Abstract
Background
Early in the coronavirus disease 2019 (COVID-19) pandemic, peak viral loads coincided with symptom onset. We hypothesized that in a highly immune population, symptom onset might occur earlier in infection, coinciding with lower viral loads.
Methods
We assessed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and influenza A viral loads relative to symptom duration in symptomatic adults (≥16 years) presenting for testing in Georgia (4/2022–4/2023; Omicron variant predominant). Participants provided symptom duration and recent testing history. Nasal swabs were tested by Xpert Xpress SARS-CoV-2/Flu/RSV assay and cycle threshold (Ct) values recorded. Nucleoprotein concentrations in SARS-CoV-2 polymerase chain reaction (PCR)-positive samples were measured by single molecule array. To estimate hypothetical antigen rapid diagnostic test (Ag RDT) sensitivity on each day after symptom onset, percentages of individuals with Ct value ≤30 or ≤25 were calculated.
Results
Of 348 newly-diagnosed SARS-CoV-2 PCR-positive individuals (65.5% women, median 39.2 years), 317/348 (91.1%) had a history of vaccination, natural infection, or both. By both Ct value and antigen concentration measurements, median viral loads rose from the day of symptom onset and peaked on the fourth/fifth day. Ag RDT sensitivity estimates were 30.0%–60.0% on the first day, 59.2%–74.8% on the third day, and 80.0%–93.3% on the fourth day of symptoms.
In 74 influenza A PCR-positive individuals (55.4% women; median 35.0 years), median influenza viral loads peaked on the second day of symptoms.
Conclusions
In a highly immune adult population, median SARS-CoV-2 viral loads peaked around the fourth day of symptoms. Influenza A viral loads peaked soon after symptom onset. These findings have implications for ongoing use of Ag RDTs for COVID-19 and influenza.
Keywords: SARS-CoV-2, COVID-19, symptom, viral load, kinetics
In a highly immune adult population, median severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral loads by cycle threshold and antigen measurements peaked around the fourth day of symptoms, with implications for testing practice. In contrast, viral loads for influenza A peaked soon after symptom onset.
Graphical Abstract
Graphical Abstract.
This graphical abstract is also available at Tidbit: https://tidbitapp.io/tidbits/the-new-normal-delayed-peak-sars-cov-2-viral-loads-relative-to-symptom-onset-and-implications-for-covid-19-testing-programs-a6c0d9ff-2c70-4d88-93fb-2bb9c5c24714/update
Early in the coronavirus disease 2019 (COVID-19) pandemic, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral loads were observed to peak at the onset of symptoms, and steadily decrease thereafter [1–4]. Subsequently, multiple evaluations of the first commercially-available antigen rapid diagnostic tests (Ag RDTs) indicated that viral loads in symptomatic adults within the first 7 days of symptoms were reliably high enough to be detected in most individuals by the Ag RDTs with strongest performance [5]. Accordingly, initial Food and Drug Administration (FDA)-approved instructions for use of Ag RDTs utilized only a single test in symptomatic individuals within the first week of symptoms. Early Ag RDT deployment across the United States similarly utilized only one test per symptomatic individual, though the utility of backup molecular testing in those with negative Ag RDT results was simultaneously emphasized [6].
More recently, and particularly during the SARS-CoV-2 Omicron variant surge in early 2022—which overlapped with markedly increased consumer access to home Ag RDTs–increased uncertainty emerged regarding the negative predictive value of Ag RDTs in newly symptomatic individuals and how to guide consumers now in charge of interpretation of their own testing results. Multiple investigations focused on the analytic capability of Ag RDTs to detect the Omicron variant, which appeared to be similar to earlier variants [7]. Omicron viral loads were shown to be only slightly lower on average than Delta viral loads [8, 9], and the clinical sensitivity of Ag RDTs (vs polymerase chain reaction [PCR] testing) for Delta versus Omicron variants was also shown to be similar [10]. In parallel, studies of home Ag RDT performance documented improvement of sensitivity with serial testing [11] and in November 2022, the FDA adjusted recommendations for home Ag RDT use to include guidance to repeat testing in symptomatic individuals 48 hours after an initial negative test, for a total of at least 2 tests [12].
Despite the shift over time toward use of serial Ag RDTs in symptomatic individuals to exclude COVID-19, the reasons for the apparent change in clinical performance of Ag RDTs in symptomatic individuals between early and late in the pandemic have not been a major focus of investigation. Although there has been much speculation that symptom onset might now be occurring earlier in the course of infection (coinciding with lower viral loads) due to acquired immunity, the current relationship between symptom duration and peak viral load in a highly immune population must be understood in detail to guide Ag RDT testing practice going forward. We therefore sought to assess SARS-CoV-2 viral loads (by PCR cycle threshold [Ct] value and directly-measured antigen concentrations) relative to symptom duration in symptomatic PCR-positive individuals presenting for COVID-19 testing in the past year (after the early 2022 Omicron surge). The use of a multiplexed PCR assay over this timeframe allowed us to also assess the relationship between viral load and symptom duration for influenza A.
METHODS
The Atlanta Center for Microsystems Engineered Point-of-Care Technologies (ACME-POCT) network utilized hospital and community-based COVID-19 testing centers to enroll participants from 1 April 2022 to 13 April 2023 for evaluation of novel viral diagnostic tests under development. Eligible participants were identified consecutively at each study site. Inclusion criteria for the parent study included any person seeking testing for upper respiratory infection. Exclusion criteria for the parent study included patients who were unable to tolerate a nasal swab or were unable to provide informed consent. Following identification, eligible participants for the parent study were approached by study personnel, who obtained informed consent. Clinical electronic case report forms were used to collect demographic and clinical variables, including age, sex, race, COVID-19-related symptoms and date of symptom onset, any COVID-19 testing in prior 14 days (and specifically any positive testing within 14 days or negative testing within 2 days; data on test method or testing date were not collected), current health conditions, and SARS-CoV-2 exposure, prior infection, and vaccine status. Clinical and demographic variables were collected in a centralized, web-based database (REDCap, Nashville, Tennessee. USA). The study protocol was approved by the Emory Institutional Review Board, Children's Healthcare of Atlanta, and the Grady Research Oversight Committee. For all participants, an FDA-approved reverse transcription polymerase chain reaction (RT-PCR) SARS-CoV-2 test was administered within 24 hours of study enrollment (anterior nasal swab sample collected with a flocked swab [PurFlock Ultra® Dry Transport System 25-3606-U BT] and diluted in 3.0 mL saline). For this analysis, only data from patients tested with the Cepheid GeneXpert Xpress SARS-CoV-2/Flu/RSV RT-PCR assay (Cepheid, Sunnyvale, California, USA) were used. Additional inclusion criteria for our analysis were the presence of symptoms consistent with COVID-19 infection at time of testing, age 16 years or older, and positive results of PCR testing for either SARS-CoV-2 or influenza A. For our analysis, participants were subdivided into those who had tested positive for SARS-CoV-2 in the 2 weeks prior to enrollment and those who had not. Exclusion criteria for this analysis included missing data for the date of symptom onset, vaccine status, or prior infection status, and patients with co-detection of SARS-CoV-2 and influenza A or B.
The sample collection date and the self-reported date of symptom onset were used to calculate the number of days of symptoms the patient had experienced at the time of sample collection (with the first day of symptoms designated as day 0). For 1 individual, a sample collection date had not been recorded, and the date of consent was used instead.
See Supplementary Methods for details of RT-PCR testing and nucleoprotein antigen concentration measurements.
Days since symptom onset and Ct values or log transformed antigen concentrations were plotted using box-and-whisker plots to represent median/interquartile range (IQR). Counts of each symptom present were plotted using stacked bar charts. To estimate the hypothetical performance of Ag RDTs in this population, we also calculated the percentage of participants with a Ct value ≤30 or ≤25. All plots and tables were generated using the ggplot2, dplyr, and r2rtf packages in R Statistical Software (v4.2.0; R Core Team 2023; Vienna, Austria) [13–15].
RESULTS
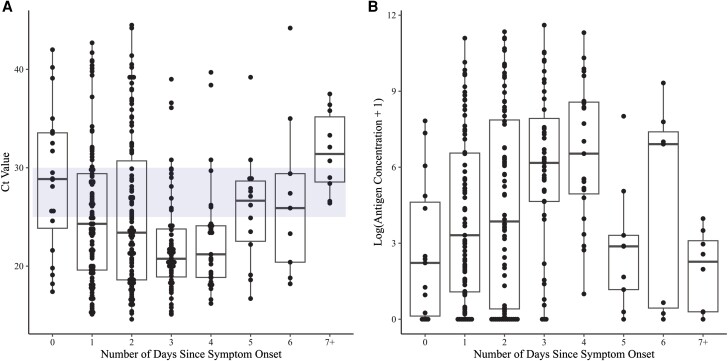
There were 348 participants that met our inclusion criteria for analysis and had not had any positive testing for COVID-19 in the 14 days prior to enrollment; 65.5% were women and the median age was 39.2 years. Of the 348 individuals, 317 (91.1%) had a history of SARS-CoV-2 vaccination, natural infection, or both, confirming a high level of SARS-CoV-2 immunity in the study population. Of the 348 participants, 89 (25.6%) reported COVID-19 testing in the prior 14 days (62/89 [69.7%] reported testing negative in the prior 2 days), 144 (41.4%) reported no testing within 14 days, and 115 (33.0%) were missing data for prior testing. Supplementary Figure 1 plots the symptom distribution in these 348 participants; the symptoms with the highest prevalence were cough (81.3%), sore throat (79.3%), and rhinorrhea/runny nose/congestion (74.7%). Figure 1A shows the SARS-CoV-2 Ct values from testing of nasal swab samples from these symptomatic PCR-positive participants plotted by the duration of symptoms at the time the sample for PCR was collected (day 0 = the first day of symptoms). Median Ct values hit their nadir (consistent with peak viral loads) on the fourth/fifth day of symptoms and then trended up on the following days. Plotting of paired antigen concentrations (measured in the same samples that received PCR testing; Methods) confirmed the same pattern, with median antigen concentrations rising from the day of symptom onset and peaking on the fourth/fifth day of symptoms (Figure 1B).
Figure 1.
SARS-CoV-2 Ct values (A) and paired log-transformed antigen concentrations (B) measured in anterior nasal swab samples plotted by days since symptom onset for PCR-positive symptomatic adults (day 0 = the first day of symptoms). The blue bar in (A) outlines the window between Ct of 30 and Ct of 25. Abbreviations: Ct, Cycle threshold; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
To estimate the percentage of PCR-positive individuals who might be expected to test positive by Ag RDT on each day after symptom onset, the percentage of participants with a Ct value ≤ 30 (a threshold chosen to represent the most sensitive Ag RDTs commercially available, based on internal data [16]) versus ≤ 25 (representing the least sensitive Ag RDTs available) on each day were calculated (Table 1; see also blue shading, Figure 1A). On the first day of symptoms, sensitivity of rapid testing (compared to PCR) was estimated to range from 30.0% to 60.0%, and on the fourth day of symptoms, from 80.0% to 93.3% (Table 1). Estimated sensitivity on the third day of symptoms (notably, corresponding to the timing of the FDA recommendation to repeat testing 48 hours after an initial negative test, [12]) was 59.2%–74.8%. For all participants within the first 7 days of symptoms, sensitivities ranged from 60.4% to 80.8%.
Table 1.
SARS-CoV-2 Cycle Threshold (Ct) Values, Number/Percent of Samples With Ct Values No More Than Ct 25 or Ct 30, and Nucleoprotein Antigen Concentrations, Grouped by Days Since Symptom Onset
| Days Since Symptom Onset | Ct, Median (IQR) | Ct ≤ 25, n (%) | Ct ≤ 30, n (%) | Ag Conc (pg/mL), Median (IQR), n |
|---|---|---|---|---|
| 0, n = 20 | 28.9 (23.9–33.5) | 6 (30) | 12 (60) | 8.2 (0.1–103.6), n = 15 |
| 1, n = 101 | 24.3 (19.6–29.4) | 54 (53.5) | 81 (80.2) | 26.6 (1.9–704.2), n = 79 |
| 2, n = 103 | 23.4 (18.6–30.7) | 61 (59.2) | 77 (74.8) | 46.6 (0.5–2614.4), n = 80 |
| 3, n = 60 | 20.8 (18.9–23.8) | 48 (80) | 56 (93.3) | 477.7 (103.6–2937.1), n = 43 |
| 4, n = 31 | 21.2 (18.9–24.1) | 25 (80.6) | 28 (90.3) | 688.5 (141.5–5270.8), n = 23 |
| 5, n = 14 | 26.6 (22.5–28.6) | 6 (42.9) | 12 (85.7) | 16.7 (2.2–26.5), n = 9 |
| 6, n = 9 | 25.9 (20.4–29.4) | 4 (44.4) | 7 (77.8) | 1002.1 (0.6–1756.4), n = 7 |
| 0–6, n = 338 | 23.4 (19.2–28.9) | 204 (60.4) | 273 (80.8) | 120 (2.8–1801), n = 256 |
| 7+, n = 10 | 31.4 (28.5–35.2) | 0 (0) | 4 (40) | 9.1 (0.3–21.8), n = 8 |
Abbreviations: Ag, antigen; Conc, concentration; Ct, cycle threshold; IQR, interquartile range.
For comparison, a similar analysis was performed in 273 individuals who reported testing positive on a COVID-19 test (by any method) in the 14 days prior to study enrollment. Of this cohort, 62.3% were women, the median age was 43.2, and 87.5% had a history of vaccination, natural infection, or both. By both Ct value and antigen concentration measurements, viral loads in this cohort peaked on the third/fourth days of symptoms (Supplementary Figure 2).
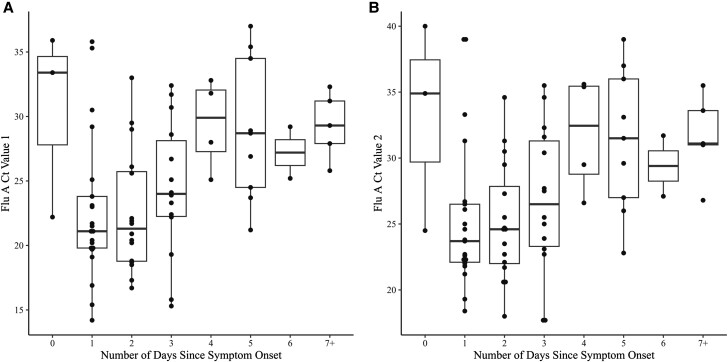
There were 74 symptomatic adults who presented for testing and were PCR-positive for influenza A. These participants had a median age of 35 years, and 55% were women. No influenza vaccination data were available. Ct value distributions (for the 2 influenza A targets from the Xpress assay) plotted by duration of symptoms at the time of testing are shown in Figure 2. Median/IQR trends (Figure 2, Supplementary Table 1) for both influenza A targets suggest that in this population, influenza A viral loads peaked shortly after symptom onset—unlike the trend observed for SARS-CoV-2. Of the 21 participants positive for influenza A on the second day of symptoms (day 1), 85.7% and 76.2% had Ct values ≤30 and ≤25, respectively (Supplementary Table 1).
Figure 2.
Influenza A Ct values measured in anterior nasal swab samples plotted by days since symptom onset for PCR-positive symptomatic adults (day 0 = the first day of symptoms). A, Ct value for influenza A target 1 (PA, a phosphoprotein subunit of influenza RNA polymerase); B, Ct value for influenza A target 2 (PB2, polymerase basic 2 subunit of influenza RNA polymerase). Abbreviations: Ct, cycle threshold; PCR, polymerase chain reaction.
DISCUSSION
Our data suggest that the relationship between SARS-CoV-2 Ct value distributions (as a well-established proxy for viral load distributions) and the timing of symptom onset in a highly immune population is very different than the relationship between these parameters observed early in the pandemic—a finding with major implications for testing practice going forward.
Early in the pandemic, multiple studies showed that viral loads were highest at the time of symptom onset and then declined steadily thereafter [1–4]. The sensitivity of rapid antigen tests within the first 7 days of symptoms was also quite high in multiple studies performed early in the pandemic, with overall sensitivities (vs PCR) > 90%–95% in the first week of symptoms in multiple studies [17–20]. In short, early in the pandemic, a single negative antigen test had reasonable negative predictive value early in the course of symptoms.
Now, in this highly immune population presenting after the early 2022 Omicron surge, our data suggest that viral loads appear to peak around 4 days after the onset of symptoms. In newly test-positive individuals, predicted rapid antigen test sensitivity peaked on day 4 at 80.0%–93.3%, whereas overall predicted sensitivity within the first 7 days of symptoms was only 60.4%–80.8%. Our choice of Ct cutoffs of 25 and 30 for predictions of hypothetical rapid antigen test sensitivity were based both on the numerous studies assessing antigen test sensitivity (vs a variety of PCR assays) at these cutoffs [17–20] and on internal data evaluating the sensitivity of commercial rapid antigen tests versus the Cepheid Xpert Xpress assay at point-of-care and using Omicron swab samples in transport media [16]. Similar findings were also observed in individuals who presented for testing and study enrollment having already tested positive elsewhere, with viral loads appearing to peak on the third/fourth days of symptoms. In this study, trends observed for directly-measured antigen concentration distributions very closely mirrored Ct value trends, providing additional support for the antigen test sensitivity predictions and suggesting that the close correlation between Ct value and Ag concentration observed in samples from early in the pandemic remains [21].
This delayed peak relative to the onset of symptoms has been observed in other studies conducted later in the pandemic [22–24], but the implications of this finding for testing practice—in particular, home antigen testing practice—have not been sufficiently highlighted. Hay et al [22] evaluated and modeled PCR test results in a cohort of mostly young healthy men (18% unvaccinated) and observed a median 2–3 day interval between onset of symptoms and peak viral loads (nadir Ct values) for both Delta and Omicron infections, but did not present Ct value distributions by day of symptoms to inform Ag RDT use. Kandel et al [23] evaluated serial testing data for 37 vaccinated adults with incident Omicron infection and found that viral load peaked 3 days after symptom onset. Interestingly, this shift to a later peak viral load relative to symptom onset may have been observed even by the second year of the pandemic, given that a study done in the first half of 2021 [24] (pre-Delta variant, with 86% of participants unvaccinated) using serial home Ag RDT testing already demonstrated that Ag RDT sensitivity in adults and children with PCR-confirmed infection peaked 4 days after symptom onset. We note that none of these prior studies included antigen concentration measurements, and our finding that the curves for Ct value and Ag concentration data were so similar suggests that the kinetics of RNA and protein clearance from infected tissue may be similar.
Although the observation of a delayed peak (relative to onset of symptoms) compared to Ct value distributions observed early in the pandemic is consistent overall with current FDA recommendations to repeat rapid antigen testing 48 hours after an initial negative test [12], our data in combination with others’ suggest that symptomatic individuals testing positive for SARS-CoV-2 by PCR currently may not reliably test positive on a rapid antigen test until the third, fourth, or even fifth day of symptoms. Although testing negative on an antigen test suggests that the person is less likely to be viral culture-positive (and presumably infectious) at that moment than someone who tests positive [25], we note that our data indicate that viral load would be expected to be rising, and not falling, in the window after that early false negative test. Additionally, we note that samples containing the Omicron variant have been observed to be culture-positive at lower viral loads than samples containing the Delta variant [26], suggesting that the reassurance provided by negative antigen tests may be even less now than earlier in the pandemic. Individuals who test negative on antigen tests on the first or second day of symptoms—or even on the third—and who remain symptomatic need to clearly understand that COVID has not been excluded. Serial testing recommendations should be updated to clarify the timing of repeat testing (potentially including a third test if tests are negative on the first and third days of symptoms) and the need to take precautions around others through at least the fourth day of symptoms, including masking when feasible. Moreover, although challenging, it will be important to find ways to educate home antigen test users about these updated recommendations. Our findings also have implication for test-to-treat programs that combine home Ag RDT use with prescriptions of antiviral treatment [27], given that treatment is optimally given as early in the course of infection as possible.
Our additional finding that influenza A viral loads were highest early in the symptom course—similar to the trends observed early in the COVID pandemic, and very different than the current trends we observed for SARS-CoV-2 infection—has important implications for test development and testing practice, although we note that we were unable to assess the impact of prior vaccine- or infection-based immunity on these findings. Multiple manufacturers are currently engaged in the development of multiplexed tests capable of simultaneous detection and distinction of SARS-CoV-2 and influenza viruses, and discordance in the timing of peak viral loads for the different viruses means that multiplexed tests will have major variance in negative predictive values at different time points within the course of one illness. Consumers, particularly those performing multiplexed tests at home, will need to be made aware of this variation so that the meaning of negative results can be more clearly understood.
Limitations of our study include that we utilized PCR Ct values as a proxy for viral loads. However, given that our goal was to assess trends in viral load kinetics relative to days of symptoms, utilized 1 PCR assay for the entire study, and were able to directly assess antigen concentrations in parallel with Ct values, we do not believe that this is an important limitation. We acknowledge that our population-level analysis may not be generalizable to all populations nor perfectly represent viral load kinetics at the per-patient level, though our findings are consistent with serial testing data [23]. We were unable to confirm the date of prior positive testing within 14 days of study enrollment and did not systematically collect data on prior negative test results, but note that viral load kinetics in the cohorts who did versus did not report a positive test prior to study enrollment were similar. We did not have enough participants to clearly evaluate the impact of varying forms of immunity (vaccine, prior infection, or both) on Ct value trends, but given that almost everyone presenting for testing in the study window had some known form of immunity, we think that our results are likely generalizable to the larger US population at this point in the pandemic. Similarly, we did not confirm the variants responsible for the infections in our study population, though local/national circulation of Omicron variant strains was well-established during the study timeframe. Our influenza data are limited by the relatively small sample size and the lack of information on vaccination, and we recognize that we are not able to clearly predict the implications for influenza A Ag RDT testing of the Ct value trends we observed.
In summary, our data remind us that viral load kinetics relative to duration of symptoms can change over time with exposure to an initially novel virus, and that data collected early in a pandemic should not be assumed to still apply in the months to years that follow. Capture of duration of symptoms data at the time of testing is both simple and high yield, and should be incorporated into testing programs so that these trends can be monitored and continue to guide testing practice going forward.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Jennifer K Frediani, Nell Hodgson Woodruff School of Nursing, Emory University, Atlanta, Georgia, USA.
Richard Parsons, Nell Hodgson Woodruff School of Nursing, Emory University, Atlanta, Georgia, USA.
Kaleb B McLendon, Emory/Children's Laboratory for Innovative Assay Development, Department of Pathology, Emory University, Atlanta, Georgia, USA.
Adrianna L Westbrook, Pediatric Biostatistics Core, Department of Pediatrics, Emory University, Atlanta, Georgia, USA.
Wilbur Lam, Department of Pediatrics, Emory University School of Medicine, Atlanta, Georgia, USA; Aflac Cancer and Blood Disorders Center of Children's Healthcare of Atlanta, Atlanta, Georgia, USA; Wallace H. Coulter Department of Biomedical Engineering, Emory University and Georgia Institute of Technology, Atlanta, Georgia, USA.
Greg Martin, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Nira R Pollock, Department of Laboratory Medicine, Boston Children's Hospital, Boston, Massachusetts, USA.
Notes
Author Contributions. J. K. F., R. P., A. L. W., and N. R. P. conceived and designed the analysis. K. B. M. contributed to data generation. J. K. F., R. P., K. B. M., A. L. W., W. L, and N. R. P. analyzed and/or interpreted the data. N. R. P., R. P., and J. K. F. drafted the manuscript. Critical revisions to the manuscript were made by all members of the study group. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data Availability Statement. Data available upon request.
Financial support. This work was supported by the National Institutes of Health (NIH NIBIB) (grant numbers U54 EB027690 02S1, U54 EB027690 03S1, U54EB027690 03S2 [to W. L. and G. S.] and UL1TR002378).
References
- 1. Singanayagam A, Patel M, Charlett A, et al. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill 2020; 25:2001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miller EH, Zucker J, Castor D, et al. Pretest symptom duration and cycle threshold values for severe acute respiratory syndrome coronavirus 2 reverse-transcription polymerase chain reaction predict coronavirus disease 2019 mortality. Open Forum Infect Dis 2021; 8:ofab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020; 26:672–5. [DOI] [PubMed] [Google Scholar]
- 4. Stankiewicz Karita HC, Dong TQ, Johnston C, et al. Trajectory of viral RNA load among persons with incident SARS-CoV-2 G614 infection (Wuhan strain) in association with COVID-19 symptom onset and severity. JAMA Netw Open 2022; 5:e2142796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pollock NR, Lee F, Ginocchio CC, Yao JD, Humphries RM. Considerations for assessment and deployment of rapid antigen tests for diagnosis of COVID-19. Open Forum Infect Dis 2021; 8:ofab110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hanson KE, Caliendo AM, Arias CA, et al. Infectious Diseases Society of America guidelines on the diagnosis of COVID-19: antigen testing. Infect Dis Soc 2021:ciad032. doi: 10.1093/cid/ciab048 [DOI] [PubMed] [Google Scholar]
- 7. Deerain J, Druce J, Tran T, et al. Assessment of the analytical sensitivity of 10 lateral flow devices against the SARS-CoV-2 Omicron variant. J Clin Microbiol 2022; 60:e0247921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laitman AM, Lieberman JA, Hoffman NG, Roychoudhury P, Mathias PC, Greninger AL. The SARS-CoV-2 Omicron variant does not have higher nasal viral loads compared to the Delta variant in symptomatic and asymptomatic individuals. J Clin Microbiol 2022; 60:e0013922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rattan A, Joerger J, Williams D, Pollock NR. Similar SARS-CoV-2 Ct value distributions in anterior nares versus nasopharyngeal samples from symptomatic children during Delta and Omicron surges. J Pediatric Infect Dis Soc 2023; 12:109–12. [DOI] [PubMed] [Google Scholar]
- 10. Soni A, Herbert C, Filippaios A, et al. Comparison of rapid antigen tests’ performance between Delta and Omicron variants of SARS-CoV-2: a secondary analysis from a serial home self-testing study. Ann Intern Med 2022; 175:1685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Soni A, Herbert C, Lin H, et al. Performance of rapid antigen tests to detect symptomatic and asymptomatic SARS-CoV-2 infection. medRxiv 22278466 [Preprint] August 06, 2022 [cited Jan 23, 2023]. Available from: 10.1101/2022.08.05.22278466[AQ9]. [DOI] [PMC free article] [PubMed]
- 12. Food and Drug Administration . At-home COVID-19 diagnostic tests: frequently asked questions. 2022. Available at: https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/home-covid-19-diagnostic-tests-frequently-asked-questions#negatives. Accessed May 5, 2023.
- 13. Wickham H. ggplot2: elegant graphics for data analysis. New York: Springer-Verlag, 2016. [Google Scholar]
- 14. Wickham H, François R, Henry L, Müller K, Vaughan D, Posit Software, PBC . dplyr: a grammar of data manipulation. R package version 1.0.9. 2022. Available at: https://cran.r-project.org/web/packages/dplyr/index.html. Accessed August 23, 2023.
- 15. Zhang Y, Wang S, Ye S, et al. r2rtf: easily create production-ready rich text format (RTF) table and figure. R package version 0.3.5. 2022. Available at: https://www.pharmasug.org/proceedings/2020/DV/PharmaSUG-2020-DV-198.pdf. Accessed August 23, 2023.
- 16. Rao A, Westbrook A, Bassit L, et al. Sensitivity of rapid antigen tests against SARS-CoV-2 Omicron and Delta variants varies by RT-PCR versus quantitative antigen levels. J Clin Microbiol 2023:e0013823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kruger LJ, Gaeddert M, Tobian F, et al. The Abbott PanBio WHO emergency use listed, rapid, antigen-detecting point-of-care diagnostic test for SARS-CoV-2-evaluation of the accuracy and ease-of-use. PLoS One 2021; 16:e0247918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pollock NR, Jacobs JR, Tran K, et al. Performance and implementation evaluation of the Abbott BinaxNOW rapid antigen test in a high-throughput drive-through community testing site in Massachusetts. J Clin Microbiol 2021; 59:e00083-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Igloi Z, Velzing J, van Beek J, et al. Clinical evaluation of Roche SD biosensor rapid antigen test for SARS-CoV-2 in municipal health service testing site, the Netherlands. Emerg Infect Dis 2021; 27:1323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pilarowski G, Marquez C, Rubio L, et al. Field performance and public health response using the BinaxNOWTM rapid severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antigen detection assay during community-based testing. Clin Infect Dis 2021; 73:e3098–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pollock NR, Savage TJ, Wardell H, et al. Correlation of SARS-CoV-2 nucleocapsid antigen and RNA concentrations in nasopharyngeal samples from children and adults using an ultrasensitive and quantitative antigen assay. J Clin Microbiol 2021; 59:e03077-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hay JA, Kissler SM, Fauver JR, et al. Quantifying the impact of immune history and variant on SARS-CoV-2 viral kinetics and infection rebound: a retrospective cohort study. Elife 2022; 11:e81849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kandel C, Lee Y, Taylor M, et al. Viral dynamics of the SARS-CoV-2 Omicron variant among household contacts with 2 or 3 COVID-19 vaccine doses. J Infect 2022; 85:666–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chu VT, Schwartz NG, Donnelly MAP, et al. Comparison of home antigen testing with RT-PCR and viral culture during the course of SARS-CoV-2 infection. JAMA Intern Med 2022; 182:701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kirby JE, Riedel S, Dutta S, et al. Sars-Cov-2 antigen tests predict infectivity based on viral culture: comparison of antigen, PCR viral load, and viral culture testing on a large sample cohort. Clin Microbiol Infect 2023; 29:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tassetto M, Garcia-Knight M, Anglin K, et al. Detection of higher cycle threshold values in culturable SARS-CoV-2 Omicron BA.1 sublineage compared with pre-Omicron variant specimens—San Francisco Bay Area, California, July 2021-March 2022. MMWR Morb Mortal Wkly Rep 2022; 71:1151–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. The White House . FACT SHEET: Biden administration outlines plan to get Americans an updated COVID-19 vaccine shot and manage COVID-19 this fall. 8 September 2022. Available at: https://www.whitehouse.gov/briefing-room/statements-releases/2022/09/08/fact-sheet-biden-administration-outlines-plan-to-get-americans-an-updated-covid-19-vaccine-shot-and-manage-covid-19-this-fall/. Accessed May 08, 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.