Abstract
The natural killer complex (NKC) is a genetic region of highly linked genes encoding several receptors involved in the control of NK cell function. The NKC is highly polymorphic, and allelic variability of various NKC loci has been demonstrated in inbred mice. Making use of BALB.B6-Cmv1r congenic mice, in which the NKC from disease-susceptible C57BL/6 mice has been introduced into the disease-resistant BALB/c background, we show here that during murine malaria infection, the NKC regulates a range of pathophysiological syndromes such as cerebral malaria, pulmonary edema, and severe anemia, which contribute to morbidity and mortality in human malaria. Parasitemia levels were not affected by the NKC genotype, indicating that control of malarial fatalities by the NKC cells does not operate through effects on parasite growth rate. Parasite-specific antibody responses and the proinflammatory gene transcription profile, as well as the TH1/TH2 balance, also appeared to be influenced by NKC genotype, providing evidence that this region, known to control innate immune responses via NK and/or NK T-cell activation, can also significantly regulate acquired immunity to infection. To date, NKC-encoded innate system receptors have been shown mainly to regulate viral infections. Our data provide evidence for critical NKC involvement in the broad immunological responses to a protozoan parasite.
The natural killer complex (NKC) is a genetic region of highly linked genes, which encodes several receptors involved in the control of NK cell function (50). This genomic region is located on distal mouse chromosome 6 and is conserved among species. Syntenic regions have been identified in rat and human chromosomes (9). The murine NKC spans a region of around 4.7 mb and comprises several genes such as Cd69 and Cd94, as well as multigene families including Nkrp1, Nkg2, and Ly49. These genes encode type II integral membrane proteins with C-lectin domains, having inhibitory or activation function on cytotoxic activity and cytokine production depending on the presence or absence of immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in their intracellular domains (7, 8). Upon ligation, these ITIMs become phosphorylated and recruit phosphatases such as the Src homology 2 domain-containing protein tyrosine phosphatase 1 (SHP1), which interferes with normal cell activation and results in the inhibition of NK cell function. In the mouse, inhibitory receptors can be divided into two groups, the Ly49 superfamily and the NKG2 molecules, which are expressed as heterodimers together with CD94. Whereas members of the Ly49 superfamily interact with major histocompatibility complex class I (MHC-I) molecules (2), CD94/NKG2 complexes bind to the nonclassical MHC-I receptor Qa-1b (45, 46). Interaction of these inhibitory receptors with their MHC-I or MHC-I-like ligands induces inhibition of cytotoxic activity by NK cells. Under certain pathophysiological conditions such as viral infections or tumors, the expression of MHC-I molecules is down-regulated, resulting in the loss of negative regulation by inhibitory receptors, subsequent NK cell activation, and killing of target cells (29). Some members of the Ly49 family lack ITIMs in their cytoplasmic domains and therefore have activation function. These receptors contain a charged amino acid in their transmembrane domains, which allows the interaction with immunoreceptor tyrosine-based activation motif-containing adaptor molecule DAP12. DAP12 activation results in tyrosine phosphorylation and downstream cell activation (33). Stimulation of activation receptors such as Ly49D (33) and Ly49H (17) leads to gamma interferon (IFN-γ) secretion and cytotoxic activity. Other activation receptors encoded within the murine NKC include CD94/NKG2C heterodimers, NKG2D, and the alloantigen NK1.1. These activation receptors (but not the Ly49 activation molecules) are expressed not only in NK cells but also in a subset of lymphocytes coexpressing some NKC markers together with a conserved T-cell receptor (TCR) (CD1-restricted NK T cells) (3). It has been shown that stimulation with anti-NK1.1 antibody preferentially stimulates IFN-γ production by CD1-restricted NK T cells (4, 22).
The NKC appears to be a highly polymorphic region. Allelic variability of various NKC loci has been demonstrated in inbred mice, providing evidence for NKC haplotypes (10, 36). Apart from genes encoding known protein products, the NKC encodes several phenotypically defined loci. For example, C57BL/6 mice are resistant to mousepox viral infection whereas BALB/c mice are susceptible. Resistance to mousepox virus has been shown to be under the control of a locus named Rmp1, which maps to the NKC region in distal mouse chromosome 6 (11, 15). Chok was initially characterized as a locus controlling C57BL/6 NK-cell-mediated killing of allogeneic Chinese hamster ovary cells. BALB/c NK cells are unable to kill Chinese hamster ovary cells (27). More recently, it was found that Chok in fact encodes the activation receptor Ly49D (28). Similarly, genetically determined resistance to murine cytomegalovirus is controlled by a gene originally designated Cmv1 that regulates viral replication in the spleens of mice (42). C57BL/6 mice express the Cmv1r allele and are resistant to this condition, whereas BALB/c mice express the Cmv1s allele and show high viral titers. Generation of congenic mouse strains has allowed the mapping of these alleles to the NKC located on the distal region of mouse chromosome 6. Recent studies show that Cmv1r maps to the NK activation receptor Ly49H (17).
C57BL/6 and BALB/c mouse strains substantially differ in their ability to mount immune responses to several pathogens, including malaria parasites. C57BL/6 mice are susceptible to the Plasmodium berghei ANKA-mediated severe malaria, whereas BALB/c mice are resistant (14). Both humans and experimental animals affected by malaria may suffer several disease syndromes, including acute respiratory distress, metabolic acidosis, renal failure, pulmonary edema, anemia, and cerebral involvement (47). Proinflammatory and counterregulatory cytokines, as well as immune system effector cells, have been shown to be involved in the control of malarial pathogenesis. In previous studies, we found that CD1-restricted NK T cells differentially regulate P. berghei-mediated cerebral malaria depending on the genetic background of the host. CD1-restricted NK T cells have a protective role against P. berghei-mediated cerebral malaria in BALB/c mice. In contrast, NK T cells induce early IFN-γ production and promote disease in C57BL/6 susceptible animals (22). Making use of BALB.B6-Cmv1r congenic mice, in which the NKC from C57BL/6 mice has been introduced in the BALB/c background (42), we found that the immunological properties of NK T cells during malaria infection vary depending on the NKC genotype. BALB.B6-Cmv1r mice were also found to be significantly more susceptible to P. berghei-mediated severe malaria than BALB/c controls (22), demonstrating that the NKC is a genetic determinant of malarial fatalities. In the present study, we analyzed the impact of the NKC genotype in the development of severe malarial pathogenesis and the nature of various immune responses to infection. We found that the NKC regulates several immunopathological responses to malaria, including cerebral pathology, pulmonary edema, and severe anemia, which are thought to contribute to morbidity and mortality in human malaria. Parasite-specific antibody responses as well as the TH1/TH2 balance were also influenced by the NKC genotype, providing evidence that this genomic region, known to control innate immune responses via NK and/or NK-T-cell activation, can also regulate acquired immunity to infection.
MATERIALS AND METHODS
Mice and infections.
Eight- to twelve-week-old BALB/c, C57BL/6, and BALB.B6-Cmv1r (F10 generation [42]) mice were used throughout the study. Groups of 10 to 15 mice were injected intraperitoneally with 106 P. berghei ANKA-infected red blood cells. In some experiments, mice were treated with chloroquine (10 mg/kg of body weight) and pyrimethamine (10 mg/kg) for 5 to 7 days starting at day 5 postinfection (p.i.). Parasitemia was assessed from Giemsa-stained smears of tail blood prepared every 2 to 3 days. Mortality was checked daily. Mice were judged as developing cerebral malaria if they displayed neurological signs such as ataxia, loss of reflex, and hemiplegia and died between days 6 and 12 p.i. with relatively low parasitemia. All experiments were performed in compliance with local Animal Ethics Committee requirements.
Histology.
For histological analysis of cerebral pathology, brains from P. berghei-infected mice were taken into 10% neutral-buffered formalin, sectioned (5 μm), and stained with hematoxylin and eosin. Slides were coded and scored blind for histological evidence of cerebral syndrome.
Pulmonary edema.
Lungs were collected from BALB/c and BALB.B6-Cmv1r mice at different time points p.i. with P. berghei ANKA. The wet weight was measured immediately after removal of the organ, and the dry weight was determined after overnight incubation at 80°C. The wet/dry weight ratio was then calculated.
Anemia.
Hemoglobin levels were assessed in 4 μl of tail blood collected from BALB/c and BALB.B6-Cmv1r mice at different time points p.i. with P. berghei. Blood was diluted in 100 μl of Drabkin's solution (Sigma, St. Louis, Mo.), and the optical density at 580 nm was determined. Hemoglobin level in blood from infected mice was calculated using bovine hemoglobin (Sigma) for preparation of standard curves.
Flow cytometry.
Spleen cells from BALB/c, C57BL/6, and BALB.B6-Cmv1r mice were incubated with anti-CD16 antibody (Fc-block), washed, and then stained with phycoerythrin-conjugated anti-CD49b (DX-5) and phycoerythrin-Cy5-conjugated anti-TCR (H57-597) antibodies. Some samples were simultaneously stained with other antibodies such as fluorescein isothiocyanate-conjugated anti-Ly49A (A1), Ly49C/I (5E6), Ly49D (4E5), Ly49G2 (Cwy-e), Ly49I (YLI-90), anti-NKG2A/C/E (20d5), or anti-NK1.1 (PK136) for 1 h on ice. Some cells were incubated with biotinylated anti-Ly49F (HBF-719), followed by incubation for 1 h on ice with a streptavidin-fluorescein isothiocyanate conjugate (all antibodies and conjugates were from Pharmingen, San Diego, Calif.). The cells were then washed two times with phosphate-buffered saline (PBS) containing 1% fetal calf serum and suspended in 200 μl of PBS. The cells were then analyzed in a FACScan cytofluorometer (Becton Dickinson, Grenoble, France) using CellQuest software. Viable lymphocytes were gated by forward and side scatter.
P. berghei ANKA lysate preparation.
P. berghei ANKA lysates were prepared as described previously (23). Briefly, 10 ml of blood collected from P. berghei ANKA-infected mice (day 14 p.i.) was diluted 1:2 in RPMI-1640 medium and passed through a Whatman CF-11 cellulose column. The erythrocytes were eluted by washing the column with 2 volumes of RPMI-1640 medium. The purified erythrocytes were centrifuged at 1,000 × g for 5 min and trypsinized for 10 min at 37°C. After washing three times with RPMI-1640 medium, the erythrocytes were lysed with PBS-0.05% saponin and centrifuged at 10,000 rpm for 10 min in a Sorvall centrifuge. The pellet was washed and resuspended in PBS. The parasites were disrupted by 5 cycles of freezing-thawing and centrifuged for 5 min at 2,000 rpm. The supernatant was stored at −20°C until use.
ELISA for detection of P. berghei-specific antibodies.
Microtiter plates were coated with P. berghei ANKA lysate (5 μg/ml) in carbonate buffer, pH 9.6, by overnight incubation at 4°C. Empty sites were blocked with 5% skim milk for 1 h at 37°C. After washing three times with 0.05% Tween 20 in PBS, plates were incubated with different antisera in serial 1:2 dilutions for 1 h at 37°C. The plates were washed three times and incubated with a peroxidase-conjugated rabbit anti-mouse antibody (Pierce, Rockford, Ill.). The isotype titers were determined by incubating for 1 h at 37°C with rabbit anti-mouse antibodies against immunoglobulin M (IgM), IgG1, IgG2a, IgG2b, and IgG3 (all antibodies were from Zymed, San Francisco, Calif.). The plates were then washed three times and incubated with a peroxidase-conjugated goat anti-rabbit antibody (Pierce). In all enzyme-linked immunosorbent assays (ELISAs), bound complexes were detected by reaction with tetramethyl-benzidine (TMB) (KBL, Gaithersburg, Md.) and H2O2. Absorbance was read at 450 nm.
ELISA for detection of total IgM and IgG content.
The following pairs of antibodies were used: monoclonal antibody LO-MM-9 (Zymed) for capture with a polyclonal rabbit anti-mouse IgM (Zymed) for detection of IgM and a polyclonal anti-mouse IgG Fab (Sigma) for capture with a peroxidase-conjugated sheep anti-mouse IgG (Amersham, Little Chalfont, United Kingdom) for detection of IgG. Ninety-six-well plates were coated with capture antibody by overnight incubation at 4°C in phosphate buffer, pH 9. Plates were then blocked with 1% bovine serum albumin for 1 h at 37°C. Sera from infected mice were tested in duplicate by incubation for 1 h 37°C. After washing three times with PBS-0.05% Tween 20, the plates were incubated for 1 h at 37°C with their respective detection antibodies. For detection of IgM antibodies, plates were further incubated for 1 h at 37°C with a peroxidase-conjugated goat anti-rabbit antibody (Pierce). Bound complexes were detected by reaction with TMB (KBL) and H2O2. Absorbance was read at 450 nm. The antibody concentration in samples was calculated using purified immunoglobulins (Sigma) for the preparation of standard curves.
In vitro T-cell stimulation.
BALB/c, C57BL/6, and BALB.B6-Cmv1r mice (n = 3) were infected with 106 P. berghei-infected erythrocytes. At day 5 p.i., mice were treated with chloroquine (10 mg/kg) and pyrimethamine (10 mg/kg) for 5 to 7 days. Seven days after we finished the treatment, mice were sacrificed and splenocyte suspensions were prepared. Spleen cells were suspended in complete RPMI-1640 medium-5% fetal calf serum and seeded in 96-well plates at a density of 2 × 106 cells/ml. Cells were then stimulated in triplicate for 3 days with P. berghei ANKA total lysate (25 μg/ml) or anti-CD3 (5 μg/ml; Pharmingen). Cells cultured in medium alone were used as background controls. The cell culture supernatants were collected to determine cytokine content by capture ELISA.
ELISAs for IL-4, IFN-γ, and TGF-β detection.
Cytokine ELISAs were carried out as described previously (22). Briefly, the following pairs of antibodies were used: 11B11 for capture and BVD6-24G2 for detection of interleukin-4 (IL-4), R4-6A2 for capture and XMG1-2 for detection of IFN-γ, and A75-2.1 for capture and A75-3.1 for detection of transforming growth factor β (TGF-β) (all antibodies from Pharmingen). Antibodies used for detection were biotinylated. Ninety-six-well plates were coated with capture antibody by overnight incubation at 4°C in phosphate buffer, pH 9. Plates were then blocked with 1% bovine serum albumin for 1 h at 37°C. Cell culture supernatants or sera from infected mice were tested in duplicate by overnight incubation at 4°C under mild agitation. Prior to addition to the plates, samples used for TGF-β detection were treated by acidification by incubation in 0.04 N HCl for 20 min at 20°C, followed by neutralization with 0.04 N NaOH. The plates were then incubated for 3 h at 20°C with the respective biotinylated antibody, followed by a 2-h incubation at 20°C with streptavidin-peroxidase conjugate (Pierce). Bound complexes were detected by reaction with TMB (KBL) and H2O2. Absorbance was read at 450 nm. The cytokine concentration in samples was calculated in picograms per milliliter by using recombinant murine cytokines (Pharmingen) for the preparation of standard curves.
Microarray analyses.
RNA was extracted from BALB/c and BALB.B6-Cmv1r mice (n = 2) at days 0 and 7 p.i. with P. berghei ANKA (106). cDNA and RNA synthesis reactions were performed according to the Affymetrix (Santa Clara, Calif.) Gene Chip Expression Analysis technical manual, and Affymetrix murine U74v2 arrays were used. Analyses were conducted using Genespring version 6.1 after importing signal measurements from Microarray Suite version 5.0. For normalizations, the 50th percentile of all measurements was used as a positive control for each sample. Each measurement for each gene was divided by this synthetic positive control, assuming this was at least 10. The bottom 10th percentile was used as a test for correct background subtraction. This was never less than the negative of the synthetic positive control. The measurement for each gene in each sample was divided by the corresponding value at the day 0 control (average of two samples), assuming that it was at least 0.01, to obtain the final normalized value for each probe set (gene). An individual probe set was said to have changed only if the ratio was greater than 2.0 or less than 0.6 in both samples at day 7. Measurements for which the signal was deemed absent by Microarray Suite version 5.0 analysis were excluded. Normalized data were grouped according to the n-fold change, and lists of genes were compiled.
Statistic analysis.
A paired-sample Student's t test was used for data evaluation.
RESULTS
Differential expression of markers encoded by the NKC in BALB/c and BALB.B6-Cmv1r mice.
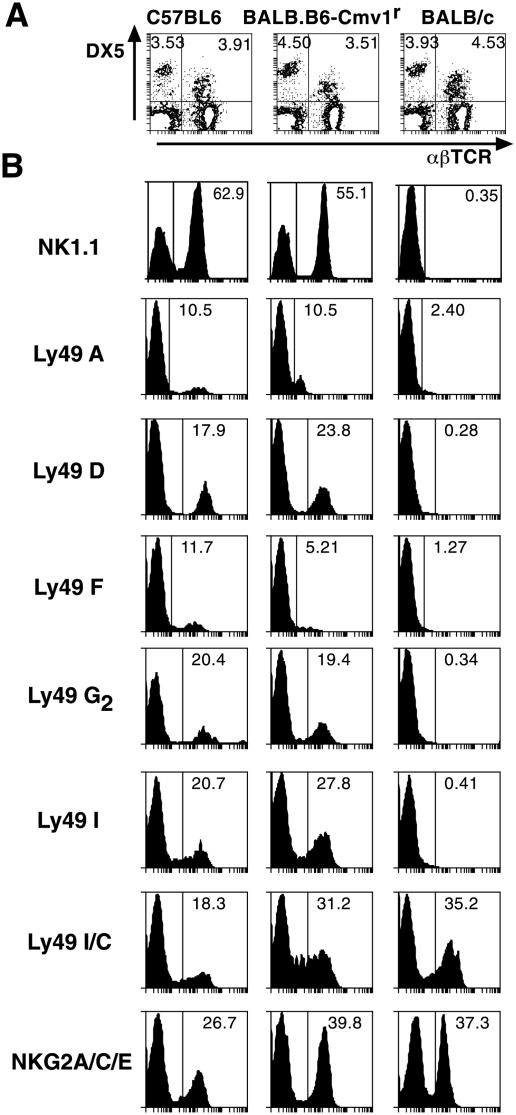
BALB.B6-Cmv1r mice are a congenic strain in which the NKC from C57BL/6 mice has been introduced in the BALB/c background (42). To determine the phenotypic properties of NK and NK T cells from wild-type and congenic mice, spleen cells from BALB/c, C57BL/6, and BALB.B6-Cmv1r mice were stained with antibodies against the pan-NK-NK-T-cell marker DX-5 and α/βTCR. Figure 1A shows that spleens of BALB/c, C57BL/6, and BALB.B6-Cmv1r mice have similar percentages of both NK and NK T cells. The expression of different NKC markers was then studied in all DX5-positive splenocytes from the three mouse strains. Antibodies directed to C57BL/6 NKC receptors such as NK1.1, Ly49A, Ly49D, Ly49F, Ly49G2, and Ly49I recognized these molecules on the surfaces of cells from wild-type C57BL/6 mice as well as congenic animals (Fig. 1B). None of these molecules could be detected in spleen cells from BALB/c mice, suggesting that they are absent in this mouse strain or that BALB/c mice express a different allelic variant which substantially differs from the C57BL/6 allele and is therefore not recognized by strain-specific monoclonal antibodies. In contrast, antibodies directed against NKG2A/C/E and Ly49C/I were able to detect these molecules on the three mouse strains tested, suggesting a higher homology level (Fig. 1B). Thus NK and/or NK T cells from BALB/c and BALB.B6-Cmv1r mice differ in the expression of NKC markers such as NK1.1 and members of the Ly49 gene superfamily.
FIG. 1.
Differential expression of NKC markers in NK and NK T cells from BALB/c and BALB.B6-Cmv1r mice. (A) Spleen cells from C57BL/6, BALB/c, and BALB.B6-Cmv1r mice were stained with anti-CD49b (DX5) and anti-αβTCR antibodies. The percentages of NK and NK T cells are indicated. (B) The expression of the NKC markers NK1.1, Ly49A, Ly49D, Ly49F, Ly49G2, Ly49I, Ly49C/I, and NKG2A/C/E was calculated on all DX5-positive cells from the three mouse strains. Representative histograms are shown.
Increased cerebral pathology, pulmonary edema, and severe anemia in BALB.B6-Cmv1r mice.
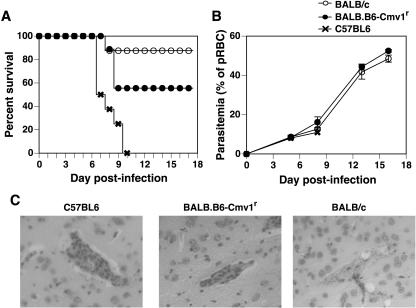
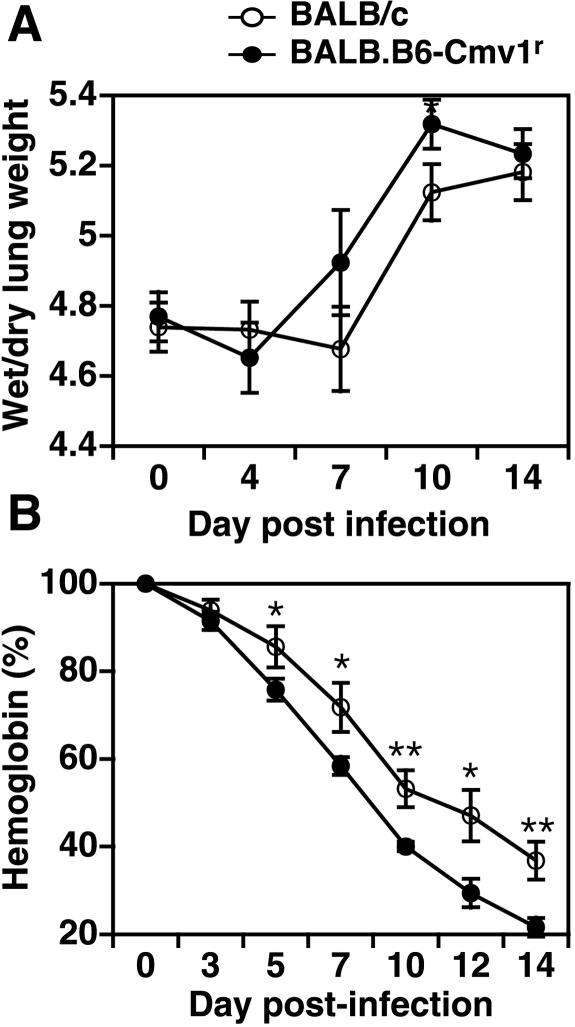
We have previously shown that BALB.B6-Cmv1r mice display increased death rates during infection with P. berghei ANKA compared to fully resistant BALB/c wild-type controls (22). To study whether the NKC genotype could in fact influence the development of severe malarial pathogenesis, BALB/c, BALB.B6-Cmv1r, and C57BL/6 mice were infected with P. berghei and different pathophysiological endpoints were analyzed. As shown before, BALB.B6-Cmv1r congenic animals succumb to P. berghei-mediated severe malaria and 54% of the animals died between days 7 and 9 p.i. (Fig. 2A). Parasitemia levels were not affected by the NKC genotype (Fig. 2B), indicating that control of malarial fatalities by the NKC cells does not operate through effects on parasite growth rates. The development of cerebral malaria was confirmed by histological examination of brains taken at day 7 p.i. Mice dying of cerebral syndrome displayed typical pathology, including high levels of vascular occlusion with both parasitized erythrocytes and macrophages (Fig. 2C). This pathology was pronounced in C57BL/6 mice and was also present, although with reduced severity, in BALB.B6-Cmv1r animals. BALB/c mice, in contrast, showed absent or very much reduced vascular occlusion despite similar parasite burdens (Fig. 2C). As with P. falciparum infection in humans, mice infected with P. berghei ANKA develop a range of disease symptoms including acute respiratory distress, coagulopathy, shock, metabolic acidosis, hypoglycaemia, renal failure, severe anemia, and pulmonary edema. We (43) and others (13) have previously shown that these disease symptoms peak at day 6 p.i. in fully susceptible C57BL/6 mice. To study whether the NKC genotype could also influence the susceptibility to some of these malarial syndromes, lung edema and hemoglobin levels were determined in BALB/c and BALB.B6-Cmv1r mice at different time points p.i. with P. berghei ANKA. BALB.B6-Cmv1r mice displayed a substantially increased pulmonary edema compared to wild-type mice which was significantly different at day 10 p.i. (Fig. 3A). Both BALB/c and BALB.B6-Cmv1r mice showed an important reduction in their hemoglobin levels as the infection developed. However, this reduction was significantly higher in BALB.B6-Cmv1r mice compared to that in wild-type animals (Fig. 3B) in spite of identical parasitemia levels. Taken together, these results indicate that the NKC regulates murine cerebral and severe malaria by a mechanism independent of parasite growth rates.
FIG. 2.
Control of malarial pathogenesis by the NKC. Groups of 10 to 12 BALB/c, C57BL/6, and BALB.B6-Cmv1r mice were infected with P. berghei ANKA. (A) The percentage of survival was monitored daily. (B) Parasitemia was assessed from Giemsa-stained blood smears. (C) Histological examination of brains from C57BL/6, BALB.B6-Cmv1r, and BALB/c mice infected with P. berghei is shown. Magnification, ×100.
FIG. 3.
Control of malarial pathogenesis by the NKC. BALB/c and BALB.B6-Cmv1r mice were infected with P. berghei ANKA. (A) Lung edema was calculated as the wet weight/dry weight ratio of the organ at different time points p.i. Each point represents the mean of six samples ± the standard error. (B) Hemoglobin levels were assessed in tail blood at different time points p.i. with P. berghei. Each point represents the mean of 6 to 10 samples ± the standard error. *, P < 0.05; **, P < 0.01.
The NKC controls systemic IFN-γ and TGF-β production in murine severe malaria.
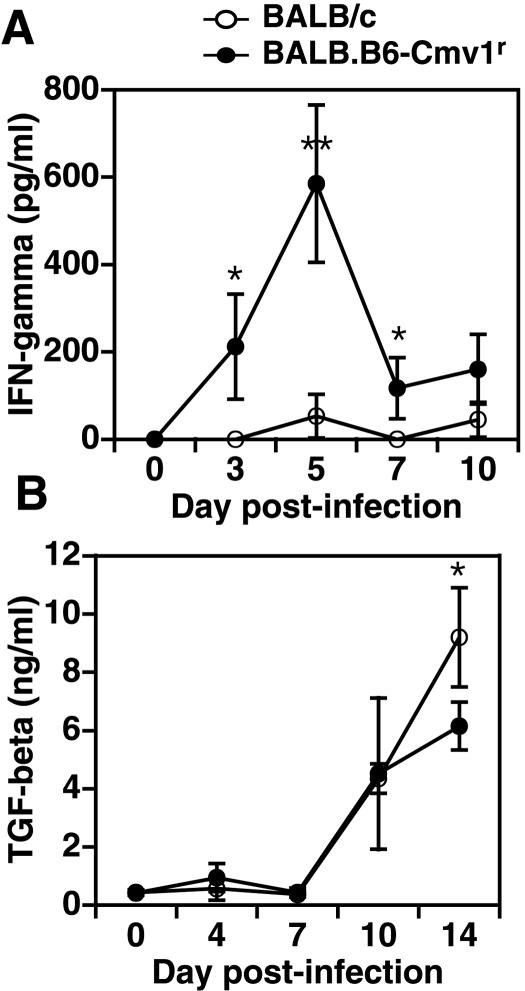
Tumor necrosis factor alpha (TNF-α) and IFN-γ are two proinflammatory cytokines described to contribute to cerebral malaria pathogenesis (20, 21). To investigate whether the NKC could regulate systemic levels of these cytokines during experimental severe malaria, sera were collected from BALB/c and BALB.B6-Cmv1r mice at different time points p.i. with P. berghei ANKA and cytokine levels were determined by ELISA. Both BALB/c and BALB.B6-Cmv1r mice had negligible levels of TNF-α in serum throughout the course of infection, indicating that the increased susceptibility observed in the congenic mice is not associated with enhanced TNF-α production (data not shown). In contrast, IFN-γ levels were significantly higher in the more susceptible BALB.B6-Cmv1r strain at days 3, 5, and 7 p.i than in the BALB/c wild-type controls (Fig. 4A). To determine whether the production of anti-inflammatory cytokines could also be influenced by the NKC genotype, the production of TGF-β was analyzed in serum samples from malaria-infected BALB/c and BALB.B6-Cmv1r mice. TGF-β was produced only during the second week of infection with P. berghei in both mouse strains. However, serum TGF-β levels were significantly lower at day 14 p.i. in the more susceptible BALB.B6-Cmv1r mice than in controls (Fig. 4B). Thus, the NKC genotype influences systemic levels of IFN-γ and TGF-β during malaria infection.
FIG. 4.
The NKC controls systemic IFN-γ and TGF-β production during malaria infection. BALB/c and BALB.B6-Cmv1r mice were infected with P. berghei ANKA. IFN-γ (A) and TGF-β (B) levels in sera collected at different time points p.i. were measured by capture ELISA. The experiment is representative of three separate infections. Each point represents the mean of three samples ± the standard error. *, P < 0.05; **, P < 0.01.
IFN-γ-regulated gene expression detected by transcriptional profiling.
Analysis of the host transcriptional response to infection could provide novel insights into the causes of severe pathogenesis, particularly when the coregulated expression of genes implicates the activation or repression of key regulatory pathways. Transcriptional analysis might eventually clarify areas of similarity and difference between humans and animal models, thereby refining the utility of the latter. Therefore, to identify IFN-γ-regulated host genes differentially expressed under the control of the NKC during malaria infection, spleens of BALB/c and BALB.B6-Cmv1r mice were taken for transcriptional analysis at 7 days p.i. We applied the Affymetrix mouse U74Av2 array, comprising 15,000 probe sets representing functionally characterized sequences (6,000) in the mouse UniGene database, plus 6,000 expressed sequence tag clusters. The intensity data for detectable transcripts from duplicate mice was very strongly correlated (day 0 BALB/c R2 = 0.925, day 7 BALB/c R2 = 0.923, day 0 BALB.B6-Cmv1r R2 = 0.934, day 7 BALB.B6-Cmv1r R2 = 0.931), revealing highly reproducible changes and suggesting a specific and directed response to malaria infection. Subjecting the microarray database to GeneSpring filter allowed the identification of a considerable number of IFN-γ-regulated or -associated genes for which mean transcript levels in the spleen at day 7 of infection differed from those at day 0 and between BALB.B6-Cmv1r and BALB/c mice (Table 1). Notable among these was Ifng (IFN-γ) itself, which was up-regulated 18-fold in BALB.B6-Cmv1r and only 4-fold in BALB/c mice. Consistent with the detection of increased IFN-γ mRNA, a number of interferon-inducible gene transcripts were more strongly increased during malaria infection in the spleens of BALB.B6-Cmv1r than in BALB/c mice. Among families of such genes were two families of GTPases, including the IFN-γ-inducible GTPases and members of the guanylate nucleotide binding protein family (Table 1). This group of proteins has been described to dominate the cellular cascade induced upon IFN-γ stimulation (6), and although their main function remains unknown, there has been evidence suggesting they are involved in defense against microorganisms and control of cell growth (19). Considerable interferon-dependent transcriptional regulation of chemokines was also apparent (Table 1). Cxcl10 (IP-10), an IFN-γ-inducible chemokine that attracts activated TH1 lymphocytes and NK cells through interaction with the CXCR3 receptor (32, 39), had increased transcripts in spleens at day 7 relative to that at resting levels (day 0), and this was more pronounced in BALB.B6-Cmv1r than BALB/c mice. The same was true of Cxcl9 (Mig), which has been shown to signal through the same receptor (31). Transcripts of the proinflammatory chemokine MIP1-α (Ccl3) were also significantly increased in BALB.B6-Cmv1r over BALB/c mice (Table 1). The expression of a number of interferon-associated genes fluctuated to an equal degree in both mouse strains (data not shown). Several interferon-associated transcripts, including the IFN-γ receptor second chain, were found to be down-regulated at this time point p.i., but more so in BALB/c than BALB.B6-Cmv1r mice (Table 1).
TABLE 1.
IFN-γ-regulated or -associated genes for which mean transcript levels in the spleen at day 7 of infection differ significantly between BALB.B6-Cmv1r and BALB/c mice
| GenBank no. | Gene | Description | Mean fold change (day 7) for:
|
|
|---|---|---|---|---|
| BALB.B6-Cmv1r mice | BALB/c mice | |||
| K00083 | Ifng | IFN-γ | 18.248 | 4.009 |
| M55544 | Gbp1 | Guanylate nucleotide binding protein 1 | 9.086 | 4.097 |
| AA914345 | Iigpa | Interferon-inducible GTPase | 8.816 | 4.804 |
| AJ007971 | Iigpa | Interferon-inducible GTPase | 6.120 | 4.250 |
| M34815 | Cxcl9 | Chemokine (C-X-C motif) ligand 9 | 5.763 | 2.589 |
| AJ007970 | Gbp2 | Guanylate nucleotide binding protein 2 | 3.925 | 2.602 |
| U53219 | Igtp | γ-IFN-induced GTPase | 3.823 | 2.708 |
| U19119 | Ifi1 | Interferon-inducible protein 1 | 3.650 | 2.504 |
| L38444 | Tgtp | T-cell-specific GTPase | 3.517 | 2.323 |
| AW047476 | Gbp3 | Guanylate nucleotide binding protein 3 | 3.400 | 2.138 |
| M33266 | Cxc110 | Chemokine (C-X-C motif) ligand 10 | 3.288 | 1.546 |
| AB019505 | Il18bp | IL-18 binding protein | 3.133 | 2.101 |
| M31419 | Ifi204 | Interferon-activated gene 204 | 3.026 | 2.080 |
| J04491 | Ccl3 | Chemokine (C-C motif) ligand 3 | 8.197 | 2.496 |
| M74123 | Ifi205 | Interferon-activated gene 205 | 0.797 | 0.311 |
| U69599 | Ifngr2 | IFN-γ receptor second chain | 0.730 | 0.449 |
| U06924 | Stat1 | Signal transducer and activator of transcription 1 | 1.733 | 0.459 |
| A1844520 | Ifi30 | IFN-γ-inducible protein 30 | 1.167 | 0.482 |
| J03368 | Mx2 | Interferon-induced Mx gene 2 | 0.879 | 0.540 |
| AF036341 | Irf3 | Interferon regulatory factor 3 | 1.066 | 0.587 |
| AV152244 | Isg15 | Interferon-stimulated protein | 1.085 | 0.588 |
| M32489 | Icsbp1 | Interferon consensus sequence binding protein 1 | 1.206 | 0.600 |
Pending.
Reduced antibody responses to malaria infection in BALB.B6-Cmv1r mice.
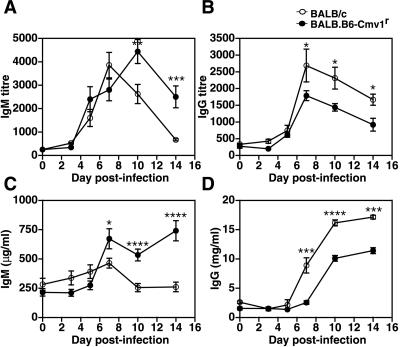
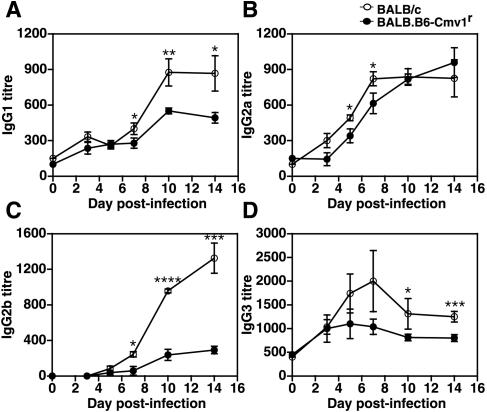
In the next series of experiments, we sought to investigate whether the NKC genotype could also affect the development of acquired immune responses to malaria parasites. To that end, BALB/c and BALB.B6-Cmv1r mice were infected with P. berghei. Serum samples were collected at different times p.i., and parasite-specific antibody responses were analyzed. The parasite-specific IgM titers were of similar magnitude in BALB/c and BALB.B6-Cmv1r mice during the first week of infection (Fig. 5A). After day 7 p.i., parasite-specific IgM antibody responses decreased in BALB/c mice but remained elevated in BALB.B6-Cmv1r animals, suggesting a delayed switch from IgM to IgG production in mice congenic for the NKC. The absolute IgM levels determined by capture ELISA followed the same kinetics as the parasite-specific responses, with increased IgM levels in BALB.B6-Cmv1r compared to that in wild-type mice (Fig. 5C). On the contrary, both P. berghei-specific IgG titers, as well as total IgG levels, were significantly higher in BALB/c wild-type animals than in BALB.B6-Cmv1r mice in the second week of infection (Fig. 5B and D). The IgG subclasses produced in response to infection were also analyzed. The parasite-specific IgG1, IgG2a, IgG2b, and IgG3 antibody titers were significantly higher in BALB/c wild-type compared to BALB.B6-Cmv1r mice (Fig. 6). Interestingly, IgG2b responses were negligible in congenic animals (Fig. 6C). Taken together, these results indicate that the NKC genotype influences parasite-specific antibody responses during malaria infection.
FIG. 5.
The antibody response to P. berghei ANKA in BALB/c and BALB.B6-Cmv1r mice. BALB/c and BALB.B6-Cmv1r mice were infected with P. berghei. Serum samples were collected at different time points p.i., and parasite-specific IgM (A) and IgG (B) antibody responses or total IgM (C) and IgG (D) contents were analyzed by ELISA as indicated in Materials and Methods. Each point represents the mean of 8 to 10 samples ± the standard error. *, P < 0.05; **, P < 0.01; ***, P < 0.005; ****, P < 0.001.
FIG. 6.
IgG subclasses produced in response to P. berghei infection in BALB/c and BALB.B6-Cmv1r mice. BALB/c and BALB.B6-Cmv1r mice were infected with P. berghei. Serum samples were collected at different time points p.i., and parasite-specific IgG1 (A), IgG2a (B), IgG2b (C), and IgG3 (D) were determined by ELISA. Each point represents the mean of 8 to 10 samples ± the standard error. *, P < 0.05; **, P < 0.01; ***, P < 0.005; ****, P < 0.001.
The NKC regulates TH1/TH2 balance during malaria infection.
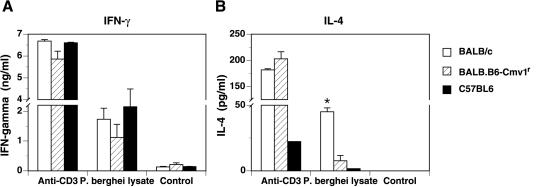
Figures 5 and 6 show that the NKC genotype can influence parasite-specific antibody responses to malaria infection. To determine whether the NKC could also influence the development of T-cell-mediated immune responses, BALB/c, BALB.B6-Cmv1r, and C57BL/6 mice were infected with P. berghei ANKA. At day 5 p.i, the animals were drug cured as described in Materials and Methods, and 1 week later, we examined cytokine production by splenocytes from malaria-infected mice. Spleen cells from BALB/c, BALB.B6-Cmv1r, and C57BL/6 mice produced similar levels of IFN-γ in response to both anti-CD3 antibody and P. berghei lysate (Fig. 7A). Anti-CD3-mediated IL-4 responses were of similar magnitude in spleen cells from BALB/c and BALB.B6-Cmv1r animals, although they were reduced in C57BL/6 mice (Fig. 7B). Splenocytes from malaria-infected BALB/c mice also produced substantial levels of IL-4 in response to a parasite-specific stimulus. In contrast, cells from infected C57BL/6 as well as BALB.B6-Cmv1r mice were virtually unable to secrete this cytokine in response to P. berghei lysate (Fig. 7B), suggesting a lack of parasite-specific memory cells producing IL-4 in these mouse strains. Thus the NKC genotype influences the development of parasite-specific TH1/TH2 responses in response to P. berghei infection.
FIG. 7.
The NKC influences the TH1/TH2 polarization in response to malaria. Spleen cells from P. berghei ANKA-infected C57BL/6, BALB/c, and BALB.B6-Cmv1r mice were stimulated for 3 days with P. berghei ANKA total lysate or anti-CD3 antibody. Cells cultured in medium alone were used as background controls. IFN-γ and IL-4 production was determined in cell culture supernatant by capture ELISA. The experiment is representative of three separate infections. Each point represents the mean of three samples ± the standard error. *, P < 0.01.
DISCUSSION
This study shows that the NKC is a significant genetic determinant of murine severe malarial pathogenesis. C57BL/6 NKC alleles appear to be associated with susceptibility to disease, as congenic BALB.B6-Cmv1r mice showed increased cerebral pathology, pulmonary edema, severe anemia, and death rates during P. berghei infection (22) compared to fully resistant BALB/c wild-type controls. The increased severity to malarial disease in mice congenic for the C57BL/6 NKC does not seem to result from higher parasite burdens, as parasitemia levels throughout infection were virtually identical in both BALB/c and BALB.B6-Cmv1r mice. Rather than changes in parasite growth rates, susceptibility to severe malaria in congenic animals correlated with a differential immune response to infection, mainly characterized by a significantly enhanced production of IFN-γ.
A large body of evidence indicates that IFN-γ plays a central role in cerebral malaria pathogenesis in mouse infection models as well as in humans (26). High IFN-γ levels were found in Southeast Asian (25) as well as African patients (40) with acute malaria infections. Heterozygosis for an IFN-γ receptor-1 polymorphism was found to be associated with lower incidence and death resulting from cerebral malaria in Gambian children (30). In the rodent model of cerebral malaria, it was found that passive transfer of anti-IFN-γ neutralizing antibodies protects susceptible mice from the development of P. berghei-mediated cerebral malaria and reduces the production of TNF-α, another proinflammatory cytokine associated with disease severity (21). IFN-γ−/− (49) as well as IFN-γ receptor α-chain-deficient mice (1) have also been found to be resistant to cerebral malaria. It is interesting to note that neutralization of IFN-γ with monoclonal antibodies as well as genetic deletion of IFN-γ receptor in mice reduced cerebral malaria severity without affecting parasitemia levels throughout infection. These results are in agreement with our observations here in which mutant mice bearing the C57BL/6 NKC on a BALB/c background (which leads to enhanced IFN-γ responses in this case) resulted in increased severity of disease by a mechanism independent of parasite growth rates.
The NKC was found to regulate not only cerebral malaria but also other disease syndromes such as pulmonary edema and severe anemia. The etiology of these disorders is not fully understood. Several mechanisms have been postulated to contribute to malarial anemia, including erythrocyte destruction, phagocytosis, sequestration of infected red blood cells, and inhibition of erythropoiesis resulting from the action of proinflammatory cytokines produced in response to infection (34). Because IFN-γ responses to P. berghei are elevated in BALB.B6-Cmv1r mice, it is reasonable to postulate that the NKC involvement in malarial anemia results from hematopoiesis inhibition due to exacerbated proinflammatory responses to infection. We are currently investigating the effect of cytokines produced by different cell lineages in both acute and chronic rodent models of malarial anemia.
The differential patterns of transcription among parental and NKC congenic mice detected here by microarray analysis are consistent with a predominant role for polymorphic NKC loci in regulating IFN-γ-dependent expression profiles for a number of genes. Overall, the majority of genes fluctuated equally in both mouse strains, including a number of interferon-associated genes. The single time point taken for analysis in this study may not be sufficient to capture all relevant fluctuations in gene expression. Of approximately 30,000 mouse genes (99% having homologues in humans), we observed only a small minority of gene transcripts that, in dependence on NKC haplotype, fluctuated differentially in response to malaria infection. Although there are limitations in the transcriptome approach in that no quantitative relationship between mRNA levels and function is guaranteed, our data are overall consistent with differential patterns in gene expression contributing significantly to the NKC dependency of severe malaria syndromes such as cerebral disease, pulmonary edema, and the inflammatory cascade. Identification of the most important genes of the mouse influencing susceptibility and resistance to malaria could speed the identification of parallel genes in humans.
The present study uses congenic mice to establish one or more NKC loci exerting 50% penetrance in the control of P. berghei-induced pathogenesis and fatality. This partial penetrance might reflect the contribution of loci other than the NKC in promoting disease in the fully susceptible C57BL/6 background. However, the NKC encodes several different receptors with opposing activities in the control of innate immune responses (50). It is therefore possible that not all genes contained in the congenic interval present in BALB.B6-Cmv1r mice contribute to disease induction. We are currently investigating that hypothesis, utilizing intracongenic recombinant mice (41) derived from the BALB.B6-Cmv1r strain and bearing smaller NKC congenic intervals.
Because the NKC comprises several genes and multigene families, the question still remains which NKC receptor(s) is responsible for susceptibility to severe malaria. Several activation receptors encoded within the murine NKC have been described to elicit IFN-γ secretion by NK and/or NK T cells upon stimulation with their ligands and are therefore candidates to mediate disease induction. Some of those receptors include Ly49D (33), Ly49H (17), NK1.1 (4), and NKG2D (24). NK1.1 and NKG2D (but not the Ly49 activatory molecules) are expressed on both NK cells and CD1-restricted NK T cells. In previous studies, we found that administration of anti-asialo GM1 antibody (which depletes NK cells without affecting NK T cells) does not reverse the susceptibility of BALB.B6-Cmv1r mice to malaria (22), suggesting that at least in this mouse strain, expression of NKC receptors in the remaining NK-T-cell population is sufficient to induce fatalities. It has been shown that cross-linking of NK1.1 preferentially induces IFN-γ (4) and no IL-4 production by CD1-restricted NK T cells, raising the possibility that activation of this NKC receptor in NK T cells is involved in the up-regulation of proinflammatory responses by a TCR-independent pathway and contributes to the increased susceptibility to malaria found here. Although NK cells did not appear to be essential to induce fatality in malaria-infected BALB.B6-Cmv1r mice (22), with the present data, we cannot exclude the possibility that IFN-γ production by activated NK cells could have an impact in the overall immunological status of BALB.B6-Cmv1r mice, influencing parasite-specific antibody responses and the TH1/TH2 balance in response to infection. In fact, activated NK T cells have been shown to stimulate NK cells to proliferate (18) and secrete IFN-γ (12). Moreover, recent in vitro studies indicate that human NK cells produce IFN-γ in response to P. falciparum-infected red blood cells (5). We are currently investigating the effect of NK cells and their NKC-encoded receptors in mouse strains other than BALB.B6-Cmv1r.
Genes encoded within the NKC are known to regulate the innate immune system through the control of NK and NK-T-cell function. In this study, we found that acquired immune responses to malaria were also influenced by the NKC since, unlike BALB/c wild-type controls, in both C57BL/6 and BALB.B6-Cmv1r mice, P. berghei infection generated only memory cells able to secrete IFN-γ but not IL-4 upon parasite-specific in vitro restimulation. Genes encoded within the C57BL/6 NKC appear therefore to favor the development of TH1 responses to infection. Because of their capacity to secrete large amounts of immunoregulatory cytokines with rapid kinetics, NK T cells have been postulated to influence TH1/TH2 differentiation of the acquired immune system (38). We have previously shown that α-galactosylceramide-stimulated NK T cells from malaria-infected BALB.B6-Cmv1r mice but not from BALB/c wild-type mice preferentially produce IFN-γ, demonstrating that expression of C57BL/6 NKC markers predisposes NK T cells to increased IFN-γ production (22). Therefore, it is reasonable to postulate that early IFN-γ secretion by NK T cells bearing C57BL/6 NKC receptors might have an impact in the downstream development of TH1 responses to infection.
Consistent with poor IL-4 production and a TH1-biased immune response to infection, BALB.B6-Cmv1r animals displayed a delayed switch from IgM to IgG production and substantially reduced parasite-specific antibody responses compared to BALB/c wild-type mice. Particularly, IgG2b responses were virtually absent in BALB.B6-Cmv1r mice. Switching to IgG2b production has been shown to be enhanced by TGF-β (35, 44). Interestingly, systemic TGF-β levels were substantially reduced in BALB.B6-Cmv1r mice compared to BALB/c controls. In previous studies, we found that CD1-restricted NK T cells from BALB/c wild-type mice enhance splenic B-cell expansion and parasite-specific antibody responses to infection. The increased B-cell-mediated response correlated with the ability of BALB/c NK T cells to influence the immune response towards TH2 (23). Consistent with those observations, we found here that the introduction of C57BL/6 NKC genes into the BALB/c strain, which results in an increased TH1 bias and susceptibility to severe malaria, leads to a down-regulated antibody response to infection.
About 2.5 million people die of P. falciparum-mediated malaria every year (48). In spite of the important public health problem, many cellular, immunological, and genetic aspects of malarial pathogenesis are not fully understood. The relevance of the P. berghei infection model for human disease is being increasingly appreciated (16, 37). Using this infection model, we established that polymorphisms within NKC loci regulate malarial pathogenesis and acquired immune responses to P. berghei. To date, NKC receptors were mainly described to regulate viral infections or cancers through their interaction with MHC-I or MHC-I ligands. Malaria parasites infect red blood cells, which lack MHC-I molecules, and our data provide evidence for critical NKC involvement in immunological responses to a nonviral infectious agent. Because the NKC is conserved among species, with syntenic regions identified in rat and human chromosomes, these findings might be relevant to the etiology of human fatal malaria. Further work is still necessary to establish whether NKC receptors are associated with malaria disease severity in human populations.
Acknowledgments
This work was supported by NIH grant AI-45548, the NH&MRC, the UNDP/World Bank/WHO TDR program, and the HFSP. L.S. is an International Research Scholar of the Howard Hughes Medical Institute.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Amani, V., A. M. Vigario, E. Belnoue, M. Marussig, L. Fonseca, D. Mazier, and L. Renia. 2000. Involvement of IFN-gamma receptor-mediated signaling in pathology and anti-malarial immunity induced by Plasmodium berghei infection. Eur. J. Immunol. 30:1646-1655. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, S. K., J. R. Ortaldo, and D. W. McVicar. 2001. The ever-expanding Ly49 gene family: repertoire and signaling. Immunol. Rev. 181:79-89. [DOI] [PubMed] [Google Scholar]
- 3.Arase, H., N. Arase, K. Ogasawara, R. A. Good, and K. Onoe. 1992. An NK1.1+ CD4+8− single-positive thymocyte subpopulation that expresses a highly skewed T-cell antigen receptor V beta family. Proc. Natl. Acad. Sci. USA 89:6506-6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arase, H., N. Arase, and T. Saito. 1996. Interferon gamma production by natural killer (NK) cells and NK1.1+ T cells upon NKR-P1 cross-linking. J. Exp. Med. 183:2391-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Artavanis-Tsakonas, K., and E. M. Riley. 2002. Innate immune response to malaria: rapid induction of IFN-gamma from human NK cells by live Plasmodium falciparum-infected erythrocytes. J. Immunol. 169:2956-2963. [DOI] [PubMed] [Google Scholar]
- 6.Boehm, U., L. Guethlein, T. Klamp, K. Ozbek, A. Schaub, A. Futterer, K. Pfeffer, and J. C. Howard. 1998. Two families of GTPases dominate the complex cellular response to IFN-gamma. J. Immunol. 161:6715-6723. [PubMed] [Google Scholar]
- 7.Brennan, J., D. Mager, W. Jefferies, and F. Takei. 1994. Expression of different members of the Ly-49 gene family defines distinct natural killer cell subsets and cell adhesion properties. J. Exp. Med. 180:2287-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan, J., F. Takei, S. Wong, and D. L. Mager. 1995. Carbohydrate recognition by a natural killer cell receptor, Ly-49C. J. Biol. Chem. 270:9691-9694. [DOI] [PubMed] [Google Scholar]
- 9.Brown, M. G., A. A. Scalzo, K. Matsumoto, and W. M. Yokoyama. 1997. The natural killer gene complex: a genetic basis for understanding natural killer cell function and innate immunity. Immunol. Rev. 155:53-65. [DOI] [PubMed] [Google Scholar]
- 10.Brown, M. G., A. A. Scalzo, L. R. Stone, P. Y. Clark, Y. Du, B. Palanca, and W. M. Yokoyama. 2001. Natural killer gene complex (Nkc) allelic variability in inbred mice: evidence for Nkc haplotypes. Immunogenetics 53:584-591. [DOI] [PubMed] [Google Scholar]
- 11.Brownstein, D. G., and L. Gras. 1997. Differential pathogenesis of lethal mousepox in congenic DBA/2 mice implicates natural killer cell receptor NKR-P1 in necrotizing hepatitis and the fifth component of complement in recruitment of circulating leukocytes to spleen. Am. J. Pathol. 150:1407-1420. [PMC free article] [PubMed] [Google Scholar]
- 12.Carnaud, C., D. Lee, O. Donnars, S.-H. Park, A. Beavis, Y. Koezuka, and A. Bendelac. 1999. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J. Immunol. 163:4647-4650. [PubMed] [Google Scholar]
- 13.Chang, W.-L., S. P. Jones, D. J. Lefer, T. Welbourne, G. Sun, L. Yin, H. Suzuki, J. Huang, D. N. Granger, and H. C. van der Heyde. 2001. CD8+-T-cell depletion ameliorates circulatory shock in Plasmodium berghei-infected mice. Infect. Immun. 69:7341-7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Kossodo, S., and G. E. Grau. 1993. Profiles of cytokine production in relation with susceptibility to cerebral malaria. J. Immunol. 151:4811-4820. [PubMed] [Google Scholar]
- 15.Delano, M. L., and D. G. Brownstein. 1995. Innate resistance to lethal mousepox is genetically linked to the NK gene complex on chromosome 6 and correlates with early restriction of virus replication by cells with an NK phenotype. J. Virol. 69:5875-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Souza, J. B., and E. M. Riley. 2002. Cerebral malaria: the contribution of studies in animal models to our understanding of immunopathogenesis. Microbes Infect. 4:291-300. [DOI] [PubMed] [Google Scholar]
- 17.Dokun, A. O., S. Kim, H. R. Smith, H. S. Kang, D. T. Chu, and W. M. Yokoyama. 2001. Specific and nonspecific NK cell activation during virus infection. Nat. Immunol. 2:951-956. [DOI] [PubMed] [Google Scholar]
- 18.Eberl, G., and H. R. MacDonald. 2000. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur. J. Immunol. 30:985-992. [DOI] [PubMed] [Google Scholar]
- 19.Gorbacheva, V. Y., D. Lindner, G. C. Sen, and D. J. Vestal. 2002. The interferon (IFN)-induced GTPase, mGBP-2. Role in IFN-gamma-induced murine fibroblast proliferation. J. Biol. Chem. 277:6080-6087. [DOI] [PubMed] [Google Scholar]
- 20.Grau, G. E., L. F. Fajardo, P. F. Piguet, B. Allet, P. H. Lambert, and P. Vassalli. 1987. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science 237:1210-1212. [DOI] [PubMed] [Google Scholar]
- 21.Grau, G. E., H. Heremans, P. F. Piguet, P. Pointaire, P. H. Lambert, A. Billiau, and P. Vassalli. 1989. Monoclonal antibody against interferon gamma can prevent experimental cerebral malaria and its associated overproduction of tumor necrosis factor. Proc. Natl. Acad. Sci. USA 86:5572-5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen, D. S., M. A. Siomos, L. Buckingham, A. A. Scalzo, and L. Schofield. 2003. Regulation of murine cerebral malaria pathogenesis by CD1d-restricted NKT cells and the natural killer complex. Immunity 18:391-402. [DOI] [PubMed] [Google Scholar]
- 23.Hansen, D. S., M. A. Siomos, T. F. de Koning-Ward, L. Buckingham, B. S. Crabb, and L. Schofield. 2003. CD1d-restricted NKT cells contribute to malarial splenomegaly and enhance parasite-specific antibody responses. Eur. J. Immunol. 33:2588-2598. [DOI] [PubMed] [Google Scholar]
- 24.Ho, E. L., L. N. Carayannopoulos, J. Poursine-Laurent, J. Kinder, B. Plougastel, H. R. Smith, and W. M. Yokoyama. 2002. Costimulation of multiple NK cell activation receptors by NKG2D. J. Immunol. 169:3667-3675. [DOI] [PubMed] [Google Scholar]
- 25.Ho, M., M. M. Sexton, P. Tongtawe, S. Looareesuwan, P. Suntharasamai, and H. K. Webster. 1995. Interleukin-10 inhibits tumor necrosis factor production but not antigen-specific lymphoproliferation in acute Plasmodium falciparum malaria. J. Infect. Dis. 172:838-844. [DOI] [PubMed] [Google Scholar]
- 26.Hunt, N. H., and G. E. Grau. 2003. Cytokines: accelerators and brakes in the pathogenesis of cerebral malaria. Trends Immunol. 24:491-499. [DOI] [PubMed] [Google Scholar]
- 27.Idris, A. H., K. Iizuka, H. R. Smith, A. A. Scalzo, and W. M. Yokoyama. 1998. Genetic control of natural killing and in vivo tumor elimination by the Chok locus. J. Exp. Med. 188:2243-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Idris, A. H., H. R. Smith, L. H. Mason, J. R. Ortaldo, A. A. Scalzo, and W. M. Yokoyama. 1999. The natural killer gene complex genetic locus Chok encodes Ly-49D, a target recognition receptor that activates natural killing. Proc. Natl. Acad. Sci. USA 96:6330-6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kärre, K. 2002. NK cells, MHC class I molecules and the missing self. Scand. J. Immunol. 55:221-228. [DOI] [PubMed] [Google Scholar]
- 30.Koch, O., A. Awomoyi, S. Usen, M. Jallow, A. Richardson, J. Hull, M. Pinder, M. Newport, and D. Kwiatkowski. 2002. IFNGR1 gene promoter polymorphisms and susceptibility to cerebral malaria. J. Infect. Dis. 185:1684-1687. [DOI] [PubMed] [Google Scholar]
- 31.Loetscher, M., B. Gerber, P. Loetscher, S. A. Jones, L. Piali, I. Clark-Lewis, M. Baggiolini, and B. Moser. 1996. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J. Exp. Med. 184:963-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loetscher, M., P. Loetscher, N. Brass, E. Meese, and B. Moser. 1998. Lymphocyte-specific chemokine receptor CXCR3: regulation, chemokine binding and gene localization. Eur. J. Immunol. 28:3696-3705. [DOI] [PubMed] [Google Scholar]
- 33.Mason, L. H., J. Willette-Brown, A. T. Mason, D. McVicar, and J. R. Ortaldo. 2000. Interaction of Ly-49D+ NK cells with H-2Dd target cells leads to Dap-12 phosphorylation and IFN-gamma secretion. J. Immunol. 164:603-611. [DOI] [PubMed] [Google Scholar]
- 34.McDevitt, M. A., J. Xie, V. Gordeuk, and R. Bucala. 2004. The anemia of malaria infection: role of inflammatory cytokines. Curr. Hematol. Rep. 3:97-106. [PubMed] [Google Scholar]
- 35.McIntyre, T. M., D. R. Klinman, P. Rothman, M. Lugo, J. R. Dasch, J. J. Mond, and C. M. Snapper. 1993. Transforming growth factor beta 1 selectivity stimulates immunoglobulin G2b secretion by lipopolysaccharide-activated murine B cells. J. Exp. Med. 177:1031-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehta, I. K., J. Wang, J. Roland, D. H. Margulies, and W. M. Yokoyama. 2001. Ly49A allelic variation and MHC class I specificity. Immunogenetics 53:572-583. [DOI] [PubMed] [Google Scholar]
- 37.Miller, L. H., D. I. Baruch, K. Marsh, and O. K. Doumbo. 2002. The pathogenic basis of malaria. Nature 415:673-679. [DOI] [PubMed] [Google Scholar]
- 38.Porcelli, S. A. 1995. The CD1 family: a third lineage of antigen-presenting molecules. Adv. Immunol. 59:1-98. [DOI] [PubMed] [Google Scholar]
- 39.Qin, S., J. B. Rottman, P. Myers, N. Kassam, M. Weinblatt, M. Loetscher, A. E. Koch, B. Moser, and C. R. Mackay. 1998. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J. Clin. Investig. 101:746-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ringwald, P., F. Peyron, J. P. Vuillez, J. E. Touze, J. Le Bras, and P. Deloron. 1991. Levels of cytokines in plasma during Plasmodium falciparum malaria attacks. J. Clin. Microbiol. 29:2076-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scalzo, A. A., M. G. Brown, D. T. Chu, J. W. Heusel, W. M. Yokoyama, and C. A. Forbes. 1999. Development of intra-natural killer complex (NKC) recombinant and congenic mouse strains for mapping and functional analysis of NK cell regulatory loci. Immunogenetics 49:238-241. [DOI] [PubMed] [Google Scholar]
- 42.Scalzo, A. A., P. A. Lyons, N. A. Fitzgerald, C. A. Forbes, W. M. Yokoyama, and G. R. Shellam. 1995. Genetic mapping of Cmv1 in the region of mouse chromosome 6 encoding the NK gene complex-associated loci Ly49 and musNKR-P1. Genomics 27:435-441. [DOI] [PubMed] [Google Scholar]
- 43.Schofield, L., M. C. Hewitt, K. Evans, M. A. Siomos, and P. H. Seeberger. 2002. Synthetic GPI as a candidate anti-toxic vaccine in a model of malaria. Nature 418:785-789. [DOI] [PubMed] [Google Scholar]
- 44.Stavnezer, J. 1995. Regulation of antibody production and class switching by TGF-beta. J. Immunol. 155:1647-1651.7636223 [Google Scholar]
- 45.Vance, R. E., A. M. Jamieson, and D. H. Raulet. 1999. Recognition of the class Ib molecule Qa-1(b) by putative activating receptors CD94/NKG2C and CD94/NKG2E on mouse natural killer cells. J. Exp. Med. 190:1801-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vance, R. E., D. M. Tanamachi, T. Hanke, and D. H. Raulet. 1997. Cloning of a mouse homolog of CD94 extends the family of C-type lectins on murine natural killer cells. Eur. J. Immunol. 27:3236-3241. [DOI] [PubMed] [Google Scholar]
- 47.White, N. J., and M. Ho. 1992. The pathophysiology of malaria. Adv. Parasitol. 31:83-173. [DOI] [PubMed] [Google Scholar]
- 48.W.H.O. 1992. World malaria situation 1990. Division of Control of Tropical Diseases. World Health Organization, Geneva. World Health Stat. Q 45:257-266. [PubMed] [Google Scholar]
- 49.Yanez, D. M., D. D. Manning, A. J. Cooley, W. P. Weidanz, and H. C. van der Heyde. 1996. Participation of lymphocyte subpopulations in the pathogenesis of experimental murine cerebral malaria. J. Immunol. 157:1620-1624. [PubMed] [Google Scholar]
- 50.Yokoyama, W. M., and B. F. Plougastel. 2003. Immune functions encoded by the natural killer gene complex. Nat. Rev. Immunol. 3:304-316. [DOI] [PubMed] [Google Scholar]