Abstract
To determine optimal strategies to induce specific-antibody-secreting cells (specific ASC) in the rectal and vaginal mucosae, we immunized monkeys with a prototype mucosal immunogen, cholera toxin (CT), given locally or via gastric or parenteral administration. Repeated rectal or vaginal CT immunizations induced strong mucosal and systemic ASC responses. The mucosal responses were, however, confined to the immunization sites and comprised high levels of both specific antitoxin immunoglobulin A (IgA) and IgG. Large numbers of specific IgA and IgG ASC were detected in cell suspensions from dissociated genital and rectal tissues, demonstrating local accumulation of effector B cells at these sites. Intragastric immunization with CT did not per se give rise to cervicovaginal or rectal ASC responses but did prime for a rectal IgA ASC response to local booster immunization. Both rectal and vaginal immunizations also induced circulating blood IgG ASC and IgA ASC. In conclusion, these results show that local administration of antigen to the rectal or vaginal mucosa results in higher ASC responses than systemic or distant mucosal delivery. Furthermore, both the vaginal and the rectal mucosae can serve as inductive sites for systemic ASC responses. These observations should be relevant to the development of vaccines against sexually transmitted diseases such as that caused by human immunodeficiency virus.
Sexually transmitted microbial infections are common worldwide, are often persistent, and in many cases involve severe and sometimes life-threatening complications. These pathogens include human immunodeficiency virus (HIV), human papillomavirus, herpes simplex virus type 2 (HSV-2), Chlamydia trachomatis, Neisseria gonorrhoeae, Treponema pallidum, Haemophilus ducreyi, and group B Streptococcus (GBS). No vaccine against any of these infections exists.
Protection against sexual transmission of most of these pathogens has been associated with local production of specific antibodies (6, 19, 20, 23, 30, 31, 33, 40, 45, 46). Both immunoglobulin G (IgG) and secretory IgA appear to be important. In this respect, IgA can protect mice against a chlamydial genital challenge (30) and reinfection (40). Protection against sexual HIV infection in humans (23) and against mucosally transmitted simian immunodeficiency virus in macaques (19) has also been associated with specific mucosal IgA production. In addition, secretory IgA has also been shown to block mucosal entry and replication of several viruses in mucosal epithelial cells (21, 22, 36, 44) and to eradicate bacteria from other mucosal surfaces, as shown for Vibrio cholerae, Helicobacter felis, and Salmonella typhimurium in the gut (1, 8, 27). In contrast, IgG appear to be the major protective isotype against, e.g., human papillomavirus (4), HSV-2 (31), and T. pallidum (3).
The development of effective immunization schemes that could evoke an antibody response in the rectal and genital tract mucosae should therefore have a major impact on the control of sexually transmitted diseases. Such mucosal antibodies could be derived from local vaginal or rectal sites and/or from transudate from serum (5, 10, 28, 29, 47). However, the latter is rarely associated with protective immunity (6, 7, 38). This means that rapid recruitment and sustained accumulation of effector B cells at mucosal sites play a critical role in immune protection. However, little is known about how such cells are induced in the genital and rectal mucosae.
We have previously shown, with rodents, that the concentration of vaccine-specific antibodies in the genital tract secretions does not necessarily correlate with the numbers of vaccine specific-antibody-secreting cells (specific ASC) at the same site (13). Whereas, e.g., nasal and vaginal immunizations gave rise to comparable levels of specific genital antibodies, vaginal immunization was superior at inducing vaginal ASC and was paramount for the appearance of ASC in the draining lymph nodes (13). Whether this is also true for larger animal species, including primates, is not known. To assess the most efficient way of inducing local rectal and vaginal ASC responses in primates, we have compared different mucosal and systemic immunization strategies with respect to induction of local genital and rectal antigen-specific ASC responses, as well as for the induction of systemic immunity. To this end, monkeys were immunized with a prototype mucosal immunogen, cholera toxin (CT), given orally, vaginally, rectally, or systemically. Local mucosal ASC responses in suspensions of mononuclear cells (MNC) from vaginal and rectal tissues were measured and were compared to the corresponding responses in blood. We also measured the amounts of specific antibodies in genital tract secretions and in protein extracts from rectal biopsy specimens.
MATERIALS AND METHODS
Animals.
Thirty-nine cynomolgus monkeys (Macaca fascicularis) were housed at the primate facilities of the Swedish National Bacteriology Laboratory, Stockholm, Sweden, and at the Department of Medical Microbiology, Göteborg, Sweden. These studies were approved by the Ethical Committees for Animal Experimentation in Stockholm and Göteborg. All animals were under mild sedation with ketamine (Ketalar; Parke-Davis, S.A., Barcelona, Spain) at the times of immunization and sample collection.
Immunizations.
Cynomolgus monkeys were immunized with CT (LIST Biological Laboratories, Inc., Campbell, Calif.) three or four times, 3 to 6 weeks apart. In a dose-escalating study involving two monkeys fed consecutively with 1, 10, and finally 50 μg of CT, we found no evidence of detectable side effects (fever, weight loss, diarrhea, or vomiting). The last dose was therefore employed for mucosal immunizations in all subsequent experiments. Four monkeys served as controls. None of the monkeys suffered from any notable side effects.
(i) Systemic immunizations.
Six monkeys (one female) received four intradermal injections with 2 μg of CT in 0.2 ml of phosphate-buffered saline (PBS). Four female monkeys received one intradermal injection with CT.
(ii) Intragastric immunizations.
Six monkeys (one female) received four intragastric doses of 50 μg of CT in sodium bicarbonate-citric acid buffer (ACO Pharmaceuticals, Stockholm, Sweden). Immunizations were performed by administering 5 ml of solution through a baby-feeding tube into the stomach.
(iii) Vaginal immunizations.
Eight female monkeys received four vaginal doses of 50 μg of CT in 0.2 ml of PBS. Immunizations were performed with a double balloon, and the vaccine was instilled for 5 min.
(iv) Rectal immunizations.
Five monkeys (two females) received four rectal doses of 50 μg of CT in 1 ml of sodium bicarbonate-citric acid buffer. Rectal immunizations were performed with a double balloon, and the vaccine was instilled for 5 min.
(v) Oral priming followed by vaginal or rectal booster.
Three male monkeys received three intragastric doses of CT followed by one rectal dose. Three female monkeys received three intragastric doses of CT followed by one vaginal dose. All of these doses and immunizations were as described above.
Collection of specimens.
Vaginal washings and heparinized venous blood were collected before the primary immunization and 7 days after each subsequent immunization. Vaginal washings were collected by rinsing the vagina with 100 to 300 μl of PBS for 1 min, and the samples were then stored at −20°C in phenylmethylsulfonyl fluoride (0.35 mg/ml; Sigma)–soybean trypsin inhibitor (0.1 mg/ml)–EDTA (0.05 M; Sigma)–bovine serum albumin (BSA) (0.1%).
Seven days after the final immunization, animals were sacrificed by an intracardiac overdose of thiopental natrium (500 mg) (Pentothal Natrium; Abbott S.p.A.-Campoverde LT., Italy). The distal part of the colon was collected from all monkeys, and the vagina, uterus, and fallopian tubes were collected from the female animals. The tissues were thoroughly rinsed in PBS containing 0.1% heparin.
Isolation of MNC suspensions.
Heparinized venous blood was mixed with 3% (wt/vol) gelatin (gelatin L936; PB Gelatins UK Ltd.) in PBS at a 3:1 (vol/vol) ratio, and the erythrocytes were allowed to sediment for 1 h at 37°C. The supernatant was diluted in PBS (1:1, vol/vol), and MNC were then isolated by standard Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) gradient centrifugation.
Vaginal and rectal tissue fragments were cut into 0.1- by 0.1-mm pieces and incubated for 30 min at 4°C in 0.5 mg of Bacillus thermoproteolyticus thermolysin (Boehringer, Mannheim, Germany) per ml in Hanks balanced salt solution (Gibco, Paisley, United Kingdom) containing 1 mM CaCl2 and 10 mM dithiothreitol. Extracted cells were separated from the remaining tissue fragments by filtration through a 150-μm nylon mesh. Undigested tissue fragments were reextracted by incubation for 45 min at 37°C with 1 mg of collagenase-dispase (Boehringer) per ml in Iscove’s medium (Gibco) supplemented with 10% fetal calf serum. Extracted cells were separated as described above. The cell suspensions were pooled and incubated for 20 min at 37°C with 2 mg of DNase (type IV; Sigma) per ml in Iscove’s medium containing 5% fetal calf serum and filtered through a 50-μm nylon mesh (34). Cell viability was >90% as determined by trypan blue staining.
Perfusion-extraction method (PERFEXT) (13).
A small piece of rectal tissue was collected at the time of sacrifice. The tissue was stored at −20°C in a PBS solution (1 ml of PBS per g of tissue) containing 2 mM phenylmethylsulfonyl fluoride, 0.1 mg of soybean trypsin inhibitor (Sigma) per ml, and 0.05 M EDTA. Prior to analysis, saponin (Sigma) was added to a final concentration of 2% (wt/vol), and the samples were incubated overnight at 4°C. Antibody measurements were performed on the collected supernatants (see below).
ELISPOT assays.
Vaginal, rectal, and blood MNC suspensions were assayed for CT-specific ASC by an amplified enzyme-linked immunospot (ELISPOT) assay (9). Briefly, nitrocellulose-bottomed 96-well plates (Millipore, Bedford, Mass.) were coated overnight with GM1 ganglioside (3 μM) (Sigma) followed by CT (2.5 μg/ml) and were then blocked with 0.5% (wt/vol) BSA. Immediately following their isolation, MNC were added to antigen-coated wells and incubated at 37°C overnight in a moist atmosphere with 5% CO2. Next, biotin-conjugated goat anti-human IgA or IgG antibodies (Medac; diluted 1:500) were added, followed by horseradish peroxidase (HRP)-labelled egg avidin (Extravidin; Sigma) (4 μg/ml), biotin-labelled goat anti-HRP antibodies (2 μg/ml), and Extravidin (4 μg/ml), in PBS containing 0.1% BSA and 0.05% Tween 20. Spots were developed by addition of 0.3 mg of 3-amino-9-ethylcarbazole (Sigma) per ml and 0.015% (vol/vol) H2O2 in 0.1 M sodium acetate, pH 5.0. Data are expressed as individual ASC numbers per 106 MNC together with the geometric mean numbers of ASC. A response was defined as >5 specific ASC per 106 blood MNC and >30 specific ASC per 106 vaginal or rectal MNC, in order to exceed (i) the geometric mean plus three standard deviations of results for nonimmunized control monkeys and (ii) three spots per well. Normally, 106 blood MNC or 105 vaginal or rectal MNC would be analyzed per well, accounting for the difference in the cutoff values.
ELISAs.
Enzyme-linked immunosorbent assay (ELISA) plates were coated overnight with 0.3 μM GM1 ganglioside followed by 0.5 μg of CT per ml. Serially diluted samples in PBS containing 0.1% BSA and 0.05% Tween 20 were added and were incubated overnight at 4°C. Biotin-conjugated goat anti-human IgA (diluted 1:5,000) or IgG (diluted 1:7,500) antibodies (Medac) were then added, followed by 2 μg of HRP-labelled egg avidin per ml. Plates were developed by addition of 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (Sigma) at 0.25% (wt/vol) in 0.1 M sodium acetate buffer (pH 4.8 to 5.0) containing 0.0075% H2O2. Color development was monitored spectrophotometrically at 405 nm. The specific-antibody titer was estimated as the interpolated sample dilution giving an absorbance of 0.4 above the background level (11, 12).
To determine total antibody contents of vaginal washes, ELISA plates were coated with 1 μg of goat anti-human IgG F(ab)2 specific antibodies (Jackson) per ml (for IgG determinations) or a mixture of monoclonal mouse anti-human kappa (6062; 2 μg/ml) and anti-human lambda (6054; 2 μg/ml) (a gift from the late Charles Reimer, Centers for Disease Control and Prevention, Atlanta, Ga.) (for IgA determinations). Following incubation of the samples, solid-phase-captured Igs were detected as described above. Purified rhesus IgG and IgA (gifts from John Eldridge, Birmingham, Ala.) were used as standards.
Antibody titers were expressed as the reciprocal sample dilution giving an absorbance of 0.4 above the background level. Data are expressed as (i) the specific antibody titer post- versus preimmunization (for plasma), where a 2-fold or greater increase in titer is regarded as a response to the vaccination (12); (ii) the specific antibody titer divided by the total Ig concentration expressed in milligrams per milliliter (for vaginal washes), where a response is a 2.3-fold or greater titer increase (11); or (iii) the specific antibody titer in 1 mg of tissue per ml (for rectal PERFEXT samples). Only vaginal washings containing at least 5 μg of IgA per ml and 5 μg of IgG per ml were used for CT-specific antibody determinations (11). Preimmunization titers for sera were <10.6 ± 3.0 for IgA and <130 ± 117 for IgG. Preimmunization titers for vaginal washings were 19 ± 25 for IgA and 48 ± 73 for IgG.
Statistical evaluations.
Pearson’s correlation coefficient (r) was determined for (i) specific antibodies in vaginal washes versus numbers of specific genital ASC, (ii) specific antibodies in vaginal washes versus titers of specific antibodies in serum (iii) titers of specific antibodies in serum versus specific genital ASC, (iv) specific antibodies in rectal tissue versus numbers of specific rectal ASC, (v) specific antibodies in rectal tissue versus titers of specific antibodies in serum, and (vi) titers of specific antibodies in serum versus specific rectal ASC.
RESULTS
Effect of immunization route on cervicovaginal CT-specific antibody responses.
An important component of the development of vaccines against sexually transmitted diseases is to determine optimal strategies to induce specific immune responses in the genital tract. To this end, macaques were immunized with CT by parenteral and mucosal routes, and the subsequent B-cell responses in the vaginal and rectal mucosae and in blood were monitored.
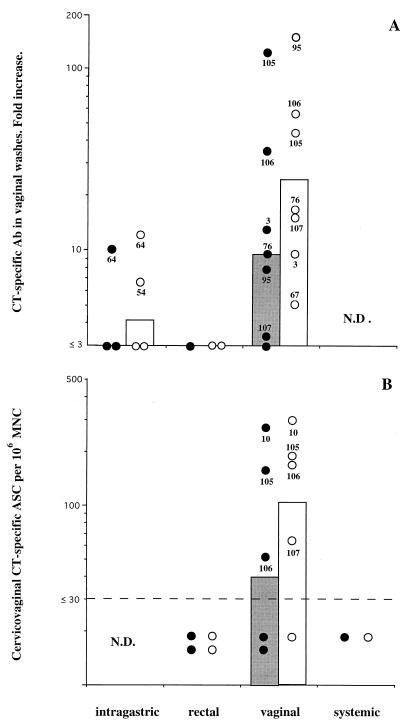
When the immunization routes applied in this study were compared, repeated vaginal administration of CT was found to be the most consistent way to induce specific antibody responses in the female genital tract. These responses were characterized by the development of high titers of specific IgA and IgG in cervicovaginal secretions. After two immunizations, all monkeys had CT-specific IgG antibodies in vaginal secretions, and 50% of these monkeys also had CT-specific IgA (not shown). After four immunizations, increased CT-specific IgG in cervicovaginal washes in all seven vaginally immunized animals was observed (Fig. 1A). Six of these monkeys also had significantly increased CT-specific IgA titers (Fig. 1A). Most importantly, the genital tracts of four of five animals examined harbored specific ASC (Fig. 1B), with considerable numbers of specific ASC in some animals.
FIG. 1.
Specific antibody (Ab) responses in the female genital tracts of macaques after CT immunization by various routes. Data are expressed as geometric mean numbers (bars) and individual values (circles) for CT-specific IgA (closed symbols) and IgG (open symbols). (A) CT-specific titer increases in cervicovaginal washes after three or four enteric, vaginal, or rectal administrations of CT. Some values are missing due to insufficient material for total IgA determinations. Titer increases are defined as the postbooster titer divided by the prepriming titer. (B) CT-specific ASC responses in the cervicovaginal mucosa after four systemic, vaginal, or rectal immunizations with CT. The dashed line denotes the limit (30 ASC per 106 MNC) below which net values were considered negative (99% confidence interval). N.D., not determined.
In contrast, neither intragastric nor rectal immunization proved as efficient for the induction of genital antibody responses. Thus, no responses were observed before the third or fourth intragastric immunization, and then only two of four animals showed an increased IgG titer and one of three animals showed an increased IgA titer (Fig. 1A). We also examined vaginal antibody responses in two animals after four rectal immunizations. Neither animal had any appreciable anti-CT antibody responses in cervicovaginal washes (Fig. 1A) or any detectable ASC in cervicovaginal suspensions (Fig. 1B).
It should be noted that we do not have enough data from systemically immunized animals to draw any conclusions about the applicability of this route of immunization for induction of cervicovaginal antibody responses. Even though a single systemic injection with CT induced increased IgG anti-CT activity, but no IgA anti-CT activity, in cervicovaginal secretions of all five monkeys examined (not shown), only one of these monkeys was further immunized systemically, and after four systemic immunizations, this animal did not have any detectable vaginal CT-specific ASC (Fig. 1B).
To evaluate the relationship between vaginal specific ASC and titers of specific antibodies in vaginal washes, we calculated Pearson’s correlation coefficient (r). There was no statistical correlation between these two parameters for either IgA or IgG; for IgA, r = 0.082 (not significant), and for IgG, r = 0.350 (not significant).
Effect of immunization route on CT-specific ASC responses in the rectal mucosa.
Several sexually transmitted pathogens can also infect the lower alimentary tract, e.g., HIV and GBS. This indicates that an efficient vaccine should, simultaneously with the induction of genital immunity, also induce immune responses in the rectal mucosa. To assess the optimal immunization route(s) for rectal ASC responses, we compared the different immunization routes (oral, rectal, vaginal, and systemic) with respect to the induction of specific ASC in the rectum.
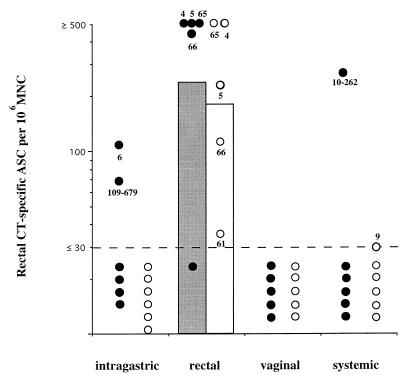
Similar to the situation for the female genital tract, repeated topical (rectal) administration of CT was the most consistent way to induce specific ASC responses in the rectum. Thus, four of five animals examined after four rectal CT administrations displayed appreciable CT-specific IgA ASC responses, and all five animals had vaccine-specific IgG ASC (Fig. 2). In contrast, only one of six systemically immunized macaques, two of six intragastrically immunized animals, and none of five vaginally immunized animals displayed local IgA ASC responses of a comparable magnitude (Fig. 2). Furthermore, none of the last three types of immunizations induced any detectable rectal CT-specific IgG ASC (Fig. 2).
FIG. 2.
Specific ASC responses in the rectums of macaques after immunization by various routes. CT-specific ASC responses in the rectal mucosa were measured after four immunizations. Data are expressed as geometric mean numbers (bars) and individual values (circles) for CT-specific IgA (closed symbols) and IgG (open symbols) measured 7 days after the last booster immunization. The dashed line denotes the limit (30 ASC per 106 MNC) below which net values were considered negative (99% confidence interval).
Due to a low antibody content, we were unfortunately unable to measure the amounts of antibodies secreted into the rectal lumen. Instead, we analyzed the relative amount of CT-specific antibodies within rectal tissue by means of the PERFEXT method (13). As seen in Table 1, four rectal immunizations induced the highest concentrations of CT-specific IgA within rectal tissue, with an average >500-fold-higher titer compared to that for naive control animals. Moderate levels of CT-specific IgA were also induced following repeated vaginal immunizations, where a >50-fold increase in average titer was observed. CT-specific IgG, on the other hand, was best induced by repeated systemic or rectal immunizations (>200- and >100-fold increases in titer, respectively, compared to naive animals), whereas oral and vaginal immunizations were poor inducers of CT-specific IgG accumulation within the rectum. To evaluate the relationship between specific rectal ASC responses and titers of specific antibodies in rectal tissue, we calculated Pearson’s correlation coefficient (r). There was a statistical correlation between these two parameters for both IgA and IgG, with r = 0.737 for IgA (P < 0.001) and r = 0.683 for IgG (P < 0.01).
TABLE 1.
CT-specific antibody concentrations in rectal tissue following four immunizations with CT
| Immunization routea | Mean titer per 1 mg of rectal tissue in saponin per ml (range)
|
|
|---|---|---|
| IgA | IgG | |
| Oral | 66 (<10–430) | 2,040 (1,040–3,990) |
| Rectal | 5,520 (3,320–8,890) | 18,490 (9,210–58,670) |
| Vaginal | 570 (120–2,680) | 2,740 (1,000–6,220) |
| Systemic | 130 (90–260) | 31,830 (31,330–32,310) |
| Naive controls | <10 (all <10) | <143 (<100–420) |
For oral, rectal, and vaginal immunizations, three monkeys received four doses of 50 μg of CT intragastrically, rectally, or vaginally, respectively. For systemic immunizations, three monkeys received four intradermal injections of 2 μg of CT.
Systemic immune responses after mucosal and systemic immunizations.
Even though local vaginal and rectal production of antibodies appears to be fundamental in the defense against several of the sexually transmitted microbial infections discussed above, the systemic levels of specific antibodies are also important. For instance, neonatal infection with GBS can be blocked by passive transfer of specific maternal IgG to the fetus (15). Perhaps systemic antibodies can also help prevent dissemination of genital infection, e.g., infection with HIV or HSV-2. In keeping with this notion, we analyzed the levels of specific antibodies as well as numbers of specific ASC in serum after systemic, oral, rectal, and vaginal immunization with CT.
All of the applied immunization schedules induced systemic B-cell responses. Thus, 2 to 3 weeks after a single systemic immunization with CT, serum IgG anti-CT antibody responses had developed in all animals, with titer increases ranging from 80- to more than 2,000-fold (Table 2), and these levels increased with each subsequent immunization (Table 2). After two systemic doses of CT, all animals also had increased serum IgA, with increases in titers ranging from 45- to 160-fold (Table 2). Furthermore, all systemically immunized animals had considerable numbers of CT-specific IgG ASC in blood after four CT immunizations (Fig. 3).
TABLE 2.
CT-specific serum responses following immunization with CT via various routes
| Response | Immunization routea | Mean CT-specific titer in serum (range)
|
||||
|---|---|---|---|---|---|---|
| Preimmunization | After dose:
|
|||||
| 1 | 2 | 3 | 4 | |||
| IgG | Oral | <116 (<100–324) | 1,766 (387–4,916) | 4,724 (1,721–9,102) | 8,975 (5,129–15,706) | |
| Rectal | <268 (<100–1,926) | 4,331 (2,007–1,923) | 3,797 (1,923–9,160) | 7,178 (3,450–21,750) | ||
| Vaginal | <124 (<100–213) | 4,150 (311–19,188) | 6,128 (1,923–9,160) | 4,752 (3,450–21,750) | ||
| Systemic | <123 (<100–371) | 39,521 (22,624–77,280) | 142,980 (73,977–218,700) | 112,326 (82,707–132,935) | >166,652 (96,769–>218,700) | |
| IgA | Oral | <10 (<10) | <12 (<10–33) | <28 (<10–320) | <48 (<10–230) | |
| Rectal | <10 (<10) | 21 (11–47) | 51 (27–75) | 295 (243–426) | ||
| Vaginal | <12 (<10–36) | <310 (<10–5,355) | <406 (<10–5,326) | 414 (40–4,654) | ||
| Systemic | <11 (<10–14) | <57 (<10–695) | 723 (451–1,598) | 808 (699–879) | 1,234 (852–1,487) | |
For oral immunizations six monkeys received four intragastric doses of 50 μg of CT, for rectal immunizations three monkeys received four rectal doses of 50 μg of CT, for vaginal immunizations six monkeys received four vaginal doses of 50 μg of CT, and for systemic immunizations six monkeys received four intradermal injections of 2 μg of CT.
FIG. 3.
Specific ASC responses in macaque peripheral blood after four CT immunizations. Data are expressed as geometric mean numbers (bars) and individual values (circles) for CT-specific IgA (closed symbols) and IgG (open symbols) measured 7 days after the last booster immunization.
After three intragastric doses of CT, all eight macaques examined had on average more than a 40-fold (range, 13- to 90-fold) increase in the serum IgG titer, of which five also had increased serum IgA titers (2.4- to 30-fold increase). However, only two of four intragastrically immunized macaques had IgG ASC and/or IgA ASC in blood after three intragastric doses, and these responses were modest (Fig. 3).
Rectal immunization induced serum IgG and IgA antibody responses to CT that were comparable to those seen after intragastric immunization (Table 2). Furthermore, three of four animals had low frequencies of circulating specific IgA ASC, and two of these also had IgG ASC (Fig. 3).
All animals receiving CT applied to the cervicovaginal mucosa displayed increased IgG and IgA antibody activity in serum after two immunizations (Table 2), and these responses were maintained following further vaginal immunizations. After four doses of CT, circulating CT-specific IgA ASC were detected in three of five animals, and CT-specific IgG ASC were detected in two of five animals (Fig. 3). However, the levels of specific ASC were relatively modest.
There was a statistically significant correlation between the titers of antibodies in vaginal washes and the titers of specific antibodies in serum. This was true for both IgA and IgG. Thus, Pearson’s correlation coefficient was 0.575 (P < 0.05) for IgA and 0.539 (P < 0.05) for IgG. The corresponding correlation coefficients for comparison between numbers of genital ASC and titers of specific antibodies in serum were 0.395 (not significant) for IgA and −0.260 (not significant) for IgG. For comparison of serum responses with rectal responses, there was a statistically significant correlation between IgG titers in serum and concentrations of CT-specific IgG in rectal tissue, with r = 0.506 (P < 0.05). However, no statistically significant correlation between these two groups was observed for IgA (r = −0.022), nor were there any significant correlations when serum and rectal ASC responses were compared (r = −0.234 for IgA and r = −0.218 for IgG). There was a statistical correlation between blood ASC responses and serum antibody responses for both IgA (r = 0.501; P < 0.05) and IgG (r = 0.934; P < 0.001).
Effect of enteric priming on genital and rectal ASC responses to a subsequent local booster with CT.
Although intragastric administrations of CT largely failed to induce an ASC response in the cervicovaginal mucosa and in the rectum, we examined whether this route of immunization could prime animals for a subsequent ASC response upon local boosting with recall antigen.
For the genital tract, three intragastric doses of CT followed by one vaginal immunization gave rise to low numbers of vaginal CT-specific IgA ASC in only one of three animals (Fig. 4A). None of the animals had any detectable CT-specific IgG ASC. Thus, the level of specific ASC following intragastric priming was very low compared to the responses obtained after four vaginal immunizations (Fig. 4A).
FIG. 4.
Effect of intragastric priming with CT on specific genital (A) and rectal (B) ASC responses after vaginal and rectal CT booster immunization, respectively. Monkeys received three intragastric doses of CT followed by one vaginal or rectal dose. ASC responses were measured 7 days after booster immunization. Data are expressed as geometric mean numbers (bars) and individual values (circles) for CT-specific IgA (closed symbols) and IgG (open symbols). The dashed lines denote the limit (30 ASC per 106 MNC) below which net values were considered negative (99% confidence interval).
For the rectum, all three monkeys that had been primed intragastrically with CT followed by one rectal booster immunization had relatively high numbers of CT-specific IgA ASC in their rectums, and one of these animals also had substantial numbers of CT-specific rectal IgG ASC (Fig. 4B). These responses were in general of lower magnitude than those seen in animals that had been both primed and repeatedly boosted in the rectum (Fig. 4B).
DISCUSSION
In developing vaccines against sexually transmitted microbial infections, it is important to explore means of inducing local genital and rectal immunity to the pathogen. Such local immunity involves the accumulation of specific immune cells at the site of infection, be these antibody-producing B cells, helper or cytotoxic T cells, or others. In this study we have focused on immunization strategies that would evoke specific B-cell responses in the female genital tracts and rectums of primates. We demonstrated that it is possible to obtain strong ASC responses in the vaginal and rectal mucosae of nonhuman primates following immunization with CT. These responses were evidenced by large numbers of CT-specific IgA as well as IgG ASC found among MNC isolated from the respective mucosal tissue. In this respect, local application of antigen was strikingly more effective than gastric or systemic administration in inducing immune responses in the genital and lower alimentary tract mucosae.
Within the clear limitations caused by the small numbers of animals that could be used for each type of immunization, our observations indicate that among the four different routes of immunization tried, topical application of CT to the cervicovaginal and rectal mucosae was the most efficient and the only consistent way of inducing both a systemic and a mucosal antibody response. The latter response was largely accounted for by local accumulation of specific ASC in the genital tract and lower alimentary tract mucosae. These data represent the first proof of vaccine-induced accumulation of specific ASC in the female genital tracts and lower alimentary tracts of primates. Previously, similar findings have been recorded for the female genital tracts of rodents (13, 25).
That vaginal and rectal immunizations give rise to vaginal and rectal antibody responses, respectively, has previously been shown with both monkeys (50) and humans (18, 28, 29, 43). However, these responses were monitored only as antibodies in washings from the respective mucosae. We and others have previously shown, with the genital tracts of rodents, that levels of specific antibodies in secretions and frequencies of specific ASC do not necessarily correlate (13, 25). The same is true in monkeys, as CT-specific IgG and, to a lesser extent, IgA were detected in the sera and cervical secretions of parenterally or enterically immunized animals in the absence of detectable ASC in the genital tract. In fact, there was a statistically significant correlation between titers of specific antibodies in sera and in vaginal washes, and this correlation was seen for both IgA and IgG. This indicates (i) that measurements of antibody concentrations in vaginal and rectal fluids are not always a good reflection of local immune induction and (ii) that the bulk of IgG and IgA detected in cervicovaginal secretions from parenterally, intragastrically, or rectally immunized macaques was derived from transudation of systemically produced antibodies. When rectal samples were analyzed, the PERFEXT method, in which specific antibody titers in extracts from whole tissue are determined (13), was evaluated. Judging from the statistical correlation analyses performed, the PERFEXT method is compatible with ELISPOT for IgA determinations, whereas for IgG, the concentration of CT-specific rectal antibodies correlates statistically with both the number of specific IgG ASC in the rectum and the levels of specific IgG in serum.
The appearance of specific ASC in the genital and rectal mucosae after local antigen delivery has several important implications. First, it confirms the notion that a mucosal immune response is usually strongest at the site of the initial encounter with antigen (25, 49). Second, this finding has important implications not only for B-cell responses, where the effector molecule, the antibody, is secreted and can reach other destinations in the body via the lymph and blood, but for cell-mediated immune reactions, where close contact between effector cells and targets is of fundamental importance. Third, efficient memory responses are induced only when the specific memory population of cells expresses the appropriate homing molecules, enabling them to constantly move through and scrutinize tissues where infection is likely to occur. It has been shown that different routes of immunization or infection induce different homing receptors on the resulting circulating effector as well as memory cells (14, 35, 37) and that these homing receptors direct the cell trafficking to different inductive or effector sites, including the genital tract (5, 16, 32, 39).
The high numbers of specific ASC detected in both genital and rectal samples show that the female genital tract mucosa and the rectum, or their respective draining lymphoid tissues, can serve as efficient inductive sites for localized as well as remote humoral immune responses. Conceptually, this has been known for decades (17, 28, 29), but with respect to the genital tract, the differences that exist between different types of antigens, different animal species, and levels of reproductive cycle hormones have only lately been appreciated. In progesterone-treated mice, but not in mice with normal hormone levels, vaginal ASC and vaginal antibodies can readily be induced by vaginal or intranasal immunization with CT or CTB (10, 13, 41, 42). In rats, specific genital ASC and secretory antibodies are induced when CTB-conjugated antigen is given together with CT adjuvant to animals that are in the proestrus or estrus stage of the cycle (25). In humans, on the other hand, rectal and vaginal antibody responses to V. cholerae vaccine (18, 43) and poliovirus vaccine (28, 29) were obtained after either rectal or vaginal inoculations, respectively, and were seemingly unrelated to the stage of the menstrual cycle. In our study, no hormone treatments or measurements of estradiol levels were performed. Therefore, we cannot rule out the possibility that the differences in responses observed were related to hormone levels.
For several sexually transmitted infections, an optimal B-cell response would involve not only the appearance of specific ASC in the mucosa but also high levels of specific antibodies in the circulation. Such circulating antibodies could prevent dissemination of an infection, e.g., HIV, and in the case of GBS could prevent neonatal infection by passive transmission of antibodies through the placenta to the fetus (15). As expected, systemic immunization was superior at increasing both levels of specific antibodies in serum and frequencies of circulating CT-specific IgG ASC, even though all routes of immunization induced moderate levels of specific IgG. However, only vaginal and rectal immunization gave rise to detectable numbers of CT-specific IgA ASC. Such specific IgA ASC could be of importance particularly for the defense of other mucosal surfaces. Thus, in keeping with the notion of a common mucosal immune system (26) whereby a fraction of B cells primed at a mucosal site can repopulate distant mucosal effector compartments, it has been shown that IgA ASC from the intestinal (mesenteric) lymph nodes of rodents home to various mucosal tissues, including the female reproductive tract, when transferred into syngeneic mice (24). Furthermore, intragastric immunization with pertinent antigens induces the appearance of specific antibodies in the female reproductive tract (6, 48) and can protect mice against vaginal challenge with C. trachomatis (6). In the present study, recruitment of IgA-secreting B cells into the genital tract mucosa from a distant, presumably gut-derived, precursor pool could be found in only one of the three orally primed macaques given a local vaginal booster dose with CT, again demonstrating the larger capacity of local antigen delivery to induce local vaginal B-cell responses. On the other hand, intragastric immunizations with CT appeared to be effective at priming for rectal IgA ASC responses. These responses were lower than those seen following rectal priming, which agrees with earlier studies showing that a maximal response to a secondary challenge usually occurs at the initial site of mucosal priming (25, 49). Furthermore, these results imply that following oral priming, there is a preferential homing of ASC within the gastrointestinal tract. Studies with rodents (13) indicate that the nasal route of antigen delivery is very efficient for the induction of genital tract antibody and ASC responses. In humans, nasal immunization gives rise to appreciable titers of specific antibodies in vaginal secretions (2). It would be interesting to determine the applicability of this route of immunization for the induction of vaginal and rectal ASC responses in human or nonhuman primates.
In summary, the results of this study indicate that specific-antibody production within the rectal and vaginal tract mucosae as well as a systemic humoral immune response can be induced by local application of antigen. These observations have obvious implications for the development of vaccines against sexually transmitted diseases, especially in view of recent data implicating mucosal IgA as a major factor in preventing sexual HIV infection (23).
ACKNOWLEDGMENTS
The help of Eva Sjögren, Inger Nordström, Maria Hjulström, Margareta Fredriksson, Annie George-Chandy, Margareta Hedin, Sten Holm, and Anders Kihlander is gratefully acknowledged.
This study was supported by grants from SIDA/SAREC’s Special Programme for AIDS and related diseases; NIH grant no. 1 RO1 A1 35543-02; European Commission (Biomed) contract no. CT 920272; the Swedish Medical Research Council (MFR) projects no. 16X-3382 and 16X-8320; the Faculty of Medicine, University of Göteborg; the Swedish Foundation of Physicians against AIDS; the Swedish Society for Medical Research; and Syntello Inc.
REFERENCES
- 1.Apter F M, Michetti P, Winner III L S, Mack J A, Mekalanos J J, Neutra M R. Analysis of the roles of antilipopolysaccharide and anti-cholera toxin immunoglobulin A (IgA) antibodies in protection against Vibrio cholerae and cholera toxin by use of monoclonal IgA antibodies in vivo. Infect Immun. 1993;61:5279–5285. doi: 10.1128/iai.61.12.5279-5285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azedegan A A, Schell R F, Alder J D, Steiner B M, Liu H, Harris O N, Coe J E. Synergistic effect of macrophage activation and immune serum, especially IgG2a, on resistance to infection with Treponema pallidum ssp. Endemicum in hamsters. Reg Immunol. 1988;1:3–8. [PubMed] [Google Scholar]
- 3.Bergquist C, Johansson E L, Lagergård T, Holmgren J, Rudin A. Intranasal vaccination of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and in the vagina. Infect Immun. 1997;65:2676–2684. doi: 10.1128/iai.65.7.2676-2684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryan J T, Jansen K U, Lowe R S, Fife K H, McClowry T, Glass D, Brown D R. Human papillomavirus type 11 neutralization in the athymic mouse xenograft system: correlation with virus-like particle IgG concentration. J Med Virol. 1997;53:185–188. doi: 10.1002/(sici)1096-9071(199711)53:3<185::aid-jmv1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 5.Butcher E C, Picket L J. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 6.Cui Z D, Tristram D, LaScolea L J, Kwiatkowski T, Jr, Kopti S, Ogra P L. Induction of antibody response to Chlamydia trachomatis in the genital tract by intragastric immunization. Infect Immun. 1991;59:1465–1469. doi: 10.1128/iai.59.4.1465-1469.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Culliton B J. AIDS against the rest of the world. Nature. 1991;352:15. doi: 10.1038/352015a0. [DOI] [PubMed] [Google Scholar]
- 8.Czinn S J, Cai A, Nedrud J G. Protection of germ-free mice from infection by Helicobacter felis after active oral or passive IgA immunization. Vaccine. 1993;11:637–642. doi: 10.1016/0264-410x(93)90309-l. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson K, Nordström I, Horal P, Jeanson S, Svennerholm B, Vahlne A, Holmgren J, Czerkinsky C. Amplified ELISPOT assay for detection of HIV-specific antibody-secreting cells in subhuman primates. J Immunol Methods. 1992;153:107–113. doi: 10.1016/0022-1759(92)90312-h. [DOI] [PubMed] [Google Scholar]
- 10.Haneberg B, Kendall D, Amerongen H M, Apter F M, Krahenbuhl J P, Neutra M R. Induction of specific immunoglobulin A in the small intestine, colon-rectum, and vagina measured by a new method for collection of secretions from local mucosal surfaces. Infect Immun. 1994;62:15–23. doi: 10.1128/iai.62.1.15-23.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jertborn M, Svennerholm A M, Holmgren J. Saliva, breast milk, and serum antibody responses as indirect measure of intestinal immunity after oral cholera vaccination or natural disease. J Clin Microbiol. 1986;24:203–209. doi: 10.1128/jcm.24.2.203-209.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jertborn M, Svennerholm A M, Holmgren J. Evaluation of different immunization schedules for oral cholera B subunit-whole cell vaccine in Swedish volunteers. Vaccine. 1993;11:1007–1012. doi: 10.1016/0264-410x(93)90125-h. [DOI] [PubMed] [Google Scholar]
- 13.Johansson E L, Rask C, Fredriksson M, Eriksson K, Czerkinsky C, Holmgren J. Antibodies and antibody-secreting cells in the female genital tract after vaginal or intranasal immunization with cholera toxin B subunit or conjugates. Infect Immun. 1998;66:514–520. doi: 10.1128/iai.66.2.514-520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kantele A, Kantele J M, Savilahti E, Westerholm M, Arvilommi H, Lazarovits A, Butcher E C, Mäkälä H P. Homing potentials of circulating lymphocytes in humans depend on the site of activation—oral, but not parenteral, typhoid vaccination induces circulating antibody-secreting cells that bear homing receptors directing them to the gut. J Immunol. 1997;158:574–579. [PubMed] [Google Scholar]
- 15.Kasper D L. Designer vaccines to prevent infections due to group B Streptococcus. Proc Assoc Am Physicians. 1995;107:369–373. [PubMed] [Google Scholar]
- 16.Kelly A K, Rank R G. Identification of homing receptors that mediate the recruitment of CD4 T cells to the genital tract following intravaginal infection with Chlamydia trachomatis. Infect Immun. 1997;65:5198–5208. doi: 10.1128/iai.65.12.5198-5208.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kerr W R, Robertson M. Active and passive sensitization of the uterus of the cow in vivo against trichomonas foetus antigen and the evidence for the local production of antibody at that site. J Hyg. 1953;51:405–415. doi: 10.1017/s0022172400015825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kowlowski P A, Cu-Uvin S, Neutra M R, Flanigan T P. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect Immun. 1997;65:1387–1394. doi: 10.1128/iai.65.4.1387-1394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehner T, Wang Y, Cranage Y M, Bergmeier M L A, Mitchell E, Tao L, Hall G, Dennis M, Cook N, Brookes R, Klavinskis L, Jones I, Doyle C, Ward R. Protective mucosal immunity elicited by targeted iliac lymph node immunization with a subunit SIV envelope and core vaccine in macaques. Nat Med. 1996;2:767–775. doi: 10.1038/nm0796-767. [DOI] [PubMed] [Google Scholar]
- 20.Lewinski M A, Miller J N, Champion C I, Walker E M, Borenstein L A, Gayek R J, Lovett M A, Blanco D R. Treponemicidal antibody measured by the assay correlates with immunity in experimental rabbit syphilis. Sex Transm Dis. 1995;22:31–38. doi: 10.1097/00007435-199501000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Mazanec M B, Coudret C L, Fletcher D R. Intracellular neutralization of influenza virus by immunoglobulin A antihemagglutinin monoclonal antibodies. J Virol. 1995;69:1339–1343. doi: 10.1128/jvi.69.2.1339-1343.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazanec M B, Kaetzel C S, Lamm M E, Fletcher D, Nedrud J G. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc Natl Acad Sci USA. 1992;89:6901–6905. doi: 10.1073/pnas.89.15.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazzoli S, Trabbatoni D, Caputo S L, Piconi S, Ble C, Meacci F, Ruzzante S, Salvi A, Semplici F, Longhi R, Fusi M L, Tofani N, Biasin M, Villa M L, Mazzotta F, Clerici M. HIV-specific mucosal and cellular immunity in HIV-seronegative partners of HIV-seropositive individuals. Nat Med. 1997;11:1250–1257. doi: 10.1038/nm1197-1250. [DOI] [PubMed] [Google Scholar]
- 24.McDermott M R, Bienenstock J. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J Immunol. 1979;122:1892–1898. [PubMed] [Google Scholar]
- 25.Menge A C, Michalek S M, Russell M W, Mestecky J. Immune response of the female rat genital tract after oral and local immunization with keyhole limpet hemocyanin conjugated to cholera toxin B subunit. Infect Immun. 1993;61:2162–2171. doi: 10.1128/iai.61.5.2162-2171.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987;7:265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- 27.Michetti P, Mahan M J, Slauch J M, Mekalanos J J, Neutra M R. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect Immun. 1992;60:1786–1792. doi: 10.1128/iai.60.5.1786-1792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogra P L, Karzon D T. Distribution of poliovirus antibody in serum, nasopharynx and lower alimentary tract following segmental immunization of lower alimentary tract with poliovaccine. J Immunol. 1969;102:1423–1430. [PubMed] [Google Scholar]
- 29.Ogra P L, Ogra S S. Local antibody response to poliovaccine in the human female genital tract. J Immunol. 1973;110:1307–1311. [PubMed] [Google Scholar]
- 30.Pal S, The I, Peterson E M, de la Maza L M. Monoclonal immunoglobulin A antibody to the major outer membrane protein of the Chlamydia trachomatis mouse pneumonitis biovar protects mice against chlamydial genital challenge. Vaccine. 1997;15:575–582. doi: 10.1016/s0264-410x(97)00206-5. [DOI] [PubMed] [Google Scholar]
- 31.Parr E L, Parr M B. Immunoglobulin G is the main protective antibody in mouse vaginal secretions after vaginal immunization with attenuated herpes simplex virus type 2. J Virol. 1997;71:8109–8115. doi: 10.1128/jvi.71.11.8109-8115.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perry L L, Feilzer K, Portis J L, Caldwell H D. Distinct homing pathways direct T lymphocytes to the genital and intestinal mucosae in Chlamydia-infected mice. J Immunol. 1998;160:2905–2914. [PubMed] [Google Scholar]
- 33.Plummer F A, Chubb H, Simonsen J N, Bosire M, Slanley L, Nagelkerke N J, Maclean I, Ndinya-Achola J O, Waiyaki P, Brunham R C. Antibodies to opacity proteins (Opa) correlate with a reduced risk of gonococcal salpingitis. J Clin Invest. 1994;93:1748–1755. doi: 10.1172/JCI117159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quiding M, Nordström I, Kilander A, Andersson G, Hansson L Å, Holmgren J, Czerkinsky C. Intestinal immune responses in humans. Oral cholera vaccination induces strong intestinal antibody responses and interferon-γ production and evokes local immunological memory. J Clin Invest. 1991;88:143–148. doi: 10.1172/JCI115270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quiding-Järbrink M, Nordström I, Granström G, Kilander A, Jertborn M, Butcher E C, Lazarovits A I, Holmgren J, Czerkinsky C. Differential expression of tissue-specific adhesion molecules on human circulating antibody-forming cells after systemic, enteric, and nasal immunizations: a molecular basis for the compartmentalization of effector B cell responses. J Clin Invest. 1997;99:1281–1286. doi: 10.1172/JCI119286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Renegar K B, Small P A. Passive transfer of local immunity to influenza-virus infection by IgA antibodies. J Immunol. 1991;146:1972–1978. [PubMed] [Google Scholar]
- 37.Rott L S, Rose J R, Bass D, Williams M B, Greenberg H B, Greenberg E C. Expression of mucosal homing receptor alpha4beta7 by circulating CD4+ cells with memory to intestinal rotavirus. J Clin Invest. 1997;100:1204–1208. doi: 10.1172/JCI119633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sabin A B. Improbability of effective vaccination against human immunodeficiency virus because of its intracellular transmission and rectal portal of entry. Proc Natl Acad Sci USA. 1992;89:8852–8855. doi: 10.1073/pnas.89.18.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salmi M, Jalkanen S. How do lymphocytes know where to go: current concepts and enigmas of lymphocyte homing. Adv Immunol. 1997;64:139–218. doi: 10.1016/s0065-2776(08)60889-5. [DOI] [PubMed] [Google Scholar]
- 40.Su H, Feilzer K, Caldwell H D, Morrison R P. Chlamydia trachomatis genital tract infection of antibody-deficient gene knockout mice. Infect Immun. 1997;65:1993–1999. doi: 10.1128/iai.65.6.1993-1999.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thapar M A, Parr E L, Bozzola J J, Parr M B. Secretory immune responses in the mouse vagina after parenteral or intravaginal immunization with an immunostimulating complex. Vaccine. 1991;9:129–132. doi: 10.1016/0264-410x(91)90269-c. [DOI] [PubMed] [Google Scholar]
- 42.Thapar M A, Parr E L, Parr M B. Secretory immune responses in mouse vaginal fluid after pelvic, parenteral or vaginal immunization. Immunology. 1990;70:121–125. [PMC free article] [PubMed] [Google Scholar]
- 43.Wassen L, Schön K, Holmgren J, Jertborn M, Lycke N. Local intravaginal vaccination of the female genital tract. Scand J Immunol. 1996;44:408–414. doi: 10.1046/j.1365-3083.1996.d01-320.x. [DOI] [PubMed] [Google Scholar]
- 44.Weltzin R, Hsu S A, Mittler E S, Georgakopoulos K, Monath T P. Intranasal monoclonal immunoglobulin A against respiratory syncytial virus protects against upper and lower respiratory-tract infections in mice. Antimicrob Agents Chemother. 1994;38:2785–2791. doi: 10.1128/aac.38.12.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whaley K J, Zeitlin L, Barratt R A, Hoen T E, Cone R A. Passive immunization of the vagina protects mice against vaginal transmission of genital herpes infections. J Infect Dis. 1994;169:647–649. doi: 10.1093/infdis/169.3.647. [DOI] [PubMed] [Google Scholar]
- 46.White W I, Wilson S D, Bonnez W, Rose R C, Koenig S, Suzich J A. In vitro infection and type-restricted antibody-mediated neutralization of authentic human papillomavirus type 16. J Virol. 1998;72:959–964. doi: 10.1128/jvi.72.2.959-964.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilkie B N, Duncan J R, Winter A J. The origin, class and specificity of immunoglobulins in bovine cervico-vaginal mucus: variation with parenteral immunization and local infection with vibrio fetus. J Reprod Fert. 1972;31:359–365. doi: 10.1530/jrf.0.0310359. [DOI] [PubMed] [Google Scholar]
- 48.Wira C R, Sandoe C P. Specific IgA and IgG antibodies in the secretions of the female reproductive tract: effects of immunization and estradiol on expression of this response in vivo. J Immunol. 1987;138:4159–4164. [PubMed] [Google Scholar]
- 49.Wira C R, Sandoe C P. Effect of uterine immunization and oestradiol on specific IgA and IgG antibodies in uterine, vaginal and salivary secretions. Immunology. 1989;68:24–30. [PMC free article] [PubMed] [Google Scholar]
- 50.Yang S L, Schumacher G F B. Immune response after vaginal application of antigens in the rhesus monkey. Fertil Steril. 1979;32:588–598. doi: 10.1016/s0015-0282(16)44365-7. [DOI] [PubMed] [Google Scholar]