Abstract
Background
Myocardial ischemia–reperfusion injury (MIRI) is widespread in the treatment of ischemic heart disease, and its treatment options are currently limited. Adiponectin (APN) is an adipocytokine with cardioprotective properties; however, the mechanisms of APN in MIRI are unclear. Therefore, based on preclinical (animal model) evidence, the cardioprotective effects of APN and the underlying mechanisms were explored.
Methods
The literature was searched for the protective effect of APN on MIRI in six databases until 16 November 2023, and data were extracted according to selection criteria. The outcomes were the size of the myocardial necrosis area and hemodynamics. Markers of oxidation, apoptosis, and inflammation were secondary outcome indicators. The quality evaluation was performed using the animal study evaluation scale recommended by the Systematic Review Center for Laboratory animal Experimentation statement. Stata/MP 14.0 software was used for the summary analysis.
Results
In total, 20 papers with 426 animals were included in this study. The pooled analysis revealed that APN significantly reduced myocardial infarct size [weighted mean difference (WMD) = 16.67 (95% confidence interval (CI) = 13.18 to 20.16, P < 0.001)] and improved hemodynamics compared to the MIRI group [Left ventricular end-diastolic pressure: WMD = 5.96 (95% CI = 4.23 to 7.70, P < 0.001); + dP/dtmax: WMD = 1393.59 (95% CI = 972.57 to 1814.60, P < 0.001); -dP/dtmax: WMD = 850.06 (95% CI = 541.22 to 1158.90, P < 0.001); Left ventricular ejection fraction: WMD = 9.96 (95% CI = 7.29 to 12.63, P < 0.001)]. Apoptosis indicators [caspase-3: standardized mean difference (SMD) = 3.86 (95% CI = 2.97 to 4.76, P < 0.001); TUNEL-positive cells: WMD = 13.10 (95% CI = 8.15 to 18.05, P < 0.001)], inflammatory factor levels [TNF-α: SMD = 4.23 (95% CI = 2.48 to 5.98, P < 0.001)], oxidative stress indicators [Superoxide production: SMD = 4.53 (95% CI = 2.39 to 6.67, P < 0.001)], and lactate dehydrogenase levels [SMD = 2.82 (95% CI = 1.60 to 4.04, P < 0.001)] were significantly reduced. However, the superoxide dismutase content was significantly increased [SMD = 1.91 (95% CI = 1.17 to 2.65, P < 0.001)].
Conclusion
APN protects against MIRI via anti-inflammatory, antiapoptotic, and antioxidant effects, and this effect is achieved by activating different signaling pathways.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-024-02028-w.
Keywords: Adiponectin, Myocardial ischemia–reperfusion injury, Apoptosis, Oxidation, Inflammation, Animals
Introduction
Ischemic heart disease (IDH) is a common cardiovascular disease that places substantial burdens on society [1]. Timely restoration of blood flow in the ischemic area is crucial to restore oxygen and nutrient supply, which is important for saving the damaged myocardium. The most effective therapeutic strategies are coronary artery bypass grafting and percutaneous coronary intervention [2]. However, this leads to disturbances in myocardial function and electrical activity, structural damage, and exacerbation of myocardial necrosis, a phenomenon termed myocardial ischemia–reperfusion injury (MIRI) [3, 4]. Treatment for MIRI is scarce, and the pathogenesis is complex. Although the exact mechanisms are not fully understood, research has indicated a potential link among oxidative stress, apoptosis, inflammation, and their mechanisms of action [5–7].
Adiponectin (APN) is an adipokine responsible for regulating the metabolism of glucose and lipids [8]. APN also has anti-inflammatory, antithrombotic, antioxidative stress, and antiatherosclerotic properties [9, 10]; it may also be involved in myocardial protection against MIRI. Braun et al. found that APN knockout mice have more severe myocardial infarctions after ischemia–reperfusion than wild-type mice [11]. Kim et al. demonstrated that the high incidence of ischemic heart disease in the diabetic population is strongly associated with a decrease in APN levels [12]. These studies suggest that APN has cardioprotective effects against MIRI.
To date, few clinical studies have revealed the protective effect of APN on MIRI, and most of the findings are limited to animal studies. Animal models have limitations in that they cannot reproduce the complex pathophysiological processes in humans, and conclusions are generally drawn from relatively small independent samples, which can be biased. Nevertheless, animal experiments are necessary, and a well-designed meta-analysis can provide compelling evidence while minimizing bias. Therefore, the present study hypothesized that APN may mitigate MIRI. A meta-analysis of relevant studies and summarizing possible mechanisms will accelerate the translation to clinical studies and provide a basis for MIRI treatment.
Methods
The present study adhered to the requirements of the Cochrane Handbook Systematic Reviews of Interventions (version 6.2) and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement [13] (Supplementary File 1). This study was registered in the Prospective Registry for Systematic Reviews platform (PROSPERO), where any modifications can be found (ID: CRD42023433357).
Literature search
A search of Web of Science, EmBase, SinoMed, PubMed, CNKI, and WanFang databases was used to collect the relevant literature on the beneficial effect of APN on MIRI without limitation of the year, language, type of article, or research object. The search time was from the creation of the database to 16 November 2023. The following search terms were utilized: [(adiponectin) OR (acrp30) OR (adipokines) OR (apM1 protein)] AND [(myocardial ischemia–reperfusion injury) OR (ischemic heart disease) OR (myocardial ischemia) OR (myocardial reperfusion)]. Citations from relevant literature were tracked to ensure the integrity of the included studies as much as possible.
Eligibility criteria
The inclusion criteria were as follows: (1) the MIRI model was established by using appropriate methods; (2) the treatment group was injected with exogenous recombinant APN (proteolytic cleavage product of APN), and the MIRI group was given normal saline or a carrier without the drug (there were no requirements for the mode of administration, drug action time, or the dose of the two groups); (3) the object of study was animals; (4) the outcomes were the size of the myocardial necrosis area and hemodynamic monitoring indicators; and (5) markers of oxidation, apoptosis, and inflammation were used as secondary outcome indicators. The exclusion criteria were as follows: (1) duplicate publications; (2) editorials, conferences, abstracts, case reports, meta-analysis, and technical reports; (3) in vitro and clinical studies; (4) lack of intervention or control group modeling; (5) use of the same set of research data; (6) APN was not the only intervention; and (7) incomplete data.
Data extraction
Two authors (HY and QZ) examined each study based on selection criteria and extracted relevant data. The following information was extracted: (1) author and year in which the article was published; (2) type, sex, weight, and weekly age of laboratory animals; (3) type of narcotic drug used; (4) methodology for the construction of the MIRI model; (5) APN treatment information, including dose, time, and method of administration; (6) time of ischemia and reperfusion; (7) presence of comorbidities; (8) outcome indicators and corresponding p-values; and (9) signaling pathways involved and corresponding mechanisms. When the sample size given by the study was not a definite value, the minimum sample size was taken to ensure accuracy. When there were differences in the dose and duration of action of APN in the intervention group, the differences were combined into a single treatment group according to the formula recommended by the Cochrane Handbook [14, 15]. For articles that only display data graphically, the data were obtained using GetData graph digitizer 2.22 (http://getdata-gra ph-digitizer.com/) based on the method of Chen et al. [16].
Quality evaluation
Quality evaluation of the 20 studies was performed by HY and QZ using the risk assessment scale recommended by the Systematic Review Center for Laboratory animal Experimentation (SYRCLE) statement according to the methodology of Hooijmans et al. [17]. A third author (WH) checked the raw data for corrections. The scale evaluates the following components: (A) whether sequence generation was described in detail; (B) whether animals were characterized in detail at baseline; (C) sufficiency of allocation sequence hiding; (D) whether the animals were randomly captive; (E) whether the researcher was blinded; (F) whether the outcome measures were randomized; (G) whether the researcher was blinded at the time of outcome assessment; (H) whether incompleteness of outcome data was adequately addressed; (I) whether the results were selectively reported; and (J) whether other sources of bias were described. The included studies were visualised using RevMan 5.4.1. A third author (WH) adjudicated when there was disagreement. At the same time, the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) system was used to assess the quality of each outcome [18].
Statistical analysis
Stata/MP 14.0 software was utilized to generate the forest plots and analyze all data. When P < 0.05, the analysis was considered meaningful. The mean ± standard deviation was used to represent the parameters of the forest map. For studies expressed as the median and interquartile range (e.g., Kondo, 2010), data were extracted after calculations based on the method stated by Wan et al. [19, 20]. Regarding the forest plots [21], the standardized mean difference (SMD) was used for pooled analysis when the methods or units of measurement were inconsistent, and the value of the confidence interval (CI) was set at 95%. When the measurement method or unit was consistent, the analysis was combined using the weighted mean difference (WMD). Because the number of animals in preclinical studies was generally small, a hedge was selected for statistical analysis of the effect size of SMD [22]. The I squared (I2) statistic assessed the variability among the studies involved. The DerSimonian and Laird method was used to develop a random-effects model, and the inverse variance method was used to develop a fixed-effects model. When I2 > 50% or P < 0.10, which indicated significant heterogeneity, the analyses were pooled using random-effect models. When I2 < 50% or P > 0.10, the choice of effect model was fixed [23]. To investigate potential variations in the studies, predefined subgroups were examined for primary outcomes displaying significant heterogeneity. The predefined subgroups mainly included various species, different methods of APN administration, and the presence or absence of comorbidities. Analyses used Begg's test, Egger's test [24], and funnel plots [25] to determine whether the included studies were biased. Sensitivity analyses determined whether the robustness of the included studies was sufficient. The approach was to exclude one study at a time and assess its impact on the overall outcome. When there were fewer than three articles on outcome measures, they were not analyzed.
Results
Study inclusion
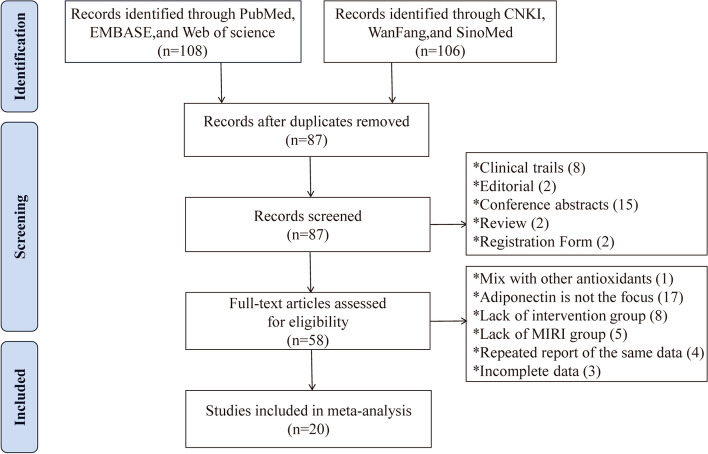
According to the search strategy, 214 articles were identified from six databases, including 106 articles in Chinese and 108 in English. First, 127 duplicate articles were removed using NoteExpress, and the abstracts and titles of the remaining 87 articles were read by HY and QZ, respectively. In total, 8 clinical studies, 2 editorials, 2 reviews, 15 conference abstracts, and 2 registration forms for scientific and technological achievements were excluded. The remaining 58 articles were read, and 38 articles were excluded due to the following reasons: 18 articles were excluded because APN was not the primary study subject or was mixed with other antioxidants; 13 articles were excluded due to the lack of intervention/MIRI group modeling; 4 articles were excluded due to the use of the same data set; and 3 articles were excluded due to incomplete data. Finally, 20 articles that met the requirements were included [26–45]. The process of searching for literature is depicted in Fig. 1.
Fig. 1.
PRISMA Literature Search Flowchart
Features of the included studies
In total, 20 papers and 27 study cohorts (seven papers involving two study cohorts [26, 28, 32, 33, 37, 39, 41]), including 10 in English and 10 in Chinese, were included, with 426 animals (9 studies used rats [30, 31, 34, 36, 38, 42–45], 10 studies used mice [26–28, 32, 33, 35, 37, 39–41], and 1 study used pigs [29]). Regarding the MIRI model, one model was generated by the Langendorff cardiac perfusion system, and the remaining models were generated by coronary artery ligation. The duration of myocardial ischemia was usually 0.5/3 h; however, in five papers, the duration was 0.75 h. The reperfusion time was 24 h in nine studies, 48 h in one study, and 1–3 h in the remaining studies, of which 3 h accounted for the most significant proportion. APN pretreatment was primarily intravenous (IV) or intraperitoneal (IP), while pretreatment ranged from 2 min before surgery to 3 days before surgery. Regarding the dose of administration, the doses differed among studies. The anesthetics were primarily isoflurane and sodium pentobarbital; however, one study used urethane, and another used chloral hydrate. Fourteen studies had no disease other than MIRI [26–30, 34–37, 40–43, 45], while six studies considered comorbidities [31–33, 38, 39, 44]. Other basic features are shown in Table 1.
Table 1.
Characteristic table of the literature
| Author and Year | Species and gender | Week old and Weight | Anesthetic | Model and method | Treatment group | MI/RI Time | Comorbidity | Outcomes and P-Value | Mechanisms | |
|---|---|---|---|---|---|---|---|---|---|---|
| APN and Duration | Administration | |||||||||
| Shibata et al. 2005 [26] | Queue a (WT): C57BL/6 mices, ND | 10 to 12 weeks, ND | Pent | MIRI, ligation of LAD | 2 × 108 PFU, 3 d | IV | 0.5 h/48 h | no | 1. Infarct size: P < 0.05 2. Apoptotic rate: P < 0.05 3. LVEDP: P < 0.05 4. + dP/dtmax: P < 0.05 5. -dP/dtmax: P < 0.05 6. TNF-α: P < 0.05 | AMPK↑/COX-2↑/apoptosis↓/proinflammatory cytokine↓ |
| Queue b (APN-KO): C57BL/6 mices, ND | 10 to 12 weeks, ND | Pent | MIRI, ligation of LAD | 2 × 108 PFU, 3 d | IV | 0.5 h/48 h | no |
1. Infarct size: P < 0.01 2. Apoptotic rate: P < 0.05 3. TNF-α: P < 0.01 |
||
| Tao et al. 2007 [27] | Adult mices, male | ND | Isoflurane | MIRI, ligation of LAD | 1 to 4 μg/g, 10 min | IP | 0.5 h/3 h, 24 h | no |
1. Infarct size: P < 0.01 2. Apoptotic rate: P < 0.01 3. LVEDP: P < 0.01 4. + dP/dtmax: P < 0.01 5. -dP/dtmax: P < 0.01 6. Caspase-3: P < 0.01 7. Superoxide production: P < 0.01 |
iNOS↓/gp91^phox protein↓/oxidative or nitrative stress↓ |
| Wang et al. 2009 [28] | Queue a (WT): D157A mices, male | ND | Isoflurane | MIRI, ligation of LAD | 2 μg/g, 10 min | IP | 0.5 h/3 h, 24 h | no | 1. Infarct size: P < 0.01 2. Apoptotic rate: P < 0.01 3. LVEDP: P < 0.05 4. + dP/dtmax: P < 0.05 5. -dP/dtmax: P < 0.05 6. Caspase-3: P < 0.01 7. Superoxide production: P < 0.01 8. LDH: P < 0.01 | apoptosis↓/oxidative or nitrative stress↓ (Note: the antioxidative and antinitrative effects of APN in MIRI hearts are not mediated by AMPK) |
| Queue b (AMPK-DN): D157A mices, male | ND | Isoflurane | MIRI, ligation of LAD | 2 μg/g, 10 min | IP | 0.5 h/3 h, 24 h | no | 1. Infarct size: P < 0.01 2. Apoptotic rate: P < 0.01 3. LVEDP: P < 0.05 4. + dP/dtmax: P < 0.05 5. -dP/dtmax: P < 0.05 6. Caspase-3: P < 0.05 7. Superoxide production: P < 0.05 8. LDH: P < 0.01 | ||
| Kondo et al. 2010 [29] | Yorkshire-Duroc pigs, female | 2 to 3 months, 29.55 to 31.95 kg | Pent | MIRI, ligation of LAD | 0.03 μg/kg, 10 min | Arterial administration | 0.75 h/24 h | no | 1. Infarct size: P < 0.05 2. Apoptotic rate: P < 0.01 3. LVEDP: P < 0.05 4. + dP/dtmax: P < 0.05 5. -dP/dtmax: P < 0.05 6. TNF-α: P < 0.05 | inflammation↓/apoptosis↓/oxidative stress↓ |
| Wang et al. 2010 [30] | SD rats, male | 8 to 10 weeks, 180 to 220 g | Urethane | MIRI, ligation of LAD | 60 ng/g, 2 min | IV | 0.5 h/1 h | no | 1. Caspase-3: P < 0.01 | AMPK↑/PPAR-γ↑/apoptosis↓ |
| Chen 2011 [31] | SD rats, male | ND, 220 to 250 g | Pent | MIRI, ligation of LAD | 60/120/180 ng/g, 30 min | IV | 0.75 h/3 h | DM | 1. Infarct size: P < 0.01 2. + dP/dtmax: P < 0.01 3. -dP/dtmax: P < 0.01 4. Caspase-3: P < 0.01 5. SOD: P < 0.01 | NOS↑/SOD↑/oxidative stress↓ |
| Liu et al. 2011 [32] | Queue a (WT): C57B1/6 J mices, male | 8 to 12 weeks, ND | Isoflurane | MIRI, ligation of LAD | 0.5 mg/kg, 10 min | IV | 0.5 h/3 h | Trauma | 1. Infarct size: P < 0.01 2. + dP/dtmax: P < 0.01 3. Caspase-3: P < 0.01 4. Superoxide production: P < 0.05 | apoptosis↓/oxidative or nitrative stress↓ |
| Queue b(APN-KO): C57B1/6 J mices, male | 8 to 12 weeks, ND | Isoflurane | MIRI, ligation of LAD | 0.5 mg/kg, 10 min | IV | 0.5 h/3 h | Trauma | 1. Infarct size: P < 0.01 2. + dP/dtmax: P < 0.01 | ||
| Ma et al. 2011 [33] | Queue a (DM1d): Swiss mices, ND | 6 to 8 weeks, ND | Isoflurane | MIRI, ligation of LAD | 2 μg/g, 10 min | IP | 0.5 h/3 h, 24 h | DM | 1. Infarct size: P > 0.05 2. Apoptotic rate: P > 0.05 3. Caspase-3: P > 0.05 4. LDH: P > 0.05 | AdipoR1↑/AMPK↑/LDH↓/apoptosis↓ |
| Queue b (DM7d): Swiss mices, ND | 6 to 8 weeks, ND | Isoflurane | MIRI, ligation of LAD | 2 μg/g, 10 min | IP | 0.5 h/3 h, 24 h | DM | 1. Infarct size: P < 0.01 2. Apoptotic rate: P < 0.01 3. Caspase-3: P < 0.01 4. LDH: P < 0.01 | ||
| Chen et al. 2011 [34] | SD rats, male | ND, 220 to 250 g | Pent | MIRI, ligation of LAD | 180 ng/g, 30 min | IV | 0.75 h/3 h | no | 1. LVEDP: P < 0.05 2. LDH: P < 0.01 | apoptosis↓/oxidative stress↓ |
| Gao et al. 2012 [35] | C57 mices, male | 4 to 6 weeks, 20 to 25 g | Isoflurane | MIRI, ligation of LAD | 0.5 mg /kg, 10 min | IP | 0.5 h/3 h, 24 h | no | 1. LVEF: P < 0.05 2. Caspase-3: P < 0.05 | ND |
| Guo 2013 [36] | SD rats, male | 7 to 8 weeks, 220 to 250 g | Pent | MIRI, ligation of LAD | 1 μg/kg, 10 min | IV | 0.75 h/3 h | no |
1. Infarct size: P < 0.05 2. Apoptotic rate: P < 0.05 3. LVEDP: P < 0.05 4. + dP/dtmax: P < 0.05 5. -dP/dtmax: P < 0.05 6. Caspase-3: P < 0.05 7. LDH: P < 0.05 |
apoptosis↓/LDH↓/CK-MB↓/arrhythmia↓ |
| Zhang et al. 2013 [37] | Queue a (Scramble): D157A mices, male | ND | Isoflurane | MIRI, ligation of LAD | 2 μg/g, 20 min | IP | 0.5 h/3 h, 24 h | no | 1. Infarct size: P < 0.01 2. LVEDP: P < 0.01 3. + dP/dtmax: P < 0.01 4. LVEF: P < 0.05 5. Caspase-3: P < 0.01 6. Apoptotic rate: P < 0.01 7. Superoxide production: P < 0.01 | PKA activity↑/oxidative stress↓/IKK, IκB↓/NF-κB↓ |
| Queue b (PKA-KO): D157A mices, male | ND | Isoflurane | MIRI, ligation of LAD | 2 μg/g, 20 min | IP | 0.5 h/3 h, 24 h | no | 1. Infarct size: P < 0.05 2. LVEDP: P < 0.05 3. + dP/dtmax: P < 0.05 4. LVEF: P > 0.05 5. Caspase-3: P > 0.05 6. Apoptotic rate: P > 0.05 7. Superoxide production: P < 0.01 | ||
| Guo et al. 2014 [38] | SD rats, ND | ND, 220 to 250 g | Pent | MIRI, ligation of LAD | 1 μg/kg, 10 min | IV | 0.75 h/3 h | DM | 1. Infarct size: P < 0.05 2. Caspase-3: P < 0.05 3. SOD: P < 0.05 | apoptosis↓/oxidative stress↓ |
| Song et al. 2014 [39] | Queue a (WT): C57B1/6 J mices, ND | 8 to 10 weeks, ND | Isoflurane | MIRI, ligation of LAD | 2 μg/g, 7 d | IP | 0.5 h/3 h, 24 h | renal failure | 1. Infarct size: P < 0.05 2. Apoptotic rate: P < 0.01 3. Caspase-3: P < 0.05 4. LVEF: P < 0.01 | AdipoR1↑/AMPK↑/iNOS↓/apoptosis↓/oxidative or nitrative stress↓ |
| Queue b (APN-KO): C57B1/6 J mices, ND | 8 to 10 weeks, ND | Isoflurane | MIRI, ligation of LAD | 2 μg/g, 7 d | IP | 0.5 h/3 h, 24 h | renal failure | 1. Infarct size: P < 0.05 2. Apoptotic rate: P < 0.01 3. Caspase-3: P < 0.05 4. LVEF: P < 0.01 | ||
| Wang et al. 2014 [40] | C57BL/6 J mices, male | 8 to 10 weeks, ND | Isoflurane | MIRI, ligation of LAD | 2 μg /g, 10 min | IP | 0.5 h/3 h, 24 h | no | 1. Infarct size: P < 0.01 2. LVEF: P < 0.01 | increased APPL1 expression |
| Gao et al. 2015 [41] | Queue a (WT): C57BL/6 mices, male | 6 to 8 weeks, 20 to 25 g | Isoflurane | MIRI, ligation of LAD | 0.5 mg/kg, 10 min | IP | 0.5 h/3 h, 24 h | no |
1. Infarct size: P < 0.01 2. Apoptotic rate: P < 0.05 3. LVEDP: P < 0.05 4. + dP/dtmax: P < 0.05 5. -dP/dtmax: P < 0.05 6. Caspase-3: P < 0.05 7. LVEF: P < 0.05 |
AMP-activated protein kinase phosphorylation↑/apoptosis↓/TNF-α↓ |
| Queue b (APN-KO): C57BL/6 mices, male | 6 to 8 weeks, 20 to 25 g | Isoflurane | MIRI, ligation of LAD | 0.5 mg/kg, 10 min | IP | 0.5 h/3 h,24 h | no | 1. Infarct size: P < 0.05 2. Apoptotic rate: P < 0.05 3. Caspase-3: P < 0.01 4. LVEF: P < 0.05 | ||
| Zhao et al. 2016 [42] | SD rats, ND | ND, 220 to 280 g | Chloral hydrate | MIRI, ligation of LAD | 1.8 μg/g, 20 min | IV | 0.5 h/2 h | no | 1. LDH: P < 0.05 2. MDA: P < 0.05 3. SOD: P < 0.05 | Lipid peroxidation↓/free radical scavenging↑ |
| Potenza et al. 2019 [43] | SD rats, male | ND, 250 to 300 g | Pent | MIRI, Langendorff perfusion system | 3 μg/mL, 3.5 h | Arterial administration | 0.5 h/3 h | no | 1. Infarct size: P < 0.05 2. LVEDP: P < 0.01 | AMPK, LKB1↑/ eNOS↑/SIRT-1↑ |
| Xing et al. 2020 [44] | SD rats, male | 8 to 10 weeks, 250 to 300 g | Pent | MIRI ligation of LAD | 5 μg /g, 15 min | IP | 0.5 h/2 h | DM | 1. Infarct size: P < 0.05 | SphK1↓/S1P↓/phosphorylation of NF-κB↓ |
| Wang et al. 2022 [45] | SD rats, male | 4 weeks, 180 to 220 g | Pent | MIRI ligation of LAD | 5 μg/g, 15 min | IP | 0.5 h/2 h | no | 1. LDH: P < 0.05 | MIF↑/autophagy↓ |
Pent Pentobarbital sodium, PFU plaque-forming units, IV Intravenous injection, IP Intraperitoneal injection, IS infarct size, AAR area at risk, ND no description, WT wild-type, APN-KO APN gene knockout, AMPK-DN a mutant AMPKα2 subunit, MIRI myocardial ischemia/reperfusion injury, d day, h hour, min minute, LAD left anterior descending branch, SD Sprague–Dawley, COX-2 Cyclooxygenase-2, iNOS inducible NO synthase, gp91^phox nicotinamide adenine dinucleotide phosphate-oxidase, LVEDP left ventricular end-diastolic pressure, + dP/dtmax maximum rate of left ventricular pressure rise, -dP/dtmax maximum rate of left ventricular pressure decrease, LVEF left ventricular ejection fraction, DM diabetes mellitus, SOD superoxide dismutase, NO nitric oxide, NOS nitric oxide synthase, PKA-KO Protein kinase A knockout, CK-MB creatine kinase isoenzymes, APPL1 Recombinant Adiponectin Receptor 1, eNOS endothelial nitric oxide synthase, LKB1 Liver kinase B1, SIRT-1 sirtuin-1, SphK1 Sheath Amino Acid Kinase 1, S1P Sphingosine-1-phosphate, TNF-α tumor necrosis factor alpha, IHC immunohistochemical staining, PPAR-γ Peroxisome proliferator activated receptor, MIF migration inhibitory factor
Quality assessment of research
The content of 20 articles was independently assessed according to the 10-item systematic review scale for animal experiments recommended by the SYRCLE statement. Sequence generation was described in detail in all studies. Animals were randomly assigned to rearing, and the incompleteness of the resulting data was adequately addressed in all studies. None of the studies provided a detailed description of the blinding of researchers or blinding during outcome assessment. Five studies did not describe (rated as high risk) the baseline characteristics of the animals in detail [29, 32, 34, 35, 38], and six studies did not adequately describe whether or not they were randomized at the time of the outcome measure [30, 31, 34, 35, 42, 43]. Moreover, nine studies adequately illustrated the limitations of the study [26–29, 32, 34–36, 42]. Overall, the risk of bias was unclear in most studies. Only a few studies were at high risk, and there was a relatively high level of confidence in the evidence for inclusion. Other specific details are provided in Fig. 2.
Fig. 2.
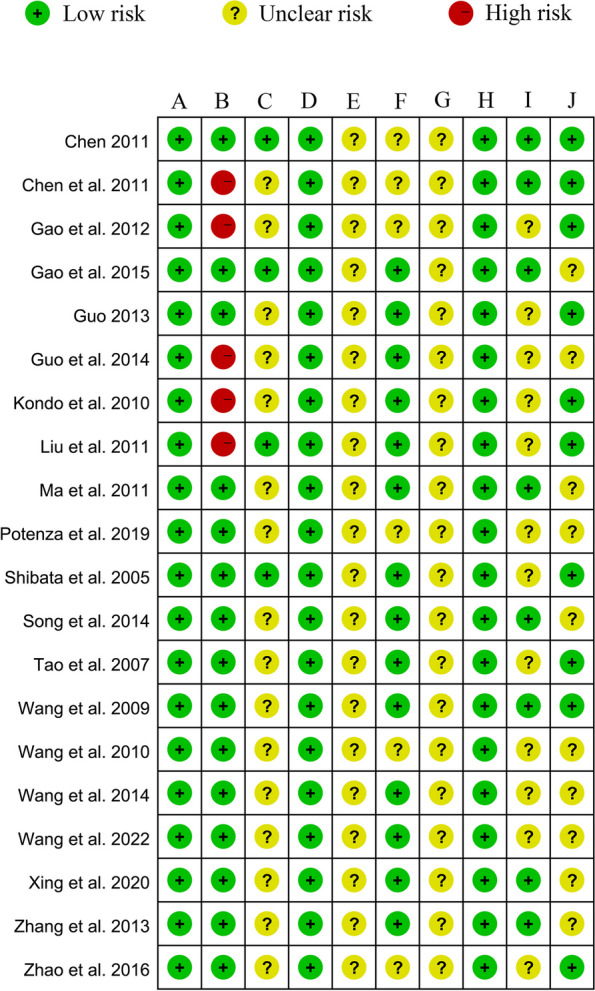
Quality evaluation of 20 animal literatures (SYRCLE tool). A Formation of sequences; B Initial characteristics of the animal; C Sequence hiding; D Randomness of animal feeding; E Personnel blinding; F: Randomness of outcome measurement; G Outcome assessment bias; H Integrity of result data; I Biased results; J Other biases
Evidence quality evaluation
Eleven outcome indicators were evaluated using the GRADE approach. Of these, 27.27% (3/11) were deemed to be of moderate quality, 36.36% (4/11) were considered to be of low quality, and the rest were rated as critically low quality (Table 2). It is important to note that inconsistencies and risk of bias were the main reasons for the downgrading of the evidence. In addition, due to significant heterogeneity in some results, we downgraded the quality of these results.
Table 2.
A GRADE summary of the protective effect of APN on MIRI
| Outcomes | Summary of finding | Relative effect (95% CI) | Limitations | Inconsistency | Indirectness | Imprecision | Publication bias | Quality | |
|---|---|---|---|---|---|---|---|---|---|
| MIRI group | APN group | ||||||||
| Infarct size | 171 animals in 15 studies | 169 animals in 15 studies | WMD, 16.67 (13.18, 20.16) | + | − | + | + | + | ⨁⨁⨁◯, Moderate |
| LVEDP | 99 animals in 9 studies | 98 animals in 9 studies | WMD, 5.96 (4.23, 7.70) | − | − | + | + | − | ⨁◯◯◯, Critically low |
| + dp/dtmax | 107 animals in 9 studies | 107 animals in 9 studies | WMD, 1393.59 (972.57, 1814.60) | + | − | + | + | − | ⨁⨁◯◯, Low |
| -dp/dtmax | 68 animals in 7 studies | 68 animals in 7 studies | WMD, 850.06 (541.22, 1158.90) | + | − | + | + | + | ⨁⨁⨁◯, Moderate |
| LVEF | 68 animals in 5 studies | 68 animals in 5 studies | WMD, 9.96 (7.29, 12.63) | + | − | + | + | + | ⨁⨁⨁◯, Moderate |
| Caspase-3 | 126 animals in 12 studies | 126 animals in 12 studies | SMD, 3.86 (2.97, 4.76) | − | − | + | + | − | ⨁◯◯◯, Critically low |
| Apoptotic rate | 95 animals in 9 studies | 95 animals in 9 studies | WMD, 13.10 (8.15, 18.05) | − | − | + | + | + | ⨁⨁◯◯, Low |
| SOD | 24 animals in 3 studies | 24 animals in 3 studies | SMD, 1.91 (1.17, 2.65) | − | + | + | + | − | ⨁⨁◯◯, Low |
| LDH | 70 animals in 6 studies | 70 animals in 6 studies | SMD, 2.82 (1.60, 4.04) | − | − | + | + | − | ⨁◯◯◯, Critically low |
| TNF-α | 13 animals in 2 studies | 13 animals in 2 studies | SMD, 4.23 (2.48, 5.98) | − | + | + | + | − | ⨁⨁◯◯, Low |
| Superoxide production | 43 animals in 4 studies | 43 animals in 4 studies | SMD, 4.53 (2.39, 6.67) | − | − | + | + | − | ⨁◯◯◯, Critically low |
− downgrade, + not downgrade, LVEDP Left ventricular end-diastolic pressure, + dp/dtmax Maximum rate of left ventricular pressure rise, -dp/dtmax Maximum rate of left ventricular pressure decrease, LVEF Left ventricular ejection fraction, SOD Superoxide dismutase, LDH lactate dehydrogenase, SMD standardized mean difference, WMD weighted mean difference
Meta-analysis results
Myocardial infarction size
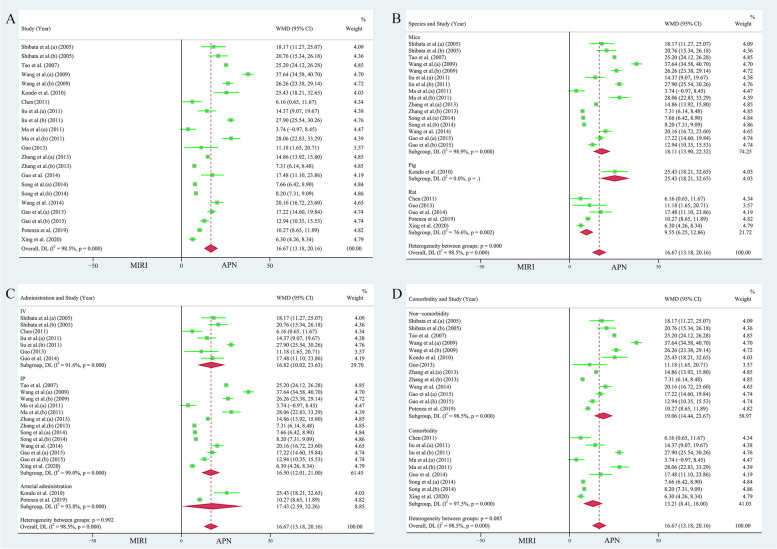
Fifteen publications (22 study cohorts), including 340 animals, reported the relationship between APN and myocardial infarct size [26–29, 31–33, 36–41, 43, 44]. Due to the heterogeneity test results (I2 = 98.50%, P = 0.000), the random effects model was used for analysis. The area of myocardial infarction decreased by 16.67% due to the application of APN (WMD = 16.67, 95% CI = 13.18 to 20.16, P < 0.001, Fig. 3A). However, the funnel plot was not symmetrical (Supplementary Fig. 1A). Egger's test (t = 1.16, P = 0.261 > 0.05) and Begg's test (Z = 1.02, P = 0.310) showed no bias. Sensitivity analyses showed (Supplementary Fig. 1B) that no study significantly changed the total effect sizes and I2 values of the pooled analyses, indicating relatively stable results.
Fig. 3.
A Forest plot of the effect of APN on myocardial infarct size. Subgroup analyses of the effect of APN on myocardial infarct size included B different species, C different modes of administration, and D presence of comorbidities
The studies were stratified according to species (mice: 246 animals in 16 studies, heterogeneity: I2 = 98.90%, P = 0.000, WMD = 18.11, 95% CI = 13.90 to 22.32, P < 0.001; rats: 83 animals in 5 studies, heterogeneity: I2 = 76.60%, P = 0.002, WMD = 9.55, 95% CI = 6.25 to 12.86, P < 0.001, Fig. 3B), different modes of administration (IV: 7 studies with 86 animals, heterogeneity: I2 = 91.60%, P = 0.000, WMD = 16.82, 95% CI = 10.02 to 23.63, P < 0.001; IP: 13 studies 230 animals, heterogeneity: I2 = 99.00%, P = 0.000, WMD = 16.50, 95% CI = 12.01 to 21.00, P < 0.001; arterial administration: 24 animals in 2 studies, heterogeneity: I2 = 93.80%, P = 0.000, WMD = 17.43, 95% CI = 2.59 to 32.26, P < 0.05, Fig. 3C), and presence of comorbidities (non-comorbidity: 204 animals in 13 studies, heterogeneity: I2 = 98.50%, P = 0.000, WMD = 19.06, 95% CI = 14.44 to 23.67, P < 0.001; comorbidity: 136 animals in 9 studies, heterogeneity: I2 = 97.50%, P = 0.000, WMD = 13.21, 95% CI = 8.41 to 18.00, P < 0.001, Fig. 3D), to further explore sources of heterogeneity. APN still significantly reduced the size of the infarcted myocardium in different subgroups, and the heterogeneity remained significant.
Hemodynamics-related indicators
Left ventricular end-diastolic pressure (LVEDP)
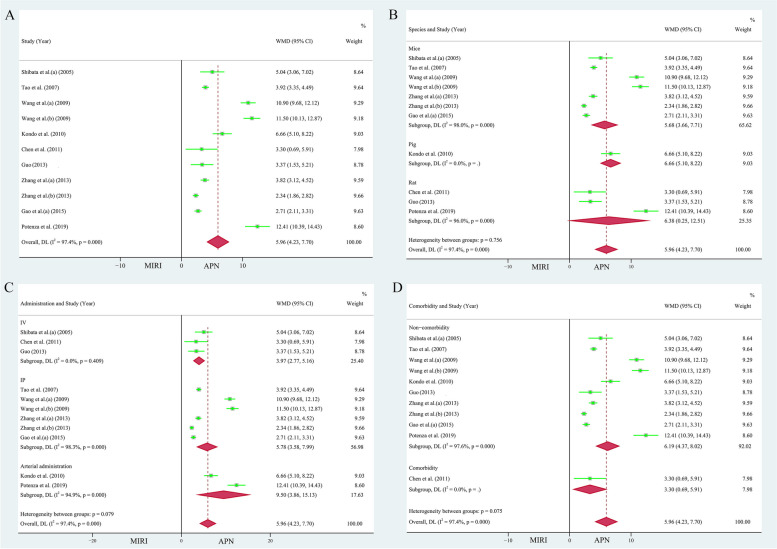
Nine papers (11 study cohorts), containing 197 animals, reported the protective effect of APN on LVEDP [26–29, 34, 36, 37, 41, 43]. Due to the heterogeneity test result (I2 = 97.40%, P = 0.000), the individual effects model selection was randomized. The comprehensive analysis showed that APN had an excellent reduction of LVEDP by 5.96 mmHg compared to the MIRI cohort (WMD = 5.96, 95% CI = 4.23 to 7.70, P < 0.001, Fig. 4A). Egger's test (t = 2.52, P = 0.033 < 0.05) and the funnel plot (Supplementary Fig. 2A) showed publication skewing. Begg's test (P = 0.350, Z = 0.93) indicated bias. Sensitivity analysis indicated the results were stable and credible (Supplementary Fig. 2C).
Fig. 4.
A Forest plot of the protective effect of APN on LVEDP. Subgroup analyses of the protective effect of APN on LVEDP included B different species, C different modes of administration, and D presence of comorbidities
The beneficial effect of APN on LVEDP was observed in the subgroups according to different species (mice: 138 animals in 7 studies, heterogeneity: I2 = 98.00%, P = 0.000, WMD = 5.68, 95% CI = 3.66 to 7.71, P < 0.001; rats: 45 animals in 3 studies, heterogeneity: I2 = 96.00%, P = 0.000, WMD = 6.38, 95% CI = 0.25 to 12.51, P < 0.05, Fig. 4B), different modes of administration (IV: 3 studies 42 animals, WMD = 3.97, 95% CI = 2.77 to 5.16, P < 0.001; IP: 6 studies with 128 animals, heterogeneity: I2 = 98.30%, P = 0.000, WMD = 5.78, 95% CI = 3.58 to 7.99, P < 0.001; arterial administration: 27 animals in 2 studies, heterogeneity: I2 = 94.90%, P = 0.000, WMD = 9.50, 95% CI = 3.86 to 15.13, P < 0.05, Fig. 4C), and the presence of comorbidities (non-comorbidity: 181 animals in 10 studies, heterogeneity: I2 = 97.60%, P = 0.000, WMD = 6.19, 95% CI = 4.37 to 8.02, P < 0.001, Fig. 4D). However, when stratifying the studies according to different modes of administration, the heterogeneity of the protective effect of APN on LVEDP was not significant in subgroup IV (I2 = 00.00%, P = 0.409).
Maximum rate of left ventricular pressure rise (+ dp/dtmax)
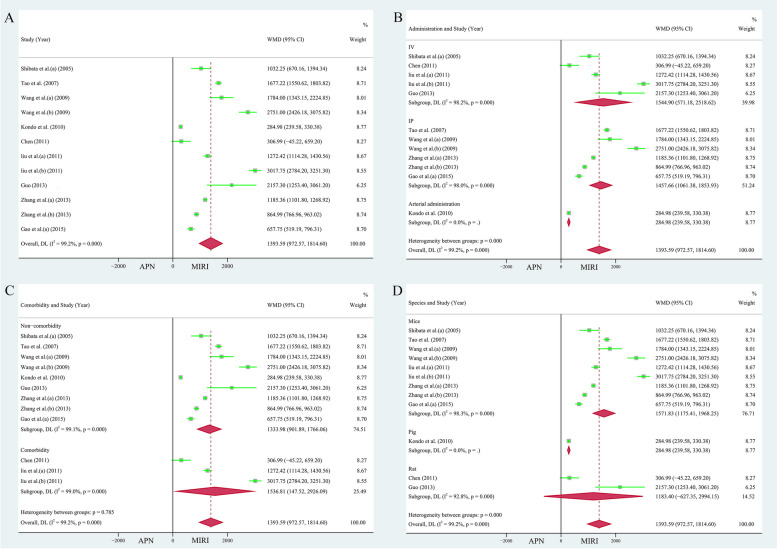
Nine publications (12 study cohorts), containing 214 animals, reported the protective effect of APN on + dp/dtmax [26–29, 31, 32, 36, 37, 41]. The heterogeneity test result (I2 = 99.20%, P = 0.000) indicated that a random effects model was required for analysis. Pooled analysis showed a significant increase in + dp/dtmax due to APN administration compared to the MIRI cohort (WMD = 1393.59, 95% CI = 972.57 to 1814.60, P < 0.001, Fig. 5A). Egger's test (t = 2.71, P = 0.022 < 0.05) and the funnel plot (Supplementary Fig. 2B) indicated bias in the included studies. In addition, Begg's test (P = 0.945, Z = 0.07) indicated bias. Sensitivity analysis indicated the results were stable and credible (Supplementary Fig. 2D).
Fig. 5.
A Forest plot of the protective effect of APN on + dp/dtmax. Subgroup analyses of the protective effect of APN on + dp/dtmax included B different modes of administration, C presence of comorbidities, and D different species
The beneficial effect of APN on + dp/dtmax was maintained when the studies were stratified according to different modes of administration (IV: 72 animals in 5 studies, heterogeneity: I2 = 98.20%, P = 0.000, WMD = 1544.90, 95% CI = 571.18 to 2518.62, P < 0.05; IP: 128 animals in 6 studies, heterogeneity: I2 = 98.00%, P = 0.000, WMD = 1457.66, 95% CI = 1061.38 to 1853.93, P < 0.001, Fig. 5B), and the presence of comorbidities (non-comorbidity: 168 animals in 9 studies, heterogeneity: I2 = 99.10%, P = 0.000, WMD = 1333.98, 95% CI = 901.89 to 1766.06, P < 0.001; comorbidity: 46 animals in 3 studies, heterogeneity: I2 = 99.00%, P = 0.000, WMD = 1536.81, 95% CI = 147.52 to 2926.09, P < 0.05, Fig. 5C). However, when the studies were stratified according to species, the protective effect of APN on + dp/dtmax was only statistically significant in mice (168 animals in 9 studies, heterogeneity: I2 = 98.30%, P = 0.000, WMD = 1571.83, 95% CI = 1175.41 to 1968.25, P < 0.001, Fig. 5D).
Maximum rate of left ventricular pressure decrease (-dp/dtmax)
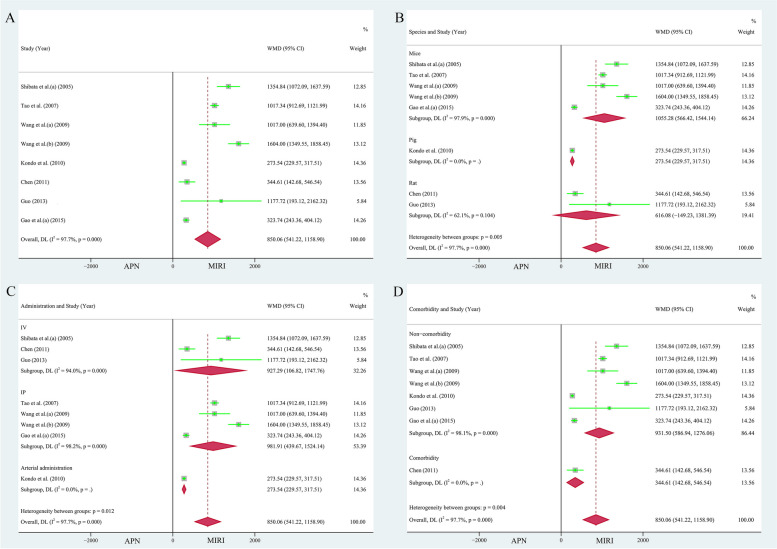
Pooled analysis of seven publications (eight study cohorts) showed that the -dp/dtmax was significantly lower in the MIRI group compared to the APN administration group [26–29, 31, 36, 41], with a difference of 850.06 mmHg/s (n = 136, heterogeneity: I2 = 97.70%, P = 0.000, WMD = 850.06, 95% CI = 541.22 to 1158.90, P < 0.001, Fig. 6A). Although the funnel plot (Supplementary Fig. 3A) was asymmetrical, Egger's (t = 2.23, P = 0.067 > 0.05) and Begg's (P = 0.536, Z = 0.62) tests did not indicate bias. Sensitivity analysis indicated the results were robust and reliable (Supplementary Fig. 3C).
Fig. 6.
A Forest plot of the protective effect of APN on -dp/dtmax. Subgroup analyses of the protective effect of APN on -dp/dtmax included B different species, C different modes of administration, and D presence of comorbidities
When stratifying the studies according to different species, the protective effect of APN on -dp/dtmax was only statistically significant in mice (46 animals in 5 studies, heterogeneity: I2 = 97.90%, P = 0.000, WMD = 1055.28, 95% CI = 566.42 to 1544.14, P < 0.001, Fig. 6B). In contrast, the protective effect of APN on -dp/dtmax, as well as the heterogeneity, remained significant when the studies were stratified according to the different modes of administration (IV: 42 animals in 3 studies, heterogeneity: I2 = 94.00%, P = 0.000, WMD = 927.29, 95% CI = 106.82 to 1747.76, P < 0.05; IP: 80 animals in 4 studies, heterogeneity: I2 = 98.20%, P = 0.000, WMD = 981.91, 95% CI = 439.67 to 1524.14, P < 0.001, Fig. 6C), and the presence of comorbidities (non-comorbidity: 120 animals in 7 studies, heterogeneity: I2 = 98.10%, P = 0.000, WMD = 931.50, 95% CI = 586.94 to 1276.06, P < 0.001, Fig. 6D).
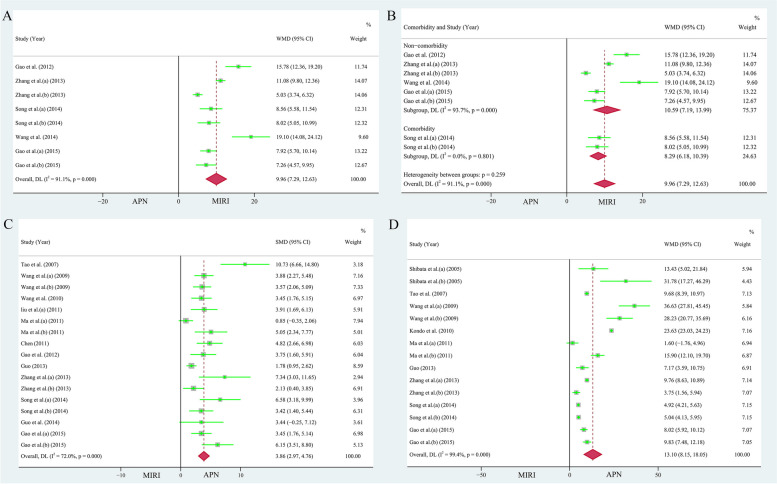
Left ventricular ejection fraction (LVEF)
A pooled analysis of five papers (eight study cohorts) showed that APN improved LVEF [35, 37, 39–41], with an increase of 9.96% compared to the MIRI group (n = 136, heterogeneity: I2 = 91.10%, P = 0.000, WMD = 9.96, 95% CI = 7.29 to 12.63, P < 0.001, Fig. 7A). Egger's test (P = 0.290 > 0.05, t = 1.17) and the funnel plot (Supplementary Fig. 3B) showed no bias. Begg's test (P = 0.266, Z = 1.11) also indicated no bias. Sensitivity analysis indicated the results were robust and reliable (Supplementary Fig. 3D).
Fig. 7.
A Forest plot of the protective effect of APN on LVEF. B Subgroup analysis of the protective effect of APN on LVEF (stratified by presence of comorbidities). C Forest plot of the effect of APN on caspase-3 expression. D Forest plot of the effect of APN on apoptosis
When stratifying the studies according to the presence of comorbidities, the increasing effect of APN on LVEF persisted (non-comorbidity: 112 animals in 6 studies, heterogeneity: I2 = 93.70%, P = 0.000, WMD = 10.59, 95% CI = 7.19 to 13.99, P < 0.001; comorbidity: 24 animals in 2 studies, WMD = 8.29, 95% CI = 6.18 to 10.39, Fig. 7B, P< 0.001). However, heterogeneity was insignificant in the comorbidity subgroup (I2 = 00.00%, P = 0.801). Because the APN administration modalities and species included in the study were the same, the results were not stratified.
Cardioprotective mechanisms of APN
Anti-apoptosis
The combined analysis of 12 published studies (consisting of 17 study cohorts) [27, 28, 30–33, 35–39, 41] revealed that the APN group had a lower expression level of caspase-3 (n = 252, P < 0.001, heterogeneity: I2 = 72.00%, P = 0.000, SMD = 3.86, 95% CI = 2.97 to 4.76, Fig. 7C). Egger's test (t = 6.07, P = 0.000 < 0.05) and the funnel plot (Supplementary Fig. 4A) showed publication bias in the selected studies. Sensitivity analysis indicated the results were robust and reliable (Supplementary Fig. 4C).
Analysis of nine papers (15 study cohorts) [26–29, 33, 36, 37, 39, 41] showed that the application of APN resulted in a significant reduction in the number of apoptotic cells (n = 190, heterogeneity: I2 = 99.40%, P = 0.000, WMD = 13.10, 95% CI = 8.15 to 18.05, P < 0.001, Fig. 7D). Egger's test (P = 0.597 > 0.05, t = -0.54) and the funnel plot (Supplementary Fig. 4B) indicated that there was no bias in publishing. Sensitivity analysis indicated that the results were reliable (Supplementary Fig. 4D).
Anti-oxidation
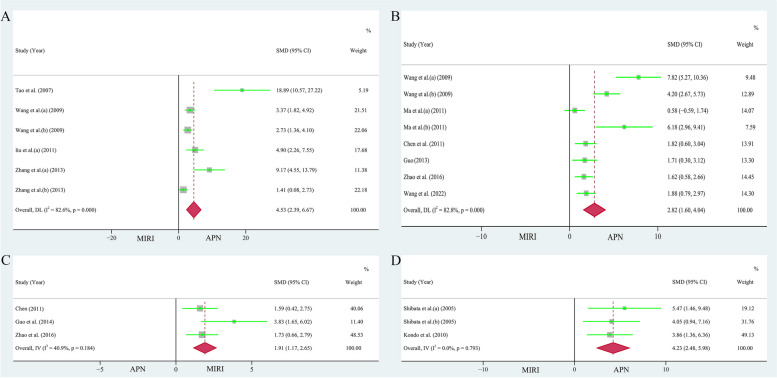
A pooled analysis of four papers (6 study cohorts) [27, 28, 32, 37] showed that the use of APN significantly reduced superoxide production in the myocardium, and the results were meaningful (n = 86, heterogeneity: I2 = 82.60%, P = 0.000, SMD = 4.53, 95% CI = 2.39 to 6.67, P < 0.001, Fig. 8A). Pooled examination of the three studies [31, 38, 42] showed that superoxide dismutase content was significantly higher in the APN cohort than in the MIRI cohort (n = 48, heterogeneity: I2 = 40.90%, P = 0.184, SMD = 1.91, 95% CI = 1.17 to 2.65, P < 0.001, Fig. 8C).
Fig. 8.
A Forest plot of APN effects on superoxide production. B Forest plot of the effect of APN on LDH content. C Forest plot of the effect of APN on SOD content. D Forest plot of the effect of APN on TNF-α levels
For studies evaluating the effect of APN on superoxide levels in the myocardium, Egger's test (P = 0.003 < 0.05, t = 6.39) and the funnel plot (Supplementary Fig. 5A) showed publication bias in the selected studies. However, sensitivity analysis suggested that the results were reliable (Supplementary Fig. 5C).
Anti-inflammatory
The combined examination of six articles (consisting of 8 study cohorts) [28, 33, 34, 36, 42, 45] revealed a significant decrease in lactate dehydrogenase (LDH) levels within the APN group (n = 140, heterogeneity: I2 = 82.80%, P = 0.000, SMD = 2.82, 95% CI = 1.60 to 4.04, Fig. 8B, P < 0.001). Analysis of two papers (3 study cohorts) [26, 29] showed that APN application significantly reduced TNF-α levels (n = 26, heterogeneity: I2 = 00.00%, P = 0.793, SMD = 4.23, 95% CI = 2.48 to 5.98, P < 0.001, Fig. 8D).
For the studies on the effect of APN on lactate dehydrogenase levels, Egger's test (P = 0.009 < 0.05, t = 3.81) and the funnel plot (Supplementary Fig. 5B) showed publication bias in the selected studies. However, the reliability of the results was supported by the sensitivity analysis (Supplementary Fig. 5D).
Discussion
The present meta-analysis included 20 studies, containing 426 animals, and the studies were high quality. The risk of bias was low, and the results were reliable. Pooled analyses showed that APN significantly reduces the size of the infarcted myocardium, regulates cardiovascular function, and suppresses the concentration of biomarkers associated with myocardial infarction. APN also improves myocardial inflammation, oxidative stress, and apoptosis.
MIRI is unavoidable in cardiac surgery, and the incidence increases yearly. Rescue from this injury remains a substantial challenge due to the lack of effective treatments [46]. The protective effect of APN on MIRI has not been reported by meta-analyses, and most of the evidence has been derived from basic science research. Interestingly, the results of some studies suggest different mechanisms. Some researchers have suggested that APN benefits MIRI through its adenine monophosphate-activated protein kinase signaling pathways [43, 47, 48]. However, some scholars believe that the cardioprotective effect of APN is not dependent on this mechanism [28, 49]. The protective effect of APN on MIRI may involve several mechanisms. A large body of evidence suggests that APN exerts cardioprotective functions through multiple molecular mechanisms [50, 51]. Understanding these protective mechanisms will help to better understand adiponectin. The currently reported mechanisms of action of APN in MIRI involve the mechanisms described below.
(1) Modulation of angiogenesis: Enhancing blood vessel formation and restoring blood supply is essential for clearing inflammation and repairing myocardial damage. APN, a chemotactic factor, has the ability to stimulate the transformation of endothelial cells into structures resembling capillaries in a laboratory setting, thereby controlling the process of angiogenesis [52]. APN ameliorates vascular damage after ischemic stress through an AMP-activated protein kinase-dependent pathway (AMPK) [53].
(2) Regulating autophagy: In recent years, autophagy has been recognized as one of the critical mechanisms underlying the cardioprotective effects of APN. The ERK/mTOR/AMPK signaling pathway regulates autophagy in cells, thereby protecting cardiomyocytes from oxidative stress [54]. In addition, APN also triggers autophagy in macrophages through the AMPK pathway and suppresses the inflammatory reaction, resulting in a reduction in cardiac fibrosis [55].
(3) Controlling the metabolism of fats and sugars: Numerous studies have indicated a connection among inflammation, oxidative stress, and disorders in the processing of glucose and lipids, ultimately affecting cardiovascular balance and damaging heart muscle [10, 56]. Protection of pancreatic β cells by APN enhances the absorption and utilization of monosaccharides by tissues, reduces sugar production, and regulates glucose metabolism [50]. APN also promotes adipocyte differentiation and facilitates fatty acid oxidation and turnover, thereby protecting the myocardium from injury [57].
Several studies have reported that APN is associated with cardiovascular disease development. Tentolouris et al. found that plasma lipoprotein levels are inversely proportional to the risk of developing IDH [58]. Numerous epidemiologic studies have demonstrated that plasma APN levels are significantly lower after myocardial infarction in obese and type 2 diabetic patients [59, 60]. It has been demonstrated that the degree of intimal hyperplasia after arterial injury is twice as high in APN knockout mice as in wild-type mice, whereas this process is markedly inhibited by supplementation with exogenous APN [61]. These findings suggest that APN is an anti-MIRI agent. Several sensitivity analyses and subgroup analyses have supported these results. In the present meta-analysis, sensitivity testing found that no studies had an impact on the results of the analysis, suggesting that the present results were stable, significant, and of high quality. Exogenous APN showed better therapeutic effects than other forms of APN. Fruebis et al. reported that exogenous APN releases more fatty acids and has a faster and more effective treatment effect in vivo after a single injection compared to full-length APN [62]. In addition, exogenous APN supplementation may be superior to full-length APN in renal insufficiency because exogenous APN lacks the NH2-terminal structural domain and does not bind to and inactivate cystatin C [63, 64].
Because APN has a better safety profile and lower toxicity than other monomers, it is necessary to continue to explore and support clinical trials. Moreover, the targets of APN for the treatment of MIRI should be explored in future studies to improve its molecular mechanism to accelerate the clinical process.
Strengths and limitations
The present meta-analysis is the first to elucidate the therapeutic effect of APN in MIRI based on animal experiments and establish a foundation for MIRI treatment with APN. The sample size of this meta-analysis was large, and many bias and sensitivity analyses were performed, demonstrating highly credible conclusions. In addition, the relevant mechanisms were summarized and elaborated, and the protective role of APN in MIRI was demonstrated, providing a basis for future research.
Although the number of animals in the present study was large and the safety and validity of the results were adequate, there were some limitations. First, the amount of data obtained from the animals was relatively small and could not be analyzed in depth. Second, due to the paucity of relevant studies, we were unable to determine whether the cardioprotective effects of APN are enhanced with further dose increases based on dose–response modeling in animal studies. Third, the results of the pooled analyses using SMD values with 95% CI should be interpreted with caution. SMD is a relative indicator and may not reflect the actual event. Fourth, only animal studies were included because only a few pertinent clinical studies have been published. In addition, the development of MIRI in real-world clinical settings is complex, thus limiting the present findings. Finally, meaningful results are easier to publish, indicating a likely overestimation of the efficacy of APN.
Conclusion
The present meta-analysis was based on preclinical studies and systematically illustrated the value of APN anti-MIRI. APN protects damaged myocardium by reducing the size of myocardial infarction and improving intracardiac hemodynamics. Moreover, APN has multiple mechanisms of action, including its ability to reduce inflammation, prevent cell death, and counteract oxidative stress. This conclusion holds despite limitations that reduce the persuasiveness of the evidence. APN is a promising anti-MIRI substance that may be incorporated into the treatment of MIRI and provide a strategy for MIRI treatment.
Supplementary Information
Additional file 1: Supplementary File 1. PRISMA 2020 Checklist. Supplementary Figure 1. Funnel plot and sensitivity analysis of myocardial infarction size. Supplementary Figure 2. Funnel plot and sensitivity analysis. (A and C) LVEDP, (B and D) +dp/dtmax. Supplementary Figure 3. Funnel plot and sensitivity analysis. (A and C) -dp/dtmax, (B and D) LVEF. Supplementary Figure 4. Funnel plot and sensitivity analysis. (A and C) Caspase-3, (B and D) TUNEL-positive cells. Supplementary Figure 5. Funnel plot and sensitivity analysis. (A and C) Superoxide content, (B and D) LDH.
Abbreviations
- IHD
Ischemic heart disease
- CVD
Cardiovascular disease
- PCI
Percutaneous coronary intervention
- CABG
Coronary artery bypass grafting
- MIRI
Myocardial ischemia–reperfusion injury
- APN
Adiponectin
- I2
I squared
- CI
Confdence interval
- SMD
Standardized mean difference
- WMD
Weighted mean difference
- IV
Intravenous
- IP
Intraperitoneal
- LAD
Left anterior descending
- LVEDP
Left ventricular end-diastolic pressure
- + dp/dtmax
Maximum rate of left ventricular pressure rise
- -dp/dtmax
Maximum rate of left ventricular pressure decrease
- LVEF
Left ventricular ejection fraction
- SOD
Superoxide dismutase
- PROSPERO
Pravastatin in elderly individuals at risk of vascular disease
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- SYRCLE
Systematic Review Center for Laboratory animal Experimentation
- GRADE
Grading of Recommendations, Assessment, Development, and Evaluations
Authors’ contributions
HY and QZ: design, studies search, data collection, analyze the results, and manuscript writing. SC, XZ, MW: studies search, data collection. WL: design and funds collection. All authors make contributions to this study and approved the submitted version.
Funding
This study was financially supported by the National Natural Science Foundation of China (NO. 81760332), the Incubation Program National Natural Science Foundation of School of Medicine of Xizang Minzu University (NO. XZMZ-M2022N02), the Tibet Natural Science Foundation (NO. XZ202101ZR0100G), Key Laboratory for Molecular Genetic Mechanisms and Intervention Research on High Altitude Disease of Tibet Autonomous Region (NO. KF2022008). postgraduate Research Innovation and Practice Project of Xizang Minzu University (NO. Y2022097), national-level training program for college students' innovation and entrepreneurship (NO. 202210695031), 2023 Intramural Research Project of Xizang Minzu University (NO. 23MDQ02), scientific Research Fund Project of Hunan Provincial Health Commission (NO. 20232752), Intramural Research Project of Xizang Minzu University (NO. XJ2023001101), Postgraduate Research Innovation and Practice Project of Xizang Minzu University (NO. Y2024006), Xizang Minzu University School-level Talent Program: Teaching and Research Leader.
Availability of data and materials
The original contributions shown in the study are selected in the article/supplementary material. Appropriate inquiries can be contacted with the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Severino P, D'Amato A, Pucci M, Infusino F, Adamo F, Birtolo LI, Netti L, Montefusco G, Chimenti C, Lavalle C, et al. Ischemic Heart Disease Pathophysiology Paradigms Overview: From Plaque Activation to Microvascular Dysfunction. Int J Mol Sci. 2020;21(21):8118. doi: 10.3390/ijms21218118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang CF. Clinical manifestations and basic mechanisms of myocardial ischemia/reperfusion injury. Ci Ji Yi Xue Za Zhi. 2018;30(4):209–215. doi: 10.4103/tcmj.tcmj_33_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curran J, Burkhoff D, Kloner RA. Beyond reperfusion: acute ventricular unloading and cardioprotection during myocardial infarction. J Cardiovasc Transl Res. 2019;12(2):95–106. doi: 10.1007/s12265-019-9863-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grech ED, Jackson MJ, Ramsdale DR. Reperfusion injury after acute myocardial infarction. BMJ. 1995;310(6978):477–478. doi: 10.1136/bmj.310.6978.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang L, Guo B, Liu S, Miao C, Li Y. Inhibition of the LncRNA Gpr19 attenuates ischemia-reperfusion injury after acute myocardial infarction by inhibiting apoptosis and oxidative stress via the miR-324-5p/Mtfr1 axis. IUBMB Life. 2020;72(3):373–383. doi: 10.1002/iub.2187. [DOI] [PubMed] [Google Scholar]
- 6.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123(1):92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Q, Guo Y, Zhang B, Liu H, Peng Y, Wang D, Zhang D. Identification of hub biomarkers of myocardial infarction by single-cell sequencing, bioinformatics, and machine learning. Front Cardiovasc Med. 2022;9:939972. doi: 10.3389/fcvm.2022.939972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khoramipour K, Chamari K, Hekmatikar AA, Ziyaiyan A, Taherkhani S, Elguindy NM, Bragazzi NL. Adiponectin: Structure, Physiological Functions, Role in Diseases, and Effects of Nutrition. Nutrients. 2021;13(4):1180. doi: 10.3390/nu13041180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, Gokce N, Pedersen AA, Kalthoff C, Tullin S, Sams A, et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem. 2010;285(9):6153–6160. doi: 10.1074/jbc.M109.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi HM, Doss HM, Kim KS. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int J Mol Sci. 2020;21(4):1219. doi: 10.3390/ijms21041219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braun M, Hettinger N, Koentges C, Pfeil K, Cimolai MC, Hoffmann MM, Osterholt M, Doenst T, Bode C, Bugger H. Myocardial mitochondrial and contractile function are preserved in mice lacking adiponectin. PLoS ONE. 2015;10(3):e0119416. doi: 10.1371/journal.pone.0119416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim Y, Lim JH, Kim MY, Kim EN, Yoon HE, Shin SJ, Choi BS, Kim YS, Chang YS, Park CW. The adiponectin receptor agonist adiporon ameliorates diabetic nephropathy in a model of type 2 diabetes. J Am Soc Nephrol. 2018;29(4):1108–1127. doi: 10.1681/ASN.2017060627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10(10):Ed000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou XT, Zou JJ, Ao C, Gong DY, Chen X, Ma YR. Renal protective effects of astragaloside IV, in diabetes mellitus kidney damage animal models: a systematic review, meta-analysis. Pharmacol Res. 2020;160:105192. doi: 10.1016/j.phrs.2020.105192. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Zhou J, Lu J, Xiong H, Shi X, Gong L. Significance of CD44 expression in head and neck cancer: a systemic review and meta-analysis. BMC Cancer. 2014;14:15. doi: 10.1186/1471-2407-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 19.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li T, Jin J, Pu F, Bai Y, Chen Y, Li Y, Wang X. Cardioprotective effects of curcumin against myocardial I/R injury: A systematic review and meta-analysis of preclinical and clinical studies. Front Pharmacol. 2023;14:1111459. doi: 10.3389/fphar.2023.1111459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan Q, Liu Y, Ma W, Kan R, Zhu H, Li D. Cardioprotective effects and possible mechanisms of luteolin for myocardial ischemia-reperfusion injury: a systematic review and meta-analysis of preclinical evidence. Front Cardiovasc Med. 2022;9:685998. doi: 10.3389/fcvm.2022.685998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 26.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11(10):1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao L, Gao E, Jiao X, Yuan Y, Li S, Christopher TA, Lopez BL, Koch W, Chan L, Goldstein BJ, et al. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation. 2007;115(11):1408–1416. doi: 10.1161/CIRCULATIONAHA.106.666941. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Gao E, Tao L, Lau WB, Yuan Y, Goldstein BJ, Lopez BL, Christopher TA, Tian R, Koch W, et al. AMP-activated protein kinase deficiency enhances myocardial ischemia/reperfusion injury but has minimal effect on the antioxidant/antinitrative protection of adiponectin. Circulation. 2009;119(6):835–844. doi: 10.1161/CIRCULATIONAHA.108.815043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kondo K, Shibata R, Unno K, Shimano M, Ishii M, Kito T, Shintani S, Walsh K, Ouchi N, Murohara T. Impact of a single intracoronary administration of adiponectin on myocardial ischemia/reperfusion injury in a pig model. Circ Cardiovasc Interv. 2010;3(2):166–173. doi: 10.1161/CIRCINTERVENTIONS.109.872044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Shao ML, Cao H, Ke YS. Protective efects of adiponectin on myocardial ischemia-reperfusion injury in rats. Chin J Cardiol. 2010;38(3):252–8. [PubMed] [Google Scholar]
- 31.Chen J: Protective effect and Mechanisms of different concentrations of Adiponectin on Oxidative Stress after Ischemia-reperfusion in Diabatic Rats. Shanxi Medical University 2011.
- 32.Liu S, Yin T, Wei X, Yi W, Qu Y, Liu Y, Wang R, Lian K, Xia C, Pei H, et al. Downregulation of adiponectin induced by tumor necrosis factor α is involved in the aggravation of posttraumatic myocardial ischemia/reperfusion injury. Crit Care Med. 2011;39(8):1935–1943. doi: 10.1097/CCM.0b013e31821b85db. [DOI] [PubMed] [Google Scholar]
- 33.Ma Y, Liu Y, Liu S, Qu Y, Wang R, Xia C, Pei H, Lian K, Yin T, Lu X, et al. Dynamic alteration of adiponectin/adiponectin receptor expression and its impact on myocardial ischemia/reperfusion in type 1 diabetic mice. Am J Physiol Endocrinol Metab. 2011;301(3):E447–455. doi: 10.1152/ajpendo.00687.2010. [DOI] [PubMed] [Google Scholar]
- 34.Chen J, Bian YF, Liu Z, Xiao CS. Studies on the protection of adiponectin against myocardial ischemia-reperfusion injury in rats. Chinese Remedies Clinics. 2011;11(02):154–155. [Google Scholar]
- 35.Gao C, Liu Y, Yang Q, Sun L, Tao L, Wang HC. Protective effect of neutralizing TNF-α against myocardial ischemia/reperfusion injury in mice is partially exerted via adiponectin upregulation. Chin Heart J. 2012;24(03):281–286. [Google Scholar]
- 36.Guo J: Protective effect and mechanism of adiponectin on myocardial ischemia-reperfusion injury in rats: inhibition of endoplasmic reticulum stress. Shanxi Medical University 2013.
- 37.Zhang Y, Wang XL, Zhao J, Wang YJ, Lau WB, Yuan YX, Gao EH, Koch WJ, Ma XL. Adiponectin inhibits oxidative/nitrative stress during myocardial ischemia and reperfusion via PKA signaling. Am J Physiol Endocrinol Metab. 2013;305(12):E1436–1443. doi: 10.1152/ajpendo.00445.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo J, Bian YF, Xiao CS, Li ZD. Adiponectin reduces apoptosis by improving myocardial anti-oxidative activity after myocardial ischemia /reperfusion injury in diabetic rats. Chinese Pharmacological Bulletin. 2014;30(05):623–627. [Google Scholar]
- 39.Song Y, Yu Q, Zhang J, Huang W, Liu Y, Pei H, Liu J, Sun L, Yang L, Li C, et al. Increased myocardial ischemia-reperfusion injury in renal failure involves cardiac adiponectin signal deficiency. Am J Physiol Endocrinol Metab. 2014;306(9):E1055–1064. doi: 10.1152/ajpendo.00428.2013. [DOI] [PubMed] [Google Scholar]
- 40.Wang Z, Jin ZX, Yi W, Yi DH. Effects of APPL1 knock-down on the cardioprotective actions of adiponectin against myocardial ischemia /reperfusion injury in mouse. Chinese Journal of Extracorporeal Circulation. 2014;12(02):117–120. [Google Scholar]
- 41.Gao C, Liu Y, Yu Q, Yang Q, Li B, Sun L, Yan W, Cai X, Gao E, Xiong L, et al. TNF-α antagonism ameliorates myocardial ischemia-reperfusion injury in mice by upregulating adiponectin. Am J Physiol Heart Circ Physiol. 2015;308(12):H1583–1591. doi: 10.1152/ajpheart.00346.2014. [DOI] [PubMed] [Google Scholar]
- 42.Zhao LJ, Deng BG, Yang BS, Zhang JY: The role of adiponectin postconditioning on myocardial ischemia reperfusion injury in rats. Chongqing Med. 2016, 45(27):3763–3765+3769.
- 43.Potenza MA, Sgarra L, Nacci C, Leo V, De Salvia MA, Montagnani M. Activation of AMPK/SIRT1 axis is required for adiponectin-mediated preconditioning on myocardial ischemia-reperfusion (I/R) injury in rats. PLoS ONE. 2019;14(1):e0210654. doi: 10.1371/journal.pone.0210654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xing XL, Zheng N, Zhang J, Hu YH, Liu Q: Role of SphK1/S1P signaling pathway in adiponectin-induced restoration of attenuation of myocardial ischemia-reperfusion injury by sevoflurane postconditioning in diabetic rats. Chin J Anesthesiol 2020(6).
- 45.Wang SJ, Tan HQ, Yuan J. Exploring the role of macrophage migration inhibitory factor regulating autophagy in myocardial ischemia-reperfusion injury based on exogenous adiponectin. J Guangxi Med Univ. 2022;39(10):1563–1570. [Google Scholar]
- 46.Mokhtari-Zaer A, Marefati N, Atkin SL, Butler AE, Sahebkar A. The protective role of curcumin in myocardial ischemia-reperfusion injury. J Cell Physiol. 2018;234(1):214–222. doi: 10.1002/jcp.26848. [DOI] [PubMed] [Google Scholar]
- 47.Pei H, Qu Y, Lu X, Yu Q, Lian K, Liu P, Yan W, Liu J, Ma Y, Liu Y, et al. Cardiac-derived adiponectin induced by long-term insulin treatment ameliorates myocardial ischemia/reperfusion injury in type 1 diabetic mice via AMPK signaling. Basic Res Cardiol. 2013;108(1):322. doi: 10.1007/s00395-012-0322-0. [DOI] [PubMed] [Google Scholar]
- 48.Guerre-Millo M. Adiponectin: an update. Diabetes Metab. 2008;34(1):12–18. doi: 10.1016/j.diabet.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Lau WB, Gao E, Tao L, Yuan Y, Li R, Wang X, Koch WJ, Ma XL. Cardiomyocyte-derived adiponectin is biologically active in protecting against myocardial ischemia-reperfusion injury. Am J Physiol Endocrinol Metab. 2010;298(3):E663–670. doi: 10.1152/ajpendo.00663.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yanai H, Yoshida H. Beneficial Effects of Adiponectin on Glucose and Lipid Metabolism and Atherosclerotic Progression: Mechanisms and Perspectives. Int J Mol Sci. 2019;20(5):1190. doi: 10.3390/ijms20051190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peng J, Chen Q, Wu C. The role of adiponectin in cardiovascular disease. Cardiovasc Pathol. 2023;64:107514. doi: 10.1016/j.carpath.2022.107514. [DOI] [PubMed] [Google Scholar]
- 52.Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279(2):1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J Biol Chem. 2004;279(27):28670–28674. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- 54.Essick EE, Wilson RM, Pimentel DR, Shimano M, Baid S, Ouchi N, Sam F. Adiponectin modulates oxidative stress-induced autophagy in cardiomyocytes. PLoS ONE. 2013;8(7):e68697. doi: 10.1371/journal.pone.0068697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qi GM, Jia LX, Li YL, Li HH, Du J. Adiponectin suppresses angiotensin II-induced inflammation and cardiac fibrosis through activation of macrophage autophagy. Endocrinology. 2014;155(6):2254–2265. doi: 10.1210/en.2013-2011. [DOI] [PubMed] [Google Scholar]
- 56.Matsuda M, Shimomura I. Roles of adiponectin and oxidative stress in obesity-associated metabolic and cardiovascular diseases. Rev Endocr Metab Disord. 2014;15(1):1–10. doi: 10.1007/s11154-013-9271-7. [DOI] [PubMed] [Google Scholar]
- 57.Nguyen TMD. Adiponectin: role in physiology and pathophysiology. Int J Prev Med. 2020;11:136. doi: 10.4103/ijpvm.IJPVM_193_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tentolouris N, Doulgerakis D, Moyssakis I, Kyriaki D, Makrilakis K, Kosmadakis G, Stamatiadis D, Katsilambros N, Stathakis C. Plasma adiponectin concentrations in patients with chronic renal failure: relationship with metabolic risk factors and ischemic heart disease. Horm Metab Res. 2004;36(10):721–727. doi: 10.1055/s-2004-826022. [DOI] [PubMed] [Google Scholar]
- 59.Goldstein BJ, Scalia RG, Ma XL. Protective vascular and myocardial effects of adiponectin. Nat Clin Pract Cardiovasc Med. 2009;6(1):27–35. doi: 10.1038/ncpcardio1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Basu R, Pajvani UB, Rizza RA, Scherer PE. Selective downregulation of the high molecular weight form of adiponectin in hyperinsulinemia and in type 2 diabetes: differential regulation from nondiabetic subjects. Diabetes. 2007;56(8):2174–2177. doi: 10.2337/db07-0185. [DOI] [PubMed] [Google Scholar]
- 61.Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, Arita Y, Okamoto Y, Shimomura I, Hiraoka H, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23(1):85–89. doi: 10.1161/01.ATV.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 62.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98(4):2005–2010. doi: 10.1073/pnas.98.4.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ix JH, Shlipak MG, Chertow GM, Whooley MA. Association of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: data from the Heart and Soul Study. Circulation. 2007;115(2):173–179. doi: 10.1161/CIRCULATIONAHA.106.644286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Komura N, Kihara S, Sonoda M, Maeda N, Tochino Y, Funahashi T, Shimomura I. Increment and impairment of adiponectin in renal failure. Cardiovasc Res. 2010;86(3):471–477. doi: 10.1093/cvr/cvp415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary File 1. PRISMA 2020 Checklist. Supplementary Figure 1. Funnel plot and sensitivity analysis of myocardial infarction size. Supplementary Figure 2. Funnel plot and sensitivity analysis. (A and C) LVEDP, (B and D) +dp/dtmax. Supplementary Figure 3. Funnel plot and sensitivity analysis. (A and C) -dp/dtmax, (B and D) LVEF. Supplementary Figure 4. Funnel plot and sensitivity analysis. (A and C) Caspase-3, (B and D) TUNEL-positive cells. Supplementary Figure 5. Funnel plot and sensitivity analysis. (A and C) Superoxide content, (B and D) LDH.
Data Availability Statement
The original contributions shown in the study are selected in the article/supplementary material. Appropriate inquiries can be contacted with the corresponding author.