Abstract
Background:
Cerebral pulsatility is thought to reflect arterial stiffness and downstream microvascular resistance. Although previous studies indicated cerebral pulsatility might closely relate to development of cerebral small vessel disease (SVD), yet evidence remain controversial and longitudinal data are rare.
Objective:
We aimed to explore relationships of cerebral pulsatility with severity and progression of various SVD imaging markers among the community-dwelling elderly.
Design:
A longitudinal cohort study.
Methods:
As part of the prospective community-based Shanghai Aging Study cohort, dementia- and stroke-free elderly were recruited for baseline assessment of cerebral pulsatility and SVD severity during 2010–2011 and traced for SVD progression during 2016–2017. Cerebral pulsatility was quantified for both anterior and posterior circulation with transcranial Doppler ultrasound. SVD imaging markers were measured with brain magnetic resonance imaging (MRI) including white matter hyperintensities (WMHs), enlarged perivascular spaces (ePVS), lacunes, and microbleeds. The cross-sectional and longitudinal relationships between cerebral pulsatility and SVD were analyzed by univariable and multivariable regression models.
Results:
Totally, 188 eligible subjects were included at baseline and out of them, 100 (53.19%) returned for a 7-year follow-up. At baseline, increased pulsatility of posterior circulation was independently associated with more periventricular WMH (PWMH) and ePVS in basal ganglia (BG-ePVS) but not with other SVD markers. Longitudinally, higher posterior pulsatility predicted greater PWMH progression in participants with hypertension (β = 2.694, standard error [SE] = 1.112, p = 0.020), whereas pulsatility of anterior circulation was shown to prevent BG-ePVS progression among followed-up elderly (β = −6.737, SE = 2.685, p = 0.012). However, no significant relationship was found between cerebral pulsatility and burden of lacunes or cerebral microbleeds.
Conclusion:
Higher pulsatility of posterior circulation could worsen PWMH progression, especially for participants with hypertension. But for development of ePVS, increased cerebral pulsatility could play a compensatory role among several healthy elderly. The distinct relationships between cerebral pulsatility and various SVD markers emphasized the importance of individualized SVD management.
Keywords: cerebral pulsatility, cerebral small vessel disease, cohort study, enlarged perivascular space, hypertension, white matter hyperintensity
Introduction
Cerebral small vessel disease (SVD) is being increasingly recognized as a leading cause of stroke and dementia worldwide.1,2 The prevalence of SVD has been reported to be nearly 50% among people over 70 years, which also increases insidiously with age. 3 Assessment of SVD severity commonly depends on typical imaging features including white matter hyperintensities (WMHs), lacunes, cerebral microbleeds (CMBs), and enlarged perivascular spaces (ePVS). It remains difficult to evaluate subtle alterations in morphology or function of cerebral small vessels prior to the emergence of imaging-based lesions, primarily due to limitations in resolution of generally used imaging techniques. Thus, to early predict and intervene SVD progression is still a great challenge.
Pulsatility of cerebral blood flow (CBF), as an indicator of arterial stiffness and downstream vascular resistance,4,5 has long been supposed to correlate with SVD pathology. Previous research has shown that flow pulsation could play a vital role in neurovascular units, including endothelial nitric oxide synthase (eNOS)-dependent cerebral artery reactivity 6 and integrity of blood brain barrier. 7 Additionally, cerebrovascular pulsation has been regarded as a key driving force of fluid movement in perivascular spaces (PVS). 8 Importantly, cilostazol and isosorbide mononitrate (ISMN) have been suggested to decrease cerebral pulsatility in patients with lacunar stroke9,10 and another trial on effect of sildenafil is ongoing. 11 Thus, cerebral pulsatility might be not only an early alarming sign for SVD pathogenesis but also a potential therapeutic target for SVD. Although many cross-sectional studies have indicated significant associations between cerebral pulsatility and SVD burden,12–14 yet evidence from longitudinal studies is contradictory.15–17 It is noteworthy that the heterogenous pathogenesis of various SVD markers located in different regions has been largely overlooked in most previous studies.18,19 Furthermore, longitudinal analyses on lacunes and microbleeds are still lacking by now.
In the current longitudinal community-based study, we measured cerebral pulsatility of both anterior and posterior circulation at baseline and assessed progression of SVD imaging markers during a 7-year follow-up. We aimed to explore whether cerebral pulsatility is predictive of SVD progression, and whether these relationships are distinct for different SVD markers.
Methods
Participant selection and data collection
The current study analyzed data from a sub-cohort derived from Shanghai Aging Study, which is a prospective community-based cohort study located in Jingansi community, Shanghai, China. 20 During the period of 2010–2011, individuals over 60 years old underwent baseline assessments of clinical characteristics, brain magnetic resonance imaging (MRI) and transcranial Doppler ultrasound (TCD), and neuropsychological tests. And participants were traced for repeated MRI and neuropsychological tests between 2016 and 2017. Potential participants were excluded if they had (1) no baseline brain MRI scans or TCD examinations; (2) baseline history of stroke or dementia; (3) baseline history of hydrocephalus, brain tumors, head trauma, or craniocerebral operations; (4) stenosis of large intracranial artery (>50%) on baseline magnetic resonance angiography (MRA).
Transcranial Doppler ultrasound
TCD evaluations were performed by an experienced sonographer using a TCD monitoring device (Sonara, Middleton, WI, USA) with a 2-MHz handheld probe. Proximal and middle segment middle cerebral arteries (mMCA) were detected through temporal windows at depth of 64 and 52 mm, respectively, whereas vertebral arteries (VA) and basilar arteries (BA) were detected through occipital windows at depth of 64 and 86 mm, respectively. Hemodynamic indices were recorded including peak systolic velocity (PSV), mean flow velocity (MFV), and end diastolic velocity (EDV). Pulsatility index (PI) was calculated to quantify cerebral pulsatility according to the Gosling’s equations (PI = (PSV − EDV)/MFV). Then mean bilateral PI was calculated for further analysis. For subjects with detectable CBF only on one side, unilateral PI was taken as the mean.
Brain MRI scans
Baseline MRI examinations were performed on 1.5T GE scanner with the following sequences, including three-dimensional T1-weighed (T1W), T2-weighted (T2W), fluid attenuated inversion recovery (FLAIR), gradient recalled echo, and MRA sequences. Repeated MRI examinations at follow-up were performed on 3.0T GE scanner with sequences including T1W, T2W, FLAIR, and T2 star weighted angiography. Detailed protocols of baseline and follow-up MRI scans had been reported previously.21,22
Evaluation of SVD imaging markers
SVD imaging markers were rated according to Standards for Reporting Vascular Changes on Neuroimaging (STRIVE) criteria 23 by two skilled neurologists, and ascertained by a senior neurologist in case of disagreement. The κ-coefficients of SVD markers between raters were as follows: 0.82 for the Fazekas score of WMH, 0.90 for the severity of ePVS, 0.80 for the presence of lacune, and 0.82 for the presence of CMB. Volume of periventricular WMH (PWMH) and deep WMH (DWMH) were automatically segmented with a deep-learning method based on U-net model as reported in our previous studies. 21 Follow-up FLAIR images were resampled with 3dresample in AFNI to match the resolution of baseline images. 24 Progression of WMH was defined as volume difference between follow-up and baseline. Additionally, Fazekas score ⩾2 in PWMH or DWMH was defined as moderate/severe PWMH or DWMH. 25 ePVS in basal ganglia (BG) and centrum semiovale (CS) was respectively scored with a validated ordinal scale (range 0–4). 26 The score ⩾2 in BG or CS was defined as extensive ePVS. The progression of ePVS was defined as an increase of score (⩾1) from baseline. The number of lacunes and CMBs were recorded manually, and incident lacune and CMB were defined as new lesions (⩾1) from baseline. The total SVD score (0–4) was graded by combining WMH, lacunes, CMBs, and ePVS 27 and moderate/severe SVD was defined if SVD score ⩾2. The progression of total SVD was defined as an increase of SVD score (⩾1) from baseline.
Statistical analysis
The Kolmogorov–Smirnov test was used to evaluate distribution of quantitative variables. Continuous variables with normal distribution were shown as mean ± SD and compared with t test or one-way analysis of variance. Skewed continuous variables were shown as median (interquartile range) and compared with the Mann–Whitney U test or the Kruskal–Wallis test. Categorical variables were shown as number (percentage) and compared with Fisher’s exact test or Pearson chi-square test. McNemar’s test and paired Wilcoxon test were used to demonstrate changes of clinical characteristics and SVD severity during follow-up.
General linear regression was used to explore relationships of cerebral pulsatility with log-transformed baseline WMH volume and WMH progression during follow-up. Binominal logistic regression was used to explore relationships of cerebral pulsatility with baseline severity and progression of CMBs, lacunes, ePVS, and total SVD. In cross-sectional analysis, variables with p < 0.05 in univariable analyses or those with potential clinical relevance were adjusted in the multivariable regression models,16,21,28,29 including age, sex, body mass index, history of smoking, medical history (hypertension, diabetes, hyperlipidemia, and cardiac disease), and MFV. And variables of interval time and baseline SVD score were additionally added for longitudinal analyses. Interaction analyses were also performed on cerebral pulsatility and other covariates for PWMH progression. Besides, we determined the optimal PI cutoff value to identify moderate/severe PWMH based on receiver operator characteristic (ROC) analysis. PI was dichotomized as high or low at the point which maximized the Youden index. All statistical analyses were performed using R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria) and two-tailed p-value < 0.05 was considered statistically significant.
Results
Participants characteristics
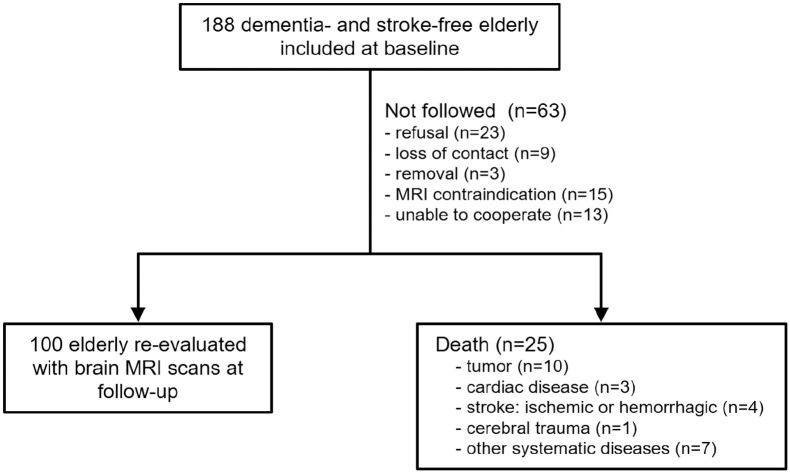
A total of 188 eligible elderly without history of dementia or stroke were recruited at baseline. Baseline characteristics of participants were summarized in Table 1. The median age was 70.00 years old, and 93 (49.47%) were men. Out of 188 participants included at baseline, 25 were deceased, 23 refused to be followed, 9 could not be reached, 3 moved to other communities, 15 had MRI contraindication, and 13 could not be cooperative for MRI scans at follow-up (Figure 1). Finally, 100 participants repeated brain MRI scans with a mean interval of 6.95 ± 0.78 years (6.00–7.00 years). Compared to participants followed with MRI scans, those not followed were older and had more SVD lesions, and their cerebral pulsatility was significantly higher at baseline (Table 1). Among the participants followed, significant progression of most SVD markers was observed during the follow-up, whereas no statistically significant changes were found in terms of lacunes and DWMH volume (Table 2).
Table 1.
Baseline characteristics of participants in our study.
| Variables | Overall | Followed with repeated MRI | Not followed with repeated MRI a | p |
|---|---|---|---|---|
| N (%) | 188 (100.00) | 100 (53.19) | 88 (46.81) | / |
| Baseline demographics | ||||
| Age, years, median (IQR) | 70.00 (64.25–74.00) | 68.50 (62.00–72.75) | 72.00 (66.25–76.00) | <0.001 |
| Male, n (%) | 93 (49.47) | 46 (46.00) | 47 (53.41) | 0.381 |
| Baseline vascular risk factors | ||||
| BMI, kg/m2, mean ± SD | 24.96 ± 3.49 | 24.90 ± 3.37 | 25.03 ± 3.65 | 0.790 |
| Smoking, n (%) | 26 (13.83) | 9 (9.00) | 17 (19.32) | 0.056 |
| Hypertension, n (%) | 104 (55.32) | 50 (50.00) | 54 (61.36) | 0.142 |
| Diabetes, n (%) | 32 (17.02) | 14 (14.00) | 18 (20.45) | 0.251 |
| Hyperlipidemia, n (%) | 80 (42.55) | 42 (42.00) | 38 (43.18) | 0.884 |
| Cardiac disease, n (%) | 26 (13.83) | 11 (11.00) | 15 (17.05) | 0.291 |
| Baseline SVD imaging markers | ||||
| WMH volume, ml, median (IQR) | 6.26 (2.84–11.99) | 3.86 (2.06–7.31) | 9.27 (5.70–18.37) | <0.001 |
| PWMH volume, ml, median (IQR) | 2.21 (1.08–4.81) | 1.56 (0.77–3.09) | 3.50 (1.41–8.59) | <0.001 |
| DWMH volume, ml, median (IQR) | 3.55 (1.59–6.52) | 2.29 (1.14–4.67) | 5.13 (2.61–9.38) | <0.001 |
| ⩾1 Lacune, n (%) | 29 (15.43) | 9 (9.00) | 20 (22.73) | 0.014 |
| ⩾1 CMB, n (%) | 26 (13.83) | 9 (9.00) | 17 (19.32) | 0.056 |
| Extensive BG-ePVS, n (%) | 20 (10.64) | 7 (7.00) | 13 (14.77) | 0.100 |
| Extensive CS-ePVS, n (%) | 7 (3.72) | 5 (5.00) | 2 (2.27) | 0.451 |
| SVD score ⩾2, n (%) | 32 (17.02) | 8 (8.00) | 24 (27.27) | 0.001 |
| Baseline cerebral pulsatility | ||||
| With temporal window, n (%) | 146 (77.66) | 82 (82.00) | 64 (72.73) | 0.161 |
| Mean mMCA PI, mean ± SD | 1.04 ± 0.19 | 1.01 ± 0.18 | 1.08 ± 0.20 | 0.029 |
| Mean pMCA PI, mean ± SD | 1.01 ± 0.20 | 0.99 ± 0.19 | 1.04 ± 0.22 | 0.152 |
| Mean VA PI, mean ± SD | 0.82 ± 0.14 | 0.80 ± 0.14 | 0.85 ± 0.14 | 0.020 |
| BA PI, mean ± SD | 0.84 ± 0.18 | 0.81 ± 0.17 | 0.87 ± 0.19 | 0.033 |
Not followed with repeated MRI: including deceased participants (n = 25) and alive participants who failed to repeat MRI scans due to other reasons (n = 63).
BA, basilar artery; BG, basal ganglia; BMI, body mass index; CMB, cerebral microbleed; CS, centrum semiovale; DWMH, deep white matter hyperintensities; ePVS, enlarged perivascular space; IQR, interquartile range; mMCA, middle segment of middle cerebral artery; PI, pulsatility index; pMCA, proximal segment of middle cerebral artery; PWMH, periventricular white matter hyperintensities; SD, standard deviation; SVD, cerebral small vessel diseases; VA, vertebral artery; WMH, white matter hyperintensities.
Figure 1.
Flow chart of the follow-up of participants recruited in our study.
Table 2.
SVD progression during 7-year follow-up (n = 100).
| Variables | Baseline | Follow-up | Change | p |
|---|---|---|---|---|
| WMH volume, ml, median (IQR) | 3.86 (2.06–7.31) | 7.34 (3.64–12.50) | 2.72 (0.68–5.51) | <0.001 |
| PWMH volume, ml, median (IQR) | 1.56 (0.77–3.09) | 3.38 (2.06–5.73) | 1.75 (0.74–3.66) | <0.001 |
| DWMH volume, ml, median (IQR) | 2.29 (1.14–4.67) | 2.94 (1.43–6.02) | 0.14 (−0.82 to 1.77) | 0.053 |
| ⩾1 Lacune, n (%) | 9 (9.00) | 14 (14.00) | 7 (7.00) a | 0.063 |
| ⩾1 CMB, n (%) | 9 (9.00) | 26 (26.00) | 22 (22.00) a | <0.001 |
| Extensive BG-ePVS, n (%) | 7 (7.00) | 16 (16.00) | 20 (20.00) a | 0.004 |
| Extensive CS-ePVS, n (%) | 5 (5.00) | 20 (20.00) | 36 (36.00) a | <0.001 |
| SVD score ⩾2, n (%) | 8 (8.00) | 22 (22.00) | 19 (19.00) a | <0.001 |
Numbers and percentages of participants with progression of respective SVD markers.
BG, basal ganglia; CMB, cerebral microbleed; CS, centrum semiovale; DWMH, deep white matter hyperintensity; ePVS, enlarged perivascular spaces; IQR, interquartile range; PWMH, periventricular white matter hyperintensities; SVD, cerebral small vessel disease; WMH, white matter hyperintensities.
Cross-sectional associations between cerebral pulsatility and SVD
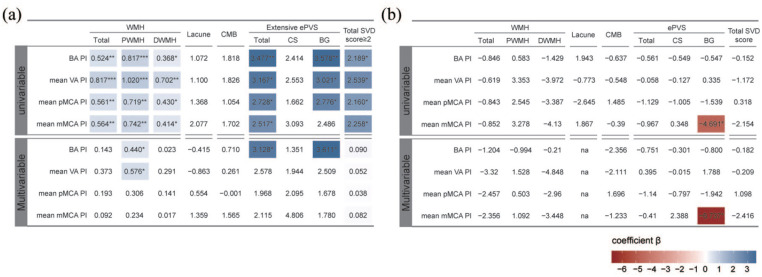
Univariable regression analyses indicated that higher cerebral pulsatility was significantly associated with greater WMH volume (both periventricular and deep) and presence of extensive ePVS (BG, but not CS) as shown in Figure 2(a). But no significant relationship between cerebral pulsatility and presence of lacune or CMB was found. After accounting for covariates including age, sex, and vascular risk factors, increased pulsatility of posterior circulation, rather than anterior, was still independently related to more PWMH volume [βBA = 0.440, SEBA = 0.205, pBA = 0.033; βVA = 0.576, SEVA = 0.257, pVA = 0.027; Figure 2(a)]. However, no independent association of pulsatility indices with DWMH volume was found. Furthermore, ROC curves demonstrated high VA-PI, instead of BA-PI, could serve as a robust indicator for identifying moderate/severe PWMH with an optimal cut-off value of 0.812 (pVA = 0.006, pBA = 0.354; Figure 3).
Figure 2.
Relationships between baseline cerebral pulsatility and severity/progression of SVD imaging markers. (a) Cross-sectional regression analyses on relationships between cerebral pulsatility and SVD severity at baseline. (b) Longitudinal regression analyses on relationships between baseline cerebral pulsatility and SVD progression during follow-up. Colored cells represent statistically significant components (p < 0.05). Color gradient reflects regression coefficients.
***p < 0.001. **p < 0.01, *p < 0.05.
BA, basilar artery; BG, basal ganglia; CMB, cerebral microbleed; CS, centrum semiovale; DWMH, deep white matter hyperintensities; ePVS, enlarged perivascular space; mMCA, middle segment of middle cerebral artery; na, not applicable; PI, pulsatility index; pMCA, proximal segment of middle cerebral artery; PWMH, periventricular white matter hyperintensities; SVD, cerebral small vessel disease; VA, vertebral artery.
Figure 3.
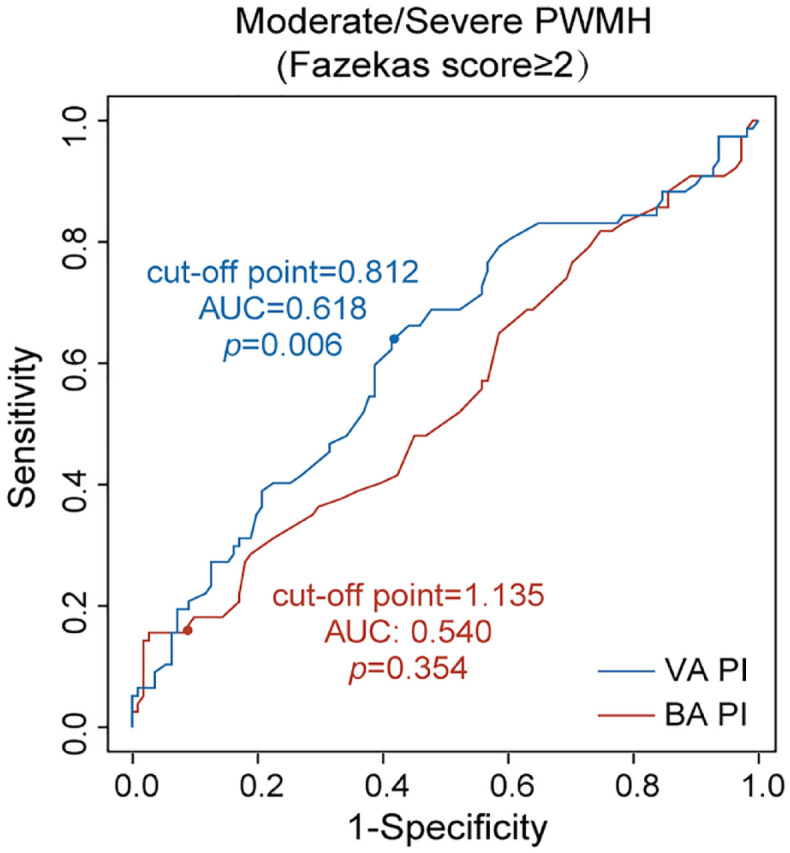
ROC analysis of posterior cerebral pulsatility for identify moderate/severe PWMH. Blue curve represented VA-PI and red curve represented BA-PI. Cut-off points were derived according to the highest Youden indices.
AUC, area under ROC curve; BA, basilar artery; PI, pulsatility index; PWMH, periventricular white matter hyperintensities; ROC, receiver operator characteristic; VA, vertebral artery.
Additionally, only increased BA-PI remained correlated with the presence of extensive BG-ePVS after adjustment [βBA = 3.611, SEBA = 1.448, pBA = 0.013; Figure 2(a)]. In terms of total SVD score, we observed a positive correlation between increased cerebral pulsatility and the presence of moderate/severe SVD, albeit non-significant after adjustment for covariates [Figure 2(a)], which might need further evaluation in larger cohort studies.
Prediction of cerebral pulsatility on SVD progression
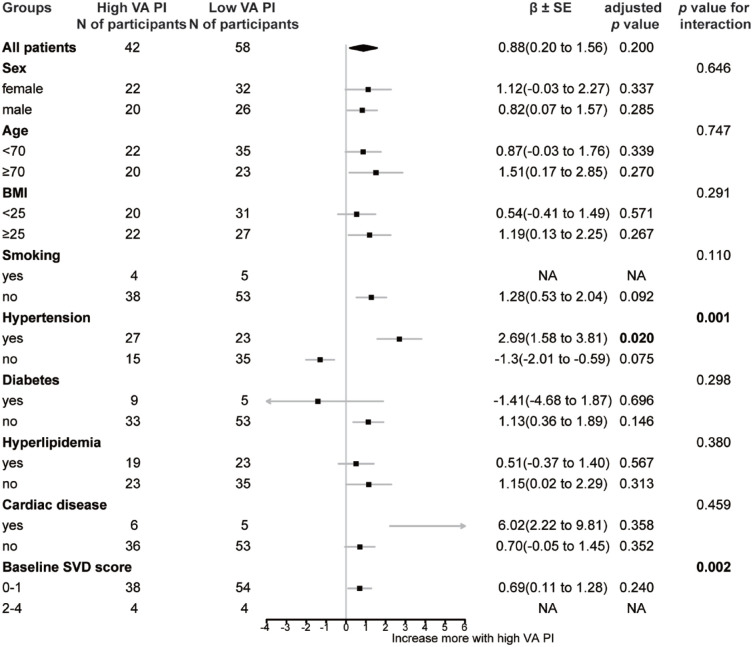
Longitudinally, we found no significant linear relationship between baseline cerebral pulsatility and WMH progression (neither PWMH nor DWMH) [all p > 0.05, Figure 2(b)]. However, we found PWMH volume of participants with baseline high VA-PI increased more than those with low VA-PI (median, 2.27 versus 1.55 ml; p = 0.032; Supplemental Figure 1). As demonstrated in Figure 4, baseline high VA-PI was found to interact with hypertension (β = 4.331, SE = 1.256, p = 0.001), and baseline moderate/severe SVD (β = 7.222, SE = 2.288, p = 0.002) for PWMH progression. Subsequent subgroup analyses revealed a positive association between high baseline VA-PI and PWMH progression in participants with hypertension (β = 2.694, SE = 1.112, p = 0.020), whereas no such association was observed in those without hypertension (Figure 4).
Figure 4.
Subgroup analysis for prediction of high VA-PI on PWMH progression during follow-up. Group of high VA-PI means participants with baseline VA-PI > 0.812. Bold represents statistically significant components (p < 0.05).
BMI, body mass index; NA, not applicable; PI, pulsatility index; PWMH, periventricular white matter hyperintensities; SVD, cerebral small vessel disease; VA, vertebral artery.
Interestingly, we found that participants with BG-ePVS progression had lower baseline mMCA-PI (0.92 ± 0.17 versus 1.04 ± 0.17, p = 0.026), whereas no significant difference in pulsatility indices was found between participants with and without CS-ePVS progression (Supplemental Figure 2). Univariable logistic regression also suggested that increased baseline mMCA-PI was negatively associated with progression of BG-ePVS [β = −4.691, SE = 2.065, p = 0.023; Figure 2(b)]. After accounting for potential confounders, higher baseline mMCA-PI was also related to reduced risk of BG-ePVS progression [β = −6.737, SE = 2.685, p = 0.012; Figure 2(b)].
The effects of baseline pulsatility on progression of lacune, CMB, and total SVD burden were also investigated. Consistent with non-significant findings observed in cross-sectional analyses, baseline PIs were not related to incident lacunes, CMBs, or an increase in SVD score [all p > 0.05, Figure 2(b); Supplemental Figures 3–5].
Discussion
We assessed cerebral pulsatility of both anterior and posterior circulation, as well as the development of various SVD imaging markers among the non-clinical stroke, non-dementia community elderly with 7-year follow-up. Our results suggested that increased PI of posterior circulation was significantly related to more PWMH and the presence of extensive BG-ePVS at baseline. Longitudinally, high VA-PI could predict greater PWMH progression of hypertensive participants, whereas increased mMCA-PI might prevent progression of BG-ePVS in relatively healthy community elderly. No significant relationship was found between cerebral pulsatility and burden of lacune and CMB either cross-sectionally or longitudinally.
A major finding of our study was that pulsatility of posterior circulation was closely associated with severity and progression of PWMH, especially for hypertensive patients. Consistent with previous evidence from two 5-year prospective cohorts conducted in Australia and Sweden, our data suggested that cerebral pulsatility of anterior circulation at baseline did not predict WMH progression.16,17 But pulsatility of posterior circulation and heterogeneity of periventricular and DWMH failed to be discussed in previous longitudinal studies. Limited cross-sectional evidence from Einstein Aging Study cohort indicated PI of vertebral and posterior cerebral arteries might relate to white matter structural integrity. 30 Recent data from UK Biobank has shown that white matter tracts supplied by posterior circulation were more susceptible to pulsatile CBF, compared to those supplied by anterior circulation, 31 which might suggest potential differences between anterior and posterior neurovascular units in response to CBF pulsatility. Furthermore, we only found the significant associations between cerebral pulsatility and PWMH, rather than DWMH. It is known that periventricular white matter is primarily supplied by short penetrating arteries from large cerebral arteries, different from long microvessels through deep white matter. 32 Thus, the former might be affected more directly by pulsatile flow pressure and velocity transmitted from large arteries. 18 Our longitudinal results also revealed that periventricular white matter in individuals with hypertension exhibited increased vulnerability to cerebral pulsatility, as compared to those without hypertension. This finding might support the implementation of personalized management for hypertensive SVD, highlighting the potential benefit from selection of medications with vasodilating properties. 33
In addition to WMH, ePVS was another SVD imaging marker correlated with cerebral pulsatility in our study. Recently, intracranial pulsatility has been regarded to play a vital role in fluid and waste movement through PVS. 34 Several cross-sectional studies have also found higher pulsatility of internal carotid arteries (ICA) and venous sinus correlated with more ePVS, especially BG-ePVS, among patients with transient ischemic attacks (TIA) or minor stroke,13,35,36 which were consistent with our baseline findings. Intriguingly, opposite to the baseline association, our data demonstrated that higher baseline mMCA PI predicted reduced risk of BG-ePVS progression, which is a novel finding on ePVS progression and cerebral pulsatility. One possible reason is that high flow pulsation within a certain range could drive arterial wall pulsation in well-compliant brain,4,14 like our followed-up participants with less baseline vascular risk factors and SVD lesions, and then further promote movement of perivascular fluid. In addition, that only mMCA-PI was related to BG-ePVS progression could be partially attributed to vasculature within BG which are mostly composed of perforating arteries from mMCA. Thus, the involvement of cerebral pulsatility in BG-ePVS progression may exhibit dual aspects. Although high flow pulsatility tends to be a destructive factor in cerebral vasculature, it might be a compensatory driving force for vessel pulsation and perivascular fluid movement in relatively healthy elderly with well-compliant brains, which will eventually be beneficial to the prevention of BG-ePVS progression.
Prior evidence on effects of cerebral pulsatility on lacune and CMB is rare. Only several cross-sectional studies have shown that higher ICA and MCA pulsatility were related to more lacunes and CMBs among patients with TIA or ischemic stroke.13,35 Associations between CMB and cerebral pulsatility were reported to be location-specific in Taiwan I-Lan cohort, with lobar CMB related to VA-PI and deep CMB related to ICA-PI. 37 We did not find any significant relationship between cerebral pulsatility and baseline or progression of lacune and CMB. The relatively small sample size and low prevalence of lacune and CMB in our study cohort might contribute partly to these non-significant results.
To date, relationships between cerebral pulsatility and SVD progression are still under debate and longitudinal data are scarce. Different from previous views from Western longitudinal cohorts,16,17 the current study confirmed significant effects of cerebral pulsatility on SVD development. But previous studies were limited to anterior circulation of MCA 16 or ICA. 17 Additionally, the underestimation of pathological heterogeneity and topographical distributions across various SVD markers in previous longitudinal studies might obscure potential influences of cerebral pulsatility, as only total volume of WMH and ePVS were investigated.16,17 To our knowledge, this is the first longitudinal study to explore the relationships between pulsatility of both anterior and posterior circulation and SVD development in the Chinese urban community. We have made comprehensive evaluations of various SVD imaging markers. Moreover, the present study performed detailed analyses of SVD markers in various anatomical locations, including periventricular or deep regions for WMH and BG or CS regions for ePVS, considering of underlying different pathological mechanisms. Our findings on baseline posterior pulsatility in relation to PWMH progression might provide additional evidence for future clinical trials on drugs such as cilostazol and ISMN to prevent SVD progression. 38 And the distinct associations of cerebral pulsatility with various SVD markers suggest that selection of suitable patients for future clinical trials on cerebral pulsatility might be necessary.
However, there are some limitations. First, the sample size of participants repeating MRI scans was relatively small, partly due to old baseline age with median of 70 years old and long interval time with 7 years. Yet we still found significant associations between baseline pulsatility and progression of PWMH and BG-ePVS among the relatively healthy elderly, compared to those dropped out with higher baseline SVD burden and CBF pulsatility. Second, differences in MRI scanners between baseline (1.5T) and follow-up (3.0T) might result in overestimation of SVD progression. However, improvements in MRI scanners are almost inevitable, especially for very long-term cohort studies. To overcome inter-scanner variation, our followed raw images have been resampled to match resolution of baseline raw images during image postprocessing. Additionally, since all participants assessed at the same time point underwent the same MRI scans, inter-scanner variation might be regarded as a limited systematic error, which partly contributed to SVD progression between follow-up and baseline MRI scans. And baseline SVD burden has been included as a covariate for adjustment in longitudinal regression analyses. Despite of probability of overestimation of BG-ePVS progression, the protective role of high mMCA-PI remained strongly significant. Another minor weakness is that we did not adjust for multiple comparisons in regression analyses. Thus, these factors might limit the generalization of our results, which need further validation in longitudinal studies with larger sample sizes and more advanced imaging tools.
Conclusion
In conclusion, we found significant relationships between cerebral pulsatility and burden of PWMH and BG-ePVS in the longitudinal community-based study. Posterior pulsatility might be more relevant to PWMH development, especially in the elderly with hypertension. Although higher cerebral pulsatility was associated with more BG-ePVS in cross-sectional analyses, it possibly decreased risk of BG-ePVS progression longitudinally in relatively healthy elderly. The distinct role of cerebral pulsatility in development of various SVD markers underlines the pathogenic heterogeneity of these SVD markers and the importance of individualized management of SVD patients. More evidence from larger cohorts and experimental studies is warranted to further interpret roles of cerebral pulsatility in different SVD imaging markers.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864241227304 for Cerebral pulsatility in relation with various imaging markers of cerebral small vessel disease: a longitudinal community-based study by Weiyi Zhong, Yiwei Xia, Yunqing Ying, Yi Wang, Lumeng Yang, Xiaoniu Liang, Qianhua Zhao, Jianjun Wu, Zonghui Liang, Xiaoxiao Wang, Xin Cheng, Ding Ding and Qiang Dong in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-docx-2-tan-10.1177_17562864241227304 for Cerebral pulsatility in relation with various imaging markers of cerebral small vessel disease: a longitudinal community-based study by Weiyi Zhong, Yiwei Xia, Yunqing Ying, Yi Wang, Lumeng Yang, Xiaoniu Liang, Qianhua Zhao, Jianjun Wu, Zonghui Liang, Xiaoxiao Wang, Xin Cheng, Ding Ding and Qiang Dong in Therapeutic Advances in Neurological Disorders
Acknowledgments
None.
Footnotes
ORCID iD: Qiang Dong  https://orcid.org/0000-0002-3874-0130
https://orcid.org/0000-0002-3874-0130
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Weiyi Zhong, Department of Neurology, National Center for Neurological Disorders, National Clinical Research Centre for Aging and Medicine, Huashan Hospital, Fudan University, Shanghai, China.
Yiwei Xia, Department of Neurology, National Center for Neurological Disorders, National Clinical Research Centre for Aging and Medicine, Huashan Hospital, Fudan University, Shanghai, China.
Yunqing Ying, Department of Neurology, National Center for Neurological Disorders, National Clinical Research Centre for Aging and Medicine, Huashan Hospital, Fudan University, Shanghai, China.
Yi Wang, Department of Neurology, National Center for Neurological Disorders, National Clinical Research Centre for Aging and Medicine, Huashan Hospital, Fudan University, Shanghai, China.
Lumeng Yang, Department of Neurology, National Center for Neurological Disorders, National Clinical Research Centre for Aging and Medicine, Huashan Hospital, Fudan University, Shanghai, China.
Xiaoniu Liang, Institute of Neurology, National Clinical Research, National Clinical Research Centre for Aging and Medicine, Huashan Hospital, Fudan University, Shanghai, China.
Qianhua Zhao, Institute of Neurology, National Clinical Research, National Clinical Research Centre for Aging and Medicine, Huashan Hospital, Fudan University, Shanghai, China.
Jianjun Wu, Department of Neurology, National Center for Neurological Disorders, National Clinical Research Centre for Aging and Medicine, Huashan Hospital, Fudan University, Shanghai, China.
Zonghui Liang, Department of Radiology, Jing’an District Center Hospital, Shanghai, China.
Xiaoxiao Wang, Center for Biomedical Engineering, University of Science and Technology of China, Hefei, Anhui, China.
Xin Cheng, Department of Neurology, National Center for Neurological Disorders, National Clinical Research Centre for Aging and Medicine, Huashan Hospital, Fudan University, Shanghai, China.
Ding Ding, Institute of Neurology, National Clinical Research, National Clinical Research Centre for Aging and Medicine, Huashan Hospital, Fudan University, No. 12 Wulumuqi Zhong Road, Shanghai 200040, China.
Qiang Dong, Department of Neurology, National Center for Neurological Disorders, National Clinical Research Centre for Aging and Medicine, Huashan Hospital, Fudan University, No. 12 Wulumuqi Zhong Road, Shanghai 200040, China; State Key Laboratory of Medical Neurobiology, Fudan University, Shanghai, China.
Declarations
Ethics approval and consent to participate: This study was approved by the Medical Ethics Committee of Huashan Hospital, Fudan University, Shanghai, China (ID: 2009-195 and 2016-359). All participants provided written informed consent.
Consent for publication: Not applicable.
Author contributions: Weiyi Zhong: Conceptualization; Data curation; Formal analysis; Methodology; Visualization; Writing – original draft.
Yiwei Xia: Data curation; Formal analysis; Investigation; Methodology; Validation; Writing – original draft.
Yunqing Ying: Data curation; Formal analysis; Validation; Writing – review & editing.
Yi Wang: Investigation; Validation; Writing – review & editing.
Lumeng Yang: Investigation; Validation; Writing – review & editing.
Xiaoniu Liang: Formal analysis; Investigation; Writing – review & editing.
Qianhua Zhao: Formal analysis; Investigation; Writing – review & editing.
Jianjun Wu: Investigation; Project administration; Resources; Writing – review & editing.
Zonghui Liang: Investigation; Project administration; Resources; Writing – review & editing.
Xiaoxiao Wang: Methodology; Resources; Software; Writing – review & editing.
Xin Cheng: Conceptualization; Funding acquisition; Writing – review & editing.
Ding Ding: Conceptualization; Data curation; Project administration; Resources; Writing – review & editing.
Qiang Dong: Conceptualization; Funding acquisition; Project administration; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Natural Science Foundation of China (81971123), Clinical Research Plan of SHDC (SHDC2020CR4016), Shanghai Municipal Committee of Science and Technology (20Z11900802), Shanghai Municipal Science and Technology Major Project (2018SHZDZX01), and ZJLab.
The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1. Pasi M, Cordonnier C. Clinical relevance of cerebral small vessel diseases. Stroke 2020; 51: 47–53. [DOI] [PubMed] [Google Scholar]
- 2. Bos D, Wolters FJ, Darweesh SKL, et al. Cerebral small vessel disease and the risk of dementia: a systematic review and meta-analysis of population-based evidence. Alzheimers Dement 2018; 14: 1482–1492. [DOI] [PubMed] [Google Scholar]
- 3. Hilal S, Mok V, Youn YC, et al. Prevalence, risk factors and consequences of cerebral small vessel diseases: data from three Asian countries. J Neurol Neurosurg Psychiatry 2017; 88: 669–674. [DOI] [PubMed] [Google Scholar]
- 4. Avolio A, Kim MO, Adji A, et al. Cerebral haemodynamics: effects of systemic arterial pulsatile function and hypertension. Curr Hypertens Rep 2018; 20: 20. [DOI] [PubMed] [Google Scholar]
- 5. Gosling RG, King DH. Arterial assessment by Doppler-shift ultrasound. Proc R Soc Med 1974; 67: 447–449. [PMC free article] [PubMed] [Google Scholar]
- 6. Raignault A, Bolduc V, Lesage F, et al. Pulse pressure-dependent cerebrovascular eNOS regulation in mice. J Cereb Blood Flow Metab 2017; 37: 413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garcia-Polite F, Martorell J, Del Rey-Puech P, et al. Pulsatility and high shear stress deteriorate barrier phenotype in brain microvascular endothelium. J Cereb Blood Flow Metab 2017; 37: 2614–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mestre H, Tithof J, Du T, et al. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun 2018; 9: 4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blair GW, Janssen E, Stringer MS, et al. Effects of cilostazol and isosorbide mononitrate on cerebral hemodynamics in the LACI-1 randomized controlled trial. Stroke 2022; 53: 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han SW, Lee SS, Kim SH, et al. Effect of cilostazol in acute lacunar infarction based on pulsatility index of transcranial Doppler (ECLIPse): a multicenter, randomized, double-blind, placebo-controlled trial. Eur Neurol 2013; 69: 33–40. [DOI] [PubMed] [Google Scholar]
- 11. Webb A, Werring D, Dawson J, et al. Design of a randomised, double-blind, crossover, placebo-controlled trial of effects of sildenafil on cerebrovascular function in small vessel disease: Oxford haemodynamic adaptation to reduce pulsatility trial (OxHARP). Eur Stroke J 2021; 6: 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shi Y, Thrippleton MJ, Marshall I, et al. Intracranial pulsatility in patients with cerebral small vessel disease: a systematic review. Clin Sci (Lond) 2018; 132: 157–171. [DOI] [PubMed] [Google Scholar]
- 13. Lau KK, Pego P, Mazzucco S, et al. Age and sex-specific associations of carotid pulsatility with small vessel disease burden in transient ischemic attack and ischemic stroke. Int J Stroke 2018; 13: 832–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pahlavian SH, Wang X, Ma S, et al. Cerebroarterial pulsatility and resistivity indices are associated with cognitive impairment and white matter hyperintensity in elderly subjects: a phase-contrast MRI study. J Cereb Blood Flow Metab 2021; 41: 670–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee WJ, Jung KH, Ryu YJ, et al. Progression of cerebral white matter hyperintensities and the associated sonographic index. Radiology 2017; 284: 824–833. [DOI] [PubMed] [Google Scholar]
- 16. Kneihsl M, Hofer E, Enzinger C, et al. Intracranial pulsatility in relation to severity and progression of cerebral white matter hyperintensities. Stroke 2020; 51: 3302–3309. [DOI] [PubMed] [Google Scholar]
- 17. Vikner T, Karalija N, Eklund A, et al. 5-Year associations among cerebral arterial pulsatility, perivascular space dilation, and white matter lesions. Ann Neurol 2022; 92: 871–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Armstrong NJ, Mather KA, Sargurupremraj M, et al. Common genetic variation indicates separate causes for periventricular and deep white matter hyperintensities. Stroke 2020; 51: 2111–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shams S, Martola J, Charidimou A, et al. Topography and determinants of magnetic resonance imaging (MRI)-visible perivascular spaces in a large memory clinic cohort. J Am Heart Assoc 2017; 6: e006279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ding D, Zhao Q, Guo Q, et al. The Shanghai Aging Study: study design, baseline characteristics, and prevalence of dementia. Neuroepidemiology 2014; 43: 114–122. [DOI] [PubMed] [Google Scholar]
- 21. Xia Y, Shen Y, Wang Y, et al. White matter hyperintensities associated with progression of cerebral small vessel disease: a 7-year Chinese urban community study. Aging 2020; 12: 8506–8522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mok V, Srikanth V, Xiong Y, et al. Race-ethnicity and cerebral small vessel disease – comparison between Chinese and White populations. Int J Stroke 2014; 9(Suppl. A100): 36–42. [DOI] [PubMed] [Google Scholar]
- 23. Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29: 162–173. [DOI] [PubMed] [Google Scholar]
- 25. Fazekas F, Chawluk JB, Alavi A, et al. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 1987; 149: 351–356. [DOI] [PubMed] [Google Scholar]
- 26. Doubal FN, MacLullich AM, Ferguson KJ, et al. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke 2010; 41: 450–454. [DOI] [PubMed] [Google Scholar]
- 27. Staals J, Makin SD, Doubal FN, et al. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 2014; 83: 1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brown R, Low A, Markus HS. Rate of, and risk factors for, white matter hyperintensity growth: a systematic review and meta-analysis with implications for clinical trial design. J Neurol Neurosurg Psychiatry 2021; 92: 1271–1277. [DOI] [PubMed] [Google Scholar]
- 29. Cai M, Jacob MA, van Loenen MR, et al. Determinants and temporal dynamics of cerebral small vessel disease: 14-year follow-up. Stroke 2022; 53: 2789–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fleysher R, Lipton ML, Noskin O, et al. White matter structural integrity and transcranial Doppler blood flow pulsatility in normal aging. Magn Reson Imaging 2018; 47: 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wartolowska KA, Webb AJ. White matter damage due to pulsatile versus steady blood pressure differs by vascular territory: a cross-sectional analysis of the UK Biobank cohort study. J Cereb Blood Flow Metab 2021; 42: 802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. De Reuck J. The human periventricular arterial blood supply and the anatomy of cerebral infarctions. Eur Neurol 1971; 5: 321–334. [DOI] [PubMed] [Google Scholar]
- 33. Webb AJS. Effects of vasodilating medications on cerebral haemodynamics in health and disease: systematic review and meta-analysis. J Hypertens 2019; 37: 1119–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wardlaw JM, Benveniste H, Nedergaard M, et al. Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol 2020; 16: 137–153. [DOI] [PubMed] [Google Scholar]
- 35. Nam KW, Kwon HM, Lee YS. Distinct association between cerebral arterial pulsatility and subtypes of cerebral small vessel disease. PLoS One 2020; 15: e0236049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shi Y, Thrippleton MJ, Blair GW, et al. Small vessel disease is associated with altered cerebrovascular pulsatility but not resting cerebral blood flow. J Cereb Blood Flow Metab 2020; 40: 85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chou KH, Wang PN, Peng LN, et al. Location-specific association between cerebral microbleeds and arterial pulsatility. Front Neurol 2019; 10: 1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wardlaw J, Bath PMW, Doubal F, et al. Protocol: The Lacunar Intervention Trial 2 (LACI-2). A trial of two repurposed licenced drugs to prevent progression of cerebral small vessel disease. Eur Stroke J 2020; 5: 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864241227304 for Cerebral pulsatility in relation with various imaging markers of cerebral small vessel disease: a longitudinal community-based study by Weiyi Zhong, Yiwei Xia, Yunqing Ying, Yi Wang, Lumeng Yang, Xiaoniu Liang, Qianhua Zhao, Jianjun Wu, Zonghui Liang, Xiaoxiao Wang, Xin Cheng, Ding Ding and Qiang Dong in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-docx-2-tan-10.1177_17562864241227304 for Cerebral pulsatility in relation with various imaging markers of cerebral small vessel disease: a longitudinal community-based study by Weiyi Zhong, Yiwei Xia, Yunqing Ying, Yi Wang, Lumeng Yang, Xiaoniu Liang, Qianhua Zhao, Jianjun Wu, Zonghui Liang, Xiaoxiao Wang, Xin Cheng, Ding Ding and Qiang Dong in Therapeutic Advances in Neurological Disorders