Abstract
Campylobacter jejuni is a major cause of human inflammatory enteritis. During the course of human disease numerous proinflammatory cytokines are produced. Little is known, however, about the cytokine responses produced during the interaction of this bacterium with the avian host. Campylobacter has been considered a commensal of the avian host. Any differences in innate responses to this pathogen between the human and avian hosts should lead to a greater understanding of the disease process in humans. We have demonstrated expression of proinflammatory cytokines and chemokines in response to Campylobacter infection in avian primary chick kidney cells and the avian macrophage cell line HD11. The data indicate that Campylobacter can stimulate the avian host in a proinflammatory manner. The data strongly suggest that the lack of pathology in vivo is not due to an inability of Campylobacter to stimulate a proinflammatory response from avian cells.
Campylobacter jejuni causes severe gastroenteritis in humans. The pathology includes severe inflammation of the intestinal mucosa with an influx of professional phagocytes (25, 41-43). Histological analysis of biopsy samples from patients with C. jejuni colitis has shown that the bacteria invade the colonic mucosa. In contrast Campylobacter infection of the chicken leads to high-level colonization of the intestinal tract in an apparently commensal association, with little or no pathology (6). Campylobacter can invade human epithelial cell monolayers (11, 12), causing disruption to the epithelium and gaining access to its basal side (41, 45). Interleukin-1β (IL-1β), IL-8, and nitric oxide (NO) are produced during human Campylobacter infections (10), and in vitro experiments with human-derived epithelial cell lines have shown that C. jejuni can induce the secretion of a range of cytokines and chemokines (2, 3, 16, 17, 19).
During human infection Campylobacter can invade and traverse the epithelial barrier (41, 42). Given the range of chemokines produced in vitro and the observed attraction of a range of leukocytes in vivo, it is probable that Campylobacter interacts with leukocytes. Human monocytes produce a range of cytokines and chemokines, including IL-1β, IL-6, tumor necrosis factor alpha, and IL-8 when infected with Campylobacter, and their stimulation could contribute to disease pathology (20, 39).
Two C. jejuni-derived factors that can induce IL-8 production from epithelial cells have been defined: the cytolethal-distending toxin (CDT) and the adhesion factor Jlp (17, 19). CDT has an effect in disease models, but it is not required for induction of cytokines by live C. jejuni during infection of either epithelial or monocytic cells (14, 17, 20).
While the production of chemokines by human cells in response to Campylobacter infection has only just been described (3), their role in infection may be crucial to the development of inflammatory disease. The chicken has a reduced chemokine repertoire compared to mammals (18) and possesses two homologues of IL-8, 9E3/CEF4 (also known as IL-8/CAF and referred to as IL-8 in this paper) and K60, both of which are found on chromosome 4 (38).
Little is known about the stimulation of avian cells by Campylobacter, but studies have been carried out with Salmonella infections. Kaiser et al. (23) used chick kidney cells (CKCs) as a model for bacterial interaction with avian epithelial cells. HD11 cells (7) can be used as a model for interactions with avian macrophages. Colonization of the chicken by Campylobacter generates an antibody response as indicated by the production of both secretory and serum antibodies (8, 32). Therefore, it could be expected that this is driven by an innate reaction. In this paper, we aimed to investigate whether Campylobacter could induce proinflammatory cytokines in HD11 cells and CKCs following in vitro invasion.
MATERIALS AND METHODS
Bacteria.
C. jejuni 11168H and NCTC11168 cdtB have been described previously (20, 24, 34, 35). NCTC11168 cdtB has an insertion mutation in the cdtB gene and lacks CDT-dependent cytotoxicity (35). C. jejuni G1 was isolated from a patient who went on to develop Guillain-Barré syndrome (30). All strains were cultured for 2 days in Mueller-Hinton broth, from which they were diluted 1/50 into fresh prewarmed Mueller-Hinton medium and grown for 12 h prior to experimentation. The optical density was measured at 600 nm, and then the bacteria were centrifuged and resuspended in prewarmed phosphate-buffered saline (PBS) to the desired cell density for inoculation of eukaryotic cell cultures at a multiplicity of infection (MOI) of 100 bacteria per cell. All cultures were grown at 37°C in a modified gas atmosphere of 10% CO2, 5% O2, and 85% N2. Heat-killed bacteria were prepared in the same way, but after suspension in PBS they were heated to 70°C for 20 min. All heat-killed cultures were assessed for nonviability by plating on sheep blood agar plates.
Production of recombinant chicken IFN-γ by COS-7 cells.
Recombinant chicken gamma interferon (IFN-γ) was produced in COS-7 cells as described previously (28). Briefly, 5 × 105 COS-7 cells/ml were transfected with 37.5 μg of DNA (pCI-neo-chIFN-γ) per ml by using a DEAE-dextran-based method. COS-7 supernatant containing recombinant chicken IFN-γ was harvested at 72 h after transfection, and bioactivity was confirmed by titration in a macrophage activation assay, using HD11 cells (28). Recombinant chicken IFN-γ (ex-COS) was used at a dilution (1/200) that induced a half-maximal NO response in HD11 cells.
Cells and culture conditions.
HD11 cells (7) were cultured in RPMI 1640 medium containing 20 mM l-glutamine (Life Technologies), 2.5% newborn calf serum, 2.5% chicken serum, and 10% tryptose phosphate broth. Cells were seeded at 4 × 106 cells/ml (1 ml per well) in 24-well tissue culture plates, and cells were incubated at 42°C for 2 days prior to use. Cells were washed three times in PBS at 37°C, and fresh antibiotic-free medium was added. All infections were carried out by inoculating bacteria as suspensions in PBS at an MOI of 100:1, unless stated otherwise. Controls consisted of mock infections using PBS alone or positive controls of Escherichia coli O55:B5 lipopolysaccharide (LPS) (Sigma, Poole, United Kingdom) at a final concentration of 5 μg/ml unless stated otherwise. Primary CKCs were prepared from the kidneys of 1- to 2-week-old Rhode Island Red chicks as previously described (5). CKCs were seeded in 24-well plates at 1.2 × 106 cells/ml (1 ml per well) in Dulbecco's modified Eagle's medium supplemented with 12.5% newborn calf serum, 10% tryptose phosphate broth, and 1% HEPES and incubated at 37°C with 5% CO2 prior to use. Human epithelial INT407 cells were cultured in RPMI 1640 medium containing 20% newborn calf serum and 10% tryptose phosphate broth. Cells were seeded at 1.2 × 106 cells/ml and cultured at 37°C for 2 days prior to use.
Intracellular bacterial counts.
The number of intracellular bacteria per eukaryotic cell culture was assessed by using a gentamicin protection assay. At 1 h postinfection, the culture supernatant was supplemented with medium containing gentamicin to give a final concentration of 100 μg/ml and incubated at 37°C and 5% CO2. The concentration of 100 μg/ml was previously found to be sufficient to kill 100% of noninternalized Campylobacter organisms by 1 h under equivalent conditions (data not shown). At set time points the cell medium was removed, and the cells were washed three times in warm PBS. The cell monolayer was lysed in cold 0.5% (vol/vol) Triton X-100. The viable bacterial counts were determined by plating serial dilutions of the lysate on sheep blood agar plates and are expressed as CFU per milliliter, where the volume of a well was 1 ml.
Quantitative RT-PCR.
RNA expression was determined by quantitative reverse transcription-PCR (RT-PCR) with the ABI PRISM 7700 sequence detection system (Perkin-Elmer Applied Biosystems, Boston, Mass.) as described previously (15, 23, 27, 44). Primers and probes for 28S rRNA, IL-1β, IL-6, IL-8, K60, and IL-10 have been described previously (Table 1) (23, 36, 44). The sequences for the inducible nitric oxide synthase (iNOS) primer and probe set were kindly provided by Bas Baaten (personal communication). RT-PCR was performed with the RT qRT-PCR Mastermix (Eurogentec, Seraing, Belgium). Amplification and detection of specific products were performed with the ABI PRISM 7700 with the following cycle profile: one cycle of 50°C for 2 min, 96°C for 5 min, 60°C for 30 min, and 95°C for 5 min and 40 cycles of 94°C for 20 s and 59°C for 1 min. Each RT-PCR experiment contained three no-template controls, test samples, and a standard log10 dilution series. Each experiment was performed in triplicate with replicates performed on different days. Regression analysis of the mean values from six replicate RT-PCRs for the log10-diluted RNA was used to generate standard curves.
TABLE 1.
Primers and probes
| Target | Probe or primera | Sequenceb | GenBank accession no. |
|---|---|---|---|
| 28S | Probe | 5′-(FAM)-AGGACCGCTACGGACCTCCACCA-(TAMRA)-3′ | X59733 |
| F | 5′-GGCGAAGCCAGAGGAAACT-3′ | ||
| R | 5′-GACGACCGATTTGCACGTC-3′ | ||
| IL-1β | Probe | 5′-(FAM)-CCACACTGCAGCTGGAGGAAGCC-(TAMRA)-3′ | AJ245728 |
| F | 5′-GCTCTACATGTCGTGTGTGATGAG-3′ | ||
| R | 5′-TGTCGATGTCCCGCATGA-3′ | ||
| IL-6 | Probe | 5′-(FAM)-AGGAGAAATGCCTGACGAAGCTCTCCA-(TAMRA)-3′ | AJ250838 |
| F | 5′-GCTCGCCGGCTTCGA-3′ | ||
| R | 5′-GGTAGGCTGAAAGGCGAACAG-3′ | ||
| IL-8 | Probe | 5′-(FAM)-GCCCTCCTCCTGGTTTCAG-(TAMRA)-3′ | AJ009800 |
| F | 5′-TGGCACCGCAGCTCATT-3′ | ||
| R | 5′-TCTTTACCAGCGTCCTACCTTGCGACA-3′ | ||
| K60 | Probe | 5′-(FAM)-TGGCTCTTCTCCTGATCTCAATG-(TAMRA)-3′ | AF277660 |
| F | 5′-GCACTGGCATCGGAGTTCA-3′ | ||
| R | 5′-TCGCTGAACGTGCTTGAGCCATACCTT-3′ | ||
| IL-10 | Probe | 5′-(FAM)-CGACGATGCGGCGCTGTCA-(TAMRA)-3′ | AJ621614 |
| F | 5′-CATGCTGCTGGGCCTGAA-3′ | ||
| R | 5′-CGTCTCCTTGATCTGCTTGATG-3′ | ||
| iNOS | Probe | 5′-(FAM)-TCCACAGACATACAGATGCCCTTCCTCTTT-(TAMRA)-3′ | U46504 |
| F | 5′-TTGGAAACCAAAGTGTGTAATATCTTG-3′ | ||
| R | 5′-CCCTGGCCATGCGTACAT-3′ |
F, forward primer; R, reverse primer.
FAM, 6-carboxyfluorescein; TAMRA, 6-carboxytetramethylrhodamine.
Data were calculated as fold changes compared to the mock-infected samples. All of the data shown are from three independent experiments and represent the averages and standard deviations (SD) of the fold changes between experiments. Statistical analysis was carried by analysis of variance between experiments.
Measurement of NO by the Griess assay.
Nitric oxide production was measured by assaying cell culture supernatant fluid for the presence of nitrite, using the standard Griess assay (9, 40). The absorbance was read at 550 nm, using an Anthos Lab Systems microtiter plate reader (Labtech, Ringmer, United Kingdom). Serial dilutions of sodium nitrite (Sigma) were used to determine a standard curve.
RESULTS
Intracellular survival of C. jejuni in cells.
C. jejuni strains 11168H, NCTC11168 cdtB, and G1 were taken up by (invaded) both HD11 and CKC cells to the same extent (Table 2). Both human INT407 and CKC cells were invaded to the same extent by C. jejuni 11168H, and the bacteria did not persist (Fig. 1), being undetectable after a period of 24 h (data not shown). To determine whether priming of macrophages would affect invasion and survival, C. jejuni 11168H infection was also assessed with HD11 cells treated with or without IFN-γ (Fig. 2) (P < 0.002). We monitored intracellular survival of C. jejuni 11168H in HD11 cells, and killing was more rapid in the presence of IFN-γ (Fig. 2) (P < 0.002). Intracellular Campylobacter was undetectable after 24 h postinfection of HD11 cells with and without IFN-γ (data not shown).
TABLE 2.
Invasion counts for bacteria
| C. jejuni strain | Intracellular bacterial counta (log10 CFU/ml) in:
|
|
|---|---|---|
| CKCs | HD11 cells | |
| 11168H | 4.46 ± 0.02 | 5.66 ± 0.08 |
| 11168 cdtB | 4.51 ± 0.01 | 5.27 ± 0.07 |
| G1 | 4.63 ± 0.06 | 5.89 ± 0.06 |
Intracellular bacterial counts at 2 h postinfection. Data represent the averages from three independent experiments ± SD.
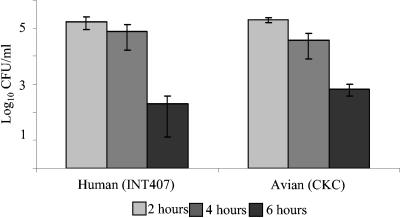
FIG. 1.
Invasion of INT407 cells and CKCs by Campylobacter. Cells seeded at equivalent levels (1.2 × 106 cells/ml) were infected at an MOI of 100:1 with C. jejuni 11168H. Internalized bacteria were assessed by gentamicin protection at 2, 4, and 6 h postinfection. Data are representative of those from three independent experiments. Values shown are averages and SD from three independent samples.
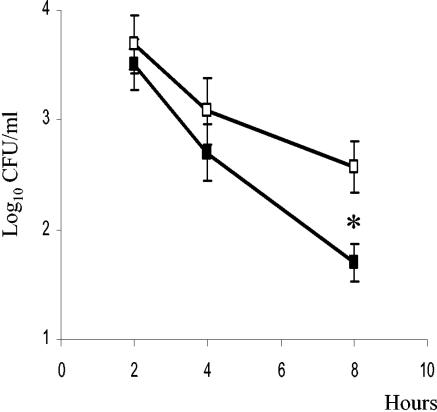
FIG. 2.
Survival of C. jejuni 11168H within HD11 cells. At 1 h prior to infection, cells were either treated with IFN-γ (closed symbols) or not treated (open symbols). Cells were infected at an MOI of 100:1. Internalized bacteria were assessed by gentamicin protection assay, and intracellular bacteria were counted at 2, 4, and 8 h postinfection. Data shown are the averages and SD from three replicate samples. *, significant difference (P < 0.002) between IFN-γ-treated and nontreated cells.
Induction of iNOS and production of NO by CKCs and HD11 cells.
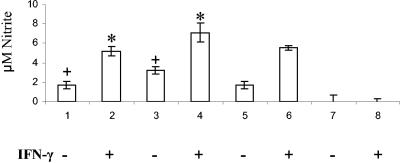
The fold change in iNOS transcripts from CKCs and HD11 cells was measured at 4 h after Campylobacter infection (MOI of 100:1). iNOS was induced by both CKCs and HD11 cells (Fig. 3). The production of NO from the infected HD11 cells and CKCs was determined by using the Griess assay. Nitric oxide was produced by both infected CKCs (Fig. 4) and HD11 cells (Fig. 5). The production of NO from infected HD11 cells pretreated with or without chicken IFN-γ was also assessed. As expected, priming of the HD11 cells with IFN-γ increased their response to Campylobacter infection. There was a significant increase in NO production during infection in the presence of IFN-γ compared to infection without IFN-γ (Fig. 5). At 24 h, maximal NO production had been reached in both IFN-γ-treated and untreated cells (data not shown). To determine whether stimulation of the cells required active invasion by the bacteria, the production of NO was also assessed after infection with heat-killed bacteria at an MOI equivalent to 100:1. No significant difference in NO production was observed between cells infected with either heat-killed or live bacteria (Fig. 4 and 5) (P > 0.05).
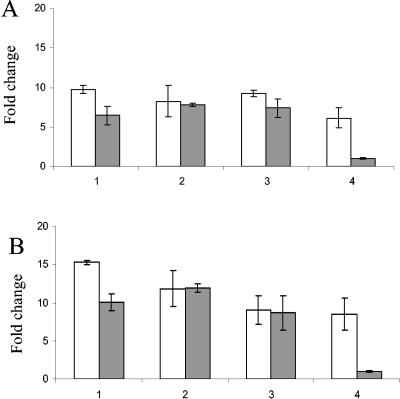
FIG. 3.
Quantification of iNOS transcripts from CKCs (A) and HD11 cells (B) at 4 h postinfection. Open bars, live bacteria; closed bars, heat-killed bacteria. Bars: 1, C. jejuni 11168H; 2, C. jejuni G1; 3, C. jejuni 11168 cdtB; 4, LPS control E. coli O55:B55 (5 μg/ml) (open bar) and mock infection (closed bar). RNA was isolated at 4 h postinfection. Data are representative of those from three independent experiments. Mean values and SD from three replicate samples are shown.
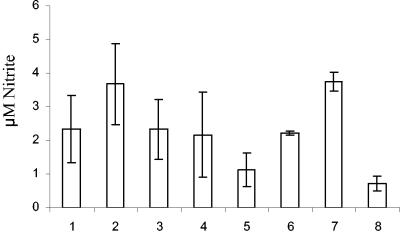
FIG. 4.
Production of NO by CKCs at 4 h postinfection. Bars: 1, live C. jejuni 11168H; 2, live C. jejuni G1; 3, live C. jejuni cdtB; 4, heat-killed C. jejuni 11168H; 5, heat-killed C. jejuni G1; 6, heat-killed C. jejuni cdtB; 7, LPS control E. coli O55:B55 (5 μg/ml); 8, mock infection. Bacterial infections were carried out at an MOI of 100:1. Data shown are averages and SD from three independent experiments.
FIG. 5.
Production of NO by HD11 cells at 6 h postinfection with and without IFN-γ. Bars: 1 and 2, live C. jejuni 11168H; 3 and 4, heat-killed C. jejuni 11168H; 5 and 6, E. coli O55:B55 LPS at 1 μg/ml (positive control); 7 and 8, mock-infected controls with no IFN-γ and IFN-γ alone, respectively. Cells for bars 2, 4, 6, and 8 were pretreated with IFN-γ for 1 h prior to infection. Bacterial infections were carried out at a MOI of 100:1. The data shown are representative of those from two independent experiments and are the averages and SD from four replicate samples. *, significant difference (P < 0.002) between IFN-γ-treated and nontreated cells; +, no significant difference (P > 0.05) between infection with heat-killed and live cells.
Production of cytokines by CKCs.
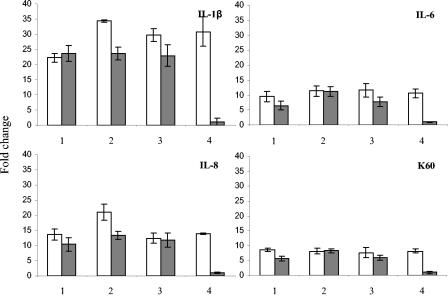
The levels of IL-1β, IL-6, K60, and IL-8 transcripts in CKCs were measured at 4 h postinfection with C. jejuni strains 11168H, G1, and NCTC11168 cdtB and heat-killed bacteria of the same strains (Fig. 6). Mock-infected cells and those infected with E. coli LPS at 5 μg/ml were used as controls throughout. As can be seen, all three bacterial strains induced the production of IL-1β, IL-6, K60, and IL-8 (Fig. 6). Heat-killed bacteria produced equivalent stimulation of the proinflammatory signals (Fig. 6). IFN-γ production was measured and, as expected, was not induced (data not shown). There was no significant difference in the level of IL-10 between infected and uninfected cells (P > 0.05) (data not shown).
FIG. 6.
Quantification of cytokine and chemokine transcripts from CKCs at 4 h postinfection. Open bars, live bacteria; closed bars, heat-killed bacteria. Bars: 1, C. jejuni 11168H; 2, C. jejuni G1; 3, C. jejuni 11168 cdtB; 4, LPS control E. coli O55:B55 (5 μg/ml) (open bar) and mock infection (closed bar). RNA was isolated at 4 h postinfection. Data are representative of those from three independent experiments. Mean values and SD from three replicate samples are shown.
Production of cytokines by HD11 cells.
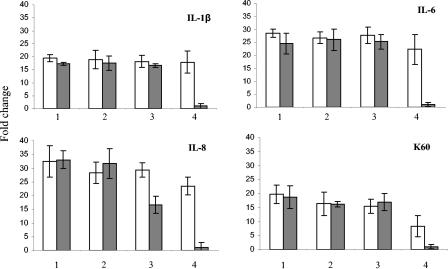
The levels of IL-1β, IL-6, K60, and IL-8 transcripts in HD11 cells were measured at 4 h postinfection with C. jejuni strains 11168H, G1, and NCTC11168 cdtB and heat-killed bacteria of the same strains (Fig. 7). Mock-infected cells and cells infected with E. coli LPS at 5 μg/ml were used as controls throughout. All three bacterial strains, whether live or heat killed, induced the production of IL-1β, IL-6, K60, and IL-8 in HD11 cells (Fig. 7). IFN-γ was measured and, as expected, was not produced (data not shown). There was no significant difference in the level of IL-10 between infected and uninfected cells (P > 0.05) (data not shown).
FIG. 7.
Quantification of cytokine and chemokine transcripts from HD11 cells at 4 h postinfection. Open bars, live bacteria; closed bars, heat-killed bacteria. Bars: 1, C. jejuni 11168H; 2, C. jejuni G1; 3, C. jejuni 11168 cdtB; 4 LPS control E. coli O55:B55 (5 μg/ml) (open bar) and mock infection (closed bar). RNA was isolated at 4 h postinfection. Data are representative of those from three independent experiments. Mean values and SD from three replicate samples are shown.
DISCUSSION
Previous studies of cytokine stimulation by C. jejuni have concentrated on the interaction of the bacterium with human cell lines (2, 16, 17, 19, 20, 31). In this study we describe the interaction of C. jejuni with avian cells. We used CKCs as a model for epithelial cells and an avian macrophage cell line, HD11, to study the potential effects on tissue macrophages if C. jejuni penetrated the epithelial layer. The results clearly show that C. jejuni can invade CKCs to a level equivalent to that seen with human enterocytes and that the rate of killing is similar.
To determine whether there was an inflammatory response to Campylobacter, we measured the induction of iNOS and the production of NO from both CKCs and HD11 cells after infection with Campylobacter. Both CKC and HD11 cells showed a measurable increased in iNOS transcription at 4 h postinfection and the production of NO at 6 h postinfection. Analysis of primary transcripts from infected CKCs shows that Campylobacter is capable of inducing the expression of the proinflammatory cytokines IL-1β and IL-6 and the proinflammatory chemokines K60 and IL-8. Thus, it would appear that CKCs, a well established model for chicken epithelial cells, are capable of being stimulated by Campylobacter in a similar manner to that for human epithelial cells. Work with Salmonella, both in vitro invasion of CKCs and in vivo infections (23, 44), strongly suggests these data can be extrapolated to responses that may occur in the intestine.
In humans, bacterial infection of epithelial cells stimulates the production of chemokines which are involved in the attraction of leukocytes (3, 21). Campylobacter can traverse human epithelial cells and therefore interact with leukocytes to further stimulate the immune response. It has been suggested that the stimulation of leukocytes is significant and could contribute to the pathology of disease (20). To investigate whether Campylobacter can stimulate avian leukocytes in a similar manner, we measured the production of cytokines and chemokines in the HD11 macrophage cell line (7). The different Campylobacter strains are taken up to the same level by HD11 cells and are rapidly killed, with live bacteria being undetectable by gentamicin protection assay at 24 h postinfection. This result is equivalent to that seen previously with peritoneal macrophages (33). IFN-γ increases the sensitivity of HD11 cells to bacterial stimulation, and bacterial killing was enhanced by the addition of IFN-γ prior to infection. Campylobacter clearly stimulates the HD11 cells in a proinflammatory manner, inducing iNOS, the production of NO, the proinflammatory cytokines IL-1β and IL-6, and the proinflammatory chemokines K60 and IL-8.
The production of cytokines by enterocytes has been associated with the active invasion of bacteria (21). To determine whether the stimulation that we observe requires active invasion, we used heat-killed bacteria at an MOI equivalent to that of live infection. The up-regulation of cytokine mRNA levels and NO was not significantly different between live and heat-killed bacterial infection (P > 0.05). This stimulation by the heat-killed bacteria could be multifaceted. Although we have ruled out a requirement for CDT by using the cdtB knockout strain, jlpA (19) may still be active, and the heat-killed bacteria will contain many potential innate ligands, such as lipooligosaccharides and flagellin, any of which may be involved in stimulation.
This paper clearly shows that Campylobacter can stimulate inflammatory responses from avian cells. The induction of IL-8 in CKCs suggests that there should be an attraction of peripheral blood mononuclear cells (4). To our knowledge, no in vivo data to indicate any leukocyte migration in response to Campylobacter colonization in the chicken have been published. The biological roles of the avian IL-8 homologues measured in this study are undefined (22), although there is circumstantial evidence for a true functional homologue of IL-8 (26). Mammalian IL-8 is involved in the attraction of neutrophils, while the avian IL-8 homologue attracts heterophils and monocytes.
Reports of invasion and pathology by Campylobacter in the chicken are limited to day-of-hatch chicks (37). Day-of-hatch birds have no established gut flora and possess an immature mucosal immune system, and analysis of published infection data suggests that the chicken develops resistance to Campylobacter invasion with age. The only published studies on the avian immune response to Campylobacter describe adaptive immunity (8, 32). To induce the adaptive response, an innate response is first required.
Even though Campylobacter colonizes the intestinal tracts of chickens to a high level (109 CFU/g), very little invasion is observed and the bacteria do not elicit inflammatory disease, through either a lack of contact with the relevant receptors or induction of tolerance. If Campylobacter does not invade in sufficient numbers to cause severe inflammation, it may still cause local inflammation, which could be sufficient to control the bacteria and also drive the adaptive immune response. This local response may be self-limiting and so not lead to severe pathology. This question will be addressed in further studies.
While iNOS, IL-1β, IL-6, and IL-8 are major markers of inflammatory disease, other factors, such as IL-10 and suppressors of cytokine signaling (1, 29), which are key in regulation of inflammation, may be modulated in different manners in humans and chickens and may play a crucial role in the development of disease. We measured the levels of IL-10 transcripts in infected and uninfected CKCs and HD11 cells and found no significant differences between samples. However, this does not rule out IL-10 production in vivo by other cell types. Recent evidence from Salmonella enterica serovar Infantis infections of gnotobitic pigs shows that polymorphonuclear leukocyte influx can be uncoupled from the disease process and preempt a virulent infection, thus protecting against challenge with virulent Salmonella (13). Obviously, therefore, factors other than the signals that attract polymorphonuclear leukocytes are required for inflammatory disease.
Evidence exists for a role of other chemokines in human infection (3), but nothing is known about their production in chickens during Campylobacter infection. Given the importance of chemokines in Salmonella infections (46), key differences in their production may dictate the outcome of the balance between colonization and disease.
Finally, we cannot rule out that there may be fundamental differences between host physiologies that preclude disease. There may be a crucial, as yet undefined, difference between human and avian hosts. The ability of the bacteria to induce inflammatory cytokines may be sufficient to prevent disease in poultry, and either the physical interaction of Campylobacter with the infected host or the regulation of those responses may be crucial to the outcome of infection.
Acknowledgments
We thank B. Wren and N. Gregson for the supply of the parental strains used in this study. We also thank D. Purdy, CAMR, United Kingdom, for supplying NCTC11168 ctdB.
We acknowledge funding from DEFRA and BBSRC.
Editor: J. T. Barbieri
REFERENCES
- 1.Alexander, W. S., and D. J. Hilton. 2004. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu. Rev. Immunol. 22:503-529. [DOI] [PubMed] [Google Scholar]
- 2.Al-Salloom, F. S., A. A. Mahmeed, A. Ismaeel, G. A. Botta, and M. Bakhiet. 2003. Campylobacter-stimulated INT407 cells produced dissociated cytokine profiles. J. Infect. 47:217-224. [DOI] [PubMed] [Google Scholar]
- 3.Bakhiet, M., F. S. Al-Salloom, A. Qareiballa, K. Bindayna, I. Farid, and G. A. Botta. 2004. Induction of α and β chemokines by intestinal epithelial cells stimulated with Campylobacter jejuni. J. Infect. 48:236-244. [DOI] [PubMed] [Google Scholar]
- 4.Barker, K. A., A. Hampe, M. Y. Stoeckle, and H. Hanafusa. 1993. Transformation-associated cytokine 9E3/CEF4 is chemotactic for chicken peripheral blood mononuclear cells. J. Virol. 67:3528-3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrow, P. A., and M. A. Lovell. 1989. Invasion of Vero cells by Salmonella species. J. Med. Microbiol. 28:59-67. [DOI] [PubMed] [Google Scholar]
- 6.Beery, J. T., M. B. Hugdahl, and M. P. Doyle. 1988. Colonization of gastrointestinal tracts of chicks by Campylobacter jejuni. Appl. Environ. Microbiol. 54:2365-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beug, H., A. von Kirchbach, G. Doderlein, J.-F. Conscience, and T. Graf. 1979. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell 18:375-390. [DOI] [PubMed] [Google Scholar]
- 8.Cawthraw, S., R. Ayling, P. Nuijten, T. Wassenaar, and D. G. Newell. 1994. Isotype, specificity, and kinetics of systemic and mucosal antibodies to Campylobacter jejuni antigens, including flagellin, during experimental oral infections of chickens. Avian Dis. 38:341-349. [PubMed] [Google Scholar]
- 9.Ding, A. H., C. F. Nathan, and D. J. Stuehr. 1988. Release of reactive nitrogen intermediates from mouse peritoneal macrophages: comparison of activating cytokines and evidence for independent production. J. Immunol. 141:2407-2412. [PubMed] [Google Scholar]
- 10.Enocksson, A., J. Lundberg, E. Weitzberg, A. Norrby-Teglund, and B. Svenungsson. 2004. Rectal nitric oxide gas and stool cytokine levels during the course of infectious gastroenteritis. Clin. Diagn. Lab. Immunol. 11:250-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everest, P. H., H. Goossens, J. P. Butzler, D. Lloyd, S. Knutton, J. M. Ketley, and P. H. Williams. 1992. Differentiated Caco-2 cells as a model for enteric invasion by Campylobacter jejuni and C. coli. J. Med. Microbiol. 37:319-325. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez, H., and L. R. Trabulsi. 1995. Invasive and enterotoxic properties in Campylobacter jejuni and Campylobacter coli strains isolated from humans and animals. Biol. Res. 28:205-210. [PubMed] [Google Scholar]
- 13.Foster, N., M. A. Lovell, K. L. Marston, S. D. Hulme, A. J. Frost, P. Bland, and P. A. Barrow. 2003. Rapid protection of gnotobiotic pigs against experimental salmonellosis following induction of polymorphonuclear leukocytes by avirulent Salmonella enterica. Infect. Immun. 71:2182-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox, J. G., A. B. Rogers, M. T. Whary, Z. Ge, N. S. Taylor, S. Xu, B. H. Horowitz, and S. E. Erdman. 2004. Gastroenteritis in NF-κB-deficient mice is produced with wild-type Campylobacter jejuni but not with C. jejuni lacking cytolethal distending toxin despite persistent colonization with both strains. Infect. Immun. 72:1116-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hackney, K., D. Cavanagh, P. Kaiser, and P. Britton. 2003. In vitro and in ovo expression of chicken gamma interferon by a defective RNA of avian coronavirus infectious bronchitis virus. J. Virol. 77:5694-5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hickey, T. E., S. Baqar, A. L. Bourgeois, C. P. Ewing, and P. Guerry. 1999. Campylobacter jejuni-stimulated secretion of interleukin-8 by INT407 cells. Infect. Immun. 67:88-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hickey, T. E., A. L. McVeigh, D. A. Scott, R. E. Michielutti, A. Bixby, S. A. Carroll, A. L. Bourgeois, and P. Guerry. 2000. Campylobacter jejuni cytolethal distending toxin mediates release of interleukin-8 from intestinal epithelial cells. Infect. Immun. 68:6535-6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes, S., A. Haynes, M. O'Regan, and N. Bumstead. 2001. Identification, mapping, and phylogenetic analysis of three novel chicken CC chemokines. Immunogenetics 53:674-683. [DOI] [PubMed] [Google Scholar]
- 19.Jin, S., Y. C. Song, A. Emili, P. M. Sherman, and V. L. Chan. 2003. JlpA of Campylobacter jejuni interacts with surface-exposed heat shock protein 90alpha and triggers signalling pathways leading to the activation of NF-kappaB and p38 MAP kinase in epithelial cells. Cell Microbiol. 5:165-174. [DOI] [PubMed] [Google Scholar]
- 20.Jones, M. A., S. Totemeyer, D. J. Maskell, C. E. Bryant, and P. A. Barrow. 2003. Induction of proinflammatory responses in the human monocytic cell line THP-1 by Campylobacter jejuni. Infect. Immun. 71:2626-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung, H. C., L. Eckmann, S. K. Yang, A. Panja, J. Fierer, E. Morzycka-Wroblewska, and M. F. Kagnoff. 1995. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Investig. 95:55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaiser, P., L. Rothwell, S. Avery, and S. Balu. 2004. Evolution of the interleukins. Dev. Comp. Immunol. 28:375-394. [DOI] [PubMed] [Google Scholar]
- 23.Kaiser, P., L. Rothwell, E. E. Galyov, P. A. Barrow, J. Burnside, and P. Wigley. 2000. Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium, Salmonella enteritidis, and Salmonella gallinarum. Microbiology 146:3217-3226. [DOI] [PubMed] [Google Scholar]
- 24.Karlyshev, A. V., D. Linton, N. A. Gregson, and B. W. Wren. 2002. A novel paralogous gene family involved in phase-variable flagella-mediated motility in Campylobacter jejuni. Microbiology 148:473-480. [DOI] [PubMed] [Google Scholar]
- 25.Ketley, J. M. 1997. Pathogenesis of enteric infection by Campylobacter. Microbiology 143:5-21. [DOI] [PubMed] [Google Scholar]
- 26.Kogut, M. H. 2002. Dynamics of a protective avian inflammatory response: the role of an IL-8-like cytokine in the recruitment of heterophils to the site of organ invasion by Salmonella enteritidis. Comp. Immunol. Microbiol. Infect. Dis. 25:159-172. [DOI] [PubMed] [Google Scholar]
- 27.Kogut, M. H., L. Rothwell, and P. Kaiser. 2003. Differential regulation of cytokine gene expression by avian heterophils during receptor-mediated phagocytosis of opsonized and nonopsonized Salmonella enteritidis. J. Interferon Cytokine Res. 23:319-327. [DOI] [PubMed] [Google Scholar]
- 28.Lawson, S., L. Rothwell, B. Lambrecht, K. Howes, K. Venugopal, and P. Kaiser. 2001. Turkey and chicken interferon-γ, which share high sequence identity, are biologically cross-reactive. Dev. Comp. Immunol. 25:69-82. [DOI] [PubMed] [Google Scholar]
- 29.Li, M. C., and S. H. He. 2004. IL-10 and its related cytokines for treatment of inflammatory bowel disease. World J. Gastroenterol. 10:620-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linton, D., A. V. Karlyshev, P. G. Hitchen, H. R. Morris, A. Dell, N. A. Gregson, and B. W. Wren. 2000. Multiple N-acetyl neuraminic acid synthetase (neuB) genes in Campylobacter jejuni: identification and characterization of the gene involved in sialylation of lipo-oligosaccharide. Mol. Microbiol. 35:1120-1134. [DOI] [PubMed] [Google Scholar]
- 31.Mellits, K. H., J. Mullen, M. Wand, G. Armbruster, A. Patel, P. L. Connerton, M. Skelly, and I. F. Connerton. 2002. Activation of the transcription factor NF-kappaB by Campylobacter jejuni. Microbiology 148:2753-2763. [DOI] [PubMed] [Google Scholar]
- 32.Myszewski, M. A., and N. J. Stern. 1990. Influence of Campylobacter jejuni cecal colonization on immunoglobulin response in chickens. Avian Dis. 34:588-594. [PubMed] [Google Scholar]
- 33.Myszewski, M. A., and N. J. Stern. 1991. Phagacytosis and intracellular killing of Campylobacter jejuni by elicited peritoneal macrophages. Avian Dis. 35:750-755. [PubMed] [Google Scholar]
- 34.Parkhill, J., B. W. Wren, K. Mungall, J. M. Ketley, C. Churcher, D. Basham, T. Chillingworth, R. M. Davies, T. Feltwell, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Moule, M. J. Pallen, C. W. Penn, M. A. Quail, M. A. Rajandream, K. M. Rutherford, A. H. van Vliet, S. Whitehead, and B. G. Barrell. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403:665-668. [DOI] [PubMed] [Google Scholar]
- 35.Purdy, D., C. M. Buswell, A. E. Hodgson, K. McAlpine, I. Henderson, and S. A. Leach. 2000. Characterisation of cytolethal distending toxin (CDT) mutants of Campylobacter jejuni. J. Med. Microbiol. 49:473-479. [DOI] [PubMed] [Google Scholar]
- 36.Rothwell, L. J. R. Young, R. Zoorob, C. A. Whittaker, P. Hesketh, A. Archer, A. L. Smith, and P. Kaiser. 2004. Cloning and characterization of the chicken IL-10 and its role in immune response to Eimeria maxima. J. Immunol. 173:2675-2682. [DOI] [PubMed] [Google Scholar]
- 37.Shane, S. M. 1992. The significance of Campylobacter jejuni infection in poultry: a review. Avian Pathol. 21:189-213. [DOI] [PubMed] [Google Scholar]
- 38.Sick, C., K. Schneider, P. Staeheli, and K. C. Weining. 2000. Novel chicken CXC and CC chemokines. Cytokine 12:181-186. [DOI] [PubMed] [Google Scholar]
- 39.Siegesmund, A. M., M. E. Konkel, J. D. Klena, and P. F. Mixter. 2004. Campylobacter jejuni infection of differentiated THP-1 macrophages results in interleukin 1β release and caspase-1-independent apoptosis. Microbiology 150:561-569. [DOI] [PubMed] [Google Scholar]
- 40.Sung, Y.-H., J. H. Hotchkiss, R. E. Austric, and R. R. Dietert. 1991. Arginine dependent production of a reactive nitrogen intermediate by macrophages of uricotelic species. J. Leukoc. Biol. 50:49-56. [DOI] [PubMed] [Google Scholar]
- 41.Walker, R. I., M. B. Caldwell, E. C. Lee, P. Guerry, T. J. Trust, and G. M. Ruiz-Palacios. 1986. Pathophysiology of Campylobacter enteritis. Microbiol. Rev. 50:81-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wallis, M. R. 1994. The pathogenesis of Campylobacter. Br. J. Biomed Sci. 51:57-64. [PubMed] [Google Scholar]
- 43.Wassennaar, T. M., and M. J. Blaser. 1999. Pathophysiology of Campylobacter jejuni infections of humans. Microbes Infect. 1:1023-1033. [DOI] [PubMed] [Google Scholar]
- 44.Withanage, G. S., P. Kaiser, P. Wigley, C. Powers, P. Mastroeni, H. Brooks, P. Barrow, A. Smith, D. Maskell, and I. McConnell. 2004. Rapid expression of chemokines and proinflammatory cytokines in newly hatched chickens infected with Salmonella enterica serovar Typhimurium. Infect. Immun. 72:2152-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wooldridge, K. G., and J. M. Ketley. 1997. Campylobacter-host cell interactions. Trends Microbiol. 5:96-102. [DOI] [PubMed] [Google Scholar]
- 46.Zhang, S., L. G. Adams, J. Nunes, S. Khare, R. M. Tsolis, and A. J. Baumler. 2003. Secreted effector proteins of Salmonella enterica serotype Typhimurium elicit host-specific chemokine profiles in animal models of typhoid fever and enterocolitis. Infect. Immun. 71:4795-4803. [DOI] [PMC free article] [PubMed] [Google Scholar]