Abstract
Ring-stage parasitized erythrocytes (RPEs) were demonstrated to interact with effector cells of the innate immune system. With receptor blockade studies and CD36-null macrophages, human and murine macrophages were shown to phagocytose RPEs through the pattern recognition receptor CD36. These in vitro data implicate scavenger receptors in the clearance of RPEs.
Phagocytic clearance of Plasmodium falciparum-parasitized erythrocytes (PEs) is an important line of innate defense against malaria (20, 25). Pattern recognition receptors, including CD36, have been implicated in innate clearance of mature-stage PEs (MPEs) by monocytes and macrophages (3, 12, 13, 15, 21, 22, 24, 26). Ring-stage PEs (RPEs) have recently been shown to express ligands capable of interacting with endothelial cells (5, 16, 23, 29). The objective of this study was to examine whether RPEs might also interact with effector cells of the innate immune system.
We demonstrate that murine and human monocyte-derived macrophages can recognize and phagocytose RPEs (see Fig. 1 to 3). To ensure that only ring-stage parasites were used in phagocytosis assays (15, 21), we used procedures (2, 10) to generate highly synchronous ring-stage cultures (purity, >99% RPEs) and used cryopreserved malaria isolates and fresh clinical isolates that only contained RPEs (confirmed by microscopic criteria) (see Fig. 4). To ensure that only RPEs were quantified in phagocytic assays, we used a strict lysis procedure to remove adherent erythrocytes and morphological criteria (12, 23) to ensure that only phagocytosed PE were counted. The slides were read blinded to the experimental conditions and confirmed by a second reader.
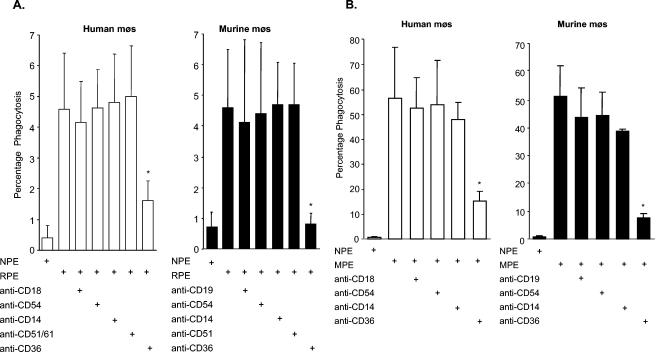
FIG. 1.
Anti-CD36 antibody reduces phagocytosis of RPEs by human and murine macrophages (mφs). Human monocyte-derived macrophages and murine macrophages were cotreated with IgG Fc fragments (20 μg/ml) to block Fc receptors and 10 μg of MAbs to human or murine macrophage receptors per ml (where indicated), followed by incubation with non-PEs (NPE) or RPEs (A) and MPEs (B) (12, 15). Phagocytosis was assessed by light microscopy by counting 500 to 1,000 cells and expressed as the percentage of macrophages that had phagocytosed at least one RPE. The results show that Fc receptor-blocked human and murine macrophages can internalize RPEs. CD36 receptor blockade with 10 μg of anti-CD36 MAb per ml resulted in a significant decrease in phagocytosis of nonopsonized RPEs by human macrophages (mean decrease of 65%, left side) and murine macrophages (mean decrease of 82%, right side). MAbs to ICAM-1 (CD54), CD18 or CD19 (isotype controls), integrins (CD51/61, CD51), and CD14 did not result in a significant decrease in phagocytosis. Experiments were performed in triplicate, and the data shown represent the mean ± the standard deviation of at least six independent experiments. (*, P < 0.05 for RPE versus anti-CD36; Student's t test).
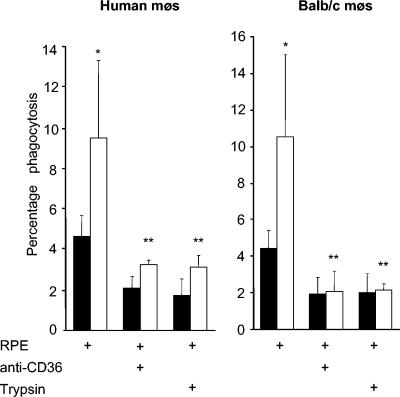
FIG. 3.
Upregulating CD36 increases phagocytosis of RPEs obtained from synchronized cultures. Human monocyte-derived macrophages (mφs) (left side) and BALB/c murine macrophages (right side) were treated with 50 μM troglitazone (□) or with dimethyl sulfoxide as a control (▪) and then used in RPE phagocytosis assays as previously described (21). Fc receptors were blocked in all assays, and where indicated, CD36 was blocked by preincubation with 10 μg of FA6-152 per ml for human macrophages and clone 63 for murine macrophages, or RPEs were pretreated with 50 μg of trypsin per ml and washed before use in phagocytosis assays. Data shown are means ± standard deviations of at least four independent experiments. Phagocytosis of RPEs was increased in macrophages treated with 50 μM troglitazone for 48 h (∼108% ± 31% and 140% ± 33% for human and murine macrophages, respectively) compared to phagocytosis by control macrophages (*, P < 0.01; Student's t test; n = 12 per group). Anti-CD36 and trypsin treatment significantly reduced RPE uptake by troglitazone-treated macrophages (**, P < 0.01).
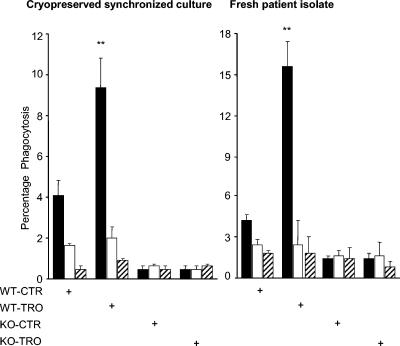
FIG. 4.
Upregulating CD36 increases phagocytosis of RPEs obtained from cryopreserved synchronized cultures and from patient isolates. CD36 wild-type (WT) and CD36-null (KO) murine macrophages were used with cryopreserved synchronized ring-stage parasite cultures (left side) and a fresh patient isolate of ring-stage P. falciparum (right side). Macrophages were treated with 50 μM troglitazone (TRO) or with dimethyl sulfoxide as a control (CTR) for 48 h and then used in RPE phagocytosis assays. Where indicated, RPE suspensions were treated with 50 μg of trypsin per ml and macrophages were treated with 10 μg of anti-CD36 (clone 63) per ml. After incubation and hypotonic lysis to remove noninternalized PEs, macrophages were fixed and stained with Diff-Quik stain and phagocytosis was assessed by light microscopy. Data show a significant increase (**, P < 0.01; Student's t test) in RPE uptake with cryopreserved synchronized RPE cultures (left side, ∼130%) and a patient isolate of RPEs (right side, ∼260%). Blocking CD36 and treating cultures with trypsin significantly reduced phagocytosis of RPEs. In all cases, results are presented as the mean ± the standard deviation of at least four independent experiments. Macrophages were treated with IgG Fc fragments and incubated with RPEs (▪) or subjected to an anti-CD36 blockade (□) or trypsin treatment (▨).
To investigate the molecular mechanisms underlying RPE phagocytosis, we undertook receptor blockade studies with monoclonal antibodies (MAbs) against macrophage receptors known to interact with PEs and against macrophage pattern recognition receptors (4, 9) (Fig. 1). Only CD36 receptor blockade resulted in a significant decrease in RPE or MPE phagocytosis. Inhibition of phagocytosis was confirmed with two separate parasite lines: 3D7 (30) and ITG (17).
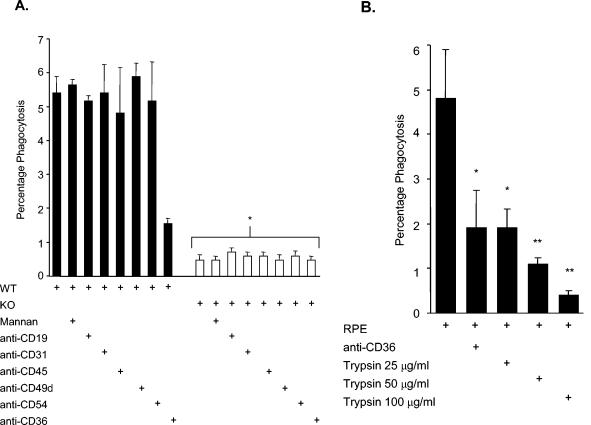
To confirm the role of CD36 in mediating RPE uptake, we examined phagocytosis by CD36 wild-type and CD36-null macrophages (7, 15). Phagocytosis of RPEs was reduced by ∼90% in CD36-null macrophages. To investigate whether other macrophage receptors might cooperate with CD36 or mediate uptake in the absence of CD36 (1, 6, 14, 18, 19, 28, 29), we performed phagocytosis assays with wild-type and CD36-null macrophages in the presence of MAbs to other macrophage surface receptors. MAb blockade of receptors other than CD36 did not significantly decrease uptake of RPEs (Fig. 2A). Since pattern recognition receptors such as the mannose receptor have also been implicated in opsonin-independent phagocytosis of P. chabaudi (26), we investigated whether the mannose receptor contributed to RPE uptake. As shown in Fig. 2A, mannan did not significantly decrease uptake of RPEs but did inhibit uptake of MPEs compared to β-glucan treatment (data not shown).
FIG. 2.
CD36 and protease-sensitive ligands mediate the uptake of RPEs by murine macrophages. (A) CD36 wild-type (WT) (▪) or CD36-null (KO [knockout]) (□) murine macrophages were incubated in the presence of a panel of MAbs (as indicated) prior to phagocytosis assays with RPEs. Fc receptor-blocked macrophages were also treated with 3 μg of mannan per ml at room temperature for 30 min to block the mannose receptor. Phagocytosis of RPEs by CD36-null murine macrophages was reduced by ∼90% compared to phagocytosis by CD36 wild-type murine macrophages (*, P < 0.01; n = ≥4). (B) Treating RPEs with trypsin reduces their phagocytosis by murine macrophages. Cultures containing nonopsonized RPEs were incubated with different concentrations of trypsin (25, 50, and 100 μg/ml), washed repeatedly, and then incubated with macrophages. Phagocytosis of trypsin-treated RPEs was reduced by ∼60% ± 23%, 77% ± 18%, and 92% ± 20% (mean ± standard deviation), respectively, by treatment with 25, 50, and 100 μg of trypsin per ml compared to phagocytosis of control nontreated RPEs. Treatment with 25 μg of trypsin per ml decreased phagocytosis of RPEs to a degree similar to that of receptor blockade by treatment with 10 μg of anti-CD36 per ml (*, P < 0.05). When the concentration of trypsin used for treatment was increased, RPE uptake decreased proportionally compared to control uptake (**, P < 0.01; n = 3).
Adhesion of MPEs to CD36 is primarily mediated by PfEMP-1. PfEMP-1 is removed from the surface of PEs following mild protease treatment, and cleaving this ligand has been shown to decrease macrophage uptake of MPEs (12, 15, 21). Cytoadhesion of RPEs to endothelial cells has also been reported to be protease sensitive (16). We investigated whether the phagocytosis of RPEs is also dependent upon protease-sensitive ligands on RPEs. Cleavage of surface ligands from RPEs with trypsin reduced the phagocytic clearance of RPEs to the level observed with CD36-null macrophages (Fig. 2B).
The CD36 gene promoter contains a response element recognized by the nuclear receptor heterodimer PPARγ-RXR (8, 27), and PPARγ-RXR agonists increase CD36 expression and uptake of MPEs (15, 21, 22). We tested the hypothesis that PPARγ agonists would increase macrophage uptake of RPEs by upregulating CD36 or other macrophage pattern recognition receptors. Phagocytosis of synchronized RPEs (Fig. 3) or RPEs from cryopreserved or fresh clinical isolates (Fig. 4) was significantly increased by pretreatment of macrophages with PPARγ agonists. The increased uptake appeared to be CD36 dependent since it was eliminated by CD36 receptor blockade.
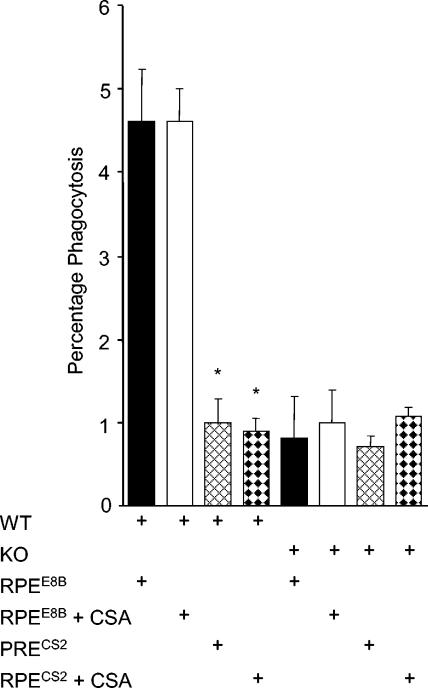
RPE adhesion has been reported in parasites that subsequently acquire the chondroitin sulfate A (CSA)-binding phenotype (16). We examined phagocytosis of RPEs from parasite lines E8B, which binds to CD36, and CS2, which binds to CSA. The uptake of CS2 RPEs was significantly lower than that of E8B RPEs (Fig. 5).
FIG. 5.
RPEs derived from CSA-binding isolate CS2 are not phagocytosed by macrophages. Phagocytosis of RPEs by Fc receptor-blocked (CD36 wild-type [WT] and CD36-null [KO]) macrophages was performed with RPEs from parasite line E8B, which binds CD36 (RPEE8B), and parasite line CS2, which binds CSA (RPECS2). Macrophages pretreated with 20 μg of Fc fragments per ml were also incubated with 1 mg of CSA per ml for 30 min where indicated. Compared to the uptake of RPEs derived from E8B, the uptake of RPEs derived from CS2 is about fivefold lower. Addition of CSA had no significant effect. Experiments were performed in duplicate, and the data shown are representative of three independent experiments. (*, P < 0.01 for RPEE8B versus RPECS2 or RPEE8B plus CSA versus RPECS2 plus CSA; Student's t test; n = 3).
Increasing evidence indicates that innate immune responses contribute to the control of blood-stage malaria infection, lighten the parasite burden, and decrease progression to severe disease (25). In this study, we examined the ability of RPEs to interact with effector cells of the innate immune system. We present evidence that ligands expressed by RPEs confer the capacity for recognition and uptake by macrophages. We demonstrate that human monocyte-derived macrophages and murine macrophages are able to phagocytose RPEs in vitro even in the absence of opsonization and in the presence of an Fc receptor blockade. A role for the scavenger receptor CD36 in mediating nonopsonic phagocytosis of RPEs was supported by the observations that uptake was inhibited by a CD36 blockade, by the cleavage of putative CD36 ligands from the RPEs, and by the elimination of uptake of RPEs by CD36-null macrophages. Furthermore, RPE uptake was significantly increased by cotreatment with the PPARγ agonists, which upregulate surface levels of CD36. These data suggest that nonopsonic uptake in vitro appears to be largely dependent on CD36 and not on other macrophage receptors such as ICAM-1, αvβ3, or PECAM-1 or pattern recognition receptors such as the mannose receptor or CD14. These observations are similar to those reported for uptake of MPEs by macrophages (12, 15, 21); however, different surface ligands may interact with CD36 in these cases. For MPEs, previous studies suggest that PfEMP-1 is the primary ligand interacting with CD36 (12, 15, 21). RPEs may express one or more surface ligands capable of interacting with CD36. Pouvelle and colleagues (16) have shown that RPE can adhere to an unknown receptor on syncytiotrophoblast and endothelial cells. Adhesion was dependent upon the presence of a parasite rhoptry-derived ring surface protein, RSP-2 (5). Their studies indicate that RSP-2 appears earlier on malaria-infected erythrocytes than does PfEMP-1, but both ligands may be simultaneously present on the same erythrocyte. In contrast, Udomsangpetch and colleagues attributed RPE cytoadherence to PfEMP-1 (29). The var genes that encode PfEMP-1 have been shown to be expressed as early as 3 h after merozoite invasion (11). Recent data indicate that PfEMP-1 is expressed in RPEs and that trafficking to the membrane is facilitated at elevated temperatures (29). Additional study is required to better characterize the RPE ligand(s) that interacts with CD36.
In summary, our data indicate that the scavenger receptor CD36 participates in clearance of RPEs in vitro and may contribute to innate control of blood-stage infection; however this will require confirmation in vivo.
Acknowledgments
This work was supported by Canadian Institutes of Health Research (CIHR) operating grant MT-13721 (K.C.K.), a CIHR Canada Research Chair (K.C.K.), and a CIHR fellowship (L.S.).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Berendt, A. R., D. L. Simmons, J. Tansey, C. K. Newbold, and K. Marsh. 1989. Intercellular adhesion molecule 1 (ICAM-1) is an endothelial cytoadherence receptor for Plasmodium falciparum. Nature 341:57-59. [DOI] [PubMed] [Google Scholar]
- 2.Brau-Breton, C., T. L. Rosenberry, and L. H. Pereira da Silva. 1988. Induction of the proteolytic activity of a membrane protein in Plasmodium falciparum by phosphatidyl inositol-specific phospholipase C. Nature 332:457-459. [DOI] [PubMed] [Google Scholar]
- 3.Chuluyan, H. E., and A. C. Issekutz. 1993. VLA-4 can mediate CD11/CD18-independent transendothelial migration of human monocytes. J. Clin. Investig. 92:2768-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deitsch, K. W., and T. E. Wellems. 1996. Membrane modifications in erythrocytes parasitized by Plasmodium falciparum. Mol. Biochem. Parasitol. 76:1-10. [DOI] [PubMed] [Google Scholar]
- 5.Douki, J. B., Y. Sterkers, C. Lépolard, B. Traore, F. T. Coasta, A. Scherf, and J. Gysin. 2003. Adhesion of normal and Plasmodium falciparum ring-infected erythrocytes to endothelial cells and the placenta involves the rhoptry-derived ring surface protein-2. Blood 101:5025-5032. [DOI] [PubMed] [Google Scholar]
- 6.Fadok, V. A., M. L. Warner, D. L. Bratton, and P. M. Henson. 1998. CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (αvβ3). J. Immunol. 161:6250-6257. [PubMed] [Google Scholar]
- 7.Febbraio, M., N. A. Abumrad, D. P. Hajjar, K. Sharma, W. Cheng, S. F. A. Pearce, and R. L. Silverstein. 1999. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J. Biol. Chem. 274:19055-19062. [DOI] [PubMed] [Google Scholar]
- 8.Gearing, K. L., M. Gottlicher, M. Teboul, E. Widmark, and J. A. Gustafsson. 1993. Interaction of the peroxisome-proliferator-activated receptor and retinoid X receptor. Proc. Natl. Acad. Sci. USA 90:1440-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmann, P. R., A. M. deCathelineau, C. A. Ogden, et al. 2001. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J. Biol. Chem. 155:649-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kutner, S., W. V. Breuer, H. Ginsburg, S. Aley, and Z. I. Cabantchik. 1985. Characterization of permeation pathways in the plasma membrane of human erythrocytes infected with early stages of Plasmodium falciparum: association with parasite development. J. Cell Physiol. 125:521-527. [DOI] [PubMed] [Google Scholar]
- 11.Kyes, S., R. Pinches, and C. Newbold. 2000. A simple RNA analysis method shows var and rif multigene family expression patterns in Plasmodium falciparum. Mol. Biochem. Parasitol. 105:311-315. [DOI] [PubMed] [Google Scholar]
- 12.McGilvray, I. D., L. Serghides, A. Kapus, O. D. Rotstein, and K. C. Kain. 2000. Nonopsonic monocyte/macrophage phagocytosis of Plasmodium falciparum-parasitized erythrocytes: a role for CD36 in malarial clearance. Blood 66:3231-3240. [PubMed] [Google Scholar]
- 13.Nathoo, S., L. Serghides, and K. C. Kain. 2003. Effect of HIV-1 antiretroviral drugs on cytoadherence and phagocytic clearance of Plasmodium falciparum-parasitised erythrocytes. Lancet 362:1039-1041. [DOI] [PubMed] [Google Scholar]
- 14.Ockenhouse, C. F., M. Ho, N. N. Tandon, G. A. Van Seventer, S. Shaw, N. J. White, G. A. Jamieson, J. D. Chulay, and H. K. Webster. 1991. Molecular basis of sequestration in severe and uncomplicated Plasmodium falciparum malaria: differential adhesion of infected erythrocytes to CD36 and ICAM-1. J. Infect. Dis. 164:163-169. [DOI] [PubMed] [Google Scholar]
- 15.Patel, N. S., L. Serghides, T. G. Smith, M. Febbraio, R. L. Silvertein, T. W. Kurtz, M. Pravenec, and K. C. Kain. 2004. CD36 mediates the phagocytosis of Plasmodium falciparum-infected erythrocytes by rodent macrophages. J. Infect. Dis. 189:205-213. [DOI] [PubMed] [Google Scholar]
- 16.Pouvelle, B., P. A. Buffet, C. Lepolard, A. Scherf, and J. Gysin. 2000. Cytoadhesion of Plasmodium falciparum ring-stage-infected erythrocytes. Nat. Med. 6:1264-1268. [DOI] [PubMed] [Google Scholar]
- 17.Roberts, D. D., J. A. Sherwood, S. L. Spitalnik, L. J. Panton, R. J. Howard, V. M. Dixit, W. A. Frazier, L. H. Miller, and V. Grinsburg. 1985. Thrombospondin binds falciparum parasitized erythrocytes and may mediate cytoadherence. Nature 318:64-66. [DOI] [PubMed] [Google Scholar]
- 18.Savill, J., L. Dransfield, N. Hogg, and C. Haslett. 1990. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature 343:170-173. [DOI] [PubMed] [Google Scholar]
- 19.Savill. J., N. Hogg, Y. Ren, and C. Haslett. 1989. Thrombospondin cooperates with CD36 and the Vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J. Clin. Investig. 90:1513-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwarzer, E., F. Turrini, D. Ulliers, G. Giribaldi, H. Ginsburg, and P. Arese. 1993. Impairment of macrophage functions after ingestion of Plasmodium falciparum-infected erythrocytes or isolated malarial pigment. J. Exp. Med. 177:1033-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serghides, L., and K. C. Kain. 2001. Peroxisome proliferator-activated receptor gamma-retinoid X receptor agonists increase CD36-dependent phagocytosis of Plasmodium falciparum-parasitized erythrocytes and decrease malaria-induced TNF-α secretion by monocytes/macrophages. J. Immunol. 166:6742-6748. [DOI] [PubMed] [Google Scholar]
- 22.Serghides, L., and K. C. Kain. 2002. Mechanism of protection induced by vitamin A in falciparum malaria. Lancet 359:1404-1406. [DOI] [PubMed] [Google Scholar]
- 23.Silamut, K., N. H. Phu, C. Whitty, G. D. Turner, K. Louwrier, N. T. Mai, J. A. Simpson, T. T. Hien, and N. J. White. 1999. A quantitative analysis of the microvascular sequestration of malaria parasites in the human brain. Am. J. Pathol. 155:395-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith, T. G., L. Serghides, S. N. Patel, M. Febbraio, R. P. Silverstein, and K. C. Kain. 2003. CD36-mediated nonopsonic phagocytosis of erythrocytes infected with stage I and IIA gametocytes of Plasmodium falciparum. Infect. Immun. 71:393-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevenson, M. M., and E. M. Riley. 2004. Innate immunity to malaria. Nat. Rev. Immunol. 4:169-180. [DOI] [PubMed] [Google Scholar]
- 26.Su, Z., A. Fortin, P. Gros, and M. M. Stevenson. 2002. Opsonin-independent phagocytopsis: an effector mechanism against acute blood-stage Plasmodium chabaudi AS infection. J. Infect. Dis. 186:1321-1329. [DOI] [PubMed] [Google Scholar]
- 27.Tontonoz, P., L. Nagy, J. G. Alvarez, V. A. Thomazy, and R. M. Evans. 1998. PPARγ promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 93:241-252. [DOI] [PubMed] [Google Scholar]
- 28.Treutiger, C. J., A. Heddini, V. Fernandez, W. A. Muller, and M. Wahlgren. 1997. PECAM-1/CD31, an endothelial receptor for binding Plasmodium falciparum-infected erythrocytes. Nat. Med. 3:1405-1408. [DOI] [PubMed] [Google Scholar]
- 29.Udomsangpetch, R., B. Pipitaporn, K. Silamut, R. Pinches, S. Kyes, S. Looareesuwan, C. Newbold, and N. J. White. 2002. Febrile temperatures induce cytoadherence of ring-stage Plasmodium falciparum-infected erythrocytes. Proc. Natl. Acad. Sci. USA 99:11825-11829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walliker, D., I. A. Quakyi, T. E. Wellems, T. F. McCutchan, A. Szarfman, W. T. London, L. M. Corcoran, T. R. Burkot, and R. Carter. 1987. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science 236:1661-1666. [DOI] [PubMed] [Google Scholar]