Abstract
YopB is a 401-amino-acid protein that is secreted by a plasmid-encoded type III secretion system in pathogenic Yersinia species. YopB is required for Yersinia spp. to translocate across the host plasma membrane a set of secreted effector proteins that function to counteract immune signaling responses and to induce apoptosis. YopB contains two predicted transmembrane helices (residues 166 to 188 and 228 to 250) that are thought to insert into the host plasma membrane during translocation. YopB is also required for pore formation and host-cell-signaling responses to the type III machinery, and these functions of YopB may also require membrane insertion. To elucidate the importance of membrane insertion for YopB function, YopB proteins containing helix-disrupting double consecutive proline substitutions in the center of each transmembrane domain were constructed. Yersinia pseudotuberculosis strains expressing the mutant YopB proteins were used to infect macrophages or epithelial cells. Effector translocation, pore formation, and host-cell-signaling responses were studied. Introduction of helix-disrupting substitutions into the second transmembrane domain of YopB resulted in a nonfunctional protein that was not secreted by the type III machinery. Introduction of helix-disrupting substitutions into the first transmembrane domain of YopB resulted in a protein that was fully functional for secretion and for interaction with YopD, another component of the translocation machinery. However, the YopB protein with helix-disrupting substitutions in the first transmembrane domain was partially defective for translocation, pore formation, and signaling, suggesting that all three functions of YopB involve insertion into host membrane.
A large number of gram-negative bacteria that cause human diseases utilize type III secretion systems (TTSSs) to export virulence factors (16, 27). Included in this group are Yersinia, Salmonella, Shigella, Escherichia, Bordetella, Chlamydia, and Pseudomonas species (16, 27). TTSSs that secrete virulence effectors are structurally similar to flagellar TTSSs, suggesting that both systems evolved from a common ancestor (8, 48). Virulence-associated TTSSs consist of a multiprotein basal body that spans the inner and outer membranes and periplasm and a rigid needle-like structure that protrudes from the bacterial surface (8).
The three Yersinia species which are pathogenic for humans (Yersinia pestis, Y. pseudotuberculosis, and Y. enterocolitica) harbor a ∼70-kb plasmid which encodes a TTSS (15, 49). This apparatus functions to transfer virulence effectors, also encoded on the 70-kb plasmid, from the interior of the bacterium into the cytosol of the targeted host cell (14). Upon entry into the host cell, these virulence effectors, termed Yops (for “Yersinia outer membrane proteins”), disrupt host-cell-signaling pathways to antagonize phagocytosis, repress cytokine synthesis, and induce apoptosis (30). For example, YopH is a protein tyrosine phosphatase that dephosphorylates several focal adhesion-associated proteins (4, 23, 46). YopE is a GTPase-activating protein that deactivates small GTPases of the Rho family (Rac1, Cdc42, and RhoA) (5, 59). YopT is a cysteine protease that inactivates Rho GTPases by removing a C-terminal prenyl group that is required for membrane anchoring (52). YopJ, a putative protease, binds to multiple mitogen-activated protein kinase (MAPK) kinases and to inhibitor of κB kinase β. YopJ is believed to induce apoptosis by inhibiting activation of the transcription factor NF-κB (1, 42).
Another group of secreted proteins, termed translocators, mediate the transfer of the effectors across the eukaryotic plasma membrane (11). Genetic studies indicate that the proteins YopB, YopD, and LcrV, which are encoded by the lcrGlcrVsycDyopBD operon, are essential translocation factors (18, 22, 32, 38, 40, 41, 47). SycD (also known as LcrH), one of the other products of this operon, functions as a chaperone for YopB and YopD (39). YopK (also known as YopQ), a 183-amino-acid protein encoded outside the lcrGlcrVsycDyopBD operon, appears to regulate the translocation process (26). Holmström et al. (26) have shown that a Y. pseudotuberculosis yopK mutant translocates increased levels of YopE into cultured mammalian cells (a process known as “hypertranslocation”).
The expression, assembly, and activation of the plasmid-encoded TTSS is regulated by several environmental cues (15, 20, 49). A temperature of 37°C stimulates expression of TTSS components and assembly of the apparatus. The effectors, translocators, and even components of the type III apparatus are secreted in abundant amounts in the absence of host cell contact when the bacteria are grown in a calcium-deficient medium at 37°C. However, during infection of cultured mammalian cells with Yersinia spp., the delivery of effectors into the host cell appears to be focused so that little or no effector protein is detected in the extracellular milieu (50), suggesting that the TTSS needle forms a continuous conduit with the translocation machinery. Interestingly, unlike the effectors, the translocators YopB, YopD, and LcrV are detected in the extracellular milieu of infected cells (19, 31, 40). It has been suggested that secreted translocators can function at a distance from the site of bacterial-host cell contact to promote pathogenesis (31). Although there is no evidence that translocators can function at a distance to mediate effector delivery, at least one of these proteins acts as a diffusible immune modulator. LcrV has immunosuppressive activity (10) as a result of its ability to stimulate signaling through toll-like receptor 2 (53).
Several lines of evidence support the idea that the translocators insert into the host cell plasma membrane to form a channel that facilitates the passage of effectors. YopB and YopD contain central hydrophobic domains that are predicted to function as transmembrane helices (21). YopB has two centrally located hydrophobic domains, while YopD has one such region (21). YopB and YopD have been shown to insert into synthetic membranes when bacterial secretion of these proteins was induced by low-calcium conditions in the presence of liposomes (56). Furthermore, insertion of YopB and YopD into synthetic membranes under these conditions was associated with channel formation (56). Purified LcrV has also been shown to have channel-forming activity in synthetic lipid bilayers (25). Yersinia mutants that are defective for the production of certain Yop proteins (e.g., yopE, yopK, or yopN mutants) exhibit pore-forming activity against erythrocytes, epithelial cells, or macrophages in vitro (22, 26, 33, 38, 40, 57). This pore-forming activity of yop mutant Yersinia strains is lost when YopB, YopD, or LcrV is inactivated, indicating that each of these proteins is required for pore formation (22, 25, 33, 38, 57).
YopB, the focus of this study, shares structural similarity with several type III translocation factors found in other bacterial pathogens, including SipB of Salmonella spp. and IpaB of Shigella spp. (27). SipB and IpaB each contain two centrally located hydrophobic domains that function as transmembrane helices (29, 34). SipB and IpaB insert into synthetic membranes with a similar topological orientation (29, 34). Each transmembrane helix spans the bilayer, creating a hairpin structure, with the N and C termini of the proteins exposed on one side of the membrane and the hydrophilic region between the helices exposed on the opposite side (29, 34).
In addition to being required for effector translocation, YopB is required to activate a host-cell-signaling response to the type III secretion machinery (58). YopB-dependent signaling is counteracted by YopE, YopH, or YopJ in epithelial cells infected by the wild-type bacteria. However, infection of epithelial cells with yopE yopH yopJ mutant Y. pseudotuberculosis results in robust activation of MAPKs and production of the cytokine interleukin-8 (IL-8). It has been suggested (58) that activation of this signaling response involves either insertion of YopB into the host plasma membrane or interaction of YopB with a host cell receptor, in a manner analogous to the interaction of IpaB with the hyaluronan receptor CD44 (54).
In this study we have taken a genetic approach to elucidate the importance of membrane insertion for the translocation, pore formation, and host-signaling functions of YopB. We reasoned that by disrupting transmembrane helices, it might be possible to separate the translocation, pore formation, and signaling functions of YopB, since translocation and pore formation are likely to require membrane insertion whereas host signaling may not. We expressed mutant YopB proteins, in which the transmembrane helices were disrupted by amino acid substitutions, in Y. pseudotuberculosis. The ability of these strains to secrete YopB, to translocate effectors, to form pores, and to stimulate signaling in infected host cells was studied. Our results suggest that all functions of YopB, translocation, pore formation, and host signaling, involve membrane insertion.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The Y. pseudotuberculosis strains used in this study (Table 1) are derived from the serogroup 03 Y. pseudotuberculosis strain YP126. Escherichia coli strains used were TUNER(DE3) pLacI (Novagen) for coexpression of YopB with SycD and SM10λpir for the conjugation of expression plasmids into Y. pseudotuberculosis. Y. pseudotuberculosis and E. coli were grown in Luria-Bertani (LB) broth or on LB plates overnight at 26 or 37°C, respectively, and in the presence of antibiotics at standard concentrations when appropriate. Induction of type III protein secretion by Y. pseudotuberculosis strains grown under low-calcium conditions was carried out as previously described (44). Secreted proteins were resolved by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and detected by staining with Coomassie brilliant blue or by immunoblotting as described below. For infection assays, cultures of Y. pseudotuberculosis grown overnight at 26°C were subcultured to an optical density at 600 nm (OD600) of 0.1 in the appropriate medium and grown with shaking as described below. The bacteria were centrifuged (6,000 × g, 5 min, room temperature) and resuspended in warm (37°C) Hank's balanced salt solution (HBSS) (GIBCO) to an OD600 of 1. The bacterial suspension was used to infect cells as described below.
TABLE 1.
Yersinia pseudotuberculosis strains used in this study
| Strain | Relevant characteristic(s) | Figure nomenclature | Source or reference |
|---|---|---|---|
| YP126 | wild type, YPIII (pIB1) | B+ | 9 |
| YP18 | yopB18 (in-frame deletion of nucleotides 496-774) | B18 | 44 |
| YP40 | yopB40 (stop codon and frameshift) | B40 | This work |
| YP36 | yopK | K− | This work |
| YP41 | yopK yopB40 | K−B40 | This work |
| YP6 | yopE | E− | 7 |
| YP29 | yopE yopH yopJ yopB18 | EHJB− | 45 |
| YP43 | yopE yopH yopJ yopB18 yopK | EHJBK− | This work |
| YP37 | yopE yopH yopJ yopO yopK yopM | Not applicable | 58 |
| YP44 | yopE yopH yopJ yopO yopK yopM yopB40 | EHJOKMB− | This work |
Mutant design.
Y. pseudotuberculosis yop mutants were constructed using allelic recombination as previously described (44). To generate the yopB mutant strain, YP40, a DNA fragment encompassing yopB, was amplified using PCR using the virulence plasmid as template and primers V2 (5′CAAATTATTTAAAGATCTCACGAGGTAATTATGCAACAAGAG3′) and B2 (5′GTTAGCACCGAGTTTCTTTGATGCGATGCCGGATTTCTT3′). The DNA fragment was cloned into pET-Blue 2 (Perfectly Blunt cloning kit; Novagen). A mutation (yopB40) corresponding to a stop codon at codon 8 of yopB followed by a frameshift and an XbaI site was generated using a QuikChange mutagenesis kit (Stratagene) and primers BFRAME1 (5′CGTTGATACCCATGTCTAGATCAACGCCAGTAACTGG3′) and BFRAME2 (5′CCAGTTACTGGCGTTGATCTAGACATGGGTATCAACG3′). The mutated DNA fragment was cloned into the pSB890 allelic exchange plasmid (44) by the use of BamHI and BglII sites. The resulting plasmid, pSB890-B40, was introduced into SM10λpir by electroporation, and the yopB40 mutation was introduced into the virulence plasmid in YP126 by allelic exchange. To create the yopK mutant strain YP36, a frameshift mutation was introduced into the yopK gene in YP126 by the use of pLP6 (44). The yopB yopK double mutant, YP41, was generated using pSB890-B40 to introduce the yopB40 mutation into YP36. The yopB40 mutation was introduced into YP37 (58) to create YP44 (yopEHJOMKB). YP43 (yopEHJK) was constructed using pLP6 to introduce the yopK mutation into YP29.
The plasmid pGEX2T-YopB was constructed to serve as a template for site-directed mutagenesis of the transmembrane sequences in yopB. A DNA fragment containing yopB was amplified by PCR using the virulence plasmid as the template and primers B1 (5′GGGATCCCATATGAGTGCGTTGATAACCCATGAC3′) and B4 (5′CGGATTCGAATTCTTAAACAGTATGGGGTCTGCCGG3′). The DNA fragment was inserted into pGEX-2T (Pharmacia) by the use of EcoRI and BamHI restriction sites. Double proline codon substitutions were introduced into pGEX2T-YopB by the use of a QuikChange mutagenesis kit. The yopB1 allele contains proline substitutions at codons 175 and 176. The yopB2 allele contains proline substitutions at codons 239 and 240. Primers for creating yopB1 were BTM1F (5′CGCCATAGCCCCACCGATTGTCGGTGCCATC3′) and BTM1R (5′GATGGCACCGACAATCGGTGGGGCTATGGCG3′). Primers for creating yopB2 were BTM2F (5′GTCGCATTGACTGTACCTCCAACCGTAATGACCTTTGGC3′) and BTM2R (5′GCCAAAGGTCATTACGGTTGGAGGTACAGTCAATGCGAC3′). Mutations were confirmed by DNA sequencing. DNA fragments carrying yopB, yopB1, and yopB2 were inserted into a derivative of the expression plasmid pMMB67HE by the use of NdeI and EcoRI sites as previously described (44). The resulting plasmids, pYopB, pYopBTM1, and pYopBTM2, were introduced into the YP29, YP40, YP41, or YP43 strain by conjugation.
Coexpression of YopB and SycD in E. coli.
A two-plasmid system was used for the coexpression of YopB and SycD in E. coli. The plasmids pYopB, pYopBTM1, and pYopBTM2 (see above) were used for expression of YopB proteins. To construct a plasmid for SycD expression, a DNA fragment encompassing sycD was amplified using PCR with the virulence plasmid as a template and primers YopB5 (5′CTCCTTAAACTTAATCATGGGTTATCAACGCACTCATG3′) and LcrV2 (5′CAAATTATTTAAAGATCTCACGAGGTAATTATGCAACAAGAG3′). The amplified DNA fragment was inserted into pET-Blue2 (Novagen) using a Perfectly Blunt cloning kit (Novagen). The resulting plasmid, pETBlue2-SycD, was digested with BglII and EcoRI, and the DNA fragment carrying sycD was isolated and inserted between the BamHI and EcoRI sites of pET28a (Novagen). Competent TUNER(DE3) pLacI cells were transformed with pET28a-SycD by following the guidelines of the supplier (Novagen). The plasmids pYopB, pYopBTM1, and pYopBTM2 were introduced into TUNER(DE3) pLacI cells by conjugation.
For induction of protein expression, overnight cultures of bacteria were subcultured to an OD600 of 0.1 and grown with 3 h of shaking at 30°C. IPTG (isopropyl-β-d-thiogalactopyranoside) (0.1 mM) was added, and cultures were grown another 2 h. Proteins were extracted from pelleted TUNER(DE3) pLacI cells by the use of Bug Buster reagent (Novagen) supplemented with EDTA-free protease inhibitor cocktail (ROCHE) and benzonase (Novagen). Following 2 min of incubation at room temperature with shaking, an aliquot was removed and this sample was saved as the total cell fraction. The remainder of the lysate was centrifuged (12,000 × g, 20 min, 4°C), and the supernatant was saved as the soluble fraction. The samples were analyzed by anti-YopB immunoblotting as described below.
Production of antibodies to YopB.
The rabbit anti-YopB antibody preparation designated SB452 was prepared in a commercial facility (Cocalico Biologicals, Inc.). A sample of Yop proteins secreted from Y. pseudotuberculosis in low-calcium medium was resolved on an SDS-8% polyacrylamide gel. After staining with Coomassie brilliant blue, a gel fragment containing the YopB protein band was excised. Rabbits were given multiple injections of the emulsified gel fragment to generate antisera against YopB. A sample of the antisera was incubated sequentially with acetone powders of TUNER(DE3) pLacI cells and YP40 cells to remove nonspecific antibodies as previously described (24).
Analysis of secreted YopB and YopD interaction.
Plasmids pHis6-YopB and pHis6-YopBTM1 were constructed as follows. YopB and YopBTM1 were amplified using PCR with pGEX2T-YopB and pGEX2T-YopBTM1, respectively, as templates and primers B1 and B4 (see above). The DNA products were digested with NdeI and EcoRI and ligated between the NdeI and EcoRI sites in pET-28a, creating a fusion between the His6 sequence and the 5′ end of the yopB open reading frame. The resulting plasmids, pET28a-His6YopB and pET28a-His6YopBTM1, were verified by sequencing. pET28a-His6YopB and pET28a-His6YopBTM1 were then digested with XbaI and EcoRI, and the inserts were isolated and inserted between the XbaI and EcoRI sites in pMMB67HE. The resulting plasmids, pHis6-YopB or pHis6-YopBTM1, were introduced into YP44 by conjugation.
Cultures of YP44 carrying pYopB, pHis6-YopB, or pHis6-YopBTM1 were grown and induced for secretion as previously described (44), with the exception that IPTG (0.05 mM final concentration) was added to the cultures at the time of the temperature shift to induce expression of the YopB proteins and the cultures were incubated at 37°C for 4.5 h. Secreted proteins in 20 ml of supernatant were concentrated using a Centriplus centrifugal device (YM-30; Amicon) and conditions provided by the supplier. Proteins were purified from the concentrated supernatant by the use of the small-scale purification batch method with nickel-coated resin and buffers provided in a His-Bind purification kit (Novagen). The concentrated supernatant and all buffers, except the charge buffer, were supplemented with 1% Triton X-100 (Sigma) before purification. Samples of the concentrated supernatant, unbound proteins, and proteins eluted from the resin were resolved by SDS-10% polyacrylamide gel electrophoresis and analyzed by immunoblotting with anti-YopB, anti-YopD, or anti-LcrV antibodies as described below.
Cell culture conditions.
J774A.1 murine macrophage-like cells were maintained as continuous cultures from passage 10 to 25. HeLa cells were maintained as continuous cultures from passage 76 to 90. Cells were seeded into wells of tissue culture plates 1 day prior to infection. J774 cells were seeded at 6.8 × 105 cells per well in a 6-well plate for the translocation assay and at 1 × 105 cells per well in a 24-well plate for the lactate dehydrogenase (LDH) release and immunofluorescence assays. HeLa cells were seeded onto 60-mm-diameter tissue culture dishes at 6 × 105 cells per well for the extracellular signal-regulated kinase (ERK) activation assay and at 1 × 105 cells per dish in a 24-well plate for the IL-8 assay. The culture medium used for both cell types was Dulbecco's modified Eagle's medium (GIBCO) supplemented with heat-inactivated 10% fetal bovine serum (HyClone) and 1 mM sodium pyruvate (GIBCO). Cells were incubated overnight at 37°C in a humidified incubator containing 5% CO2, infected with bacteria, and returned to the incubator for the time period specified below.
Translocation assay.
Bacteria were grown in LB broth containing 2.5 mM CaCl2 for 2 h at 37°C and used to infect J774A.1 cells at a multiplicity of infection (MOI) of 50. The plates were centrifuged (100 × g, 1 min, room temperature) to facilitate bacterial contact with macrophages and then placed at 37°C in a 5% CO2 incubator for 2 h. The tissue culture plate was placed on ice, the medium was removed, and the infected cells were washed twice with ice-cold HBSS containing 10 mM NaF and 1 mM NaVO4. To each well 0.5 ml of 1% Triton X-100 lysis buffer (1% [vol/vol] Triton X-100, 0.01 M Tris [pH 7.6], 0.15 M NaCl, 10% glycerol) containing EDTA-free protease inhibitor cocktail (ROCHE) was added. After 15 min on ice, the cells were scraped from the plates, and the soluble and insoluble fractions of the lysates were separated by centrifugation (12,000 × g, 10 min, 4°C). The insoluble pellets were washed once with lysis buffer. The soluble protein concentrations were calibrated using a protein assay (Bio-Rad) and normalized by adjusting volumes with lysis buffer. The protein samples were analyzed using immunoblotting as described below.
ERK activation assay.
At 30 min prior to infection the HeLa cell culture medium was aspirated from each well and replaced with warm (37°C) Dulbecco modified Eagle medium lacking serum. In some cases the medium was supplemented with IPTG at a concentration of 0.1 mM. The cells were infected as described for the LDH assay for 1 h. The dishes were then placed on ice and washed three times with ice-cold phosphate-buffered saline containing 1 mM Na3VO4 and 10 mM NaF. The cells were incubated in 0.5 ml of cold 1% Triton X-100 lysis buffer containing 1 mM Na3VO4 and 10 mM NaF for 15 min on ice. The cell lysates were scraped into microcentrifuge tubes and centrifuged (12,000 × g, 10 min, 4°C). The supernatants were collected, and samples were analyzed by α-Phospho-ERK and α-ERK immunoblotting.
Electrophoresis and immunoblotting.
Protein samples were boiled for 5 min in Laemmli sample buffer containing 0.1 M 1,4-dithiothreitol (DTT) prior to electrophoresis (24). Proteins were separated on SDS-10% polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes for immunoblot analysis. The membranes were blocked in Tris-buffered saline (24) containing 0.05% Tween 20 (TBST) and 1% bovine serum albumin for the Yop detection or TBST containing 5% nonfat milk for ERK detection. YopB was detected with a rabbit polyclonal antibody (provided by O. Schneewind, University of California at Los Angeles) diluted 1:1,000 in TBST or with the SB452 antibody (see above) diluted 1:20,000 in TBST. Rabbit polyclonal antibodies specific for YopD (60) and LcrV (40) have been described previously and were used at dilutions of 1:20,000 and 1:5000, respectively. YopE was detected with rabbit polyclonal antibodies (5) diluted 1:1,000 in TBST. Src-kinase-associated protein (SKAP) was detected with rabbit polyclonal anti-SKAP antibody diluted 1:10,000 in TBST (6). ERK was detected with a phospho-p44/42 MAPK (α-P-ERK) antibody or a p44/42 MAPK (α-ERK) antibody under conditions specified by the manufacturer (Cell Signaling Technology). Secondary horseradish peroxidase-conjugated anti-rabbit antibodies (Jackson Immunoresearch Laboratories) were diluted 1:10,000 in TBST for Yop detection or in TBST containing 5% nonfat milk for ERK detection. Immunoblots were developed by chemiluminescence (Perkin Elmer).
Densitometry.
Densitometry was carried out using a Bio-Rad GS-710 imaging densitometer. Bands on films were quantified as trace quantities in units of OD and diameter in millimeters. These values were normalized to the signals of loading controls (SKAP for the translocation assay and total ERK for the ERK activation assays). For ERK activation assays, the signals from the different ERK isoforms (p44/p42) within each lane were summed.
Immunofluorescence microscopy.
J774A.1 cells seeded on glass coverslips were infected as described for the translocation assay. The cells were processed for immunofluorescence microscopy using rabbit anti-YopE antibodies and fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG as previously described (5). Phase-contrast and epifluorescence microscopy was performed using a Zeiss AxioPlan 2 microscope equipped with appropriate filters and a 40× (NA 0.75) objective. Images were captured with a Diagnostic Instruments Spot digital camera and processed using Adobe Photoshop 5.5. To quantify the YopE signal in infected J774 cells, the green-pixel intensity of each cell was measured as follows. The area of each cell was defined using the circular marque tool in Adobe Photoshop (version 5.5). The size of the defined region was held constant for all measurements. The histogram function in Adobe Photoshop was used to measure the average brightness value within the defined region. For YP41/pYopB infections, images of 78 cells were evaluated. For YP41/pBTM1 infections, images of 112 cells were evaluated.
LDH release assay.
Bacteria were grown in LB broth containing 20 mM MgCl2 and 20 mM C2O4Na2 at 26°C for 1 h and at 37°C for 2 h. J774A.1 cells were infected at an MOI of 50 for 2 h. Gentamicin was then added (100 μg/ml final concentration), and the incubation was then continued for an additional 3 h. HeLa cells were infected at an MOI of 100 for 3 h. Where indicated, IPTG was present at 0.1 mM to increase YopB expression. Uninfected cells were lysed by a freeze-thaw cycle to obtain the total lactate dehydrogenase (LDH) values. Culture medium from infections and controls were collected and centrifuged (12,000 × g, 10 min, 4°C) to eliminate bacteria and cellular debris. Aliquots from each supernatant sample were transferred to a 96-well plate and analyzed for LDH by the use of a CytoTox96 nonradioactive cytotoxicity assay kit (Promega) and an MRX microplate reader (DYNA TECH Laboratories). OD values for each condition were averaged, and percent LDH release was calculated as follows: (LDH released during infection/total LDH) × 100.
IL-8 assay.
Bacteria were grown as described for the LDH release assay and used to infect HeLa cells at an MOI of 100 for 1 h. The medium was then removed and replaced with fresh medium containing gentamicin (100 μg/ml), and the incubation was continued an additional 4 h. The infection medium was collected and centrifuged (12,000 × g, 10 min, 4°C), and duplicate samples of each supernatant were analyzed for IL-8 by enzyme-linked immunosorbent assay (ELISA) (Antigenix America).
Sequence analysis of YopB.
The primary sequence of YopB was entered into the online search engine TMHMM version 2 (www.cbs.dtu.dk/services/TMHMM/) to identify transmembrane regions. The data for the location of coiled-coil domains in YopB were derived from a primary article (43).
Statistical analysis.
Statistical analysis of data was performed by one-way analysis of variance (ANOVA) using InStat 2.01. The Tukey-Kramer multiple-comparisons test was used to determine significance in comparisons of individual treatments. P values of <0.05 were considered significant.
RESULTS
YopB functions in cis to mediate YopE translocation by wild-type and yopK mutant Y. pseudotuberculosis.
YopB is predicted from sequence analysis to contain two transmembrane helices (21) and two coiled-coil domains (43) (Fig. 1). The primary goal of this study was to investigate the importance of the transmembrane helices for YopB function. Before proceeding with this analysis, initial studies were carried out to better characterize the role of YopB in the translocation process. YopB is required for Y. pseudotuberculosis to translocate YopE into HeLa cells during infection (22, 41). We confirmed that YopB is required for translocation of YopE into macrophages. J774A.1 cells were infected with a wild-type Y. pseudotuberculosis strain (YP126) or with an isogenic mutant (YP40) (Table 1), which contains a stop codon at codon 8 of yopB, followed by a frameshift mutation. The infected cells were analyzed for YopE translocation by a detergent solubility assay (Materials and Methods). Samples of the detergent-soluble fractions (containing translocated protein) and the detergent-insoluble fractions (containing bacterium-associated protein) were analyzed for the presence of YopE by immunoblotting (Fig. 2). YopE was translocated into cells infected with the wild-type strain but not into cells infected with the yopB mutant (Fig. 2A; compare lanes 2 and 3). Similar amounts of bacterial-associated YopE were detected in the insoluble fractions of the cells infected with either strain (Fig. 2A, lanes 7 and 8).
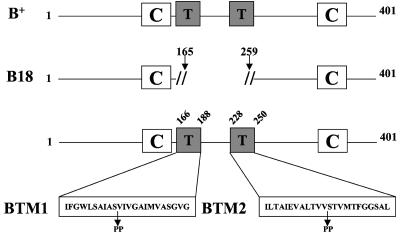
FIG. 1.
Schematic diagram of YopB primary structure and mutant design. The structure of wild-type YopB protein (B+) is indicated at the top. Boxes represent structural elements, corresponding to predicted transmembrane domains (T) and coiled-coil (C) regions. The middle structure represents YopB18 (B18), which results from an in-frame deletion of amino acids 166 to 258, removing both transmembrane domains. The lower structure shows the transmembrane domains expanded to single-letter code, and the positions of double proline substitutions are indicated. YopBTM1 (BTM1) contains prolines substituted for serine 175 and valine 176. YopBTM2 (BTM2) contains prolines substituted for valine 239 and serine 240.
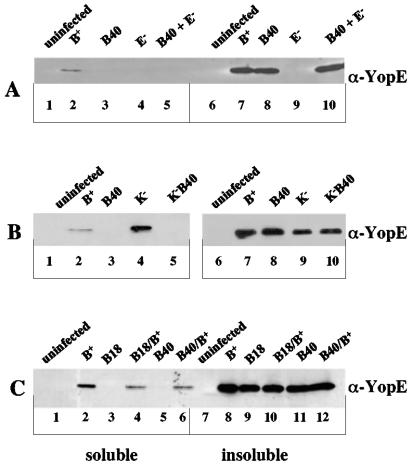
FIG. 2.
Analysis of YopE translocation by detergent solubility assays. J774A.1 cells were left uninfected or were infected with the indicated Y. pseudotuberculosis strains for 2 h. YopE translocation was assayed using detergent solubility. Samples of soluble proteins and insoluble proteins were analyzed by anti-YopE immunoblotting. Bands detected in the soluble fractions represent translocated YopE. (A) YopB is required in cis for YopE translocation. J774A.1 cells were infected with YP126 (B+; lanes 2 and 7), YP40 (B40; lanes 3 and 8), YP6 (E−; lanes 4 and 9), or YP40 and YP6 (B40 + E−; lanes 5 and 10). The final multiplicity of infection for the mixed infection was 100. (B) YopB is required for YopE translocation into macrophages infected with wild-type or yopK Y. pseudotuberculosis. J774A.1 cells were infected with YP126 (B+; lanes 2 and 7), YP40 (B40; lanes 3 and 8), YP36 (K−; lanes 4 and 9), or YP41 (K−B40; lanes 5 and 10). (C) Complementation analysis of yopB mutants. J774A.1 cells were infected with YP126 (B+; lanes 2 and 8), YP18 (B18; lanes 3 and 9), YP18/pYopB (B18/B+; lanes 4 and 10), YP40 (B40; lanes 5 and 11), or YP40/pYopB (B40/B+; lanes 6 and 12).
YopB is secreted into the extracellular milieu during infection (31), suggesting that YopB secreted by one bacterium could function to translocate YopE secreted by another bacterium. To test whether YopB can function in trans to promote translocation, we carried out a trans-complementation experiment in which we coinfected J774A.1 cells with the yopB mutant YP40 and the yopE mutant YP6 (Table 1). Translocation of YopE was not detected when the cells were coinfected (Fig. 2A, lane 5). We also analyzed the infected cells for the cell-rounding activity of YopE by phase-contrast microscopy. Rounding was observed in cells infected with wild-type Y. pseudotuberculosis but not in cells infected with the yopB mutant or the yopE mutant or in cells coinfected with both strains (data not shown). We conclude that the translocator, YopB, and the effector, YopE, must be produced by the same bacterium for translocation of the effector to occur.
Håkansson et al. (22) have demonstrated that YopB is required for pore formation by yopK mutant Y. pseudotuberculosis, but it has not specifically been shown that YopB is required for the hypertranslocation phenotype. To determine whether hypertranslocation is YopB dependent, J774A.1 cells were infected with a yopK mutant (YP36) or a yopK yopB double mutant (YP41) (Table 1), and YopE translocation was assayed as before. As shown in Fig. 2B, YopE was translocated at high levels into cells infected with the yopK mutant (compare lanes 2 and 4), while no such translocation occurred in cells infected with the yopK yopB double mutant (lane 5). Thus, hypertranslocation requires YopB.
To confirm that the mutation introduced into yopB in YP40 is nonpolar, the plasmid pYopB carrying wild-type yopB under control of the tac promoter was introduced into YP40. YopB is produced constitutively at a low level from this plasmid in the absence of the inducer (IPTG). The resulting strain (YP40/pYopB) was used to infect macrophages, and YopE translocation was assayed as before. YopE was translocated when YopB was expressed in YP40 (Fig. 2C; compare lanes 5 and 6). Taken together, these results indicate that YopB is required in cis for YopE translocation into macrophages infected with wild-type or yopK mutant Y. pseudotuberculosis.
Mutation of predicted transmembrane helices in YopB.
The Y. pseudotuberculosis strain YP18 (Table 1) results from an in-frame deletion in yopB that removes codons 166 to 258 and both transmembrane domains of the protein (Fig. 1) (44). As shown previously (45), removal of both transmembrane domains in YopB results in loss of translocation activity (Fig. 2C, lane 3). Complementation analysis confirmed that the yopB18 allele is a nonpolar mutation (Fig. 2C, lane 4).
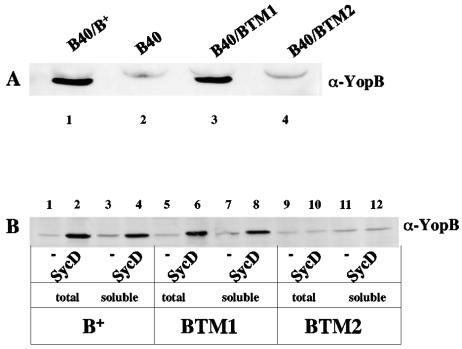
The functional importance of the individual transmembrane domains in YopB was investigated by the insertion of double proline substitutions near the center of each 23-amino-acid helix. The altered YopB designated YopBTM1 has substitutions S175P and V176P, and YopBTM2 has substitutions V239P and S240P (Fig. 1). The proline's rigid conformation, due to the bonding of the last atom of its side chain to the main chain, produces kinks in α-helices. The distortion created by a double consecutive proline substitution decreases, but does not fully abolish, the ability of model hydrophobic peptides to form transmembrane structures (12). We first investigated whether the double proline substitutions affected the stability or secretion of YopB. Plasmids that express YopBTM1 or YopBTM2 under control of the tac promoter were constructed and introduced into the yopB mutant YP40. The supernatants from cultures of YP40/pYopB, YP40/pYopBTM1, and YP40/pYopBTM2 grown under low-calcium conditions were analyzed by anti-YopB immunoblotting. YopB and YopBTM1 were secreted under these conditions, while secretion of YopBTM2 could not be detected (Fig. 3A; compare lanes 1, 3, and 4). To determine why YopBTM2 was not secreted, the bacterial pellets of low-calcium-induced cultures were analyzed by anti-YopB immunoblotting. Wild-type YopB and YopBTM1 were detected in the bacterial pellets, while YopBTM2 was not detected (data not shown), suggesting that YopBTM2 is unstable when expressed in Y. pseudotuberculosis.
FIG. 3.
Analysis of YopB proteins containing double consecutive proline substitutions in transmembrane domain 1 or 2. (A) Immunoblot analysis of YopB, YopBTM1, and YopBTM2 secreted by Y. pseudotuberculosis under low-calcium conditions. YP40/pYopB (B40/B+; lane 1), YP40 (B40; lane 2), YP40/pYopBTM1 (B40/BTM1; lane 3), and YP40/pYopBTM2 (B40/BTM2; lane 4) were grown under low-calcium conditions in the absence of IPTG. Cultures were centrifuged, and proteins secreted into the supernatants were analyzed by anti-YopB immunoblotting. (B) Immunoblot analysis of YopB, YopBTM1, and YopBTM2 expressed in E. coli in the presence or absence of SycD. TUNER(DE3) pLacI cells transformed with pYopB (B+), pYopBTM1 (BTM1), or pYopBTM2 (BTM2) alone or with pET28a-SycD (+SycD) were grown to log phase, induced with IPTG, and lysed with detergent. Samples of total bacterial cell lysate (lanes 1, 2, 5, 6, 9, and 10) or soluble fractions (lanes 3, 4, 7, 8, 11, and 12) were analyzed by immunoblotting with anti-YopB antibody.
Neyt and Cornelis (39) have shown that steady-state levels of YopB are very low in a sycD mutant background, suggesting that interaction with SycD is important for YopB stability in Yersinia spp. In addition, ectopic coexpression of SycD with YopB increases YopB stability in E. coli (39). YopB, YopBTM1, and YopBTM2 were produced in E. coli, with or without coexpression of SycD. As shown by immunoblotting results, steady-state levels of YopB and YopBTM1 increased in the presence of SycD, indicating that these proteins interact with the chaperone (Fig. 3B, lanes 1 to 8). However, the steady-state level of YopBTM2 remained low in the presence of SycD, suggesting that this protein is unable to bind SycD.
Role of transmembrane domain 1 in YopD interaction.
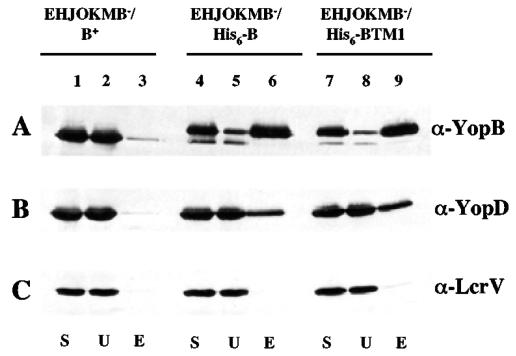
YopB has been shown to interact with YopD in crude extracts of E. coli and in the Yersinia cytoplasm (39). In addition, it is believed that after secretion, YopB and YopD interact to form a translocation channel in the host cell membrane. We developed an assay for detecting interaction of secreted YopB and YopD to determine whether the double proline substitutions in transmembrane domain 1 interfere with this interaction. Plasmids that express YopB or YopBTM1 proteins with six additional N-terminal His residues (His6) were constructed. These plasmids were introduced into the multi-yop mutant strain YP44 (yopEHJOKMB; Table 1). Cultures of YP44/pHis6-YopB and YP44/pHis6-YopBTM1 were grown in the presence of IPTG to induce His6-YopB and His6-YopBTM1 expression and in the absence of calcium to induce type III protein secretion. YP44 expressing YopB without the His6 sequence (YP44/pYopB) was cultured in parallel as a control. Supernatants of the bacterial cultures were concentrated and subjected to small-scale affinity chromatography using a Ni2+-coated resin (Materials and Methods). The results of the purification were analyzed by immunoblotting with antibodies specific for YopB, YopD, or LcrV. As shown in Fig. 4A, YopB, His6-YopB, and His6-YopBTM1 were secreted into the bacterial growth medium at similar levels (lanes 1, 4, and 7), showing that the presence of the N-terminal His6 sequence did not interfere with secretion of His6-YopB or His6-YopBTM1. Both YopB proteins with N-terminal His6 sequences were selectively adsorbed to and eluted from the resin (Fig. 4B, lanes 2, 3, 5, 6, 8, and 9). In addition, similar amounts of YopD copurified with His6-YopB and His6-YopBTM1 (Fig. 4B, lanes 6 and 9), suggesting that the presence of the double proline substitution in transmembrane domain 1 did not interfere with YopD interaction. Interestingly, LcrV did not copurify with His6-YopB or His6-YopBTM1 (Fig. 4C), suggesting that LcrV does not interact with YopB or YopD under these conditions.
FIG. 4.
Disruption of transmembrane domain 1 in YopB does not prevent YopD interaction. Immunoblot analysis of proteins secreted by Y. pseudotuberculosis and affinity purification of His6-YopB and His6-YopBTM1. YP44/pYopB (EHJOKMB−/B+; lanes 1 to 3), YP44/pHis6-YopB (EHJOKMB−/His6-B; lanes 4 to 6), and YP44/pHis6-YopBTM1 (EHJOKMB−/His6-BTM1; lanes 7 to 9) were grown under low-calcium conditions in the presence of IPTG. Cultures were centrifuged, and concentrated culture supernatants were subjected to affinity chromatography using Ni2+-coated resin. Samples of starting material (S), unbound protein (U), and eluted protein (E) were analyzed by immunoblotting with anti-YopB (A), anti-YopD (B), or anti-LcrV (C) antibodies.
Role of transmembrane domain 1 in translocation.
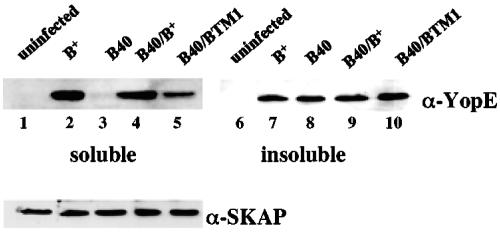
J774A.1 cells were infected with YP126, YP40/pYopB, or YP40/pYopBTM1, and translocation of YopE was assayed as before to determine whether disruption of transmembrane domain 1 affects the translocation function of YopB. YopE was translocated into J774A.1 cells by YP40/pYopBTM1 at a level that was reduced (∼4-fold) compared to that seen with the control strains (Fig. 5; compare lanes 2, 4, and 5). We next carried out the translocation assay with yopK mutant strains to determine whether YopBTM1 was defective for hypertranslocation. Less YopE was translocated into cells infected with YP41/pYopBTM1 than into cells infected with YP41/pYopB (data not shown), suggesting that disruption of the first transmembrane helix in YopB affects hypertranslocation.
FIG. 5.
Disruption of transmembrane domain 1 of YopB is associated with decreased YopE translocation by Y. pseudotuberculosis. J774A.1 cells were left uninfected (lanes 1 and 6) or were infected for 2 h with strain YP126 (B+; lanes 2 and 7), YP40 (B40; lanes 3 and 8), YP40/pYopB (B40/B+; lanes 4 and 9), or YP40/pYopBTM1 (B40/BTM1; lanes 5 and 10). The infected cells were lysed with detergent, and samples of the soluble and insoluble fractions were analyzed by anti-YopE immunoblotting. Bands in the soluble fraction represent translocated YopE. Densitometric analysis of the bands in lanes 4 and 5 indicated that the signal in lane 5 is 25% of the signal in lane 4. The blot containing soluble samples was stripped and analyzed with anti-SKAP antibody as a loading control (bottom panel).
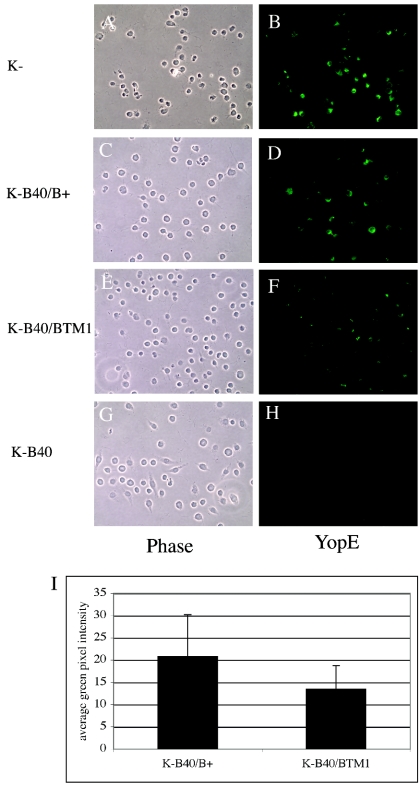
To confirm the results of the detergent solubility assay, immunofluorescence microscopy was used to examine the amount of YopE translocated into J774A.1 cells by different yopK mutant Y. pseudotuberculosis strains. J774A.1 cells were infected with the yopK mutant strain (YP36), the yopK yopB double-mutant strain (YP41), or YP41 carrying pYopB or pYopBTM1. After 2 h, the infected cells were fixed, permeabilized, and labeled with anti-YopE antibody and a FITC-conjugated secondary antibody. The labeled cells were viewed by phase and fluorescence microscopy. Strong anti-YopE staining was observed in cells infected with the yopK mutant, while only background signal was seen in the cells infected with the yopK yopB double mutant (Fig. 6), confirming that YopB is required for YopE translocation in the yopK mutant background. In addition, a higher (∼2-fold) level of anti-YopE staining was seen in cells infected with YP41/pYopB than in cells infected with YP41/pYopBTM1 (Fig. 6I), again suggesting that disruption of transmembrane domain 1 in YopB decreases the efficiency of YopE translocation.
FIG. 6.
Analysis of YopE translocation into J774 cells by immunofluorescence microscopy. J774A.1 cells were infected for 2 h with indicated strains of Y. pseudotuberculosis. The infected cells were fixed, permeabilized, and incubated sequentially with primary rabbit anti-YopE antibody and FITC-conjugated anti-rabbit secondary antibody. (A to H) Samples were viewed using phase-contrast (left panels) or epifluorescence (right panels) microscopy under a 40× objective. Representative images were captured by digital photography. (I) Quantification of the YopE signal in cells infected with YP41/pYopB (K−B40/B+) or YP41/pYopBTM1 (K−B40/BTM1). The green-pixel intensity, representing translocated YopE, was measured for each cell in multiple images and averaged. Means and standard deviations of the values obtained are plotted.
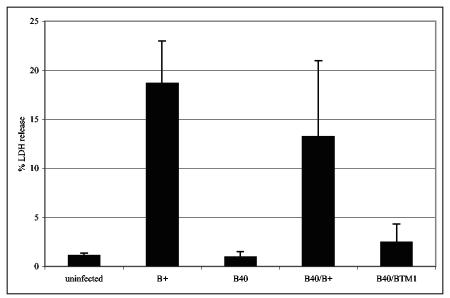
To further characterize the role of YopB membrane insertion for effector translocation, translocation of YopJ into macrophages was measured by its ability to induce apoptosis. J774 cells were infected with various Y. pseudotuberculosis strains, and apoptosis was measured by an LDH release assay (Materials and Methods). LDH release reflects cell lysis that occurs in the late stages of apoptosis. As shown previously (36), very little LDH was detected in the supernatants of uninfected cells or cells infected with a yopJ mutant (1 to 2% of total LDH) (Fig. 7 and data not shown). LDH release increased significantly when the macrophages were infected with YP126 or YP40/pYopB but not when they were infected with YP40 or YP40/pYopBTM1 (Fig. 7), suggesting that the first transmembrane helix in YopB is important for translocation of YopJ into macrophages.
FIG. 7.
Disruption of transmembrane domain 1 is associated with decreased apoptosis. J774A.1 cells were left uninfected or were infected with Y. pseudotuberculosis strain YP126 (B+), YP40 (B40), YP40/pYopB (B40/B+), or YP40/pYopBTM1 (B40/BTM1) for a total of 5 h. Apoptosis was measured by LDH release. Percentages of LDH released into culture supernatants were calculated by dividing the amount of LDH released from infected cells by the amount of LDH released from uninfected cells that were lysed by a freeze-thaw cycle. Means and standard deviations of the values obtained from four different infections performed in two independent experiments are plotted. The difference between B40/B+ and B40/BTM1 results was statistically significant (P < 0.05; ANOVA).
Role of transmembrane domain 1 in pore formation.
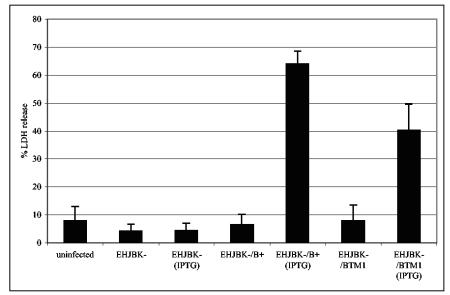
To determine whether disruption of transmembrane domain 1 affects the pore-forming activity of YopB, HeLa cells were infected with a multiple yop mutant (yopEHJBK) strain of Y. pseudotuberculosis (YP43) (Table 1), and pore-induced osmotic lysis was measured by an LDH release assay. HeLa cells were used for this assay, because they do not undergo apoptosis upon Yersinia infection (51); therefore, any membrane damage we observed could be specifically attributed to pore formation. Infections were carried out with YP43, YP43/pYopB, or YP43/pYopBTM1 for 3 h, and the release of LDH was quantified (Materials and Methods). Low levels of LDH were present in the supernatants of uninfected cells or cells infected with YP43 (Fig. 8). Interestingly, LDH release resulting from pore formation was observed when the cells were infected with YP43/pYopB in the presence of IPTG but not in the absence of IPTG (Fig. 8), suggesting that high-level expression of YopB from the pYopB vector was required for pore formation. In addition, release of LDH when HeLa cells were infected with YP43/pYopBTM1 was reduced ∼2-fold relative to YP43/pYopB results in the presence of IPTG (Fig. 8), suggesting that membrane insertion is important for YopB-dependent pore-forming activity.
FIG. 8.
Disruption of transmembrane domain 1 of YopB is associated with decreased pore formation. HeLa cells were left uninfected or were infected with the multiple yop mutant Y. pseudotuberculosis strain YP43 (EHJBK−), YP43/pYopB (EHJBK−/B+), or YP43/pYopBTM1 (EHJBK−/BTM1) for 3 h in the presence of IPTG. LDH released from HeLa cells was quantitated using a colorimetric assay. Percent LDH release was calculated by dividing the units of LDH in the medium of infected cells by total LDH units in uninfected HeLa cells and multiplying by 100. The means and standard deviations (error bars) of values obtained from four different infections in two independent experiments are shown. The results are representative of two independent experiments. The difference between the results obtained for YP43/pYopB (IPTG) and YP43/pYopBTM1 (IPTG) was statistically significant (P < 0.001, ANOVA).
Role of YopB membrane insertion for host signaling.
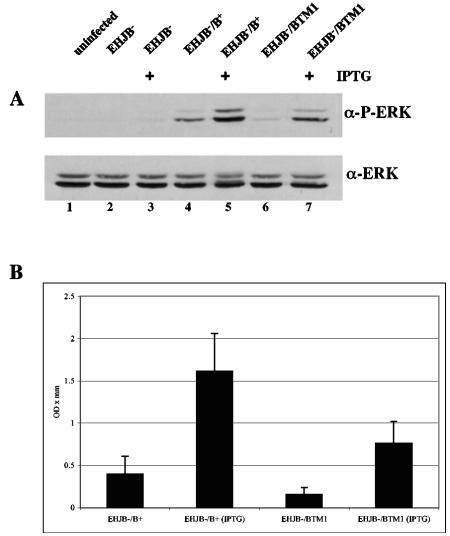
Activation of the MAPK ERK in infected HeLa cells was measured to determine whether membrane insertion is important for YopB-dependent activation of host signaling (Materials and Methods). HeLa cells were infected with YP29 (yopEHJB mutant) (Table 1), YP29/pYopB, or YP29/pYopBTM1, and lysates of the infected cells were analyzed by immunoblotting to detect activated ERK (phospho-ERK) or total ERK. Some infections were carried out in the presence of IPTG to increase expression of YopB. P-ERK was not detected in uninfected cells or in cells infected with YP29 (Fig. 9A, lanes 1 to 3). However, P-ERK was detected in cells infected with YP40/pYopB (lane 4), and increased levels of P-ERK were seen in the presence of IPTG (lane 5). P-ERK was also detected in cells infected with YP29/pYopBTM1 (lanes 6 and 7), although at lower levels than those seen in YP40/pYopB-infected cells. Densitometric analysis of the data from four independent experiments showed that ERK activation was ∼2-fold lower in cells infected with YP40/pYopBTM1than in cells infected with YP40/pYopB (Fig. 9B).
FIG. 9.
Disruption of transmembrane domain 1 is associated with decreased ERK activation. (A) HeLa cells were left uninfected (lane 1) or were infected with Y. pseudotuberculosis strain YP29 (EHJB−; lanes 2 and 3), YP29/pYopB (EHJB−/B+; lanes 4 and 5), or YP29/pYopBTM1 (EHJB−/BTM1; lanes 6 and 7) for 1 h in the presence (+) or absence of IPTG. Cells were lysed, and samples of the soluble fractions were analyzed by anti-P-ERK immunoblotting (top) or anti-ERK immunoblotting (bottom). (B) Densitometric analysis was used to compare the signals in lanes 4 to 7 of four experimentally independent blots. Band intensities (expressed in OD values and diameters in millimeters) for P-ERK were normalized to total ERK results and plotted as means with standard deviations. The difference between the results obtained with EHJB−/B+ (IPTG) and EHJB−/BTM1 (IPTG) was statistically significant (P < 0.01; ANOVA).
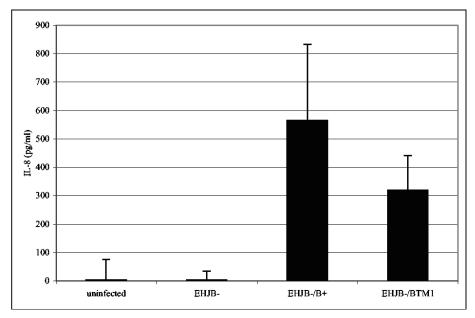
To determine whether membrane insertion of YopB is important for IL-8 production, HeLa cells were infected with YP29, YP29/pYopB, or YP29/pYopBTM1 in the absence of IPTG, and IL-8 release was measured by ELISA (Materials and Methods). As shown in Fig. 10 very little IL-8 was released from uninfected cells or cells infected with YP29, whereas cells infected with YP29/pYopB released high levels of IL-8. Cells infected with YP29/pYopBTM1 also released IL-8 but at a significantly lower (twofold) level than cells infected with YP29/pYopB, suggesting that membrane insertion is important for the host-cell-signaling function of YopB.
FIG. 10.
Disruption of transmembrane domain 1 is associated with reduced IL-8 release. HeLa cells were left uninfected or were infected with Y. pseudotuberculosis strain YP29 (EHJB−), YP29/pYopB (EHJB−/B+), or YP29/pYopBTM1 (EHJB−/BTM1) for a total of 5 h. Culture supernatants from seven different infections were analyzed for the presence of IL-8 by an ELISA performed for three independent experiments. Means and standard deviations are plotted. The difference between the results obtained with EHJB−/B+ and EHJB−/BTM1 was significant (P < 0.05; ANOVA).
DISCUSSION
This study was undertaken to investigate the role of the transmembrane domains in YopB for translocation, pore formation, and host signaling. Using nonpolar yopB mutants of Y. pseudotuberculosis we first showed that YopB is required in cis to promote translocation of YopE into J774A.1 cells (Fig. 2). Therefore, YopB that is secreted into the extracellular milieu during cell culture infection (19, 31) is not able to facilitate effector translocation at a distance. Although these results do not rule out the possibility that YopB could act as a diffusible factor to promote pathogenesis (31), they are consistent with the idea that YopB must remain in the vicinity of the needle from which it is secreted to promote efficient effector translocation. Perhaps a high local concentration of YopB or continuous physical contact with the needle is required for translocation.
Our data also show that YopB is required for hypertranslocation of effectors by a Y. pseudotuberculosis yopK mutant (26) (Fig. 2). While YopB and the other translocators have homologues in several other pathogen-associated TTSSs, YopK is unique to Yersinia spp. YopK does not share significant similarity at the primary sequence level with any protein in the GenBank database. Using the yeast two-hybrid system we have been unable to detect a direct interaction between YopK and YopB (data not shown). Therefore, how YopK functions to modulate the translocation mechanism remains to be determined (26).
YopB is structurally and functionally similar to SipB and IpaB. It is therefore likely that the hydrophobic domains in YopB insert into host membranes in a hairpin conformation (29, 34). The Y. pseudotuberculosis yopB18 allele produces a variant of YopB lacking both transmembrane domains, and strains carrying this mutation are defective for translocation (45) (Fig. 2) and pore formation (57). To investigate the importance of the individual transmembrane domains in YopB, we introduced amino acid substitutions into each predicted helix with the aim of disrupting stable insertion into host membrane. Proline is somewhat unfavorable for membrane insertion, probably because its structure leaves an unsatisfied backbone H-bond. Introduction of a proline into a transmembrane helix also tends to introduce a kink, which shortens the effective length of the hydrophobic sequence and can make it more difficult for short-to-medium length sequences to span the hydrophobic core of the bilayer. Nevertheless, while not abundant, individual proline residues are not rare in transmembrane helices. On the other hand, studies of hydrophobic polyleucine helices showed that two consecutive proline residues strongly destabilize transmembrane insertion relative to a state in which the helix locates at the membrane surface (12). It should be noted that even a double consecutive proline substitution did not fully disrupt transmembrane insertion of hydrophobic helices in model membranes (12). Introduction of such substitutions into transmembrane sequences may be more useful than introduction of substitutions that completely disrupt transmembrane insertion (for example, introduction of charged double consecutive lysines) (12). Mild substitutions are more likely to induce only local conformational perturbations, while severe mutations might alter global protein folding and thus make it difficult to specify function of a specific membrane-inserting segment. Therefore, a double consecutive proline substitution was chosen to disrupt the transmembrane domains in YopB.
The mutant proteins containing the double consecutive proline substitutions were initially tested for SycD binding, type III secretion, and YopD interaction. YopBTM1, containing a double consecutive proline substitution in the first transmembrane domain, was similar to wild-type YopB in terms of its ability to bind SycD, to be secreted, and to bind YopD (Fig. 3 and 4). Introduction of a double consecutive proline insertion into the second transmembrane domain of YopB resulted in a protein that appeared to be unstable in Y. pseudotuberculosis and was not secreted (Fig. 3). Thus, although our strategy of using consecutive proline substitutions to disrupt transmembrane domains was devised to avoid complete disruption of protein function, this strategy clearly failed in the case of transmembrane domain 2. In this context, it is somewhat surprising that removal of both transmembrane domains of YopB has a different outcome. YopB lacking both transmembrane domains retains the ability to bind SycD and is stable in Yersinia spp. (39) (unpublished data). It is possible that the double proline substitution in transmembrane domain 2 disrupts a global conformation in YopB that is important for SycD binding. An alternative hypothesis that cannot be excluded is that YopBTM2 does bind SycD but that the resulting complex is susceptible to proteolysis in situ. In either case, the results suggest that overall structural stability of YopB is highly sensitive to perturbations in transmembrane domain 2.
Infection of macrophages with yopK+ or yopK mutant Y. pseudotuberculosis strains producing YopB or YopBTM1 showed that YopBTM1 is partially defective for effector translocation (Fig. 5 to 7) and pore formation (Fig. 8). This likely reflects a requirement for YopB insertion to form translocation channels and pores. We have shown that Yersinia-induced pore formation in epithelial cells requires YopB and is inhibited by the catalytic activity of YopE (57). YopE prevents pore formation by blocking host cell actin polymerization (57), suggesting that host signaling and YopB insertion are required for pore formation. It is possible that YopB insertion activates a signaling or stress response leading to the conversion of translocation channels into pores, or alternatively, that host signaling leads to opening of host-derived pores. Interestingly, high expression of YopB was required to observe pore formation, while translocation and host-signaling activities could be detected under conditions of lower YopB expression. This dependence on YopB levels may indicate that a large number of pores need to be generated before osmotic lysis can occur or that a high concentration of YopB must be reached to trigger pore formation.
IpaB interacts with the CD44 receptor, and this interaction is thought to play a role in activation of early signaling events required for Shigella entry into epithelial cells (54). Interaction of IpaB with CD44 does not require membrane insertion of IpaB (54). We found that YopBTM1 is partially defective for signaling (Fig. 9 and 10), suggesting that membrane insertion of YopB is important for signaling and making it is less likely that binding of YopB to a receptor is responsible for signaling. This conclusion assumes that disruption of transmembrane domain 1 does not alter the interaction of YopB with a receptor. The fact that signaling, but not translocation, can occur in the absence of YopD (58, 60) argues against the possibility that decreased signaling by YopBTM1 results from decreased translocation of an unknown effector that is responsible for signaling. Therefore, the data are consistent with the idea that YopB is directly responsible for signaling and that membrane insertion of YopB is important to activate the signaling response in the host cell.
It is important to point out that although residues 166 to 188 are predicted to form a transmembrane helix, a sequence immediately following 166 to 188 (residues 189 to 208) is relatively hydrophobic as well (as are the corresponding sequences in its relatives IpaB and SipB). This means that residues 166 to 208 in YopB have the potential to form a transmembrane helix 43 residues in length. In fact, this region is long enough to form a two-helix hairpin by itself (37). However, data showing that the corresponding region of SipB (residues 320 to 353) forms a single transmembrane helix (34) makes formation of a hairpin by residues 166 to 208 in YopB unlikely.
There are several possible consequences of having a very long hydrophobic sequence. One is that the entire hydrophobic sequence is inserted in the form of a highly tilted transmembrane sequence. Another is that the transmembrane segment is derived from a relatively untilted 20-residue portion of the hydrophobic sequence and that the rest of the hydrophobic sequence protrudes from the membrane. In any case, the identity of the residues that penetrate the bilayer may depend on the functional state of the protein. For Tar and related bacterial chemoreceptors a shift between two states in which different residues in a transmembrane helix are membrane embedded has been found to be important for function (3, 13, 17, 28, 35). In the case of Neu receptor tyrosine kinase a valine-to-glutamic acid mutation within a transmembrane segment induces a shift in which residues are buried in the bilayer and alters function (55). In the case of a human integrin, a functionally important switch of a transmembrane helix between a tilted and untilted state has been proposed (2). Thus, the double consecutive proline substitution at residues 175 and 176 of YopB might create a situation in which only residues 177 to 208 of the first hydrophobic region can form a stable transmembrane segment, resulting in partial impairment of YopB function. Further studies of the structure and function of membrane-inserted YopB will be needed to examine this possibility.
Acknowledgments
This research was funded by Public Health Service grants RO1 AI43389 from the National Institute for Allergy and Infectious Diseases (NIAID) (to J.B.B.) and U54 AI57158 from the Northeast Biodefense Center and the NIAID (to J.B.B.). E. L. was supported by GM31986. M.B.R. was supported in part by a training grant (T32 AI07539) from the NIAID. M.B.R. and H.C. were also supported in part by Howard Hughes Medical Institute Summer Research Scholar Awards.
We thank O. Schneewind for providing the antibody to Y. enterocolitica YopB, Sue Straley for antibodies to YopD and LcrV, and members of the Bliska laboratory for reviewing the manuscript.
Editor: J. T. Barbieri
REFERENCES
- 1.Aepfelbacher, M., R. Zumbihl, K. Ruckdeschel, C. A. Jacobi, C. Barz, and J. Heesemann. 1999. The tranquilizing injection of Yersinia proteins: a pathogen's strategy to resist host defense. Biol. Chem. 380:795-802. [DOI] [PubMed] [Google Scholar]
- 2.Armulik, A., I. Nilsson, G. von Heijne, and S. Johansson. 1999. Determination of the border between the transmembrane and cytoplasmic domains of human integrin subunits. J. Biol. Chem. 274:37030-37034. [DOI] [PubMed] [Google Scholar]
- 3.Beel, B. D., and G. L. Hazelbauer. 2001. Signalling substitutions in the periplasmic domain of chemoreceptor Trg induce or reduce helical sliding in the transmembrane domain. Mol. Microbiol. 40:824-834. [DOI] [PubMed] [Google Scholar]
- 4.Black, D. S., and J. B. Bliska. 1997. Identification of p130Cas as a substrate of Yersinia YopH (Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. EMBO J. 16:2730-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black, D. S., and J. B. Bliska. 2000. The RhoGAP activity of the Yersinia pseudotuberculosis cytotoxin YopE is required for antiphagocytic function and virulence. Mol. Microbiol. 37:515-527. [DOI] [PubMed] [Google Scholar]
- 6.Black, D. S., A. Marie-Cardine, B. Schraven, and J. B. Bliska. 2000. The Yersinia tyrosine phosphatase YopH targets a novel adhesion-regulated signalling complex in macrophages. Cell. Microbiol. 2:401-414. [DOI] [PubMed] [Google Scholar]
- 7.Bliska, J. B., M. C. Copass, and S. Falkow. 1993. The Yersinia pseudotuberculosis adhesin YadA mediates intimate bacterial attachment to and entry into HEp-2 cells. Infect. Immun. 61:3914-3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blocker, A., K. Komoriya, and S. Aizawa. 2003. Type III secretion systems and bacterial flagella: insights into their function from structural similarities. Proc. Natl. Acad. Sci. USA 100:3027-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolin, I., L. Norlander, and H. Wolf-Watz. 1982. Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infect. Immun. 37:506-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brubaker, R. R. 2003. Interleukin-10 and inhibition of innate immunity to yersiniae: roles of Yops and LcrV (V antigen). Infect. Immun. 71:3673-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buttner, D., and U. Bonas. 2002. Port of entry—the type III secretion translocon. Trends Microbiol. 10:186-192. [DOI] [PubMed] [Google Scholar]
- 12.Caputo, G. A., and E. London. 2003. Cumulative effects of amino acid substitutions and hydrophobic mismatch upon the transmembrane stability and conformation of hydrophobic alpha-helices. Biochemistry 42:3275-3285. [DOI] [PubMed] [Google Scholar]
- 13.Chervitz, S. A., and J. J. Falke. 1995. Lock on/off disulfides identify the transmembrane signaling helix of the aspartate receptor. J. Biol. Chem. 270:24043-24053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornelis, G. R. 2002. Yersinia type III secretion: send in the effectors. J. Cell Biol. 158:401-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornelis, G. R. 2002. The Yersinia Ysc-Yop “type III” weaponry. Nat. Rev. Mol. Cell Biol. 3:742-752. [DOI] [PubMed] [Google Scholar]
- 16.Cornelis, G. R., and F. Van Gijsegem. 2000. Assembly and function of type III secretory systems. Annu. Rev. Microbiol. 54:735-774. [DOI] [PubMed] [Google Scholar]
- 17.Falke, J. J., and G. L. Hazelbauer. 2001. Transmembrane signaling in bacterial chemoreceptorsa. Trends Biochem. Sci. 26:257-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fields, K. A., M. L. Nilles, C. Cowan, and S. C. Straley. 1999. Virulence role of V antigen of Yersinia pestis at the bacterial surface. Infect. Immun. 67:5395-5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fields, K. A., and S. C. Straley. 1999. LcrV of Yersinia pestis enters infected eukaryotic cells by a virulence plasmid-independent mechanism. Infect. Immun. 67:4801-4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Francis, M. S., H. Wolf-Watz, and A. Forsberg. 2002. Regulation of type III secretion systems. Curr. Opin. Microbiol. 5:166-172. [DOI] [PubMed] [Google Scholar]
- 21.Hakansson, S., T. Bergman, J. Vanooteghem, G. Cornelis, and H. Wolf-Watz. 1993. YopB and YopD constitute a novel class of Yersinia Yop proteins. Infect. Immun. 61:71-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hakansson, S., K. Schesser, C. Persson, E. E. Galyov, R. Rosqvist, F. Homble, and H. Wolf-Watz. 1996. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact-dependent membrane disrupting activity. EMBO J. 15:5812-5823. [PMC free article] [PubMed] [Google Scholar]
- 23.Hamid, N., K. Gustavsson, K. Anderson, K. McGee, C. Persson, C. E. Rudd, and M. Fallman. 1999. YopH dephosphorylates Cas and Fyn-binding protein in macrophages. Microb. Pathog. 27:231-242. [DOI] [PubMed] [Google Scholar]
- 24.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 25.Holmstrom, A., J. Olsson, P. Cherepanov, E. Maier, R. Nordfelth, J. Pettersson, R. Benz, H. Wolf-Watz, and A. Forsberg. 2001. LcrV is a channel size-determining component of the Yop effector translocon of Yersinia. Mol. Microbiol. 39:620-632. [DOI] [PubMed] [Google Scholar]
- 26.Holmstrom, A., J. Pettersson, R. Rosqvist, S. Hakansson, F. Tafazoli, M. Fallman, K.-E. Magnusson, H. Wolf-Watz, and A. Forsberg. 1997. YopK of Yersinia pseudotuberculosis controls translocation of Yop effectors across the eukaryotic cell membrane. Mol. Microbiol. 24:73-91. [DOI] [PubMed] [Google Scholar]
- 27.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hughson, A. G., and G. L. Hazelbauer. 1996. Detecting the conformational change of transmembrane signaling in a bacterial chemoreceptor by measuring effects on disulfide cross-linking in vivo. Proc. Natl. Acad. Sci. USA 93:11546-11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hume, P. J., E. J. McGhie, R. D. Hayward, and V. Koronakis. 2003. The purified Shigella IpaB and Salmonella SipB translocators share biochemical properties and membrane topology. Mol. Microbiol. 49:425-439. [DOI] [PubMed] [Google Scholar]
- 30.Juris, S. J., F. Shao, and J. E. Dixon. 2002. Yersinia effectors target mammalian signalling pathways. Cell. Microbiol. 4:201-211. [DOI] [PubMed] [Google Scholar]
- 31.Lee, V. T., and O. Schneewind. 1999. Type III machines of pathogenic yersiniae secrete virulence factors into the extracellular milieu. Mol. Microbiol. 31:1619-1629. [DOI] [PubMed] [Google Scholar]
- 32.Lee, V. T., C. Tam, and O. Schneewind. 2000. LcrV, a substrate for Yersinia enterocolitica type III secretion, is required for toxin targeting into the cytosol of HeLa cells. J. Biol. Chem. 275:36869-36875. [DOI] [PubMed] [Google Scholar]
- 33.Marenne, M. N., L. Journet, L. J. Mota, and G. R. Cornelis. 2003. Genetic analysis of the formation of the Ysc-Yop translocation pore in macrophages by Yersinia enterocolitica: role of LcrV, YscF and YopN. Microb. Pathog. 35:243-258. [DOI] [PubMed] [Google Scholar]
- 34.McGhie, E. J., P. J. Hume, R. D. Hayward, J. Torres, and V. Koronakis. 2002. Topology of the Salmonella invasion protein SipB in a model bilayer. Mol. Microbiol. 44:1309-1321. [DOI] [PubMed] [Google Scholar]
- 35.Miller, A. S., and J. J. Falke. 2004. Side chains at the membrane-water interface modulate the signaling state of a transmembrane receptor. Biochemistry 43:1763-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monack, D. M., J. Mecsas, N. Ghori, and S. Falkow. 1997. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc. Natl. Acad. Sci. USA 94:10385-10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monne, M., I. Nilsson, A. Elofsson, and G. von Heijne. 1999. Turns in transmembrane helices: determination of the minimal length of a “helical hairpin” and derivation of a fine-grained turn propensity scale. J. Mol. Biol. 293:807-814. [DOI] [PubMed] [Google Scholar]
- 38.Neyt, C., and G. Cornelis. 1999. Insertion of a Yop translocation pore into the macrophage plasma membrane by Yersinia enterocolitica: requirement for translocators YopB and YopD, but not LcrG. Mol. Microbiol. 33:971-981. [DOI] [PubMed] [Google Scholar]
- 39.Neyt, C., and G. Cornelis. 1999. Role of SycD, the chaperone of the Yersinia Yop translocators YopB and YopD. Mol. Microbiol. 31:143-156. [DOI] [PubMed] [Google Scholar]
- 40.Nilles, M. L., K. A. Fields, and S. C. Straley. 1998. The V antigen of Yersinia pestis regulates Yop vectorial targeting as well as Yop secretion through its effects on YopB and LcrG. J. Bacteriol. 180:3410-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nordfelth, R., and H. Wolf-Watz. 2001. YopB of Yersinia enterocolitica is essential for YopE translocation. Infect. Immun. 69:3516-3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orth, K. 2002. Function of the Yersinia effector YopJ. Curr. Opin. Microbiol. 5:38-43. [DOI] [PubMed] [Google Scholar]
- 43.Pallen, M. J., G. Dougan, and G. Frankel. 1997. Coiled-coiled domains in proteins secreted by type III secretion systems. Mol. Microbiol. 25:423-425. [DOI] [PubMed] [Google Scholar]
- 44.Palmer, L. E., S. Hobbie, J. E. Galan, and J. B. Bliska. 1998. YopJ of Yersinia pseudotuberculosis is required for the inhibition of macrophage TNFα production and the downregulation of the MAP kinases p38 and JNK. Mol. Microbiol. 27:953-965. [DOI] [PubMed] [Google Scholar]
- 45.Palmer, L. E., A. R. Pancetti, S. Greenberg, and J. B. Bliska. 1999. YopJ of Yersinia spp. is sufficient to cause downregulation of multiple mitogen-activated protein kinases in eukaryotic cells. Infect. Immun. 67:708-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Persson, C., N. Carballeira, H. Wolf-Watz, and M. Fallman. 1997. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130Cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesions. EMBO J. 16:2307-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pettersson, J., A. Holmstrom, J. Hill, S. Leary, E. Frithz-Lindsten, A. von Euler-Matell, E. Carlsson, R. Titball, A. Forsberg, and H. Wolf-Watz. 1999. The V-antigen of Yersinia is surface exposed before target cell contact and involved in virulence protein translocation. Mol. Microbiol. 32:961-976. [DOI] [PubMed] [Google Scholar]
- 48.Plano, G. V., J. B. Day, and F. Ferracci. 2001. Type III export: new uses for an old pathway. Mol. Microbiol. 40:284-293. [DOI] [PubMed] [Google Scholar]
- 49.Ramamurthi, K. S., and O. Schneewind. 2002. Type III protein secretion in Yersinia species. Annu. Rev. Cell Dev. Biol. 18:107-133. [DOI] [PubMed] [Google Scholar]
- 50.Rosqvist, R., K.-E. Magnusson, and H. Wolf-Watz. 1994. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 13:964-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruckdeschel, K., S. Harb, A. Roggenkamp, M. Hornef, R. Zumbihl, S. Kohler, J. Heesemann, and B. Rouot. 1998. Yersinia enterocolitica impairs activation of transcription factor NF-κB: involvement in the induction of programmed cell death and in the suppression of the macrophage tumor necrosis factor α production. J. Exp. Med. 187:1069-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shao, F., P. O. Vacratsis, Z. Bao, K. E. Bowers, C. A. Fierke, and J. E. Dixon. 2003. Biochemical characterization of the Yersinia YopT protease: cleavage site and recognition elements in Rho GTPases. Proc. Natl. Acad. Sci. USA 100:904-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sing, A., D. Rost, N. Tvardovskaia, A. Roggenkamp, A. Wiedemann, C. J. Kirschning, M. Aepfelbacher, and J. Heesemann. 2002. Yersinia V-antigen exploits toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J. Exp. Med. 196:1017-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skoudy, A., J. Mounier, A. Aruffo, H. Ohayon, P. Gounon, P. Sansonetti, and G. Tran Van Nhieu. 2000. CD44 binds to the Shigella IpaB protein and participates in bacterial invasion of epithelial cells. Cell. Microbiol. 2:19-33. [DOI] [PubMed] [Google Scholar]
- 55.Smith, S. O., C. S. Smith, and B. J. Bormann. 1996. Strong hydrogen bonding interactions involving a buried glutamic acid in the transmembrane sequence of the neu/erbB-2 receptor. Nat. Struct. Biol. 3:252-258. [DOI] [PubMed] [Google Scholar]
- 56.Tardy, F., F. Homble, C. Neyt, R. Wattiez, G. R. Cornelis, J. M. Ruysschaert, and V. Cabiaux. 1999. Yersinia enterocolitica type III secretion-translocation system: channel formation by secreted Yops. EMBO J. 18:6793-6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Viboud, G. I., and J. B. Bliska. 2001. A bacterial type III secretion system inhibits actin polymerization to prevent pore formation in host cell membranes. EMBO J. 20:5373-5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Viboud, G. I., S. S. So, M. B. Ryndak, and J. B. Bliska. 2003. Proinflammatory signalling stimulated by the type III translocation factor YopB is counteracted by multiple effectors in epithelial cells infected with Yersinia pseudotuberculosis. Mol. Microbiol. 47:1305-1315. [DOI] [PubMed] [Google Scholar]
- 59.Von Pawel-Rammingen, U., M. V. Telepnev, G. Schmidt, K. Aktories, H. Wolf-Watz, and R. Rosqvist. 2000. GAP activity of the Yersinia YopE cytotoxin specifically targets the Rho pathway: a mechanism for disruption of actin microfilament structure. Mol. Microbiol. 36:737-748. [DOI] [PubMed] [Google Scholar]
- 60.Williams, A. W., and S. C. Straley. 1998. YopD of Yersinia pestis plays a role in negative regulation of the low-calcium response in addition to its role in translocation of Yops. J. Bacteriol. 180:350-358. [DOI] [PMC free article] [PubMed] [Google Scholar]