Abstract
Cytokine gene expression in cells migrating in afferent and efferent intestinal lymph was monitored for extended time periods in individual sheep experimentally infected with the nematode Trichostrongylus colubriformis. Animals from stable selection lines with increased levels of either genetic resistance (R) or susceptibility (S) to nematode infection were used. Genes for interleukin-5 (IL-5), IL-13, and tumor necrosis factor alpha (TNF-α), but not for IL-4, IL-10, or gamma interferon (IFN-γ), were consistently expressed at higher levels in both afferent and efferent lymph cells of R sheep than in S sheep. However, only minor differences were observed in the surface phenotypes and antigenic and mitogenic responsiveness of cells in intestinal lymph between animals from the two selection lines. The IL-4 and IL-10 genes were expressed at higher levels in afferent lymph cells than in efferent lymph cells throughout the course of the nematode infection in animals of both genotypes, while the proinflammatory TNF-α gene was relatively highly expressed in both lymph types. These relationships notwithstanding, expression of the IL-10 and TNF-α genes declined significantly in afferent lymph cells but not in efferent lymph cells during infection. Collectively, the results showed that R-line sheep developed a strong polarization toward a Th2-type cytokine profile in immune cells migrating in lymph from sites where the immune response to nematodes was initiated, although the IFN-γ gene was also expressed at moderate levels. Genes or alleles that predispose an animal to develop this type of response appear to have segregated with the R selection line and may contribute to the increased resistance of these animals.
Our present understanding of the immunological interactions occurring between intestinal nematodes and their hosts is derived largely from laboratory models, although the precise effector mechanisms that lead to parasite removal remain ill defined (14, 17). Cytokine profiles associated with nematode resistance vary in detail depending on the particular host-parasite combination, but there is typically a dominant secretion of Th2 type cytokines (3, 16, 19, 23, 24, 26, 32, 33, 53). Similar tendencies toward Th2 cytokine responses have been observed in cattle (2, 20, 21) and sheep (22, 39, 43, 48) although the polarization of the response has been less pronounced. In most of these studies, however, observations were made on cells taken from an animal at only one or a few time points and then cultured in vitro with specific stimuli, leaving uncertainties about how accurately they reflect the dynamic changes that occur over extended time periods in vivo.
Trichostrongylus colubriformis is an important nematode parasite of sheep. Following ingestion of an infective third-stage larva (L3), it takes almost 3 weeks for the parasite to progress through several developmental stages within the small intestine and to achieve patancy. In animals raised to be nematode naïve, multiple or extended infections are normally needed to stimulate protective immunity, although sheep with an appropriate genotype can become immune after shorter exposure times (18, 50). In the work reported here, we used lymphatic cannulation procedures to access continuously immune cells migrating in lymph directly from the small intestinal mucosa and from the regional mesenteric lymph nodes of sheep. This procedure enabled us to monitor immunological events over long periods of time while animals were subjected to experimental infection with T. colubriformis. Two groups of selection line animals were used, one with significantly enhanced genetic resistance (R) to nematodes and another with increased susceptibility (S). The two sheep lines differ in fecal egg counts by at least 36-fold under natural challenge (6, 36). This approach allowed us to monitor, without any need for further in vitro stimulation, the in vivo expression levels of several cytokine genes in cells migrating in lymph directly from those sites where antinematode immune responses are generated. We wanted to address a number of questions. Are there differences in the cytokine responses between animals selected for various levels of genetically determined resistance? Do the afferent and efferent mucosal immune compartments sustain similar or different cytokine profiles? Do cytokine profiles induced by nematodes fluctuate over time, as the parasite progresses through its life cycle?
MATERIALS AND METHODS
Surgical procedures.
All animal experiments and surgical procedures were approved by the Animal Ethics Committee of the Wallaceville Animal Research Centre. Methods for long-term collection and characterization of afferent and efferent lymph draining from the small intestine of sheep have been described in detail elsewhere (25). The collection of afferent lymph involved a two-stage surgical procedure. Briefly, in the first stage, mesenteric lymph nodes were removed from sites adjacent to the jejunum, and in a second-stage operation 2 months later, a reconstituted pseudo-afferent lymphatic vessel was located and cannulated. A similar procedure was used to cannulate efferent lymphatic vessels draining from lymph nodes in the region of the upper jejunum in normal intact sheep. All cannulae were exteriorized from the abdominal cavity and led into a collection flask attached to the animal, enabling continuous sampling of lymph.
Animals and experimental design.
The overall aim of the study was to detect whether there were differences in the cytokine gene expression of afferent and efferent intestinal lymph cells (AILC and EILC, respectively) between two lines of sheep with different levels of genetic resistance or susceptibility to nematodes during experimental infection with T. colubriformis. Cytokine genes that are important in mediating resistance or susceptibility to nematode infection were included in this study. These cytokines are indicative of either a Th1-type (gamma interferon [IFN-γ]) or a Th2-type (interleukin-4 [IL-4], IL-5, and IL-13) response or are regulatory (IL-10) or proinflammatory (tumor necrosis factor alpha [TNF-α]). A further aim was to examine in which anatomical compartment the polarization toward a Th1- or Th2-type response occurs.
Data from a total of 18 New Zealand wethered male Romney sheep, between 1 and 2 years old, that had a patent cannula for more than 18 days after oral infection with 50,000 infective larvae of the nematode T. colubriformis were included in the cytokine expression study. For the phenotypic analysis of intestinal lymphatic cells, additional animals (one animal for efferent lymph collection and two animals for afferent lymph collection) that had a patent cannula for less than 18 days were included. All sheep were derived from the Wallaceville nematode-resistant (R) or nematode-susceptible (S) sheep lines that differ by at least 36-fold in autumn fecal egg counts (FEC) under natural challenge (6, 36). Within each line, lambs were allocated into nematode-naïve (Rn and Sn) and nematode-primed (Rp and Sp) groups. Nematode-naïve animals were raised from 24 h after birth in pens under nematode-free conditions, but nematode-primed sheep were kept on pasture and therefore experienced natural nematode challenge, of which T. colubriformis formed the major part. The Rn, Sn, Rp, and Sp groups were randomly divided further into subgroups and fitted with either afferent or efferent lymphatic cannulae. Genotype, preinfection status, and type of intestinal lymph cannulation of the 18 animals analyzed are listed in Table 1.
TABLE 1.
Genotype, preinfection status, and type of intestinal lymph collected from the 18 animals included in the cytokine gene expression study
| Type of lymph | No. of animals by genotype and preinfection status
|
|||
|---|---|---|---|---|
| S-line sheep (n = 9)
|
R-line sheep (n = 9)
|
|||
| Naïve | Primed | Naïve | Primed | |
| Afferent | 1 | 2 | 3 | 2 |
| Efferent | 3 | 3 | 3 | 1 |
| Total | 4 | 5 | 6 | 3 |
Animals were infected on day 0 orally with 50,000 infective larvae of T. colubriformis, and the infection was terminated at day 21 by drenching with oxfendazole (5 mg/kg of body weight; Systamex; Coopers).
Parasitology.
Infective T. colubriformis larvae were cultured under standard procedures from eggs obtained from feces of a monospecifically infected sheep. Fecal egg counts were performed on each animal at weekly intervals throughout the duration of the experiment by using a modified McMaster method (55).
Sampling.
Lymph was collected continuously into sterile flasks containing heparin (Sigma) and antibiotics (penicillin and streptomycin; Sigma). In addition, 5 to 15 ml of fresh lymph was collected over a period of 30 to 60 min daily into sample tubes under aseptic conditions to allow sampling of cells and lymph plasma. Cells were promptly separated from lymph by centrifugation at 400 × g for 3 min, washed once in cold phosphate-buffered saline (PBS) and resuspended in RNAlater (Ambion) to preserve the integrity of mRNA. Samples were stored at −20°C until subjected to RNA extraction and reverse transcription.
At specified intervals, cells obtained from lymph were subjected to phenotypic analysis of cell differentiation markers or used for cell culture experiments. Cells obtained from lymph before experimental infection, and thereafter at weekly intervals for up to 4 weeks, were used for cell culture. Phenotyping of AILC and EILC was done prior to experimental infection and thereafter at intervals of 6 to 8 days throughout the experiment.
RNA extraction and reverse transcription.
RNAlater was removed prior to RNA extraction by centrifugation at 3,300 × g for 5 min. Total RNA was isolated, according to the manufacturer's protocol, from cells by using TRI-REAGENT-LS (Molecular Research Center, Inc.) that combines phenol and guanidine thiocyanate to facilitate cell lysis and inhibition of RNase activity. The lysate was separated into aqueous and organic phases by bromochloropropane addition and centrifugation. RNA was precipitated from the aqueous phase by the addition of isopropanol, and the RNA pellet was washed in ethanol and solubilized. Total RNA yield was calculated based on the spectrophotometric measurement of absorption at 260 to 280 nm. First-strand cDNA synthesis was performed by using SuperScriptII RNase H− reverse transcriptase (Invitrogen) and poly(A) · oligo(dT)12-18 primer to reverse transcribe up to 5 μg of total RNA, according to the manufacturer's protocol, and adjusted to an initial concentration of 20 ng of RNA per ml. cDNA was stored at −20°C until used for real time PCR.
Primer design.
For each target gene a primer pair was selected by using Primer Express software (Applied Biosystems) for use in a SYBRGreen real-time PCR assay. Oligonucleotides were designed based on known ovine mRNA sequences to amplify a product with the size of 51 bp, with a melting temperature of 58 to 60°C and one of the two primers spanning the junction of two exons. In cases where the ovine genomic sequence was not known (IL-4, IL-13, IFN-γ, and TNF-α), exon-intron boundaries were empirically identified by comparison of the ovine mRNA sequence with the corresponding very closely related bovine genomic sequence. A validation experiment that demonstrated exclusive amplification of cDNA was conducted for each primer pair prior to the start of the experiment (data not shown). Primer sequences and the melting temperatures of the corresponding amplicons were reported previously (25).
Real-time PCR and quantification of gene expression.
Cytokine gene expression was detected by real-time PCR by using a GeneAmp 5700 sequence detection system in combination with sequence detector software (Applied Biosystems, Foster City, Calif.). All reactions were assembled in 25 μl in duplicates in optical 96-well reaction plates by using AmpErase uracil N-glycosylase as carryover prevention. The reaction mixture consisted of SYBRGreen PCR master mix (Applied Biosystems) containing SYBRGreen 1 dye, AmpliTaq Gold, and deoxynucleoside triphosphates with dUTP in optimized buffer components, a 0.2 μM concentration of each gene-specific primer, and 1 μl of cDNA template. For each gene of interest and on each plate, negative and positive controls were included. Forty cycles of amplification with denaturation at 95°C for 15 sec, followed by annealing and extension at 60°C for 1 min, were performed after an initial incubation at 50°C for 2 min, followed by incubation at 95°C for 10 min. For each sample a melting curve was generated after completion of amplification and analyzed in comparison to the positive and negative controls. Mean cycle threshold (CT) values of duplicate samples were used for analysis.
The comparative CT method was employed for relative quantification where the amount of target is normalized to an endogenous reference (housekeeping gene) (User Bulletin 2, ABI PRISM 7700 Sequence Detection System; Applied Biosystems). The CT value indicates the fractional cycle number at which the amount of amplified target reaches a fixed threshold, and ΔCT represents the difference in threshold cycles for the target and housekeeping genes. A validation experiment that demonstrated the approximately equal amplification efficiency for the target (IL-4, IL-5, IL-10, IL-13, IFN-γ, and TNF-α) and housekeeping (GAPDH [glyceraldehyde-3-phosphate dehydrogenase]) gene was conducted prior to commencement of the experiment (data not shown).
Flow cytometry.
Conjugated monoclonal antibodies (MAbs) that recognize ovine major histocompatibility complex class II (MHC-II; clone Du6-30-19), CD4 (clone 17D-13), CD8 (clone 7C-2), γδ T-cell receptor (TCR; clone H1-176), CD21 (clone Du14-24), CD11c (clone OM1[38]), and CD1b (clone CC14 [30]) were used at a dilution of 1:100. These MAbs were conjugated to Alexa 488 (Molecular Probes), a fluorochrome with spectral properties similar to fluorescein isothiocyanate, or to phycoerythrin (Molecular Probes) according to the instructions of the manufacturer. The anti-mouse CD25 MAb (clone CACT116A) that cross-reacts with ovine CD25 was obtained from VMRD. The MAb SBUT6 that reacts with ovine CD1c (31) was obtained from E. Meeusen, The University of Melbourne. The cell lines for OM1 and CC14 were kindly provided by M. Pepin, Institut National de la Recherche Agronomique, Laboratoire de Pathologie Infectieuse et Immunologie, Nouzilly, France, and C. J. Howard, Institute for Animal Health, Compton, Newbury, Berkshire, United Kingdom, respectively. The MAbs Du6-30-19, 17D-13, 7C-2, H1-176, and Du14-24 were produced and characterized by the sheep immunology group at the Basel Institute for Immunology and shown to have the indicated specificities by immunoprecipitation and immunohistology and comparison with known standard antibodies.
A combination of indirect and direct staining procedures was used according to standard methods to label about 106 cells. All incubation steps were performed at room temperature for 10 min, washed in between with PBS supplemented with 0.01% sodium azide and 0.5% fetal calf serum (PBS-sodium azide-FCS), and resuspended in 300 μl of PBS-sodium azide-FCS and analyzed on a FACSCalibur flow cytometer (BD Biosciences, San Jose, Calif.) by using the CELLQuest software package. Live gates were set on forward scatter and side scatter profiles to include the entire live cell population present in AIL or EIL in the analysis, and results were expressed as a proportion of positively stained cells.
Lymphocyte proliferation assays (LPAs).
Cells obtained from AIL or EIL were washed twice in RPMI 1640 medium (Gibco) and adjusted to a concentration of 106 cells/ml in RPMI 1640 medium plus l-glutamine (Invitrogen), supplemented with 0.2% sodium bicarbonate (BDH), 50 mM mercaptoethanol (Sigma), 100 U of penicillin (Sigma) per ml, 100 μg of streptomycin (Sigma) per ml, 0.05 μg of gentamicin (Sigma) per ml, 0.5 μg of amphotericin B (Sigma) per ml, and 5% FCS (Invitrogen). Viability was always higher than 95% as determined by trypan blue dye exclusion.
Approximately 2 × 105 AILC or EILC were cultured per well in triplicate in the presence of 10 μg of T. colubriformis larval excretory-secretory antigen (TcL3ESag) per ml, with 5 μg of concanavalin A (ConA; Sigma) per ml, or with medium alone (nonstimulated). TcL3ESag was obtained from exsheathed infective larvae by using standard procedures (15). Optimal concentrations of antigen or mitogen were established prior to the start of the experiment. Cultures were incubated for 96 h, including a final 18-h pulse with 1 Ci of [methyl-3H]thymidine (Amersham, Chalfont St. Giles, United Kingdom) at 37°C in a humidified atmosphere of 5% CO2 in air and harvested onto glass fiber filter paper (Tomtec), and radioactivity was measured in a liquid scintillation counter (MicroBeta Trilux; Wallac). Results were expressed as mean counts per minute (cpm) of triplicate cultures, and for mitogen- and antigen-stimulated cultures a stimulation index (SI) was calculated according to the following formula: SI = mean cpm of stimulated cell cultures/mean cpm of nonstimulated cell cultures.
Statistical analysis.
The nonparametric Kruskal test was used to compare FEC data from the R and S groups. Cell phenotyping data were analyzed by using the unbalanced treatment structure three-way analysis of variance including interactions in Genstat by using lymph compartment (afferent or efferent), genotype (R or S), and preinfection status (nematode naïve or primed) as variables. The analysis of LPA data was performed on log-transformed, triplicate observations, and analysis of variance was used for analysis of dose responses. In all cases, P < 0.05 was considered to be significant.
Cytokine gene expression data.
Cytokine gene expression data were analyzed on ΔCT values by using the following procedures for (i) pulse analysis and (ii) analysis of group means.
(i) Pulse analysis.
Pulse analysis for individual cytokine gene expression profiles was done by using the variables control chart method, which is used for tracking processes over time and is able to detect special cases not fitting into the baseline variation. In this setting the special cases looked for were any pulses of cytokine gene expression. The control chart for individual observations was used here.
The individual data points were plotted against day and the average (xbar) was calculated and drawn in; then the variance (sigma2) was estimated by using the average of the moving range (of length 2) divided by an unbiasing constant (in Minitab 13.31), and upper and lower limits were drawn in at 3 sigmas from the average. The tests used to look for pulses from the average were the following: (a) any point more than 3 sigmas from mean; (b) two consecutive points out of three more than 2 sigmas, on the same side, from the mean; and (c) four consecutive points out of five more than 1 sigma, on the same side, from the mean.
Once the first run had been done and any pulses found, these points were removed from the calculations of the mean and sigma, and the process was repeated with the remaining points. This was continued until all the remaining points fitted into the baseline variation. This baseline could be shown to fit a normal distribution.
(ii) Analysis for group means.
Residual maximum likelihood analysis for group means (37) fits both fixed effects for comparisons between treatments and estimates the random effects, the standard deviation, and any correlation coefficient for that particular model. It was used for analyzing this data because of the possibility of the readings over time being correlated, and by using residual maximum likelihood we can fit or test different models for this. In most cases the ΔCT profiles of the animals remained either reasonably constant or increased or decreased in a roughly linear way with correlated variation about a best fit line, with, for example, several samples above the line followed by several beneath it.
Fixed effects were genotype (R or S), the preinfection status (naïve or primed), the compartment (afferent or efferent), day of infection, and all the interactions of these. Random effect elements were the individual animals and their responses over time. Out of several different models tested (unstructured, autoregressive [AR], RC [random coefficients] AR+RC, and spline models) the AR model appeared the most consistent as it gave the largest decrease in deviance from the unstructured model, with there being little difference between this and the AR+RC model for most variables.
The AR model supposes that future observations will be correlated with the present one. The next observation per animal is correlated r with the present one, the observation 2 time points away will be correlated r*r, the observation 3 time points out will be correlated r*r*r with the present one, etc. The absolute size of r is less than 1, so the correlation with the present point decreases as the new observation is more time points away from the present.
By using the AR model, a full four-factor model with all interactions was fitted. This calculated pooled estimates both for within and between animal variation and for the correlation coefficient. These were used to test the significance levels for all the main effects and their interactions. Where higher-order interactions were significant, lower-order differences are not reported. The results in the report include the predicted means of each of the groups at day 10 along with the standard errors (SE) of these predictions.
RESULTS
Parasitology.
FEC performed 3 weeks after experimental infection with 50,000 L3 T. colubriformis larvae revealed that R-line sheep had significantly (P = 0.034) lower FEC than the S-line sheep. For R-line groups (n = 9), the first quartile, median, and third quartile for FEC of each of groups were 300, 1,700, and 3,350, respectively; for S-line groups (n = 9), the corresponding values were 2,900, 3,400, and 5,350, respectively. This demonstrates that in R- but not S-line sheep the immune response to nematode infection was partly protective. As expected, FEC performed 1 and 2 weeks after infection were always 0.
Background cytokine gene expression prior to experimental nematode infection.
Analysis of cell samples obtained from lymph prior to the time of experimental nematode challenge (day −3 to day 0) revealed that expression of the IL-4, IL-5, IL-10, IFN-γ, and TNF-α genes did not differ significantly between the R-line (n = 9) and S-line (n = 9) sheep (Table 2). For both AILC and EILC, expression of the IL-13 gene tended to be higher in R-line than in S-line sheep, although these differences did not quite achieve statistical significance. Therefore, among all animals used in this work, there were no significant differences in the background expression levels of these cytokine genes in either lymph compartment between R- and S-line sheep prior to experimental nematode challenge.
TABLE 2.
Cytokine gene expression in AILC and EILC of R- and S-line sheep prior to experimental infection with T. colubriformis
| Cytokine | ΔCT (mean ± SE)a
|
|||
|---|---|---|---|---|
| R-line sheep
|
S-line sheep
|
|||
| AILC (n = 5) | EILC (n = 4) | AILC (n = 3) | EILC (n = 6) | |
| IL-4 | 10.1 ± 0.76 | 11.0 ± 0.96 | 8.9 ± 1.02 | 11.4 ± 0.68 |
| IL-5 | 12.3 ± 1.92 | 15.7 ± 2.43 | 16.1 ± 2.57 | 15.8 ± 1.72 |
| IL-10 | 7.6 ± 1.22 | 11.1 ± 1.54 | 9.3 ± 1.63 | 11.9 ± 1.09 |
| IL-13 | 10.8 ± 1.57 | 10.1 ± 1.98 | 13.0 ± 2.10 | 12.1 ± 1.40 |
| IFN-γ | 9.7 ± 1.53 | 8.7 ± 1.94 | 7.3 ± 2.05 | 9.3 ± 1.37 |
| TNF-α | 5.8 ± 0.94 | 7.2 ± 1.18 | 6.2 ± 1.26 | 7.3 ± 0.84 |
Values were derived from lymph samples collected over the 3 days immediately preceding challenge. ΔCT represents the difference in threshold cycles between the target and house keeping genes. Therefore, low ΔCT values represent high gene expression levels and high ΔCT values represent low gene expression levels. n, number of sheep in group.
This analysis also showed that AILC obtained prior to nematode challenge (day −3 to day 0) had a higher level of IL-10 gene expression than did EILC (P < 0.01). There was a tendency for the IL-4 and TNF-α genes to be expressed at higher levels in AILC than in EILC; however, the differences did not quite achieve statistical significance. IL-10 gene expression was also significantly higher in both lymph compartments of primed sheep of both genotypes (n = 8) in comparison to levels in pen-raised, nematode-naïve sheep (n = 10; P < 0.05; data not shown). This suggests that primed, but not pen-raised, animals experienced stimuli that caused a long-lasting up-regulation of the IL-10 gene in recirculating intestinal lymph cells.
Effect of genotype on cytokine gene expression during experimental nematode infection.
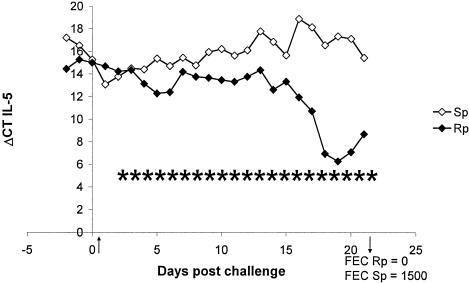
The genotype of the animal had a significant influence on the level of cytokine gene expression during nematode challenge (Table 3). AILC and EILC of R sheep (n = 9) showed a significantly higher expression of the IL-5 gene than cells of S sheep (n = 9; P < 0.05). In addition, there were opposing trends of IL-5 gene expression in AILC between Rp and Sp sheep: gene up-regulation occurred during infection in the Rp group (P < 0.01; n = 2), while gene down-regulation occurred in the Sp group (P < 0.01; n = 2) (Fig. 1). These effects were not seen in EILC (data not shown). Little or no IL-5 gene expression was observed in AILC and EILC of the Sn group during primary nematode infection.
TABLE 3.
Mean ΔCT and mean slope of cytokine gene expression in AILC and EILC of R- and S-line sheep during experimental infection with T. colubriformisa
| Cytokine | Value for R-line sheep (n = 9)
|
Value for S-line sheep (n = 9)
|
||
|---|---|---|---|---|
| Mean ΔCT ± SE | Mean slope ± SE | Mean ΔCT ± SE | Mean slope ± SE | |
| IL-5 | 13.6 ± 1.06b | −0.07 ± 0.029 | 16.7 ± 1.06 | 0.03 ± 0.029 |
| IL-13 | 9.0 ± 0.69b | −0.16 ± 0.034c | 12.8 ± 0.69 | 0.03 ± 0.034 |
| TNF-αd | 7.3 ± 0.53 | 0.00 ± 0.023 | 8.0 ± 0.53 | 0.10 ± 0.230 |
| IL-10 | 10.3 ± 0.50 | 0.01 ± 0.032 | 10.5 ± 0.47 | −0.01 ± 0.031 |
| IFN-γ | 9.7 ± 0.78 | −0.03 ± 0.022 | 8.7 ± 0.78 | 0.01 ± 0.022 |
| IL-4 | 10.5 ± 0.44 | 0.00 ± 0.019 | 11.0 ± 0.44 | 0.04 ± 0.019 |
For each animal samples were collected daily over a period of 18 to 21 days. The lymphatic compartment and the preinfection status of the animals were not taken into consideration. ΔCT represents the difference in threshold cycles for the target and housekeeping genes. Therefore, low ΔCT values represent high gene expression levels and high ΔCT values represent low gene expression levels.
Significantly (P < 0.05 for IL-5; P < 0.01 for IL-13) higher expression in R-line than S-line animals during T. colubriformis infection.
Significant (P < 0.01) trend for up-regulation of the IL-13 gene in R-line sheep during infection with T. colubriformis.
Significant (P < 0.01) difference in the trend for TNF-α gene expression between R- and S-line animals during T. colubriformis infection.
FIG. 1.
IL-5 gene expression in AILC of a representative Rp- and Sp-line sheep. Animals were experimentally infected on day 0 with 50,000 L3 T. colubriformis larvae. FEC determined at day 21 revealed a nematode immunity phenotype in the Rp and a nonimmunity phenotype in the Sp animal. IL-5 is expressed at a significantly (P < 0.01) higher level in Rp than Sp sheep with a significant (P < 0.01) trend for up-regulation in Rp and down-regulation in Sp sheep. Results are presented as ΔCT values, which is the difference in threshold cycles for the target and housekeeping genes. Therefore, low ΔCT values represent high gene expression and high ΔCT values represent low gene expression. An asterisk indicates time points of significant increases in gene expression within the Rn sheep.
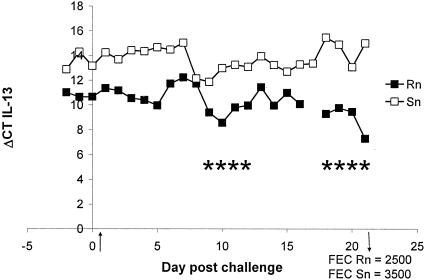
In both lymph compartments, IL-13 gene expression was higher during nematode infection in R (n = 9) than in S animals (n = 9) (P < 0.01). There was also a difference in the trend between the two selection lines; again, in both lymph compartments of R-line sheep, the expression of the IL-13 gene progressively increased over time (P < 0.01), while in S-line animals it did not change (Table 3 and Fig. 2).
FIG. 2.
IL-13 gene expression in AILC of a representative Rn- and Sn-line sheep. Animals were experimentally infected on day 0 with 50,000 L3 T. colubriformis larvae. FEC determined at day 21 revealed a nematode nonimmunity phenotype in both animals. IL-13 is expressed at a significantly (P < 0.01) higher level in the Rn than Sn sheep. Results are presented as ΔCT values, which is the difference in threshold cycles for the target and housekeeping genes. Therefore, low ΔCT values represent high gene expression and high ΔCT values represent low gene expression. An asterisk indicates time points of significant increases in gene expression within the Rn sheep.
The TNF-α gene was expressed abundantly in all animals. However, the levels achieved in both lymph compartments of R-line sheep (n = 9) consistently exceeded those in S-line sheep (n = 9), and the overall slopes differed significantly between the R and S groups (P < 0.01) (Table 3). This result can be attributed to the fact that during primary infection of nematode-naïve animals, TNF-α gene expression remained approximately constant in the EILC of S-line sheep (n = 3) but showed a strong trend for up-regulation in R-line sheep (n = 3; P < 0.01; data not shown). In addition AILC of primed S-line sheep (n = 2) showed a significant trend for down-regulation during infection (P < 0.01; data not shown), which was not seen in the corresponding R group.
The IL-4, IL-10, and IFN-γ genes were expressed in cells from both lymphatic compartments of R- and S-line sheep. There were no consistent or significant differences in either the levels or trends of IL-4 and IL-10 gene expression between R-line (n = 9) and S-line (n = 9) animals during experimental nematode infection (Table 3). Similarly, there were no genotype-related differences in IFN-γ gene expression levels between the R and S lines (Table 3).
Taken together, these data clearly demonstrate that the regional immune response of R-line sheep was characterized by a highly up-regulated Th2-type and proinflammatory cytokine profile, which far exceeded expression observed in the S-line sheep, although in both line animals and both lymphatic compartments the IFN-γ gene was expressed at moderate levels. In vivo, cytokine polarization evidently occurred at the mucosal site of infection and in the regional draining lymph nodes, since immune cells migrating from these sites exhibited the characteristics of a dominant Th2 cytokine gene profile. In contrast to R-line animals, even primed S-line sheep failed to develop comparable IL-13 and IL-5 responses in cells migrating out of these sites.
Cytokine gene expression in different lymph compartments during nematode infection.
Significant differences occurred in the expression of some cytokine genes in immune cells taken from either the afferent or efferent lymphatic compartments. Throughout the course of nematode infection, IL-4 gene expression was higher in AILC (n = 8) than EILC (n = 10), regardless of the genotype of the animal (P < 0.05) (Table 4). A corresponding relationship was observed for the IL-10 gene (P < 0.01) (Table 4). For both the R- and S-line animals, the trend of IL-10 gene expression declined in AILC (n = 8; P < 0.01), but increased in EILC (n = 10; P < 0.01) after challenge (Table 4), suggesting that, irrespective of genotype, experimental nematode infection down-regulated IL-10 gene expression in cells migrating in afferent but not efferent lymph. During nematode infection, IFN-γ gene expression showed an inclining trend in EILC (n = 10; P < 0.05) but not AILC (n = 8) (Table 4).
TABLE 4.
Mean ΔCT and mean slope, of cytokine gene expression in AILC and EILC of sheep during experimental infection with T. colubriformisa
| Cytokine | Value in AILC (n = 8)
|
Value in EILC (n = 10)
|
||
|---|---|---|---|---|
| Mean ΔCT ± SE | Mean slope ± SE | Mean ΔCT ± SE | Mean slope ± SE | |
| IL-5 | 14.0 ± 1.20 | −0.01 ± 0.032 | 16.1 ± 1.07 | −0.03 ± 0.029 |
| IL-13 | 11.0 ± 1.02 | −0.06 ± 0.042 | 10.8 ± 0.91 | −0.08 ± 0.037 |
| TNF-α | 7.1 ± 0.54 | 0.10 ± 0.024 | 8.1 ± 0.49 | 0.01 ± 0.022 |
| IL-10 | 9.2 ± 0.60b | 0.09 ± 0.026c | 11.3 ± 0.50 | −0.06 ± 0.022d |
| IFN-γ | 9.3 ± 0.84 | 0.04 ± 0.022 | 9.1 ± 0.75 | −0.05 ± 0.020d |
| IL-4 | 9.9 ± 0.40b | 0.02 ± 0.021 | 11.4 ± 0.35 | 0.02 ± 0.019 |
For each animal samples were collected daily over a period of 18 to 21 days. The genotype and the prechallenge status of the animals were not taken into consideration. ΔCT represents the difference in threshold cycles for the target and housekeeping genes. Therefore, low ΔCT values represent high gene expression levels, and high ΔCT values represent low gene expression levels.
Significantly (P < 0.05 for IL-4; P < 0.01 for IL10) higher expression in AILC than in EILC during T. colubriformis infection.
Significant (P < 0.01) trend for down-regulation of the IL-10-gene in AILC during infection with T. colubriformis.
Significant trend for IFN-γ (P < 0.05) and IL-10 (P < 0.01) gene up-regulation in EILC but not AILC during infection with T. colubriformis.
Cytokine gene expression in sheep with a nematode-naïve or primed prechallenge status during nematode infection.
The gene expression levels of IL-5, IL-13, TNF-α, IFN-γ, and IL-4 did not differ significantly between groups of animals with a naïve and primed preinfection status during experimental infection (Table 5). The only interaction observed with higher-order significance was the expression of the IL-10 gene at higher levels in primed (n = 8) than naïve sheep (n = 10, P < 0.01) (Table 5). For both selection lines, TNF-α gene expression was lower in EILC of pen-raised nematode-naïve animals (n = 6) than in primed animals (n = 4; P < 0.01; data not shown).
TABLE 5.
Mean ΔCT and mean slope of cytokine gene expression in intestinal lymph cells of nematode-naïve and-primed sheep during experimental infection with T. colubriformisa
| Cytokine | Value for naive sheep (n = 10)
|
Value for primed sheep (n = 8)
|
||
|---|---|---|---|---|
| Mean ΔCT ± SE | Mean slope ± SE | Mean ΔCT ± SE | Mean slope ± SE | |
| IL-5 | 16.4 ± 1.11 | −0.03 ± 0.030 | 13.9 ± 1.11 | −0.01 ± 0.030 |
| IL-13 | 11.0 ± 0.96 | −0.07 ± 0.039 | 10.7 ± 0.96 | −0.07 ± 0.039 |
| TNF-α | 8.2 ± 0.51 | −0.01 ± 0.022 | 7.1 ± 0.51 | 0.11 ± 0.022 |
| IL-10 | 11.3 ± 0.71 | −0.02 ± 0.026 | 9.5 ± 0.75b | 0.03 ± 0.027 |
| IFN-γ | 9.3 ± 0.80 | −0.04 ± 0.021 | 9.0 ± 0.80 | 0.02 ± 0.021 |
| IL-4 | 11.2 ± 0.41 | 0.02 ± 0.020 | 10.2 ± 0.41 | 0.02 ± 0.020 |
For each animal samples were collected daily over a period of 18 to 21 days. The lymphatic compartment and the genotype of the animals were not taken into consideration. ΔCT represents the difference in threshold cycles for the target and house keeping genes. Therefore, low ΔCT values represent high gene expression levels, and high ΔCT values represent low gene expression levels.
Significantly (< 0.01) higher IL-10 gene expression in nematode-primed than in nematode-naïve sheep during infection with T. colubriformis.
LPAs.
Cells from both afferent and efferent intestinal lymph proliferated when stimulated in vitro by nematode antigens or ConA, regardless of the genotype or preinfection status of the animal. The proliferative responses induced by nematode antigens were very small in comparison to those induced by ConA.
The SI of the response of AILC from primed animals to 10 μg of TcL3ESag per ml increased from 2.5 ± 1.4 at week 0 to 4.4 ± 0.7 at week 3 (mean SI ± standard deviation of the mean [SDM]; n = 3) after challenge and was higher at weeks 2 and 3 than the response seen with cells from naïve animals (n = 3), but none of these differences achieved statistical significance. Responsiveness of AILC to stimulation by 5 μg of ConA per ml was high in both naïve and primed animals. No significant differences between groups and time points were seen (data not shown). The in vitro proliferative activity induced in EILC by TcL3ESag and ConA was also assayed at different time points after nematode infection. Concentration-dependent responses comparable to those seen in AILC were measured (data not shown). For example, 1 week after nematode challenge, the response of EILC to 10 μg of TcL3ESag per ml was 3.2 ± 0.4 (mean SI ± SDM; n = 4).
Phenotypic analysis of AILC and EILC.
The surface phenotype of cells in afferent and efferent intestinal lymph was determined by fluorescence-activated cell sorting analysis at different time points after nematode challenge. The results of single-color analyses are summarized in Table 6. The percentage of CD4+ cells in efferent lymph decreased significantly from 1 week after challenge and remained depressed thereafter. Apart from this, there was large animal-to-animal variability, and no other cell subset showed any significant change in frequency after nematode challenge in either of the compartments analyzed. The frequency of γδ TCR+ cells in AIL and EIL tended to increase continually after nematode challenge, as did the proportion of MHC-II+ cells in AIL, but none of these differences achieved statistical significance.
TABLE 6.
Expression of cell differentiation markers on leucocytes in afferent and efferent intestinal lymph cells after experimental infection with T. colubriformis
| Marker | % of cells positive (mean ± SDM) at:a
|
||||
|---|---|---|---|---|---|
| Time 0 | Week 1 | Week 2 | Week 3 | Week 4 | |
| Marker in EILC | |||||
| CD4 | 51.5 ± 11.3 | 43.5 ± 8.4b | 37.5 ± 9.4b | 38.1 ± 7.0b | 37.6 ± 4.4b |
| CD8 | 31.4 ± 12.6 | 29.4 ± 7.9 | 24.7 ± 5.1 | 23.4 ± 5.2 | 26.0 ± 5.4 |
| γδ TCR | 5.6 ± 2.0 | 6.4 ± 2.0 | 7.9 ± 4.1 | 8.0 ± 2.6 | 9.3 ± 5.6 |
| CD21 | 20.4 ± 11.9 | 19.3 ± 10.0 | 15.4 ± 7.5 | 16.6 ± 7.0 | 15.3 ± 6.3 |
| Marker in AILC | |||||
| CD4 | 54.5 ± 9.6 | 55.9 ± 8.5 | 54.1 ± 8.7 | 53.8 ± 8.0 | NT |
| CD8 | 29.0 ± 9.2 | 29.4 ± 5.0 | 28.3 ± 7.5 | 21.8 ± 7.8 | NT |
| γδ TCR | 3.9 ± 1.4 | 5.1 ± 2.5 | 5.7 ± 3.8 | 6.9 ± 3.1 | NT |
| CD21 | 8.9 ± 6.5 | 10.6 ± 4.1 | 10.0 ± 5.1 | 11.8 ± 5.4 | NT |
| MHC-II | 34.3 ± 8.3 | 37.2 ± 9.0 | 43.0 ± 11.4 | 40.4 ± 11.7 | NT |
| CD11c | 11.2 ± 3.2 | 15.0 ± 1.1 | 14.7 ± 2.1 | 14.8 ± 3.4 | NT |
| CD1b | 1.5 ± 1.0 | 3.0 ± 1.6 | 2.6 ± 0.8 | 2.4 ± 0.1 | NT |
| CD25 | 10.8 ± 4.2 | 12.8 ± 3.5 | 11.3 ± 5.3 | 15.1 ± 7.5 | NT |
Data from R-line and S-line sheep have been combined, since no genotype-related differences in cell subset frequencies occurred after experimental infection (see text). For EILC, n = 10 to 11; for AILC, n = 6 to 10. NT, not tested.
Values different from time zero (P < 0.05).
Prior to nematode challenge, Sn sheep had significantly higher proportions of γδ TCR+ cells in lymph than did Rn sheep, but this difference was not detected postchallenge. No other genotype-related differences in lymphocyte phenotypes were observed.
The AILC population was distinguished by the occurrence of CD11c+, CD1b+, CD1c+, CD11c+MHC-II+, and CD11c+CD1c+ cells, demonstrating the presence of antigen presenting cells (APCs), which collectively constituted around 10 to 15% of AILC. No significant differences between the frequencies of these APC phenotypes or of activated CD4+ T cells (CD4+CD25+) were detected in afferent intestinal lymph from the R and S lines (Table 6 and data not shown).
DISCUSSION
Cannulation models in sheep have been successfully employed to study a range of different aspects in immunity like adjuvant properties (34, 45), dendritic cell physiology (40), and dendritic cell function (46, 54), as well as immunological responses to various diseases (9, 13, 44). Our results demonstrate that cannulation of pseudo-afferent and -efferent lymphatic vessels draining from the jejunum can be used to investigate in vivo intestinal nematode immune responses over extended periods of time. One of the key findings of this study was that a range of cytokine genes was differentially expressed in AILC and EILC during experimental T. colubriformis infection of nematode-naïve and -primed R- and S-line sheep.
Breeding of sheep for resistance and susceptibility to natural nematode infection, in which T. colubriformis forms a major part of the challenge, resulted in two sheep lines that differ largely in their ability to reject nematode infection (6, 7), without compromising the overall immune responsiveness (42). For these two sheep lines, the responsiveness of lymphocytes obtained from mesenteric lymph nodes to larval or adult T. colubriformis-specific antigens was demonstrated previously (10). In this study, cells obtained from AIL and EIL were responsive to antigenic and mitogenic stimulation. The levels of response of AILC and EILC were similar in R- and S-line sheep and did not change significantly during nematode challenge, although for primed animals, the SI tended to be higher at week 3 than prior to challenge. However, these results are compromised by the small group size and large animal-to-animal variation and need to be validated in a larger group size, preferably with fractionated soluble larval antigens. In random-bred sheep the responsiveness of lymphocytes obtained from blood to larval or adult T. colubriformis antigen is documented, although no correlation between immunity and lymphoproliferative response was found (11). In contrast, differences in the proliferative responses to soluble Haemonchus contortus larval or adult antigen between sheep that are immune to reinfection and naïve lambs are well established (47, 51).
R-line sheep had slightly elevated gene expression levels of Th2-type cytokines in AILC and EILC prior to challenge in comparison to levels in S-line sheep, but these differences did not achieve statistical significance. This observation indicates that the breeding procedure had no major influence on the background cytokine gene expression. However, during experimental nematode infection, when afferent and efferent lymph cells were directly subjected to gene expression studies without any in vitro stimulation, a range of differentially expressed cytokine genes was detected that could be related to either the nematode resistance and susceptibility genotype, to the prechallenge status, or to distinct lymphatic compartments.
The critical role of IL-13 in mediating immunity to nematode infection was recently identified in mice (3, 4, 23, 35, 52, 56) where IL-13 mediates smooth muscle hypercontraction (1), goblet cell hyperplasia (27), and altered intestinal epithelial cell function (32) through activation of STAT-6. Although the immunological mechanisms that underlie nematode rejection in sheep are not well understood, our study clearly shows that the IL-13 gene is consistently expressed at a higher level in cells recirculating in afferent and efferent lymph of R-line sheep compared to levels in cells of S-line sheep during experimental infection with T. colubriformis. However, the initial IL-13-associated immune response in Rn sheep was not consistently fully protective but did result in lower FEC in comparison to counts in the Sn sheep. IL-13 gene expression in Rp sheep was consistently highly up-regulated and the associated immune response was protective; neither of these features was consistently seen in the Sp sheep. Single experimental T. colubriformis infection of Sp sheep did not result in a significant up-regulation of the IL-13 gene. It remains to be determined whether S-line sheep are able to mount an IL-13-dependent immune response when challenged repeatedly.
IL-5 mediates nematode immunity via induction of eosinophilia, mastocytosis, and immunoglobulin E (IgE) production, which is well established in both sheep (5, 22) and rodents (57). In a parallel study of the same animals used in the present experiment, we found that R-line sheep had higher concentrations than S-line sheep of total IgE in lymph, and primed animals had higher total IgE levels than nematode-naïve animals (41). In the present study, the IL-5 gene was found to be expressed in particular in AILC and EILC of Rp sheep, with a strong trend for up-regulation in AILC during challenge. In contrast, even primed S-line sheep failed to develop an IL-5 response in cells recirculating in afferent lymph. This result could be taken as evidence for a persistent local suppression of, or nonresponsiveness to, IL-5 gene expression in S-line animals, but this requires further study.
Recently, it became clear that TNF-α is involved in the intestinal pathology of Trichinella spiralis infection of mice (29), and it might also have an effector function responsible for the expulsion of Trichuris muris (23). In our study, TNF-α mRNA was highly expressed in both AILC and EILC but overall was higher in R- than in S-line sheep, which might account for the previously observed more acute inflammatory response of R-line sheep to natural nematode challenge (43). In addition, the overall slopes of the TNF-α gene expression differed significantly between the R and S animals during nematode challenge. In R-line sheep TNF-α gene expression tended to remain steady or even increased in EILC of naïve sheep but tended to decline in all the S-groups. This indicates that in S-line sheep the TNF-α-associated pathology is somehow controlled during nematode challenge. Whether this decline in TNF-α gene expression is actively mediated by nematode antigens so as to prevent damage through TNF-α-associated pathology or whether this occurs through another mechanism remains unclear and requires further study.
Differences observed in the cytokine gene expression profiles between AILC and EILC may occur as a consequence of different functional properties and/or different cell compositions. AILC consistently expressed higher levels of the IL-10 and IL-4 genes during nematode challenge than did EILC, which was not related to the genotype of the animal. Because mononuclear phagocytic cells and other APCs are an important source of IL-10 (28), the higher IL-10 gene expression seen in AILC may be due to the contribution made by these types of APCs. Further clarification will require detailed gene expression studies on a cellular level. Interestingly, nematode challenge, regardless of the preinfection status of both line animals, resulted in a significant decline in IL-10 gene expression in AILC. This is of interest as it became clear from knock-out studies in mice that IL-10 is critical for the polarization of a Th2 immune response and for development of resistance to Trichuris muris infection (49). It remains to be determined how nematode infection maintains a down-regulation of IL-10 gene expression and therefore leads to an immunological environment that favors nematode survival.
CD4+ T cells formed the major cell subset in both lymphatic compartments, and their frequency tended to be constant in afferent lymph but decreased significantly in efferent lymph during experimental nematode infection. Whether these changes in the frequency of CD4+ T cells accounted for differences in IL-4 gene expression between AILC and EILC or whether there is a localized nematode infection-dependent mechanism for selective IL-4 induction in afferent lymph remains to be elucidated. It also remains to be established whether the cellular changes seen in efferent lymph during nematode infection account for or contribute to any of the observed changes in gene expression levels.
Different parasite antigen-specific Th cell clones, which constituted a spectrum of cell phenotypes, are also seen in cattle (2, 8). Primary Ostertagia ostertagii infection in cattle resulted in elevated expression of the IL-4 and IFN-γ genes in lamina propria lymphocytes (2) as well as in abomasal lymph node cells (12) of cattle. Our data on gene expression profiles, taken together, demonstrate that R-line sheep are able to respond to experimental nematode infection in the presence of IFN-γ, with an early up-regulation of Th2-type and proinflammatory cytokines, which far exceeded expression levels observed in the S-line sheep. Our data also provide evidence that in vivo, the polarization toward a Th2-type response occurs at the site of the infection. The possible presence of different subtypes of Th cell clones in AIL and EIL is suggested by the different cytokine gene expression levels observed in the two lymph compartments.
Acknowledgments
This work was supported by the Foundation for Research, Science and Technology.
We thank Roger Littlejohn for advice about the statistical analyses used and Bryce Buddle for critical reading of the manuscript.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Akiho, H., P. Blennerhassett, Y. Deng, and S. M. Collins. 2002. Role of IL-4, IL-13, and STAT6 in inflammation-induced hypercontractility of murine smooth muscle cells. Am. J. Physiol. Gastrointest. Liver Physiol. 282:G226-G232. [DOI] [PubMed] [Google Scholar]
- 2.Almeria, S., A. Canals, D. S. Zarlenga, and L. C. Gasbarre. 1997. Quantification of cytokine gene expression in lamina propria lymphocytes of cattle following infection with Ostertagia ostertagi. J. Parasitol. 83:1051-1055. [PubMed] [Google Scholar]
- 3.Bancroft, A. J., D. Artis, D. D. Donaldson, J. P. Sypek, and R. K. Grencis. 2000. Gastrointestinal nematode expulsion in IL-4 knockout mice is IL-13 dependent. Eur. J. Immunol. 30:2083-2091. [DOI] [PubMed] [Google Scholar]
- 4.Bancroft, A. J., A. N. McKenzie, and R. K. Grencis. 1998. A critical role for IL-13 in resistance to intestinal nematode infection. J. Immunol. 160:3453-3461. [PubMed] [Google Scholar]
- 5.Bao, S., S. J. McClure, D. L. Emery, and A. J. Husband. 1996. Interleukin-5 mRNA expressed by eosinophils and gamma/delta T cells in parasite-immune sheep. Eur. J. Immunol. 26:552-556. [DOI] [PubMed] [Google Scholar]
- 6.Bisset, S. A., C. A. Morris, J. C. McEwan, and A. Vlassoff. 2001. Breeding sheep in New Zealand that are less reliant on anthelmintics to maintain health and productivity. N. Z. Vet. J. 49:236-246. [DOI] [PubMed] [Google Scholar]
- 7.Bisset, S. A., A. Vlassoff, P. G. C. Douch, W. E. Jonas, C. J. West, and R. S. Green. 1996. Nematode burdens and immunological responses following natural challenge in Romney lambs selectively bred for low or high faecal worm egg count. Vet. Parasitol. 61:249-263. [DOI] [PubMed] [Google Scholar]
- 8.Brown, W. C., A. C. Rice-Ficht, and D. M. Estes. 1998. Bovine type 1 and type 2 responses. Vet. Immunol. Immunopathol. 63:45-55. [DOI] [PubMed] [Google Scholar]
- 9.Buxton, D., K. M. Thomson, S. Maley, J. M. Wastling, E. A. Innes, W. R. Panton, and S. Nicoll. 1994. Primary and secondary responses of the ovine lymph node to Toxoplasma gondii: cell output in efferent lymph and parasite detection. J. Comp. Pathol. 111:231-241. [DOI] [PubMed] [Google Scholar]
- 10.Cabaj, W., M. Stankiewicz, S. A. Bisset, A. Pernthaner, C. J. West, and E. Hadas. 1996. Immunisation of nematode resistant or susceptible Romney lambs with Oxfendazole abbreviated infections against pasture challenge. Acta Parasitol. 41:159-165. [Google Scholar]
- 11.Cabaj, W., M. Stankiewicz, W. E. Jonas, A. Pernthaner, and S. Lawrence. 1996. The effect of immunization of sheep with drug-abbreviated infections of Trichostrongylus colubriformis on in vitro lymphocyte blastogenic responses. Acta Parasitol. 41:97-102. [Google Scholar]
- 12.Canals, A., D. S. Zarlenga, S. Almeria, and L. C. Gasbarre. 1997. Cytokine profile induced by a primary infection with Ostertagia ostertagi in cattle. Vet. Immunol. Immunopathol. 58:63-75. [DOI] [PubMed] [Google Scholar]
- 13.Dandie, G. W., S. J. Ragg, and H. K. Muller. 1992. Migration of Langerhans cells from carcinogen-treated sheep skin. J. Investig. Dermatol. 99:S51-S53. [DOI] [PubMed] [Google Scholar]
- 14.Dehlawi, M. S., and P. K. Goyal. 2003. Responses of inbred mouse strains to infection with intestinal nematodes. J. Helminthol. 77:119-124. [DOI] [PubMed] [Google Scholar]
- 15.Douch, P. G. C., R. S. Green, and P. L. Risdon. 1994. Antibody responses of sheep to challenge with Trichostrongylus colubriformis and the effect of dexamethasone treatment. Int. J. Parasitol. 24:921-928. [DOI] [PubMed] [Google Scholar]
- 16.Else, K. J., G. M. Entwistle, and R. K. Grencis. 1993. Correlations between worm burden and markers of Th1 and Th2 cell subset induction in an inbred strain of mouse infected with Trichuris muris. Parasite Immunol. 15:595-600. [DOI] [PubMed] [Google Scholar]
- 17.Else, K. J., and F. D. Finkelman. 1998. Intestinal nematode parasites, cytokines and effector mechanisms. Int. J. Parasitol. 28:1145-1158. [DOI] [PubMed] [Google Scholar]
- 18.Emery, D. L., S. J. McClure, B. M. Wagland, and W. O. Jones. 1992. Studies of stage-specific immunity against Trichostrongylus colubriformis in sheep: immunization by normal and truncated infections. Int. J. Parasitol. 22:215-220. [DOI] [PubMed] [Google Scholar]
- 19.Garside, P., M. W. Kennedy, D. Wakelin, and C. E. Lawrence. 2000. Immunopathology of intestinal helminth infection. Parasite Immunol. 22:605-612. [DOI] [PubMed] [Google Scholar]
- 20.Gasbarre, L. C. 1997. Effects of gastrointestinal nematode infection on the ruminant immune system. Vet. Parasitol. 72:327-337. [DOI] [PubMed] [Google Scholar]
- 21.Gasbarre, L. C., E. A. Leighton, and T. Sonstegard. 2001. Role of the bovine immune system and genome in resistance to gastrointestinal nematodes. Vet. Parasitol. 98:51-64. [DOI] [PubMed] [Google Scholar]
- 22.Gill, H. S., K. Altmann, M. L. Cross, and A. J. Husband. 2000. Induction of T helper 1- and T helper 2-type immune responses during Haemonchus contortus infection in sheep. Immunology 99:458-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grencis, R. K. 2001. Cytokine regulation of resistance and susceptibility to intestinal nematode infection—from host to parasite. Vet. Parasitol. 100:45-50. [DOI] [PubMed] [Google Scholar]
- 24.Grencis, R. K. 1997. Th2-mediated host protective immunity to intestinal nematode infections. Philos. Trans. R. Soc. Lond. B 352:1377-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hein, W. R., T. Barber, S. Cole, L. Morrison, and A. Pernthaner. Long-term collection and characterization of afferent lymph from the ovine small intestine. J. Immunol. Methods 293:153-168. [DOI] [PubMed]
- 26.Ishikawa, N., P. K. Goyal, Y. R. Mahida, K. F. Li, and D. Wakelin. 1998. Early cytokine responses during intestinal parasitic infections. Immunology 93:257-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan, W. I., P. Blennerhasset, C. Ma, K. I. Matthaei, and S. M. Collins. 2001. Stat6 dependent goblet cell hyperplasia during intestinal nematode infection. Parasite Immunol. 23:39-42. [DOI] [PubMed] [Google Scholar]
- 28.Lalani, I., K. Bhol, and A. R. Ahmed. 1997. Interleukin-10: biology, role in inflammation and autoimmunity. Ann. Allergy Asthma Immunol. 79:469-483. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence, C. E., J. C. Paterson, L. M. Higgins, T. T. MacDonald, M. W. Kennedy, and P. Garside. 1998. IL-4-regulated enteropathy in an intestinal nematode infection. Eur. J. Immunol. 28:2672-2684. [DOI] [PubMed] [Google Scholar]
- 30.MacHugh, N. D., A. Bensaid, W. C. Davis, C. J. Howard, K. R. Parsons, B. Jones, and A. Kaushal. 1988. Characterization of a bovine thymic differentiation antigen analogous to CD1 in the human. Scand. J. Immunol. 27:541-547. [DOI] [PubMed] [Google Scholar]
- 31.Mackay, C. R., J. F. Maddox, K. J. Gogolin-Ewens, and M. R. Brandon. 1985. Characterization of two sheep lymphocyte differentiation antigens, SBU-T1 and SBU-T6. Immunology 55:729-737. [PMC free article] [PubMed] [Google Scholar]
- 32.Madden, K. B., L. Whitman, C. Sullivan, W. C. Gause, J. F. Urban, Jr., I. M. Katona, F. D. Finkelman, and T. Shea-Donohue. 2002. Role of STAT6 and mast cells in IL-4- and IL-13-induced alterations in murine intestinal epithelial cell function. J. Immunol. 169:4417-4422. [DOI] [PubMed] [Google Scholar]
- 33.Matsuda, S., R. Uchikawa, M. Yamada, and N. Arizono. 1995. Cytokine mRNA expression profiles in rats infected with the intestinal nematode Nippostrongylus brasiliensis. Infect. Immun. 63:4653-4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McClure, S. J., B. M. Wagland, and D. L. Emery. 1991. Effects of Freund's adjuvants on local, draining and circulating lymphocyte populations in sheep. Immunol. Cell Biol. 69:361-367. [DOI] [PubMed] [Google Scholar]
- 35.McKenzie, G. J., A. Bancroft, R. K. Grencis, and A. N. McKenzie. 1998. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Curr. Biol. 8:339-342. [DOI] [PubMed] [Google Scholar]
- 36.Morris, C. A., A. Vlassoff, S. A. Bisset, R. L. Baker, T. G. Watson, C. J. West, and M. Wheeler. 2000. Continued selection of Romney sheep for resistance or susceptibility to nematode infection: estimates of direct and correlated responses. Animal Sci. 70:17-27. [Google Scholar]
- 37.Patterson, H. D., and R. Thompson. 1971. Recovery of inter-block information when block sizes are unequal. Biometrika 58:545-554. [Google Scholar]
- 38.Pepin, M., D. Cannella, J. J. Fontaine, J. C. Pittet, and A. Le Pape. 1992. Ovine mononuclear phagocytes in situ: identification by monoclonal antibodies and involvement in experimental pyogranulomas. J. Leukoc. Biol. 51:188-198. [DOI] [PubMed] [Google Scholar]
- 39.Pernthaner, A., W. Cabaj, M. Stankiewicz, J. Davies, and D. Maass. 1997. Cytokine mRNA expression and IFN-gamma production of immunised nematode resistant and susceptible lambs against natural poly-generic challenge. Acta Parasitol. 42:180-186. [Google Scholar]
- 40.Pernthaner, A., S. A. Cole, T. Gatehouse, and W. R. Hein. 2002. Phenotypic diversity of antigen-presenting cells in ovine-afferent intestinal lymph. Arch. Med. Res. 33:405-412. [DOI] [PubMed] [Google Scholar]
- 41.Pernthaner, A., R. J. Shaw, M. M. McNeill, L. Morrison, and W. R. Hein. 2005. Total and nematode-specific IgE responses in intestinal lymph of genetically resistant and susceptible sheep during infection with Trichostrongylus colubriformis. Vet. Immunol. Immunopathol. 104:69-80. [DOI] [PubMed] [Google Scholar]
- 42.Pernthaner, A., M. Stankiewicz, W. Cabaj, and W. Jonas. 1995. Immune responsiveness of Romney sheep selected for resistance or susceptibility to gastrointestinal nematodes: field studies. Vet. Immunol. Immunopathol. 48:97-103. [DOI] [PubMed] [Google Scholar]
- 43.Pernthaner, A., A. Vlassoff, P. G. C. Douch, and D. R. Maass. 1997. Cytokine mRNA expression and IFN-γ production in nematode resistant and susceptible line lambs artificially infected with gastro-intestinal nematodes. Acta Parasitol. 42:55-61. [Google Scholar]
- 44.Ragg, S. J., G. W. Dandie, G. M. Woods, and H. K. Muller. 1995. Abrogation of afferent lymph dendritic cell function after cutaneously applied chemical carcinogens. Cell Immunol. 162:80-88. [DOI] [PubMed] [Google Scholar]
- 45.Rothel, J. S., L. A. Corner, M. W. Lightowlers, H. F. Seow, P. McWaters, G. Entrican, and P. R. Wood. 1998. Antibody and cytokine responses in efferent lymph following vaccination with different adjuvants. Vet. Immunol. Immunopathol. 63:167-183. [DOI] [PubMed] [Google Scholar]
- 46.Ryan, S., L. Tiley, I. McConnell, and B. Blacklaws. 2000. Infection of dendritic cells by the Maedi-visna lentivirus. J. Virol. 74:10096-10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schallig, H., M. A. van Leeuwen, and W. M. L. Hendrikx. 1994. Immune responses of Texel sheep to excretory/secretory products of adult Haemonchus contortus. Parasitology 108:351-357. [DOI] [PubMed] [Google Scholar]
- 48.Schallig, H. D. 2000. Immunological responses of sheep to Haemonchus contortus. Parasitology 120(Suppl.):S63-S72. [DOI] [PubMed] [Google Scholar]
- 49.Schopf, L. R., K. F. Hoffmann, A. W. Cheever, J. F. Urban, Jr., and T. A. Wynn. 2002. IL-10 is critical for host resistance and survival during gastrointestinal helminth infection. J. Immunol. 168:2383-2392. [DOI] [PubMed] [Google Scholar]
- 50.Stankiewicz, M., W. Cabaj, A. Pernthaner, W. Jonas, and B. Rabel. 1996. Drug-abbreviated infections and development of immunity against Trichostrongylus colubriformis in sheep. Int. J. Parasitol. 26:97-103. [DOI] [PubMed] [Google Scholar]
- 51.Torgerson, P. R., and S. Lloyd. 1993. The B cell dependence of Haemonchus contortus antigen-induced lymphocyte proliferation. Int. J. Parasitol. 23:925-930. [DOI] [PubMed] [Google Scholar]
- 52.Urban, J., H. Fang, Q. Liu, M. J. Ekkens, S. J. Chen, D. Nguyen, V. Mitro, D. D. Donaldson, C. Byrd, R. Peach, S. C. Morris, F. D. Finkelman, L. Schopf, and W. C. Gause. 2000. IL-13-mediated worm expulsion is B7 independent and IFN-gamma sensitive. J. Immunol. 164:4250-4256. [DOI] [PubMed] [Google Scholar]
- 53.Urban, J. F., Jr., R. Fayer, C. Sullivan, J. Goldhill, T. Shea-Donohue, K. Madden, S. C. Morris, I. Katona, W. Gause, M. Ruff, L. S. Mansfield, and F. D. Finkelman. 1996. Local TH1 and TH2 responses to parasitic infection in the intestine: regulation by IFN-gamma and IL-4. Vet. Immunol. Immunopathol. 54:337-344. [DOI] [PubMed] [Google Scholar]
- 54.Watkins, C., S. Lau, R. Thistlethwaite, J. Hopkins, and G. D. Harkiss. 1999. Analysis of reporter gene expression in ovine dermis and afferent lymph dendritic cells in vitro and in vivo. Vet. Immunol. Immunopathol. 72:125-133. [DOI] [PubMed] [Google Scholar]
- 55.Whitlock, H. V. 1948. Some modifications of the McMaster helminth egg-counting techniques and apparatus. J. Counc. Sci. Ind. Res. 21:177-180. [Google Scholar]
- 56.Wynn, T. A. 2003. IL-13 effector functions. Annu. Rev. Immunol. 21:425-456. [DOI] [PubMed] [Google Scholar]
- 57.Zhou, Y., S. Bao, T. L. Rothwell, and A. J. Husband. 1996. Differential expression of interleukin-5 mRNA+ cells and eosinophils in Nippostrongylus brasiliensis infection in resistant and susceptible strains of mice. Eur. J. Immunol. 26:2133-2139. [DOI] [PubMed] [Google Scholar]