Abstract
Background:
Real-world data assessing treatment outcomes in patients with hemophilia A in routine clinical practice are limited.
Objective:
To evaluate the effectiveness and safety of octocog alfa in patients with moderate/severe hemophilia A receiving treatment in clinical practice.
Design:
The international Antihemophilic Factor Hemophilia A Outcome Database study is an observational, noninterventional, prospective, multicenter study.
Methods:
This planned interim data read-out was conducted following 7 years of observation of patients receiving octocog alfa (cut-off, 30 June 2020). The primary endpoint was joint health status, assessed by the Gilbert Score. Secondary endpoints included annualized bleeding rates (ABRs), Hemophilia Joint Health Score (HJHS), health-related quality of life, consumption, and safety. This post hoc analysis stratified data by hemophilia severity at baseline [moderate, factor VIII (FVIII) 1–5%; severe, FVIII <1%].
Results:
Of the 711 patients in this analysis, 582 (82%) were receiving prophylaxis with octocog alfa at enrollment, and 498 (70%) had severe disease. Median Gilbert Scores were higher with on-demand therapy versus prophylaxis and scores were comparable in moderate and severe disease. In patients receiving prophylaxis, there was an improvement in HJHS Global Gait Score over 7 years of follow-up overall and in patients with severe disease. ABRs and annualized joint bleeding rates were low across all 7 years. An ABR of zero was reported in 34–56% of prophylaxis patients versus 20–40% in the on-demand group. ABRs were similar in severe and moderate disease. In total, 13/702 (1.9%) patients experienced 18 treatment-related adverse events.
Conclusion:
These data demonstrate the long-term effectiveness and safety of octocog alfa in patients with moderate and severe hemophilia A, especially in those receiving prophylaxis. The high number of patients receiving on-demand treatment experiencing zero bleeds could be due to selection bias within the study, with patients with less severe disease more likely to be receiving on-demand treatment.
Trial registration:
ClinicalTrials.gov: NCT02078427.
Keywords: bleeding, hemarthrosis, hemophilia A, observational study, recombinant factor VIII
Introduction
Joint arthropathy and poor joint health status due to recurrent joint bleeding is a hallmark of hemophilia A and has a deleterious impact on the quality of life (QoL) of people with hemophilia A.1–4 Standard of care for prevention of bleeding and arthropathy in people with hemophilia A has traditionally involved the intravenous replacement of factor VIII (FVIII) either during a bleeding event (on-demand treatment) or continuously to prevent bleed occurrences (referred to as prophylaxis). 1 In clinical trials, FVIII prophylaxis is associated with improved joint health and significantly reduces the incidence of joint and total hemorrhage.5–7
Octocog alfa (recombinant antihemophilic factor; ADVATE®; Baxalta US Inc., a Takeda Company, Lexington, MA, USA) is a recombinant, human, full-length DNA coagulation FVIII. Prophylactic and on-demand administration have been shown to be effective for the prevention and treatment of bleeding episodes, including during surgery, in patients with moderately severe or severe hemophilia A.8–11 Rurioctocog alfa pegol was designed to extend the half-life of octocog alfa by including a covalently conjugated polyethylene glycol polymer. Clinical data have demonstrated equivalent efficacy and safety of rurioctocog alfa pegol in previously treated patients with hemophilia A.12–14
However, real-world data examining the long-term outcomes of FVIII replacement, including the impact on joint health and patient QoL, are limited. Therefore, the international, observational Antihemophilic Factor (recombinant rAHF) Hemophilia A Outcome Database (AHEAD) study was established to evaluate the effectiveness and safety of FVIII replacement in patients with hemophilia A receiving octocog alfa or rurioctocog alfa pegol from 22 countries. This study aims to capture data on patients of all ages with or without a history of inhibitors who are receiving treatment as routine clinical practice (on demand or prophylaxis) and are followed for up to 7 years. A previous analysis investigating bleeding patterns in patients treated with octocog alfa conducted after 3 years of observation has already been published. 15 This analysis presents effectiveness, including joint health status, and safety data from an interim read-out in patients receiving octocog alfa conducted after an additional 4 years, for a total of up to 7 years, of follow-up.
Methods
Objectives
The primary objective of the AHEAD international study is to describe joint health outcomes, assessed using pain, bleeding, and physical exam parameters of the Gilbert Score, in patients receiving octocog alfa or rurioctocog alfa pegol in a routine clinical practice setting. Secondary objectives include hemostatic effectiveness in a variety of clinical settings, hemophilia-related comorbidities, drug-utilization, health-related QoL (HRQoL) assessments, as well as safety and immunogenicity. The analysis described in this manuscript focuses only on data collected during octocog alfa treatment, as there were very few patients receiving rurioctocog alfa pegol during this study period.
Patients
Eligible patients have either moderate (FVIII 1–5%) or severe hemophilia A (FVIII <1%) and had been prescribed octocog alfa or rurioctocog alfa pegol, in accordance with their respective indications, by their treating physician before study enrollment. Patients of any age, gender, and ethnicity are eligible for the study. Patients included those who were previously treated as well as previously untreated patients, defined as naïve to FVIII exposure at the start of the study, and minimally treated patients, defined as patients with 1–4 prior exposure days to FVIII at the start of the study. Exclusion criteria include known hypersensitivity to the active substance or any of the excipients, known allergic reaction to mouse or hamster proteins, or participation in another clinical study involving an investigational product or device within 30 days before study enrollment.
Study design
AHEAD international is an ongoing observational, post-authorization, noninterventional, prospective, multicenter, database study (ClinicalTrials.gov: NCT02078427). The treatment regimen, including frequency and dosing of on-demand and prophylaxis using standardized regimens or individual pharmacokinetic (PK)-guided dosing regimens, or immune tolerance induction (ITI) therapy, as well as the occurrence of laboratory, radiologic, and clinical monitoring, is decided by the treating clinician. Study visits coincide with routinely scheduled and emergency visits. Joint health assessments were conducted according to routine clinical practice in each country. From February 2018, patients who were receiving octocog alfa at baseline were permitted to switch to rurioctocog alfa pegol during the study. Data for the primary endpoint of joint health outcomes are collected for up to approximately 12 years from the time of study enrollment, with secondary outcome data collected for up to 8 years for patients receiving octocog alfa or rurioctocog alfa pegol alone. Patients who switch from octocog alfa to rurioctocog alfa pegol during the study are followed up for at least 4 years after the switch, for a total of up to approximately 12 years follow-up. Patients who switch to other factor concentrates or non-factor therapies stop being followed at the time of this switch. Full details of the study design have been published previously. 15 Study completion (last patient, last visit) is expected on 16 January 2024.
Endpoints
The primary endpoint of joint health outcome was assessed physically using only the pain (score: 0–3), bleeding (score: 0–3), and physical examination (score: 0–12) parameters of the Gilbert Score. Higher scores for each of these categories represent worsening conditions. Secondary endpoints reported for this interim data read-out include annualized bleeding rate (ABR) and annualized joint bleeding rate (AJBR), HRQoL using the 12-item Short Form Health Survey, version 2 (SF-12v2) questionnaire, 16 status of joint health assessed using the Hemophilia Joint Health Score (HJHS), including both the Global Gait and Total Scores, and factor FVIII consumption. All adverse events (AEs), including serious AEs (SAEs) and treatment-related AEs, were monitored and recorded throughout the study, including development of FVIII inhibitors.
Statistical analysis
Statistical hypothesis tests or comparisons for safety and effectiveness endpoints were not planned for this study. Therefore, no powered samples size calculations were performed. Patients were enrolled based on feasibility and the planned final sample size (~1130 patients) was selected as a reasonable number for a noninterventional study that can practically be recruited within the planned duration of recruitment (5 years).
Due to the noninterventional nature of this study, missing values were expected, and no statistical imputations were conducted. Therefore, all analyses were performed using non-missing data. Continuous variables are expressed as mean (standard deviation, SD) and median (interquartile range). Categorical variables are expressed as frequencies and percentages. Stratifications by age were based on age at each study time point. This interim report summarizes observational data for enrolled patients who received octocog alfa for up to 7 years (cut-off, 30 June 2020). Patients who switched to rurioctocog alfa pegol during the study were excluded from the safety analysis, and for all other outcomes only data for octocog alfa treatment periods are included in the analysis. The study is ongoing and patient data have been collected continuously. Data were stratified by patients’ hemophilia severity at baseline, with moderate hemophilia defined as FVIII 1–5% and severe hemophilia defined as FVIII <1%.
Results
Between June 2011 and June 2020, 711 patients who received octocog alfa were enrolled from 22 countries (Australia, Austria, Belgium, Brazil, Canada, China, Colombia, Czech Republic, Denmark, France, Greece, Hungary, Italy, Norway, Poland, Portugal, Russia, Slovenia, Spain, Sweden, Switzerland, and United Kingdom). Nine patients switched from octocog alfa to rurioctocog alfa pegol and were subsequently excluded from the safety analyses. Eleven patients receiving rurioctocog alfa pegol at baseline were enrolled in the study but are not included in the present analyses which focus only on patients receiving octocog alfa. Of the 711 patients included in this analysis, 76 (10.7%) had completed 7 years of follow-up at the time of the data cut-off, including 59/582 patients (10.1%) receiving octocog alfa prophylactically and 17/112 patients (15.2%) receiving it on-demand. As of the data cut-off, 267 (75.9%) patients discontinued the study early and 85 (12.0%) completed the study according to the protocol. Of the patients discontinuing the study early, 145 patients had switched to another FVIII product, 29 withdrew consent, 28 were lost to follow-up, 8 discontinued because the study was terminated by the sponsor, 4 for non-compliance with the study protocol, 4 due to death, 3 unsatisfactory therapeutic response, and 1 AE; 45 discontinued for non-specified other reasons. The mean (SD) total study duration was 3.55 (1.88) years and the median (range) duration was 3.45 (0.10–8.49) years.
Baseline characteristics
Baseline characteristics for the full patient cohort (n = 711) are shown in Table 1. All but one patient in the study was male and the median age of patients at enrollment was 14 years (range, 0–78 years). Children and adolescents under 18 years comprised 55.7% of the population and 69.2% of patients were of White ethnicity. Of the 711 patients, 582 (81.9%) were receiving prophylactic therapy at baseline, 112 (15.8%) were receiving on-demand therapy, and 17 (2.4%) were receiving ITI therapy. Most patients [n = 498 (70.0%)] had severe hemophilia A (FVIII <1%), whereas 210 (29.5%) had moderate disease (FVIII 1–5%). A hemophilia severity grading was not available for three patients (0.4%).
Table 1.
Baseline demographics.
| Characteristic | All patients (n = 711) |
|---|---|
| Age, median (range), years | 14 (0–78) |
| Age category, n (%) | |
| 0 to <1 month | 2 (0.3) |
| 1 month to <2 years | 48 (6.8) |
| 2 to <12 years | 262 (36.8) |
| 12 to <18 years | 84 (11.8) |
| ⩾18 years | 315 (44.3) |
| Ethnic group, n (%) | |
| White | 492 (69.2) |
| Black/African American | 40 (5.6) |
| Asian | 5 (0.7) |
| Other | 88 (12.4) |
| Not reported | 86 (12.1) |
| Treatment regimen, n (%) | |
| Prophylaxis | 582 (81.9) |
| FVIII 1–5% a | 153 (21.5) |
| FVIII <1% a | 427 (60.1) |
| FVIII unknown | 2 (0.3) |
| On-demand | 112 (15.8) |
| Immune tolerance induction | 17 (2.4) |
Moderate disease = FVIII 1–5%; severe disease = FVIII <1%.
FVIII, factor VIII.
Joint health outcomes
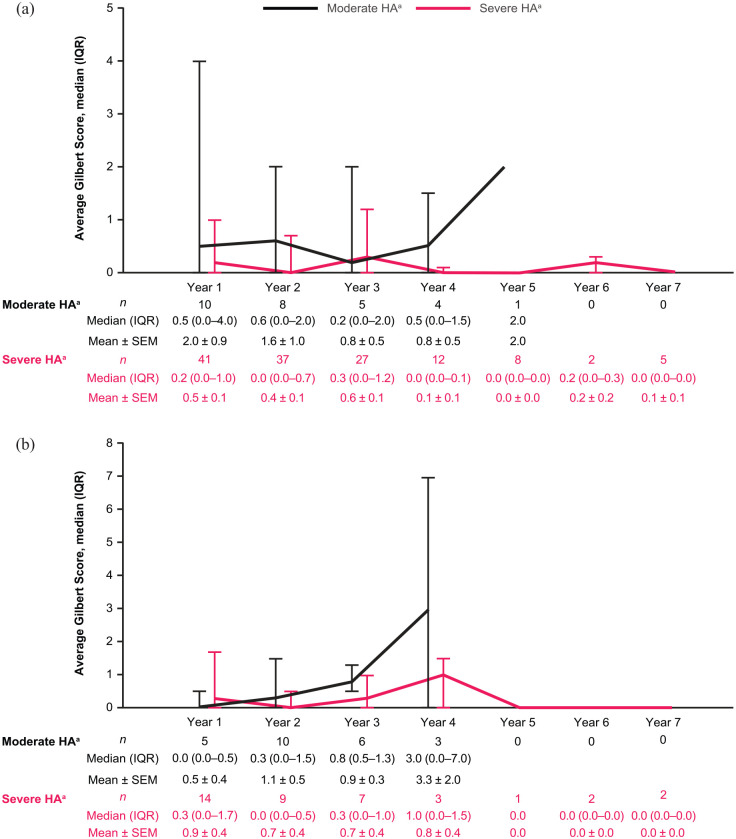
Median average Gilbert Scale Scores over the follow-up period (up to 7 years) stratified by treatment regimen and disease severity are presented in Supplemental Figure S1. Median Gilbert Scores ranged from 3.6 to 13.0 in adult patients (aged 18 years or older) with severe disease receiving on-demand therapy and 1.7 to 2.8 in those receiving prophylaxis during the study. In patients with moderate disease, median Gilbert Scores ranged from 0.3 to 2.1 in those receiving on-demand therapy and 0.8 to 2.2 in those receiving prophylaxis (Supplemental Figure S1). Median Gilbert Scores ranged from 0.0 to 0.3 in children aged 2 to under 12 years with severe disease and from 0.2 to 0.6 in those with moderate disease. In patients aged 12 to under 18 years, median Gilbert Scores ranged from 0.0 to 1.0 in those with severe disease, and 0.0 to 3.0 in those with moderate disease, although patient numbers were low (Figure 1).
Figure 1.
Average Gilbert Score (three-dimensional; all joints) over time in patients (a) aged 2 to under 12 years and (b) aged 12 to under 18 years, with severe or moderate disease. All patients were receiving prophylaxis.
aModerate disease = FVIII 1–5%; severe disease = FVIII <1%.
FVIII, factor VIII; HA, hemophilia A; IQR, interquartile range; SEM, standard error of the mean.
The mean HJHS Global Gait Score over time in patients receiving prophylaxis stratified by disease severity is shown in Supplemental Figure S2. In patients receiving prophylaxis, there was a numerical decrease in HJHS Global Gait Score over the 7-year follow-up in patients with severe disease, who represent the majority of such patients. In patients receiving on-demand therapy there was no clear trend in HJHS Global Gait Score, but the numbers of patients were very small (Supplemental Figure S3). Results for HJHS Total Score in patients receiving prophylaxis and on-demand therapy (Supplemental Figure S4) were similar to those for HJHS Global Gait Score in the overall population.
Effectiveness
ABRs and AJBRs over the study follow-up stratified by treatment regimen and disease severity are shown in Supplemental Figures S5 and S6, respectively. Median overall ABRs ranged from 1.8 to 10.4 in patients receiving on-demand therapy and 0.0 to 1.3 in those receiving prophylaxis. Median overall AJBRs ranged from 0.9 to 5.4 in patients receiving on-demand therapy and 0.0 to 0.0 in those receiving prophylaxis. The percentage of patients with an ABR of zero was 34–56% over the study follow-up in those treated with prophylaxis and 20–40% in those receiving on-demand therapy (Supplemental Figure S7). When categorized according to disease severity, more patients achieved an ABR of zero with prophylaxis compared with on-demand therapy in both the moderate and severe groups although the numbers of patients receiving on-demand therapy was small (Supplemental Figure S7). In addition, there was a trend for decreasing bleeding rates and an increasing proportion of patients experiencing zero bleeds over time (Supplemental Figures S7 and S8).
The mean annual consumption of octocog alfa per observation period, including bleed events, was higher in patients treated with prophylaxis (4356–4752 IU/kg/year) compared with on-demand therapy (393–856 IU/kg/year). The mean (SD) weekly number of infusions, including bleed events, ranged from 2.9 (0.9) to 3.1 (1.0) in patients receiving prophylaxis compared with 0.3 (0.2) to 0.6 (1.1) in those receiving on-demand treatment across the study follow-up period.
Health-related QoL
The physical component scores and mental component scores of the SF-12v2 questionnaire for patients aged 18 years or older with severe or moderate hemophilia A are presented in Supplemental Figure S9. An SF-12v2 score of approximately 50 is indicative of a good QoL, whereas a score of less than 50 denotes a poorer QoL. Consistent physical and mental composite scores were reported during the 7-year follow-up period for both patients receiving on-demand therapy and for those receiving prophylaxis. Patients with severe hemophilia A receiving prophylaxis reported numerically higher physical component scores than those receiving on-demand therapy. However, mental component scores were similar between treatment groups.
Safety
The safety analysis set comprised the 702 patients who received octocog alfa throughout the study. Of these patients, 414 (59.0%) experienced a total of 2234 AEs, including 13 patients (1.9%) who experienced 18 AEs considered by the treating clinician to be possibly/probably related to octocog alfa. The only octocog alfa-related AE that occurred in more than one patient was FVIII inhibitor development, which was reported in nine patients. FVIII inhibitor development was considered as an SAE. Rash, adverse drug reaction, treatment failure, nausea, allergic rhinitis, and hyperhidrosis were reported in one patient each. SAEs were reported in 141 patients (20.1%), including 12 patients (1.7%) who each experienced one SAE considered to be possibly/probably related to octocog alfa. The octocog alfa-related SAEs were all cases of FVIII inhibition.
Discussion
Long-term treatment outcomes data in a real-world cohort of patients with hemophilia A are significantly lacking. For this reason, the noninterventional, prospective AHEAD international study was initiated in June 2011. The results of an interim analysis conducted after 3 years of follow-up in 522 patients found an ABR of 1.7–2.2 for patients receiving prophylaxis with octocog alfa and 8.9–13.0 in those receiving on-demand treatment. In addition, approximately 42% of patients on prophylaxis and 12% of those treated with on-demand therapy experienced zero annual joint bleeds. 15 This suggested that the goal of achieving zero bleeds with prophylactic treatment was possible for many patients. However, the data included in this previous 3-year interim analysis did not provide information on long-term outcomes such as joint health. The results of this 7-year interim analysis of the AHEAD international study show that the long-term effectiveness of octocog alfa is maintained, regardless of age or disease severity, compared with earlier analyses15,17 and provides data assessing joint health outcomes in these patients.
During the study there was a tendency for numerically lower ABRs with prophylaxis than with on-demand therapy. An ABR of zero was achieved by 34–56% of patients receiving prophylaxis compared with 20–40% of those receiving on-demand therapy. This numerical difference was not as much as might be expected given the known clinical advantages of a prophylaxis-based regimen. This could be due to selection bias within the study, with patients with severe disease, and, therefore, more likely to experience bleeding events, being those most likely to be receiving a prophylaxis regimen. It is important to note, however, that the difference between prophylaxis and on-demand therapy was smallest in year 1 and generally increased over time. The numerical difference in zero bleed rate was more pronounced in patients with severe hemophilia A, with 32–54% of patients treated with prophylaxis and 17–40% of patients treated with on-demand therapy achieving an ABR of zero. The ABRs in the present study represent an improvement compared with the 3-year analysis of the AHEAD study, in which 42% of patients receiving prophylaxis and 12% of patients receiving on-demand therapy overall achieved an ABR of zero. 15 This may be related to optimization of treatment as patients continue therapy, although it may also be related to the larger sample size in the present analysis compared with the 3-year analysis. To put the bleeding rates in context with other factor and non-factor hemophilia treatments, a recent multicenter, observational study reporting bleeding outcomes and safety among patients with hemophilia A treated with emicizumab before 15 May 2019, an ABR of 0.4 was reported at the end of follow-up. 18 In a retrospective, observational study of patients in the USA who had received previous treatment with a prophylactic recombinant FVIII for 12 months or longer before switching to rurioctocog alfa pegol, a median ABR of 0.8 was reported in 56 patients after a median of 12 months’ treatment. 19 In a study of recombinant FVIII (BAY 81-8973; Bayer, Berkeley, CA, USA), interim data from 89 patients followed for 6 months or longer showed a median ABR of 3.3 and median AJBR of 1.1. 20 In a subset of 40 patients aged less than 12 years, median spontaneous ABR was 1.5 and traumatic ABR was 0.9. 21 Consistent with ABR results, median Gilbert Scores in adults with severe hemophilia A in the present analysis were higher in those receiving on-demand therapy than in those receiving prophylaxis. Scores were similar in adults with moderate and severe hemophilia A, whereas Gilbert Scores in children and adolescents were low. This is not unexpected, as the increased availability of prophylaxis has led to an increased number of younger patients being started on prophylaxis earlier compared with more senior patients with hemophilia A. In addition, joint arthropathy develops slowly over time, especially in patients receiving primary prophylaxis. This subtle development of disease, coupled with the limitations of its early detection by available tools, hamper timely diagnosis and treatment. This further highlights the need to explore alternative early diagnostics tools, such as joint health biomarkers, for arthropathy detection.
Most patients in the present study (52.3%) were treated with non-PK-guided prophylaxis. However, recent data from the phase III PROPEL study suggests that personalization of prophylaxis regimens based on individual patients’ characteristics and PK profile may provide improved response. PROPEL compared two different FVIII troughs using a PK-guided approach and showed that targeting a higher FVIII trough was associated with fewer bleeding events. 22 One of the secondary objectives of the AHEAD study is to collect PK parameters and assess the relationship between PK-guided prophylactic treatment and bleeding rates to help further improve patient outcomes. This will be explored in future analyses.
Overall, AEs were reported in 59% of patients, with SAEs in 20.1% of patients. In 1.7% of patients, SAEs were considered to be possibly/probably related to octocog alfa treatment. The types of AEs reported were consistent with the known safety profile of octocog alfa.
The AHEAD international study is the first prospective study carried out in a large cohort with the aim of evaluating long-term treatment outcomes and safety in patients with hemophilia A. Strengths of the AHEAD study include the global scope and real-world nature of the study, with patients included who would not normally be considered for clinical trials, including those with present, or a history of, inhibitors or with poor adherence to therapy. In addition, stratification of patients by disease severity and by treatment type allowed more detailed analysis of individual patient cohorts.
As an observational, noninterventional study, AHEAD has several limitations, including the lack of randomization and a control group, potentially leading to selection bias. As a noninterventional trial, patients are treated according to their perceived treatment needs, hence patients with a ‘milder phenotype’ may be more likely to be managed on demand, rather than using prophylaxis. In parallel, patients with severe disease are more likely to receive prophylaxis. This confounds comparisons of outcome measures when comparing on-demand with prophylaxis. Additionally, as a noninterventional, observational real-world study, compliance with therapy is invariably likely to be less rigorous than in a clinical trial setting. In addition, there was no standardized treatment protocol. Indeed, the international scope of the study means that patients could be receiving a wide range of treatment regimens. Importantly, most safety and effectiveness parameters were based on patient recall or self-reported information, leading to potential recall bias, and data completion was challenging as not all endpoints are available for every patient. As a result, it is possible that some bleeds were not recorded. Joint health was assessed by AJBR, three-dimensional Gilbert Scores (without X-ray assessment), and HJHS. Imaging analysis would have provided more accurate information on joint damage. 23 However, joint health assessments were conducted according to routine clinical practice in each country. Despite the advantages of radiological imaging in terms of sensitivity and early detection of joint disease, this is offset by cost, the training required by physicians to interpret radiographs, and inter-rater variability. It should also be noted that, while the Gilbert Score was the predominant assessment method when the AHEAD study was initiated, the newer HJHS is optimized for use in children with no or minimal joint disease as well as patients on primary prophylaxis, and is better suited than the Gilbert Score for use in children and adolescents.24,25 The increasing preference for HJHS over the Gilbert Score may explain in part the low ‘N’ values for the Gilbert Score data.
Furthermore, as the study is ongoing, patients do not necessarily contribute data at all timepoints during the 7-year follow-up as a result of dynamic enrollment (i.e. patients enrolled at different timepoints). This could be addressed by future analyses focusing on patients who were followed for a specific period (e.g. 5 years). There was also an imbalance in the number of patients receiving prophylaxis versus on-demand therapy, which makes comparison of these two regimens difficult. Withdrawal bias may also potentially arise due to the withdrawal of patients with higher bleeding rates from the study who consequently choose to move to some other treatment, leading to an apparent improvement in outcomes over time.
The primary analysis included all patients, regardless of disease severity, and interpretation of these results is therefore challenging because of differences between severity groups. To address this, a post hoc analysis was conducted in patients classified at baseline as having moderate or severe disease. Importantly, these classifications were prespecified and assessed at baseline based on FVIII activity rather than clinician opinion. Stratifying patients in this way also increases the transparency of the data, as outcomes are generally better in moderate disease compared with severe disease. It is important to note, however, that some patient subgroups were very small, such as the group of on-demand patients with available joint health score data. It should also be noted that no data on treatment nonadherence – which can lead to underestimation of effectiveness, particularly relating to prophylaxis – were collected.
Conclusion
These 7-year interim data corroborate previous analyses demonstrating the long-term effectiveness of octocog alfa for bleed prevention and for maintenance of long-term joint health in patients with moderate and severe hemophilia A, regardless of patient age. In particular, patients receiving octocog alfa as prophylaxis in the AHEAD study experienced a low number of bleeds, demonstrating that a low ABR can be maintained using a standard half-life product. No new safety signals were observed when compared with the 3-year follow-up. 15 The only octocog alfa-related SAE that occurred in more than one patient in both analyses was FVIII inhibitor development.
Supplemental Material
Supplemental material, sj-docx-1-tah-10.1177_20406207231218624 for The effectiveness and safety of octocog alfa in patients with hemophilia A: up to 7-year follow-up of the real-world AHEAD international study by Margareth C. Ozelo, Cedric Hermans, Manuel Carcao, Benoît Guillet, Joan Gu, Randy Guerra, Leilei Tang and Kate Khair in Therapeutic Advances in Hematology
Acknowledgments
The authors thank Jaco Botha (Takeda Pharmaceuticals International AG, Zurich, Switzerland), who provided statistical analysis support. The AHEAD international study was funded by Baxalta Innovations GmbH, a Takeda Company, Vienna, Austria. Medical writing support for this manuscript was provided by Laura Harrison, PhD, of Excel Medical Affairs (Fairfield, CT, USA), and was funded by Takeda Development Center Americas, Inc., Lexington, MA, USA.
Footnotes
ORCID iDs: Margareth C. Ozelo  https://orcid.org/0000-0001-5938-0675
https://orcid.org/0000-0001-5938-0675
Cedric Hermans  https://orcid.org/0000-0001-5429-8437
https://orcid.org/0000-0001-5429-8437
Manuel Carcao  https://orcid.org/0000-0001-5350-1763
https://orcid.org/0000-0001-5350-1763
Benoît Guillet  https://orcid.org/0000-0003-2938-8013
https://orcid.org/0000-0003-2938-8013
Randy Guerra  https://orcid.org/0000-0002-7683-4429
https://orcid.org/0000-0002-7683-4429
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Margareth C. Ozelo, Hemocentro UNICAMP, University of Campinas, Campinas, São Paulo, Brazil
Cedric Hermans, St-Luc University Hospital, Université catholique de Louvain, Louvain, Belgium.
Manuel Carcao, Division of Haematology/Oncology, Hospital for Sick Children, Toronto, ON, Canada.
Benoît Guillet, Haemophilia Treatment Center, Univ Rennes, CHU Rennes, Inserm, EHESP, Irset (Institut de recherche en santé, environnement et travail) - UMR_S 1085, F-35000 Rennes, France.
Joan Gu, Takeda Development Center Americas, Inc., Cambridge, MA, USA.
Randy Guerra, Takeda Development Center Americas, Inc., 500 Kendal Street, Cambridge, MA 02142, USA.
Leilei Tang, Takeda Pharmaceuticals International AG, Zurich, Switzerland.
Kate Khair, Haemnet, London, UK.
Declarations
Ethics approval and consent to participate: The study was approved by the independent ethics committees of each study center (Supplemental Table S1). Informed consent/assent was provided by each patient or their legally authorized representative.
Consent for publication: Not applicable.
Author contributions: Margareth C. Ozelo: Formal analysis; Writing – review & editing.
Cedric Hermans: Formal analysis; Writing – review & editing.
Manuel Carcao: Formal analysis; Writing – review & editing.
Benoît Guillet: Formal analysis; Writing – review & editing.
Joan Gu: Formal analysis; Writing – review & editing.
Randy Guerra: Formal analysis; Writing – review & editing.
Leilei Tang: Formal analysis; Methodology; Writing – review & editing.
Kate Khair: Formal analysis; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The AHEAD International study was funded by Baxalta Innovations GmbH, a Takeda Company, Vienna, Austria.
MO: Grant/Research support – BioMarin, Novo Nordisk, Pfizer, Roche, Sanofi, Shire (a Takeda Company); Consultant – BioMarin, Novo Nordisk, Roche, Pfizer, Sanofi, Shire (a Takeda Company); Speaker Bureau – Bayer, BioMarin, Novo Nordisk, Roche, Shire (a Takeda Company). CH has received grants and research support from Shire/Takeda, Bayer, Pfizer, OctaPharma, Novo Nordisk, CSL Behring, and CAF-DCF; and honoraria or consultation fees from Shire/Takeda, Bayer, Pfizer, OctaPharma, Novo Nordisk, Sobi-Biogen, LFB, CAF-DCF, Roche, CSL Behring, Uniqure, and BioMarin. MC has received grants and research support from Bayer, Bioverativ/Sanofi, CSL Behring, Novo Nordisk, Octapharma, Pfizer, Roche, Shire/Takeda; and honoraria or consultation fees from Bayer, Bioverativ/Sanofi, CSL Behring, Grifols, LFB, Novo Nordisk, Pfizer, Roche, Shire/Takeda. BG: Grant/research support (CSL Behring, Octapharma); consultancy/speaker bureau (Bayer, Novo Nordisk, Shire, Sobi-Biogen). JG: Employee – Takeda Pharmaceuticals International AG; Shareholder – Takeda. RG: Employee – Takeda Development Center Americas, Inc.; Shareholder – Takeda. LT: Employee – Takeda Pharmaceuticals International AG; Shareholder – Takeda. KK: Grant/research support (Baxalta/Shire, CSL Behring, Novo Nordisk, Pfizer, Sobi uniQure); speaker bureau (Bayer, BioMarin, CSL Behring, Novo Nordisk, Roche, Shire, Sobi, Takeda).
Availability of data and materials: The datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participants data supporting the results of the study, will be made available after the publication of study results within 3 months from initial request to researchers who provide a methodologically sound proposal. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.
References
- 1. Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia 2020; 26(Suppl. 6): 1–158. [DOI] [PubMed] [Google Scholar]
- 2. Knobe K, Berntorp E. Haemophilia and joint disease: pathophysiology, evaluation, and management. J Comorb 2011; 1: 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klamroth R, Pollmann H, Hermans C, et al. The relative burden of haemophilia A and the impact of target joint development on health-related quality of life: results from the ADVATE Post-Authorization Safety Surveillance (PASS) study. Haemophilia 2011; 17: 412–421. [DOI] [PubMed] [Google Scholar]
- 4. O’Hara J, Walsh S, Camp C, et al. The impact of severe haemophilia and the presence of target joints on health-related quality-of-life. Health Qual Life Outcomes 2018; 16: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med 2007; 357: 535–544. [DOI] [PubMed] [Google Scholar]
- 6. Manco-Johnson MJ, Kempton CL, Reding MT, et al. Randomized, controlled, parallel-group trial of routine prophylaxis vs. on-demand treatment with sucrose-formulated recombinant factor VIII in adults with severe hemophilia A (SPINART). J Thromb Haemost 2013; 11: 1119–1127. [DOI] [PubMed] [Google Scholar]
- 7. Tiede A, Oldenburg J, Lissitchkov T, et al. Prophylaxis vs. on-demand treatment with Nuwiq((R)) (Human-cl rhFVIII) in adults with severe haemophilia A. Haemophilia 2016; 22: 374–380. [DOI] [PubMed] [Google Scholar]
- 8. Tarantino MD, Collins PW, Hay CR, et al.; RAHF-PFM Clinical Study Group. Clinical evaluation of an advanced category antihaemophilic factor prepared using a plasma/albumin-free method: pharmacokinetics, efficacy, and safety in previously treated patients with haemophilia A. Haemophilia 2004; 10: 428–437. [DOI] [PubMed] [Google Scholar]
- 9. Blanchette VS, Shapiro AD, Liesner RJ, et al.; rAHF-PFM Clinical Study Group. Plasma and albumin-free recombinant factor VIII: pharmacokinetics, efficacy and safety in previously treated pediatric patients. J Thromb Haemost 2008; 6: 1319–1326. [DOI] [PubMed] [Google Scholar]
- 10. Negrier C, Shapiro A, Berntorp E, et al. Surgical evaluation of a recombinant factor VIII prepared using a plasma/albumin-free method: efficacy and safety of Advate in previously treated patients. Thromb Haemost 2008; 100: 217–223. [PubMed] [Google Scholar]
- 11. Valentino LA, Mamonov V, Hellmann A, et al.; Prophylaxis Study Group. A randomized comparison of two prophylaxis regimens and a paired comparison of on-demand and prophylaxis treatments in hemophilia A management. J Thromb Haemost 2012; 10: 359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Konkle BA, Stasyshyn O, Chowdary P, et al. Pegylated, full-length, recombinant factor VIII for prophylactic and on-demand treatment of severe hemophilia A. Blood 2015; 126: 1078–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mullins ES, Stasyshyn O, Alvarez-Roman MT, et al. Extended half-life pegylated, full-length recombinant factor VIII for prophylaxis in children with severe haemophilia A. Haemophilia 2017; 23: 238–246. [DOI] [PubMed] [Google Scholar]
- 14. Chowdary P, Mullins ES, Konkle BA, et al. Long-term safety and efficacy results from the phase 3b, open-label, multicentre continuation study of rurioctocog alfa pegol for prophylaxis in previously treated patients with severe haemophilia A. Haemophilia 2020; 26: e168–e178. [DOI] [PubMed] [Google Scholar]
- 15. Khair K, Mazzucconi MG, Parra R, et al. Pattern of bleeding in a large prospective cohort of haemophilia A patients: a three-year follow-up of the AHEAD (Advate in HaEmophilia A outcome Database) study. Haemophilia 2018; 24: 85–96. [DOI] [PubMed] [Google Scholar]
- 16. Ware J, Jr, Kosinski M, Keller SD. A 12-item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996; 34: 220–233. [DOI] [PubMed] [Google Scholar]
- 17. Tsakiris DA, Oldenburg J, Klamroth R, et al. Effectiveness and safety outcomes in patients with hemophilia A receiving antihemophilic factor (recombinant) for at least 5 years in a real-world setting: 6-year interim analysis of the ahead international and German studies. Blood 2020; 136: 1.32430499 [Google Scholar]
- 18. McCary I, Guelcher C, Kuhn J, et al. Real-world use of emicizumab in patients with haemophilia A: bleeding outcomes and surgical procedures. Haemophilia 2020; 26: 631–636. [DOI] [PubMed] [Google Scholar]
- 19. Aledort L, Milligan S, Watt M, et al. A retrospective observational study of rurioctocog alfa pegol in clinical practice in the United States. J Manag Care Spec Pharm 2020; 26: 492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Santoro C, Fuh B, Le PQ, et al. BAY 81-8973 prophylaxis and pharmacokinetics in haemophilia A: interim results from the TAURUS study. Eur J Haematol 2020; 105: 164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Le PQ, Maes P, Mingot-Castellano ME, et al. BAY 81-8973 prophylaxis in patients with haemophilia A: interim findings for children <12 years from the TAURUS real-world study. Haemophilia 2020; 26: P165. [Google Scholar]
- 22. Klamroth R, Windyga J, Radulescu V, et al. Rurioctocog alfa pegol PK-guided prophylaxis in hemophilia A: results from the phase 3 PROPEL study. Blood 2021; 137: 1818–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oldenburg J. Optimal treatment strategies for hemophilia: achievements and limitations of current prophylactic regimens. Blood 2015; 125: 2038–2044. [DOI] [PubMed] [Google Scholar]
- 24. Feldman BM, Funk S, Lundin B, et al. Musculoskeletal measurement tools from the International Prophylaxis Study Group (IPSG). Haemophilia 2008; 14(Suppl. 3): 162–169. [DOI] [PubMed] [Google Scholar]
- 25. Feldman BM, Funk SM, Bergstrom BM, et al. Validation of a new pediatric joint scoring system from the International Hemophilia Prophylaxis Study Group: validity of the hemophilia joint health score. Arthritis Care Res (Hoboken) 2011; 63: 223–230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tah-10.1177_20406207231218624 for The effectiveness and safety of octocog alfa in patients with hemophilia A: up to 7-year follow-up of the real-world AHEAD international study by Margareth C. Ozelo, Cedric Hermans, Manuel Carcao, Benoît Guillet, Joan Gu, Randy Guerra, Leilei Tang and Kate Khair in Therapeutic Advances in Hematology