darveau, R. P., S. MacIntyre, J. T. Buckley, and R. E. Hancock. 1983. Purification and reconstitution in lipid bilayer membranes of an outer membrane, pore-forming protein of Aeromonas salmonicida. J. Bacteriol. 156:1006-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ernst, R. K., T. Guina, and S. I. Miller. 2001. Salmonella typhimurium outer membrane remodeling: role in resistance to host innate immunity. Microbes Infect. 3:1327-1334. [DOI] [PubMed] [Google Scholar]
- 13.Ernst, R. K., E. C. Yi, L. Guo, K. B. Lim, J. L. Burns, M. Hackett, and S. I. Miller. 1999. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science 286:1561-1565. [DOI] [PubMed] [Google Scholar]
- 14.Fields, P. I., E. A. Groisman, and F. Heffron. 1989. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science 243:1059-1062. [DOI] [PubMed] [Google Scholar]
- 15.Folch, J., M. Lees, and G. H. S. Stanley. 1957. A simple method for the isolation and purification of lipids from animal tissue. J. Biol. Chem. 226:497-509. [PubMed] [Google Scholar]
- 16.Fruman, D. A., S. B. Snapper, C. M. Yballe, F. W. Alt, and L. C. Cantley. 1999. Phosphoinositide 3-kinase knockout mice: role of p85alpha in B cell development and proliferation. Biochem. Soc. Trans. 27:624-629. [DOI] [PubMed] [Google Scholar]
- 17.Ganz, T. 2003. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3:710-720. [DOI] [PubMed] [Google Scholar]
- 18.Gorin, G., S. F. Wang, and L. Papapavlou. 1971. Assay of lysozyme by its lytic action on M. lysodeikticus cells. Anal. Biochem. 39:113-127. [DOI] [PubMed] [Google Scholar]
- 19.Gunn, J. S., W. J. Belden, and S. I. Miller. 1998. Identification of PhoP-PhoQ activated genes within a duplicated region of the Salmonella typhimurium chromosome. Microb. Pathog. 25:77-90. [DOI] [PubMed] [Google Scholar]
- 20.Gunn, J. S., R. K. Ernst, A. J. McCoy, and S. I. Miller. 2000. Constitutive mutations of the Salmonella enterica serovar Typhimurium transcriptional virulence regulator phoP. Infect. Immun. 68:3758-3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunn, J. S., S. S. Ryan, J. C. Van Velkinburgh, R. K. Ernst, and S. I. Miller. 2000. Genetic and functional analysis of a PmrA-PmrB-regulated locus necessary for lipopolysaccharide modification, antimicrobial peptide resistance, and oral virulence of Salmonella enterica serovar Typhimurium. Infect. Immun. 68:6139-6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo, L., K. B. Lim, C. M. Poduje, M. Daniel, J. S. Gunn, M. Hackett, and S. I. Miller. 1998. Lipid A acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell 95:189-198. [DOI] [PubMed] [Google Scholar]
- 23.Hill, C. P., J. Yee, M. E. Selsted, and D. Eisenberg. 1991. Crystal structure of defensin HNP-3, an amphiphilic dimer: mechanisms of membrane permeabilization. Science 251:1481-1485. [DOI] [PubMed] [Google Scholar]
- 24.Hirschfeld, M., Y. Ma, J. H. Weis, S. N. Vogel, and J. J. Weis. 2000. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J. Immunol. 165:618-622. [DOI] [PubMed] [Google Scholar]
- 25.Hooper, L. V., P. G. Falk, and J. I. Gordon. 2000. Analyzing the molecular foundations of commensalism in the mouse intestine. Curr. Opin. Microbiol. 3:79-85. [DOI] [PubMed] [Google Scholar]
- 26.Hooper, L. V., and J. I. Gordon. 2001. Commensal host-bacterial relationships in the gut. Science 292:1115-1118. [DOI] [PubMed] [Google Scholar]
- 27.Hooper, L. V., T. S. Stappenbeck, C. V. Hong, and J. I. Gordon. 2003. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat. Immunol. 4:269-273. [DOI] [PubMed] [Google Scholar]
- 28.Hugot, J. P., M. Chamaillard, H. Zouali, S. Lesage, J. P. Cezard, J. Belaiche, S. Almer, C. Tysk, C. A. O'Morain, M. Gassull, V. Binder, Y. Finkel, A. Cortot, R. Modigliani, P. Laurent-Puig, C. Gower-Rousseau, J. Macry, J. F. Colombel, M. Sahbatou, and G. Thomas. 2001. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411:599-603. [DOI] [PubMed] [Google Scholar]
- 29.Lala, S., Y. Ogura, C. Osborne, S. Y. Hor, A. Bromfield, S. Davies, O. Ogunbiyi, G. Nunez, and S. Keshav. 2003. Crohn's disease and the NOD2 gene: a role for Paneth cells. Gastroenterology 125:47-57. [DOI] [PubMed] [Google Scholar]
- 30.Lehrer, R. I., and T. Ganz. 2002. Defensins of vertebrate animals. Curr. Opin. Immunol. 14:96-102. [DOI] [PubMed] [Google Scholar]
- 31.Macfarlane, E. L., A. Kwasnicka, and R. E. Hancock. 2000. Role of Pseudomonas aeruginosa PhoP-PhoQ in resistance to antimicrobial cationic peptides and aminoglycosides. Microbiology 146:2543-2554. [DOI] [PubMed] [Google Scholar]
- 32.Macfarlane, E. L., A. Kwasnicka, M. M. Ochs, and R. E. Hancock. 1999. PhoP-PhoQ homologues in Pseudomonas aeruginosa regulate expression of the outer-membrane protein OprH and polymyxin B resistance. Mol. Microbiol. 34:305-316. [DOI] [PubMed] [Google Scholar]
- 33.Manthey, C. L., P. Y. Perera, B. E. Henricson, T. A. Hamilton, N. Qureshi, and S. N. Vogel. 1994. Endotoxin-induced early gene expression in C3H/HeJ (Lpsd) macrophages. J. Immunol. 153:2653-2663. [PubMed] [Google Scholar]
- 34.Mazzotta, A. S., and T. J. Montville. 1997. Nisin induces changes in membrane fatty acid composition of Listeria monocytogenes nisin-resistant strains at 10 degrees C and 30 degrees C. J. Appl. Microbiol. 82:32-38. [DOI] [PubMed] [Google Scholar]
- 35.McGovern, D. P., D. A. van Heel, T. Ahmad, and D. P. Jewell. 2001. NOD2 (CARD15), the first susceptibility gene for Crohn's disease. Gut 49:752-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller, S. I., W. S. Pulkkinen, M. E. Selsted, and J. J. Mekalanos. 1990. Characterization of defensin resistance phenotypes associated with mutations in the phoP virulence regulon of Salmonella typhimurium. Infect. Immun. 58:3706-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogura, Y., D. K. Bonen, N. Inohara, D. L. Nicolae, F. F. Chen, R. Ramos, H. Britton, T. Moran, R. Karaliuskas, R. H. Duerr, J. P. Achkar, S. R. Brant, T. M. Bayless, B. S. Kirschner, S. B. Hanauer, G. Nunez, and J. H. Cho. 2001. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411:603-606. [DOI] [PubMed] [Google Scholar]
- 38.Ogura, Y., S. Lala, W. Xin, E. Smith, T. A. Dowds, F. F. Chen, E. Zimmermann, M. Tretiakova, J. H. Cho, J. Hart, J. K. Greenson, S. Keshav, and G. Nunez. 2003. Expression of NOD2 in Paneth cells: a possible link to Crohn's ileitis. Gut 52:1591-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ouellette, A. J. 1999. IV. Paneth cell antimicrobial peptides and the biology of the mucosal barrier. Am. J. Physiol. 277:G257-G261. [DOI] [PubMed] [Google Scholar]
- 40.Ouellette, A. J., and C. L. Bevins. 2001. Paneth cell defensins and innate immunity of the small bowel. Inflamm. Bowel Dis. 7:43-50. [DOI] [PubMed] [Google Scholar]
- 41.Ouellette, A. J., R. M. Greco, M. James, D. Frederick, J. Naftilan, and J. T. Fallon. 1989. Developmental regulation of cryptdin, a corticostatin/defensin precursor mRNA in mouse small intestinal crypt epithelium. J. Cell Biol. 108:1687-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peeters, T., and G. Vantrappen. 1975. The Paneth cell: a source of intestinal lysozyme. Gut 16:553-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 44.Qu, X. D., K. C. Lloyd, J. H. Walsh, and R. I. Lehrer. 1996. Secretion of type II phospholipase A2 and cryptdin by rat small intestinal Paneth cells. Infect. Immun. 64:5161-5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rumio, C., D. Besusso, M. Palazzo, S. Selleri, L. Sfondrini, F. Dubini, S. Menard, and A. Balsari. 2004. Degranulation of Paneth cells via toll-like receptor 9. Am. J. Pathol. 165:373-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salzman, N. H., M. M. Chou, H. de Jong, L. Liu, E. M. Porter, and Y. Paterson. 2003. Enteric salmonella infection inhibits Paneth cell antimicrobial peptide expression. Infect. Immun. 71:1109-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Satchell, D. P., T. Sheynis, Y. Shirafuji, S. Kolusheva, A. J. Ouellette, and R. Jelinek. 2003. Interactions of mouse Paneth cell α-defensins and α-defensin precursors with membranes: prosegment inhibition of peptide association with biomimetic membranes. J. Biol. Chem. 278:13838-13846. [DOI] [PubMed] [Google Scholar]
- 48.Satoh, Y. 1988. Effect of live and heat-killed bacteria on the secretory activity of Paneth cells in germ-free mice. Cell Tissue Res. 251:87-93. [DOI] [PubMed] [Google Scholar]
- 49.Satoh, Y., Y. Habara, K. Ono, and T. Kanno. 1995. Carbamylcholine- and catecholamine-induced intracellular calcium dynamics of epithelial cells in mouse ileal crypts. Gastroenterology 108:1345-1356. [DOI] [PubMed] [Google Scholar]
- 50.Satoh, Y., K. Ishikawa, Y. Oomori, S. Takeda, and K. Ono. 1992. Bethanechol and a G-protein activator, NaF/AlCl3, induce secretory response in Paneth cells of mouse intestine. Cell Tissue Res. 269:213-220. [DOI] [PubMed] [Google Scholar]
- 51.Satoh, Y., K. Ishikawa, Y. Oomori, M. Yamano, and K. Ono. 1989. Effects of cholecystokinin and carbamylcholine on Paneth cell secretion in mice: a comparison with pancreatic acinar cells. Anat. Rec. 225:124-132. [DOI] [PubMed] [Google Scholar]
- 52.Schutte, B. C., and P. B. McCray, Jr. 2002. Beta-defensins in lung host defense. Annu. Rev. Physiol. 64:709-748. [DOI] [PubMed] [Google Scholar]
- 53.Selsted, M. E., and S. S. Harwig. 1989. Determination of the disulfide array in the human defensin HNP-2. A covalently cyclized peptide. J. Biol. Chem. 264:4003-4007. [PubMed] [Google Scholar]
- 54.Somerville, J. E., Jr., L. Cassiano, B. Bainbridge, M. D. Cunningham, and R. P. Darveau. 1996. A novel Escherichia coli lipid A mutant that produces an antiinflammatory lipopolysaccharide. J. Clin. Investig. 97:359-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Somerville, J. E., Jr., L. Cassiano, and R. P. Darveau. 1999. Escherichia coli msbB gene as a virulence factor and a therapeutic target. Infect. Immun. 67:6583-6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ueki, K., D. A. Fruman, C. M. Yballe, M. Fasshauer, J. Klein, T. Asano, L. C. Cantley, and C. R. Kahn. 2003. Positive and negative roles of p85 alpha and p85 beta regulatory subunits of phosphoinositide 3-kinase in insulin signaling. J. Biol. Chem. 278:48453-48466. [DOI] [PubMed] [Google Scholar]
- 57.Yethon, J. A., J. S. Gunn, R. K. Ernst, S. I. Miller, L. Laroche, D. Malo, and C. Whitfield. 2000. Salmonella enterica serovar Typhimurium waaP mutants show increased susceptibility to polymyxin and loss of virulence in vivo. Infect. Immun. 68:4485-4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389-395. [DOI] [PubMed] [Google Scholar]
- 59.Zhou, Z., A. A. Ribeiro, S. Lin, R. J. Cotter, S. I. Miller, and C. R. Raetz. 2001. Lipid A modifications in polymyxin-resistant Salmonella typhimurium: pmrA-dependent 4-amino-4-deoxy-l-arabinose, and phosphoethanolamine incorporation. J. Biol. Chem. 276:43111-43121. [DOI] [PubMed] [Google Scholar]