Abstract
The C-terminal third of intimin binds to its translocated receptor (Tir) to promote attaching and effacing lesion formation during infection with enteropathogenic Escherichia coli (EPEC). We observed that the adherence of EPEC strains to HEp-2 cells was reduced and that actin polymerization was blocked by antibody raised against the C-terminal third of intimin α.
Enteropathogenic Escherichia coli (EPEC) strains are pediatric pathogens associated with acute and protracted infantile diarrhea, particularly in developing countries. Outbreaks of disease among children and adults also occur sporadically worldwide (19). EPEC strains target the small intestinal mucosa where they adhere, efface the microvilli, and induce cytoskeletal changes and pedestal formation in the underlying enterocytes. These lesions, described in 1983 by Moon et al. as attaching and effacing (A/E) lesions, are the hallmark pathology of EPEC infection (18). The bacterial surface protein intimin is necessary for A/E lesion formation and for the full virulence of EPEC in human volunteer infections (3, 4, 11). Since the carboxyl-terminal 190 amino acids of intimin bind the translocated intimin receptor, Tir (1, 15), we reasoned that antibody directed against the binding domain of intimin might block lesion formation.
Intimins expressed by various E. coli strains that produce A/E lesions in humans and animals have been divided into at least five distinct types based on the antigenic variability of the 280 C-terminal amino acids that comprise the eukaryotic binding domain. Previous studies in our laboratory showed that antibody directed against the binding domain of intimin γ effectively reduces binding of enterohemorrhagic E. coli (EHEC) O157:H7, but not EPEC strain E2348/69, to HEp-2 cells (5). Other investigators used rabbit polyclonal antibodies against a histidine-tagged 280-amino-acid portion of the C terminus of intimin α to monitor intimin expression in the HEp-2 cell EPEC adherence model (12). Here we evaluated the capacity of antibody specific for the carboxyl-terminal binding domain of EPEC intimin α to interfere with the binding of EPEC strains and to block actin polymerization in the HEp-2 cell adherence assay.
The portion of the eae gene that encodes the C-terminal 286 amino acids of intimin α was amplified by PCR from pCVD438 (Table 1) with the forward primer MW5 (GTACGGATCCTGATCAAACCAAGGCCAGCATTAC; the incorporated BamHI site is underlined) and the reverse primer JKK11 (GTACGGTACCTTATTTTACACAAGTGGCATAAGC; the incorporated KpnI site is underlined). The resulting amplicon was digested with restriction enzymes BamHI and KpnI purchased from New England Biolabs (Beverly, Mass.). The 863-bp DNA fragment was ligated into the histidine tag-generating protein expression vector, pQE31 (QIAGEN, Inc., Chatsworth, Calif.), at the corresponding restriction sites with T4 DNA ligase purchased from U.S. Biochemical Corp. Laboratories (Cleveland, Ohio). The ligation product was transformed into XL1-Blue E. coli (Stratagene, La Jolla, Calif.). The resulting plasmid, designated pJKint3/3, was purified with the QIAGEN mini prep kit (QIAGEN, Inc.). The putative intimin gene fragment was sequenced at the Uniformed Services University Biomedical Instrumentation Center from samples generated with the ABI PRISM Big Dye Terminator sequencing kit (Perkin-Elmer, Foster City, Calif.) to verify that it encoded the truncated 286-amino-acid C-terminal portion of intimin (subsequently named His-Int286). Plasmid pJKint3/3 was transformed into E. coli strain L172, a slyD::kan mutant host strain that was derived as described by Gansheroff et al. (5). This SlyD mutant strain was used to produce His-Int286 for immunization because the E. coli SlyD protein contains a metal binding motif that causes it to be coprecipitated with histidine-tagged proteins on nickel affinity resin columns (20, 23).
TABLE 1.
Bacterial strains and plasmids used in these experiments
| Bacterial strain or plasmid | Relevant genotype and/or description | Reference or source |
|---|---|---|
| E2348/69 | Wild-type EPEC (O127:H7), produces intimin α | 14, 22 |
| JPN15 | E2348/69 spontaneously cured of pEAF | 9 |
| CVD206 | E2348/69, TnphoA insertion in eae | 3 |
| 86-24 | Wild-type EHEC (O157:H7) produces intimin γ | 8 |
| 86-24Δ10 | 86-24 with an internal deletion of 1,125 bp in eae | 16 |
| TOP 10 | Chemically competent E. coli host strain | Invitrogen |
| L172 | slyD E. coli host background for nickel affinity protein purification | 20, 23 |
| pCVD438 | eae gene from E2348/69 in pACYC184 background, Cmr | 10 |
| pEGFP | pUC19 vector with IPTG-inducible wild-type green fluorescent protein gene, Amr | Clontech |
| pJKint3/3 | pQE31 (QIAGEN) expression vector encoding histidine-tagged C-terminal 286 amino acids of intimin α, Amr | This study |
The truncated intimin protein was obtained from late log-phase cultures of E. coli strain L172 (pJKint3/3) in which protein expression was induced with 0.01 mM of isopropyl-B-d-1-thiogalactopyranoside (IPTG) according to the vector manufacturer's protocol. His-Int286 was purified over Ni-nitrilotriacetic acid agarose resin (QIAGEN), as previously described (17), and dialyzed in phosphate-buffered saline. We screened sera from several goats raised at Duncroft, Inc. (Lovettsville, Va.) by Western blotting against full-length intimin α and selected an animal with no detectable preexisting immune response to intimin α for immunization with purified His-Int286. The goat was immunized with His-Int286 emulsified in Freund's adjuvant at days 1, 14, 21, and 49, and serum was collected on day 56.
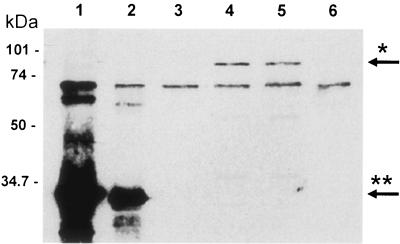
We tested the immunized goat serum by enhanced chemiluminescence Western blotting (Amersham, Little Chalfont Buckinghamshire, England) with secondary horseradish peroxidase-labeled rabbit anti-goat serum (Bio-Rad, Hercules, Calif.) to verify that antibodies specific for His-Int286 were present (Fig. 1.). The serum recognized bands of the predicted sizes for both the purified C-terminal fragment of EPEC intimin (32 kDa) and the full-length EPEC intimin (94 kDa) from lysates of overnight cultures of wild-type EPEC (strain E2348/69). In addition, the goat anti-EPEC intimin286 recognized the purified C-terminal third (281 amino acids) of intimin γ and the full-length intimin γ (of E. coli O157:H7 strain 86-24) from overnight culture lysates. The intensity of the binding of anti-His-Int286 to bacterial culture lysates of EPEC or EHEC and the intensity of the binding of anti-His-Int286 to truncated C-terminal intimin α and intimin γ appeared the same in a Western blot. We presume that the cross-reactivity of this anti-His-Int286 could be attributed to the inclusion of conserved amino acids in the N-terminal region of the truncated intimin protein that we used for immunization. The polyclonal antibody did not recognize proteins from cultures of intimin-negative derivatives of the EPEC or EHEC strains, as expected. The goat antisera also reacted with an unknown protein, at 70 kDa, that was present in all the samples. The polyclonal anti-intimin antibody, named anti-His-Int286, was used to attempt to block intimin-mediated adherence in subsequent experiments.
FIG. 1.
Western blots with primary goat anti-His-Int286 and secondary rabbit anti-goat horseradish peroxidase-conjugated antibody. Lane 1, purified EPEC His-Int286; lane 2, purified, histidine-tagged C-terminal third 281 amino acids of intimin γ; lane 3, Δeae EHEC strain 86-24Δ10; lane 4, wild-type strain 86-24; lane 5, wild-type E2348/69 strain; lane 6, Δeae CVD206 strain. Arrows indicate the reaction with full-length intimin α or intimin γ (*) from lysates of wild-type EPEC and EHEC strains, respectively, and the reaction with the purified C-terminal fragment of intimin α or intimin γ (**). Intimin was not detected in lysates of Δeae strains CVD206 or 86-24Δ10, as expected (lanes 3 and 6).
Although intimin is essential for A/E lesion formation, bundle-forming pili (BFP) mediate localized adherence of EPEC in the HEp-2 adherence assay (6). The BFP operon, along with several other genes, including the positive regulator of BFP synthesis, per (plasmid encoded regulator) (7), is located on the pEAF plasmid of EPEC. We reasoned that intimin-mediated binding and the potential reduction of such binding by anti-intimin antibody would be more readily observed in a BFP-negative background. Therefore, the effects of the anti-His-Int286 on binding of EPEC wild-type strain E2348/69 and JPN15, the plasmid-cured derivative (Table 1), were compared in both the conventional HEp-2 cell slide culture adherence assay and a newer flow cytometric method. The conventional visual adherence assay was done as previously described (5) with slight modifications. The EPEC strains were grown statically overnight, and then 2.5 μl of culture was preincubated for 30 min in 300 μl of adherence medium that contained a 1:50 dilution of preimmune goat serum or immune serum. The adherence medium inoculum was then added to 90%-confluent HEp-2 cell monolayers in eight-well chamber slides and incubated for 3 h. The monolayers were washed three times with Dulbecco's phosphate buffer solution (Cambrex, Walkersville, Md.), and fresh adherence medium with antiserum or normal goat serum was added for a second 3-h incubation. Finally, the wells were washed five times with Dulbecco's phosphate buffer solution, and the slides were stained with Hema 3 staining solution (Biochemical Sciences, Inc., Swedesboro, N.J.).
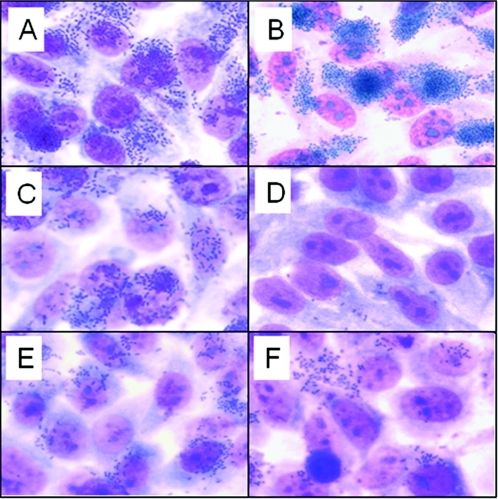
EPEC adherences following the preincubation with normal goat serum or anti-His-Int286 were compared microscopically (Fig. 2). No reduction in the binding of wild-type EPEC strains was visibly evident following the preincubation with normal serum or anti-His-Int286 (Fig. 2A and B). However, the plasmid-cured strain JPN15 showed markedly reduced adherence to HEp-2 cells following incubation with anti-His-Int286 (Fig. 2D) compared to JPN15 incubated with normal goat serum (Fig. 2C). No reduction in binding was observed with the intimin-negative mutant strain CVD206 treated with or without anti-His-Int286 (Fig. 2E and F), as expected. From these findings, we reasoned that other adherence factors, such as bundle-forming pili, were the predominant determinants of binding to HEp-2 cells for wild-type EPEC strains, and the role of intimin-mediated adherence was difficult to discern visually. In contrast, in the plasmid-cured mutant that lacks BFP, intimin played a greater role in adherence, hence, a reduction in binding following the preincubation of JPN15 with anti-His-Int286, compared to that of JPN15 preincubated with normal serum, was readily apparent.
FIG. 2.
Inhibition of binding of EPEC strains to HEp-2 cells in the presence of anti-His-Int286. Shown are wild-type strain E2348/69 treated with preimmune serum (A) and anti-His-Int286 (B), plasmid-cured strain JPN15 treated with preimmune serum (C) and anti-His-Int286 (D), and intimin mutant strain CVD206 treated with preimmune serum (E) and anti-His-Int286 (F). The binding of the plasmid-cured strain was greatly reduced following the preincubation of bacteria with anti-intimin antibody as observed by light microscopy.
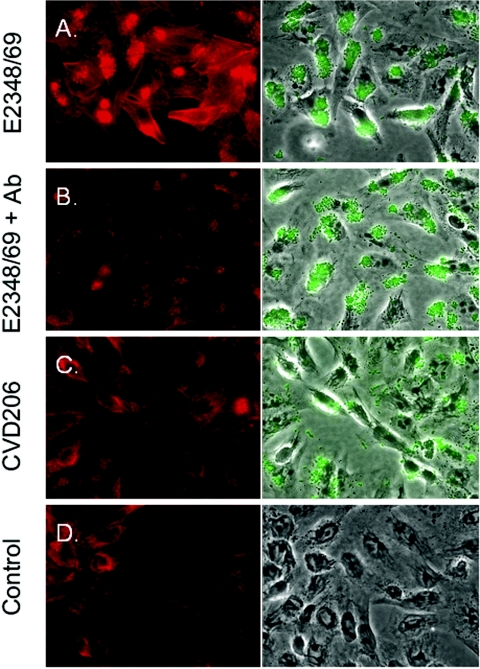
The observation that actin polymerization associated with adherent EPEC strains in cell culture is indicative of the cytoskeletal rearrangement that results in A/E lesion formation at the submicroscopic level in vivo. To test whether anti-Int286 would prevent actin polymerization at the sites where the bacteria adhered to HEp-2 cells, fluorescent actin staining was done with Alexa Fluor 595 phalloidin (Molecular Probes, Eugene, Oreg.) as described by Knutton et al. in 1989 (Fig. 3) (13). Although no reduction of binding of wild-type E2348/69 was observed microscopically, when the bacteria were pretreated as described above with anti-His-Int286, actin polymerization was inhibited in the HEp-2 cells to which the antibody-treated EPEC strains were bound.
FIG. 3.
Inhibition of actin polymerization in HEp-2 cells following incubation of EPEC strains with anti-Int286 polyclonal antibody. The cells shown on the left of each panel were stained with Alexa Fluor 595 phalloidin. On the right, phase contrast images of the same fields are shown. The corresponding green fluorescence images were merged with the phage contrast images to localize adherent GFP-expressing EPEC strains. Contrast and coloration have been improved with the Image-J program (National Institutes of Health) to highlight the location of the bacteria. (A) Wild-type EPEC strains adhered and promoted actin polymerization in HEp-2 cells. (B) Pretreatment of EPEC strains with anti-His-Int286 prevented actin polymerization at the sites where the bacteria adhered to HEp-2 cells. (C) Intimin-negative EPEC strain CVD206 adhered but did not induce actin polymerization. (D) The negative control showed no actin staining on HEp-2 cells in the absence of bacteria.
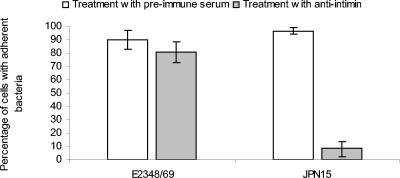
Flow cytometry was used, as previously described with modifications (2), to compare the numbers of HEp-2 cells to which green fluorescent protein (GFP)-expressing EPEC strains adhered following preincubation with normal serum or anti-His-Int286. The flow cytometry was performed at the Uniformed Services University Biomedical Instrumentation Center. We seeded 2.2 × 105 HEp-2 cells per well in 12-well plates (Nunc, Inc., Naperville, Ill.) and incubated them for 24 h with Eagle's minimum essential medium (Cambrex) supplemented with 10% fecal calf serum (BioSource International, Camarillo, Calif.), 2 mM l-glutamine (Cambrex), 0.2 U of penicillin-0.2 μg of streptomycin (Invitrogen Corporation, Grand Island, N.Y.), and 80 μg of gentamicin (Quality Biological Inc., Gaithersburg, Md.)/ml. The pEGFP-transformed strains (Clontech, Palo Alto, Calif.) were grown statically overnight in Luria-Bertani broth with ampicillin and then added to the adherence medium with normal or immune serum as described above. In addition, we added ampicillin and IPTG to the adherence medium to maintain pEGFP and induce the expression of GFP. After the final washing of the wells, 200 μl of a 0.25% solution of trypsin (Cambrex) was added to each well. The plates were gently rotated for 30 s, then the trypsin solution was removed by aspiration, and the plates were incubated at 37°C for 8 min. The cells were resuspended with 300 μl of phosphate-buffered saline and added to a 5.0-ml polystyrene round-bottomed Falcon tube (Becton Dickinson Labware, Franklin Lakes, N.J.) that contained 300 μl of a 6% solution of Formalde-fresh (Fisher Scientific). A total of 10,000 cells per sample were acquired with an EPICS XL-MCL flow cytometer (Beckman Coulter, Miami, Fla.). Both scatter plots and histogram plots were analyzed, and the percentage of cells with adherent bacteria versus the percentage of cells without adherent bacteria was calculated for each sample (Fig. 4). The percent reductions of adherence between the normal serum- or antibody-treated EPEC strains were compared statistically by paired t tests after the transformation of the proportions with the arcsine square root transformation (21).
FIG. 4.
Flow cytometric measurement of adherence of GFP-transformed EPEC strains to HEp-2 cells after treatment with preimmune serum or anti-His-Int286. The bars indicate the ranges from three experiments. The adherence of wild-type EPEC strain E2348/69 was consistently reduced by 10%, but the difference between the normal serum-treated strain and the immune serum-treated strain was not statistically significant as assessed by paired t tests following the conversion of proportions by the arcsine square root transformation. The binding of the plasmid-cured strain JPN15 was dramatically reduced (P < 0.001) following anti-His-Int286 treatment compared to the same strain treated with normal serum.
When pEGFP-transformed EPEC strains were treated with preimmune goat serum, we detected bound fluorescent bacteria on 90% of HEp-2 cells infected with wild-type EPEC strains and 90.6% of cells infected with the plasmid-cured strain JPN15. When the strains were treated with a 1:50 dilution of anti-His-Int286 prior to mixing with HEp-2 cells, we detected a 10.6% reduction in the number of HEp-2 cells to which fluorescent wild-type EPEC bacteria were bound. A 90.9% reduction in the number of HEp-2 cells with adherent plasmid-cured JPN15 was seen. A statistical comparison of the treated and untreated wild-type strains showed a consistent but not statistically significant reduction of adherence following anti-intimin treatment (P = 0.133). The reduction of the adherence of the plasmid-cured strain with anti-His-Int286 was highly significant (P < 0.001) and corresponded to our visual interpretation of the slide culture adherence assay for strain JPN15.
We expected that the level of adherence of the plasmid-cured JPN15 would be substantially less than that exhibited by wild-type strain E2348/69 due to the lack of BFP. To the contrary, the plasmid-cured strain adhered well in our studies. In the absence of the pEAF-borne per regulatory region, which upregulates BFP expression and intimin expression via ler (locus of enterocyte effacement-encoded regulator), the temporal expression of intimin may have been altered. Others have shown that intimin expression in E2348/69 is downregulated after 3 h in cell culture, whereas intimin production in the plasmid-cured strain JPN15 does not diminish unless the regulator per is supplied in trans (12). We hypothesized that the duration of our assays (6 h) permitted sustained intimin expression and increased intimin-mediated binding in the plasmid-cured strain. We did not observe a difference in total intimin expression by Western blot analysis between the wild-type and plasmid-cured strains in the adherence assay (data not shown).
The anti-C-terminal third intimin antibody effectively reduced intimin-mediated binding of the intimin-producing strains tested and, more importantly, blocked actin polymerization in HEp-2 cells. Actin polymerization in response to the binding of intimin-expressing organisms is the surrogate model for A/E lesion formation in vivo. Therefore, we conclude that because the anti-His-Int286 antibody interfered with this classic intimin-host cell interaction, the antibody might also block A/E lesions in vivo. Further studies are under way to test the potential for an antibody-mediated reduction of A/E lesion formation and pathogenesis in an animal model.
Acknowledgments
We extend our thanks to James Kaper and Ann Jerse for supplying the EPEC strains for our studies. We acknowledge the work of Stephen Darnell in reference database management.
These studies were funded by a Public Health Service grant from the National Institutes of Health (2 RO1 AI20148-22).
The opinions or assertions contained herein are those of the authors and are not to be construed as the views of the Department of Defense.
Editor: J. T. Barbieri
REFERENCES
- 1.Batchelor, M., S. Prasannan, S. Daniell, S. Reece, I. Connerton, G. Bloomberg, G. Dougan, G. Frankel, and S. Matthews. 2000. Structural basis for recognition of the translocated intimin receptor (Tir) by intimin from enteropathogenic Escherichia coli. EMBO J. 19:2452-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boullier, S., C. Tasca, and A. Milon. 2003. New flow cytometric method to quantify the inhibition of enteropathogenic Escherichia coli adhesion by anti-adhesin antibodies. Cytometry 53A:79-87. [DOI] [PubMed] [Google Scholar]
- 3.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donnenberg, M. S., C. O. Tacket, S. P. James, G. Losonsky, J. P. Nataro, S. S. Wasserman, J. B. Kaper, and M. M. Levine. 1993. Role of the eaeA gene in experimental enteropathogenic Escherichia coli infection. J. Clin. Investig. 92:1412-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gansheroff, L. J., M. R. Wachtel, and A. D. O'Brien. 1999. Decreased adherence of enterohemorrhagic Escherichia coli to HEp-2 cells in the presence of antibodies that recognize the C-terminal region of intimin. Infect. Immun. 67:6409-6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girón, J. A., A. S. Ho, and G. K. Schoolnik. 1993. Characterization of fimbriae produced by enteropathogenic Escherichia coli. J. Bacteriol. 175:7391-7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gómez-Duarte, O. G., and J. B. Kaper. 1995. A plasmid-encoded regulatory region activates chromosomal eaeA expression in enteropathogenic Escherichia coli. Infect. Immun. 63:1767-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffin, P. M., S. M. Ostroff, R. V. Tauxe, K. D. Greene, J. G. Wells, J. H. Lewis, and P. A. Blake. 1988. Illnesses associated with Escherichia coli O157:H7 infections. A broad clinical spectrum. Ann. Intern. Med. 109:705-712. [DOI] [PubMed] [Google Scholar]
- 9.Jerse, A. E., K. G. Gicquelais, and J. B. Kaper. 1991. Plasmid and chromosomal elements involved in the pathogenesis of attaching and effacing Escherichia coli. Infect. Immun. 59:3869-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jerse, A. E., and J. B. Kaper. 1991. The eae gene of enteropathogenic Escherichia coli encodes a 94-kilodalton membrane protein, the expression of which is influenced by the EAF plasmid. Infect. Immun. 59:4302-4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jerse, A. E., J. Yu, B. D. Tall, and J. B. Kaper. 1990. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc. Natl. Acad. Sci. USA 87:7839-7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knutton, S., J. Adu-Bobie, C. Bain, A. D. Phillips, G. Dougan, and G. Frankel. 1997. Down regulation of intimin expression during attaching and effacing enteropathogenic Escherichia coli adhesion. Infect. Immun. 65:1644-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knutton, S., T. Baldwin, P. H. Williams, and A. S. McNeish. 1989. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 57:1290-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levine, M. M., J. P. Nataro, H. Karch, M. M. Baldini, J. B. Kaper, R. E. Black, M. L. Clements, and A. D. O'Brien. 1985. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J. Infect. Dis. 152:550-559. [DOI] [PubMed] [Google Scholar]
- 15.Liu, H., P. Radhakrishnan, L. Magoun, M. Prabu, K. G. Campellone, P. Savage, F. He, C. A. Schiffer, and J. M. Leong. 2002. Point mutants of EHEC intimin that diminish Tir recognition and actin pedestal formation highlight a putative Tir binding pocket. Mol. Microbiol. 45:1557-1573. [DOI] [PubMed] [Google Scholar]
- 16.McKee, M. L., A. R. Melton-Celsa, R. A. Moxley, D. H. Francis, and A. D. O'Brien. 1995. Enterohemorrhagic Escherichia coli O157:H7 requires intimin to colonize the gnotobiotic pig intestine and to adhere to HEp-2 cells. Infect. Immun. 63:3739-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKee, M. L., and A. D. O'Brien. 1996. Truncated enterohemorrhagic Escherichia coli (EHEC) O157:H7 intimin (EaeA) fusion proteins promote adherence of EHEC strains to HEp-2 cells. Infect. Immun. 64:2225-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moon, H. W., S. C. Whipp, R. A. Argenzio, M. M. Levine, and R. A. Giannella. 1983. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect. Immun. 41:1340-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roof, W. D., S. M. Horne, K. D. Young, and R. Young. 1994. slyD, a host gene required for φX174 lysis, is related to the FK506-binding protein family of peptidyl-prolyl cis-trans-isomerases. J. Biol. Chem. 269:2902-2910. [PubMed] [Google Scholar]
- 21.Snedecor, G. W., and W. G. Cochran. 1989. Statistical methods. Iowa State University Press, Ames.
- 22.Taylor, J. 1970. Infectious infantile enteritis, yesterday and today. Proc. R. Soc. Med. 63:1297-1301. [PMC free article] [PubMed] [Google Scholar]
- 23.Wülfing, C., J. Lombardero, and A. Plückthun. 1994. An Escherichia coli protein consisting of a domain homologous to FK506-binding proteins (FKBP) and a new metal binding motif. J. Biol. Chem. 269:2895-2901. [PubMed] [Google Scholar]