Abstract
Escherichia coli strains are responsible for many cases of gastrointestinal disease and represent a serious health problem worldwide. An essential step in the pathogenesis of such strains involves recognition and attachment to host intestinal surfaces. TibA is a potent bacterial adhesin associated with a number of enterotoxigenic E. coli strains and mediates bacterial attachment to a variety of human cells; additionally, it promotes invasion of such cells. This adhesin is a surface-displayed autotransporter protein and belongs to the exclusive group of bacterial glycoproteins; only the glycosylated form confers binding to and invasion of mammalian cells. Here we characterized TibA and showed that it possesses self-association characteristics and can mediate autoaggregation of E. coli cells. We demonstrated that intercellular TibA-TibA interaction is responsible for bacterial autoaggregation. Also, TibA expression significantly enhances biofilm formation by E. coli on abiotic surfaces.
Escherichia coli strains are responsible for many cases of gastrointestinal disease throughout the world, notably in infants in third world countries, where these pathogens are a major cause for concern (37). According to World Health Organization sources, more than 2 million humans, mainly infants, die from E. coli-associated diarrhea each year. Enterotoxigenic E. coli (ETEC) strains are, if not the most important diarrhea-causing strains, among the primary diarrhea-causing types. ETEC strains produce at least one of two enterotoxins, heat-labile enterotoxin and heat-stable enterotoxin, which are the principal causes of the diarrhea (37). However, additional virulence factors are required for ETEC strains to become fully virulent. A crucial first step in the pathogenesis of ETEC strains is the initial recognition of and attachment to intestinal tissue surfaces. Bacterial attachment occurs through specific adhesins protruding from the bacterial surface. A multitude of bacterial adhesins exist, and they can be crudely divided into two groups: long organelle-type adhesins (typified by fimbriae) and short, nonfimbrial adhesins (26, 27).
The TibA protein, a nonorganelle adhesin, was originally found in the classical ETEC serotype O78:H11 strain H10407 (14). The tib locus directs the synthesis of a 104-kDa outer membrane protein, the product of the tibA gene. Although strain H10407 harbors many plasmid-encoded virulence factors, the tib locus is chromosomally encoded (13). Introduction of the tib locus into nonadherent E. coli K-12 hosts directs adhesion to epithelial cell line cells (13). Although cell invasion is not a general trait of ETEC strains, strain H10407 is capable of invading human intestinal epithelial cell line cells (13). It has also been shown that the presence of the tib locus directs not only cell adhesion but also cell invasion with high efficacy (13, 14, 30). TibA was found to be responsible for this capacity.
TibA belongs to the autotransporter protein family. For this group of proteins, which encompasses many virulence factors, the protein itself contains all the information required for traversing the bacterial membrane system and routing to the bacterial cell surface (19, 20). TibA is produced as a precursor consisting of 989 amino acids, which subsequently undergoes extended posttranslational modifications. Initially, it is processed by removal of a 54-amino-acid signal peptide during transit to the periplasm. TibA belongs to an autotransporter subfamily of proteins that contain repetitive amino acid sequence motifs; other members of this subfamily are AIDA, antigen 43 (Ag43), and filamentous hemagglutinin (2, 21, 24). Like these proteins, TibA consists of two domains, a C-terminal translocator domain and an N-terminal passenger domain. The translocator moiety forms a β-barrel porin in the outer membrane, through which the adhesin moiety gains access to the surface (29). Additional modifications occur since the fully mature TibA adhesin is a glycoprotein. Immediately upstream of the tibA gene is a second gene, tibC, which encodes a 406-residue glycosyltransferase, presumably a heptosyltransferase, that modifies the TibA protein by addition of glycosyl residues (29). Without this modification TibA does not bind to human cells (14, 30).
TibA has been demonstrated to mediate bacterial binding to a range of human cell types that are exemplified by epithelial cell lines such as HEp-2 larynx cells, HuTu80 duodenal cells, HCT8 ileocecal cells, and HCT116 colonic cells (14). This suggests that the molecular motif(s) with which TibA interacts is quite common in the human digestive tract. This versatility is also reflected in the fact that although TibA was originally isolated from H10407, it seems to be widespread among ETEC strains (14).
Several adhesins, such as type 1 fimbriae and curli, have been shown to confer bacterial autoaggregation and/or to enhance biofilm formation on abiotic surfaces in addition to their receptor recognition faculty (16, 43, 46). These phenotypes are recognized as bona fide virulence properties (3, 8, 15). Here we investigated whether the TibA adhesin, in addition to its ability to promote bacterial binding to and invasion of human cells, possesses alternative virulence properties, including autoaggregation of bacterial cells and biofilm formation.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The strains, plasmids, and primers used in this study are listed in Tables 1, 2, and 3, respectively. Cells were grown at 37°C on solid Luria-Bertani medium or in liquid Luria-Bertani medium supplemented with the appropriate antibiotics unless indicated otherwise.
TABLE 1.
Bacterial strains
| Strain | Relevant characteristic(s) | Reference |
|---|---|---|
| K. pneumoniae strains | ||
| C105 | K35 capsule | 49 |
| C105NCV | Noncapsulated variant of C105 | 49 |
| PKL1019 | C105 containing pACYC184 | This study |
| PKL1024 | C105NCV containing pACYC184 | This study |
| RMV22 | C105 containing pRMV1 (TibA+) | This study |
| RMV23 | C105NCV containing pRMV1 (TibA+) | This study |
| E. coli strains | ||
| MG1655 | E. coli K-12 reference strain | 1 |
| MS427 | MG1655 Δflu | 23 |
| MS528 | MG1655 Δflu Δfim | 23 |
| OS56 | MS427 attB::bla-PA1/04/03-gfpmut3b*-T0 (Gfp+) | This study |
| OS60 | MS427 containing pACYC184 and pBAD | This study |
| OS70 | OS56 containing pACYC184 (Gfp+) | This study |
| OS82 | MS427 containing pAR163 (DsRed:T3+) | This study |
| OS108 | OS56 containing pRMV1 (Gfp+ TibA+) | This study |
| OS109 | OS82 containing pRMV1 (DsRed:T3+ TibA+) | This study |
| OS136 | OS56 containing pRMV4b (Gfp+ TibCA+) | This study |
| RMV3 | MS427 containing pRMV1 (TibA+) | This study |
| RMV4 | MS427 containing pRMV1 and pPKL4 (TibA+ Fim+) | This study |
| RMV6 | MS427 containing pRMV1 and pOS38 (TibA+ TibC+) | This study |
| RMV7 | MS427 containing pRMV1 and pBR322 (TibA+) | This study |
| RMV8 | MS427 containing pRMV1 and pBAD (TibA+) | This study |
| RMV10 | MS427 containing pACYC184 and pPKL4 (Fim+) | This study |
| RMV11 | MS427 containing pACYC184 and pBR322 | This study |
| RMV12 | MS427 containing pACYC184 and pOS38 (TibC+) | This study |
| RMV19 | MS528 containing pMW119 | This study |
| RMV20 | MS528 containing pRMV3 (TibA+) | This study |
| RMV24 | MS528 containing pACYC184 and pBR322 | This study |
| RMV25 | MS528 containing pRMV1 and pBR322 (TibA+) | This study |
| RMV26 | MS528 containing pACYC184 and pKT274 (K1+) | This study |
| RMV27 | MS528 containing pRMV1 and pKT274 (TibA+ K1+) | This study |
TABLE 2.
Plasmids used in this study
| Plasmid | Relevant genotype or phenotype | Reference or source |
|---|---|---|
| pPKL4 | fim gene cluster from E. coli PC31 in pBR322 | 25 |
| pRL27 | Mini-Tn5 delivery vector, source for PtetA | 28 |
| pSM1690 | PrrnB,P1-RBSII-gfpmut3b+-T0-T1 NotI fragment in LOW2, Kmr | 48 |
| pTP801 | cat gene in BamHI site of pUC19 | 35 |
| pTP809 | pBR322 derivative with multiple cloning site introduced between EcoRI and NdeI sites | 35 |
| pAR90 | cfp* inserted between SphI and HindIII sites in pJBA27 | 42 |
| pDsRed.T3 | Encodes a rapidly maturating DsRed.T3 variant | 4 |
| pKKJ128 | flu gene from E. coli MG1655 in pACYC184 | 22 |
| pKT274 | E. coli K1 capsule operon in pHC79 | 44 |
| pLDR8 | Temperature-sensitive helper plasmid carring the λ int gene | 11 |
| pLDR11 | Vector containing the attP sequence for integrating DNA into the lambda attachment site attB | 11 |
| pMW119 | Low-copy-number cloning vector | Nippon Gene |
| pAR81 | cat gene from pTP801 ligated into BamHI-digested pTP809, Cmr | This study |
| pAR94 | PrrnB,P1 containing SacI-XbaI fragment of pSM1690 inserted into pAR90 digested with SacI and XbaI | This study |
| pAR163 | Amplification of dsred2.T3 with primers Dsred2d and UDsRed2.T3 and ligation into XbaI-HindIII-digested pAR94; results in replacement of cfp* with dsred2.T3, controlled by PrrnB,P1 | This study |
| pAR176 | Amplification of PtetA with primers ar047 and ar048 from pRL27 and ligation into SacI-XbaI-digested pSM1690; results in replacement of PrrnB,P1 with PtetA | This study |
| pAR178 | Amplification of cat gene with primers ar059 and ar060 from pAR81 and ligation with ClaI-SalI-digested p15A ori-containing fragment from pSM1690 (amplified by PCR with primers ar057 and ar058) | This study |
| pAR179 | Insertion of PrrnB,P1-RBSII-dsred2.T3-T0 containing NotI cassette from pAR163 into | This study |
| the NotI site of pAR178 | ||
| pOS32 | gfpmut3b gene from plasmid pAR176 ligated to XbaI-HindIII-digested pAR179; construct has gfpmut3b gene under transcriptional control of the E. coli rrnB-P1 promoter | This study |
| pOS38 | tibC gene from E. coli H10407 PCR amplified with primers 531 and 532 and cloned into XhoI-KpnI-digested pBAD | This study |
| pRMV1 | tibA gene from E. coli H10407 PCR amplified with primers 535 and 536 and cloned into BamHI-XmaIII-digested pACYC184 | This study |
| pRMV3 | tibA gene from E. coli H10407 PCR amplified with primers 563 and 564 and cloned into HindIII-BamHI-digested pMW119 | This study |
| pRMV4b | tibC and tibA genes from E. coli H10407 PCR amplified with primers 573 and 536 and cloned into BamHI-XmaIII-digested pACYC184 | This study |
TABLE 3.
Primers used in this study
| Primer | Sequence |
|---|---|
| DsRed2d | 5′-CCTCAAGCTTCCCGGGTTACAGGAACAGGTGGTGGCG-3′ |
| UDsRed2.T3 | 5′-GGTCTAGAATTAAAGAGGAGAAATTAAGCATGGCCTCCTCCGAG-3′ |
| ar047 | 5′-GTCGAGCTCAATGATTCTCCGCCAGCA-3′ |
| ar048 | 5′-GCTCTAGAATATGTGGCCTCCGGACC-3′ |
| ar057 | 5′-GCATCGATGACCAAAATCCCTTAACG-3′ |
| ar058 | 5′-GCGGTCGACGGAAATGGCTTACGAA-3′ |
| ar059 | 5′-GCGGTCGACTCCTTACGCATCTGTGC-3′ |
| ar060 | 5′-GCATCGATAGGCGTATCACGAGGC-3′ |
| 531 | 5′-CGCGCTCGAGATAATAAGGATCATTTAATGTCAACGCTGAAG-3′ |
| 532 | 5′-CGCGCCGGTACCTTCATTGCTTACTCCTT-3′ |
| 535 | 5′-CGCGGGATCCATAATAAGGATCATTTAATGAATAAGGTCTAT-3′ |
| 536 | 5′-CGGCGCGGCCGTCAGAAGTTGATTCGG-3′ |
| 563 | 5′-CGCGAAGCTTATAATAAGGATCATTTAATGAATAAGGTCTAT-3′ |
| 564 | 5′-CGGCGGGATCCTCAGAAGTTGATTCGG-3′ |
| 573 | 5′-CGCGGGATCCATAATAAGGATCATTTAATGTCAACGCTGAAG-3′ |
E. coli strain MS427 was genetically marked by insertion of gfpmut3b* into the chromosomal attachment site of bacteriophage λ (attB) essentially as previously described (11). gfpmut3b* containing a NotI fragment from pOS32 was ligated to the bla-attP-containing NotI fragment of plasmid pLDR11. The ligation mixture was transformed into MS427(pLDR8) cells expressing λ Int. Correct chromosomal insertion of gfpmut3b* into an ampicillin-resistant, green fluorescent transformant (OS56) was verified by PCR. E. coli MS427 was labeled with DsRed by transformation with plasmid pAR163, and the resulting strain was designated OS82. The differently labeled fluorescent strains were used as hosts for the expression of TibA via plasmid-encoded genes.
DNA manipulations and genetic techniques.
Isolation of plasmid DNA was carried out by using a QIAprep Spin Miniprep kit (QIAGEN). Restriction endonucleases were used according to the manufacturer's specifications (Biolabs). Purification of chromosomal DNA from strain H10407 was completed by using a GenomicPrep cell and tissue DNA isolation kit (Amersham Pharmacia Biotech Inc.). All PCRs were performed with the Expand High Fidelity polymerase system (Roche) essentially as previously described (47). The primers used are listed in Table 3. Amplified products were sequenced to ensure fidelity of the PCR (MWG Biotech, Ebersberg, Germany).
Autoaggregation assay.
In order to monitor differences in autoaggregation, we used an assay to monitor bacterial settling kinetics over time. Overnight cultures were standardized and mixed vigorously for 15 s prior to the start of the assay. At regular time intervals, a 150-μl sample was taken approximately 0.5 cm from the liquid surface and transferred into a microtiter plate maintained on ice. At the end of the experiment the optical densities at 600 nm were determined by using a microtiter plate reader. When the influence of pH was investigated, the cells were harvested and resuspended in phosphate-buffered saline with different pH values.
Electrophoresis of proteins and detection of glycoproteins.
Electrophoresis of whole-cell extracts was performed under denaturing conditions by using a previously described method (47). Cultures that were grown to the exponential phase and were the same optical density were harvested by centrifugation at 10,000 × g for 2 min. Samples were prepared for electrophoresis by resuspending the pelleted bacterial cells in sodium dodecyl sulfate (SDS) sample buffer, followed by boiling for 3 min; aliquots were subjected to SDS-polyacrylamide gel electrophoresis (PAGE). For Western blotting, a polyclonal rabbit antiserum that recognized TibA was used. For glycoprotein detection staining, samples treated for electrophoresis as described above were transferred to nitrocellulose filters as described previously (24). Glycoprotein staining was carried out by using a Roche Molecular Biochemicals digoxigenin glycan detection kit according to the manufacturer's instructions.
Immunofluorescence microscopy.
Surface presentation of TibA was assessed by immunofluorescence microscopy by using a polyclonal rabbit antiserum that recognized TibA. A fluorescein isothiocyanate-labeled anti-rabbit serum (Sigma) was used as the secondary antibody. Cell fixation, immunolabeling, and microscopy were carried out as previously described (18).
Biofilm flow chamber experiments.
Flow chamber experiments were performed essentially as previously described (7); the major exception was that cells were grown in ABT minimal media (39). Briefly, biofilms were allowed to form on glass surfaces in a multichannel flow system that permitted online monitoring of community structures. Flow cells were inoculated with cultures with a standardized optical density at 600 nm that were pregrown overnight in ABT medium containing chloramphenicol. Glucose was used as the sole carbon source at a concentration of 0.002%.
Microscopy and image analysis.
Microscopic observation and image acquisition were performed with a scanning confocal laser microscope (SCLM) (TCS4D; Leica Lasertechnik, GmbH, Heidelberg, Germany) equipped with detectors and filters for monitoring green fluorescent protein (Gfp) and DsRed. Vertical cross sections through the biofilms were generated by using the IMARIS software package (Bitplane AG, Zürich, Switzerland) running on a Silicon Graphics Indigo2 workstation (Silicon Graphics, Mountain View, Calif.). Images were further processed for display by using Photoshop software (Adobe, Mountain View, Calif.).
RESULTS
Cloning and characterization of the Tib system.
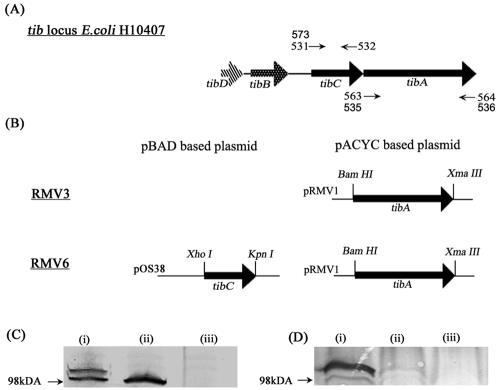
The tib locus has been described to contain four genes, tibABCD (29). In order to define what gene(s) is required for TibA display and to permit functional analysis of TibA with and without glycosylation, we cloned different segments of the tib locus from E. coli H10407 (Fig. 1A). Plasmid pRMV1 containing the tibA gene alone was prepared by PCR amplification by employing H10407 genomic DNA as the target and was cloned into vector pACYC184 (Fig. 1B). Transformation of pRMV1 into E. coli MS427 resulted in strain RMV3. In a similar manner a plasmid containing the glycosyltransferase-encoding tibC gene was constructed based on the pBAD vector; this plasmid, pOS38, was transformed into strain RMV3, resulting in RMV6 (Fig. 1B). Constructs were verified by restriction enzyme mapping and DNA sequencing. To confirm correct processing (RMV3) and glycosylation of TibA (RMV6), the total cell protein was examined by SDS-PAGE and Western blotting (Fig. 1C), followed by protein staining or glycoprotein staining (Fig. 1D). As expected, proteins with apparent molecular masses of approximately 104 kDa (glycosylated TibA) (RMV6) and 98 kDa (TibA) (RMV3) were visible (Fig. 1C). That TibA was glycosylated in the presence of TibC was also evident; a 104-kDa glycoprotein was present in strain RMV6 but not in RMV3 (TibA+) or OS60 (vector control) (Fig. 1D).
FIG. 1.
(A) Genetic organization of the chromosomally encoded tib locus from E. coli H10407, adapted from the study of Lindenthal and Elsinghorst (29). The locations of PCR primers used for amplification of tibC and tibA are indicated by numbers and arrows. (B) Overview of the plasmid constructs used for expression of TibA (pRMV1) and glycosylated TibA (pRMV1 and pOS38). (C and D) Western blot of E. coli total cell protein (C) and glycoprotein staining of E. coli total cell protein (D) isolated from E. coli MS427 harboring pOS38 and pRMV1 (TibC+ TibA+) (lane i), strain RMV6 harboring pRMV1 (TibA+) (lane ii), and strain RMV3 harboring pACYC184 and pBAD (control) (strain OS60) (lane iii).
TibA is surface exposed and mediates autoaggregation, flocculation, and settling of cells in a glycosylation-independent manner.
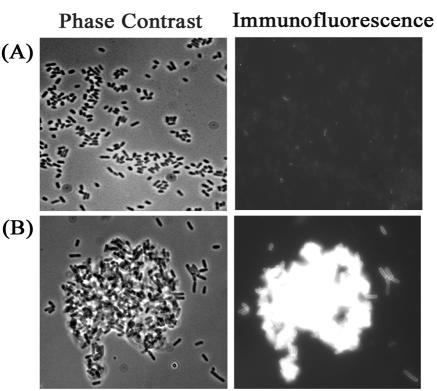
Having demonstrated the presence of TibA in whole-cell lysates, we verified the surface presentation of TibA. To do this, phase-contrast microscopy coupled with immunofluorescence microscopy was used. The results showed that while cells of control strain OS60 produced no immunofluorescence signal (Fig. 2A), cells of RMV6 gave a clear fluorescent signal (Fig. 2B) indicative of surface-located TibA.
FIG. 2.
Surface presentation of TibA results in bacterial cell-cell aggregation. Phase-contrast microscopy (left) and immunofluorescence (right) of E. coli vector control strain OS60 (A) and E. coli strain RMV6 harboring pOS38 and pRMV1 (TibC+ TibA+) (B).
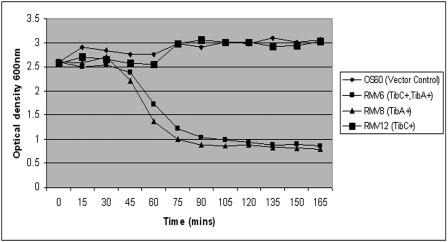
Cells of strain OS60 (Δflu) did not aggregate or settle from liquid suspensions (Fig. 3A). However, cells of strain RMV6 (tibC+ tibA+) were observed to aggregate, and when liquid suspensions of cells were left standing, they flocculated and settled (Fig. 3B). TibA-mediated attachment to and invasion of human cells has been reported to be dependent on glycosylation of the protein; i.e., in the absence of the glycosylation conferred by the tibC heptosyltransferase gene product, TibA does not confer binding to or invasion of human cells (29). To determine if glycosylation played a role in bacterial autoaggregation, we compared cells of strain RMV6 (TibC+ TibA+) and strain RMV3 (TibA+). In both cases the cells aggregated and settled from liquid suspensions (Fig. 3B and C). To obtain a more detailed picture of the aggregation activity of TibA and the role of glycosylation, we compared the autoaggregation profiles of MS427 hosts expressing combinations of the tibA and tibC gene products, viz., RMV12 (TibC+), RMV8 (TibA+), and RMV6 (TibC+ TibA+) (Fig. 4). Strain RMV6 (TibC+ TibA+) was observed to flocculate and settle in a manner similar to RMV8 (TibA+). However, strain RMV12 (TibC+) did not aggregate or settle from liquid suspensions, suggesting that the TibA protein but not the TibC protein is required for bacterial autoaggregation. Taken together, these results indicate that TibA mediates bacterial aggregation and that, in contrast to attachment to human cells, glycosylation of TibA does not play a role in this phenotype.
FIG. 3.
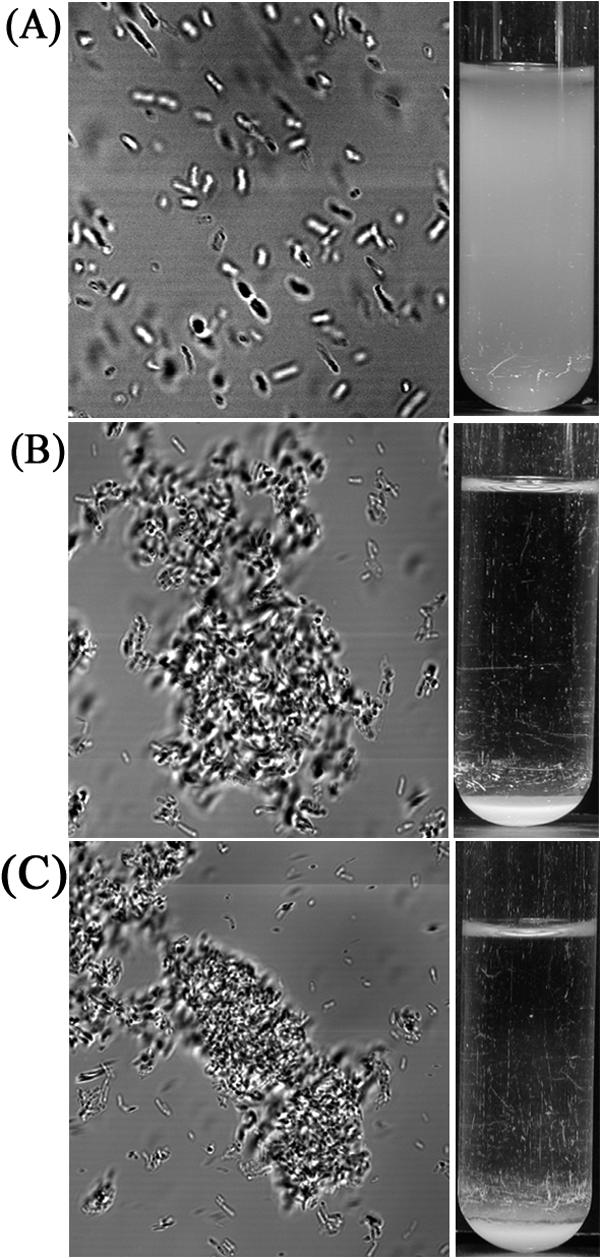
Cell-cell autoaggregation (left) and settling (right) of E. coli vector control strain OS60 (A), E. coli strain RMV6 harboring pOS38 and pRMV1 (TibC+ TibA+) (B), and E. coli strain RMV3 harboring pRMV1(TibA+) (C).
FIG. 4.
Settling patterns for liquid suspensions of E. coli MS427 (MG21655 Δflu) derivative strains harboring (i) pBAD and pACYC184 (control strain OS60), (ii) pOS38 and pRMV1 (TibC+ TibA+) (strain RMV6), (iii) pBAD and pRMV1 (TibA+) (strain RMV8), and (iv) pOS38 and pACYC184 (TibC+) (strain RMV12).
Finally, in order to rule out any potential influence of copy number on TibA-mediated aggregation, we subcloned the tibA gene into the low-copy-number vector pMW119, resulting in plasmid pRMV3. Cells containing pRMV3 (i.e., RMV20 cells) were found to aggregate and settle in comparison to the control RMV19 cells (data not shown). The autoaggregation was essentially like that observed with other tibA-containing constructs, suggesting that TibA-mediated cell aggregation was not due to high gene dosage. Furthermore, TibA-induced cell aggregation was not restricted to a particular host strain as it was observed in a range of E. coli K-12 strains. These data also support the notion that only the tibA gene is required for surface presentation of the TibA protein. TibC is required for glycosylation of TibA but is not per se involved in surface presentation. The two additional genes in the tib locus, tibB and tibD, do not seem to play any direct role in directing TibA to the surface of E. coli.
Intercellular TibA-TibA interaction is responsible for bacterial autoaggregation.
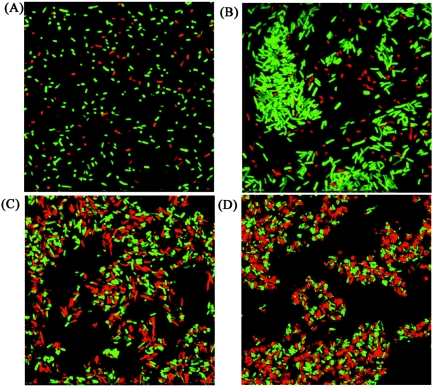
The observed TibA-mediated cell aggregation could have been due to either (i) intercellular TibA-TibA interaction or (ii) intercellular interaction between TibA and some other cell surface component. In order to distinguish between these possibilities, plasmid pRMV1, containing the tibA gene, was transformed into the MS427 derivatives OS56 and OS82 expressing the Gfp and DsRed fluorescent proteins, respectively. The resulting strains were designated OS108 (TibA+ Gfp+) and OS109 (TibA+ DsRed+). When aliquots of OS56 (Gfp+) and OS82 (DsRed+) were mixed, no interaction between these strains was observed, as expected (Fig. 5A). When OS108 cells (TibA+ Gfp+) were mixed with OS82 cells (DsRed+), aggregates composed only of Gfp-tagged OS108 cells were evident, and no interaction between these cells and DsRed-tagged OS82 cells was evident (Fig. 5B). When OS108 (TibA+ Gfp+) and OS109 (TibA+ DsRed+) were mixed, aggregates consisting of both Gfp- and DsRed-tagged cells were produced (Fig. 5C). This suggests that an intercellular TibA-TibA interaction was responsible for the observed cell aggregation. Finally, to determine if glycosylation interfered with or influenced the TibA-TibA interaction, a new construct was prepared. The tibCA fragment from E. coli H10407 was cloned into plasmid pACYC184, resulting in plasmid pRMV4b. Plasmid pRMV4b was transformed into OS56, resulting in strain OS136 (TibCA+ Gfp+). The OS136 cells were mixed with OS109 cells (TibA+ DsRed+). It was clear that mixed aggregates were formed, suggesting that glycosylation is of no consequence in TibA-TibA interactions (Fig. 5D).
FIG. 5.
Intercellular TibA-TibA interactions are responsible for bacterial autoaggregation. SCLM images of E. coli MS427 derivative strains OS56 (Gfp+) and OS82 (DsRed+) (A), OS108 (TibA+ Gfp+) and OS82 (DsRed+) (B), OS109 (TibA+ DsRed+) and OS108 (TibA+ Gfp+) (C), and OS136 (TibCA+ Gfp+) and OS109 (TibA+ DsRed+) (D).
TibA-mediated cell aggregation was found to be sensitive to pH extremes. For example, when TibA-expressing cells were subjected to pH 11, little aggregation took place, while aggregation was most efficient at pH 4. It is therefore tempting to imagine that charged amino acid side chains could participate in the aggregation process.
TibA-mediated cell aggregation is abolished by concomitant expression of fimbriae.
TibA belongs to the class of short nonorganelle adhesins and has been predicted to protrude about 10 nm from the bacterial surface (21). Consequently, TibA-mediated cell aggregation requires close cell-to-cell contact. We speculated whether expression of type 1 fimbriae, which are ∼1-μm-long rigid organelles, could physically mask and block the close cell-to-cell contact required for TibA-mediated cell aggregation. To test this hypothesis, we introduced plasmid pPKL4, conferring expression of type 1 fimbriae, into MS427 cells expressing TibA, resulting in strain RMV4. Production of fimbriae was confirmed by agglutination of yeast cells. When cultures of RMV4 (tibA+ fim+), RMV7 (tibA+), RMV10 (fim+), and RMV11 (vector control) were left standing, only RMV7 settled, suggesting that fimbriation blocked TibA-mediated aggregation (Fig. 6A). We previously observed a similar phenomenon in the case of fimbrial elimination of Ag43-mediated cell aggregation (17).
FIG. 6.
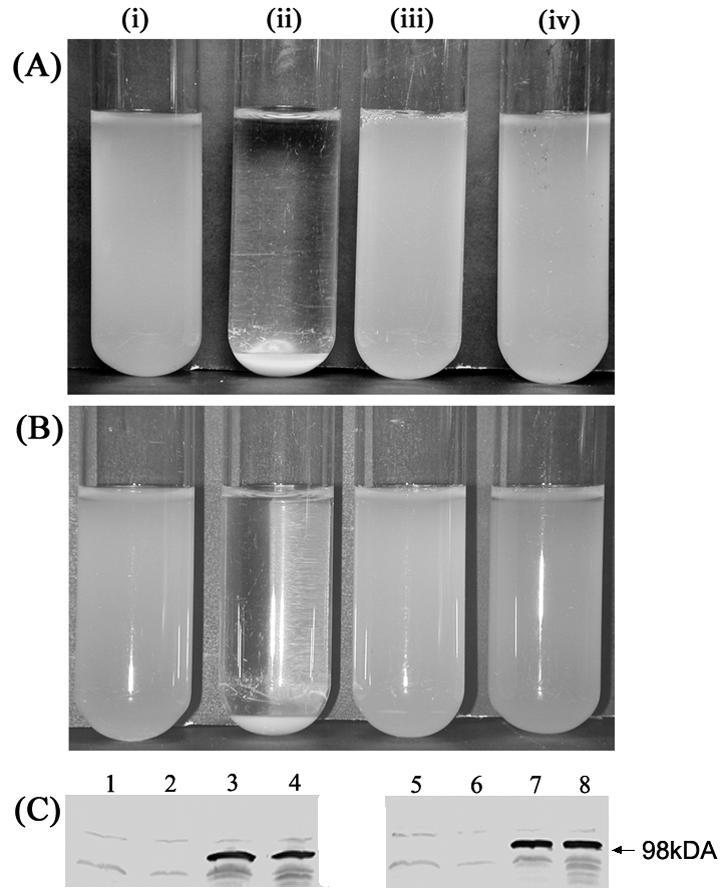
(A and B) Cell-cell aggregation imparted by TibA and interference with expression of either type 1 fimbriae (A) or K1 capsule (B). (A) Bacterial settling in static broth mediated by E. coli MS427 harboring pACYC184 and pBR322 (vector control) (tube i), strain RMV11 harboring pRMV1 and pBR322 (TibA+) (tube ii), strain RMV7 harboring pRMV1 and pPKL4 (TibA+ Fim+) (tube iii), or strain RMV4 harboring pPKL4 and pACYC184 (Fim+) (strain RMV10) (tube iv). (B) Bacterial settling in static broth mediated by E. coli MS528 harboring pACYC184 and pBR322 (vector control) (tube i), strain RMV24 harboring pRMV1 and pBR322 (TibA+) (tube ii), strain RMV25 harboring pRMV1 and pKT274 (TibA+ K1+) (tube iii), or strain RMV27 harboring pKT274 and pACYC184 (K1+) (strain RMV26) (tube iv). (C) Western blot of E. coli total cell protein liberated from E. coli MS427 host strains, showing that overexpression of type 1 fimbriae and K1 capsule does not affect surface presentation of TibA. Lane 1, pACYC184 and pBR322 (vector control) (strain RMV11); lane 2, pPKL4 and pACYC184 (Fim+) (strain RMV10); lane 3, pRMV1 and pBR322 (TibA+) (strain RMV7); lane 4, pRMV1 and pPKL4 (TibA+ Fim+) (strain RMV4); lane 5, pACYC184 and pBR322 (vector control) (strain RMV24); lane 6, pKT274 and pACYC184 (K1+) (strain RMV26); lane 7, pRMV1 and pBR322 (TibA+) (strain RMV25); lane 8, pRMV1 and pKT274 (TibA+ K1+) (strain RMV27).
TibA function is blocked upon capsule expression.
All members of the Enterobacteriaceae can elaborate a layer of surface-associated polysaccharides called the capsule, which may be either a K antigen or an O antigen (51). The capsule can extend 0.2 to 1.0 μm from the bacterial surface. Unlike wild-type strains, E. coli K-12 strains normally are not capable of producing extended surface-associated polysaccharides; their polysaccharide has a complete core but no O antigen due to the insertion of an IS5 element in the rfb gene cluster controlling O-antigen biosynthesis (31). This makes K-12 strains ideal for monitoring potential interference of capsular polysaccharides with other surface structures (44), in this case TibA. Strain RMV25 was constructed by transformation of MS528 with plasmid pRMV1 (TibA+), while strain RMV27 was constructed by transformation of MS528 with plasmid pKT274 encoding K1 capsule production and plasmid pRMV1 (TibA+). Comparison of these strains demonstrated that the TibA-expressing strain RMV25 aggregated and settled from static liquid suspensions, while cells of RMV27, forced to concomitantly express both K1 capsule and TibA, were unable to aggregate; i.e., the function of TibA was abolished (Fig. 6B). This suggests that the TibA function is physically masked by both fimbriae and the capsule.
To confirm that the surface location of TibA was not affected by type 1 fimbriae or K1 capsule expression, the total cell proteins of strains expressing TibA and TibA plus fimbriae or TibA plus capsule were prepared and examined by SDS-PAGE and Western blotting (Fig. 6C). Clearly, similar quantities of TibA were produced on the surfaces of cells expressing type 1 fimbriae and the K1 capsule, indicating that fimbriae and capsule expression did not affect surface presentation of TibA.
We also examined whether types of capsule other than K1 could mask TibA activity. Introduction of pRMV1 (TibA+) into an isogenic pair of Klebsiella pneumoniae strains, C105 and C105NCV, which differed only in the ability to express K35 capsule, resulted in strains RMV22 and RMV23, respectively. Cells of the noncapsulated strain RMV23 (TibA+) aggregated, while cells of RMV22 (K35+ TibA+) and the vector controls, PKL1019 and PKL1024, did not aggregate (data not shown). Taken together, the results indicate that a capsule indeed blocks the close cell contact required for intercellular TibA-TibA interaction and that this appears to be a general characteristic of capsules.
TibA enhances biofilm formation under continuous-flow growth conditions.
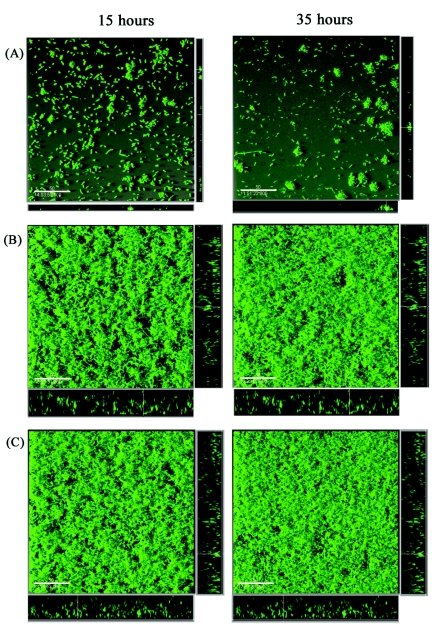
Given the cell aggregation characteristics of TibA, we speculated whether this facility could influence biofilm formation. To evaluate the role of TibA in biofilm formation, we compared strains OS108 (TibA+ Gfp+) and OS136 (TibCA+ Gfp+) with the vector control strain OS70 (Gfp+) in flow chamber biofilms. The experimental design enabled us to monitor bacterial distribution within an evolving biofilm under continuous-flow conditions. Furthermore, the spatial distribution of the bacteria could be assessed at the single-cell level due to the combination of fluorescence-tagged cells and scanning confocal laser microscopy. Biofilms were established on glass surfaces in separate flow cells and analyzed. Image analysis of optical sections was performed to examine the distribution and thickness of each established biofilm. The control strain, OS70, produced small, flat microcolonies (Fig. 7A). However, a distinct change in the surface colonization pattern was observed with glycosylated TibA (OS136) and nonglycosylated TibA (OS108) expression (Fig. 7B and C). Initially, loose aggregates were evident in the flow chamber, which developed into thick biofilms by the end of the analysis. No marked difference between the glycosylated and nonglycosylated TibA biofilms was evident.
FIG. 7.
Spatial distribution of biofilm formation for gfp-labeled E. coli MS427 derivative strains. (A) pACYC184 (vector control) (strain OS70). (B) pRMV4b (TibCA+) (strain OS136). (C) pRMV1 (TibA+) (strain RMV3). Biofilm development was monitored by SCLM at 15 h (left images) and 35 h (right images) after inoculation. The images are three-dimensional projections collected for the biofilms and vertical sections (to the right and below for each individual panel) representing the yz plane and the xz plane, respectively, at the positions indicated by the white lines. Scale bars = 50 μm.
DISCUSSION
A number of bacterial phenotypes are often linked to pathogenesis; these phenotypes include the ability to recognize and attach to host tissue, the ability to invade host cells, the ability to aggregate, and the ability to form biofilms. In nature most bacteria adhere to or live in close association with a surface. It has been estimated that at least 90% of all bacteria in the environment are attached to a surface, and many of these bacteria form sessile communities called biofilms (8). Biofilm formation can be divided into different stages. The first stage is attachment of the bacterium to the surface in question. After the initial attachment there are several possibilities. If the bacterium is attached to a eukaryotic cell, it can sometimes initiate invasion of that cell. Alternatively, it can follow the path that ultimately leads to biofilm formation. Bacterial aggregation and microcolony formation are often a prelude to biofilm formation. However, aggregation can also be considered a defense mechanism in its own right. Thus, it is becoming apparent that the ability to form aggregates seems to be a common theme among many bacterial pathogens. A diverse range of bacteria, such as Bordetella pertussis (33), Mycobacterium tuberculosis (34), Staphylococcus aureus (32), and Streptococcus pyogenes (6, 15), can form aggregates. Such aggregates are known to be able to resist various host defenses and unfavorable environmental conditions (e.g., complement attack, phagocytosis, hydrogen peroxide treatment, etc.) more efficiently than solitary bacteria (3, 40, 45). These observations lend strong support to the notion that aggregation is an important virulence mechanism. Formation of aggregates usually takes place through autoaggregation of cells.
Many E. coli strains are capable of aggregation and employ a range of different systems to this end. A subgroup of type 1 fimbriae, often found in uropathogenic E. coli strains, confer efficient aggregation (43). In enteroaggregative E. coli strains, two thin 2- to 3-nm-wide fimbrial types designated aggregative adherence fimbriae I and II have been identified (9, 36, 37). Expression causes prominent autoaggregation of bacterial cells (38). Also, curli, which are thin surface fibers formed by extracellular precipitation of secreted soluble subunit proteins, confer autoaggregation (16). Likewise, bundle-forming pili mediate autoaggregation (5). Interestingly, many aggregation factors (for example, type 1 fimbriae [46] and curli [50]) also confer biofilm formation.
In contrast to these organelle-type aggregating systems, the self-associating TibA adhesin is anchored directly to the outer membrane. Consequently, TibA-mediated aggregation results in more intimate cell-cell contact than that seen with systems in which the intercellular interactions are based on polymeric structures that reach far from the bacterial surface, like fimbriae. The group of autotransporters that includes TibA, Ag43, and AIDA belongs to a subfamily of E. coli autotransporters that is defined by sequence homology and the presence of repetitive sequence motifs (2, 24, 29). Ag43 was previously shown to be a self-associating molecule that caused bacterial aggregation when it was expressed (10, 17). These shared characteristics prompted us to investigate whether TibA, like Ag43, is a self-recognizing molecule capable of conferring bacterial autoaggregation. Our observations indicate that it does have these properties.
It was originally suggested that the tib locus harbors four genes (29). Here, we demonstrated that only the tibA gene is required for surface display of the TibA protein and that the tibC product is required for glycosylation of TibA. This observation is in line with the fact that TibA belongs to the autotransporter protein family. The other genes present in the tib locus do not seem to be involved in this process but could have regulatory roles, as suggested previously (29). In the present study we showed that TibA expression confers bacterial autoaggregation via intercellular TibA-TibA interaction. TibA belongs to a small, exclusive group of bacterial glycoproteins, and glycosylation of TibA is critical for its binding to human cells (30). Meanwhile, glycosylation is not required for interbacterial TibA-TibA interaction. However, on the other hand, glycosylation does not block this interaction, as demonstrated in this study. The molecular mechanism involved in TibA self recognition is not clear but could involve intermolecular interaction between charged amino acid side chains since pH influenced aggregation.
TibA is a nonorganelle adhesin, and like its distant relatives, Ag43 and AIDA, it probably does not protrude more than ∼10 nm from the bacterial surface (21). Because of this the biological activity of TibA is masked by more extensive surface structures, like fimbriae and capsules, as demonstrated here. Many ETEC strains are able to express capsule and fimbriae (41, 37). Arguably, the expression of TibA must be coordinated with the expression of more extended surface structures. Future studies on the expressional regulation of tibA might reveal some interesting characteristics.
TibA appears to be a multipurpose protein. In addition to being a bona fide adhesin and invasin (with human cells), it also is an autoaggregator and biofilm enhancer, as demonstrated in this study. At this point it is not clear what these novel TibA-associated phenotypes contribute to the pathogenicity or general environmental behavior of E. coli strains like H10407. Bacteria expressing the TibA aggregating phenotype may exist as tight communities of cells that enjoy all of the benefits of this type of existence. In this respect it is interesting to speculate that TibA-mediated aggregation of E. coli cells may be a tool that aids survival of the organism on route to a mammalian host. Also, aggregation may be a way to assist transfer of bacteria across the gastric acid barrier on the way to the intestines. It is interesting that bacterial aggregation helps passage through the stomach and greatly enhances the infectivity of Vibrio cholerae (52). Furthermore, we demonstrated that TibA expression enhanced biofilm formation very efficiently. The ability to form biofilms is a trait that is closely associated with bacterial persistence and virulence, and many persistent and chronic bacterial infections, including periodontitis, otitis media, biliary tract infections, and endocarditis, are now believed to be linked to the formation of biofilms (8, 12).
A novel and differentiated picture of the TibA autotransporter is emerging. Indeed, this molecule seems to be a highly versatile virulence factor that has multiple potential roles in bacterial pathogenesis, and no less than the following four phenotypes linked to bacterial pathogenicity are associated with TibA: (i) it is a potent adhesin with affinity for a number of different human cells; (ii) it is an efficient invasin that results in bacterial invasion of human cells; (iii) it is capable of mediating bacterial aggregation via intercellular self recognition; and (iv) it is a highly efficient initiator of biofilm formation.
Acknowledgments
We thank Birthe Jul Jorgensen for expert technical assistance. W. W. Metcalf, K. C. Murphy, B. Glick, and I. Roberts are acknowledged for providing plasmids.
This work was supported by grant 26-02-0183 from the Danish Technical Research Council.
Editor: A. D. O'Brien
References
- 1.Bachmann, B. 1996. Derivations and genotypes of some mutant derivatives of Escherichia coli K-12, p. 2460-2488. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 2.Benz, I., and M. A. Schmidt. 1992. AIDA-I, the adhesin involved in diffuse adherence of the diarrhoeagenic Escherichia coli strain 2787(O126:H27), is synthesized via a precursor molecule. Mol. Microbiol. 6:1539-1546. [DOI] [PubMed] [Google Scholar]
- 3.Berge, A., B.-M. Kihlberg, A. G. Sjöholm, and L. Björck. 1997. Streptococcal protein H forms soluble complement activating complexes with IgG, but inhibits complement activation by IgG-coated targets. J. Biol. Chem. 272:20774-20774. [DOI] [PubMed] [Google Scholar]
- 4.Bevis, B. J., and B. S. Glick. 2002. Rapidly maturing variants of the Discosoma red fluorescent protein (DsRed). Nat. Biotechnol. 20:83-87.11753367 [Google Scholar]
- 5.Bieber, D., S. W. Ramer, C.-Y. Wu, W. J. Murray, T. Tobe, R. Fernandez, and G. K. Schoolnik. 1998. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science 280:2114-2118. [DOI] [PubMed] [Google Scholar]
- 6.Caparon, M. G., D. S. Stephens, A. Olsen, and J. R. Scott. 1991. Role of M protein in adherence of group A streptococci. Infect. Immun. 59:1811-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen, B. B., C. Sternberg, J. B. Andersen, L. Eberl, S. Møller, M. Givskov, and S. Molin. 1998. Establishment of new genetic traits in a microbial biofilm community. Appl. Environ. Microbiol. 64:2247-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Biofilms: a common cause of persistent infections. Science 284:2137-2142. [DOI] [PubMed] [Google Scholar]
- 9.Czeczulin, J. R., S. Balepur, S. Hicks, A. Phillips, R. Hall, M. H. Mahendra, F. Navarro-Garcia, and J. P. Nataro. 1997. Aggregative adherence fimbria II, a second fimbrial antigen mediating aggregative adherence in enteroaggregative Escherichia coli. Infect. Immun. 65:4135-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diderichsen, B. 1980. flu, a metastable gene controlling surface properties of Escherichia coli. J. Bacteriol. 141:858-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diederich, L., L. J. Rasmussen, and W. Messer. 1992. New cloning vectors for the integration into the λ attachment site attB of the Escherichia coli chromosome. Plasmid 28:14-24. [DOI] [PubMed] [Google Scholar]
- 12.Donlan, R. M., and J. W. Costerton. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elsinghorst, E. A., and D. J. Kopecko. 1992. Molecular cloning of epithelial cell invasion determinants from enterotoxigenic Eschericha coli. Infect. Immun. 60:2409-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elsinghorst, E. A., and J. A. Weitz. 1994. Epithelial cell invasion and adherence directed by the enterotoxigenic Escherichia coli tib locus is associated with a 104-kilodalton outer membrane protein. Infect. Immun. 62:3463-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frick, I.-M., M. Mörgelin, and L. Björck. 2000. Virulent aggregates of Streptococcus pyogenes are generated by homophilic protein-protein interactions. Mol. Microbiol. 37:1232-1247. [DOI] [PubMed] [Google Scholar]
- 16.Hammar, M., Z. Bian, and S. Normark. 1996. Nucleator dependent intercellular assembly of adhesive curli organelles in Escherichia coli. Proc. Natl. Acad. Sci. USA 93:6562-6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasman, H., T. Chakraborty, and P. Klemm. 1999. Antigen-43-mediated autoaggregation of Escherichia coli is blocked by fimbriation. J. Bacteriol. 181:4834-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasman, H., M. A. Schembri, and P. Klemm. 2000. Antigen 43 and type 1 fimbriae determine colony morphology of Escherichia coli K-12. J. Bacteriol. 182:1089-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henderson, I. R., and J. P. Nataro. 2001. Virulence functions of autotransporter proteins. Infect. Immun. 69:1231-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henderson, I. R., F. Navarro-Garcia, and J. P. Nataro. 1998. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 6:370-378. [DOI] [PubMed] [Google Scholar]
- 21.Kajava, A. V., N. Cheng, R. Cleaver, M. Kessel, M. N. Simon, E. Willery, F. Jacob-Dubuisson, C. Locht, and A. C. Steven. 2001. Beta-helix model for the filamentous haemagglutinin adhesin of Bordetella pertussis and related bacterial secretory proteins. Mol. Microbiol. 42:279-292. [DOI] [PubMed] [Google Scholar]
- 22.Kjærgaard, K., M. A. Schembri, H. Hasman, and P. Klemm. 2000. Antigen 43 from Escherichia coli induces inter- and intraspecies cell aggregation and changes in colony morphology of Pseudomonas fluorescens. J. Bacteriol. 182:4789-4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kjærgaard, K., M. A. Schembri, C. Ramos, M. Molin, and P. Klemm. 2000. Antigen 43 facilitates formation of multispecies biofilms. Environ. Microbiol. 2:695-702. [DOI] [PubMed] [Google Scholar]
- 24.Klemm, P., L. Hjerrild, M. Gjermansen, and M. A. Schembri. 2004. Structure-function analysis of the self-recognizing antigen 43 autotransporter protein from Escherichia coli. Mol. Microbiol. 51:283-296. [DOI] [PubMed] [Google Scholar]
- 25.Klemm, P., B. J. Jorgensen, I. van Die, H. de Ree, and H. Bergmans. 1985. The fim genes responsible for synthesis of type 1 fimbriae in Escherichia coli, cloning and genetic organization. Mol. Gen. Genet. 199:410-414. [DOI] [PubMed] [Google Scholar]
- 26.Klemm, P., and M. A. Schembri. 2000. Bacterial adhesins: structure and function. Int. J. Med. Microbiol. 290:27-35. [DOI] [PubMed] [Google Scholar]
- 27.Klemm, P., and M. A. Schembri. 2004. Type 1 fimbriae, curli, and antigen 43: adhesion, colonization, and biofilm formation, p. 8.3.2.6. In R. Curtiss III, J. L. Ingraham, J. B. Kaper, S. Maloy, F. C. Neidhardt, M. M. Riley, C. L. Squires, B. L. Wanner, and A. L. Boeck (ed.), Escherichia coli and Salmonella: cellular and molecular biology, EcoSal, 3rd ed. ASM Press, Washington, D.C. [DOI] [PubMed]
- 28.Larsen, R. A., M. M. Wilson, A. M. Guss, and W. W. Metcalf. 2002. Genetic analysis of pigment biosynthesis in Xanthobacter autotrophicus Py2 using a new, highly efficient transposon mutagenesis system that is functional in a wide variety of bacteria. Arch. Microbiol. 178:193-201. [DOI] [PubMed] [Google Scholar]
- 29.Lindenthal, C., and E. A. Elsinghorst. 1999. Identification of a glycoprotein produced by enterotoxigenic Escherichia coli. Infect. Immun. 67:4084-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindenthal, C., and E. A. Elsinghorst. 2001. Enterotoxigenic Escherichia coli TibA glycoprotein adheres to human intestine epithelial cells. Infect. Immun. 69:52-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu, D., and P. R. Reeves. 1994. Escherichia coli K12 regains its O antigen. Microbiology 140:49-57. [DOI] [PubMed] [Google Scholar]
- 32.McDevitt, D., P. Francois, P. Vaudaux, and T. J. Foster. 1994. Molecular characterization of the clumping factor (fibronectin receptor) of Staphylococcus aureus. Mol. Microbiol. 11:237-248. [DOI] [PubMed] [Google Scholar]
- 33.Menozzi, F. D., P. E. Boucher, G. Riveau, C. Gantiez, and C. Locht. 1994. Surface-associated filamentous hemagglutinin induces autoagglutination of Bordetella pertussis. Infect. Immun. 62:4261-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menozzi, F. D., J. H. Rouse, M. Alawi, M. Laude-Sharp, J. Muller, R. Bischoff, M. J. Brennan, and C. Locht. 1996. Identification of a heparin-binding hemagglutinin present in mycobacteria. J. Exp. Immunol. 184:993-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy, K. C., K. G. Campellone, and A. R. Poteete. 2000. PCR-mediated gene replacement in Escherichia coli. Gene 246:321-330. [DOI] [PubMed] [Google Scholar]
- 36.Nataro, J. P., Y. Deng, D. R. Maneval, A. L. German, W. C. Martin, and M. M. Levine. 1992. Aggregative adherence fimbriae I of enteroaggregative Escherichia coli mediate adherence to HEp-2 cells and hemagglutination of human erythrocytes. Infect. Immun. 60:2297-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nataro, J. P., J. B. Kaper, R. Robins-Browne, V. Prado, P. Vial., and M. M. Levine. 1987. Patterns of adherence of diarrheagenic Escherichia coli. Pediatr. Infect. Dis. J. 6:829-831. [DOI] [PubMed] [Google Scholar]
- 39.Neidhardt, F. C., P. L. Bloch, and D. F. Smith. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ochiai, K., T. Kurita-Ochiai, Y. Kamino, and T. Ikeda. 1993. Effect of co-aggregation on the pathogenicity of oral bacteria. J. Med. Microbiol. 39:183-190. [DOI] [PubMed] [Google Scholar]
- 41.Ørskov, I., and F. Ørskov. 1977. Special O:K:H serotypes among enterotoxigenic E. coli strains from diarrhea in adults and children. Med. Microbiol. Immunol. 163:99-110. [DOI] [PubMed] [Google Scholar]
- 42.Reisner, A., S. Molin, and E. L. Zechner. 2002. Recombinogenic engineering of conjugative plasmids with fluorescent marker cassettes. FEMS Microbiol. Ecol. 42:251-259. [DOI] [PubMed] [Google Scholar]
- 43.Schembri, M. A., G. Christiansen, and P. Klemm. 2001. FimH-mediated auto-aggregation of Escherichia coli. Mol. Microbiol. 41:1419-1430. [DOI] [PubMed] [Google Scholar]
- 44.Schembri, M. A., D. Dalsgaard, and P. Klemm. 2004. Capsule shields the function of short bacterial adhesins. J. Bacteriol. 186:1249-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schembri, M. A., L. Hjerrild, M. Gjermansen, and P. Klemm. 2003. Differential expression of the Escherichia coli autoaggregation factor antigen 43. J. Bacteriol. 185:2236-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schembri, M. A., and P. Klemm. 2001. Biofilm formation in a hydrodynamic environment by novel FimH variants and ramifications for virulence. Infect. Immun. 69:1322-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stentebjerg-Olesen, B., L. Pallesen, L. B. Jensen, G. Christiansen, and P. Klemm. 1997. Authentic display of a cholera toxin epitope by chimeric type 1 fimbriae: effects of insert position and host background. Microbiology 143:2027-2038. [DOI] [PubMed] [Google Scholar]
- 48.Sternberg, C., B. B. Christensen, T. Johansen, A. Toftgaard Nielsen, J. B. Andersen, M. Givskov, and S. Molin. 1999. Distribution of bacterial growth activity in flow-chamber biofilms. Appl. Environ. Microbiol. 65:4108-4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Struve, C., and K. A. Krogfelt. 2003. Role of capsule in Klebsiella pneumoniae virulence: lack of correlation between in vitro and in vivo studies. FEMS Microbiol. Lett. 218:149-154. [DOI] [PubMed] [Google Scholar]
- 50.Vidal, O., R. Longin, C. Prigent-Combaret, C. Dorel, M. Hooreman, and P. Lejeune. 1998. Isolation of an Escherichia coli K-12 mutant able to form biofilms on inert surfaces: involvement of a new ompR allele that increases curli expression. J. Bacteriol. 180:2442-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whitfield, C., and I. S. Roberts. 1999. Structure, assembly and regulation of capsules in Escherichia coli. Mol. Microbiol. 31:1307-1319. [DOI] [PubMed] [Google Scholar]
- 52.Zhu, J., and J. J. Mekalanos. 2001. Quorum sensing-dependant biofilms enhance colonization in Vibrio cholerae. Dev. Cell 5:647-656. [DOI] [PubMed] [Google Scholar]