Abstract
The binding of bacteria to platelets is a postulated central event in the pathogenesis of infective endocarditis. Platelet binding by Streptococcus gordonii is mediated in large part by GspB, a high-molecular-mass cell wall glycoprotein. Although Staphylococcus aureus has a GspB homolog (SraP), little is known about its function. SraP has a calculated molecular mass of 227 kDa and, like GspB, is predicted to contain an atypical N-terminal signal sequence, two serine-rich repeat regions (srr1 and srr2) separated by a nonrepeat region, and a C-terminal cell wall anchoring motif (LPDTG). To assess whether SraP contributes to platelet binding, we compared the binding to human platelets of S. aureus strain ISP479C and of an isogenic variant (strain PS767) in which sraP had been disrupted by allelic replacement. Platelet binding in vitro by PS767 was 47% ± 17% (mean ± standard deviation) lower than that of ISP479C (P < 0.001). In addition, a recombinant fragment of SraP containing srr1 and the nonrepeat region was found to bind platelets directly. Binding was saturable, suggesting a receptor-ligand interaction. When tested in a rabbit model of endocarditis, in which each animal was simultaneously infected with ISP479C and PS767 at a ratio of approximately 1:1, the titers of the mutant strain within vegetations were significantly lower than those of the parent strain at 1 and 24 h postinfection. These results indicate that SraP can mediate the direct binding of S. aureus to platelets and that the platelet-binding domain of this glycoprotein is located within its N-terminal region. Moreover, the expression of SraP appears to be a virulence determinant in endovascular infection.
The binding of bacteria to platelets is a postulated central event in the pathogenesis of infective endocarditis (18). Initial colonization of damaged cardiac valves may be mediated by the attachment of blood-borne bacteria to platelets bound at the site of endovascular injury (6, 19, 22). Platelets may be recruited subsequently to the infected endocardium through their direct adhesion to immobilized bacteria (4). These events, in combination with bacterial proliferation, are thought to produce the hallmark lesion of infective endocarditis, the macroscopic vegetation.
Staphylococcus aureus can bind to human platelets through a number of adhesins. Previous studies showed that protein A, clumping factor A, clumping factor B, and fibronectin-binding protein (FnbA) all bind to proteins on the platelet surface (9, 12, 13, 16). In addition to recognizing major receptors (i.e., FnbA interacting with fibronectin), many of these adhesins are bifunctional. For example, clumping factor A is able to interact with a 118-kDa platelet membrane protein in addition to its major ligand, fibrinogen (16). Protein A binds to both immunoglobulin G and the gC1qR/p33 protein on platelet membranes (12). Thus, S. aureus has a number of multifunctional proteins that allow it to interact with human platelets through multiple redundant mechanisms.
Bensing and Sullam recently identified GspB, a large surface glycoprotein that mediates the binding of Streptococcus gordonii to human platelets (2). This adhesin is encoded within an operon that also encodes proteins required for the glycosylation and secretion of GspB (21). Of note, the gspB-sec operon appears to be conserved among a number of gram-positive bacteria. The staphylococcal homolog of GspB, which we have designated SraP (serine-rich adhesin for platelets), has several features in common with this protein. Like GspB, SraP is predicted to have a relatively large mass (227 kDa) and a similar overall organization of its domains. The N terminus contains an atypical putative signal peptide, followed by a short serine-rich region (srr1), a nonrepeat region (nrr), a second serine-rich region (srr2), and a cell wall anchoring motif (LPDTG) (Fig. 1A). As was found for the gspB-sec operon, the S. aureus genes immediately downstream of sraP appear to encode accessory secretion proteins and glycosyltransferases (Fig. 1B).
FIG. 1.
(A) Schematic representation of GspB and SraP. The putative functional domains are delineated within the individual proteins. sp, signal peptide; cw, cell wall anchoring domain. The region of the protein expressed in recombinant form is indicated below the full-length protein. a.a., amino acids. (B) Schematic representation of the gspB and sraP operons. The positions of the primers used in this study are shown: 1, Sra28Bup; 2, Sra28down; 3, SraLU; 4, SraLD; 5, SraRU; 6, SraRD; 7, SASECY2U; 8, SASECY2D; and 9, SASECA2D. See Table 1 for details.
Aside from these structural features, little is known about SraP. The gene encoding SraP is present in the genomes of all six S. aureus strains sequenced to date, indicating that it is highly conserved. Although SraP has been identified from database searches as a possible cell wall protein (SasA) (15), SraP remains otherwise uncharacterized in terms of function. While it has structural similarities to GspB, suggesting that it may have similar biologic properties, SraP does differ from GspB in several important ways. In particular, the nonrepeat domains of GspB and SraP share little homology (27% identity). Moreover, the SraP operon lacks two genes (gly and nss) that affect the carbohydrate composition of GspB. Differences in glycosylation could affect the function of SraP, as seen with other srr proteins (21). In view of these differences, it is not clear whether SraP and GspB have similar binding specificities.
To address these issues, we examined the role of SraP in mediating the binding of S. aureus to human platelets. Our results indicate that SraP binds to platelets through a mechanism that resembles a receptor-ligand interaction. Moreover, SraP appears to contribute to the virulence of S. aureus, as measured in an animal model of infective endocarditis.
MATERIALS AND METHODS
Bacterial strains, plasmids, and primers.
The bacterial strains, plasmids, and primers used in this study are shown in Table 1. S. aureus ISP479C is a commonly used laboratory strain. PS767 is an isogenic variant of ISP479C in which sraP has been disrupted by insertion of ermB. The 21 S. aureus clinical isolates were from patients with endocarditis and various other staphylococcal infections at San Francisco General Hospital. Primers were designed on the basis of the sequence of the sraP-secA2 locus from S. aureus Col. The sequence of sraP in Col is identical to that of this gene in S. aureus N315 (GenBank accession no. NC_002745-ORF2447).
TABLE 1.
Strains, plasmids, and primers used in this study
| Bacterial strain, plasmid, or primer | Genotype or descriptiona | Reference or sourceb |
|---|---|---|
| Strains | ||
| S. aureus | ||
| ISP479C | 8325-4 pig-131 | 14 |
| RN4220 | Restriction-deficient strain that can be transformed with DNA from E. coli | 11 |
| PS735 | Endocarditis-associated clinical isolate | SFGH clinical stock |
| PS736 | Endocarditis-associated clinical isolate | SFGH clinical stock |
| PS737 | Endocarditis-associated clinical isolate | SFGH clinical stock |
| PS738 | Endocarditis-associated clinical isolate | SFGH clinical stock |
| PS739 | Endocarditis-associated clinical isolate | SFGH clinical stock |
| PS740 | Endocarditis-associated clinical isolate | SFGH clinical stock |
| PS741 | Endocarditis-associated clinical isolate | SFGH clinical stock |
| PS742 | Endocarditis-associated clinical isolate | SFGH clinical stock |
| PS743 | Endocarditis-associated clinical isolate | SFGH clinical stock |
| PS745 | Endocarditis-associated clinical isolate | SFGH clinical stock |
| PS746 | Endocarditis-associated clinical isolate | SFGH clinical stock |
| PS747 | Endocarditis-associated clinical isolate | SFGH clinical stock |
| PS748 | Endocarditis-associated clinical isolate | SFGH clinical stock |
| PS749 | Endocarditis-associated clinical isolate | SFGH clinical stock |
| PS750 | Non-endocarditis-associated clinical isolate | SFGH clinical stock |
| PS751 | Non-endocarditis-associated clinical isolate | SFGH clinical stock |
| PS752 | Non-endocarditis-associated clinical isolate | SFGH clinical stock |
| PS753 | Non-endocarditis-associated clinical isolate | SFGH clinical stock |
| PS754 | Non-endocarditis-associated clinical isolate | SFGH clinical stock |
| PS755 | Non-endocarditis-associated clinical isolate | SFGH clinical stock |
| PS756 | Non-endocarditis-associated clinical isolate | SFGH clinical stock |
| PS767 | ISP479C sraP::ermB | This work |
| PS891 | PS750 sraP::ermB | This work |
| E. coli | ||
| DH5α | F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 endA1 recA1 hsdR17 (rK− mK+) deoR thi-1 supE44 λ−gyr A96 relA1 | 7 |
| BL21 | F−hsdSB (rB− mB−) gal dcm | 17 |
| BL21(DE3) | F−hsdSB (rB− mB−) gal dcm (DE3) | 17 |
| Plasmids | ||
| pET28A | T7 expression vector for His6 fusion | Novagen |
| pAP1-2EX | E. coli-S. aureus temperature-sensitive shuttle vector | Jean C. Lee |
| pRS405 | pET28A containing the sraP sequence encoding amino acids 90-723 as an in-frame fusion with the His6 and T7 tags | This work |
| pRS407 | pAP1-2EX containing the sraP coding sequence flanking the ermB gene | This work |
| Primers | ||
| Sra28Bup | 5′-CCGGATCCGCTTCTGATGCACCATTA-3′ | Primer for construction of His6-SraP90-723 |
| Sra28down | 5′-CACAAGCTTTTAAATGTTTGTTGGTGTACC-3′ | Primer for construction of His6-SraP90-723 |
| SraLU | 5′-ACCGAATTCCTTTCGATGCACCATTA-3′ | sraP-specific primer for left flank of construct pRS407 |
| SraLD | 5′-AAAGGATCCAAAGGCAAAACCGATACC-3′ | sraP-specific primer for left flank of construct pRS407 |
| SraRU | 5′-TGGTCTAGATTAAGTAACGCATTTGGC-3′ | sraP-specific primer for right flank of construct pRS407 |
| SraRD | 5′-CTCTCTAGATGTATTTGTCACAGTCCC-3′ | sraP-specific primer for right flank of construct pRS407 |
| SASECY2U | 5′-GATATAAAAGAGGTGCAAC-3′ | Upstream secY2-specific primer |
| SASECY2D | 5′-CCTCCTTACCAATACTGG-3′ | Downstream secY2-specific primer |
| SASECA2D | 5′-AAATAGTCGACTTGTTCTAACC-3′ | secA2-specific primer for production of cDNA |
Underlining indicates restriction sites.
SFGH, San Francisco General Hospital.
Media and antibiotics.
Tryptic soy (TS) broth and agar or blood agar plates were used as culture media for S. aureus. Escherichia coli was cultured with Luria-Bertani (LB) broth and agar. Kanamycin and carbenicillin were used at a concentration of 50 μg/ml where appropriate. Erythromycin was used at a concentration of 15 μg/ml for selection of S. aureus and at 400 μg/ml for E. coli.
Mutagenesis of sraP by allelic replacement.
A recombinant plasmid was constructed to insert the ermB gene into the sraP coding sequence. Two specific portions of sraP were amplified from the 5′ end region of the gene by PCR. The PCR primer pairs used for amplification (Table 1 and Fig. 1) were designed to encode unique restriction sites at the ends of the amplification products. The products were digested with the appropriate enzymes and purified after agarose gel electrophoresis.
The gene replacement vector was constructed in a two-step process. The temperature-sensitive E. coli-staphylococcal shuttle vector pAP1-2EX (kindly provided by Jean C. Lee) was cut upstream of the ermB gene with EcoRI and BamHI, gel purified, and ligated to the left-end sraP fragment. The ligation product was electroporated into E. coli DH5α, and transformants were selected on LB agar supplemented with erythromycin. The vector containing the left-end flank was recovered from a single transformant, cut downstream of ermB with XbaI, and gel purified. The vector then was ligated to the right-end sraP fragment and isolated after selection as described above. To select constructs containing the right-end flank in the correct orientation, mapping was done by restriction digestion. The resultant temperature-sensitive vector, designated pRS407, was used to transform S. aureus RN4220 by electroporation. The mixture was plated on TS agar supplemented with 15 μg of erythromycin/ml and grown at 30°C to select for transformants. pRS407 then was transferred to ISP479C and PS750 (a clinical isolate) by transduction with phage 80α.
To disrupt the sraP gene by allelic replacement, overnight cultures of ISP479C or PS750 containing pRS407 were grown at 30°C, diluted 1:100 in TS broth supplemented with 15 μg of erythromycin/ml, grown at 42°C for 8 h, and then plated on TS agar-erythromycin. The plates were incubated at 37°C to select for the growth of organisms in which the ermB gene had been inserted into the sraP coding sequence by homologous recombination. Recombinant clones were picked, and the insertion of the ermB gene into the correct chromosomal site was confirmed by Southern blotting. The stability of the mutation was assessed by repeated subculturing of the mutant in nonselective media followed by growth under selective conditions.
Assay for staphylococcal binding to platelet monolayers.
Staphylococcal binding to human platelet monolayers was assayed as described previously (16). In brief, 18-h cultures of S. aureus were washed twice with Dulbecco's phosphate-buffered saline (PBS), sonicated for 15 s to disperse clumps, and adjusted to a final A600 of 0.125. Fifty-microliter samples of each bacterial suspension were added to 6-mm-diameter microtiter wells that had been previously coated with fixed human platelets and a casein-based solution (blocking solution; Roche Diagnostics) to reduce nonspecific binding. The plates were incubated on a rotary shaker (45 rpm) at room temperature for 1 h. The wells were washed three times to remove any nonadherent bacteria and then were treated with trypsin (1 mg/ml) for 30 min at room temperature to release the attached organisms. The number of bound bacteria was determined by plating serial dilutions of the recovered organisms on blood agar plates, and binding of the strains was calculated as a percentage of the inoculum. Values for binding by the wild-type strain were normalized to 100%; binding by the mutant strain was reported relative to binding by the wild-type strain. Differences in platelet binding were compared by using the Student t test. P values of ≤0.05 were considered significant.
RT-PCR.
Overnight cultures of strains ISP479C and PS767 were diluted 1:25 and grown to the mid-log phase. Bacteria were lysed in 100 μl of a solution containing 10 mM Tris-HCl and 1 mM EDTA (pH 8.0) and supplemented with lysozyme (3 mg/ml; Sigma), lysostaphin (50 μg/ml; Sigma), and SuperRNasin (20 U; Ambion), followed by treatment with 10 U of RNase-free DNase at 37°C for 15 min. RNA was isolated from the reaction mixtures by using an RNeasy minikit (Qiagen). To prepare cDNA, 5-μg samples of RNA were mixed with 500 nM SASECA2D, a primer specific for the secA2 gene (Fig. 1B), heated at 70°C for 10 min, and then cooled to room temperature. The template-primer mixtures were combined with 4 μl of 5× reaction buffer (Invitrogen), 2 μl of 0.1 M dithiothreitol, 1 μl of 10 mM deoxynucleoside triphosphate (Roche), and 200 U of SuperscriptII reverse transcriptase (RT; Invitrogen) in a final reaction volume of 20 μl. The mixtures were incubated at 42°C for 1 h, and the reactions were stopped by incubation at 70°C for 10 min. As a control for DNA contamination, the reactions were also performed in the absence of RT. The cDNA products were used as templates in PCRs specific for secY2. Five microliters of the products of the RT reactions were combined with a 300 nM concentration each of primers SASECY2U and SASECY2D (Fig. 1B) and amplified by using the Expand long-template PCR reagent (Roche) according to the manufacturer's instructions. PCR products were separated by electrophoresis on 0.75% agarose gels and visualized by UV illumination after ethidium bromide staining.
Expression and purification of recombinant SraP90-723.
A fragment of the sraP gene encoding the region of the protein immediately downstream of the putative signal peptide cleavage site to just upstream of srr2 (SraP residues 90 to 723 [SraP90-723]) (Fig. 1A) was amplified by PCR and cloned into pET28A. The resultant vector, which encodes a His6-tagged portion of the N terminus of SraP, was used to transform E. coli strain BL21(DE3) by electroporation. The resulting transformants were recovered by plating on LB agar supplemented with kanamycin. Recombinant proteins were expressed and purified by affinity chromatography by using a His-Bind purification kit (Novagen) according to the manufacturer's instructions. Purified proteins were dialyzed against PBS and stored at −70°C.
Production of anti-SraP serum.
Rabbit anti-SraP serum was produced by Covance Research Products Inc. with purified His6-SraP90-723 as an immunogen.
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), Western blotting, and glycan detection.
Proteins were prepared for electrophoresis and separated on 4 to 12% gradient polyacrylamide gels by using a NOVEX Tris-acetate gel system (Invitrogen). Proteins were transferred electrophoretically to BioTrace NT membranes (Pall Corporation) in Towbin buffer. Membranes were treated with blocking solution and incubated overnight with polyclonal rabbit anti-His6-SraP90-723 serum (1:1,000 in blocking solution). SraP was detected by chemiluminescence with goat ant-rabbit immunoglobulin G coupled to horseradish peroxidase and SuperSignal PicoWest (Pierce).
To assess the prevalence of SraP among staphylococcal clinical isolates, 14 endocarditis-associated and 7 non-endocarditis-associated isolates of S. aureus were tested for the presence of SraP. Cell wall proteins were isolated from the bacteria by the method of Hartford et al. (8). In brief, cultures of bacteria were grown at 37°C for 18 h in a rotary shaking incubator (250 rpm), diluted 1:25 in TS broth, and grown to the mid-log phase. The bacteria were washed twice with PBS and then incubated for 30 min at 37°C in protoplasting buffer (50 mM Tris-HCl, 30% [wt/vol] raffinose, 145 mM NaCl [pH 7.5]) containing lysostaphin (20 μg/ml), DNase I (80 μg/ml; Sigma), and complete protease inhibitors (Roche). The protoplasts were removed by centrifugation (12,000 × g, 10 min), and the supernatants were assessed for SraP by Western blotting. As a control, cell wall proteins from ISP479C (SraP+) and PS767 (SraP−) were isolated as described above and tested for the presence of SraP.
Glycosylation of SraP was assessed with cell wall extracts prepared from ISP479C and PS767. Proteins were separated by electrophoresis and transferred to membranes as described above. The presence of carbohydrates was determined by using a periodate-based assay (glycan detection kit; Roche) as described previously (2).
Binding of SraP90-723 to human platelets.
SraP90-723 was purified as described above and labeled with Alexa488 (Molecular Probes) according to the manufacturer's instructions. Formalin-fixed human platelets were prepared as described previously (16). To assess the binding of SraP90-723, 107 platelets and increasing concentrations of the labeled protein were incubated at room temperature for 1 h, and the samples were diluted 100-fold and analyzed by flow cytometry. A total of 10,000 events were collected for each sample. The binding of SraP90-723 to platelets was detected as the presence of Alexa488-positive cells and was expressed as a percentage of the total platelet population. Percent binding was plotted as a function of adhesin concentration by using Prism (GraphPad Software Inc.). To assess the rapidity of binding, aliquots were removed from the binding reaction mixtures at preselected time points (30 s to 60 min) and analyzed by flow cytometry as described above.
Animal model of infective endocarditis.
A coinfection model of endocarditis was developed to assess the virulence of ISP479C and the virulence of PS767. Aortic valve endocarditis was established in rabbits by standard methods (5). Valve injury was induced by passing a polyethylene catheter via the carotid artery into the left ventricle. The catheter was secured in place by suturing. After 24 h, 1 ml of a bacterial suspension containing ISP479C and PS767 at a ratio of ∼1:1 (as measured by plating on TS agar with or without 15 μg of erythromycin/ml) was injected into the marginal ear vein of each animal. The total number of CFU in the inoculum ranged from 105 to 107 CFU. Rabbits were sacrificed either 1 h or 24 h after injection of bacteria. A 200-μl volume of blood was obtained for culturing prior to sacrificing of the animals at the 24-h time point. Valves and vegetations were aseptically removed, weighed, homogenized in 0.5 ml of sterile 0.9% saline, and quantitatively inoculated onto TS agar. A 200-μl volume was inoculated onto TS agar for the undiluted vegetation homogenate; a 100-μl volume was used for each serial 10-fold dilution. Cultures were incubated for 24 h, and CFU were counted. Replica plating on TS agar-erythromycin was performed to determine the relative numbers of CFU for the parent and mutant strains.
To assess the virulence of ISP479C and the virulence of PS767, we compared the ratios of the two organisms in the inoculum with their ratios in vegetations or in blood. We reasoned that if PS767 were significantly less virulent than the parent strain, then the parent/mutant ratio in vegetations should be significantly higher than the ratio in the inoculum. Means ± standard deviations (SDs) were calculated for the inoculum (expressed as CFU) and for bacterial densities in vegetations (log10 CFU per gram of tissue) or in blood (log10 CFU per milliliter). For each animal, the arithmetic ratio of ISP479C to PS767 in vegetations or in blood was determined. To adjust for interexperiment variability of the inoculum, these ratios were normalized by dividing each value by the upper 95% confidence limit of the parent/mutant ratio in the inoculum. A paired t test was used to compare mean normalized ratios for blood and vegetations to 1, which was the no-effect numerical value corresponding to the parent/mutant ratio in the test sample that is identical to that in the inoculum. Log transformation was performed before use of the t test for nonnormally distributed data.
RESULTS
SraP is a cell wall-anchored, glycosylated protein.
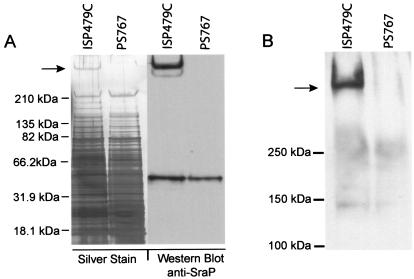
To confirm that SraP is expressed on the surface of ISP479C and that disruption of sraP results in the loss of its expression, we compared cell wall extracts from this strain with extracts from PS767. The latter strain contains an ermB antibiotic resistance gene inserted into the srr1-nrr segment of the sraP coding sequence. When assessed by SDS-PAGE and staining with silver, numerous proteins could be detected in the cell walls of both strains. However, a single protein with a very high molecular mass (≫250 kDa) was present in the cell wall of ISP479C but absent from that of PS767 (Fig. 2A). When these preparations were examined by Western blotting with an SraP-specific antiserum, the high-molecular-mass protein was present in the wild-type sample but absent from the cell wall of the mutant (Fig. 2A). These results indicate that, as predicted, sraP encodes a large, cell wall-associated protein.
FIG. 2.
Cell wall proteins of ISP479C and PS767. Cell wall proteins of the wild-type and mutant strains were prepared and separated by SDS-PAGE. (A) (Left) The total cell wall contents of the two strains are visualized on silver-stained gels. Note that a high-molecular-mass protein is present in the ISP479C cell wall but absent from the PS767 cell wall (arrow). (Right) Western blot analysis of the same preparations with anti-SraP serum shows that the high-molecular-mass protein is SraP. The approximately 50-kDa protein common to both preparations is protein A. (B) Proteins prepared from the cell walls of ISP479C and PS767 were probed for carbohydrates. Note that a single, high-molecular-mass band corresponding to SraP is present in ISP479C but absent from PS767 (arrow).
The cell wall proteins then were analyzed for carbohydrate contents by using a glycan detection assay. As shown in Fig. 2B, a single high-molecular-mass protein was present in the cell wall of ISP479C. The observed molecular mass of this protein corresponded to that of SraP. In contrast, no such glycoprotein could be detected in the cell wall of PS767. These results indicate that SraP is glycosylated. Moreover, the lack of other glycoproteins revealed by this assay suggests that SraP is the predominant glycoprotein on the surface of ISP479C.
Disruption of sraP is not transcriptionally polar.
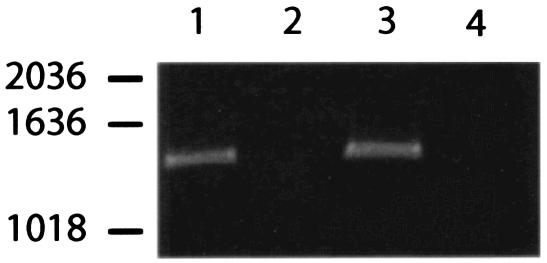
To confirm that the disruption of sraP is not associated with polar effects, we examined the transcription of secY2, the gene immediately downstream of sraP, in ISP479 and PS767. To assay for transcription, cDNA products from the parent and mutant strains were generated as described above. Control PCRs with products of the cDNA reaction from which RT had been omitted showed no amplification, indicating that the RNA preparations were not contaminated with DNA (Fig. 3). However, specific secY2 transcripts were detected in PCRs with cDNAs generated from both the wild type and the mutant. Thus, disruption of sraP with ermB does not affect downstream transcription, indicating that reduced platelet binding by PS767 is indeed dependent on sraP.
FIG. 3.
RT-PCR analysis of mRNAs isolated from ISP479C and PS767. The PCR products were amplified with secY2-specific primers and visualized after agarose gel electrophoresis. The cDNA templates used in the amplifications were generated by using a secA2-specific primer. To control for DNA contamination of the RNA preparations, RT was omitted from some reactions. Lanes: 1, ISP479C with RT; 2, ISP479C without RT; 3, PS767 with RT; 4, PS767 without RT. Numbers at left indicate sizes in basepairs.
Platelet binding by PS767.
Previous research showed that in S. gordonii, the expression of the SraP ortholog (GspB) was associated with platelet binding. To determine whether SraP has a similar function in S. aureus, we compared the binding of ISP479C and the binding of PS767 to immobilized human platelets. As shown in Fig. 4, the binding of PS767 was significantly decreased compared to that of the wild-type strain, with a mean ± SD reduction in binding of 47% ± 17% (P < 0.001). Furthermore, when platelet binding by a staphylococcal clinical isolate (PS750) and its SraP− derivative (PS891) were tested, the mutant showed a reduction in binding of 67% ± 4.3% (P < 0.0001). Thus, these results indicate that SraP mediates in part the adhesion of S. aureus to human platelets.
FIG. 4.
Platelet binding by ISP479C and PS767. Binding is expressed as a percentage of the level of binding achieved by the wild-type parent strain (mean ± SD). The asterisk indicates that the level of binding of PS767 was significantly lower than that of ISP479C (P < 0.001).
Binding of recombinant SraP to platelets.
To assess directly the interaction of SraP with platelets, we evaluated by flow cytometry the binding of Alexa488-labeled His6-SraP90-723 to human platelets. As shown in Fig. 5, His6-SraP90-723 bound platelets directly and could be detected on the surface of platelets at concentrations as low as 10 nM. Binding was very rapid; maximum levels of binding were detected within 30 s of mixing platelets and bacteria. Initial binding was concentration dependent. However, at higher concentrations of SraP (>1 μM), binding became saturable. These properties demonstrate that SraP can bind platelets directly. Moreover, the saturability of binding is consistent with a receptor-ligand interaction.
FIG. 5.
Binding of Alexa488-labeled SraP90-723 to human platelets. Binding of fluorescence-labeled SraP was assessed by flow cytometry. (A) Platelets were incubated with 0 (control), 100, and 1,000 nM concentrations of labeled proteins for 1 h, followed by analysis of 10,000 events. Data are shown as dot plots. Note that with increasing concentration of SraP, an increasing portion of the platelet population is detected in the upper section (Alexa488 fluorescence emission), indicating that SraP is bound. (B) Mean ± SD triplicate binding data plotted as a function of protein concentration.
Role of SraP in the pathogenesis of endocarditis.
To assess the impact of SraP on virulence, we compared ISP479C with PS767 in a coinfection model of infective endocarditis. Two sets of experiments were performed with two inocula: 5.75 ± 0.27 log10 CFU (mean ± SD; five replicate samples of the inoculum) and 7.01 ± 0.09 log10 CFU (three replicate samples). The parent/mutant ratios in the inocula were similar in the two trials, as measured by replica plating: 0.74 ± 0.37 (mean ± SD) with a 95% upper confidence limit of 1.2 and 0.75 ± 0.10 with a 95% upper confidence limit of 0.98.
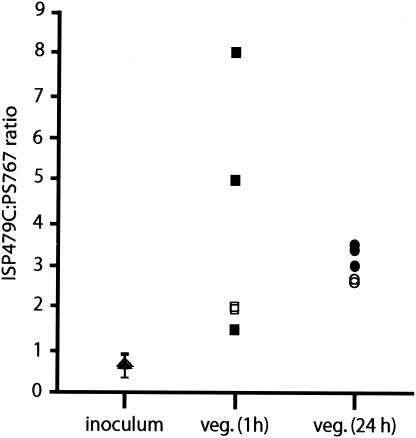
Animals were infected intravenously with the above inocula and then sacrificed either 1 h or 24 h later. Five of six rabbits sacrificed 1 h after inoculation had organisms present within their vegetations; the mean ± SD bacterial burden was 38 ± 34 CFU per vegetation (3.26 ± 0.28 log10 CFU per g of vegetation). In all five animals, the ratio of wild-type to mutant organisms in the vegetations was above that observed for the inoculum (Fig. 6). The normalized ratio of parent to mutant in the vegetations was 3.21 ± 2.22, a value which was significantly different from the no-effect value of 1 (P = 0.031).
FIG. 6.
Effect of SraP expression on virulence in a rabbit model of endocarditis. Shown are the ratios of ISP479C to PS767 present in the inoculum and vegetations (veg.) of the test animals at 1 and 24 h postinfection. Note that in all animals, the ratios are increased compared with the inoculum. The open and closed symbols represent results from two independent experiments.
Five of six rabbits sacrificed at 24 h were infected, with a mean ± SD bacterial burden of 6.91 ± 1.32 log10 CFU per g of vegetation. The ratio of wild-type to mutant organisms in the vegetations was above that observed for the inoculum in all five animals (Fig. 6). The normalized ratio of parent to mutant in the vegetations was 2.72 ± 0.15, a value which was significantly different from the no-effect value (P < 0.001). Interestingly, the ratio of organisms in blood cultures, for which the total bacterial burden was 128 ± 75 CFU/ml, was 1.82 ± 1.82; this value was not significantly different from 1, suggesting continued seeding of the blood by the mutant, either preferentially from the vegetations or from some other site of infection.
SraP is prevalent in S. aureus clinical isolates.
To assess the prevalence of SraP in S. aureus clinical isolates, we performed Western blotting of cell wall extracts that were prepared from 21 clinical isolates (14 endocarditis-associated isolates and 7 isolates associated with infections at other sites). Eighty-five percent of the isolates (12 endocarditis-associated isolates and 6 non-endocarditis-associated isolates) had cell wall proteins that were recognized by anti-SraP90-723 serum (Fig. 7). In all instances, the proteins migrated at molecular masses higher than those predicted, as was observed with SraP extracted from ISP479C. The masses of SraP from the six isolates not associated with endocarditis were identical to that of SraP recovered from ISP479C. In contrast, the masses of SraP from the endocarditis-associated isolates varied somewhat, with only 50% of the isolates having proteins with masses identical to that of SraP recovered from ISP479C. Thus, it would appear that most isolates of S. aureus express SraP but that the protein may exist in a variety of isoforms.
FIG. 7.
Prevalence of SraP in clinical isolates of S. aureus. Cell wall preparations from 23 isolates were analyzed by Western blotting with SraP-specific polyclonal antiserum. Lanes: 1, ISP479C; 2, PS767; 3 to 16, infective endocarditis (IE) isolates; 17 to 23, non-IE clinical isolates.
DISCUSSION
Our results indicate that SraP is a newly identified cell wall protein of S. aureus that mediates binding to human platelets. Nonpolar disruption of sraP was associated with a loss of SraP surface expression and a significant reduction in platelet binding by strain ISP479C. The same phenotypic changes were produced when this mutation was transduced into an endocarditis-associated clinical isolate (PS750). These data confirm the role of SraP in platelet binding and indicate that SraP-mediated binding is not an anomaly of a laboratory strain. Rather, it may be a well-conserved property of S. aureus.
To further assess the role of SraP in platelet binding, we evaluated the ability of recombinant SraP to binding platelets in vitro. We found that SraP could bind platelets directly and that binding was rapid. Moreover, platelet binding was saturable, suggesting that SraP may bind one or more receptors on the platelet surface. As yet, we have not identified any specific platelet ligands for SraP. Unlike the expression of GspB, which we recently showed to bind the platelet membrane glycoprotein GPIbα (1), the expression of SraP does not appear to enhance binding by S. aureus ISP479C to this receptor (data not shown). This finding is not entirely surprising, since the nonrepeat regions of SraP and GspB, which are likely to be the binding domains of these proteins, differ considerably at the amino acid level.
SraP is encoded within an operon that is conserved among many medically relevant gram-positive bacteria, including S. gordonii, Streptococcus pneumoniae, and Streptococcus agalactiae (21). In addition to encoding GspB, the gspB-sec operon of S. gordonii encodes numerous proteins involved in its glycosylation (including GtfA and GtfB) and export (SecY2, SecA2, and Asp1 to Asp3). Immediately downstream of sraP are homologs of the above genes, suggesting that they encode proteins with similar functions. Our finding that SraP is glycosylated further suggests that GtfA or GtfB homologs may mediate the posttranslational modification of this protein. Although the impact of glycosylation on SraP is unknown, we have demonstrated that GtfA and GtfB are essential for the solubility and export of GspB (20). Therefore, it is possible that these proteins serve a similar function for SraP. Of note, the gspB-sec operon contains two additional genes (gly and nss) that also participate in the glycosylation of GspB. Although not essential for GspB export and function, these genes are required for optimal GspB-mediated binding to platelets. Interestingly, homologs of gly and nss are not present in the sraP operon. Despite the absence of these genes, the protein appears to be fully functional.
The expression of SraP is also associated with increased virulence, as measured by our coinfection model of infective endocarditis. We elected to assess virulence with this model because such competition-based assays have proven to be sensitive and cost-efficient means of discerning differences in virulence (3, 10). When tested in this model, the SraP mutant strain was significantly impaired in its ability to initiate infection compared with the parent strain, as measured by CFU per gram of vegetation, both at 1 h and at 24 h after inoculation. This difference could not be attributed to differences in the levels of bacteremia, as both ISP479C and PS767 achieved comparable titers within the bloodstream. We postulate that the loss of SraP expression results in a decreased ability to initiate endovascular infection due to a reduction in the binding of bacteria to platelets on the damaged valve surface. In addition, reduced platelet binding could also diminish reseeding of the endocardium, i.e., the reattachment of bacteria released from the infected valve surface back onto platelets that have collected at the site of infection.
Although numerous potential virulence determinants have been identified in well-characterized laboratory strains, such as ISP479C, the relevance of these factors in clinical isolates often has not been addressed. For this reason, we examined the prevalence of SraP in 21 randomly selected clinical isolates recovered from patients with S. aureus infections. We found that 85% of the S. aureus isolates expressed SraP on their cell walls, indicating that this is a highly prevalent surface component of this organism and suggesting that it may have a broad role in virulence. Of note, the SraP adhesins showed a high degree of heterogeneity in structure among the infective endocarditis-associated clinical isolates tested. However, SraP adhesins from non-infective endocarditis-associated isolates were relatively invariant. These data may reflect a selective pressure placed upon the non-infective endocarditis isolates or may reveal a clonal origin for those isolates.
Acknowledgments
This work was supported by grants RO1 AI041513 (to P.M.S.), RO1 AI057433 (to P.M.S.), and RO AI46610 (to H.F.C.) from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and by the Merit Review Program of the Department of Veterans Affairs (grant to P.M.S.).
We thank Li Basuino for technical assistance and Gordon Archer, Barbara Bensing, and Daisuke Takamatsu for helpful discussions.
Editor: V. J. DiRita
REFERENCES
- 1.Bensing, B. A., J. A. Lopez, and P. M. Sullam. 2004. The Streptococcus gordonii surface proteins GspB and Hsa mediate binding to sialylated carbohydrate epitopes on the platelet membrane glycoprotein Ibα. Infect. Immun. 72:6528-6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bensing, B. A., and P. M. Sullam. 2002. An accessory sec locus of Streptococcus gordonii is required for export of the surface protein GspB and for normal levels of binding to human platelets. Mol. Microbiol. 44:1081-1094. [DOI] [PubMed] [Google Scholar]
- 3.Dhawan, V. K., A. S. Bayer, and M. R. Yeaman. 1998. In vitro resistance to thrombin-induced platelet microbicidal protein is associated with enhanced progression and hematogenous dissemination in experimental Staphylococcus aureus infective endocarditis. Infect. Immun. 66:3476-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durack, D. T. 1975. Experimental bacterial endocarditis. IV. Structure and evolution of very early lesions. J. Pathol. 115:81-89. [DOI] [PubMed] [Google Scholar]
- 5.Durack, D. T., and P. B. Beeson. 1972. Experimental bacterial endocarditis. I. Colonization of a sterile vegetation. Br. J. Exp. Pathol. 53:44-49. [PMC free article] [PubMed] [Google Scholar]
- 6.Gong, K., D. Y. Wen, T. Ouyang, A. T. Rao, and M. C. Herzberg. 1995. Platelet receptors for the Streptococcus sanguis adhesin and aggregation-associated antigens are distinguished by anti-idiotypical monoclonal antibodies. Infect. Immun. 63:3628-3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 8.Hartford, O., P. Francois, P. Vaudaux, and T. J. Foster. 1997. The dipeptide repeat region of the fibrinogen-binding protein (clumping factor) is required for functional expression of the fibrinogen-binding domain on the Staphylococcus aureus cell surface. Mol. Microbiol. 25:1065-1076. [DOI] [PubMed] [Google Scholar]
- 9.Heilmann, C., S. Niemann, B. Sinha, M. Herrmann, B. E. Kehrel, and G. Peters. 2004. Staphylococcus aureus fibronectin-binding protein (FnBP)-mediated adherence to platelets, and aggregation of platelets induced by FnBPA but not by FnBPB. J. Infect. Dis. 190:321-329. [DOI] [PubMed] [Google Scholar]
- 10.Jones, A. L., K. M. Knoll, and C. E. Rubens. 2000. Identification of Streptococcus agalactiae virulence genes in the neonatal rat sepsis model using signature-tagged mutagenesis. Mol. Microbiol. 37:1444-1455. [DOI] [PubMed] [Google Scholar]
- 11.Kreiswirth, B. N., S. Lofdahl, M. J. Betley, M. O'Reilly, P. M. Schlievert, M. S. Bergdoll, and R. P. Novick. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305:709-712. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen, T., B. Ghebrehiwet, and E. I. Peerschke. 2000. Staphylococcus aureus protein A recognizes platelet gC1qR/p33: a novel mechanism for staphylococcal interactions with platelets. Infect. Immun. 68:2061-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Brien, L., S. W. Kerrigan, G. Kaw, M. Hogan, J. Penades, D. Litt, D. J. Fitzgerald, T. J. Foster, and D. Cox. 2002. Multiple mechanisms for the activation of human platelet aggregation by Staphylococcus aureus: roles for the clumping factors ClfA and ClfB, the serine-aspartate repeat protein SdrE and protein A. Mol. Microbiol. 44:1033-1044. [DOI] [PubMed] [Google Scholar]
- 14.Pattee, P. A. 1981. Distribution of Tn551 insertion sites responsible for auxotrophy on the Staphylococcus aureus chromosome. J. Bacteriol. 145:479-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roche, F. M., R. Massey, S. J. Peacock, N. P. Day, L. Visai, P. Speziale, A. Lam, M. Pallen, and T. J. Foster. 2003. Characterization of novel LPXTG-containing proteins of Staphylococcus aureus identified from genome sequences. Microbiology 149:643-654. [DOI] [PubMed] [Google Scholar]
- 16.Siboo, I. R., A. L. Cheung, A. S. Bayer, and P. M. Sullam. 2001. Clumping factor A mediates binding of Staphylococcus aureus to human platelets. Infect. Immun. 69:3120-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Studier, F. W., and B. A. Moffatt. 1986. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189:113-130. [DOI] [PubMed] [Google Scholar]
- 18.Sullam, P. M. 1994. Host-pathogen interactions in the development of bacterial endocarditis. Curr. Opin. Infect. Dis. 4:304-309. [Google Scholar]
- 19.Sullam, P. M., D. G. Payan, P. F. Dazin, and F. H. Valone. 1990. Binding of viridans group streptococci to human platelets: a quantitative analysis. Infect. Immun. 58:3802-3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takamatsu, D., B. A. Bensing, and P. M. Sullam. 2004. Four proteins encoded in the gspB-secY2A2 operon of Streptococcus gordonii mediate the intracellular glycosylation of the platelet-binding protein GspB. J. Bacteriol. 186:7100-7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takamatsu, D., B. A. Bensing, and P. M. Sullam. 2004. Genes in the accessory sec locus of Streptococcus gordonii have three functionally distinct effects on the expression of the platelet-binding protein GspB. Mol. Microbiol. 52:189-203. [DOI] [PubMed] [Google Scholar]
- 22.Yeaman, M. R., P. M. Sullam, P. F. Dazin, D. C. Norman, and A. S. Bayer. 1992. Characterization of Staphylococcus aureus-platelet binding by quantitative flow cytometric analysis. J. Infect. Dis. 166:65-73. [DOI] [PubMed] [Google Scholar]