Abstract
Abnormal inflammatory states in the brain are associated with a variety of brain diseases. The dynamic changes in the number and function of immune cells in cerebrospinal fluid (CSF) are advantageous for the early prediction and diagnosis of immune diseases affecting the brain. The aggregated factors and cells in inflamed CSF may represent candidate targets for therapy. The physiological barriers in the brain, such as the blood‒brain barrier (BBB), establish a stable environment for the distribution of resident immune cells. However, the underlying mechanism by which peripheral immune cells migrate into the brain and their role in maintaining immune homeostasis in CSF are still unclear. To advance our understanding of the causal link between brain diseases and immune cell status, we investigated the characteristics of immune cell changes in CSF and the molecular mechanisms involved in common brain diseases. Furthermore, we summarized the diagnostic and treatment methods for brain diseases in which immune cells and related cytokines in CSF are used as targets. Further investigations of the new immune cell subtypes and their contributions to the development of brain diseases are needed to improve diagnostic specificity and therapy.
Keywords: Brain disease, Immune microenvironment, Cerebrospinal fluid, Disease diagnosis and therapeutics
Background
It was previously believed that the brain was an “immune privileged” site under the protection of the blood‒brain barrier (BBB). However, with the progressive understanding of brain immunity and the rediscovery of meningeal lymphatic vessels [1], the relationships between immune cells and various brain disorders, such as aging, neurodegenerative diseases and multiple sclerosis (MS), have received increasing attention [2, 3]. For instance, studies have shown a close association between aging, neurodegenerative diseases, and impaired microglial function [4, 5]. In addition, abnormal increases in the numbers of CD4+ T cells, B cells, CD8+ T cells and dendritic cells (DCs) [6, 7] are involved in the pathogenesis and progression of MS. Other immune cells in the central nervous system (CNS), including boundary-associated macrophages (BAMs), have also been increasingly recognized in brain diseases [8]. These findings highlight the importance of investigating the immune microenvironment in the brain to understand the development of related brain diseases and design effective treatments or therapies [4–7].
The population and quantities of immune cells within the CNS differ among regions [9]. The distribution of immune cells in different regions of the brain is responsible for maintaining the homeostatic balance between brain structure and functions. In addition to the dura and pia membrane layers that cover the brain surface, the brain possesses several barriers: (i) the pia blood-cerebrospinal fluid (CSF) barrier, (ii) the endothelial BBB, (iii) the choroid plexus (ChP) and the blood-cerebrospinal fluid barrier (BCSFB) [10]. As shown in Fig. 1, the permeability of each barrier varies, and the entry of immune cells into CSF is limited by tight junctions (TJs) in the ChP basement membrane and brain microvascular endothelial cells. Normally, the pia meninges prevent the infiltration of patrolling immune cells into CSF, resulting in a decrease in the diversity and quantity of immune cells closer to the parenchyma [9]. Although the brain is rather sequestered, CSF interacts with the peripheral circulation and has advantages in terms of sample collection, and the ability to sample and analyze CSF may improve the diagnosis of brain disorders. Furthermore, the immune/inflammatory state of the brain may change before symptoms occur [11]. Studies have shown that the immune microenvironment changes during late–middle age prior to obvious signs of aging and the development of neurodegenerative diseases, suggesting that the immune microenvironment may be one of the causes of aging and neurodegenerative diseases. The role of immune cells, especially CD8+ T cells and B cells, as promising therapeutic targets warrants further investigation. However, the correlation between immune cell alterations in CSF and brain disorders has not been definitively established. In this study, we primarily reviewed the alterations in microglia, T cells, B cells, DCs, BAMs and other immune cells in the brain parenchyma, limbic space and CSF associated with aging, neurodegenerative diseases, MS, infectious diseases, and other conditions that affect the brain. We propose that the immune cell alterations within CSF have remarkable potential for predicting the onset and progression of brain disorders. Simultaneously, we summarize the potential origins and migration mechanisms of immune cells into CSF in both physiological and disordered states, aiming to enhance our understanding of these changes to improve the diagnosis and treatment of brain disorders.
Fig. 1.
Main resident immune cells in the brain and their distribution in different brain regions. a Skull bone meninges. Lymphatic vessels shuttle through the dura mater, and immune cells in the dura mater are relatively numerous and abundant. The subarachnoid space is filled with CSF, which contains T cells, a small number of B cells, BAMs/monocytes and DCs. b Choroid plexus. Immune cells can enter the ChP matrix through fenestrated microvessels, but the tight junctions of the ChP basement membrane restrict immune cells in the ChP stroma from entering the CSF, decreasing the number and diversity of these cells in the CSF. c Perivascular space. A barrier formed by the endfeet of astrocytes separates the perivascular space from the brain parenchyma, with a small number and variety of immune cells present. Created with BioRender.com
Alterations in immune cell subpopulation behind several brain disorders
Aging and neurodegenerative diseases
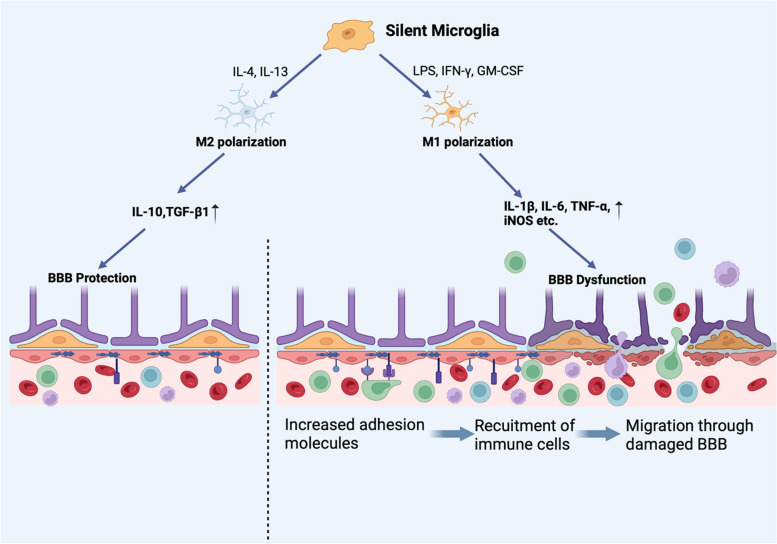
Aging leads to the functional and structural degeneration of many organs, including the brain. Extensive research has been conducted to investigate the promotive effects of inflammation and immune microenvironment disorders on aging and the development of neurodegenerative diseases [2, 4, 12–16]. Microglia, the main resident immune cells in the brain, have been found to play an important role in aging and neurodegenerative diseases. Microglia have several functions in the brain, including executing immune surveillance against infiltrating immune cells, maintaining neuronal function and homeostasis, and activating other immune cell types [17, 18]. Microglia can differentiate into M1 and M2 subsets in response to different stimuli (this will be discussed later in the next section) [19]. M1 microglia induce inflammation and neurotoxicity, whereas M2 microglia exert anti-inflammatory and neuroprotective effects [20]. Thus, alterations in the quantity and function of microglial subsets can result in damage to the brain. Over time, microglia become hyperactivated, and the balance of microglial subsets shifts toward the M1 subset [21]. Moreover, aging neurons exhibit heightened responsiveness to Toll-like receptors (TLRs), which are expressed on neurons and glial cells [22] and are specific markers of the M1 subset, potentially leading to neuronal damage within the brain parenchyma through the activation of immune cell-mediated cytotoxicity and BBB dysfunction (Fig. 2) [2, 23]. In addition, the disruption of microglial sensing functions can initiate or exacerbate neurodegenerative diseases by increasing amyloid-β (Aβ) deposition [18]. The Aβ protein can in turn bind to TLR4 on microglia and trigger the secretion of inflammatory factors [24], which can lead to neuronal damage [19]. and impair the ability of microglia to clear Aβ [18, 25]. Moreover, hyperactivated microglia were found to improperly load pathogenic proteins onto major histocompatibility complex (MHC)-II molecules, initiating adaptive immune-mediated neuronal damages. [26]. In summary, as the most abundant immune cells in the brain, overactivated M1 microglia can promote aging and neurodegenerative diseases through direct neuronal damage, BBB impairment (Fig. 2) and Aβ deposition promotion.
Fig. 2.
Dual effects of microglia on the BBB. Microglia can polarize into M1 and M2 subtypes under different stimuli and can exert destructive and protective effects on the BBB, respectively. Proinflammatory cytokines and iNOS upregulate the expression of adhesion molecules on endothelial cells and cause direct damage to the BBB (TJ, astrocyte endfeet and pericyte degradation), promoting the recruitment and migration of immune cells from the blood. Created with BioRender.com
In addition to microglia, T cells, B cells and monocytes have also been linked to the development of neurodegenerative diseases [2]. CD4+ T cells have been identified as mediators of neurodegeneration [26]. Furthermore, the oversecretion of IFN-γ by CD4+ T cells promotes the infiltration of circulating monocytes into the CNS and causes damage to peripheral neurons [27]. In addition, CD8+ T cells exert a substantial influence on CNS neurodegeneration through MHC-I-mediated activation and through their combined effects with CD4+ T cells [26]. Evidence has also shown increased IgG levels in the CSF of Parkinson's disease (PD) patients [28, 29]. and elevated B-cell infiltration in the brain parenchymas of AD patients, which promotes the deposition of immunoglobulin around Aβ plaques, suggesting a potential role of B cells in the development of neurodegenerative diseases [30].
MS
MS is an autoimmune disease of the CNS characterized by inflammation-induced demyelination, axonal loss and neuronal damage. The pathogenesis of MS is mediated mainly by myelin-reactive CD4+T helper (Th) cells [31]. Under steady-state conditions, the CNS is continuously monitored for damaging agents by T cells in CSF, but T-cell entry into the brain parenchyma is restricted by the BBB [32]. The infiltration of CD4+ T cells into the brain is considered a prominent feature of MS that precedes the onset of symptoms because the accumulation of CD4+ T cells damages the brain parenchyma and contributes to the development of MS. CD4+ T cells encompass multiple subsets, such as Th1, Th2, Th17, follicular T (Tfh) and regulatory T (Treg) cells, among which Th1 and Th17 cells play major roles in MS progression by secreting proinflammatory cytokines (e.g., IFN-γ) [33]. Evidence has suggested that Th1 cells can induce brain tissue damage by activating downstream microglial polarization to the M1 subset (which will be discussed later) [34]. In addition to Th cells, clonal expansion and activation of CD8+T cells have been observed in the CSF of MS patients [35, 36], and some of these cells persist in the CSF for a long time. Moreover, the substantial clonal expansion of CD8+ T cells in CSF may be a sign of disease aggravation. However, the factors that promote the clonal proliferation of CD8+ T cells have not been fully elucidated [37]. Numerous studies have provided evidence of CD8+ T-cell activation and expansion in the CSF, suggesting that these cells contribute to tissue damage [38].
In addition to T cells, DCs and B cells are also involved in the pathogenesis of MS due to their role in antigen presentation [39]. Experimental studies have shown substantial DC infiltration into the brain during experimental allergic encephalomyelitis (EAE) [40]. Moreover, DCs enhance the severity of MS [7]. by activating Th17 cells [41]. Studies have shown that B cells are absent in normal CSF but accumulate in CSF when neuroinflammation occurs [42]. The presence of IgG oligoclonal bands produced by B cells in the brains of MS patients can serve as a diagnostic marker for MS and an indicator of disease severity. Moreover, antibody-secreting B cells can potentially serve as diagnostic markers for relapsing–remitting MS. Memory B cells have also been found to promote the progression of MS by mediating the proliferation of autoreactive CD4+ T cells in a human leukocyte antigen DR (HLA-DR)-dependent manner [43]. B cells in the peripheral immune system can migrate into the brain. Upon reactivation by CD8+ T cells in the brain, they further differentiate into plasma cells that secrete antibodies that damage neuronal myelin proteins, which may account for the presence of oligoclonal bands in CSF [44–48].
Furthermore, microglia and BAMs also play important roles in MS [49]. Microglia can serve as downstream targets of inflammatory factors and directly regulate immune responses [34]. Proinflammatory cytokines and ROS produced by M1 microglia can damage the BBB, which effectively promotes the migration of autoimmune cells into the brain. Previous studies have shown that BAMs have dual effects on MS [50]. On the one hand, they can promote the development of MS by secreting inflammatory factors and recruiting T lymphocytes to induce neurotoxicity in neurons; on the other hand, BAMs have also been found to alleviate MS symptoms by assisting axon regeneration, clearing inhibitory myelin debris and secreting neurotrophic factors.
Infectious diseases
Infectious diseases in the brain can trigger both inflammatory and immune responses. Leukocytes are recruited to the infected CNS through the action of innate immune molecules expressed by various brain cells (e.g., resident macrophages and microglia) [51]. This recruitment facilitates inflammatory responses and promotes pathogen clearance by multiple cell types. Although neuronal damage occurs during acute inflammatory responses, neurological dysfunction caused by infectious diseases may be primarily attributable to the long-term immune response mediated by T cells after pathogen clearance [51]. After the invasion of pathogens into the brain, microglia respond by recognizing pathogen-associated molecular patterns (PAMPs) on the pathogenic microorganisms through their pattern recognition receptors (PRRs) and produce death signals or activate inflammatory mediators such as interleukin 6 (IL-6) family cytokines [52]. This phenomenon is further supported by the finding that a reduction in the expression level of caspase-1, an inflammasome component that when activated leads to the release of IL-1β, has a protective effect on neurons [53]. The relative abundance and activation of microglia vary across different brain regions during nervous system infection [51]. The overactivation of microglia is conducive to local neurodegeneration and contributes to infection-related sequelae. Variations in the relative abundance and activation of microglia during the infection stage may aid in the identification of specific infection sites. However, whether and how microglia mediate infection in the brain remain unclear.
T cells have been implicated in the neurological dysfunction that arises from nervous system infection. Th1-biased cytokines, including IL-1β, tumor necrosis factor (TNF), and IFN-γ, may contribute to persistent cognitive impairment following bacterial meningitis [54]. However, the mechanisms underlying cognitive impairment during acute meningitis remain incompletely understood. It has been shown that neutrophils in the CSF of patients with tuberculous meningitis (TBM) are initially replaced by T and B cells during disease progression and persist for a long period after bacterial clearance [51]. We also observed a significant positive correlation between the quantity of T cells and the clinical severity of disease [55]. While Th1-polarized immune responses contribute to pathogen clearance, the production of IFN-γ by these cells also promotes the development of severe neurological diseases [51]. A comprehensive assessment of the function and antigen specificity of persistent T cells could lead to the establishment of new connections between microbial-specific immunity and psychiatric disorders. Importantly, T cells exhibit a sustained increase even after infection, and their levels correlate with the degree of neurological dysfunction after infection, suggesting that T cells may be new predictive markers for chronic neurodegenerative diseases caused by infection.
Pathogen-induced neuroinflammation can lead to a substantial increase in B cells within CSF [56, 57]. and B-cell accumulation in the periparenchymal and perivascular space [58]. B cells may play multifaceted roles in viral infection, as their protective function and involvement in antibody-mediated viral particle clearance have been demonstrated [59]. In progressive multifocal leukoencephalopathy caused by John Cunningham virus (JCV) infection, B cells not only act as a potential viral reservoir but also activate T cells by secreting cytokines and producing antibodies to promote virus clearance [60]. In conclusion, during the acute inflammatory phase of infectious brain diseases, the activation and recruitment of various immune cells are important for pathogen clearance.
Other brain disorders
Glioblastoma (GBM)
GBM is one of the most common malignant primary tumors in the brain and is known for its high mortality and recurrence rates. Studies have shown that the immunosuppressive tumor microenvironment (TME) plays an important role in GBM treatment failure [61]. The formation of the TME is associated with the microglia, CD8+ T cell and DC dysfunction. Microglia promote the formation of the TME by secreting Th2-biased cytokines [62]. Furthermore, a special subset of microglia known as high-grade glioma-associated microglia (HGG-AM) has been identified as a mediator that shapes the cytokine microenvironment and promotes tumorigenesis [63]. These cells exhibit activated states and display proinflammatory and proliferative characteristics mediated by inflammasomes. GBM also impairs the integrity of the BBB, thereby facilitating the infiltration of CD8+ T cells into the CNS immune microenvironment. Conversely, the accumulation of regulatory T cells and suppressor immune cells in the brains of GBM patients suppresses the function of CD8+ T cells [64]. T-cell activation also occurs through the programmed death-1 receptor and its ligand (PD-1/PDL) pathway and through the cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) pathway [65]. In addition, although DC numbers are elevated in GBM patients, their antigen presentation capacity is attenuated. This inhibitory effect was found to be achieved by GBM cell-derived exosomal LGALS9 (lectin, galactoside-binding soluble 9), which targets DCs in CSF and suppresses the antitumor immunity of CD8+ T cells [66]. Taken together, these findings indicate that the accumulation of HGG-AM, regulatory and inhibitory immune cells, and exosomal LGALS9 in CSF may serve as markers for assessing the development and progression of GBM as well as a target for reversing its immunosuppressive TME status [64].
Autism spectrum disorder (ASD)
The etiology of ASD is complex and not fully understood; however, genetic and environmental risk factors play a role [67, 68]. Studies have indicated that abnormal maternal immune activation (MIA), a common environmental risk factor, causes inflammation and oxidative stress in the placenta and fetal brain, resulting in neurodevelopmental impairments in the developing fetal brain and subsequently causing behavioral symptoms in offspring [69–71]. Inflammatory cytokines produced by maternal infection have persistent effects on fetal development by acting on the placenta or crossing the placenta into the fetal compartment [71–73]. For example, increased levels of IL-6 during pregnancy induced the secretion of IL-17a, which increased the IL-17 receptor levels in the brains of offspring, leading to ASD-related cortical and behavioral abnormalities [72, 74]. In addition, epidemiological and experimental studies have shown that maternal autoantibodies [75]. cross the placental barrier to recognize proteins in the developing fetal brain and affect the development of the nervous system. The ASD subtype resulting from this pathogenesis is termed maternal autoantibody-related (MAR) ASD. In addition to prenatal risk factors, immune dysfunction has also been observed in the brains of children with ASD [71]. In this case, immune dysfunction is manifested by a shift in cell subsets caused by increased inflammatory factors, including eotaxin, IL-6, IL-1 IFN-γ, macrophage inflammatory protein-1α (MIP-1α) and MIP-1 [76–78]. Additionally, there is an increase in baseline natural killer (NK) cell activity. Among these factors, the concentrations of MIP-1α and MIP-1β have been reported to be associated with social behavior disorders [77], and the IL-1β concentration has been reported to be positively correlated with social and nonverbal communication disorder behavior [78]. However, the causal relationship between these inflammatory factors and ASD has not been fully elucidated. Other diseases, such as anxiety-like behavior and acute injury, have also been found to be associated with brain immune status [79, 80], further underscoring its importance.
In summary, numerous brain disorders are accompanied by brain inflammation; however, the causal relationship remains unclear. Notably, there are common immune cell alterations that occur across different brain disorders. For example, in various brain disorders and inflammatory states, there is a prevalent pattern of microglial overactivation accompanied by increased numbers of key immune cell populations, such as CD4+ T cells, CD8+ T cells, B cells, DCs, and BAMs, in the brain (Table 1). Therefore, elucidating the mechanisms underlying these immune cell increases in inflammatory states is important for understanding the etiology of disorders in the brain and for developing therapeutic strategies.
Table 1.
Changes in the number of immune cells in the brain of different diseases
| Diseases | Cell types | Changes | Ref. |
|---|---|---|---|
| Aging and Neurodegenerative Diseases | Microglia |
M1 increase M2 decrease |
[21] [21] |
| CD4+ T cells | Increase | [81] | |
| B cells | Increase | [30] | |
| MS | CD4+ T cells | Increase | [31] |
| CD8+ T cells | Increase | [35, 36] | |
| DCs | Increase | [40] | |
| B cells | Increase | [42] | |
| Infectious Diseases | T cells | Increase in early stages and persist after pathogen clearance | [51] |
| B cells | increase | [57] | |
| GBM | DCs | Increased, with decreased activity | [66] |
| CD8+ T cells | Increased, with decreased activity | [64] | |
| Tregs | Increased | [64] |
Identification of immune cells in CSF to elucidate brain immune changes
The number and functions of immune cells are dramatically altered in patients with various brain disorders. Moreover, the infiltration of peripheral immune cells into CSF and alterations in immunological homeostasis in the brain may occur before disease symptom onset, which suggests that the identification of such alterations may be used for early diagnosis [11]. In addition, analyzing CSF from patients at different stages may be an effective means to determine disease progression. Thus, several diagnostic methods for brain diseases based on immune cell changes in CSF have been proposed [82, 83], providing candidate targets for the early diagnosis and treatment of brain diseases.
Microglia
Microglia are among the most abundant resident immune cells in the brain parenchyma. Recent studies have shown that microglia are derived from CD45−c-kit+ (c-kit proto-oncogene protein) red marrow progenitor cells generated from the yolk sac [84]. During the early stages of embryonic development, differentiated CD45+c-kit−CX3CR1+ microglial progenitors colonize the brain through the blood circulation [84, 85]. The process of microglial development and homeostasis is regulated by a variety of factors, including the transcription factor PU.1 (a hematopoietic cell-specific family transcription factor), unit-related transcription factor 1 (Runx1), interferon regulator factor 8 (IRF-8), colony-stimulating factor 1 receptor 1 (CSF1R), musculoaponeurotic fibrosarcoma oncogene homolog B (MAFB) and its ligands, and noncoding small RNAs (ncRNAs) [86–88]. After being recruited to the brain through the IL-34-CSF1Ra pathway, macrophages subsequently colonize the CNS compartment under the regulation of the short-range signals Lys phosphatidylcholine (LPC) and adenosine triphosphate (ATP), which are generated by neuronal apoptosis [89].
Under normal circumstances, microglia undergo self-renewal to maintain homeostasis, and this process does not involve peripheral cells [90, 91]. Although the mechanism of microglial self-renewal has not been fully elucidated, it has been suggested that microglia maintain longevity and renewal by competing for the trophic factor IL-34. [92]. Microglia polarize into two distinct functional subtypes via a variety of receptors and signaling pathways [34, 93]. The proinflammatory cytokines IFN-γ, TNF-α and GM-CSF, as well as LPS alone, can promote M1 polarization through the Toll-like receptor-4 (TLR-4)/nuclear factor (NF)-κB, Janus tyrosine kinase-signal transducer and activator of transcription 1 (JAK-STAT1)/STAT3 and members of the mitogen-activated protein kinase (MAPK)/NF-κB pathways. M2 polarization can be activated by Th2-biased cytokines such as IL-4 and IL-13. The activation of microglia is also associated with several other receptors, including CX3C chemokine receptor 1 (CX3CR1), triggering receptor expressed on myeloid cells 2 (TREM2), P2X7R and P2Y12R (two purinergic receptors in microglia) [94–97]. The CX3CR1-CX3CL1 pathway can inhibit the activation of microglia (M1) and the release of inflammatory factors upon lipopolysaccharide (LPS) treatment [98]. In an EAE model, elevated expression levels of CX3CR1 in M1 microglia suppressed their excessive activation. In the AD brain, TREM2 is involved in the overactivation of microglia [99]. Microglial proliferation, migration and cytokine secretion can be activated by Aβ, which is subsequently cleared by binding to TREM2 on the surface of microglia. Soluble TREM2 (sTREM2) was found in the CSF of patients with AD and MS [100]. P2X7R is regarded as a key molecule on the surface of the M1 subset and is involved in neuroinflammation and neurodegeneration [96]. However, P2Y12 was found to be downregulated in M1 microglia [101]. and to regulate the translocation of microglia in the brain, which ensures the patrolling of microglia in the brain [97].
CD4+ T cells
Although T cells, especially memory CD4+ T cells [102, 103], are present in the CNS of both healthy individuals and individuals with noninflammatory diseases, their levels are significantly increased in the CSF of individuals with inflammatory diseases [104]. In the inflamed CNS, the expression of cell adhesion molecules (CAMs) and chemokines, including P-selectin, leukocyte function-associated antigen 1 (LFA-1), intercellular adhesion molecule 1 (ICMA-1), α4 integrin, chemokines and their receptors, such as atypical chemokine receptor 1 (ACKR1), are upregulated in vascular endothelial cells, facilitating the recruitment of CD4+T cells to the vascular endothelium to eventually cross the BBB [102, 104, 105]. Moreover, activated CD4+ T cells can cross the perivascular glial boundary and reach the parenchyma through a process mediated by activated matrix metalloproteinase (MMP)-2, MMP-9 and chemokines [10]. Current research has shown that a wide variety of surface receptors, including C–C chemokine receptor (CCR) 6, CCR2 and CCR4, are associated with the migration of CD4+ T cells [106]. Th17 cells can enter CSF through the ChP in a CCR6/CCL20 (C–C chemokine ligand 20)-dependent manner to initiate EAE [107]. After entry, cells independent of CCR6 may be recruited by chemokines released by Th17 cells that previously entered.
Additionally, c-Met+ (a tyrosine kinase receptor) CD4+ T cells are more susceptible to pathogenicity and migration due to their polarization toward proinflammatory phenotypes and upregulated expression of integrin α4 [108]. However, whether c-Met is merely a surface marker of T cells or plays a regulatory function and the pathway that regulates the production of CD4+ c-Met+ subsets remain unclear. Moreover, evidence suggests that aggressive T cells may be activated in the lungs before entering the brain and subsequently upregulate the expression of chemokine receptors and adhesion receptors with enhanced transferability [109], which may also enhance T-cell entry into the CNS and induce autoimmune disease.
CD8+ T cells
Unlike that of CD4+ T cells, the migration of CD8+ T cells into the brain has not been fully elucidated, but their increased numbers and direct damage to neurons illustrate the need to elucidate their migration process. The migration of T cells at different stages is regulated by various chemokines and adhesion molecules. Previous studies have reported that the level of C-X-C chemokine ligand 13 (CXCL13) is significantly increased in CSF and can recruit CXCR5+ (C-X-C chemokine receptor type 5) CD8+ T cells to the brain [110]. Previous studies have also shown that antigen-specific clonal expansion of CD8+ memory T cells occurs in the inflamed brain and that the presence of antigens promotes the migration of CD8+ T cells [37]. These molecules may serve as targets for inhibiting inflammatory damage related to CD8+ T-cell migration in the brain.
DCs
In brain inflammation, the number of myeloid and plasmacytoid DCs increases to varying degrees [111], and most of these DCs have been recruited from peripheral cells. The migration of DCs into the brain is affected by a variety of chemokines and their corresponding receptors [112]. Immature DCs expressing CCR2, CCR3 and CCR5, migrate to CSF and then drain out of CSF to cervical lymph nodes [40]. The increased migration of immature DCs to the CNS is mediated by a variety of inflammatory factors, such as TNF-α and IL-1β [113]. In mature DCs, CCR5 is not expressed, while CCR7 is upregulated [114]. The migration of mature DCs is mediated by the interaction of CCL19 and CCL21 with CCR7 and the CCL12/CXCR4 pathway [115]. Current studies report that immature DCs can interact more effectively with adhesion molecules such as α4β1 integrin expressed by inflamed endothelial cells, increasing the migratory potential of DCs to cross the vascular endothelium and enter the CNS [113]. Thus, immature DCs are more likely to be recruited into brain tissue to cross the BBB and then achieve maturation and promote antigen presentation in the brain [114]. In addition, the subpopulation of recruited DCs differs. CCR5 specifically recruits myeloid DCs (mDCs) to the CSF of patients with MS and acute optic neuritis but has no influence on plasmacytoid DCs [116], suggesting that mDCs may play specific functions in MS pathology. In addition to the increased recruitment of DCs, enhancing the antigen presentation capacity of DCs also contributes to inflammation in the brain. For instance, reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 2 (also known as CYBB/NOX2) in DCs regulates endocytic myelin oligodendrocyte protein (MOG) antigen processing and supports MOG antigen presentation to CD4+ T cells [117]. Based on the importance of DCs in T-cell activation and migration, we speculated that blocking the pathway related to the migration of immature DCs into the brain may be an effective means to inhibit MS [118]. Although the upstream mechanism of DC activation and migration has yet to be explored, the different chemotactic pathways related to mature DCs, immature DCs and other DCs are mediated by different chemokines, indicating that these factors could be used to determine disease stage and type.
B cells
B cells have been found in multiple regions of the CNS in MS patients, including the meninges, lesioned and normal white matter, the cortex, and CSF [119]. Although the distribution of B-cell clones in different brain compartments provides evidence that B cells can cross different CNS barriers, the mechanisms underlying how and where B cells cross these barriers have not been fully elucidated [47]. B-cell recruitment depends on a combination of adhesion-dependent signals and cytokines in the brain, such as very late antigen (VLA)-4, α4β1 integrin, CD44, CXCL13, CXCL12/SDF-1, IL-6, and TNF-α [47]. Among these chemokines, CXCL13 and VLA-4 play important roles. Blocking VLA-4 was found to reduce the number of B cells crossing the CNS barrier, whereas antibodies against vascular cell adhesion molecule 1 (VCAM-1, a ligand of VLA-4) did not [120, 121], indicating that the binding of other alternative CAMs to VLA-4 may be involved in B-cell migration. Moreover, the time-dependent changes in chemokines in the brain may influence the subsets of B cells that enter the CNS at different stages of disease, as B cells display distinct chemotactic signatures in the inflammatory state of the CNS [60]. Studies have shown that B cells in the meninges likely originate from the skull and reach the meninges from the skull through specialized vascular connections, suggesting that the meninges play an important role in the recruitment of B cells [122, 123].
BAMs
Macrophages present in brain boundary regions, known as BAMs, originate from the same primitive macrophages produced by red myeloid progenitor cells in the yolk sac as microglia and are disseminated to the brain in the early stage of embryonic development [124]. Thus, receptors that mediate the development and migration of BAMs largely overlap with those of microglia and include PU.1, IRF8 and CX3CR1 [125]. However, recent studies have shown that only meningeal macrophages and microglia have the same prenatal progenitor cell, while perivascular macrophages originate from meningeal macrophages [126]. CCR1, CCR2 and CCR5 mediate the migration of macrophages to sites of inflammation, but the specific underlying mechanisms have not been elucidated and require further investigation [127].
Moreover, BAMs can be divided into dura, subdural, perivascular and choroid plexus macrophages according to their location. [8]. Recent studies have shown that BAMs have different regeneration capacities and prominent population heterogeneity according to their location [8, 125]. Dural and choroid plexus macrophages are partially dependent on the recruitment of peripheral blood monocytes, while subdural macrophages are rarely replaced by monocytes [128]. Thus, macrophages in the dura and choroid plexus have shorter life spans and are renewed more frequently. Two subgroups of BAMs, MHCIIlo and MHCIIhi, were identified and characterized by different molecular characteristics. The former can be found in the dura and subdura, while the latter is found only in the dura [8]. The MHCIIlo subset has been suggested to have a negative regulatory effect on the immune response [128], while the MHCIIhi subset may be involved in immune monitoring in the dura [8]. Overall, the heterogeneity of BAMs in different regions needs to be fully elucidated to determine their contribution to brain diseases.
Other immune cells
In addition to the abovementioned immune cells, several other cells, including NK cells and astrocytes, also affect immune homeostasis in the CNS. Studies have shown that NK cells exert multiple effects on the CNS [129]. NK cells are characterized by their ability to destroy targeted cells after being recruited by the CX3CL1/CX3CR1 pathway [129]. Therefore, the accumulation of NK cells in the nervous system increases the risk of developing autoimmune inflammatory diseases. In contrast, NKs can inhibit the development of autoimmune diseases in the brain, such as MS, as evidenced by decreased NK cell activity and number in the peripheral blood of MS patients [129, 130]. However, the mechanism by which NK cells suppress autoreactive diseases is not fully understood. Several studies have shown that NK cells can cause T-cell apoptosis by producing IFN-γ [131]. The harmful effect of NK cells is mediated by their ability to damage peripheral nerve cells through bystander effects, providing evidence that NKs promote the development of neurodegenerative diseases [132]. Additionally, IFN-γ secreted by NK cells enhances the antigen presentation ability of APCs by increasing the expression of MHC class II molecules, which promotes the activation of T cells and enhances the inflammatory response [129].
Future studies should also address the interactions of other cells with different subsets of astroglia cells and find ways to stabilize and control their subsets and functional balance, which is highly important for the treatment of neurological diseases.
In conclusion, due to the continuous excavation of the complex structure and cellular function of the brain, an increasing number of immune cells has been found to have an impact on brain diseases. For example, a novel glial progenitor cell (GPC) was recently identified to have both astrocyte and oligodendrocyte gene expression signatures [133]. This study provides a framework for future studies on the function of these two cell types. However, there are still other unidentified immune cells that play a role in brain disease. Moreover, due to the heterogeneity of brain immune cells and immune factors as a result of different regions and sources, clarifying and isolating the multiple functions of known immune cells is also an important direction for future research. Elucidating these scientific mysteries may lead to new strategies for the precise targeted therapy of neurological diseases.
Targeting immune cells for the diagnosis and treatment of brain diseases
The immune microenvironment in the brain is altered in the early stage of many diseases, even before the onset of symptoms. Excessive inflammation in the brain causes nerve damage and functional disorders. Based on the advantages of identifying early changes in the immune microenvironment and the sampling methods mentioned above, we focused on immune cell and immunomodulatory factor alterations in CSF, which may be beneficial for the early diagnosis of neurological diseases. We also summarized the current treatment methods and potential therapeutic targets for the brain disorders mentioned above.
Diagnosis
Many immune cells, factors and chemokines are significantly increased in the CSF of patients with various brain diseases (Tables 1, 2). For example, quantifying B-cell numbers in CSF may be a potential strategy for diagnosing MS, as B-cell clone amplification is a common characteristic of the disease [45]. A diagnostic method based on the monocyte/lymphocyte ratio has been proposed to predict cognitive conditions in aging and neurodegenerative diseases [82]. IgG oligoclonal bands in CSF have also been used to diagnose MS [83]. However, immune diagnosis based on changes in immune cells is currently used as an auxiliary diagnostic method and not as a standard diagnostic therapy because the specific roles of these cells in different diseases have not yet been confirmed. Further elucidation of the contribution of each immune cell type to different diseases may help to improve the specificity of such diagnostic methods.
Table 2.
Chemokines and receptors involved in the migration of immune cells in the brain
| Chemokines | Microglia [134] | Lymphocytes [107, 110, 135] | DCs [7, 114, 136, 137] | B cells [47, 138] | BAMs [27] |
|---|---|---|---|---|---|
| IL-34i | CSF1R | ||||
| CCL20m | CCR6 | ||||
| CCL2i | CCR2 | CCR2 | CCR2 | ||
| CXCL13i | CXCR5 | CXCR5 | |||
| CXCL12i | CXCR4 | CXCR4 | |||
| CCL3i | CCR3 | ||||
| CCL5m | CCR5 | ||||
| CCL9/CCL21i | CCR7 |
I increase in inflammatory state
m increase first and then decrease in inflammatory state
Receptors are expressed on the corresponding immune cells
Although T-cell infiltration in the early stage is present in MS patients and mouse models of EAE, the current diagnosis of MS relies mainly on magnetic resonance imaging (MRI) data combined with the increased levels of oligoclonal IgG and monocytes in CSF [139, 140]. Early diagnosis strategies based on T cells have not yet been established. Importantly, the accumulation of cells characterized by the expression of TNF-α, IFN-γ, IL-2, CXCR4 and VLA4 in the blood of MS patients also provides candidate targets for the early diagnosis of MS [34].
Treatment targets
Microglia
As illustrated above, the balance of microglial polarization is disrupted in the inflamed brain. M1-type microglia secrete pro-inflammatory cytokines and neurotoxic mediators, which worsen neuronal damage. On the other hand, M2-type microglia promote an anti-inflammatory response that aids in repairing. It is crucial to finely regulate the activation of M1 and M2 microglia to minimize damage and maximize protection, as this has significant therapeutic potential. Currently, the main treatment strategy is to inhibit the signaling pathway of M1 microglia and promote the transformation of the M1 subtype to the M2 subtype (Fig. 3). Therefore, reducing the levels of IFN-γ and IFN-β is a potential way to alleviate inflammation in the brain [93]. In addition, antibody neutralization and microRNA (miRNA) interference are major means to specifically inhibit microglial activation and proliferation. miR-124, a highly expressed noncoding small RNA in quiescent microglia, can slow the progression of EAE by promoting microglial quiescence [141]. In addition, promoting Aβ clearance induced by activated TREM2 provides new insight into AD treatment [99]. Moreover, TREM2 has also been found to play an anti-inflammatory role in EAE, but its specific mechanism has not been fully elucidated [98]. Based on the role of P2X7R in microglial development [96]. genetic depletion or pharmacological inhibition of P2X7R was found to ameliorate the symptoms of AD in model mice [142]. However, its effectiveness needs to be further verified in AD patients.
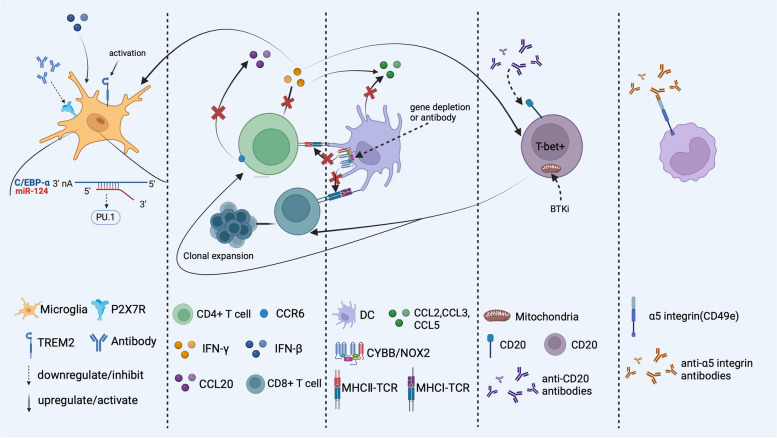
Fig. 3.
Therapeutic targets for brain disorders Inhibition of P2X7R and activation of TREM2 can promote the transition of microglia to the M2 subtype. miRNA-124 inhibits PU.1-induced microglial proliferation by reducing the synthesis of C/EBP-α. CCR2 is a target for reducing the recruitment of CD4+ T cells to the brain. IFN-γ secreted by CD4 + T cells can induce the activation of the M1 subset, while IFN-β promotes the activation of M2 and B cells and increases the levels of CCL3 and CCL5, which are responsible for immature DC recruitment. CYBB/NOX2, which can improve the antigen presentation capability of DCs, is another target for an excessive immune response and can be inhibited by gene depletion or antibody neutralization. Anti-CD20 antibodies can reduce the number of T-bet+ B cells induced by IFN-γ. BTKis can also inhibit the function of B cells by inhibiting mitochondrial respiration and thus reduce the activation of T cells. The α5 integrin is a target for reducing the recruitment and infiltration of monocytes in the CNS. Created with BioRender.com
T cells
Since CD4+ T cells play a major role in MS, reducing the recruitment of overactivated CD4+ T cells to the brain is an important strategy for alleviating MS. Moreover, as adhesion molecules help T cells and other immune cells migrate to the brain [105], targeting the inhibition of adhesion molecules may be a new therapy [143]. In addition, chemokine receptors related to T-cell migration, such as CCR6 and CCR2 (Fig. 3), can also be targeted to reduce T-cell infiltration in the brain [107, 108, 144]. Inflammatory factors secreted by T cells, such as IFN-γ (Fig. 3), TNF-α and IL-1β, also affect the infiltration and function of other immune cells, suggesting the importance of suppressing T-cell hyperactivation [113, 145–148]. In addition to traditional antibody targeting and gene silencing, current studies have shown that nanomedicine also exhibits a suitable inhibitory effect on the expression of inflammatory factors [149]. However, IFN-γ has been shown to indirectly aid in the early treatment of EAE [150], which is contradictory to the known effects of IFN-γ, and the mechanism underlying the regulation or inhibition of neuroinflammation needs to be further explored. Based on the hypothesis that clonal proliferation of CD8+ T cells in MS may be associated with EBV infection [37], preventing viral infection or peripheral escape may also be effective at inhibiting the onset of MS. Recent studies have shown that ferroptosis is an early event in EAE and can promote T-cell activation in vitro and in vivo; thus, ferroptosis is a potential therapeutic target for MS [151].
DCs
Strategies for eliminating DCs include reducing the recruitment of peripheral DCs, especially immature DCs, to the brain and reducing the antigen-presenting ability of DCs. Depletion of chemokines has been shown to reduce DC recruitment to the brain and slow the progression of EAE [115] (Fig. 3). Moreover, genetic depletion of Cybb in cDCs has also been shown to reduce the excessive entry of T cells into the CNS and thus slow the progression of EAE [117] (Fig. 3).
B cells
In response to immunoglobulin production and B-cell lineage expansion in the CSF of MS patients, B-cell antigen-driven maturation and B-cell depletion therapy have been used to treat MS [152]. Antibody-induced depletion of B cells has been found to be effective at alleviating disease symptoms and has gradually attracted increasing amounts of attention [30, 47, 153]. Bruton's tyrosine kinase (BTK) is an important downstream molecule of B-cell receptors. BTK inhibitor (BTKi) has been found to limit the proinflammatory activation of B cells and T cells by inhibiting mitochondrial respiration in B cells [154] (Fig. 3). The enrichment of CD20 on IFN-γ-induced T-bet-expressing IgG+ B cells indicates that T-bet-expressing IgG+ T-cell subsets are important therapeutic targets [6].
Monocytes/BAMs
Because the number of monocytes recruited to the brain increases significantly under inflammatory conditions, reducing the number of migration receptors on the surface of monocytes is a promising inhibitory effect. CD49e, an α5 integrin, is specifically expressed on peripheral monocytes but not on the resident myeloid cell population. Treatment with the anti-CD40e antibody blocked the migration of peripheral monocytes into the brain and significantly slowed the progression and severity of EAE [155]. In addition, transient depletion or suppression of Tregs facilitates monocyte/macrophage recruitment to the brain and the clearance of amyloid, which further ameliorates neuroinflammation and reverses cognitive decline [156].
In summary, most current therapies tend to inhibit B cells and target receptors and factors that cause the abnormal activation, proliferation, or recruitment of immune cells to the treatment site using antibodies. The method of silencing genes through miRNAs has also been used [157]. However, the specificity and efficacy of these targets cannot be guaranteed at present.
Overall, the source and specific pathway through which immune cells enter the CNS have not been fully elucidated, and the contributions of different immune cell abnormalities to neurodegenerative diseases is also unclear. Moreover, whether treatment against these immune targets causes other side effects remains to be explored. More work is needed to address these knowledge gaps and to improve the diagnostic specificity and treatment efficacy.
Conclusions
The immune microenvironment plays an important role in maintaining the normal physiological functions of the brain. As outlined in the Introduction, even under physiological conditions, the distribution of immune cells in the brain is not homogeneous because of the regions formed by barriers. The CSF can provide fundamental information about the inflammatory processes circumscribed to the CNS and reflects changes in the immunological pattern due to the progression of the pathology, and hence be used as a relatively non-invasive liquid biopsy allowing the most accurate measurement of the degree of blood-CSF barrier permeability. The abnormalities in the number, function, location and the excessive activation state of immune cells in CSF, including those in microglia, T cells, B cells, DCs, and BAMs/monocytes cause the promoted inflammatory state of the brain, resulting in direct or indirect damage to neurons and facilitating the progression of symptoms in a variety of brain diseases. These findings highlight the utility of measuring CSF immune changes to identify disease-associated neuroinflammation and to reflect the pathobiological events of brain disorders.
Moreover, because the analysis of immune cells of the CSF can provide a non-invasive alternative to predict the onset of brain disorders, immune cells appear to be potential targets for the diagnosis and treatment of certain diseases. Several researchers have proposed that brain diseases can be predicted or diagnosed by determining the number or fraction of immune cells in CSF. Although further research is needed to be performed to enhance the specificity and sensitivity of these indices, CSF immunophenotyping may be useful to gain further insight into the regulator cells and cytokines involved in the pathophysiology of barrier and function impairment. Treatment-related therapies targeting Tregs, B cells, and DCs have shown some efficacy in animal models, inspiring investigations to identify immune cells that could be new diagnostic and therapeutic targets, and to elucidate the unknown mechanisms and significance underlying immune cell migration, cell proliferation and activation in the brain microenvironment.
Acknowledgements
We thank members of our laboratory for advice and consultation for this work.
Abbreviations
- CSF
Cerebrospinal fluid
- DCs
Dendritic cells
- MS
Multiple sclerosis
- BAMs
Boundary-associated macrophages
- BBB
Blood-brain barrier
- ChP
Choroid plexus
- BCSFB
Blood-cerebrospinal fluid barrier
- CNS
Central nerves system
- TJ
Tight junction
- TLRs
Toll-like receptors
- JAK-STAT
Janus tyrosine Kinase- Signal Transducer and Activator of Transcription
- IFN-γ
Interferon gamma
- Aβ
Amyloid-β
- AD
Alzheimer's disease
- MHC II
Major Histocompatibility Complex II
- PD
Parkinson's Disease
- Th
T helper cells
- Tfh
Follicular T cells
- Treg
Regulatory T cells
- EAE
Experimental allergic encephalomyelitis
- HLA-DR
Human leukocyte Antigen DR
- EBV
Epstein-Barr-Virus
- PAMPs
Pathogen-associated molecular patterns
- PRRs
Pattern recognition receptors
- L-6
Interleukin 6
- TNF
Tumor necrosis factor
- TBM
Tuberculous meningitis
- JCV
John Cunningham virus
- GBM
Glioblastoma
- TME
Tumor microenvironment
- HGG-AM
High-grade glioma-associated microglia
- PD-1/ PDL
Programmed death-1 receptor and its ligand
- CTLA-4
Cytotoxic T-lymphocyte-associated antigen 4
- LGALS9
Lectin, Galactoside-Binding Soluble 9
- ASD
Autism Spectrum Disorder
- MAR
Maternal autoantibody-related
- NK
Natural killer cells
- c-kit
C-kit: proto-oncogene protein
- PU.1
A hematopoietic cell–specific ets family transcription factor
- Runx1
Unt-related transcription factor 1
- IRF-8
Interferon regulator factor 8
- CSF1R
Colony-stimulating factor 1 receptor 1
- MAFB
Musculoaponeurotic fibrosarcoma oncogene homolog B
- NcRNAs
Non-coding small RNAs
- LPC
Lys phosphatidylcholine
- ATP
Adenosine triphosphate
- Msr1
Macrophage scavenger receptor 1
- DAMPs
Damage associated molecular patterns
- Mafba and Mafbb
Analogues of mammalian transcription factor MAFB in zebrafish
- Gpr34a
G-protein-coupled receptor 34a
- CX3CR1
CX3C chemokine receptor1
- TREM2
Triggering receptor expressed on myeloid cells 2
- LPS
Lipopolysaccharide
- P2X7R, P2Y12R
Purinergic receptor in microglia
- sTREM2
Soluble TREM2
- CAMs
Cell adhesion molecules
- LFA
Leukocyte Function-associated Antigen
- ICMA-1
Intercellular adhesion molecule-1
- ACKR1
Atypical Chemokine Receptor 1
- MMP
Matrix metalloproteinase
- CCR
C-C chemokine receptor
- CCL
C-C chemokine ligand
- c-Met
A tyrosine kinase receptor
- CXCR5
C-X-C chemokine receptor type 5
- APCs
Antigen presenting cells
- mDC
Myeloid DCs
- NADPH
Nicotinamide adenine dinucleotide phosphate
- CYBB/NOX2
Nicotinamide adenine dinucleotide phosphate oxidase 2
- MOG
Myelin oligodendrocyte protein
- VLA
Very late antigen
- GPC
Glial progenitor cell
- MRI
Magnetic Resonance Imaging
- miRNA
Micro-RNA
- BTK
Bruton's Tyrosine Kinase
- BTKi
BTK inhibitor
Authors’ contributions
XT X conducted the initial literature search and drafted the manuscript; SJ L provided guidance and expertise in the field; J M and Y H checked and edited the manuscript for accuracy and clarity; BL Z and QZ R revised the manuscript for important intellectual content. All authors have read and approved the final version of the manuscript and have taken full responsibility for its content.
Funding
This work was supported under grants from the National Natural Science Foundation of China (grant numbers: 22076210, 22150006 and 22021003), the Youth Innovation Promotion Association of Chinese Academy of Science (2022042), the National Key Research and Development Program of China (grant number: 2018YFA0901101 and 2021YFE0101500).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yi Han, Email: hanyi@bucm.edu.cn.
Juan Ma, Email: juanm@rcees.ac.cn.
References
- 1.Alves de Lima K, Rustenhoven J, Kipnis J. Meningeal Immunity and Its Function in Maintenance of the Central Nervous System in Health and Disease. Annu Rev Immunol. 2020;38:597–620. doi: 10.1146/annurev-immunol-102319-103410. [DOI] [PubMed] [Google Scholar]
- 2.Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64:110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasper LH, Shoemaker J. Multiple sclerosis immunology: The healthy immune system vs the MS immune system. Neurology. 2010;74(Suppl 1):S2–8. doi: 10.1212/WNL.0b013e3181c97c8f. [DOI] [PubMed] [Google Scholar]
- 4.Czirr E, Wyss-Coray T. The immunology of neurodegeneration. J Clin Invest. 2012;122:1156–1163. doi: 10.1172/JCI58656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patterson SL. Immune dysregulation and cognitive vulnerability in the aging brain: Interactions of microglia, IL-1β. BDNF and synaptic plasticity Neuropharmacology. 2015;96:11–18. doi: 10.1016/j.neuropharm.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Langelaar J, Rijvers L, Smolders J, van Luijn MM. B and T Cells Driving Multiple Sclerosis: Identity. Mechanisms and Potential Triggers Front Immunol. 2020;11:760. doi: 10.3389/fimmu.2020.00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sagar D, Lamontagne A, Foss CA, Khan ZK, Pomper MG, Jain P. Dendritic cell CNS recruitment correlates with disease severity in EAE via CCL2 chemotaxis at the blood-brain barrier through paracellular transmigration and ERK activation. J Neuroinflammation. 2012;9:245. doi: 10.1186/1742-2094-9-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mildenberger W, Stifter SA, Greter M. Diversity and function of brain-associated macrophages. Curr Opin Immunol. 2022;76:102181. doi: 10.1016/j.coi.2022.102181. [DOI] [PubMed] [Google Scholar]
- 9.Mundt S, Greter M, Becher B. The CNS mononuclear phagocyte system in health and disease. Neuron. 2022;110:3497–3512. doi: 10.1016/j.neuron.2022.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Mapunda JA, Tibar H, Regragui W, Engelhardt B. How Does the Immune System Enter the Brain? Front Immunol. 2022;13:805657. [DOI] [PMC free article] [PubMed]
- 11.Cribbs DH, Berchtold NC, Perreau V, Coleman PD, Rogers J, Tenner AJ, Cotman CW. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: a microarray study. J Neuroinflammation. 2012;9:1–18. doi: 10.1186/1742-2094-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chitnis T, Weiner HL. CNS inflammation and neurodegeneration. J Clin Investig. 2017;127:3577–3587. doi: 10.1172/JCI90609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/S0197-4580(00)00124-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhusal A, Rahman MH, Suk K. Hypothalamic inflammation in metabolic disorders and aging. Cell Mol Life Sci. 2021;79:32. doi: 10.1007/s00018-021-04019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stephenson J, Nutma E, van der Valk P, Amor S. Inflammation in CNS neurodegenerative diseases Immunology. 2018;154:204–219. doi: 10.1111/imm.12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- 17.Matt SM, Johnson RW. Neuro-immune dysfunction during brain aging: new insights in microglial cell regulation. Curr Opin Pharmacol. 2016;26:96–101. doi: 10.1016/j.coph.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hickman S, Izzy S, Sen P, Morsett L, El Khoury J. Microglia in neurodegeneration. Nat Neurosci. 2018;21:1359–1369. doi: 10.1038/s41593-018-0242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown GC, Vilalta A. How microglia kill neurons. Brain Res. 2015;1628:288–297. doi: 10.1016/j.brainres.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 20.Guo S, Wang H, Yin Y. Microglia Polarization From M1 to M2 in Neurodegenerative Diseases. Front Aging Neurosci. 2022;14:815347. doi: 10.3389/fnagi.2022.815347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Y, Le W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol Neurobiol. 2016;53:1181–1194. doi: 10.1007/s12035-014-9070-5. [DOI] [PubMed] [Google Scholar]
- 22.Letiembre M, Hao W, Liu Y, Walter S, Mihaljevic I, Rivest S, Hartmann T, Fassbender K. Innate immune receptor expression in normal brain aging. Neuroscience. 2007;146:248–254. doi: 10.1016/j.neuroscience.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Haruwaka K, Ikegami A, Tachibana Y, Ohno N, Konishi H, Hashimoto A, Matsumoto M, Kato D, Ono R, Kiyama H, et al. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat Commun. 2019;10:5816. doi: 10.1038/s41467-019-13812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walter S, Letiembre M, Liu Y, Heine H, Penke B, Hao W, Bode B, Manietta N, Walter J, Schulz-Schuffer W, Fassbender K. Role of the toll-like receptor 4 in neuroinflammation in Alzheimer's disease. Cell Physiol Biochem. 2007;20:947–956. doi: 10.1159/000110455. [DOI] [PubMed] [Google Scholar]
- 25.Gold M, El Khoury J. β-amyloid, microglia, and the inflammasome in Alzheimer's disease. Semin Immunopathol. 2015;37:607–611. doi: 10.1007/s00281-015-0518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams GP, Schonhoff AM, Jurkuvenaite A, Gallups NJ, Standaert DG, Harms AS. CD4 T cells mediate brain inflammation and neurodegeneration in a mouse model of Parkinson's disease. Brain. 2021;144:2047–2059. doi: 10.1093/brain/awab103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drevets DA, Dillon MJ, Schawang JE, Stoner JA, Leenen PJ. IFN-gamma triggers CCR2-independent monocyte entry into the brain during systemic infection by virulent Listeria monocytogenes. Brain Behav Immun. 2010;24:919–929. doi: 10.1016/j.bbi.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Horvath I, Iashchishyn IA, Forsgren L, Morozova-Roche LA. Immunochemical Detection of α-Synuclein Autoantibodies in Parkinson's Disease: Correlation between Plasma and Cerebrospinal Fluid Levels. ACS Chem Neurosci. 2017;8:1170–1176. doi: 10.1021/acschemneuro.7b00063. [DOI] [PubMed] [Google Scholar]
- 29.Akhtar RS, Licata JP, Luk KC, Shaw LM, Trojanowski JQ, Lee VM. Measurements of auto-antibodies to α-synuclein in the serum and cerebral spinal fluids of patients with Parkinson's disease. J Neurochem. 2018;145:489–503. doi: 10.1111/jnc.14330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim K, Wang X, Ragonnaud E, Bodogai M, Illouz T, DeLuca M, McDevitt RA, Gusev F, Okun E, Rogaev E, Biragyn A. Therapeutic B-cell depletion reverses progression of Alzheimer's disease. Nat Commun. 2021;12:2185. doi: 10.1038/s41467-021-22479-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sawcer S, Hellenthal G, Pirinen M, Spencer CCA, Patsopoulos NA, Moutsianas L, Dilthey A, Su Z, Freeman C, Hunt SE, et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476:214–219. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smolders J, van Luijn MM, Hsiao CC, Hamann J. T-cell surveillance of the human brain in health and multiple sclerosis. Semin Immunopathol. 2022;44:855–867. doi: 10.1007/s00281-022-00926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Høglund RA, Maghazachi AA. Multiple sclerosis and the role of immune cells. World J Exp Med. 2014;4:27–37. doi: 10.5493/wjem.v4.i3.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krishnarajah S, Becher B. T(H) Cells and Cytokines in Encephalitogenic Disorders. Front Immunol. 2022;13:822919. doi: 10.3389/fimmu.2022.822919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neumann H, Medana IM, Bauer J, Lassmann H. Cytotoxic T lymphocytes in autoimmune and degenerative CNS diseases. Trends Neurosci. 2002;25:313–319. doi: 10.1016/S0166-2236(02)02154-9. [DOI] [PubMed] [Google Scholar]
- 36.Skulina C, Schmidt S, Dornmair K, Babbe H, Roers A, Rajewsky K, Wekerle H, Hohlfeld R, Goebels N. Multiple sclerosis: brain-infiltrating CD8+ T cells persist as clonal expansions in the cerebrospinal fluid and blood. Proc Natl Acad Sci U S A. 2004;101:2428–2433. doi: 10.1073/pnas.0308689100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gate D, Saligrama N, Leventhal O, Yang AC, Unger MS, Middeldorp J, Chen K, Lehallier B, Channappa D, De Los Santos MB, et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer's disease. Nature. 2020;577:399–404. doi: 10.1038/s41586-019-1895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mars LT, Saikali P, Liblau RS, Arbour N. Contribution of CD8 T lymphocytes to the immuno-pathogenesis of multiple sclerosis and its animal models. Biochim Biophys Acta. 2011;1812:151–161. doi: 10.1016/j.bbadis.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weissert R. The immune pathogenesis of multiple sclerosis. J Neuroimmune Pharmacol. 2013;8:857–866. doi: 10.1007/s11481-013-9467-3. [DOI] [PubMed] [Google Scholar]
- 40.Hatterer E, Touret M, Belin MF, Honnorat J, Nataf S. Cerebrospinal fluid dendritic cells infiltrate the brain parenchyma and target the cervical lymph nodes under neuroinflammatory conditions. PLoS ONE. 2008;3:e3321. doi: 10.1371/journal.pone.0003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, Noelle RJ, Becher B. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- 42.Cepok S, von Geldern G, Grummel V, Hochgesand S, Celik H, Hartung H, Hemmer B. Accumulation of class switched IgD-IgM- memory B cells in the cerebrospinal fluid during neuroinflammation. J Neuroimmunol. 2006;180:33–39. doi: 10.1016/j.jneuroim.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 43.Jelcic I, Al Nimer F, Wang J, Lentsch V, Planas R, Jelcic I, Madjovski A, Ruhrmann S, Faigle W, Frauenknecht K, et al. Memory B Cells Activate Brain-Homing, Autoreactive CD4(+) T Cells in Multiple Sclerosis. Cell. 2018;175:85–100.e123. doi: 10.1016/j.cell.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veroni C, Aloisi F. The CD8 T Cell-Epstein-Barr Virus-B Cell Trialogue: A Central Issue in Multiple Sclerosis Pathogenesis. Front Immunol. 2021;12:665718. doi: 10.3389/fimmu.2021.665718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Owens GP, Ritchie AM, Burgoon MP, Williamson RA, Corboy JR, Gilden DH. Single-cell repertoire analysis demonstrates that clonal expansion is a prominent feature of the B cell response in multiple sclerosis cerebrospinal fluid. J Immunol. 2003;171:2725–2733. doi: 10.4049/jimmunol.171.5.2725. [DOI] [PubMed] [Google Scholar]
- 46.Petzold A. Intrathecal oligoclonal IgG synthesis in multiple sclerosis. J Neuroimmunol. 2013;262:1–10. doi: 10.1016/j.jneuroim.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 47.Meinl E, Krumbholz M, Hohlfeld R. B lineage cells in the inflammatory central nervous system environment: migration, maintenance, local antibody production, and therapeutic modulation. Ann Neurol. 2006;59:880–892. doi: 10.1002/ana.20890. [DOI] [PubMed] [Google Scholar]
- 48.Enose-Akahata Y, Azodi S, Smith BR, Billioux BJ, Vellucci A, Ngouth N, Tanaka Y, Ohayon J, Cortese I, Nath A, Jacobson S. Immunophenotypic characterization of CSF B cells in virus-associated neuroinflammatory diseases. PLoS Pathog. 2018;14:e1007042. doi: 10.1371/journal.ppat.1007042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Papiri G, D'Andreamatteo G, Cacchiò G, Alia S, Silvestrini M, Paci C, Luzzi S, Vignini A. Multiple Sclerosis: Inflammatory and Neuroglial Aspects. Curr Issues Mol Biol. 2023;45:1443–1470. doi: 10.3390/cimb45020094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rawji KS, Yong VW. The benefits and detriments of macrophages/microglia in models of multiple sclerosis. Clin Dev Immunol. 2013;2013:948976. doi: 10.1155/2013/948976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klein RS, Garber C, Howard N. Infectious immunity in the central nervous system and brain function. Nat Immunol. 2017;18:132–141. doi: 10.1038/ni.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 53.Gerber J, Nau R. Mechanisms of injury in bacterial meningitis. Curr Opin Neurol. 2010;23:312–318. doi: 10.1097/WCO.0b013e32833950dd. [DOI] [PubMed] [Google Scholar]
- 54.Barichello T, Generoso JS, Simões LR, Sharin VG, Ceretta RA, Dominguini D, Comim CM, Vilela MC, Teixeira AL, Quevedo J. Interleukin-1β Receptor Antagonism Prevents Cognitive Impairment Following Experimental Bacterial Meningitis. Curr Neurovasc Res. 2015;12:253–261. doi: 10.2174/1567202612666150605122200. [DOI] [PubMed] [Google Scholar]
- 55.Park KH, Lee MS, Lee SO, Choi SH, Kim YS, Woo JH, Kang JK, Lee SA, Kim SH. Kinetics of T-cell-based assays on cerebrospinal fluid and peripheral blood mononuclear cells in patients with tuberculous meningitis. Korean J Intern Med. 2014;29:793–799. doi: 10.3904/kjim.2014.29.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuenz B, Lutterotti A, Ehling R, Gneiss C, Haemmerle M, Rainer C, Deisenhammer F, Schocke M, Berger T, Reindl M. Cerebrospinal fluid B cells correlate with early brain inflammation in multiple sclerosis. PLoS ONE. 2008;3:e2559. doi: 10.1371/journal.pone.0002559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kowarik MC, Grummel V, Wemlinger S, Buck D, Weber MS, Berthele A, Hemmer B. Immune cell subtyping in the cerebrospinal fluid of patients with neurological diseases. J Neurol. 2014;261:130–143. doi: 10.1007/s00415-013-7145-2. [DOI] [PubMed] [Google Scholar]
- 58.Sabatino JJ, Jr, Pröbstel AK, Zamvil SS. B cells in autoimmune and neurodegenerative central nervous system diseases. Nat Rev Neurosci. 2019;20:728–745. doi: 10.1038/s41583-019-0233-2. [DOI] [PubMed] [Google Scholar]
- 59.Dörner T, Radbruch A. Antibodies and B cell memory in viral immunity. Immunity. 2007;27:384–392. doi: 10.1016/j.immuni.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 60.Durali D. de Goër de Herve MG, Gasnault J, Taoufik Y: B cells and progressive multifocal leukoencephalopathy: search for the missing link. Front Immunol. 2015;6:241. doi: 10.3389/fimmu.2015.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Caragher SP, Hall RR, Ahsan R, Ahmed AU. Monoamines in glioblastoma: complex biology with therapeutic potential. Neuro Oncol. 2018;20:1014–1025. doi: 10.1093/neuonc/nox210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu SY, Watabe K. The roles of microglia/macrophages in tumor progression of brain cancer and metastatic disease. Front Biosci (Landmark Ed) 2017;22:1805–1829. doi: 10.2741/4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu H, Sun Y, Zhang Q, Jin W, Gordon RE, Zhang Y, Wang J, Sun C, Wang ZJ, Qi X, et al. Pro-inflammatory and proliferative microglia drive progression of glioblastoma. Cell Rep. 2021;36:109718. doi: 10.1016/j.celrep.2021.109718. [DOI] [PubMed] [Google Scholar]
- 64.Sokratous G, Polyzoidis S, Ashkan K. Immune infiltration of tumor microenvironment following immunotherapy for glioblastoma multiforme. Hum Vaccin Immunother. 2017;13:2575–2582. doi: 10.1080/21645515.2017.1303582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang H, Dai Z, Wu W, Wang Z, Zhang N, Zhang L, Zeng WJ, Liu Z, Cheng Q. Regulatory mechanisms of immune checkpoints PD-L1 and CTLA-4 in cancer. J Exp Clin Cancer Res. 2021;40:184. doi: 10.1186/s13046-021-01987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang M, Cai Y, Peng Y, Xu B, Hui W, Jiang Y. Exosomal LGALS9 in the cerebrospinal fluid of glioblastoma patients suppressed dendritic cell antigen presentation and cytotoxic T-cell immunity. Cell Death Dis. 2020;11:896. doi: 10.1038/s41419-020-03042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Usui N, Kobayashi H, Shimada S. Neuroinflammation and Oxidative Stress in the Pathogenesis of Autism Spectrum Disorder. Int J Mol Sci. 2023;24:5487. [DOI] [PMC free article] [PubMed]
- 68.Hughes HK, Moreno RJ, Ashwood P. Innate immune dysfunction and neuroinflammation in autism spectrum disorder (ASD) Brain Behav Immun. 2023;108:245–254. doi: 10.1016/j.bbi.2022.12.001. [DOI] [PubMed] [Google Scholar]
- 69.Cotter D, Takei N, Farrell M, Sham P, Quinn P, Larkin C, Oxford J, Murray RM, O'Callaghan E. Does prenatal exposure to influenza in mice induce pyramidal cell disarray in the dorsal hippocampus? Schizophr Res. 1995;16:233–241. doi: 10.1016/0920-9964(94)E0082-I. [DOI] [PubMed] [Google Scholar]
- 70.Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meltzer A, Van de Water J The Role of the Immune System in Autism Spectrum Disorder. Neuropsychopharmacology. 2017;42:284–298. doi: 10.1038/npp.2016.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Estes ML, McAllister AK. IMMUNOLOGY. Maternal TH17 cells take a toll on baby's brain. Science. 2016;351:919–920. doi: 10.1126/science.aaf2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, Hoeffer CA, Littman DR, Huh JR. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351:933–939. doi: 10.1126/science.aad0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jones KL, Van de Water J Maternal autoantibody related autism: mechanisms and pathways. Mol Psychiatry. 2019;24:252–265. doi: 10.1038/s41380-018-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011;25:40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shen Y, Ou J, Liu M, Shi L, Li Y, Xiao L, Dong H, Zhang F, Xia K, Zhao J. Altered plasma levels of chemokines in autism and their association with social behaviors. Psychiatry Res. 2016;244:300–305. doi: 10.1016/j.psychres.2016.07.057. [DOI] [PubMed] [Google Scholar]
- 78.Enstrom AM, Onore CE, Van de Water JA, Ashwood P Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain Behav Immun. 2010;24:64–71. doi: 10.1016/j.bbi.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alves de Lima K, Rustenhoven J, Da Mesquita S, Wall M, Salvador AF, Smirnov I, Martelossi Cebinelli G, Mamuladze T, Baker W, Papadopoulos Z, et al. Meningeal γδ T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nat Immunol. 2020;21:1421–1429. doi: 10.1038/s41590-020-0776-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ito M, Komai K, Mise-Omata S, Iizuka-Koga M, Noguchi Y, Kondo T, Sakai R, Matsuo K, Nakayama T, Yoshie O, et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature. 2019;565:246–250. doi: 10.1038/s41586-018-0824-5. [DOI] [PubMed] [Google Scholar]
- 81.Brochard V, Combadière B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brochard V, Combadière B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, et al: Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119:182–92. [DOI] [PMC free article] [PubMed]
- 83.Stangel M, Fredrikson S, Meinl E, Petzold A, Stüve O, Tumani H. The utility of cerebrospinal fluid analysis in patients with multiple sclerosis. Nat Rev Neurol. 2013;9:267–276. doi: 10.1038/nrneurol.2013.41. [DOI] [PubMed] [Google Scholar]
- 84.Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald HR. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nayak D, Roth TL, McGavern DB. Microglia development and function. Annu Rev Immunol. 2014;32:367–402. doi: 10.1146/annurev-immunol-032713-120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mendes MS, Majewska AK. An overview of microglia ontogeny and maturation in the homeostatic and pathological brain. Eur J Neurosci. 2021;53:3525–3547. doi: 10.1111/ejn.15225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, Sylvestre V, Stanley ER. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111–120. doi: 10.1182/blood.V99.1.111. [DOI] [PubMed] [Google Scholar]
- 89.Koshida R, Oishi H, Hamada M, Takahashi S. MafB antagonizes phenotypic alteration induced by GM-CSF in microglia. Biochem Biophys Res Commun. 2015;463:109–115. doi: 10.1016/j.bbrc.2015.05.036. [DOI] [PubMed] [Google Scholar]
- 90.Bruttger J, Karram K, Wörtge S, Regen T, Marini F, Hoppmann N, Klein M, Blank T, Yona S, Wolf Y. Genetic cell ablation reveals clusters of local self-renewing microglia in the mammalian central nervous system. Immunity. 2015;43:92–106. doi: 10.1016/j.immuni.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 91.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 92.Yu T, Kuang H, Wu X, Huang Y, Wang J, Wen Z: Cell competition for neuron-derived trophic factor controls the turnover and lifespan of microglia. Sci Adv 2023, 9:eadf9790. [DOI] [PMC free article] [PubMed]
- 93.Li YF, Ren X, Zhang L, Wang YH, Chen T. Microglial polarization in TBI: Signaling pathways and influencing pharmaceuticals. Front Aging Neurosci. 2022;14:901117. doi: 10.3389/fnagi.2022.901117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liang KJ, Lee JE, Wang YD, Ma W, Fontainhas AM, Fariss RN, Wong WT. Regulation of dynamic behavior of retinal microglia by CX3CR1 signaling. Invest Ophthalmol Vis Sci. 2009;50:4444–4451. doi: 10.1167/iovs.08-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zheng H, Jia L, Liu CC, Rong Z, Zhong L, Yang L, Chen XF, Fryer JD, Wang X, Zhang YW, et al. TREM2 Promotes Microglial Survival by Activating Wnt/β-Catenin Pathway. J Neurosci. 2017;37:1772–1784. doi: 10.1523/JNEUROSCI.2459-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Monif M, Burnstock G, Williams DA. Microglia: proliferation and activation driven by the P2X7 receptor. Int J Biochem Cell Biol. 2010;42:1753–1756. doi: 10.1016/j.biocel.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 97.Eyo UB, Mo M, Yi MH, Murugan M, Liu J, Yarlagadda R, Margolis DJ, Xu P, Wu LJ. P2Y12R-Dependent Translocation Mechanisms Gate the Changing Microglial Landscape. Cell Rep. 2018;23:959–966. doi: 10.1016/j.celrep.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mecca C, Giambanco I, Donato R, Arcuri C. Microglia and Aging: The Role of the TREM2-DAP12 and CX3CL1-CX3CR1 Axes. Int J Mol Sci. 2018;19:318. [DOI] [PMC free article] [PubMed]
- 99.Zhao Y, Wu X, Li X, Jiang LL, Gui X, Liu Y, Sun Y, Zhu B, Piña-Crespo JC, Zhang M, et al. TREM2 Is a Receptor for β-Amyloid that Mediates Microglial Function. Neuron. 2018;97:1023–1031.e1027. doi: 10.1016/j.neuron.2018.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Piccio L, Deming Y, Del-Águila JL, Ghezzi L, Holtzman DM, Fagan AM, Fenoglio C, Galimberti D, Borroni B, Cruchaga C. Cerebrospinal fluid soluble TREM2 is higher in Alzheimer disease and associated with mutation status. Acta Neuropathol. 2016;131:925–933. doi: 10.1007/s00401-016-1533-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang Q, Yao H, Liu W, Ya B, Cheng H, Xing Z, Wu Y. Microglia Polarization in Alzheimer's Disease: Mechanisms and a Potential Therapeutic Target. Front Aging Neurosci. 2021;13:772717. doi: 10.3389/fnagi.2021.772717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kivisäkk P, Mahad DJ, Callahan MK, Trebst C, Tucky B, Wei T, Wu L, Baekkevold ES, Lassmann H, Staugaitis SM, et al. Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci U S A. 2003;100:8389–8394. doi: 10.1073/pnas.1433000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.de Graaf MT, Smitt PA, Luitwieler RL, van Velzen C, van den Broek PD, Kraan J, Gratama JW. Central memory CD4+ T cells dominate the normal cerebrospinal fluid. Cytometry B Clin Cytom. 2011;80:43–50. doi: 10.1002/cyto.b.20542. [DOI] [PubMed] [Google Scholar]
- 104.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 105.Engelhardt B, Ransohoff RM. The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol. 2005;26:485–495. doi: 10.1016/j.it.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 106.Cheng W, Chen G. Chemokines and chemokine receptors in multiple sclerosis. Mediators Inflamm. 2014;2014:659206. doi: 10.1155/2014/659206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A, Engelhardt B, Sallusto F. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 108.Benkhoucha M, Tran NL, Breville G, Senoner I, Bradfield PF, Papayannopoulou T, Merkler D, Korn T, Lalive PH. CD4(+)c-Met(+)Itgα4(+) T cell subset promotes murine neuroinflammation. J Neuroinflammation. 2022;19:103. doi: 10.1186/s12974-022-02461-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Odoardi F, Sie C, Streyl K, Ulaganathan VK, Schläger C, Lodygin D, Heckelsmiller K, Nietfeld W, Ellwart J, Klinkert WE, et al. T cells become licensed in the lung to enter the central nervous system. Nature. 2012;488:675–679. doi: 10.1038/nature11337. [DOI] [PubMed] [Google Scholar]
- 110.Zhou L, Kong G, Palmisano I, Cencioni MT, Danzi M, De Virgiliis F, Chadwick JS, Crawford G, Yu Z, De Winter F, et al: Reversible CD8 T cell-neuron cross-talk causes aging-dependent neuronal regenerative decline. Science 2022, 376:eabd5926. [DOI] [PubMed]
- 111.Pashenkov M, Huang YM, Kostulas V, Haglund M, Söderström M, Link H. Two subsets of dendritic cells are present in human cerebrospinal fluid. Brain. 2001;124:480–492. doi: 10.1093/brain/124.3.480. [DOI] [PubMed] [Google Scholar]
- 112.Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Aït-Yahia S, Brière F, Zlotnik A, Lebecque S, Caux C. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chui R, Dorovini-Zis K. Regulation of CCL2 and CCL3 expression in human brain endothelial cells by cytokines and lipopolysaccharide. J Neuroinflammation. 2010;7:1. doi: 10.1186/1742-2094-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sagar D, Foss C, El Baz R, Pomper MG, Khan ZK, Jain P. Mechanisms of dendritic cell trafficking across the blood-brain barrier. J Neuroimmune Pharmacol. 2012;7:74–94. doi: 10.1007/s11481-011-9302-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ricart BG, John B, Lee D, Hunter CA, Hammer DA. Dendritic cells distinguish individual chemokine signals through CCR7 and CXCR4. J Immunol. 2011;186:53–61. doi: 10.4049/jimmunol.1002358. [DOI] [PubMed] [Google Scholar]
- 116.Pashenkov M, Teleshova N, Kouwenhoven M, Kostulas V, Huang YM, Soderstrom M, Link H. Elevated expression of CCR5 by myeloid (CD11c+) blood dendritic cells in multiple sclerosis and acute optic neuritis. Clin Exp Immunol. 2002;127:519–526. doi: 10.1046/j.1365-2249.2002.01779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Keller CW, Kotur MB, Mundt S, Dokalis N, Ligeon LA, Shah AM, Prinz M, Becher B, Münz C, Lünemann JD. CYBB/NOX2 in conventional DCs controls T cell encephalitogenicity during neuroinflammation. Autophagy. 2021;17:1244–1258. doi: 10.1080/15548627.2020.1756678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jain P, Coisne C, Enzmann G, Rottapel R, Engelhardt B. Alpha4beta1 integrin mediates the recruitment of immature dendritic cells across the blood-brain barrier during experimental autoimmune encephalomyelitis. J Immunol. 2010;184:7196–7206. doi: 10.4049/jimmunol.0901404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::AID-ANA3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 120.Alter A, Duddy M, Hebert S, Biernacki K, Prat A, Antel JP, Yong VW, Nuttall RK, Pennington CJ, Edwards DR, Bar-Or A. Determinants of human B cell migration across brain endothelial cells. J Immunol. 2003;170:4497–4505. doi: 10.4049/jimmunol.170.9.4497. [DOI] [PubMed] [Google Scholar]
- 121.Michel L, Grasmuck C, Charabati M, Lécuyer MA, Zandee S, Dhaeze T, Alvarez JI, Li R, Larouche S, Bourbonnière L, et al: Activated leukocyte cell adhesion molecule regulates B lymphocyte migration across central nervous system barriers. Sci Transl Med 2019, 11. [DOI] [PubMed]
- 122.Brioschi S, Wang WL, Peng V, Wang M, Shchukina I, Greenberg ZJ, Bando JK, Jaeger N, Czepielewski RS, Swain A, et al: Heterogeneity of meningeal B cells reveals a lymphopoietic niche at the CNS borders. Science 2021, 373. [DOI] [PMC free article] [PubMed]
- 123.Schafflick D, Wolbert J, Heming M, Thomas C, Hartlehnert M, Börsch AL, Ricci A, Martín-Salamanca S, Li X, Lu IN, et al. Single-cell profiling of CNS border compartment leukocytes reveals that B cells and their progenitors reside in non-diseased meninges. Nat Neurosci. 2021;24:1225–1234. doi: 10.1038/s41593-021-00880-y. [DOI] [PubMed] [Google Scholar]
- 124.Utz SG, See P, Mildenberger W, Thion MS, Silvin A, Lutz M, Ingelfinger F, Rayan NA, Lelios I, Buttgereit A, et al. Early Fate Defines Microglia and Non-parenchymal Brain Macrophage Development. Cell. 2020;181:557–573.e518. doi: 10.1016/j.cell.2020.03.021. [DOI] [PubMed] [Google Scholar]
- 125.Kierdorf K, Masuda T, Jordão MJC, Prinz M. Macrophages at CNS interfaces: ontogeny and function in health and disease. Nat Rev Neurosci. 2019;20:547–562. doi: 10.1038/s41583-019-0201-x. [DOI] [PubMed] [Google Scholar]
- 126.Masuda T, Amann L, Monaco G, Sankowski R, Staszewski O, Krueger M, Del Gaudio F, He L, Paterson N, Nent E, et al. Specification of CNS macrophage subsets occurs postnatally in defined niches. Nature. 2022;604:740–748. doi: 10.1038/s41586-022-04596-2. [DOI] [PubMed] [Google Scholar]
- 127.Eltayeb S, Berg AL, Lassmann H, Wallström E, Nilsson M, Olsson T, Ericsson-Dahlstrand A, Sunnemark D. Temporal expression and cellular origin of CC chemokine receptors CCR1, CCR2 and CCR5 in the central nervous system: insight into mechanisms of MOG-induced EAE. J Neuroinflammation. 2007;4:14. doi: 10.1186/1742-2094-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Van Hove H, Martens L, Scheyltjens I, De Vlaminck K, Pombo Antunes AR, De Prijck S, Vandamme N, De Schepper S, Van Isterdael G, Scott CL, et al. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat Neurosci. 2019;22:1021–1035. doi: 10.1038/s41593-019-0393-4. [DOI] [PubMed] [Google Scholar]
- 129.Shibatomi K, Ida H, Yamasaki S, Nakashima T, Origuchi T, Kawakami A, Migita K, Kawabe Y, Tsujihata M, Anderson P, Eguchi K. A novel role for interleukin-18 in human natural killer cell death: high serum levels and low natural killer cell numbers in patients with systemic autoimmune diseases. Arthritis Rheum. 2001;44:884–892. doi: 10.1002/1529-0131(200104)44:4<884::AID-ANR145>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 130.Hertwig L, Hamann I, Romero-Suarez S, Millward JM, Pietrek R, Chanvillard C, Stuis H, Pollok K, Ransohoff RM, Cardona AE, Infante-Duarte C. CX3CR1-dependent recruitment of mature NK cells into the central nervous system contributes to control autoimmune neuroinflammation. Eur J Immunol. 2016;46:1984–1996. doi: 10.1002/eji.201546194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sanmarco LM, Wheeler MA, Gutiérrez-Vázquez C, Polonio CM, Linnerbauer M, Pinho-Ribeiro FA, Li Z, Giovannoni F, Batterman KV, Scalisi G, et al. Gut-licensed IFNγ(+) NK cells drive LAMP1(+)TRAIL(+) anti-inflammatory astrocytes. Nature. 2021;590:473–479. doi: 10.1038/s41586-020-03116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Backström E, Chambers BJ, Ho EL, Naidenko OV, Mariotti R, Fremont DH, Yokoyama WM, Kristensson K, Ljunggren HG. Natural killer cell-mediated lysis of dorsal root ganglia neurons via RAE1/NKG2D interactions. Eur J Immunol. 2003;33:92–100. doi: 10.1002/immu.200390012. [DOI] [PubMed] [Google Scholar]
- 133.Liu DD, He JQ, Sinha R, Eastman AE, Toland AM, Morri M, Neff NF, Vogel H, Uchida N, Weissman IL. Purification and characterization of human neural stem and progenitor cells. Cell. 2023;186:1179–1194.e1115. doi: 10.1016/j.cell.2023.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, Barrow AD, Diamond MS, Colonna M. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol. 2012;13:753–760. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Heng AHS, Han CW, Abbott C, McColl SR, Comerford I. Chemokine-Driven Migration of Pro-Inflammatory CD4(+) T Cells in CNS Autoimmune Disease. Front Immunol. 2022;13:817473. doi: 10.3389/fimmu.2022.817473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Barratt-Boyes SM, Zimmer MI, Harshyne LA, Meyer EM, Watkins SC, Capuano S, 3rd, Murphey-Corb M, Falo LD, Jr, Donnenberg AD. Maturation and trafficking of monocyte-derived dendritic cells in monkeys: implications for dendritic cell-based vaccines. J Immunol. 2000;164:2487–2495. doi: 10.4049/jimmunol.164.5.2487. [DOI] [PubMed] [Google Scholar]
- 137.Zozulya AL, Reinke E, Baiu DC, Karman J, Sandor M, Fabry Z. Dendritic cell transmigration through brain microvessel endothelium is regulated by MIP-1alpha chemokine and matrix metalloproteinases. J Immunol. 2007;178:520–529. doi: 10.4049/jimmunol.178.1.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kowarik MC, Cepok S, Sellner J, Grummel V, Weber MS, Korn T, Berthele A, Hemmer B. CXCL13 is the major determinant for B cell recruitment to the CSF during neuroinflammation. J Neuroinflammation. 2012;9:93. doi: 10.1186/1742-2094-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Brownlee WJ, Hardy TA, Fazekas F, Miller DH. Diagnosis of multiple sclerosis: progress and challenges. Lancet. 2017;389:1336–1346. doi: 10.1016/S0140-6736(16)30959-X. [DOI] [PubMed] [Google Scholar]
- 140.Hauser SL, Cree BAC. Treatment of Multiple Sclerosis: A Review. Am J Med. 2020;133:1380–1390.e1382. doi: 10.1016/j.amjmed.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α-PU.1 pathway. Nat Med. 2011;17:64–70. doi: 10.1038/nm.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Francistiová L, Bianchi C, Di Lauro C, Sebastián-Serrano Á, de Diego-García L, Kobolák J, Dinnyés A, Díaz-Hernández M. The Role of P2X7 Receptor in Alzheimer's Disease. Front Mol Neurosci. 2020;13:94. doi: 10.3389/fnmol.2020.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Elovaara I, Lällä M, Spåre E, Lehtimäki T, Dastidar P. Methylprednisolone reduces adhesion molecules in blood and cerebrospinal fluid in patients with MS. Neurology. 1998;51:1703–1708. doi: 10.1212/WNL.51.6.1703. [DOI] [PubMed] [Google Scholar]
- 144.Louveau A, Herz J, Alme MN, Salvador AF, Dong MQ, Viar KE, Herod SG, Knopp J, Setliff JC, Lupi AL, et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci. 2018;21:1380–1391. doi: 10.1038/s41593-018-0227-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Murphy AC, Lalor SJ, Lynch MA, Mills KH. Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav Immun. 2010;24:641–651. doi: 10.1016/j.bbi.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 146.Heppner FL, Greter M, Marino D, Falsig J, Raivich G, Hövelmeyer N, Waisman A, Rülicke T, Prinz M, Priller J, et al. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med. 2005;11:146–152. doi: 10.1038/nm1177. [DOI] [PubMed] [Google Scholar]
- 147.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 148.Browne TC, McQuillan K, McManus RM, O'Reilly JA, Mills KH, Lynch MA. IFN-γ Production by amyloid β-specific Th1 cells promotes microglial activation and increases plaque burden in a mouse model of Alzheimer's disease. J Immunol. 2013;190:2241–2251. doi: 10.4049/jimmunol.1200947. [DOI] [PubMed] [Google Scholar]
- 149.Shunhao W, Jingchao L, Mei C, Liting R, Wenya F, Lining X, Xiaolan C, Tian X, Nanfeng Z, Sijin L: Palladium nanoplates scotch breast cancer lung metastasis by constraining epithelial-mesenchymal transition. National Science Review 2020.
- 150.Gran B, Chu N, Zhang GX, Yu S, Li Y, Chen XH, Kamoun M, Rostami A. Early administration of IL-12 suppresses EAE through induction of interferon-gamma. J Neuroimmunol. 2004;156:123–131. doi: 10.1016/j.jneuroim.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 151.Luoqian J, Yang W, Ding X, Tuo QZ, Xiang Z, Zheng Z, Guo YJ, Li L, Guan P, Ayton S, et al. Ferroptosis promotes T-cell activation-induced neurodegeneration in multiple sclerosis. Cell Mol Immunol. 2022;19:913–924. doi: 10.1038/s41423-022-00883-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sellebjerg F, Börnsen L, Khademi M, Krakauer M, Olsson T, Frederiksen JL, Sørensen PS. Increased cerebrospinal fluid concentrations of the chemokine CXCL13 in active MS. Neurology. 2009;73:2003–2010. doi: 10.1212/WNL.0b013e3181c5b457. [DOI] [PubMed] [Google Scholar]
- 153.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 154.Li R, Tang H, Burns JC, Hopkins BT, Le Coz C, Zhang B, de Barcelos IP, Romberg N, Goldstein AC, Banwell BL, et al. BTK inhibition limits B-cell-T-cell interaction through modulation of B-cell metabolism: implications for multiple sclerosis therapy. Acta Neuropathol. 2022;143:505–521. doi: 10.1007/s00401-022-02411-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ajami B, Samusik N, Wieghofer P, Ho PP, Crotti A, Bjornson Z, Prinz M, Fantl WJ, Nolan GP, Steinman L. Single-cell mass cytometry reveals distinct populations of brain myeloid cells in mouse neuroinflammation and neurodegeneration models. Nat Neurosci. 2018;21:541–551. doi: 10.1038/s41593-018-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Baruch K, Rosenzweig N, Kertser A, Deczkowska A, Sharif AM, Spinrad A, Tsitsou-Kampeli A, Sarel A, Cahalon L, Schwartz M. Breaking immune tolerance by targeting Foxp3(+) regulatory T cells mitigates Alzheimer's disease pathology. Nat Commun. 2015;6:7967. doi: 10.1038/ncomms8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang Y, Han JJ, Liang XY, Zhao L, Zhang F, Rasouli J, Wang ZZ, Zhang GX, Li X. miR-23b Suppresses Leukocyte Migration and Pathogenesis of Experimental Autoimmune Encephalomyelitis by Targeting CCL7. Mol Ther. 2018;26:582–592. doi: 10.1016/j.ymthe.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Giovannoni F, Quintana FJ. The Role of Astrocytes in CNS Inflammation. Trends Immunol. 2020;41:805–819. doi: 10.1016/j.it.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Adler O, Zait Y, Cohen N, Blazquez R, Doron H, Monteran L, Scharff Y, Shami T, Mundhe D, Glehr G, et al. Reciprocal interactions between innate immune cells and astrocytes facilitate neuroinflammation and brain metastasis via lipocalin-2. Nature Cancer. 2023;4:401–418. doi: 10.1038/s43018-023-00519-w. [DOI] [PubMed] [Google Scholar]
- 161.Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- 162.Norden DM, Fenn AM, Dugan A, Godbout JP. TGFβ produced by IL-10 redirected astrocytes attenuates microglial activation. Glia. 2014;62:881–895. doi: 10.1002/glia.22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sanmarco LM, Polonio CM, Wheeler MA, Quintana FJ: Functional immune cell-astrocyte interactions. J Exp Med 2021, 218. [DOI] [PMC free article] [PubMed]
- 164.McAlpine CS, Park J, Griciuc A, Kim E, Choi SH, Iwamoto Y, Kiss MG, Christie KA, Vinegoni C, Poller WC, et al. Astrocytic interleukin-3 programs microglia and limits Alzheimer's disease. Nature. 2021;595:701–706. doi: 10.1038/s41586-021-03734-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mayo L, Trauger SA, Blain M, Nadeau M, Patel B, Alvarez JI, Mascanfroni ID, Yeste A, Kivisäkk P, Kallas K, et al. Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nat Med. 2014;20:1147–1156. doi: 10.1038/nm.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.