Abstract
Previous studies indicated that inorganic pyrophosphatase of Ascaris suum (AsPPase) plays an important role in larval survival in the host. Here we describe a precise role for AsPPase in larval molting and development and also describe the potential role of recombinant AsPPase (rAsPPase) in protective immunity to A. suum infection. Using reverse transcriptase PCR analysis, we found that disruption of AsPPase gene function by RNA interference resulted in suppression of AsPPase mRNA levels. RNA interference also caused inhibition of molting of third-stage larvae (31%) and suppression of native protein expression, as demonstrated by a 56% reduction in enzyme activity and quantified by immunoblot and immunofluorescence analyses, suggesting that AsPPase has a role in the molting process. The anatomic location of the AsPPase native enzyme in the hypodermis of larvae along with its elevated expression prior to and during the molting process supports such a role. Anti-rAsPPase immunoglobulin G (IgG) also resulted in 57% inhibition of molting of A. suum lung-stage third-stage larvae to fourth-stage larvae in vitro with developmental arrest. Antigenic epitopes of AsPPase overlapped the enzyme active sites. Mice immunized with rAsPPase exhibited high antigen-specific IgG antibody responses and were protected (>70%) against a challenge A. suum migratory-phase infection. Splenic T cells from rAsPPase-immunized mice produced low levels of T helper 1-type cytokines (gamma interferon and interleukin-2) in vitro but exhibited an elevated interleukin-10 response. A significantly high level of IgG1 subclass antibodies was found in immunized mice. Our results establish that AsPPase has a critical role in the molting and development of Ascaris roundworms and suggest the potential of AsPPase for use as a candidate vaccine against ascariasis.
Soluble inorganic pyrophosphatases (PPases) (EC 3.6.1.1), which catalyze the hydrolysis of inorganic pyrophosphate (PPi) to inorganic orthophosphate (Pi), are ubiquitous enzymes that have been shown to be essential for bacterial cell growth (4, 26). There are two currently recognized families of soluble PPases, and family I soluble PPases have distinctive catalytic features and active site structures that are highly conserved evolutionarily (7). In transgenic tobacco and potato plants, soluble PPase has been reported to alter metabolism, growth, and development (24, 42), while in barley grains it stimulates germination (50). In contrast, very little is known about the metabolic significance of PPases in animals in general (17, 51), and virtually nothing is known about PPases in metazoan parasites except for the recent report of Islam et al. (23).
Ascariasis due to Ascaris lumbricoides (Linnaeus, 1758) remains a significant health problem mostly in the developing nations and affects an estimated 1.5 billion people worldwide; one million new cases occur annually. A. lumbricoides has profound effects on infected children that lead to retarded growth, deficiencies of nutrients, damage of the small intestine mucosa, and lethal hypersensitivity responses (19, 29, 44). The developmental cycle of A. lumbricoides involves two distinct patterns: a tissue-migratory phase involving the liver with infective third-stage larvae (L3) and the lungs with L3 and fourth-stage larvae (L4), and a noninvasive phase with adult worms that finally reside in the small intestine. The pig homologue Ascaris suum (Goeze, 1782) has become a suitable model for the human worm because this parasite is morphologically and antigenically indistinguishable from A. lumbricoides, has an identical life cycle, and can develop in human hosts (1, 2, 34), suggesting its zoonotic significance.
Gastrointestinal parasitic infections induce strong T helper 2 (Th2)-biased immune responses in the hosts (8, 21, 30, 31). Cytokine regulation of host defense against intestinal nematodes has been determined by using various experimental host-parasite models (3, 9, 10, 40). There is scant information concerning the immune responses to human A. lumbricoides infections, but recent studies have shown that human ascariasis induces a highly polarized Th2-type response (6, 16, 29, 49). Furthermore, it has been suggested that Ascaris roundworms have an immunomodulatory effect on the host immune system and may impair protective responses to oral vaccine antigens, such as the live oral cholera vaccine CVD 103-HgR (5, 33). Nevertheless, relatively little is known about the precise effector mechanisms of Ascaris-expressed potent immunoreactive molecules that trigger and maintain Th2-biased responses to A. lumbricoides infection. Recently, we cloned a cDNA from adult female A. suum encoding a family I soluble PPase (23). This enzyme has been shown to be highly expressed in all developmental stages, including adult worms. Homologues of the enzyme were detected in human and dog roundworms (A. lumbricoides and Toxocara canis, respectively), indicating that A. suum PPase (AsPPase) can be used as a molecule with the same potential for human and dog ascariasis. The native enzyme was intensely localized in the hypodermis and in reproductive tissues of adult worms. AsPPase-catalyzed inorganic pyrophosphate hydrolysis activity was shown to be blocked by sodium fluoride (NaF), a well-known PPase inhibitor, and by anti-mouse immunoglobulin G (IgG) purified from immune sera. Notably, blocking of the native enzyme by NaF and imidodiphosphate, a PPi analogue, has been shown to inhibit the molting of A. suum larvae, suggesting that the PPase has a role in the worm's molting processes.
In the present study, we determined the precise role(s) of the enzyme PPase in the normal molting and development of A. suum lung-stage L3 (AsLL3) to L4 in vitro using both molecular genetic and biochemical tools. For example, we employed RNA interference (RNAi), a phenomenon that is mediated by long double-stranded RNA (dsRNA) or small interfering RNAs, which trigger disruption of the cognate mRNA (13, 43). Recent reports have shown the usefulness of RNAi for abrogating gene function in the model nematode Caenorhabditis elegans (18), as well as in parasitic helminths (22, 41) and arthropods (32). We found that dsRNA-mediated interference disrupts the AsPPase gene function in AsLL3 in vivo, as reflected by suppression of AsPPase mRNA levels in reverse transcriptase PCR (RT-PCR) analysis. RNAi also caused inhibition of larval molting and suppression of native protein expression, as demonstrated by a reduction in enzyme activity and quantified by immunoblot and immunofluorescence analyses. We also used affinity-purified anti-recombinant AsPPase (rAsPPase) IgG antibody to block native AsPPase functions in A. suum larval parasites. Interestingly, our results show that anti-rAsPPase IgG antibody inhibited molting and caused an arrest of development of AsLL3 to L4 in vitro. Additionally, we evaluated the potential of AsPPase for use as a vaccine candidate and the underlying protective mechanisms induced by rAsPPase against A. suum migratory-phase infection.
MATERIALS AND METHODS
Parasites.
A. suum adults were obtained from infected pigs at a slaughterhouse in Shimotsuma, Japan. Unembryonated and embryonated eggs were obtained essentially as described elsewhere (45). A. suum infective L3 from embryonated eggs and lung-stage L3 from an infected rabbit were collected as described previously (23).
Animals.
Eight-week-old male specific-pathogen-free BALB/c mice were used in this study. The mice were housed in sterile cages in a barrier environment in the laboratory animal house of the National Institute of Animal Health. Mice were fed pelleted food and water ad libitum. All animals were acclimatized to these conditions for 1 week prior to the experiment. Animal experiments were conducted in accordance with the protocols approved by the National Institute of Animal Health Animal Care and Use Committee (approval no. 23).
Production of recombinant proteins.
An Escherichia coli-expressed, catalytically active, histidine-tagged rAsPPase was prepared as described previously (23). Briefly, genes expressing protein were cloned into the XhoI and HindIII sites of pTrcHisB. Recombinant protein was expressed by transforming E. coli strain TOP10F′ with the plasmid. Transformed cells were grown to an optical density at 600 nm at 37°C in SOB medium. Isopropylthiogalactose was added to cultures at a final concentration of 1 mM, and cells were grown for 4 h. Cells were harvested by centrifugation, and the resultant pellet was resuspended in lysis buffer. The cell suspension was disrupted by freeze-thaw cycles, followed by sonication. The lysate was centrifuged at 26,000 × g for 30 min at 4°C. The supernatant containing recombinant proteins was purified by using ProBond resin under nondenaturing conditions and was subsequently eluted with a stepwise gradient of imidazole (50, 200, 350, and 500 mM; pH 8.0). The eluted fractions were concentrated and dialyzed against 20 mM Tris-HCl (pH 7.5) and decreasing amounts of NaCl at 4°C. Protein concentrations were determined by using the micro-BCA protein assay reagent (Pierce).
Measurement of native enzyme activity in A. suum at various stages of development.
To explore the possible role of AsPPase in the molting of the worm, we measured the native enzyme activity in soluble extracts of A. suum at various stages of development. The stages included were A. suum embryonated eggs, infective L3, lung-stage L3, lung-stage L3 that were further cultured in vitro for 5 days and removed after they had started molting to L4 (this stage contained a mixed population of L3 and L4), and adult gravid female worm. Parasite extracts were prepared in 20 mM Tris-HCl (pH 7.5) (45). Enzyme activity was measured by using a molybdate blue-based colorimetric assay with a standard 200-μl reaction mixture containing 5 mM Mg2+, 100 mM Tris-HCl (pH 7.5), and 1 mM PPi (Na4P2O7) as described previously (23).
Production of AsPPase-specific double-stranded RNA and RNA interference.
The RNAi procedure was carried out by using dsRNA as described elsewhere (13, 43). The coding sequence of mature AdR44 cDNA (GenBank accession no. AB091401) was cloned into the pBluescript II SK(+) plasmid, and the inserted sequence was PCR amplified by using oligonucleotide primers T7 (5′-GTAATACGACTCACTATAGGGC-3′) and CMo422 (5′-GCGTAATACGACTCACTATAGGGAACAAAAGCTGGAG-CT-3′) to attach T7 promoter recognition sites to both the 5′ and 3′ ends. The PCR products were purified with a gel extraction kit (QIAGEN). dsRNA complementary to the DNA insert was synthesized by in vitro transcription by using the T7 RNA polymerase (Ribomax Express large-scale RNA production system; Promega) according to the manufacturer's protocol. About 3 μg of DNA was used as a template, which allowed synthesis of 200 to 300 μg of dsRNA. The dsRNA was purified and quantified spectroscopically. The RNAi experiments were performed by soaking AsLL3 in AsPPase dsRNA (43). The larvae (∼100 AsLL3) were incubated in 25 μl of serum-supplemented RPMI 1640 containing dsRNA at a final concentration of 2 μg/μl. After 24 h of soaking in 96-well flat-bottom multiwell tissue culture plates (MS-8096F; Sumitomo), the larvae were transferred to 500 μl of culture medium and were incubated in 24-well culture plates for an additional 9 days as described above. In some experiments, Lipofectin (catalog no. 18292-011; Invitrogen) was used in the culture medium with dsRNA or alone. Target gene disruption by dsRNA in these larvae was then evaluated by molting assay, enzyme activity, immunoblot, and immunofluorescence analyses.
Quantitative RT-PCR analysis.
We also examined AsPPase mRNA levels to verify that target gene disruption by dsRNA had been achieved by RT-PCR analysis. Total mRNA was isolated with a QuickPrep micro mRNA purification kit (Amersham Pharmacia) as described in the protocols of the manufacturer. cDNA was then synthesized with 30 μg of mRNA by using an RNA PCR kit (AVM, version 3.0; Takara) and following the manufacturer's instructions. The PCR was performed by using AsPPase-specific oligonucleotides (23) and oligonucleotides specific for L2R37 cDNA encoding an A. suum 16-kDa antigen (46), with 500 ng of cDNA as the template in a 50-μl (final volume) mixture. The PCR products were resolved by 1% agarose gel electrophoresis.
Epitope mapping.
The Novatope system (Novagen R and D Systems, Abingdon, United Kingdom) was used to map sites for B-cell epitopes of the recombinant AsPPase. A PCR product spanning the complete open reading frame of the AsPPase gene was digested with DNase I, and the resulting oligonucleotides (average length, 50 to 100 bp) were ligated into the plasmid. The plasmid DNA was transformed into E. coli. The positive inserts expressed by transformed cells were screened by a colony immunoassay (36) by using a pool serum obtained from protectively immune mice.
In vitro molting assay for A. suum lung-stage larvae to assess the functional significance of anti-rAsPPase IgG.
Anti-mouse rAsPPase IgG from immune sera and mouse preimmune IgG were affinity purified by using UltraLink immobilized protein G according to the manufacturer's instructions (Pierce) in order to evaluate the functional significance for the molting and development of AsLL3 to L4 in vitro. AsLL3 (∼100 worms) were cultured in 1 ml of RPMI 1640 (GIBCO-BRL) (pH 6.8) supplemented with 10% (vol/vol) fetal bovine serum (Sigma) and antibiotics (penicillin and streptomycin, each at 100 μg/ml) in 24-well flat-bottom tissue culture plates (Costar). The cultures were incubated at 37°C in humidified 5% CO2 in the presence of increasing concentrations of anti-mouse rAsPPase IgG and mouse preimmune IgG (as a control) for 10 days. The number of L4 which had molted from L3 in the culture wells was determined (23).
Immunofluorescence staining for detection of native AsPPase in lung-stage larvae.
Lung-stage A. suum L3 obtained from an infected rabbit were washed in cool phosphate-buffered saline (PBS) containing 0.1% Tween 20 (PBS-T) three times and fixed in cool acetone for 2 min. After the fixed larvae were washed in PBS-T, they were blocked with 10% goat serum (Wako) for 1 h and incubated with primary anti-rAsPPase IgG antibody (1:50) overnight at 4°C. Fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Sigma) was used as a secondary antibody at a dilution of 1:100. After labeling, larvae were washed in PBS-T, and whole larvae were mounted on slides with 70% glycerol. The larvae were analyzed and photographed with an Axiophot fluorescence microscope (Carl Zeiss) by using appropriate filter sets.
Ultrastructure of A. suum lung-stage larvae that did not molt during in vitro culture.
The lung-stage L3 that did not molt in the presence of anti-rAsPPase IgG were collected at day 6 of in vitro culture and fixed overnight at 4°C with 3% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4); then they were washed in the same buffer and processed for electron microscopy examination (48). To examine the ultrastructure of larvae, larvae undergoing normal molting were collected at day 3 of in vitro culture before the molting was complete and were processed as described above.
Immunization and challenge infection.
Mice were divided into four groups of 10 animals each. Five mice from each group were immunized subcutaneously by using a 25-gauge needle in the abdominal region with 50 μg of rAsPPase mixed with TiterMax Gold adjuvant (CytRx) in a total volume of ∼400 μl, and the other five animals were inoculated with adjuvant only and served as controls. Beginning 3 weeks after the primary immunization, the mice were boosted twice by using the same dose and route at 2-week intervals. The mice in the first and second groups received a primary immunization and a single booster immunization, respectively, while the mice in the third and fourth groups received two booster immunizations. All of the mice in first, second, and third groups were sacrificed at various times (for example, 3, 5, and 7 weeks postimmunization, respectively), and their serum antibodies were measured. Mice were bled before autopsy, and sera were collected and frozen at −20°C until they were used. Spleens from selected mice (only the third group) were removed aseptically in order to culture cells for cytokine analysis. Mice in fourth group were challenged 1 week after the second booster immunization with 2,500 A. suum embryonated eggs containing L3 in 200 μl of PBS by using a stomach catheter. The mice were sacrificed 1 week after the challenge, and the tissue-migrating L3 from the lungs (i.e., AsLL3) were recovered (47).
Evaluation of total serum IgG, IgG subclass, and IgE responses.
The recombinant AsPPase-specific IgG, IgE, and IgG subclass (IgG1, IgG2a, IgG2b, and IgG3) antibodies in serum from immune mice were measured by enzyme-linked immunosorbent assays (ELISAs) (46). The IgG and IgG subclass antibody levels were determined with horseradish peroxidase-conjugated goat anti-mouse IgG and IgG subclass antibodies (Bethyl Laboratories Inc.). Affinity-purified goat anti-mouse IgG, IgG1, IgG2a, IgG2b, and IgG3 (Bethyl) were used as standards. For rAsPPase-specific IgE measurement, anti-mouse IgE was used as a capture antibody, and IgE was detected by using biotinylated anti-mouse IgE (Bethyl). Rat monoclonal anti-mouse IgE antibody diluted 1:10,000 (American Research Products) was used as the standard.
ELISAs were performed in 96-well ELISA plates (MS-8496F; Sumitomo) by using 50-μl reaction mixtures with rAsPPase antigen coated at a concentration of 2 μg/ml of 0.1 M carbonate buffer (pH 9.6). The plates were incubated at 4°C for 14 h and washed three times with Tris-buffered saline (TBS) containing 0.05% Tween 20 (TBS-T). Wells were blocked with 100 μl of TBS-1% bovine serum albumin (Sigma) for 1 h at 37°C. After the wells were washed five times with TBS-T, serial dilutions of the serum were added, and this was followed by incubation at 37°C for 1 h. After incubation, the wells were washed five times with TBS-T, and 100-μl portions of horseradish peroxidase-conjugated anti-mouse IgG, IgE, or IgG subclass antibodies were added to the wells. The plates were incubated at 37°C for 1 h and washed five times with TBS-T. Mouse sera were diluted 1:1,000. Conjugates were used at a dilution of 1:10,000. Both test sera and conjugates were diluted in TBS (pH 8.0). Antibody was detected at 37°C with 100 μl of a 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) substrate solution (Kirkegaard Laboratories), and the coloring reaction was terminated with 100 μl of 1% sodium dodecyl sulfate (SDS). Plates were examined at 405 nm by using a microplate reader (Spectrafluor; Wako). The concentrations of antibodies were calculated by reference to a standard curve generated by using reference sera (calibrator).
Spleen cell culture for cytokine analysis.
Spleen cell suspensions were prepared by crushing spleens through a sterile stainless steel mesh (Sigma) and suspending the cells in cool Hanks balance salt solution (GIBCO/BRL) supplemented with 5% (vol/vol) heat-inactivated fetal calf serum (Sigma) and antibiotics (penicillin and streptomycin, 100 μg/ml). Erythrocytes in cell suspensions were lysed with 0.2% sodium chloride. The suspended cells were washed three times in Hanks balance salt solution with centrifugation at 500 × g at 4°C for 5 min each time. Finally, the spleen cells, mostly T lymphocytes, were resuspended in complete RPMI 1640 (GIBCO/BRL) supplemented with heat-inactivated 10% (vol/vol) fetal calf serum, 10 mM HEPES, 2 mM l-glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 1 mM sodium pyruvate, 1 mM nonessential amino acids, 1 mg of flucytosine per ml, and 5 × 10−5 M 2-mercaptoethanol. The concentrations of splenic T cells obtained from individual mice were adjusted to 4 × 106 cells/ml of complete RPMI 1640. For in vitro stimulation, splenic cells were seeded on 96-well flat-bottom tissue culture plates (MS-8096F; Sumitomo) in 100 μl of medium per well. The cells were stimulated alone or with rAsPPase antigens (23 μg/ml) for 72 h at 37°C in a humidified atmosphere containing 5% CO2, and supernatants were removed and frozen at −80°C until they were assayed to determine their cytokine content.
Cytokine ELISA.
Cytokines produced by activated spleen cells were measured by a standard sandwich ELISA by using purified anti-interleukin-4 (IL-4), anti-IL-10, anti-IL-2, and anti-gamma interferon (IFN-γ) as capture antibodies and the corresponding biotinylated antibodies as reporter antibodies according to the manufacturer's instructions (eBioscience). The concentrations of primary capture antibodies and biotinylated reporter antibodies used were the concentrations recommended in the protocol (eBioscience). Purified recombinant IL-4, IL-10, IL-2, and IFN-γ were used as standards (eBioscience). Tetramethylbenzidine microwell peroxidase was used as the substrate, and the reaction was terminated with 1 M H3PO4. Plates were read at 450 nm by using a microplate reader (Spectrafluor). Cytokine levels were calculated by reference to standard curves constructed with supernatants containing known amounts of each molecule.
Statistics.
The data were expressed as means ± standard deviations for the experimental groups. The statistical significance (P < 0.05) was determined by Student's t test.
RESULTS
Specificity and purity of E. coli-expressed recombinant AsPPase.
An E. coli-expressed recombinant AsPPase was shown to be specific and was almost 99% pure, as judged by SDS-polyacrylamide gel electrophoresis (PAGE) (data not shown). The recombinant AsPPase was shown to have a specific activity of 940 μmol/min/mg of protein and was used for mouse polyclonal antibody production, immunization, and immunofluorescence studies.
Native AsPPase activity detected throughout A. suum development and elevated prior to and during molting.
To investigate the possible function of PPase in the molting of the worm, we measured native AsPPase activity in soluble extracts of A. suum at various developmental stages, including adult gravid female worms. As shown in Table 1, AsPPase native enzyme activity was present in all developmental stages of A. suum, and the lowest activity was recorded in embryonated eggs. Interestingly, enzyme activity was elevated prior to molting of the developing lung-stage L3 recovered from an infected rabbit. These L3 could initiate molting within 3 days when they were cultured in vitro. A further increase in enzyme activity was noticed during the molting of lung-stage L3 to L4 in vitro. The enzyme activity in fully mature gravid females was, however, found to be much lower than that in lung-stage L3. An increase in AsPPase activity prior to and during the L3 molting suggests that this activity is involved in the molting of the parasite.
TABLE 1.
AsPPase native enzyme activity in A. suum at various stages of developmenta
| Parasite developmental stage | Native enzyme activity (μmol/min/mg of protein) |
|---|---|
| Embryonated eggs | 0.86 ± 0.09 |
| Infective L3 | 1.53 ± 0.04 |
| Lung-stage L3 | 5.74 ± 0.11 |
| Lung-stage L3 cultured for 5 days | 7.75 ± 0.12 |
| Adult gravid females | 1.80 ± 0.04 |
Parasite soluble extracts in 20 mM Tris-HCl (pH 7.5) were used in the standard reaction mixture for enzyme assays, as described in Materials and Methods. The data are means ± standard deviations from five independent experiments.
RNAi-mediated inhibition of molting of A. suum lung-stage L3 to L4 and reduction in enzyme activity.
To analyze the effects of RNAi-mediated AsPPase gene suppression on molting, AsLL3 soaked in dsRNA were evaluated by using the in vitro molting assay. Soaking larvae in dsRNA inhibited the molting from L3 to L4 by 31% compared with controls (Table 2) but did not affect the larval viability or cause any morphological defect as determined by light microscopy; however, the unmolted L3 showed developmental arrest. The use of Lipofectin with dsRNA or alone had no influence on L3 molting. The reason for the poor inhibition of L3 molting observed with the soaking method is unclear. We assume that soaking may be inferior to the feeding and injection method of dsRNA treatment in RNAi technology. This hypothesis is supported by a recent report of Hashmi et al. (18), who observed that feeding with bacteria expressing dsRNA of cathepsin Z-like cysteine protease caused an 80% decrease in the molting of C. elegans L3 to L4, compared to the 23% decrease observed when the soaking method was used. To elucidate the potential functions of AsPPase in vivo, feeding of AsLL3 with bacteria expressing AsPPase dsRNA or injection of such bacteria into adult worms would be of interest for future studies. RNAi-mediated gene suppression was also evaluated by measuring native enzyme activity in AsLL3 cultured for 5 days with or without dsRNA. Our results revealed a 56% reduction in enzyme activity in treated larvae compared with untreated controls (Table 2). We repeated the RNAi experiments at least three times and obtained similar results for larval molting and enzyme activity.
TABLE 2.
RNAi-mediated disruption of AsPPase gene function measured by molting and native enzyme activity in A. suum lung-stage larvaea
| Treatment | Molting rate (%) | Native enzyme activity (μmol/min/mg of protein) | Reduction (% of control)
|
|
|---|---|---|---|---|
| Molting | Activity | |||
| dsRNA | 33.22 ± 5.13 | 3.20 ± 0.28 | 31.38 | 56.28 |
| Control | 48.41 ± 3.38 | 7.32 ± 0.23 | ||
Lung-stage A. suum L3 were cultured in RPMI 1640 in the presence of dsRNA and in the absence of dsRNA (control). Molting from L3 to L4 was evaluated at day 10 of culture as described in Materials and Methods. To measure enzyme activity, larvae were removed from the culture after 5 days, and soluble extracts prepared in 20 mM Tris-HCl (pH 7.5) were used in the standard reaction mixture for enzyme assays, as described in Materials and Methods. The data are means ± standard deviations from three independent experiments.
RNAi-mediated gene disruption suppressed native protein expression and mRNA levels in A. suum lung-stage larvae, as determined by immunofluorescence, immunoblot, and RT-PCR analyses.
To verify that RNAi effectively suppressed the AsPPase gene, we also performed an immunofluorescence analysis of whole mounted AsLL3 cultured for 5 days with or without dsRNA. Larvae were washed in cool PBS-0.1% Tween 20, fixed in acetone, and incubated with primary anti-rAsPPase antibody diluted 1:20. FITC-labeled goat anti-mouse IgG (1:100) was used as a secondary antibody. Figure 1A to D shows clear evidence of suppression of native AsPPase expression in the dsRNA-treated larvae, while the untreated larvae exhibited intense expression of native protein bound to the specific antibodies. Protein extracts of dsRNA-treated lung-stage L3 after they had been cultured for 5 days were prepared for an immunoblot analysis. Equivalent amounts (25 μg/lane) of proteins extracted from treated and control larvae were separated by SDS-12.5% PAGE and were transferred to a nitrocellulose membrane. Native AsPPase protein reacted with mouse sera raised against rAsPPase, as detected by nitroblue tetrazolium—5-bromo-4-chloro-3-indolylphosphate (Promega). We observed significant suppression of native AsPPase expression in dsRNA-treated larvae compared with controls (Fig. 1E). Furthermore, we performed RT-PCR using cDNA synthesized from treated larvae as the template to determine that RNAi really did diminish AsPPase mRNA synthesis. Our results clearly showed that the target gene was completely abolished in the treated larvae (Fig. 1F, lane 2), while the L2R37 genes remained unaffected in both the treated larvae (Fig. 1F, lane 3) and the untreated control larvae (Fig. 1F, lane 4). These data suggested that the target gene had been disrupted in the treated larvae.
FIG. 1.
Effects of dsRNA treatment on AsPPase gene disruption. A. suum lung-stage larvae soaked in dsRNA were cultured in RPMI 1640 for 5 days and then removed from the medium for analysis. (A to D) Immunofluorescence analysis. Whole mounts of treated and untreated larvae were prepared. The larvae were washed in cool PBS—0.1% Tween 20, fixed in acetone, and incubated with primary anti-rAsPPase antibody. FITC-labeled goat anti-mouse IgG was used as a secondary antibody. dsRNA-mediated suppression of native protein was clearly seen in treated larva (C and D). Untreated larvae showed intense expression of native protein (A and B) (arrowheads). Panels B and D are the highlighted regions of panels A and C, respectively (magnification, ×200). Larvae were observed with an Axiophot fluorescence microscope by using appropriate filter sets and were photographed. (E) Immunoblot analysis. Equal amounts of protein prepared from treated and untreated larvae were separated by SDS-PAGE and transferred to a nitrocellulose membrane. Native AsPPase proteins expressed in dsRNA-treated larvae (lane 2) and untreated larvae (lane 1) bound to the rAsPPase-specific mouse sera were detected by using nitroblue tetrazolium—5-bromo-4-chloro-3-indolylphosphate. (F) Reverse transcriptase PCR analysis. PCR was performed by using cDNA synthesized either from AsPPase dsRNA-treated larvae (lane 2) or from nontreated controls (lane 1). An L2R37 cDNA encoding the A. suum 16-kDa antigen (46) was not affected in both dsRNA-treated larvae (lane 3) and the untreated control (lane 4). Lane M contained molecular mass markers.
Epitopes are spread across the whole AsPPase molecule.
Epitope mapping was carried out to determine the potential role of anti-rAsPPase IgG in immunological cross-reactivity with the active site of AsPPase. Immunoreactive plasmids were selected by using the Novatope system, and their inserts were sequenced for alignment with the open reading frame of AsPPase. The IgG binding epitopes recognized by anti-mouse sera mapped to residues 107-HDIPLFADEAKKVYNMIVEIPRWTNAKM-134, 267-RVYKIPTGKPANQFGFDGQYKD-288, 296-IAETHEFWKKLIKEASPSLNTESNV-320, and329-QEAWKKIVDSQPAIGKPHEIPATL-DRWHFIKE-360 in the AsPPase sequence. These data showed that rAsPPase-immunized mouse sera recognized epitopes that were spread across the whole antigen, overlapping the enzyme active site E, K, Y, and K residues (indicated by boldface type) located at positions 125, 133, 269, and 270, respectively. These results strongly suggested that anti-IgG antibodies have the potential to drive both protection and enzyme inhibition and are consistent with our previous observation that the AsPPase native enzyme in larval extracts was sensitive to inhibition by anti-IgG antibodies (25%) (23). The binding of antibody to AsPPase active site residues indicates that the antibody can be used in analyses of the functional role of native protein in the molting of Ascaris parasites.
Anti-rAsPPase IgG blocked molting and development of A. suum lung-stage larvae in vitro.
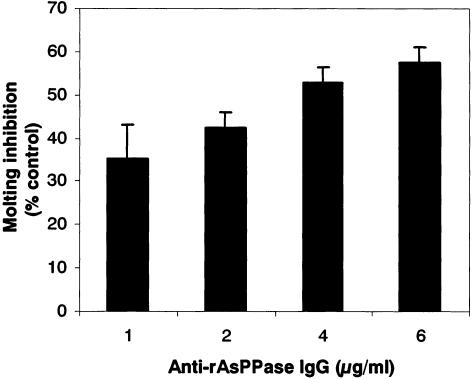
We assessed the functional significance of anti-rAsPPase IgG in the molting and development of Ascaris larvae. We hypothesized that if the native AsPPase plays a potential role in larval molting and development, then its neutralization by antigen-specific antibody might lead to blockade of larval molting and development. We developed a visual molting assay using cell culture medium that permitted molting and normal development of AsLL3 to L4 in vitro (23). We employed this assay to evaluate the ability of anti-rAsPPase IgG to block the molting of AsLL3 to L4. As shown in Fig. 2, affinity-purified anti-mouse rAsPPase IgG blocked the molting of AsLL3 to L4 in a dose-dependent manner, which clearly supports our hypothesis. Up to 57% of the molting was blocked at an anti-mouse rAsPPase IgG concentration of 6 μg/ml compared with the results obtained with mouse preimmune IgG, which was used as a control. The mean molting percentage in the control culture was 56.70% ± 3.16%. Native AsPPase activity was previously shown to be inhibited by NaF at micromolar concentrations, and larval molting was found to be blocked by NaF and imidodiphosphate at millimolar concentrations (23)
FIG. 2.
Inhibition of molting of A. suum lung-stage L3 to L4 in vitro by anti-mouse rAsPPase IgG. AsLL3 obtained from an infected rabbit were cultured in serum-supplemented RPMI 1640 in the presence of affinity-purified anti-mouse rAsPPase IgG as described in Materials and Methods. Molting from L3 to L4 was evaluated at day 10 of culture. The molting percentages are percentages based on the value for the control in the presence of mouse preimmune IgG (100%). In the control cultures the molting percentages were 56.70% ± 3.16%. The bars indicate means, and the error bars indicate standard deviations (n = 3).
Moreover, we found that treatment with anti-rAsPPase IgG caused arrested development of AsLL3 due to incomplete molting, as measured by a decrease in body length (1,564.50 ± 108.22 μm) and body width (66.20 ± 6.21 μm), but did not affect larval viability. In contrast, mouse preimmune IgG-treated AsLL3 grew well and molted to L4 with significant increases in body length (2,317.50 ± 228.03 μm) and body width (69.80 ± 8.13 μm). The AsLL3 obtained from an infected rabbit on day 7 postinfection had a body length and a body width of 1,273.00 ± 146.02 and 63.00 ± 7.94 μm, respectively.
Localization of native AsPPase in the hypodermis of A. suum lung-stage larvae by immunofluorescence.
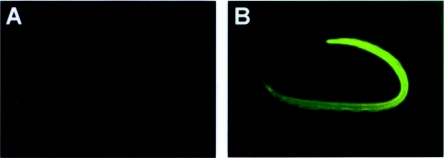
To localize the AsPPase native enzyme, lung-stage L3 were reacted with anti-rAsPPase IgG and then incubated with FITC-labeled goat anti-mouse IgG. Immunofluorescence microscopy revealed the bound IgG along the whole length of anti-rAsPPase IgG-treated larvae (Fig. 3A), while no reactivity was seen in the larvae treated with mouse preimmune IgG (Fig. 3B). The binding of anti-rAsPPase antibodies with the native enzyme intensely localized in the hypodermal tissues supports the hypothesis that the native enzyme is sensitive to inhibition by antigen-specific antibodies.
FIG. 3.
Localization of the native AsPPase in lung-stage A. suum larvae by immunofluorescence. Immunofluorescent staining of whole mounted AsLL3 was performed. (A) AsLL3 incubated with mouse preimmune IgG antibody was used as a control. Anti-mouse IgG antibody bound to the native enzyme was localized in the hypodermis along the whole length of the larva, as observed by using an Axiophot fluorescence microscope equipped with the appropriate filter sets, and was photographed. (B) AsLL3 were washed in cool PBS—0.1% Tween 20, fixed in acetone, and incubated with anti-rAsPPase IgG antibody. FITC-labeled goat anti-mouse IgG was used as a secondary antibody. Magnification, ×100.
Ultrastructure of A. suum lung-stage larvae that did not molt in the presence of anti-rAsPPase IgG.
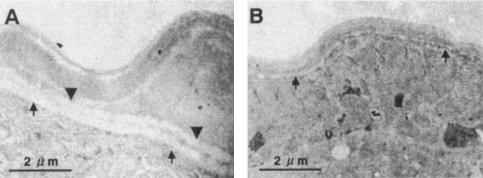
Lung-stage A. suum L3 that did not molt in the presence of anti-rAsPPase IgG were collected at day 6 postculture and processed for electron microscopy analysis. As shown in Fig. 4B, in the larvae that did not molt in the presence of anti-rAsPPase IgG there was not separation of the L3 cuticle. The larvae collected at day 3 postculture during normal molting exhibited separation of the L3 cuticle with a distinct L4 epicuticle (Fig. 4A).
FIG. 4.
Ultrastructures of A. suum lung-stage larvae during normal molting and of larvae that did not molt in the presence of anti-mouse rAsPPase IgG. Lung-stage L3 cultured in RPMI 1640 were collected at day 3 before molting was completed, and the larvae that did not molt in the presence of anti-rAsPPase IgG were collected at day 6 of in vitro culture. (A) Thin section of larva during normal development and molting. (B) Thin section of larva that did not molt in the presence of anti-rAsPPase IgG. Separation between the L4 epicuticle (arrows) and the L3 cuticle (arrowheads) is indicated.
Recombinant AsPPase-immunized mice were protected from migration of challenge A. suum larvae through the lungs.
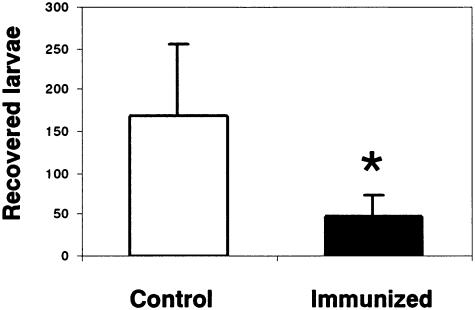
The efficacy of the rAsPPase molecule for protection against A. suum migratory-phase infection was investigated by using BALB/c mice that received two booster immunizations, followed by an oral challenge 1 week later with 2,500 A. suum embryonated eggs. The data showed that in rAsPPase-immunized mice there was a significant (P < 0.027) reduction in the number of recovered larvae (48.6 ± 26.1 larvae) compared with controls (168 ± 86.4 larvae) (Fig. 5). Thus, the level of protection was calculated to be 71.07% based on the reduction in the number of larvae recovered from immunized animals compared with controls. To test whether mouse protective immunity could also arrest larval development, we measured 50 challenge AsLL3 from immunized and control mice to determine their body lengths and body widths. We observed stunted development in this analysis; the body length (906.5 ± 85.54 μm) and body width (57.35 ± 7.22 μm) of challenge A. suum worms recovered from rAsPPase-immunized mice were less than the body length (1,207.5 ± 99.78 μm) and body width (59.22 ± 7.21 μm) of the worms grown in the control mice. The protective response induced by rAsPPase against infection by intestinal-stage A. suum (i.e., adult worms) was, however, not examined in the present study.
FIG. 5.
Protective responses induced by rAsPPase against challenge A. suum migratory-phase infection. Mice were immunized three times subcutaneously with 50 μg of rAsPPase mixed with TiterMax Gold adjuvant as described in Materials and Methods and were challenged with 2,500 A. suum embryonated eggs 1 week after the last immunization. Mice were sacrificed 1 week after the challenge, and the larvae migrating from the lungs (i.e., AsLL3) were recovered. Protective responses were evaluated by determining the reduction in the number of challenge AsLL3 recovered from rAsPPase-immunized mice compared with the nonimmunized control. The bars indicate means and the error bars indicate standard deviations for groups of five mice. The asterisk indicates that the P value is <0.05.
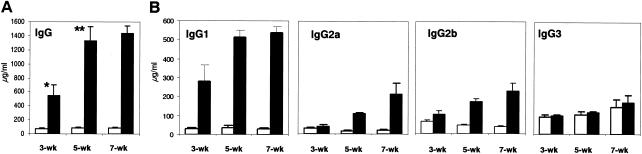
Increases in the levels of total serum IgG and IgG1 subclass antibodies and undetectable IgE responses in rAsPPase-immunized mice.
To assess the potential of rAsPPase for eliciting an antibody response in immunized mice, we measured rAsPPase-specific total IgG and IgG subclass antibodies at various times (3, 5, and 7 weeks postimmunization). The results showed that mice immunized with rAsPPase had a significantly (P < 0.014) higher level of serum IgG, and the level increased further significantly (P < 0.001) twofold when mice received the first booster immunization; however, the second booster did not induce significantly higher antibody responses (Fig. 6A). Interestingly, little or no detectable rAsPPase-specific IgE responses were seen in these mouse sera even after the first and second booster immunizations (data not shown). Histological sections of parasitized lung tissue from rAsPPase-immunized mice did not show any eosinophilic infiltration, while sections from control mice revealed moderate infiltration of eosinophils (data not shown). Together, these data indicate that rAsPPase may be a nonallergenic molecule for the host. To further assess the efficacy of rAsPPase in induction of an in vivo Th2 response, we measured IgG subclass antibody levels. Our results demonstrated that rAsPPase immunization induced a clear pattern of IgG1 response (Fig. 6B).
FIG. 6.
Serum IgG and IgG subclass responses following immunization with rAsPPase. Mice were immunized three times subcutaneously with 50 μg of rAsPPase mixed with TiterMax Gold adjuvant and were sacrificed at 3, 5, and 7 weeks postimmunization. Serum levels of anti-rAsPPase IgG (A) and IgG subclass (B) antibodies from immunized mice (solid bars) and control mice (open bars) were determined by ELISAs. The bars indicate means and the error bars indicate standard deviations for groups of five mice. One asterisk indicates that the P value is <0.05, and two asterisks indicate that the P value is <0.01.
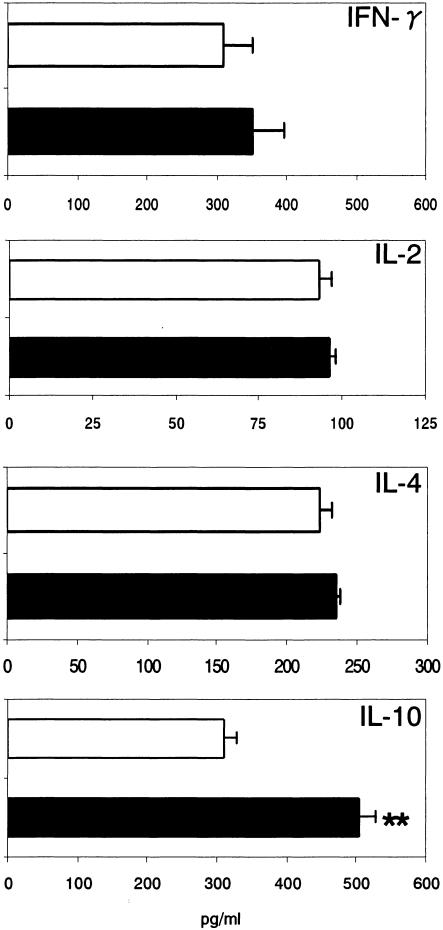
Elevated levels of IL-10 and relatively little production of IFN-γ and IL-2 in stimulated splenic cell culture supernatants.
We examined the cytokine levels in rAsPPase-stimulated splenic cell culture supernatants to determine the T-cell response to the rAsPPase molecule in immunized mice. The in vitro-stimulated splenic T cells from rAsPPase-immunized mice exhibited an elevated level of Th2-type cytokine IL-10; however, only a modest increase in the IL-4 level was observed compared with the level in stimulated T cells from nonimmunized controls (Fig. 7). By contrast, stimulated T cells from immunized mice showed only small increases in both IFN-γ and IL-2 (Th1-type cytokines) compared with T cells from nonimmunized controls (Fig. 7). These data suggest that the rAsPPase molecule induces predominantly a Th2-type immune response in mice.
FIG. 7.
Th1- and Th2-type cytokine responses in rAsPPase-stimulated splenic cell culture supernatants. Splenic T cells were isolated from mice 2 weeks after the last immunization. Levels of Th1-type (IFN-γ and IL-2) and Th2-type (IL-4 and IL-10) cytokines were determined by cytokine-specific ELISAs of cell culture supernatants from rAsPPase-immunized mice (solid bars) and control mice (open bars) after 72 h of in vitro stimulation with 10 μg of rAsPPase per ml. The bars indicate means and the error bars indicate standard deviations for groups of five mice. Each sample was examined in triplicate. Two asterisks indicate that the P value is <0.01.
DISCUSSION
PPases are ubiquitous in living cells and are known to play a role in energy metabolism, providing a thermodynamic pull for many biosynthetic reactions (26). Previously, we identified PPase in the roundworm Ascaris and proposed that the enzyme may be involved in the worm's molting processes (23). In this study we obtained evidence that AsPPase is essential for molting and normal development of A. suum larvae. Furthermore, we found that AsPPase mediates a distinct host protective mechanism and may be a potential vaccine candidate for use against A. suum infection.
The basic structure of the body wall of parasitic nematodes consists of the cuticle, an underlying syncytial or cellular layer called the hypodermis, and the longitudinally oriented somatic musculature. The entire cuticle is shed at each of four molts and is replaced with a new cuticle during the life cycle of all nematodes. Molting precedes in three major steps: (i) the old cuticle separates from the hypodermis (apolysis); (ii) components of the new cuticle are synthesized in the hypodermis and are secreted across the hypodermal membrane into the space between it and the old cuticle; and (iii) the old cuticle is ruptured and shed (ecdysis) (27). In nematode parasites, molting is crucial for development to maturity. Nevertheless, the molecular mechanisms regulating this complex process in human and animal parasitic nematodes are poorly understood. Although aminopeptidase, cysteine protease, and hyaleuronidase were previously proposed to be associated with the molting of A. suum L3, none of the associations has been confirmed (35). The cuticle of a parasitic nematode is considered to be a dynamic structure with important absorptive, secretory, and enzymatic activities (28) and not merely an inert protective covering, as was once believed. The presence of AsPPase in the hypodermis of both adult worms (23) and larvae (Fig. 3) suggests that this enzyme may play a functional role in the synthesis of components of the new cuticle required for molting. This suggestion is supported by the biochemical finding that PPase-activated PPi hydrolysis is essential for maintaining the forward direction of many biosynthetic reactions, like synthesis of DNA, RNA, proteins, and polysaccharides (26). Elevated expression of native AsPPase prior to and during molting (Table 1) also implies that it acts as a molting enzyme. These results suggest that roundworm PPase is a very important enzyme for molting. Based on the findings described above, we disrupted the target gene by RNAi to confirm the precise role of AsPPase in the molting of A. suum larvae. Our results demonstrated that treatment with dsRNA inhibited the molting of L3 to L4 (31%) and caused developmental arrest as well. Liposome-mediated transfection, however, did not enhance the inhibition of larval molting, and the reasons for this remain unclear. Expression of the native protein was found to be suppressed, as measured by native enzyme activity (Table 2) and by immunoblotting of larval extracts and immunofluorescence of whole mounted larvae (Fig. 1A to E). Furthermore, complete disruption of AsPPase mRNA levels was observed in the RT-PCR analysis (Fig. 1F). These results suggest that posttranscriptional gene silencing was achieved in A. suum larvae treated with sequence-specific dsRNA. Thus, our results established a clear in vivo function of the PPase enzyme in the molting and development of parasitic roundworms. Recently, Hashmi et al. (18) showed the usefulness of the RNAi technology for conclusively confirming the role of cathepsin Z-like cysteine proteinase in the molting of the model nematode C. elegans. Attempts were also made to determine whether neutralization of the target enzyme by a specific antibody provides a link between native AsPPase and molting. Encouragingly, our data showed that treatment with anti-rAsPPase IgG resulted in an impressive level of inhibition of molting of AsLL3 to L4 in vitro (Fig. 2). Electron microscopy data also supported this. The treated larvae showed developmental arrest as well. These data are consistent with previous observations that anti-rAsPPase IgG could partially inhibit native AsPPase activity in larval extracts (23) and are supported by the binding of anti-rAsPPase IgG to the larval hypodermal tissues, as observed by immunofluorescence. These data further suggest that AsPPases play a role in the molting and development of the parasitic roundworm Ascaris.
Unlike other nematodes, Ascaris has a complex life cycle that includes a tissue-invasive phase involving the liver and lung and migrating L3 and L4 and a noninvasive phase involving adult worms in the gut lumen of the natural host. Specific events in these two phases may provoke different host immune responses against the larval stages in the tissues and the adult worms in the small intestine of the natural host. The development of A. suum in mice includes passage through the larval stages before development into adult worms. Our results clearly show that rAsPPase-mediated immune responses are capable of protecting (>70%) against a challenge A. suum migratory-phase infection (i.e., the tissue-invasive phase) (Fig. 5). Recent studies in our laboratory in which an A. suum-mouse model was used revealed that immunization with A. suum recombinant antigens designated rAs14 and rAs16 induced 58 to 64% protection against A. suum migration to the lungs (46, 47). Whether the protective responses to rAsPPase operate against the intestinal adult stage should be studied further with swine as the natural host. In fact, immune responses against tissue-dwelling helminths are different from immune responses against gastrointestinal parasites (15, 20).
Protective immunity to A. lumbricoides infection has been shown to be associated with high levels of serum IgG and IgE responses (6, 16, 29). Similar antibody responses have also been reported in animals immunized either by repeated inoculation of A. suum embryonated eggs or by parasite crude or recombinant antigens (25, 38, 46, 47). However, no one has addressed the functional significance of the antigen-specific antibodies in protective responses to Ascaris infection. We found that mice immunized with rAsPPase developed high levels of antigen-specific IgG antibody; however, no antigen-specific IgE response or tissue eosinophilia was detected in these mice. The protective mechanism directed against larval migration and stunted development are thought to be mediated through neutralization of the native AsPPase enzyme by antigen-specific IgG and not by tissue eosinophils. This hypothesis is supported by the sensitivity of the native enzyme to binding and inhibition by specific IgG antibody and epitope analysis data (Fig. 2 and 3) (23). Since the nematode cuticle is a dynamic structure with an absorptive function (28), we hypothesized that an antibody gains access through the cuticle of larvae during incubation with it. The absence of tissue eosinophils and other inflammatory cells (data not shown) in the parasitized lungs of immunized mice suggests that these cells play no role in protection against A. suum migratory infection. Further studies are required to elucidate in detail the mechanisms of protection directed against migrating A. suum larvae. Analysis of the IgG subclass profile showed that rAsPPase induces a clear pattern of IgG1 responses, suggesting that induction by rAsPPase of systemic antibody is involved in distinct mechanisms of host protection. Furthermore, epitope analysis showed that rAsPPase-immunized mice recognize epitopes spread across the whole antigen, overlapping 4 of 13 enzyme active site residues. These results suggest that anti-IgG antibodies have the potential for both host protection and enzyme inhibition. These findings are consistent with our previous data showing that anti-IgG antibodies can partially inhibit native AsPPase activity (25%), compared with the 80% inhibition observed with NaF (23). The difference between enzyme inhibition by anti-IgG antibodies and by NaF might be due to the fact that anti-IgG antibodies bind to 4 active site residues, while NaF binds to all 13 active site residues of the AsPPase.
The observations for antibody responses are consistent with the cytokine profiles which showed that in vitro stimulation of splenic T cells from rAsPPase-immunized mice resulted in low levels of the Th1-type cytokines IFN-γ and IL-2 but an elevated level of the Th2-type cytokine IL-10 and a modest increase in the level of IL-4. Elevated production of IL-10 by CD4+ T cells might suppress synthesis of IFN-γ and IL-2 by Th1 cells and CD8+ lymphocytes in rAsPPase-immunized mice, since IL-10 is known to inhibit the production of IFN-γ and IL-2 (12, 39). It is likely that a low level of IL-4 synthesis in these mice accounts for little or no IgE response and tissue eosinophilia (11). Dominant IL-10 production together with production of IFN-γ and IL-2 but not IL-4 has been reported for BALB/c mice immunized with rAs16 (46). It would be interesting to examine whether IL-10 production in rAsPPase-immunized mice down regulates Th1 cytokine synthesis by in vitro stimulated splenic T cells by using neutralizing anti-IL-10 antibody. We hypothesized that rAsPPase may play an important role as an IL-10-expressing immunomodulatory molecule in Ascaris infection and that IL-10 may be crucial for the development host protective immunity since such immunity is thought to be instrumental in immunoglobulin heavy chain switching (14). Our hypothesis is also supported by the observation that Ascaris roundworms appear to have potential immunomodulatory effects on the host immune system (5, 33). IL-10 was recently shown to be important for the development of resistance and to be critical for the survival of Trichuris muris-infected mice (37).
In summary, we show the usefulness of RNAi for abrogating the AsPPase gene function in vivo in molting of the parasitic roundworm Ascaris. Moreover, AsPPase was found to be a potent immunogenic protein which can induce a distinct mechanism for host protection and may become a potential target for the development of a vaccine and/or chemotherapeutic agent for ascariasis.
Acknowledgments
We thank Y. Ando and T. Fujisawa for their excellent technical assistance.
This work was supported in part by a grant from the Zoonosis Control Program (grant ZCP22) of the Ministry of Agriculture, Forestry and Fisheries and by a grant from the Program for Promotion of Basic Research Activities for Innovative Biosciences (PROBRAIN). M.K.I. was supported by a JSPS postdoctoral fellowship.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Abebe, W., N. Tsuji, H. Kasuga-Aoki, T. Miyoshi, T. Isobe, T. Arakawa, Y. Matsumoto, and Y. Yoshihara. 2002. Lung-stage protein profile and antigenic relationship between Ascaris lumbricoides and Ascaris suum. J. Parasitol. 88:811-816. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, T. J., M. E. Romero-Abal, and J. Jaenike. 1993. Genetic structure and epidemiology of Ascaris populations: patterns of host affiliation in Guatemala. Parasitology 107:319-334. [DOI] [PubMed] [Google Scholar]
- 3.Bancroft, A. J., and R. K. Grencis. 1998. Th1 and Th2 cells and immunity to intestinal helminths. Chem. Immunol. 71:192-208. [DOI] [PubMed] [Google Scholar]
- 4.Chen, J., A. Brevet, M. Fromant, F. Lévêque, J. M. Schmitter, S. Blanquet, and P. Plateau. 1990. Pyrophosphatase is essential for growth of Escherichia coli. J. Bacteriol. 172:5686-5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper, P. J., M. Chico, C. Sandoval, I. Espinel, A. Guevara, M. M. Levine, G. E. Griffin, and T. B. Nutman. 2001. Human infection with Ascaris lumbricoides is associated with suppression of the interleukin-2 response to recombinant cholera toxin B subunit following vaccination with the live oral cholera vaccine CVD 103-HgR. Infect. Immun. 69:1574-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper, P. J., M. E. Chico, C. Sandoval, I. Espinel, A. Guevara, M. W. Kennedy, J. F. Urban, Jr., G. E. Griffin, and T. B. Nutman. 2000. Human infection with Ascaris lumbricoides is associated with a polarized cytokine response. J. Infect. Dis. 182:1207-1213. [DOI] [PubMed] [Google Scholar]
- 7.Cooperman, B. S., A. A. Baykov, and R. Lahti. 1992. Evolutionary conservation of the active site residue of soluble inorganic pyrophosphatase. Trends Biochem. Sci. 17:262-266. [DOI] [PubMed] [Google Scholar]
- 8.Cox, E. F. G., and F. Y. Liew. 1992. T-cell subsets and cytokines in parasitic infections. Immunol. Today 13:445-448. [DOI] [PubMed] [Google Scholar]
- 9.Finkelman, F. D., T. A. Wynn, D. D. Donaldson, and J. F. Urban, Jr. 1999. The role of IL-13 in helminth-induced inflammation and protective immunity against nematode infections. Curr. Opin. Immunol. 11:420-426. [DOI] [PubMed] [Google Scholar]
- 10.Finkelman, F. D., T. Shea-Donohue, J. Goldhill, C. A. Sullivan, S. C. Morris, K. B. Madden, W. C. Gause, and J. F. Urban, Jr. 1997. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu. Rev. Immunol. 15:505-534. [DOI] [PubMed] [Google Scholar]
- 11.Finkelman, F. D., E. J. Pearce, J. F. Urban, Jr., and A. Sher. 1991. Regulation and biological function of helminth-induced cytokine responses. Immunol. Today 12:A62-66. [DOI] [PubMed] [Google Scholar]
- 12.Fiorentino, D. F., M. A. Bond, and T. R. Mosman. 1989. Two types of T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 170:2081-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fire, A., S. Xu, M. K. Montgomery, S. A. Kostas, S. E. Driver, and C. C. Mello. 1998. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391:806-811. [DOI] [PubMed] [Google Scholar]
- 14.Garraud, O., R. Perraut, G. Riveau, and T. B. Nutman. 2003. Class and subclass selection in parasite-specific antibody responses. Trends Parasitol. 19:300-304. [DOI] [PubMed] [Google Scholar]
- 15.Garside, P., M. W. Kennedy, D. Wakelin, and C. E. Lawrence. 2000. Immunopathology of intestinal helminth infection. Parasite Immunol. 22:605-612. [DOI] [PubMed] [Google Scholar]
- 16.Geiger, S. M., C. L. Masssara, J. Bethony, P. T. Soboslay, O. S. Carvalho, and R. Corrêa-Oliveira. 2002. Cellular responses and cytokine profiles in Ascaris lumbricoides and Trichuris trichiura infected patients. Parasite Immunol. 24:499-509. [DOI] [PubMed] [Google Scholar]
- 17.Hakki, S., and A. Sitaramayya. 1990. Guanylate cyclase from bovine rod outer segments: solubilization, partial purification, and regulation by inorganic pyrophosphatase. Biochemistry 29:1088-1094. [DOI] [PubMed] [Google Scholar]
- 18.Hashmi, S., J. Zhang, Y. Oksov, and S. Lustigman. 2003. The Caenorhabditis elegans cathepsin Z-like protease, Ce-CPZ-1, has a multifunctional role during the worm's development. J. Biol. Chem. 279:6035-6045. [DOI] [PubMed] [Google Scholar]
- 19.Hlaing, T. 1993. Ascariasis and childhood malnutrition. Parasitology 107:S125-S136. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann, K. F., A. W. Cheever, and T. A. Wynn. 2000. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J. Immunol. 164:6406-6416. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann, K. F., T. A. Wynn, and D. W. Dunne. 2002. Cytokine-mediated host responses during schistosome infections: walking the fine line between immunological control and immunopathology. Adv. Parasitol. 52:265-307. [DOI] [PubMed] [Google Scholar]
- 22.Hussein, A. S., K. Kichenin, and M. E. Selkirk. 2002. Suppression of secreted acetylcholinesterase expression in Nippostrongylus brasiliensis by RNA interference. Mol. Biochem. Parasitol. 122:91-94. [DOI] [PubMed] [Google Scholar]
- 23.Islam, M. K., T. Miyoshi, H. Kasuga-Aoki, T. Isobe, T. Arakawa, Y. Matsumoto, and N. Tsuji. 2003. Inorganic pyrophosphatase in the roundworm Ascaris and its role in the development and molting process of the larval stage parasites. Eur. J. Biochem. 270:2814-2826. [DOI] [PubMed] [Google Scholar]
- 24.Jelitto, T., U. Sonnewald, L. Willmitzer, M. Hajirezeai, and M. Stitt. 1992. Inorganic pyrophosphate content and metabolites in potato and tobacco plants expressing Escherichia coli pyrophosphatase in their cytosol. Planta 188:238-244. [DOI] [PubMed] [Google Scholar]
- 25.Jungersen, G., L. Eriksen, A. Roepstorff, P. Lind, E. N. Meeusen, T. Rasmussen, and P. Nansen. 1999. Experimental Ascaris suum infection in the pig: protective memory response after three immunizations and effect of intestinal adult worm population. Parasite Immunol. 21:619-630. [DOI] [PubMed] [Google Scholar]
- 26.Kornberg, A. 1962. On the metabolic significance of phosphorolytic and pyrophosphorolytic reactions, p. 251-264. In M. Kasha and B. Pullman (ed.), Horizons in biochemistry. Academic Press, Inc., New York, N.Y.
- 27.Lee, D. L. 2002. Cuticle, moulting and exsheathment, p. 171-209. In D. L. Lee (ed.), The biology of nematodes. Taylor & Francic, London, United Kingdom.
- 28.Maizels, R. M., M. L. Blaxter, and M. E. Selkirk. 1993. Forms and functions of nematode surfaces. Exp. Parasitol. 77:380-384. [DOI] [PubMed] [Google Scholar]
- 29.McSharry, C., Y. Xia, C. V. Holland, and M. W. Kennedy. 1999. Natural immunity to Ascaris lumbricoides associated with immunoglobulin E antibody to ABA-1 allergen and inflammation indicators in children. Infect. Immun. 67:484-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mosmann, T. R., and R. L. Coffman. 1989. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145-173. [DOI] [PubMed] [Google Scholar]
- 31.Mosmann, T. R., and S. Sad. 1996. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol. Today 17:138-146. [DOI] [PubMed] [Google Scholar]
- 32.Narasimhan, S., R. R. Montgomery, K. dePonte, C. Tschudi, N. Marcantonio, J. F. Anderson, J. R. Sauer, M. Cappello, F. S. Kantor, and E. Fikrig. 2004. Disruption of Ixodes scapularis anticoagulation by using RNA interference. Proc. Natl. Acad. Sci. USA 101:1141-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paterson, J. C. M., P. Garside, M. W. Kennedy, and C. E. Lawrence. 2002. Modulation of a heterologous immune response by the products of Ascaris suum. Infect. Immun. 70:6058-6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng, W., T. J. Anderson, X. Zhou, and M. W. Kennedy. 1998. Genetic variation in sympatric Ascaris populations from humans and pigs in China. Parasitology 117:355-361. [DOI] [PubMed] [Google Scholar]
- 35.Rhoads, M. L., R. H. Fetterer, and J. F. Urban. 2001. Release of hyaluronidase during in vitro development of Ascaris suum from third to fourth larval stage. Parasitol. Res. 87:693-697. [DOI] [PubMed] [Google Scholar]
- 36.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 37.Schopf, L. R., K. F. Hoffmann, A. W. Cheever, J. F. Urban, Jr., and T. A. Wynn. 2002. IL-10 is critical for host resistance and survival during gastrointestinal helminth infection. J. Immunol. 168:2383-2392. [DOI] [PubMed] [Google Scholar]
- 38.Serrano, F. J., D. Reina, E. Frontera, A. Roepstorff, and I. Navarrete. 2001. Resistance against migrating Ascaris suum larvae in pigs immunized with infective eggs or adult worm antigens. Parasitology 122:699-707. [DOI] [PubMed] [Google Scholar]
- 39.Sher, A., D. Fiorentino, P. Caspar, E. Pearce, and T. Mosmann. 1991. Production of IL-10 by CD4+ T lymphocytes correlates with down regulation of Th1 cytokine synthesis in helminth infection. J. Immunol. 147:2713-2716. [PubMed] [Google Scholar]
- 40.Sher, S., and R. L. Coffman. 1992. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu. Rev. Immunol. 10:385-409. [DOI] [PubMed] [Google Scholar]
- 41.Skelly, P. J., A. Da'dara, and D. A. Harn. 2003. Suppression of cathepsin B expression in Schistosoma mansoni by RNA interference. Int. J. Parasitol. 33:363-369. [DOI] [PubMed] [Google Scholar]
- 42.Sonnewald, U. 1992. Expression of Escherichia coli inorganic pyrophosphatase in transgenic plants alters photoassimilate partitioning. Plant J. 2:571-581. [PubMed] [Google Scholar]
- 43.Tabara, H., A. Grishok, and C. C. Mello. 1998. RNAi in Caenorhabditis elegans: soaking in the genome sequence. Science 282:430-431. [DOI] [PubMed] [Google Scholar]
- 44.Tripathy, K., E. Duque, O. Bolanos, H. Lotero, and L. G. Mayoral. 1972. Malabsorption syndrome in ascariasis. Am. J. Clin. Nutr. 25:1276-1281. [DOI] [PubMed] [Google Scholar]
- 45.Tsuji, N., H. Kasuga-Aoki, T. Isobe, and S. Yoshihara. 2000. Cloning and characterization of a peroxiredoxin from the swine roundworm Ascaris suum. Int. J. Parasitol. 30:125-128. [DOI] [PubMed] [Google Scholar]
- 46.Tsuji, N., K. Suzuki, H. Kasuga-Aoki, T. Isobe, T. Arakawa, and Y. Matsumoto. 2003. Mice intranasally immunized with a recombinant 16-kilodalton antigen from roundworm Ascaris parasites are protected against larval migration of Ascaris suum. Infect. Immun. 71:5314-5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsuji, N., K. Suzuki, H. Kasuga-Aoki, Y. Matsumoto, T. Arakawa, K. Ishiwata, and T. Isobe. 2001. Intranasal immunization with recombinant Ascaris suum 14-kilodalton antigen coupled with cholera toxin B subunit induces protective immunity to A. suum infection in mice. Infect. Immun. 69:7285-7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsuji, N., T. H. Morales, V. V. Ozols, A. B. Carmody, and R. Chandrashekar. 2000. Cloning and preliminary characterization of a novel cuticular antigen from the filarial parasite Dirofilaria immitis. Parasitol. Int. 49:321-325. [DOI] [PubMed] [Google Scholar]
- 49.Turner, J. D., H. Faulkner, J. Kamgno, F. Cormont, J. Van Snick, K. J. Else, R. K. Grencis, J. M. Behnke, M. Boussinesq, and J. E. Bradley. 2003. Th2 cytokines are associated with reduced worm burdens in a human intestinal helminth infection. J. Infect. Dis. 188:1768-1775. [DOI] [PubMed] [Google Scholar]
- 50.Visser, K., S. Heimovaara-Dijkstra, J. W. Kijne, and M. Wang. 1998. Molecular cloning and characterization of an inorganic pyrophosphatase from barley. Plant Mol. Biol. 37:131-140. [DOI] [PubMed] [Google Scholar]
- 51.Yang, Z., and T. G. Wensel. 1992. Inorganic pyrophosphatase from bovine rod outer segments. J. Biol. Chem. 267:24634-24640. [PubMed] [Google Scholar]