Abstract
Strongyloides stercoralis causes chronic asymptomatic infections which can be maintained in the human host for many decades. Identification and treatment of S. stercoralis-infected individuals is required because immunosuppression can lead to fatal hyperinfection. In this study, human immunoglobulin G (IgG) that had previously been shown to transfer protective immunity to mice was used to identify potential protective antigens. Three antigens or genes from S. stercoralis larvae were identified as tropomyosin (Sstmy-1), Na+-K+ATPase (Sseat-6), and LEC-5 (Sslec-5). The genes were cloned into plasmids for DNA immunization, and mice were immunized intradermally with the three plasmids individually in combination with a plasmid containing murine granulocyte-macrophage colony-stimulating factor. Only Na+-K+ATPase induced a significant reduction in larval survival after DNA immunization. Immunization with a combination of all three plasmids, including Na+-K+ATPase, did not induce protective immunity. Serum from mice immunized with DNA encoding Na+-K+ATPase was transferred to naïve mice and resulted in partial protective immunity. Therefore, DNA immunization with Na+-K+ATPase induces protective immunity in mice, and it is the first identified vaccine candidate against infection with larval S. stercoralis.
Strongyloides stercoralis, a nematode infecting humans, primates, and dogs, causes a range of relatively benign symptoms in the intestine during acute infection (18). Chronic infections may persist for the lifetime of the host and are commonly clinically asymptomatic (22). However, chronically infected individuals who become immunosuppressed due to a variety of causes, including corticosteroid treatment (40, 45) or infection with human T-cell leukemia virus 1 (HTLV-1) (38), can disseminate S. stercoralis, a condition that can be life threatening (3). Although chemotherapy is available for acute and chronic infections, treatment of the potentially lethal hyperinfection syndrome remains problematic. Thus, given the potential for fatal disease associated with S. stercoralis infection and the difficulty in treatment of hyperinfection, there remains a need for prophylactic vaccines against this infection.
Protective immunity against larval S. stercoralis, as measured by decreased larval survival to challenge infections contained within diffusion chambers, has been studied in mice (2, 5, 25, 26, 42, 43). Immunization of mice with live infective third-stage larvae (L3) resulted in high levels of protective immunity which was dependent on antibody, complement, and neutrophils (5, 34). Protective immunity also developed in mice immunized with deoxycholate (DOC)-soluble larval proteins administered in alum (27). Passive transfer of immunity to mice has been achieved with immunoglobulin M (IgM) and IgG from mice immunized with live L3 (34) and with IgG from mice immunized with DOC-soluble larval proteins. Protective IgG from mice immunized with DOC-soluble larval proteins was used to purify a limited subset of antigens from the DOC-soluble larval proteins which induced high levels of protective immunity in mice (27).
Passive transfer of immunity to mice was also accomplished with IgG purified from S. stercoralis-infected humans. The human IgG mediated larval killing in mice through a mechanism dependent on complement and neutrophils but not through antibody-dependent cellular cytotoxicity (ADCC). The protective human IgG was used to purify, by affinity chromatography, a small group of antigens from the DOC-soluble antigen pool. Immunization of mice with the human IgG-specific antigen pool in the presence of alum-induced protective immunity in mice (31).
Although there has been success in immunizing mice against infection with S. stercoralis using small numbers of antigens identified either with protective mouse (27) or human (31) IgG, this approach has limited clinical application because of the scarce availability of native L3 proteins. Recombinant antigen vaccines against helminth infections have been tested with limited success (8, 28, 35, 36), which in part may be due to the altered conformation and/or glycosylation of the recombinant antigens. An alternative vaccination approach has been to immunize mice with DNA, where the protein product is produced in vivo from the plasmid DNA. This approach has been used successfully against both protozoan (7, 16, 29) and helminth (10, 46, 53, 54) infections resulting in measurable immune responses, some of which mediate protective immunity.
The goal of the present study was to determine if protective human IgG could be used to identify specific antigens that could then be tested as possible vaccine candidates. To this end, two-dimensional (2-D) immunoblotting was performed to identify antigens specifically recognized by the protective IgG, and mass spectroscopy was used to sequence specific proteins. Banked cDNA clones, archived from a large-scale nematode sequencing project (37), encoding the proteins identified by protein sequencing were subcloned into DNA-immunization vectors. These plasmids were then injected intradermally into mice to determine if the mice would develop protective immunity to infection with S. stercoralis.
MATERIALS AND METHODS
Protective human IgG.
Outdated plasma from a Haitian blood bank was screened for reactivity with recombinant S. stercoralis L3 antigen 5a (41). A single seropositive plasma sample was identified from an anonymous donor. Infection status with S. stercoralis was therefore unknown, although the serum tested negative for circulating filarial antigen. The plasma was treated with 5 U of thrombin (JPI Jones Daniels Pharmaceuticals, St. Petersburg, Fla.) for 30 min at 37°C to obtain serum. This serum has been shown to transfer protective immunity to mice (31) and is referred to as immune serum. Control serum was obtained from an individual not infected with S. stercoralis.
Antigen preparation.
Deoxycholic acid (DOC)-soluble larval proteins were prepared as described previously (27). Briefly, L3 were washed in phosphate-buffered saline (PBS) supplemented with 100 U of penicillin and 100 μg of streptomycin per ml and stored at −80°C. L3 were thawed and homogenized in the presence of a protease inhibitor cocktail (Sigma, St. Louis, Mo.) and then sonicated. The homogenized and sonicated L3 were incubated in PBS at 4°C for 18 h with continuous mixing. PBS-soluble proteins were removed, and the PBS-insoluble proteins were resuspended in 20 mM Tris-HCl-0.5% deoxycholic acid (Sigma) with continuous mixing for 12 h at 4°C. The DOC-soluble proteins were then dialyzed against PBS for 12 h, concentrated, filter sterilized, and stored at −80°C. Protein concentration was quantitated by a Micro BCA Protein Assay kit (Peirce, Rockford, Ill.).
Identification of differentially recognized parasite antigens.
Two-dimensional electrophoresis (Kendrick Laboratories, Madison, Wis.) was performed on DOC-soluble larval antigens as follows. Isoelectric focusing was carried out in glass tubes with an inner diameter of 2.0 mm by using 2.0% ampholines (pH 4 to 8; Hoefer Scientific Instruments, San Francisco, Calif.) at 9,600 V per h. After incubation for 10 min in equilibration buffer (10% glycerol, 50 mM dithiothreitol, 2.3% sodium dodecyl sulfate [SDS], and 0.0625 M Tris, pH 6.8), the tube was sealed to the top of a stacking gel layered on a 10% SDS-acrylamide slab gel (0.75 mm thick). SDS-polyacrylamide gel electrophoresis (PAGE) was then carried out for approximately 4 h at 12.5 mA/gel. Proteins from two of the gels, each loaded with 400 μg of protein, were transferred onto polyvinylidene fluoride (PVDF) membranes, incubated with IgG from immune and control human sera, and revealed using chemiluminescence. The immune and control Western blotting images were compared, and spots recognized by immune sera but not by control sera were identified. A third 2-D electrophoresis gel loaded with an equivalent amount of protein was stained with Coomassie blue, and gel spots uniquely recognized by the immune serum were cleanly excised, washed in sterile water and 50% acetonitrile, and submitted for identification by mass spectrometry (Harvard Microchemistry, Boston, Mass.). Protein internal sequencing was done by reverse-phase microcapillary high-performance liquid chromatography nanoelectrospray tandem mass spectrometry (μLC-MS-MS) on a Micromass qTof1 hybrid quadruple time-of-flight mass spectrometer. Protein samples excised from gels were reduced, carboxyamidomethylated, and digested with trypsin. Ten to 30% of the digested samples were analyzed by μLC-MS-MS, and resultant MS-MS spectra were correlated with known sequences from protein and nucleotide databases using the SEQUEST algorithm (14). Any uncorrelated MS-MS spectra from 2-D gel samples were manually de novo sequenced to obtain novel peptide sequences (9).
Construction of DNA vaccines.
Based on the amino acid sequences obtained by mass spectrometry, partial coding sequences of the genes were identified by homology to banked cDNAs from libraries of S. stercoralis rhabditiform larvae (TBN95TM-SSR). These were constructed previously by the Helminth Immunology Section of the National Institute of Allergy and Infectious Disease (NIAID) and partially sequenced by the Washington University Genome Sequencing Center. The banked cDNAs were generated as part of a nematode expressed sequence tag (EST) project (37). Glycerol stocks of Escherichia coli containing these identified genes in a Lambda Uni-zap XR vector were amplified overnight in Luria-Bertani (LB) media, and the plasmid DNA was purified using the QiaPrep Spin Mini-Prep kit (QIAGEN, Valencia, Calif.). These plasmids were sequenced in their entirety (SeqWright, Houston, Tex.). The coding portions of the cDNA constructs were amplified using PCR with sequence-specific primers and inserted into VR1020 (Vical, San Diego, Calif.), which contained an upstream signal sequence (MDAMKRGLCCVLLLCGAVFVSPSGTGSTL) to enhance extracellular secretion, and engineered to have a TOPO-TA cloning site (Invitrogen Life Technologies, Carlsbad, Calif.). The plasmid was then transformed into Top10 cells (Invitrogen). Directional and in-frame insertion was verified by primer extension sequencing through the insertion site of the resulting plasmid. An ultrapure preparation of vaccine plasmids (Aldevron, Fargo, N.D.) as well as the plasmid VC1701 containing murine granulocyte-macrophage colony-stimulating factor (GM-CSF) (generously provided by Walter Weiss, Naval Medical Research Annex) were used for the vaccinations.
Mice and parasites.
BALB/cJ female mice, 6 to 8 weeks of age and obtained from the Jackson Laboratory (Bar Harbor, Maine), were housed in filter-top microisolator boxes under light- and temperature-controlled conditions.
S. stercoralis L3 were obtained from the cultures of fresh stools from a laboratory dog infected with the parasite according to methods previously described (2). Larvae were collected from 7-day charcoal cultures, washed, and resuspended in a 1:1 mixture of IMDM (Sigma) and NCTC-135 (Sigma) with a mixture of 100 U of penicillin and 100 μg of streptomycin (Gibco, Grand Island, N.Y.) per ml.
DNA immunizations.
Mice were immunized with plasmids containing the identified sequences for larval S. stercoralis LEC-5 (Sslec-5), tropomyosin (Sstmy-1), and Na+-K+ATPase (Sseat-6). All groups, including the control groups, received plasmid containing the murine GM-CSF sequence. GM-CSF was coadministered with experimental plasmids, because it has been shown to increase the IgG response after DNA immunization (44, 49). Mice were immunized intradermally in the ear with 20 μg of the antigen DNA/μl and 10 μg of GM-CSF/μl in a total volume of 20 μl (10 μl/ear) every 2 weeks for a total of six immunizations. Mice were challenged with 50 S. stercoralis L3 contained within diffusion chambers which were subcutaneously implanted dorsally in the mice. Construction of diffusion chambers covered with 2.0-μm-pore-size Isopore membranes (Millipore, Bedford, Mass.) followed previously described methods (2). Diffusion chambers were removed from the mice 96 h after challenge, and larval viability was determined based on motility and morphology. Cells recovered from the diffusion chambers were quantitated, centrifuged onto slides with a Cytospin 3 centrifuge (Shandon, Pittsburgh, Pa.), and stained for differential counts with DiffQuik (Baxter Healthcare, Miami, Fla.).
Parasite-specific and peptide enzyme-linked immunosorbent assay (ELISA).
To measure the parasite-specific IgG response following DNA immunization, Maxisorp 96-well plates (Nunc, Inc., Naperville, Ill.) were coated with 10 μg of DOC-soluble larval proteins/ml for 12 h, and plates were blocked with borate-blocking buffer solution (BBS; 0.17 M boric acid, 0.12 M NaCl, 0.05% Tween 20, 0.025% bovine serum albumin [BSA], 1 mM EDTA, pH 8.2). Serum collected from DNA-immunized mice was serially diluted in BBS and placed in duplicate wells. A single dilution (1:1,600) from a linear part of the curve was selected for all groups to allow comparison of titers.
Based on algorithms known to predict immunogenic peptides (19), two 15-mer peptides (both unconjugated and keyhole limpet hemacyanin (KLH) conjugated) from each of the identified possible immunogens were synthesized by the NIAID peptide synthesis core facility. The predicted antibody-binding peptide regions for each gene are as follows: ATP1 (amino acids 116 to 127; CPESYDDNEVPSE), ATP2 (amino acids 89 to 101; CSKENDYGFKEGK), LEC1 (amino acids 42 to 53; CEKPKRIDFNFHK), LEC2 (amino acids 221 to 233; CGEEDRTGKFPL), TRP1 (amino acids 8 to 19; CTANQQLEEKEKK), and TRP2 (amino acids 186 to 197; CRSVQKLQKEVDR). To measure specific IgG responses to each peptide, plates were coated with 10 μg of each peptide/ml separately, and plates were blocked with 5% BSA-0.05% Tween 20-PBS. Serum samples were diluted in 1% BSA-0.05% Tween 20-PBS and placed in duplicate wells. Biotinylated anti-mouse IgG (γ-chain-specific) antibody (Sigma) was added to the wells, followed by avidin peroxidase (Sigma) and finally by the peroxidase substrate 2,2′-azinodi(3-ethylbenzthiazoline-6-sulfonate) (ABTS; Kirkgaard & Perry Laboratories, Gaithersburg, Md.). Antibody responses were uniformly low. Therefore, in all analyses the peptide ELISA data are shown as the optical density (OD) of a 1:100 dilution of serum.
Serum transfer.
Serum was collected from mice immunized with the different DNA preparations at the time that the challenge infections were recovered. The serum was pooled from each mouse in the group in proportions such that each mouse was equally represented in the serum pool. The pooled sera were then injected in naïve mice into the subcutaneous pocket surrounding the implanted diffusion chamber at the time of challenge. Each mouse was given 100 μl of serum diluted with 100 μl of PBS. Diffusion chambers were recovered 24 h after implantation.
Statistical analyses.
Experiments consisted of 5 to 12 mice per group, which is noted in the text. The data shown are the combined results of two experiments. Statistical analysis of the data was performed in Systat (version 10.2) using multiple general linear hypothesis multifactorial analysis of variance. Values were considered significantly different when P ≤ 0.05.
Nucleotide sequence accession numbers.
The GenBank accession numbers of sequences determined in the course of this study are BE579623 (tropomyosin; Sstmy-1), BE581796 (Na+-K+ATPase; Sseat-6), and BG227948 (LEC-5; Sslec-5).
RESULTS
Identification of S. stercoralis L3 antigens with protective human IgG.
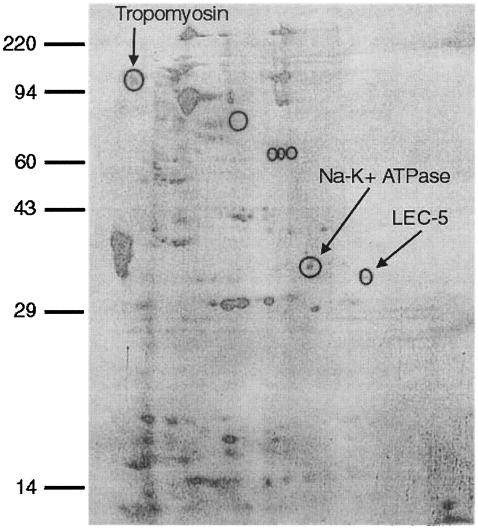
2-D immunoblotting against DOC-soluble S. stercoralis larval antigens was performed using human IgG previously shown to passively transfer protective immunity to mice (31). Seven proteins were specifically recognized by the protective IgG antibody in comparison to control human IgG (Fig. 1). 2-D SDS-PAGE gels run simultaneously were used in parallel as a source of the protein spots for amino acid sequencing. Three antigens, with molecular masses of approximately 120, 34, and 32 kDa, were uniquely recognized by immune serum and were present in sufficient quantity for sequencing. The recognized antigens were sequenced, and homology search was performed to identify matching sequences in the S. stercoralis EST databases. The three vaccine candidates were identified as tropomyosin (120 kDa), Na+-K+ATPase (34 kDa), and LEC-5 (32 kDa), a galectin. These were then cloned into plasmids and used for subsequent immunization experiments.
FIG. 1.
2-D immunoblot with immune human IgG. Seven proteins (circled) were differentially recognized by immune serum and were sent for sequencing. Three proteins (tropomyosin, Na+-K+ATPase, and LEC-5; arrows) were identified by database analyses of mass spectroscopy results.
Immunization of mice with Na+-K+ATPase, LEC-5, and tropomyosin DNA.
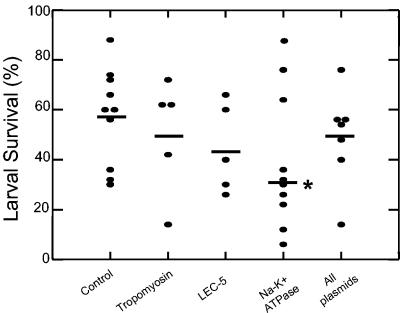
BALB/cJ mice were immunized with plasmids encoding Sseat-6, Sslec-5, or Sstmy-1 individually or as a mixture in the presence of a plasmid containing GM-CSF. Parasite survival was compared to that for control mice that had only been exposed to a plasmid containing GM-CSF. The Sseat-6-containing plasmid was the only antigen to induce protective immunity (P = 0.05) against S. stercoralis L3 (Fig. 2). There was, on average, a mean reduction in larval survival of 35% compared to that for control vaccinated mice. Mice immunized with all three plasmids, including Sseat-6, did not develop protective immunity, suggesting that the protection induced by Sseat-6 was lost when it was combined with other genes during DNA immunization.
FIG. 2.
Larval survival after DNA immunization with tropomyosin, LEC-5, and the Na+-K+ATPase. Results depicted are the combination of two experiments. Data shown are the means (horizontal bars) of the groups, with 12 (Na+-K+ATPase), 5 (tropomyosin), 5 (LEC-5), 7 (All plasmids), and 11 (control) mice per group. The asterisk denotes statistical significance (P = 0.05) from the control.
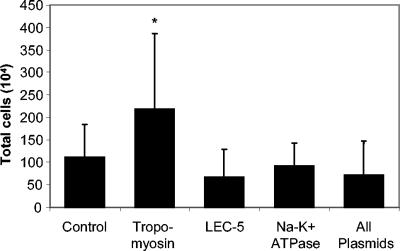
Mice receiving a DNA immunization with tropomyosin had statistically significantly higher numbers of cells migrating into the diffusion chambers than the control group and the other immunized groups (Fig. 3). Differential cell analyses were performed on the cells found in the diffusion chambers, and a statistically significant increase was seen in the percentage of eosinophils in mice immunized with Sslec-5 (Table 1). Although DNA immunization caused an increase in the total number of cells (Sstmy-1) or an increase in eosinophilia (Sslec-5), protective immunity only developed in mice immunized with Sseat-6.
FIG. 3.
Cellular recruitment into diffusion chambers during challenge infection after DNA immunization. Data shown represent the means and standard deviations of two combined experiments. The asterisk denotes statistical significance (P ≤ 0.05) from the control.
TABLE 1.
Differentials of cells recruited into the diffusion chamber in DNA-immunized mice at the time of challenge infectiona
| Antigen | Neutrophil | Lymphocyte | Monocyte | Eosinophil |
|---|---|---|---|---|
| Control | 55 ± 11 | 0.1 ± 0.3 | 44 ± 11 | 1.2 ± 1.5 |
| Tropomyosin | 68 ± 11 | 0.3 ± 0.5 | 30 ± 11 | 1.8 ± 1.0 |
| Galectin | 51 ± 20 | 0.0 ± 0.0 | 44 ± 20 | 4.2 ± 1.6* |
| Na+-K+ATPase | 64 ± 13 | 0.4 ± 1.3 | 34 ± 12.5 | 1.6 ± 2.1 |
| All plasmids | 61 ± 15 | 0.0 ± 0.0 | 39 ± 15 | 0.3 ± 0.6 |
Data shown represent the means (%) and standard deviations of two combined experiments. The asterisk denotes statistical significance (P ≤ 0.05) from the control.
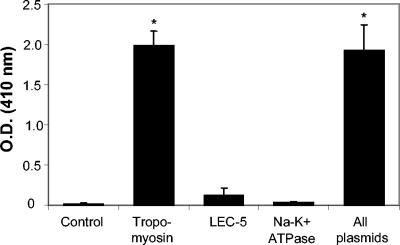
The genes for tropomyosin, LEC-5, and Na+-K+ATPase were identified in the present study from a DOC-soluble pool of larval antigens. To evaluate the antibody responses against the three identified antigens, ELISAs were performed using serum from the DNA-immunized mice against DOC-soluble larval antigens. Mice immunized with Sstmy-1, either alone or in combination with Sseat-6 and Sslec-5, developed positive antibody responses against the DOC-soluble L3 antigens. Mice exposed to the control GM-CSF plasmid, Sseat-1, or Sslec-5 had low or undetectable antibody titers to the DOC-soluble larval antigens (Fig. 4).
FIG. 4.
DOC-specific IgG titers from mice immunized with each individual plasmid or with all three plasmids. Significant antibody titers, compared to those of the control, were detected only in mice receiving the plasmid containing the tropomyosin sequence and in mice receiving all plasmids.
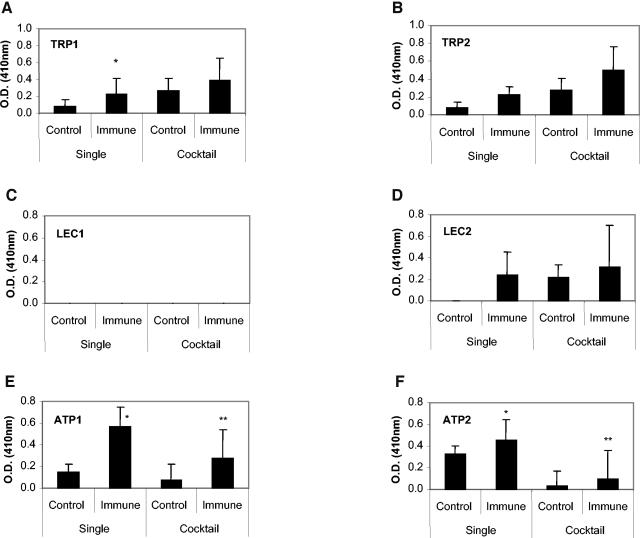
Two synthetic peptides were created for each antigen based on predicted antibody-binding regions. The IgG response was measured from mice immunized with a particular antigen against the peptides created for that antigen (Fig. 5). Statistically significant IgG responses were measured in mice immunized with Sstmy-1 to the TRP1 peptide and in mice immunized with Sseat-6 to the ATP1 and ATP2 peptides. There were no significant IgG responses generated in mice immunized with Sslec-5 to the LEC1 and LEC2 peptides in mice. After immunization with the mixture of plasmids, only antibody responses against ATP1 and ATP2 peptides were statistically elevated compared to those of controls, although these responses were statistically lower than that seen in mice immunized with Sseat-6 alone (Fig. 5).
FIG. 5.
IgG response against synthetic peptides that were predicted to be antibody-binding regions from tropomyosin, LEC-5, and Na+-K+ATPase. The peptide-specific IgG response after immunization with Sstmy-1 (A and B), Sslec-5 (C and D), and Sseat-6 (E and F) was measured by reactivity against the synthetic peptides conjugated to KLH. Single refers to groups immunized with each gene sequence separately, and cocktail refers to the group receiving all gene sequences simultaneously. Serum was diluted 1:100. The response to KLH is subtracted from the values above. *, Statistical significance from the control group (P ≤ 0.05); **, statistical significance from the single immune group (P ≤ 0.05).
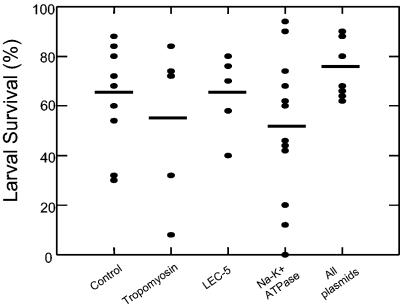
Sera from mice immunized with individual plasmids for Sseat-6, Sslec-5, or Sstmy-1 and the combination of all three plasmids were used in passive serum transfer experiments to determine if protective immunity could be transferred with antibody to naïve mice. Only partial protection was transferred with serum from mice immunized with Sseat-6 (Fig. 6). There were individual mice that were clearly protected; however, statistical significance with the group was not achieved (P = 0.187).
FIG. 6.
Larval survival after passive transfer of serum from mice immunized with DNA sequences for tropomyosin, LEC-5, Na+-K+ATPase, or all plasmids. Serum was pooled from the DNA-immunized mice from the respective group and transferred at the time of challenge into the pocket surrounding the diffusion chamber in naïve mice (n = 5). The bars represent the mean of each group, and the percent larval survival for each individual mouse in each group is depicted (•). Statistically significant protection was not reached in any group.
DISCUSSION
The goal of this study was to identify proteins with potential vaccine efficacy against larval S. stercoralis and, secondly, to determine whether DNA immunization with the identified gene sequences would induce protective immunity against S. stercoralis in mice. IgG from a seropositive individual which was previously shown to transfer protective immunity to mice (31) was used to identify antigens recognized by infected humans. Previous work has shown that mice immunized with DOC-soluble S. stercoralis antigens are protected against infection with S. stercoralis L3 (27). Furthermore, based on 1-D electrophoresis, approximately 13 DOC-soluble L3 proteins were purified using the immune human IgG, which also induced protective immunity in mice (31). In the present study, only seven proteins were differentially recognized by the same immune human IgG after 2-D electrophoresis. A possible explanation for why a greater number of antigens were uniquely recognized by the immune serum in the original study was that the antigens in that study were purified with the immune human IgG on an immunoaffinity column and then concentrated before being visualized by 1-D electrophoresis. In the present study the whole DOC-soluble larval protein pool was run in the 2-D electrophoresis. Therefore, the quantity of antigens specific for the immune human serum loaded on the gels in the two studies was surely different. Using concentrated human IgG-specific DOC-soluble larval proteins in 2-D electrophoresis immunoblot analyses would likely result in the identification of many more antigens recognized by the protective antibodies. The proteins identified in the present study therefore probably represent the most abundant reactive proteins, not necessarily the most active for use in a vaccine.
Three genes, Sseat-6, Sslec-5, and Sstmy-1, were identified in this study after 2-D immunoblot analysis of the DOC-soluble larval antigens recognized by protective human IgG followed by amino acid sequence analysis of the recognized antigens. The Sseat-6-containing plasmid was the only gene that induced protective immunity against S. stercoralis after DNA immunization. Concurrent immunization with all three plasmids eliminated the protective immune response mediated by Sseat-6 compared to immunization with Sseat-6 alone. This suggests that the immune response against Sslec-5 and Sstmy-1 reduced the specific response to Sseat-6. Indeed, data from the peptide-specific antibody response to the TRP1, TRP2, and LEC2 peptides after concurrent immunization with all three antigens were similar to that seen after immunization with each plasmid separately but was not significantly higher than that of the control group. In contrast, the peptide-specific IgG response to ATP1 and ATP2 peptides was almost completely eliminated when mice were immunized with all three constructs, but it still remained significantly higher than that of the control group. Cocktail vaccines often show enhanced protective effects compared to those of single antigens and genes (15, 58). However, in other studies it has been observed that immunization with a cocktail vaccine gave no better protection than that seen with single antigens, and in fact the antibody responses to individual antigens was reduced after immunization with multiple antigens simultaneously (1). Immunization with Sseat-6, using the present immunization protocol, did not induce sterile immunity. Therefore, a combination of more than one protective antigen may be required for the development of an effective vaccine that takes into consideration the possibility of competitive or dominant antigens.
Tropomyosin was the only antigen which induced a strong antibody response in the ELISA using DOC-soluble proteins, which might reflect the relative concentrations of the three antigens in the DOC-soluble protein pool. It has previously been reported that induction of high antibody responses after DNA immunization does not guarantee that the antibody generated will mediate a protective immune response (33). Furthermore, immunization with tropomyosin was the only antigen which induced elevated cellular infiltration into the parasite microenvironment. However, the presence of a strong antibody response and cell recruitment still did not translate into a protective immune response. DNA immunization with Ovtmy-1, the gene for tropomyosin from Onchocerca volvulus, induced a potent humoral response but failed to induce protection against O. volvulus L3 (23), whereas immunization with recombinant protein did result in protection against adult Acanthocheilonema viteae and microfilariae O. lienalis (51). Tropomyosin also has been successful at inducing a protective immune response against Trichostrongylus colubriformis (39) and A. viteae (24). Therefore, tropomyosin may still prove to be an effective protective antigen against S. stercoralis if administered in a manner that stimulates the appropriate immune response.
LEC-5 was also identified by the human immune serum. The antibody response after immunization with Sslec-5 consistently was weak regardless of the method used to detect the IgG response. An antibody response to the LEC2 peptide was detected after immunization with Sslec-5 alone but not after cocktail vaccination; no response was detected against the LEC1 peptide. Elevated eosinophil recruitment into the microenvironment of the larvae provided some evidence that immunization with Sslec-5 induced an immune response. Galectins are a family of lectins diverse in their carbohydrate-binding specificity (52) and in their functions (32, 55). Galectins are expressed by a variety of immune cells (52), and the mammalian galectin OVGAL11 is upregulated in gastrointestinal tissue during infection with the nematode parasite Haemonchus contortus, suggesting that mammalian galectins could be involved in the immune response to intestinal nematodes (13). Galectins have been identified in a variety of nematodes, including Caenorhabditis elegans (4), Haemonchus contortus (21), Trichostrongylus colubriformis (20), and Onchocerca volvulus (30). The galectin Ov-GBP-2 from O. volvulus is developmentally regulated, and the protein was detected in different areas of the worm as the larvae developed. There is presently no evidence that LEC-5 is developmentally regulated, but as the function of galectins is diverse, LEC-5 may be involved in any number of processes and therefore remains an interesting vaccine candidate.
Failure in the present study to induce a protective immune response with either Sslec-5 or Sstmy-1 may be reflective of the inability of the DNA immunization method used to induce the proper effector arms of the immune response. DNA immunization often leads to Th1-mediated immune response (12), and in the absence of a Th2-mediated immune response, protective immunity against S. stercoralis is lost (42). Therefore, immunization with recombinant proteins, a prime/boost approach with DNA and recombinant protein (49), or use of appropriate adjuvants may lead to a stronger and more effective protective immune response with these two antigens.
The results from ELISA using the synthetic peptides and the passive serum transfer data suggest that an antibody response is generated against Na+-K+ATPase in mice after DNA immunization and that the antibody can partially mediate larval killing. The role of cells in the killing process was not clear, as there was neither an increase in cell numbers nor a change in the distribution of the infiltrating cells following immunization. The human IgG used to identify Na+-K+ATPase was shown to bind to the cuticle, the glands surrounding the esophagus, and (very weakly) to muscles of S. stercoralis L3 (31), thereby suggesting that the Na+-K+ATPase may be located in one of those regions. Na+-K+ATPases are heterodimeric integral membrane proteins, consisting of alpha and beta subunits, that couple ATP hydrolysis to Na+ and K+ ion transport and maintenance of the electrochemical gradients across the plasma membrane (47). Na+-K+ATPases have not been shown to induce protective immunity against other helminth parasites, although in mice immunized against Ancylostoma caninum, challenge infection worms were found to have lower ATPase activities than worms recovered from naïve mice (56), thus suggesting that the Na+-K+ATPase may be a good potential target for the immune response. Therefore, antibodies against the Na+-K+ATPase may function in killing the larvae through one of the following mechanisms. First, a mutation in the eat-6 gene, which encodes the alpha subunit of the Na+-K+ATPase in C. elegans, causes a disruption in excitable cell function, resulting in weak contraction and slow relaxations of the pharyngeal muscle (11) and an abnormal feeding behavior (47). Because the protective human IgG binds to the glands surrounding the esophagus, it is possible that antibodies may inhibit the function of the Na+-K+ATPase, which causes a disruption of the ion concentration gradient within the pharyngeal region, leading to the death of the worm. Alternatively, antibody may bind to Na+-K+ATPases located in the cuticle and produce a disruption in the ion concentration gradient across the cuticle, leading to the death of the worm.
A second possible mechanism is based on the finding that the Na+-K+ATPase has been implicated in resistance of Schistosoma mansoni cercaria to complement-mediated killing (50). Because complement is required for immune-mediated killing of larval S. stercoralis (5, 31, 34), it is possible that disruption of the S. stercoralis Na+-K+ATPase in any location in the worm leads to a decreased ability to defend against complement-mediated larval killing.
A third possible mechanism would suggest that the location of the Na+-K+ATPase may be prime for an attack mediated by neutrophils, which are the cells required to mediate killing by human IgG (31) and mouse IgG (34) in mice. Thus, antibody to the Na+-K+ATPase may simply provide an attachment anchor for neutrophils and/or other Fcγ receptor-bearing cells.
Finally, it is possible that an immune response directed at the Na+-K+ATPase can induce paralysis of the pharyngeal musculature similar to that seen with ivermectin. Ivermectin is presently accepted as one of the most effective drugs to treat strongyloidiasis (57). It has been shown to diminish the Na+-K+ATPase activity in O. volvulus in a dose-dependent fashion (48), and it can induce paralysis of the pharyngeal musculature in Ascaris suum and Haemonchus contortus (6, 17). The immune response might interact with the Na+-K+ATPase in a similar manner, resulting in death of the parasite.
In conclusion, human serum was used to discover potential vaccine candidates; three were identified, of which only Na+-K+ATPase could induce protective immunity. Na+-K+ATPase is the first defined protective antigen against S. stercoralis in mice, and it is a promising potential vaccine candidate against S. stercoralis in humans, because it was identified with protective human antibody. The two antigens that failed to induce protective immunity via DNA immunization may have the potential to induce protective immunity if administered in a different form or manner. Finally, combining the three antigens in a single immunization proved detrimental for the development of protective immunity. This observation suggests that care must be taken in developing cocktail vaccines such that the component parts are synergistic in the induction of protective immunity.
Acknowledgments
We acknowledge support from NIH grants RO1 AI 47189 and RO1 AI 22662.
We also thank Ann Marie Galioto, Jessica Ligas, Gilberto Santiago, and Juergen Landmann for expert technical assistance.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Abraham, D., O. Leon, S. Leon, and S. Lustigman. 2001. Development of a recombinant antigen vaccine against infection with the filarial worm Onchocerca volvulus. Infect. Immun. 69:262-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham, D., H. L. Rotman, H. F. Haberstroh, W. Yutanawiboonchai, R. A. Brigandi, O. Leon, T. J. Nolan, and G. A. Schad. 1995. Strongyloides stercoralis: protective immunity to third-stage larvae in BALB/cByJ mice. Exp. Parasitol. 80:297-307. [DOI] [PubMed] [Google Scholar]
- 3.Adedayo, A. O., G. A. Grell, and P. Bellot. 2001. Case study: fatal strongyloidiasis associated with human T-cell lymphotropic virus type 1 infection. Am. J. Trop. Med. Hyg. 65:650-651. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed, H., M. A. Bianchet, L. M. Amzel, J. Hirabayashi, K. Kasai, Y. Giga-Hama, H. Tohda, and G. R. Vasta. 2002. Novel carbohydrate specificity of the 16-kDa galectin from Caenorhabditis elegans: binding to blood group precursor oligosaccharides (type 1, type 2, Tα, and Tβ) and gangliosides. Glycobiology 12:451-461. [DOI] [PubMed] [Google Scholar]
- 5.Brigandi, R. A., H. L. Rotman, W. Yutanawiboonchai, O. Leon, T. J. Nolan, G. A. Schad, and D. Abraham. 1996. Strongyloides stercoralis: role of antibody and complement in immunity to the third stage of larvae in BALB/cByJ mice. Exp. Parasitol. 82:279-289. [DOI] [PubMed] [Google Scholar]
- 6.Brownlee, D. J., L. Holden-Dye, and R. J. Walker. 1997. Actions of the anthelmintic ivermectin on the pharyngeal muscle of the parasitic nematode, Ascaris suum. Parasitology 115(Part 5):553-561. [DOI] [PubMed] [Google Scholar]
- 7.Campbell, K., H. Diao, J. Ji, and L. Soong. 2003. DNA immunization with the gene encoding P4 nuclease of Leishmania amazonensis protects mice against cutaneous Leishmaniasis. Infect. Immun. 71:6270-6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capron, A., M. Capron, and G. Riveau. 2002. Vaccine development against schistosomiasis from concepts to clinical trials. Br. Med. Bull. 62:139-148. [DOI] [PubMed] [Google Scholar]
- 9.Chittum, H. S., W. S. Lane, B. A. Carlson, P. P. Roller, F. D. Lung, B. J. Lee, and D. L. Hatfield. 1998. Rabbit beta-globin is extended beyond its UGA stop codon by multiple suppressions and translational reading gaps. Biochemistry 37:10866-10870. [DOI] [PubMed] [Google Scholar]
- 10.Da'dara, A. A., P. J. Skelly, M. M. Wang, and D. A. Harn. 2001. Immunization with plasmid DNA encoding the integral membrane protein, Sm23, elicits a protective immune response against schistosome infection in mice. Vaccine 20:359-369. [DOI] [PubMed] [Google Scholar]
- 11.Davis, M. W., D. Somerville, R. Y. Lee, S. Lockery, L. Avery, and D. M. Fambrough. 1995. Mutations in the Caenorhabditis elegans Na, K-ATPase alpha-subunit gene, eat-6, disrupt excitable cell function. J. Neurosci. 15:8408-8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnelly, J. J., J. B. Ulmer, J. W. Shiver, and M. A. Liu. 1997. DNA vaccines. Annu. Rev. Immunol. 15:617-648. [DOI] [PubMed] [Google Scholar]
- 13.Dunphy, J. L., A. Balic, G. J. Barcham, A. J. Horvath, A. D. Nash, and E. N. Meeusen. 2000. Isolation and characterization of a novel inducible mammalian galectin. J. Biol. Chem. 275:32106-32113. [DOI] [PubMed] [Google Scholar]
- 14.Eng, J. K., A. L. McCormick, and J. R. Yates III. 1994. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 5:976-989. [DOI] [PubMed] [Google Scholar]
- 15.Fachado, A., A. Rodriguez, S. O. Angel, D. C. Pinto, I. Vila, A. Acosta, R. R. Amendoeira, and J. Lannes-Vieira. 2003. Protective effect of a naked DNA vaccine cocktail against lethal toxoplasmosis in mice. Vaccine 21:1327-1335. [DOI] [PubMed] [Google Scholar]
- 16.Garg, N., and R. L. Tarleton. 2002. Genetic immunization elicits antigen-specific protective immune responses and decreases disease severity in Trypanosoma cruzi infection. Infect. Immun. 70:5547-5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geary, T. G., S. M. Sims, E. M. Thomas, L. Vanover, J. P. Davis, C. A. Winterrowd, R. D. Klein, N. F. Ho, and D. P. Thompson. 1993. Haemonchus contortus: ivermectin-induced paralysis of the pharynx. Exp. Parasitol. 77:88-96. [DOI] [PubMed] [Google Scholar]
- 18.Genta, R. M. 1989. Global prevalence of strongyloidiasis: critical review with epidemiologic insights into the prevention of disseminated disease. Rev. Infect. Dis. 11:755-767. [DOI] [PubMed] [Google Scholar]
- 19.Grant, G. 2003. Synthetic peptides for production of antibodies that recognize intact proteins, p.9.2.2-9.2.16. In K. A. Coligan, J. E. Marguilies, D. H. Shevach, and E. M. Strober (ed.), Current protocols in immunology. John Wiley and Sons, New York, N.Y. [DOI] [PubMed]
- 20.Greenhalgh, C. J., S. A. Beckham, and S. E. Newton. 1999. Galectins from sheep gastrointestinal nematode parasites are highly conserved. Mol. Biochem. Parasitol. 98:285-289. [DOI] [PubMed] [Google Scholar]
- 21.Greenhalgh, C. J., A. Loukas, D. Donald, S. Nikolaou, and S. E. Newton. 2000. A family of galectins from Haemonchus contortus. Mol. Biochem. Parasitol. 107:117-121. [DOI] [PubMed] [Google Scholar]
- 22.Grove, D. I. 1996. Human strongyloidiasis. Adv. Parasitol. 38:251-309. [DOI] [PubMed] [Google Scholar]
- 23.Harrison, R. A., and A. E. Bianco. 2000. DNA immunization with Onchocerca volvulus genes, Ov-tmy-1 and OvB20: serological and parasitological outcomes following intramuscular or GeneGun delivery in a mouse model of onchocerciasis. Parasite Immunol. 22:249-257. [DOI] [PubMed] [Google Scholar]
- 24.Hartmann, S., R. Adam, T. Marti, C. Kirsten, S. Seidinger, and R. Lucius. 1997. A 41-kDa antigen of the rodent filaria Acanthocheilonema viteae with homologies to tropomyosin induces host-protective immune responses. Parasitol. Res. 83:390-393. [DOI] [PubMed] [Google Scholar]
- 25.Herbert, D. R., J. J. Lee, N. A. Lee, T. J. Nolan, G. A. Schad, and D. Abraham. 2000. Role of IL-5 in innate and adaptive immunity to larval Strongyloides stercoralis in mice. J. Immunol. 165:4544-4551. [DOI] [PubMed] [Google Scholar]
- 26.Herbert, D. R., T. J. Nolan, G. A. Schad, and D. Abraham. 2002. The role of B cells in immunity against larval Strongyloides stercoralis in mice. Parasite Immunol. 24:95-101. [DOI] [PubMed] [Google Scholar]
- 27.Herbert, D. R., T. J. Nolan, G. A. Schad, S. Lustigman, and D. Abraham. 2002. Immunoaffinity-isolated antigens induce protective immunity against larval Strongyloides stercoralis in mice. Exp. Parasitol. 100:112-120. [DOI] [PubMed] [Google Scholar]
- 28.Hotez, P. J., B. Zhan, J. M. Bethony, A. Loukas, A. Williamson, G. N. Goud, J. M. Hawdon, A. Dobardzic, R. Dobardzic, K. Ghosh, M. E. Bottazzi, S. Mendez, B. Zook, Y. Wang, S. Liu, I. Essiet-Gibson, S. Chung-Debose, S. Xiao, D. Knox, M. Meagher, M. Inan, R. Correa-Oliveira, P. Vilk, H. R. Shepherd, W. Brandt, and P. K. Russell. 2003. Progress in the development of a recombinant vaccine for human hookworm disease: the Human Hookworm Vaccine Initiative. Int. J. Parasitol. 33:1245-1258. [DOI] [PubMed] [Google Scholar]
- 29.Ismael, A. B., D. Sekkai, C. Collin, D. Bout, and M. N. Mevelec. 2003. The MIC3 gene of Toxoplasma gondii is a novel potent vaccine candidate against toxoplasmosis. Infect. Immun. 71:6222-6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joseph, G. T., T. Huima, A. Klion, and S. Lustigman. 2000. A novel developmentally regulated galectin of Onchocerca volvulus. Mol. Biochem. Parasitol. 106:187-195. [DOI] [PubMed] [Google Scholar]
- 31.Kerepesi, L. A., T. J. Nolan, G. A. Schad, S. Lustigman, D. R. Herbert, P. B. Keiser, T. B. Nutman, A. J. Krolewiecki, and D. Abraham. 2004. Human immunoglobulin G mediates protective immunity and identifies protective antigens against larval Strongyloides stercoralis in mice. J. Infect. Dis. 189:1282-1290. [DOI] [PubMed] [Google Scholar]
- 32.Kuwabara, I., H. Sano, and F. T. Liu. 2003. Functions of galectins in cell adhesion and chemotaxis. Methods Enzymol. 363:532-552. [DOI] [PubMed] [Google Scholar]
- 33.Li, B. W., S. Zhang, K. C. Curtis, and G. J. Weil. 1999. Immune responses to Brugia malayi paramyosin in rodents after DNA vaccination. Vaccine 18:76-81. [DOI] [PubMed] [Google Scholar]
- 34.Ligas, J. A., L. A. Kerepesi, A. M. Galioto, S. Lustigman, T. J. Nolan, G. A. Schad, and D. Abraham. 2003. Specificity and mechanism of immunoglobulin M (IgM)- and IgG-dependent protective immunity to larval Strongyloides stercoralis in mice. Infect. Immun. 71:6835-6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lightowlers, M. W., and C. G. Gauci. 2001. Vaccines against cysticercosis and hydatidosis. Vet. Parasitol. 101:337-352. [DOI] [PubMed] [Google Scholar]
- 36.Lustigman, S., E. R. James, W. Tawe, and D. Abraham. 2002. Towards a recombinant antigen vaccine against Onchocerca volvulus. Trends Parasitol. 18:135-141. [DOI] [PubMed] [Google Scholar]
- 37.Mitreva, M., J. P. McCarter, J. Martin, M. Dante, T. Wylie, B. Chiapelli, D. Pape, S. W. Clifton, T. B. Nutman, and R. H. Waterston. 2004. Comparative genomics of gene expression in the parasitic and free-living nematodes Strongyloides stercoralis and Caenorhabditis elegans. Genome Res. 14:209-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newton, R. C., P. Limpuangthip, S. Greenberg, A. Gam, and F. A. Neva. 1992. Strongyloides stercoralis hyperinfection in a carrier of HTLV-I virus with evidence of selective immunosuppression. Am. J. Med. 92:202-208. [DOI] [PubMed] [Google Scholar]
- 39.O'Donnell, I. J., J. K. Dineen, B. M. Wagland, S. Letho, J. A. Werkmeister, and C. W. Ward. 1989. A novel host-protective antigen from Trichostrongylus colubriformis. Int. J. Parasitol. 19:327-335. [DOI] [PubMed] [Google Scholar]
- 40.Potter, A., D. Stephens, and B. De Keulenaer. 2003. Strongyloides hyper-infection: a case for awareness. Ann. Trop. Med. Parasitol. 97:855-860. [DOI] [PubMed] [Google Scholar]
- 41.Ramachandran, S., R. W. Thompson, A. A. Gam, and F. A. Neva. 1998. Recombinant cDNA clones for immunodiagnosis of strongyloidiasis. J. Infect. Dis. 177:196-203. [DOI] [PubMed] [Google Scholar]
- 42.Rotman, H. L., S. Schnyder-Candrian, P. Scott, T. J. Nolan, G. A. Schad, and D. Abraham. 1997. IL-12 eliminates the Th-2 dependent protective immune response of mice to larval Strongyloides stercoralis. Parasite Immunol. 19:29-39. [DOI] [PubMed] [Google Scholar]
- 43.Rotman, H. L., W. Yutanawiboonchai, R. A. Brigandi, O. Leon, T. J. Nolan, G. A. Schad, and D. Abraham. 1995. Strongyloides stercoralis: complete life cycle in SCID mice. Exp. Parasitol. 81:136-139. [DOI] [PubMed] [Google Scholar]
- 44.Scheerlinck, J. P., G. Casey, P. McWaters, J. Kelly, D. Woollard, M. W. Lightowlers, J. M. Tennent, and P. J. Chaplin. 2001. The immune response to a DNA vaccine can be modulated by co-delivery of cytokine genes using a DNA prime-protein boost strategy. Vaccine 19:4053-4060. [DOI] [PubMed] [Google Scholar]
- 45.Sen, P., C. Gil, B. Estrellas, and J. R. Middleton. 1995. Corticosteroid-induced asthma: a manifestation of limited hyperinfection syndrome due to Strongyloides stercoralis. South Med. J. 88:923-927. [PubMed] [Google Scholar]
- 46.Shalaby, K. A., L. Yin, A. Thakur, L. Christen, E. G. Niles, and P. T. LoVerde. 2003. Protection against Schistosoma mansoni utilizing DNA vaccination with genes encoding Cu/Zn cytosolic superoxide dismutase, signal peptide-containing superoxide dismutase and glutathione peroxidase enzymes. Vaccine 22:130-136. [DOI] [PubMed] [Google Scholar]
- 47.Shima, Y., Y. Tada, M. Furuki, Y. Hara, and H. Ohta. 1998. A missense mutation of the gene for Na+, K(+)-ATPase alpha-subunit causes abnormal feeding behavior in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 248:778-782. [DOI] [PubMed] [Google Scholar]
- 48.Shu, E. N., P. O. Okonkwo, W. O. Batey, and J. Onyeanusi. 2000. Ivermectin: concentration-dependent effects on adenosine triphosphatases in adult worms of Onchocerca volvulus. Acta Trop. 74:7-11. [DOI] [PubMed] [Google Scholar]
- 49.Siddiqui, A. A., T. Phillips, H. Charest, R. B. Podesta, M. L. Quinlin, J. R. Pinkston, J. D. Lloyd, M. Paz, R. M. Villalovos, and J. Pompa. 2003. Induction of protective immunity against Schistosoma mansoni via DNA priming and boosting with the large subunit of calpain (Sm-p80): adjuvant effects of granulocyte-macrophage colony-stimulating factor and interleukin-4. Infect. Immun. 71:3844-3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tarrab-Hazdai, R., M. Camacho, F. Mendelovic, and D. Schechtman. 1997. An association between activity of the Na/K-pump and resistance of Schistosoma mansoni towards complement-mediated killing. Parasite Immunol. 19:395-400. [DOI] [PubMed] [Google Scholar]
- 51.Taylor, M. J., R. E. Jenkins, and A. E. Bianco. 1996. Protective immunity induced by vaccination with Onchocerca volvulus tropomyosin in rodents. Parasite Immunol. 18:219-225. [DOI] [PubMed] [Google Scholar]
- 52.Vasta, G. R., M. Quesenberry, H. Ahmed, and N. O'Leary. 1999. C-type lectins and galectins mediate innate and adaptive immune functions: their roles in the complement activation pathway. Dev. Comp. Immunol. 23:401-420. [DOI] [PubMed] [Google Scholar]
- 53.Wang, Q. M., S. H. Sun, Z. L. Hu, D. Wu, and Z. C. Wang. 2003. Immune response and protection elicited by DNA immunisation against Taenia cysticercosis. Vaccine 21:1672-1680. [DOI] [PubMed] [Google Scholar]
- 54.Wedrychowicz, H., M. Lamparska, M. Kesik, G. Kotomski, J. Mieszczanek, L. Jedlina-Panasiuk, and A. Plucienniczak. 2003. The immune response of rats to vaccination with the cDNA or protein forms of the cysteine proteinase of Fasciola hepatica. Vet. Immunol. Immunopathol. 94:83-93. [DOI] [PubMed] [Google Scholar]
- 55.Yang, R. Y., and F. T. Liu. 2003. Galectins in cell growth and apoptosis. Cell Mol. Life Sci. 60:267-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuanqing, Y., X. Shuhua, P. J. Hotez, and W. Jiadong. 1999. Histochemical alterations of infective third-stage hookworm larvae (L3) in vaccinated mice. Southeast Asian J. Trop. Med. Public Health 30:356-364. [PubMed] [Google Scholar]
- 57.Zaha, O., T. Hirata, F. Kinjo, and A. Saito. 2000. Strongyloidiasis-progress in diagnosis and treatment. Intern. Med. 39:695-700. [DOI] [PubMed] [Google Scholar]
- 58.Zhang, Y., M. G. Taylor, M. V. Johansen, and Q. D. Bickle. 2001. Vaccination of mice with a cocktail DNA vaccine induces a Th1-type immune response and partial protection against Schistosoma japonicum infection. Vaccine 20:724-730. [DOI] [PubMed] [Google Scholar]