Abstract
Despite knowledge of the effects of toxic shock syndrome (TSS) toxin 1 (TSST-1) on the adaptive immune system, little is known about stimulation of the innate immune system, particularly epithelial cells. This study investigated the interactions of TSS Staphylococcus aureus and TSST-1 with human vaginal epithelial cells (HVECs) and porcine mucosal surfaces. When cocultured with HVECs for 6 h, TSS S. aureus MN8 proliferated, formed aggregates on the HVEC surfaces, and produced exotoxins. Receptor binding studies showed that 35S-TSST-1 bound to 5 × 104 receptors per HVEC, with saturation at 15 min. Affymetrix Human GeneChip U133A microarray analysis determined S. aureus MNSM (100 bacteria/HVEC) caused at least twofold up- or down-regulation of 410 HVEC genes by 6 h; these data were also confirmed with S. aureus MN8. TSST-1 (100 μg/ml) caused up- or down-regulation of 2,386 HVEC genes by 6 h. In response to S. aureus, the HVEC genes most up-regulated compared to those in controls were those coding for chemokines or cytokines—MIP-3α, 478-fold; GRO-α, 26-fold; GRO-β, 14-fold; and GRO-γ, 30-fold—suggesting activation of innate immunity. TSST-1 also caused up-regulation of chemokine/cytokine genes. Chemokine/cytokine gene up-regulation was confirmed by enzyme-linked immunosorbent assays measuring the corresponding proteins induced by S. aureus and TSST-1. S. aureus MN8, when incubated with porcine vaginal tissue, increased the flux of 35S-TSST-1 across the mucosal surface. This was accompanied by influx of lymphocytes into the upper layers of the tissue. These data suggest innate immune system activation through epithelial cells, reflected in chemokine/cytokine production and influx of lymphocytes, may cause changes in vaginal mucosa permeability, facilitating TSST-1 penetration.
Staphylococcus aureus is a human pathogen that causes infections by initial colonization of skin and mucosal surfaces. S. aureus causes nearly any type of infection, ranging from relatively benign furuncles and skin abscesses to potentially life-threatening necrotizing pneumonia and toxic shock syndrome (TSS) (16, 42). Development of antibiotic resistance is particularly problematic in this organism, especially in hospital settings and more recently in community settings, where the prevalence of methicillin resistance is increasing significantly (11, 17). There are two major categories of TSS caused by S. aureus: nonmenstrual, which may follow any type of S. aureus skin or mucous membrane infection (48); and menstrual (mTSS), in which the S. aureus cells remain localized on cervical-vaginal and/or oral mucosal surfaces (14, 46, 58). mTSS occurs during or within 2 days of onset or termination of menses, with the symptoms of mTSS dependent on the exotoxin TSS toxin 1 (TSST-1), having effects systemically on cells of the immune system as a superantigen (8, 39, 56).
The superantigen effects of TSST-1 on T lymphocytes and macrophages have been extensively studied. TSST-1 was determined to induce massive cytokine release from both T cells and macrophages by cross-bridging major histocompatibility complex (MHC) class II molecules on macrophages with T-cell receptors (TCRs) on CD4+ T cells, which is dependent on the composition of the β chain of the TCR variable region (22, 35-37, 39, 41, 43, 44). Despite the extensive knowledge of superantigen effects on the adaptive immune system, the TSST-1 interaction with the vaginal epithelium and the mechanism of TSST-1 penetration across the human vaginal mucosa remain unresolved. One recent collaborative study suggested that purified internally 35S-labeled TSST-1 (≤50 μg/ml) was unable to penetrate intact vaginal mucosal tissue in sufficient amounts to induce TSS (13). In addition, prior studies suggested that TSST-1 at doses of 10 and 20 μg/ml had the ability to slow the growth of isolated conjunctival epithelial cells (31, 32).
In this study, we began investigations of the interaction between purified TSST-1 and TSST-1-producing S. aureus with an immortalized human vaginal epithelial cell (HVEC) line isolated from a premenopausal woman. Epithelial cells represent the predominant cell types that initially come into contact with TSST-1 and S. aureus strains. This study utilized human genome microarray analysis to determine altered HVEC gene expression in response to TSST-1 and TSST-1-producing S. aureus and enzyme-linked immunosorbent assay (ELISA) to confirm the changes in protein levels associated with altered gene expression. Results of our studies suggest that vaginal epithelial cells are components of the innate immune system. Following stimulation with TSST-1 and TSST-1-producing S. aureus, vaginal epithelial cells up-regulate the expression of chemokine and cytokine genes, which may lead subsequently to activation of the adaptive immune system. In addition, our data demonstrate that viable TSS S. aureus cells actively growing on intact vaginal porcine mucosal surfaces increase the flux of TSST-1 across the intact vaginal tissue, with accompanying influx of lymphocytes into the upper epithelial layers. Our long-term goal is to describe the mechanisms by which S. aureus remains localized on the vaginal mucosal surface yet causes systemic TSS disease manifestations through the effects of TSST-1.
MATERIALS AND METHODS
Bacteria.
S. aureus strains MNSM and MN8 were used in these experiments. The strains were isolated from patients with mTSS. MNSM and MN8, representatives of the major class of mTSS S. aureus isolates, are tryptophan auxotrophs, with S. aureus pathogenicity island 2 (SaPI2) containing tst (the gene for TSST-1) inserted within the tryptophan operon, bacteriophage type (29/52), and the same multilocus enzyme electrophoresis profile as the majority of TSS isolates (45). In vitro S. aureus MNSM and MN8 produced the same approximate concentration of TSST-1 as other mTSS isolates. (The range of TSST-1 production by strains was 3 to 100 μg/ml.) MNSM and MN8 were positive for the staphylococcal enterotoxin A (SEA) gene by PCR, but made less than 75 pg of SEA per ml when cultured in a dialyzable beef heart medium (10). S. aureus RN4220(pCE107) was cultured in a pyrogen-free dialyzable beef heart medium containing erythromycin (5 μg/ml) for production of TSST-1 (43). Strain RN4220 has been shown to lack endogenous superantigen production. The plasmid pCE107 is a high-copy-number plasmid containing tst. S. aureus strain MNNJ was used for production of staphylococcal enterotoxin B (SEB) (63), and Escherichia coli containing pET28b with a speA insert was used for production of streptococcal pyrogenic exotoxin A (SPE A) (40).
Superantigen purification.
TSST-1 was partially purified by ethanol precipitation (75% final volume) from late-stationary-phase culture fluids of S. aureus RN4220(pCE107) grown at 37°C with high aeration (200 rpm) and resolubilization in pyrogen-free water (10). TSST-1 was purified to homogeneity by thin-layer isoelectric focusing in pH gradients: first from 3 to 10 and then from 6 to 8 (10). SEB was comparably purified from S. aureus strain MNNJ, except that the second isoelectric focusing gradient was 7 to 9 (63). SPE A was prepared from a pET28b clone (40); in this case, the second isoelectric focusing gradient was 4 to 6. The superantigens migrated as visible, clear bands in the opaque background of the gradients with pIs of 7.2 for TSST-1, 8.5 for SEB, and 5.0 for SPE A. After isoelectric focusing, ampholytes were removed following 4 days of dialysis against pyrogen-free water. Purified toxins (5 μg) were homogeneous when tested by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and silver staining (33). In addition, the proteins, at concentrations of 1 mg/ml, were negative for lipopolysaccharide (endotoxin), as tested by Limulus assay (Sigma Chemical Company, St. Louis, Mo.). The TSST-1 and SEB proteins lacked detectable hemolysins (α, β, and δ), protease, and lipase as tested in bioassays and compared to standards (described below).
Characterization and cell culture of an immortalized HVEC line.
Primary normal human epithelial cells were isolated from premenopausal vaginal hysterectomy tissue, obtained from a woman who did not have cancer, by methods that have been previously described for the isolation of human foreskin epithelial cells (25, 30). Cells were grown in keratinocyte serum-free medium (KSFM) (Gibco, Invitrogen, Carlsbad, Calif.) on plastic and passaged with a 1:4 split using trypsin-EDTA. Early-passage cells were doubly transduced with retroviruses expressing human papillomavirus 16 (HPV-16) E6/E7 (a gift from D. Galloway, Seattle, Wash.) and the reverse transcriptase component of telomerase, hTERT (obtained from the Geron Corporation, Menlo Park, Calif.), and selected with 50 μg of the antibiotic G418 per ml (Sigma). The E6/E7/TERT cells (V428) had high levels of telomerase and became immortal without a crisis, whereas normal untransduced cells senesced around passage 9.
For all experiments, HVECs were cultured at 37°C in a 7% CO2 incubator in KSFM supplemented with bovine pituitary extract and epidermal growth factor as provided by the manufacturer and a 2% final volume of penicillin-streptomycin-amphotericin B (Fungizone; Gibco, Invitrogen). The HVECs were stained with a mixture of immunoglobulin G monoclonal antibodies AE1 and AE7 (Chemicon International, Temecula, Calif.) to characterize their cytokeratin production. Clone AE1 detects the high-molecular-weight cytokeratins 10, 14, 15, and 16 and the low-molecular-weight cytokeratin 19. Clone AE3 detects the high-molecular-weight cytokeratins 1 to 6 and the low-molecular-weight cytokeratins 7 and 8.
TSST-1 receptor determination on HVECs.
35S-labeled TSST-1 was prepared from the RN4220 clone as described previously (10, 13). Briefly, the cells were grown to a cell density of 5 × 108/ml in 50 ml of beef heart dialysate medium, and then 10 mCi of [35S]methionine was added. The culture was then incubated overnight at 37°C with high aeration (200 rpm). Finally, TSST-1 was purified by ethanol precipitation, resolubilization in water, and isoelectric focusing. Experiments to assess TSST-1 binding to HVECs were performed at 4°C to prevent toxin internalization by the cells. HVECs were cultured in 75-cm2 flasks (BD Falcon, Bedford, Mass.) until confluent (approximately 107cells/flask). The cells were then scraped from the flasks with rubber spatulas, the contents of multiple flasks were combined, and the cells were resuspended in PBS for use. Initial experiments assessed the time required to saturate the receptors on the HVECs for TSST-1, with time points including 0, 5, 15, 30, and 60 min. At each time point, the cells were incubated with TSST-1, washed three times by centrifugation at 4°C, and both the cells and supernatants were counted for radioactivity with a scintillation counter. Scatchard analysis was performed to determine receptor numbers per HVEC.
Bacterial virulence factor assays in KSFM.
TSST-1 was quantified by a sandwich ELISA procedure (64). δ hemolysin was estimated by a competition ELISA procedure. Briefly, intact δ hemolysin was synthesized by the University of Minnesota Microchemical Facility; it was chromatographically purified and verified to have hemolytic activity. A rabbit was hyperimmunized against the hemolysin, and serum was collected and verified as reactive with δ hemolysin. The immunoglobulins were collected by 33% ammonium sulfate precipitation and resolubilization in phosphate-buffered saline (PBS). A competition ELISA was set up in which microtiter plate wells were coated with δ hemolysin, different concentrations of δ hemolysin (or culture fluids) plus a predetermined dilution of anti-δ hemolysin antibodies were added, and the plates were developed with antirabbit-horseradish peroxidase conjugate (64).
α hemolysin, lipase (glycerol ester hydrolase), and protease were measured in bioassays compared to the activities of known concentrations of purified control proteins (55). Briefly, rabbit erythrocytes (α hemolysin), tributyrin (lipase), or casein (protease) was added to 45°C agarose (0.75% in PBS), and 4 ml of homogeneous mixtures was applied to microscope slides. After solidifying, 4-mm wells were punched in the agarose, and 20-μl volumes of culture fluids or control purified proteins were added to the wells. Finally, the slides were incubated humidified at 37°C for 8 h. The areas of the zones of clearing were determined to be proportional to protein concentrations.
SEM and confocal microscopy.
Single-cell suspensions of S. aureus MN8 were cultured in KSFM (without antibiotics) with HVECs previously grown to confluence in transparent cell culture inserts (Becton Dickenson Labware, Franklin Lakes, N.J.) at 37°C for up to 6 h to assess adherence and growth of the strain on the epithelial cells. After 6 h, the surface was washed with PBS to remove nonadherent cells, and the HVECs with bound staphylococci were fixed for scanning electron microscopy (SEM) according to instructions provided by the University of Minnesota SEM facility. A Hitachi S-800 instrument was utilized to visualize S. aureus MN8 adherence and replication on HVECs. HVECs were also examined by confocal microscopy at the University of Minnesota Biomedical Process Imaging Laboratory to assess TSST-1 effects on cell morphology following coculture with TSST-1 at concentrations of both 10 and 100 μg/ml in KSFM at 37°C.
Microarray experiments of HVECs incubated with TSS S. aureus and TSST-1.
Affymetrix GeneChip Human Genome U133A (Santa Clara, Calif.) was used to analyze HVEC gene up- or down-regulation in the presence of S. aureus MNSM (109 CFU/ml in KSFM without antibiotics) (100 bacteria per epithelial cell) at 3 and 6 h and S. aureus MN8 (109 CFU/ml in KSFM without antibiotics) (100 bacteria per epithelial cell) at 6 h. The 6-h maximum incubation time was chosen, since this is the approximate time that women wear a tampon (59). In addition, similar analyses were performed following incubation of HVECs with purified TSST-1 (10 and 100 μg/ml) for 3 and 6 h. Control flasks contained KSFM alone without antibiotics and were comparably incubated for 3 and 6 h. The protocol entailed culture of the HVECs in 75-cm2 flasks (BD Falcon, Bedford, Mass.) until confluent (determined to be approximately 107 HVECs/flask). Prior to initiation of the experiments, the medium was then changed to include KSFM alone (without antibiotics), KSFM plus purified TSST-1 (10 or 100 μg/ml), or KSFM plus 109 bacteria/ml, that had grown to the late exponential phase in KSFM. In vitro, S. aureus cell counts of 109/ml correspond to the time of bacterial growth when secreted virulence factors such as TSST-1 are typically made and cell surface virulence factor genes, such as the gene for protein A, are down-regulated (63).
After 3 and 6 h of incubation, supernatants were removed from the flasks and frozen at −20°C for later chemokine and cytokine analysis by ELISA and to quantify exotoxin production. The HVECs were removed from the flasks by trypsinization (5 to 8 min at 37°C) and collected by centrifugation (250 × g for 5 min). The cell pellets were used for RNA extraction, which was accomplished with an RNeasy Mini kit from QIAGEN (Valencia, Calif.). RNA samples were processed for use in hybridization assays as described by Affymetrix, Inc. (Santa Clara, Calif.). Hybridization analyses were performed at the University of Minnesota Biomedical Genomics Center. The data obtained were analyzed by programs provided by both Affymetrix Microarray Suite 4.0 and Microsoft (Excel). Only those genes that were definitively present and statistically determined to be up- or down-regulated by twofold or more were considered in these studies.
ELISAs for chemokines and cytokines.
To confirm the data from microarray analysis, ELISAs were performed on the supernatants saved at −20°C after the 3- and 6-h incubation periods. Representative chemokines and cytokines were chosen for assay. Kits were purchased from R and D Systems, Minneapolis, Minn., and included assays for chemokine ligand 20 (macrophage inflammatory protein 3α [MIP-3α]), interleukin 1β [IL-1β], IL-6, IL-8, tumor necrosis factor alpha [TNF-α], and gamma interferon. All assays, including standard curve generation, were performed according to the manufacturer's specification. Absorbance values and calculated concentration of chemokines and cytokines in the supernatants were derived from the linear parts of the standard curves. In addition, supernatants from HVECs incubated for 6 h with two other superantigens, SEB (100 μg/ml) and SPE A (100 μg/ml), and with ovalbumin (100 μg/ml; Sigma, as an irrelevant protein control) were assayed for MIP-3α and IL-8 for confirmation of TSST-1 activation of HVECs.
Finally, studies were conducted in an attempt to inhibit competitively the ability of TSST-1 to bind to the HVEC receptor and induce chemokine and cytokine release. Briefly, TSST-1 (100 μg/ml), alone or with the dodecapeptide [Tyr-Asn-Lys-Lys-Lys-Ala-Thr-Val-Gln-Glu-Leu-Asp] (1,000 μg/ml), synthesized, purified, and verified to have the correct sequence by the University of Minnesota Microchemical Facility, was incubated with HVECs for 6 h, and then chemokines and cytokines were assayed by ELISA. The dodecapeptide previously was used by others to inhibit both superantigen activity and transport of superantigens across mucosal surfaces (5, 60). The dodecapeptide region lies outside the region of TSST-1 that interacts either with MHC class II or TCR (5).
Measurement of porcine vaginal tissue permeability to TSST-1 ex vivo.
Specimens of normal, porcine vaginal mucosa were excised from animals at slaughter, transported to the laboratory in sealed plastic bags, and utilized within 3 h of harvest. Tissue disks (8 to 10 mm in diameter) were mounted in continuous flow perfusion chambers (5-mm-diameter orifice) that were maintained at 37°C on water-jacketed blocks. An epithelial surface of 0.20 cm2 was exposed to the donor compartment. PBS (pH 7.4) was pumped through the receiving chamber at 1.8 ml/h as a collection fluid. The chambers were incorporated into an automated continuous flow system to permit regular sampling over a 12-h period. Seven to nine replicates were prepared for each sample application.
Permeability to 35S-labeled TSST-1 was assessed as previously described (13). Viable (1.6 × 109/ml) or heat-killed TSS S. aureus MN8 cells (both approximately 100 bacteria/epithelial cell) together with 35S-TSST-1 were applied to the epithelial surface in KSFM without antibiotics. Perfusate was collected into scintillation vials at 1-h intervals for up to 12 h. The mean of three 100-μl aliquots of the labeled donor chamber was used to determine total applied radiolabel. Flux was calculated for each sampling interval from the relationship flux = Q/At, where Q is the quantity of radiolabel traversing the tissue (disintegrations per minute) in time t (minutes) and A is the area of epithelial surface exposed in square centimeters. Units of flux are disintegrations per minute per square centimeter per minute.
Biopsies of intact porcine mucosa were incubated at 37°C with either PBS or with topically applied S. aureus MN8 in KSFM for up to 12 h. Following experimentation, the biopsies were fixed in formalin, wax embedded, cross-sectioned, and stained with hematoxylin and eosin for histological examination.
Informed consent was obtained from the human tissue donor, and all institutional guidelines regarding human research were followed according to The University of Iowa Institutional Review Board.
RESULTS
This study was undertaken to characterize the interactions of purified TSST-1 and TSS S. aureus with an immortalized vaginal epithelial cell line and ex vivo porcine vaginal tissue to characterize activation of the innate immune system and elucidate the mechanism(s) of TSST-1 penetration across the vaginal mucosa.
Characterization of cytokeratin production by HVECs.
A mixture of monoclonal antibodies (AE1 and AE3), raised against human cytokeratins, reacted as expected (47) with the HVECs (data not shown), indicating the cells were epithelial in nature.
Adherence of TSST-1 and S. aureus MN8 to HVECs.
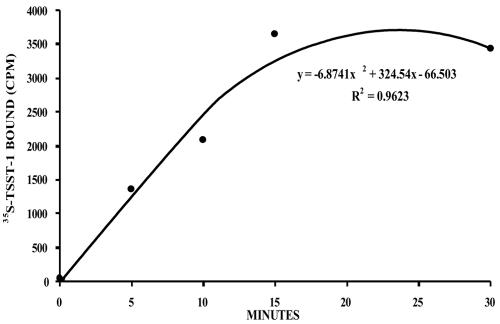
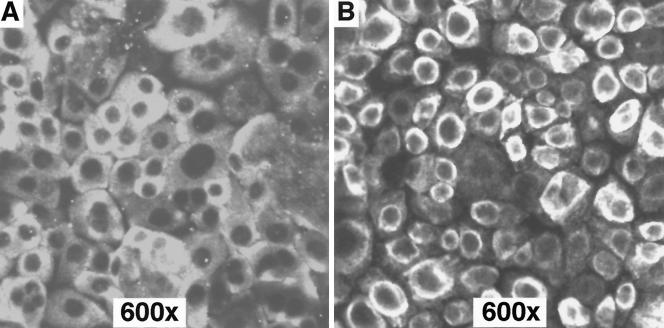
35S-TSST-1 binding experiments determined 35S-TSST-1 bound to the HVECs at approximately 5 × 104 receptors/epithelial cell with saturation of receptors occurring within 15 min of incubation at 4°C (Fig. 1). Gross morphological effects of purified TSST-1 (10 and 100 μg/ml) on the HVECs were examined by confocal microscopy. TSST-1 at 10 μg/ml had no observable effects on the HVECs (data not shown). However, HVECs appeared to lose cell-to-cell contact and contract following 6 h of exposure to TSST-1 at 100 μg/ml (Fig. 2). S. aureus MN8, cocultured at 109 CFU/ml with HVECs in KSFM at 37°C for up to 6 h, was found to adhere, proliferate, form aggregates on the HVECs (Fig. 3), and make exotoxins.
FIG. 1.
Binding of TSST-1 to HVECs. TSST-1 was internally radiolabeled with [35S]methionine. The toxin was incubated with epithelial cells for the indicated times at 4°C to prevent possible internalization, and the cells were then washed and counted in a scintillation counter.
FIG. 2.
Comparison of control untreated HVECs (A) with epithelial cells treated with TSST-1 (100 μg/ml) (B). Toxin was purified from an S. aureus clone and did not contain detectable lipopolysaccharide or other staphylococcal secreted virulence factors. Cells were treated with TSST-1 in KSFM for 6 h at 37°C and then washed with PBS, fixed with formalin, and examined by confocal microscopy.
FIG. 3.
Growth of TSS S. aureus MNSM on HVECs. (A) Control epithelial cells cultured for 6 h in KSFM in cell culture inserts at 37°C and then prepared for SEM (magnification, ×2,500); (B) epithelial cells incubated with single-cell suspensions of S. aureus MNSM in KSFM at 37°C, washed with PBS, and then prepared for microscopy (magnification, ×2500); and (C) magnification of one region of epithelial cells shown in panel B demonstrating growth of S. aureus MNSM in aggregates (magnification, ×15,000).
Characterization of secreted virulence factors of S. aureus MNSM and MN8.
MNSM and MN8, typical TSST-1-producing S. aureus strains from cases of mTSS, produced and secreted the following virulence factors when grown in KSFM medium over the 6-h incubation period with HVECs: TSST-1 (up to 80 μg/ml in broth culture), α hemolysin, δ hemolysin, protease, and lipase. Although MNSM and MN8 were determined by PCR to possess the gene for SEA, the toxin could not be detected by protein analysis.
Microarray analyses of HVEC response to TSST-1 and S. aureus MNSM and MN8.
Since gross morphology changes in HVEC were observed by confocal microscopy following incubation with TSST-1 (100 μg/ml) for 6 h, human genome microarray analyses of the epithelial cell response to TSST-1 (10 and 100 μg/ml) were conducted with the Affymetrix Human GeneChip U133A. (Table 1 shows data from analysis of TSST-1 at 100 μg/ml.) TSST-1 at 10 μg/ml caused only 60 and 61 genes to be up- or down-regulated by twofold or more at 3 and 6 h, respectively (data not shown; see website at http://www.micab.umn.edu/faculty/Schlievert.html). There was no discernible pattern to the genes that were significantly up- or down-regulated. In contrast, TSST-1 at 100 μg/ml caused significant up- or down-regulation of 1,472 and 2,386 genes by twofold or more at 3 and 6 h, respectively (examples shown in Table 1). Notably, genes for chemokines were significantly up-regulated at 6 h, such as MIP-3α up-regulated by 169-fold, CXCL1 (GRO-α) up-regulated by 84-fold, CXCL2 (GRO-β) up-regulated by 13-fold, and CXCL3 (GRO-γ) up-regulated by 32-fold. In addition, genes for cytokines were also significantly up-regulated, such as those coding for TNF-α and IL-1β, with changes of 2.5- and 2.0-fold, respectively. MHC class I classical genes (A, B, and C) and F and G genes were modestly up-regulated in response to the higher dose of TSST-1 (100 μg/ml) by 6 h; these genes were expressed at 3 h but not by greater than twofold when compared to controls. There were also large numbers of hypothetical proteins and cell regulatory genes affected by TSST-1 at 100 μg/ml (data not shown). Finally, the gene for Toll-like receptor 3 (TLR-3) was up-regulated by fourfold at 3 h (data not shown); no other TLR genes were significantly up- or down-regulated by TSST-1 (100 μg/ml).
TABLE 1.
Predominant immune genes up-regulated by TSST-1 and S. aureus strains MNSM and MN8
| Gene product | Fold change from control
|
||||
|---|---|---|---|---|---|
| TSST-1 (100 μg/ml)
|
MNSM
|
MN8 (6 h) | |||
| 3 h | 6 h | 3 h | 6 h | ||
| CCL20 (MIP-3α) | —a | 168.9 | 207.9 | 477.7 | 274.4 |
| CXCL1 (GRO-α) | 16.0 | 84.4 | 26.0 | 17.1 | 21.1 |
| CXCL2 (GRO-β) | 4.9 | 13.0 | 13.9 | 13.0 | 27.9 |
| CXCL3 (GRO-γ) | 4.0 | 32.0 | 29.9 | 19.7 | 14.9 |
| CXCL14 (BRAK/bolekine) | 12.1 | 5.3 | — | — | — |
| Colony-stimulating factor 2 (granulocyte-macrophage) | — | — | 18.4 | 119.4 | — |
| Colony-stimulating factor 3 (granulocyte) | — | — | — | 11.3 | 4.3 |
| IL-1α | — | 3.2 | 4.6 | 7.0 | 7.5 |
| IL-1β | — | 2.0 | 2.1 | 4.0 | 6.1 |
| IL-6 | — | 2.3 | 8.6 | 4.9 | 2.8 |
| IL-8 | 8.0 | 48.5 | 11.3 | 13.9 | 64.0 |
| IL-15 | 4.3 | 9.8 | — | — | 2.1 |
| Lymphotoxin-β (TNF-β) | — | — | 2.1 | 4.6 | 5.3 |
| MHC class I-A | — | 2.1 | — | — | — |
| MHC class I-B | — | 2.3 | — | — | — |
| MHC class I-C | — | 3.2 | — | — | — |
| MHC class I-F | 2.1 | 6.1 | — | 2.3 | — |
| MHC class I-G | — | 2.0 | — | — | — |
| Pentaxin-related geneb | — | — | 27.9 | 32.0 | 16.0 |
| TNF-α | — | 2.5 | 7.5 | 10.6 | 2.5 |
—, no significant change from control. Significant genes were definitively present and statistically determined to be up- or down-regulated by ≥2-fold.
Related to C-reactive protein.
Cells of S. aureus strain MNSM (100 bacteria per epithelial cell) were compared to untreated controls at 3 and 6 h. The S. aureus MNSM data were confirmed by similar studies with TSS isolate MN8 at 6 h. S. aureus MNSM stimulated the up- or down-regulation of 341 and 410 genes by twofold or more compared to controls following 3 and 6 h of incubation with HVECs, respectively (Table 1). As observed with TSST-1 at 100 μg/ml, S. aureus MNSM also up-regulated chemokine and cytokine genes. The genes most up-regulated at 6 h were MIP-3α, up-regulated by 478-fold; CXCL1 (GRO-α), up-regulated by 17-fold; CXCL2 (GRO-β), up-regulated by 13-fold; and CXCL3 (GRO-γ), up-regulated by 20-fold. Cytokine genes, such as those coding for TNF-α and IL-1β, were also up-regulated at 6 h, with changes of 11- and 4-fold, respectively. No significant up-regulation of TLR genes following 3- and 6-h incubations of HVECs with S. aureus MNSM was observed; however, modest TLR-2 gene expression was detected with up-regulation of 1.2- and 1.5-fold at 3 and 6 h, respectively (data not shown). Similar to TSST-1 (100 μg/ml), an up-regulation of MHC class I F gene expression by 1.9- and 2.3-fold was produced following incubation with S. aureus MNSM for 3 and 6 h, respectively. The data concerning up-regulation of chemokine and cytokine genes were reproduced by evaluating a second mTSS S. aureus strain (MN8) following 6 h of incubation with HVECs; similar results were observed (Table 1). S. aureus MN8 significantly expressed the TLR-2 gene, which was up-regulated by TLR-2 at 6 h by 2.5-fold (data not shown). A complete listing of genes that were affected by TSST-1 at 10 and 100 μg/ml and by S. aureus strains MNSM and MN8 can be found at http://www.micab.umn.edu/faculty/Schlievert.html.
ELISAs of HVEC response to TSST-1, SEB, and SPE A.
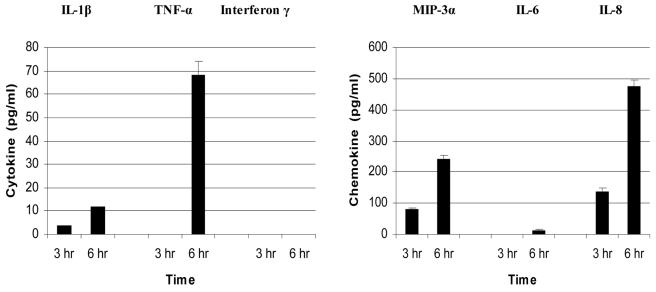
Representative chemokine and cytokine genes up-regulated following HVECs exposures to TSST-1 (100 μg/ml), as determined by microarray analysis, were confirmed by protein analysis with ELISAs. Specifically, cytokine (IL-1β, TNF-α, and gamma interferon) and chemokine (MIP-3α, IL-6, and IL-8) concentrations were determined. Both IL-1β and TNF-α were detected in the supernatant following incubation of HVECs with TSST-1 at 100 μg/ml for 6 h (12 and 68 pg/ml, respectively), which were generally consistent with the changes in gene expression at 6 h for these cytokines of 2.0- and 2.5-fold, respectively (Fig. 4). In contrast, gamma interferon was not detected in the supernatants following incubation of HVECs with TSST-1 (100 μg/ml) from 3 and 6 h, which was consistent with the lack of activation of this gene by TSST-1 compared to that of the control. Three chemokines (MIP-3α, IL-6, and IL-8) were tested and detected in the supernatants following incubation of HVECs with TSST-1 (100 μg/ml) for 6 h, with concentrations of 240, 15, and 475 pg/ml, respectively.
FIG. 4.
Production of cytokine and chemokine proteins by HVECs after exposure to 100 μg of TSST-1 per ml at 3 and 6 h as measured by ELISA. Control HVECs exposed to medium alone did not produce detectable cytokines and chemokines.
Additionally, HVECs were again incubated for 6 h with TSST-1 (100 μg/ml), ovalbumin (100 μg/ml), SEB (100 μg/ml), and SPE A (100 μg/ml), and then ELISAs were performed with supernatants for MIP-3α and IL-8 (Table 2). TSST-1 (100 μg/ml) induced similar production of the two chemokines, as was observed to be produced by HVECs incubated for 6 h with TSST-1 (100 μg/ml) in the microarray studies. Ovalbumin (100 μg/ml) did not stimulate production of MIP-3α and IL-8, suggesting the TSST-1 effect was not the result of simply having exposure to any exogenous foreign protein (Table 2). The other two superantigens at 100 μg/ml (SEB and SPE A) induced similar production of MIP-3α and IL-8 to TSST-1 (100 μg/ml), confirming those data (Table 2).
TABLE 2.
Chemokines produced by HVECs in response to TSST-1 and other superantigens and inhibition by dodecapeptide
| Protein tested (100 μg/ml) | Amt of chemokine produced (pg/ml)a
|
|
|---|---|---|
| MIP-3α | IL-8 | |
| None | 9.0 ± 1.5 | 16 ± 1.8 |
| Ovalbumin | 12 ± 1.8 | 36 ± 1.5 |
| TSST-1 | 320 ± 4 | 500 ± 33 |
| SEB | 470 ± 7 | 620 ± 7 |
| SPEA | 396 ± 8 | 470 ± 10 |
| Dodecapeptide only | 9 ± 1 | 15 ± 1 |
| TSST-1 only | 250 ± 7 | 550 ± 8 |
| TSST-1 + dodecapeptideb | 37 ± 2 | 40 ± 3 |
Values are means ± standard error.
Dodecapeptide concentration was 1,000 μg/ml.
Studies were also performed to further characterize the TSST-1 receptor on the HVECs that was involved in stimulation of chemokines and cytokines. In these studies, 10-fold excess amounts (in micrograms per microgram) of the dodecapeptide[Tyr-Asn-Lys-Lys-Lys-Ala-Thr-Val-Gln-Glu-Leu-Asp], which previously had been shown to inhibit both superantigenicity and mucosal transport by a TCR- and MHC II-independent mechanism (5, 60), were incubated with HVECs for 6 h and TSST-1 (100 μg/ml) and then ELISAs were performed to detect MIP-3α and IL-8. The dodecapeptide competitively inhibited TSST-1-induced release of both MIP-3α and IL-8 (Table 2).
ELISAs of HVEC response to S. aureus MNSM and MN8.
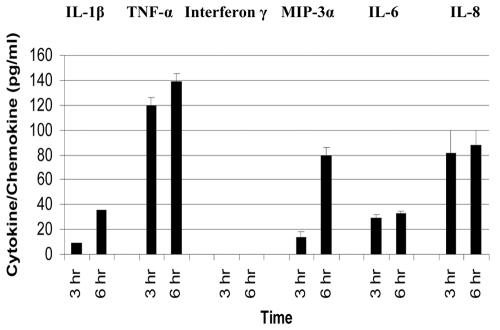
TNF-α and IL-1 concentrations of 139 and 36 pg/ml, respectively, were detected in the supernatants from experiments with HVECs incubated with S. aureus MNSM for 6 h (Fig. 5). The detection of TNF-α and IL-1β in the supernates at 6 h correlated with up-regulation of the genes for these cytokines by 11- and 4-fold, respectively (Table 1). Chemokines, MIP-3α, IL-8 and IL-6, were also detected in supernatants from experiments with HVECs incubated with S. aureus MNSM at 3 and 6 h. At 6 h, the concentrations of tested chemokines were 80 pg/ml for MIP-3α, 33 pg/ml for IL-6, and 88 pg/ml for IL-8. The amounts generally correlated with the up-regulation of the genes for these chemokines at 6 h (Table 1). However, the correlation of up-regulation (fold) of the MIP-3α gene with MIP-3α protein detected by ELISA is generally in the same direction, but the protein concentration was not as high as expected based on the microarray data. We have shown subsequently that proteases made by TSS S. aureus strains MNSM and MN8 degrade MIP-3α protein (data not shown), and this likely contributed to the lack of complete concordance between gene and ELISA data for MIP-3α and possibly other chemokines. Gamma interferon was not detected in the supernatants from experiments with HVECs incubated with S. aureus MNSM at either time point, again consistent with the lack of up-regulation of this gene. HVEC controls (without TSST-1 or S. aureus MNSM) showed no detectable cytokines or chemokines at 3 and 6 h. The lower limit of detection for all cytokines and chemokines was 4 to 16 pg/ml and depended on the cytokine or chemokine. Data obtained by ELISAs of S. aureus strain MN8 incubated for 6 h also confirmed the same chemokine and cytokine release by the HVECs (data not shown).
FIG. 5.
Production of cytokine and chemokine proteins by HVECs after exposure to 109 CFU of TSST-1-producing S. aureus MNSM per ml at 3 and 6 h as measured by ELISA. Control HVECs exposed to medium alone did not produce detectable cytokines and chemokines.
35S-labeled TSST-1 penetration of porcine vaginal tissue ex vivo in the presence of TSS S. aureus MN8.
Previous research has demonstrated that porcine vaginal tissue is an excellent model of human vaginal tissue in that the cellular architecture and permeability barriers are similar (13). Our studies also provided evidence that TSST-1 penetrated normal intact porcine vaginal mucosa poorly, suggesting that other factors, such as inflammation, may facilitate TSST-1 penetration (Table 3). The total amount of 35S-labeled TSST-1 penetrating across porcine vaginal tissue was increased significantly in the presence of viable TSS S. aureus MN8 (200 ng) over the 12-h period compared to that in untreated control tissue (36 ng). Killed MN8 cells also facilitated significant penetration of radiolabeled TSST-1 across the porcine vaginal tissue (115 ng) compared to that of untreated control tissue, but not to the same extent as viable S. aureus MN8 (Table 3). The data suggested that secreted factors from the viable organisms contributed significantly to the increased radiolabeled TSST-1 penetration. Histological examination of the tissue incubated with viable TSS S. aureus indicated desquamation of superficial epithelial cells and separation of the upper epithelium from the basement membrane region so as to create subepithelial blistering. This was accompanied by a marked subepithelial lymphocytic infiltrate (Fig. 6), which was surprising in an ex vivo specimen isolated from vascular perfusion.
TABLE 3.
Penetration of 35S-TSST-1 across intact porcine vaginal mucosa in the presence of TSS S. aureus
| Treatment | No. of tissue samples tested | Total toxin penetrating through tissuea (ng)a | Maximum flux (ng/cm2/min)a,b |
|---|---|---|---|
| MN8 | |||
| Live cells | 9 | 200 ± 18 | 2.68 ± 0.40 |
| Killed cells | 9 | 115 ± 20 | 1.66 ± 0.41 |
| Untreated controls | 7 | 36 ± 7.8 | 0.44 ± 0.01 |
Values are means ± standard error.
Maximum flux was defined as the greatest amount of radiolabeled TSST-1 penetrating across the entire porcine vaginal mucosa per square centimeter of tissue per minute.
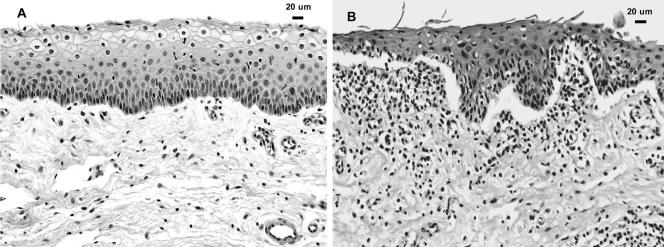
FIG. 6.
(A) Porcine vaginal mucosa incubated in control KFSM for 10 h. The tissue is well preserved, with little evidence of inflammatory cell infiltration or necrosis. (B) Porcine vaginal mucosa preincubated for 1 h with MN8 S. aureus live cells prior to topical application of radiolabeled TSST-1 and incubation for 10 h. Note the significant subepithelial lymphocytic infiltration and separation of the epithelium from the connective tissue at the basement membrane region and the desquamation of epithelial cells at the surface.
DISCUSSION
TSS is an acute-onset illness that typically begins with colonization of mucosal surfaces by TSST-1-producing S. aureus (14, 46, 58). The majority of S. aureus strains that cause mTSS are highly related and clonally derived from the same ancestral strain as the two used in our studies, MNSM and MN8 (45). Once S. aureus colonizes the mucosal surface, the organism must produce TSST-1 in order to cause TSS (8, 56). Although the precise in vivo factors that regulate TSST-1 production have not been determined, numerous in vitro studies have identified important environmental conditions that favor toxin production, such as 37°C, at least 2% O2 and 7% CO2, minimal glucose, protein, and pH near 7.0 (29, 53).
TSST-1 is recognized today as a member of the large superantigen family of proteins, which also includes staphylococcal enterotoxins (SEs) and SPEs (39). Superantigens cross-bridge MHC class II molecules on antigen-presenting cells with variable region β chains of TCRs (16, 35, 39, 41, 42). The consequence of superantigen cross-bridging of T cells with antigen-presenting cells is a massive systemic release of chemokines and cytokines from both the T cells, dominated by Th1 cell activation, and macrophages (16, 35, 39, 41, 42). The significant release of chemokines and cytokines results in the clinical features of TSS (1, 49, 51). A skewing toward Th1 responses might also be expected to cause concurrent suppression of Th2 cell responses, manifested in suppression of antibody production (1, 49, 51). A characteristic of women who develop mTSS is the failure to develop neutralizing antibody responses to TSST-1 (46).
In the case of mTSS, TSST-1 must first cross the vaginal mucosal barrier. Previous collaborative studies using ex vivo porcine vaginal tissue suggested that purified TSST-1 alone (concentrations ≤50 μg/ml) is unlikely to penetrate the vaginal mucosa in sufficient amounts to cause TSS (13). Porcine vaginal mucosa has a nonkeratinized stratified squamous epithelium and a superficial permeability barrier that is identical in structure and function to that of humans (61). The studies described in this article were initiated in an attempt to characterize the interaction between S. aureus or TSST-1 and HVECs. The results of our microarray studies of the HVEC response to S. aureus and TSST-1 at 6 h suggested that significant activation of the innate immune system occurred in part by an up-regulation of genes for chemokines and cytokines. Ex vivo studies with porcine vaginal tissue were also initiated to assess whether or not activation of the innate immune system occurs in response to TSS S. aureus and whether activation may facilitate TSST-1 transport. These studies demonstrated that incubation of the porcine vaginal tissue with TSS S. aureus resulted in both influx of lymphocytes into the tissue, consistent with innate immune system activation, and increased TSST-1 flux across the intact vaginal mucosa compared to that in untreated control tissue.
Initial studies determined that both TSS S. aureus MN8 and purified TSST-1 bound to immortalized, premenopausal HVECs. Although these HVECs were immortalized, they retained important properties (47) of normal human vaginal epithelial cells, such as growth inhibition by contact and synthesis of the appropriate cytokeratins. TSS S. aureus MN8 was shown to adhere to HVECs and replicate, suggesting interactions between staphylococcal surface adhesins with receptors on HVECs. There were also clear epithelial cell morphological changes induced by the high dose of TSST-1 (100 μg/ml) following 6 h of incubation at 37°C, consistent with toxin interaction with the cells.
The observation of approximately 5 × 104 TSST-1 receptors/HVEC is consistent with prior studies in which findings of similar receptor numbers (approximately 104/conjunctival epithelial cell) were obtained (31, 32). The two characterized receptors for TSST-1 on human cells are MHC class II molecules and TCRs (35, 39). Studies suggest only 5 to 7% of epithelial cells that have not been stimulated with cytokines are positive for MHC class II molecules; however, the number increases substantially following exposure to gamma interferon (2, 27). Potentially, MHC II molecules on the surface of the HVECs were receptors for TSST-1. However, this seems unlikely, since gamma interferon, which would lead to up-regulation of MHC class II genes, was not detected in the cell culture supernatants from any of the TSST-1 experiments, nor was there evidence by microarray analyses of significant up-regulation of MHC class II gene expression. Similarly, epithelial cells are unlikely to have TCR molecules, suggesting the toxin is binding to a novel receptor. The existence of a novel receptor for TSST-1 on the cell surface of epithelial cells has been previously suggested. Arad et al. (5) showed that all superantigens have the ability to stimulate cross-immunity against each other through interaction of a dodecapeptide region of the molecules with a host cell receptor not involving MHC II or TCR. Later, Shupp and colleagues suggested that this receptor was important for superantigen transcytosis across mucosal surfaces (60). In addition, the SEs, which are members of the superantigen family, have receptors on intestinal cells that lead to emesis and diarrhea associated with food poisoning; these biological effects are independent of superantigenicity (26, 54). Finally, we showed in the present studies with use of the same dodecapeptide that the peptide competitively inhibits TSST-1-induced chemokine release from HVECs.
Microarray analyses examined the vaginal epithelial genes that were differentially regulated by coculture of immortalized HVECs with purified TSST-1, S. aureus MNSM, and S. aureus MN8. The TSST-1 concentrations, 10 and 100 μg/ml, are in the approximate range of TSST-1 produced in vitro by S. aureus MNSM and MN8 grown in KSFM. The TSST-1 10-μg/ml dose caused minimal effects on gene expression. These data did not suggest a mechanism by which TSST-1 alone could alter the mucosal surface. However, extensive up-regulation of chemokine and cytokine genes by the higher dose of TSST-1 (100 μg/ml) was observed. Chemokines and cytokines released by the HVECs are likely to have the following primary effects: (i) recruitment and activation of cells involved in local inflammation and (ii) participation in activation of the adaptive immune system (6, 50).
The effect of TSS S. aureus MNSM and MN8 on epithelial cell gene expression demonstrated, that like the high dose of TSST-1, S. aureus caused significant up-regulation of chemokine and cytokine genes. Although real-time PCR was not performed to confirm the microarray data, the up-regulation of chemokine and cytokine genes resulted in increased production and secretion of both chemokine and cytokine proteins at 3 and 6 h, providing more definitive confirmation of the microarray findings. These data, demonstrating up-regulation of chemokine and cytokine genes in HVECs in response to TSS S. aureus, combined with the skewed Th1 response seen in mTSS, raise three important possibilities: (i) TSST-1 will be able to cross the mucosa as a result of inflammation induced by the presence of TSS S. aureus; (ii) the local manifestations of TSS, including development of the rash and mucosal sloughing, occur due to the initial chemokine and cytokine response of the epithelial cells and subsequent TSST-1 activation of CD4+ T cells and macrophages; and (iii) microbes, such as S. aureus, produce virulence factors, including superantigens, that preferentially promote Th1 adaptive immune responses at the expense of the host developing Th2 responses that would lead to development of neutralizing antibody responses. This skewed response is initiated by the microbial interaction with epithelial cells.
The major categories of genes that were up-regulated in response to S. aureus MNSM, S. aureus MN8, and TSST-1 (100 μg/ml) at both the 3- and 6-h time points were those that encode chemokines and cytokines that promote inflammation and have the potential to skew adaptive T-cell immune responses to Th1. For example, MIP-3α was induced by 208- and 478-fold in response to S. aureus MNSM at 3 and 6 h, respectively. MIP-3α is a C-C chemokine that is known to direct maturation specifically of Langerhan's cells into dendritic cells in the epidermis and to regulate T-lymphocyte development (12, 15). The CXC chemokine genes for CXCL1 (GRO-α), CXCL2 (GRO-β), and CXCL3 (GRO-γ), which have effects on neutrophil attraction and regulate T-cell development (28, 38), were highly significantly up-regulated (20- to 50-fold) in response to the organism at both 3 and 6 h. Many of the manifestations of TSS, including rash and hypotension, are consistent with a dominant Th1 response. Hypothetically, vaginal epithelial cell responses to TSS S. aureus may lead to the production of specific chemokines that skew the adaptive response to Th1. This has also recently been suggested in the case of skin exposure to certain allergens that mediate contact hypersensitivity (3, 7).
The reason for the high up-regulation of MIP-3α specifically in response to the TSS strains MNSM and MN8 and to TSST-1 is unknown. However, the chemokine is one of the most significant molecules released by damaged epithelium to signal adaptive immune responses (57). Other mucosal surface pathogenic microorganisms and agents that induce inflammation have been studied for induction of innate immunity, and there is abundant evidence for induction of chemokines such as IL-6 and IL-8 (4, 19-21). In addition, although not always seen in our studies, there are data to suggest that HVECs constitutively produce low levels of chemokines such as IL-6 and IL-8 (18).
An up-regulation of the chemokine CCL2 (MCP-1) gene was not observed by microarray analysis. CCL2 (MCP-1) is also known to be a monocyte chemoattractant protein (62). Previously, a study reported that transformation of vaginal epithelial cells with HPV E6/E7 genes suppressed expression of CCL2 (MCP-1), while not having effects on IL-6 and IL-8 gene expression (20). As the HVECs used in the present study were immortalized with the HPV E6/E7 genes, the suppressive effects of these genes may have prevented the up-regulation of the CCL2 gene.
A critical, yet unexplained, aspect of TSS is the mechanism(s) of TSST-1 penetration across the vaginal mucosal surface into the circulation, where T cells and macrophages are induced to produce cytokines, such as IL-1-β and TNF-α. These two cytokines are the primary causes of fever and hypotension in TSS, respectively (16, 39, 42). Examination of our microarray data, collectively, suggests that TSS S. aureus cells induce local inflammatory responses as a result of interaction with the epithelium, with release of potent chemokines and cytokines potentially resulting in the increased permeability in the mucosal barrier to facilitate TSST-1 penetration. The microarray studies led to examination of the response of ex vivo porcine vaginal tissue to TSS S. aureus. The observation that TSS S. aureus caused an influx of lymphocytes into the upper epithelium of the ex vivo porcine tissue is consistent with innate immune system activation. The accompanying tissue damage, including cell desquamation and epithelial blistering, likely facilitated the significant increase in TSST-1 penetration across the intact tissue that was measured. This tissue provides an excellent model for the study of TSST-1 penetration across the mucosal barrier. The changes seen in the tissue, loss of surface cells and separation of the epithelium at the basement membrane, are both consistent with changes seen on autopsy of TSS patients (34). The histologic effects were due primarily to secreted virulence factors, since heat-killed TSS S. aureus did not have as large an effect on radiolabeled TSST-1 penetration of the epithelium as viable cells.
Staphylococcal exoproteins may provoke inflammatory responses. Hemolysins, such as α hemolysin, are known to induce inflammation and dermonecrosis of the skin (16). The strains used in this study, MNSM and MN8, were determined to produce small amounts of α hemolysin. S. aureus strains that grow on mucosal surfaces usually make low levels of α hemolysin, sufficient to induce local low-level inflammation, but insufficient to induce massive inflammation and significant epithelial necrosis (55). It is also likely that the high levels of TSST-1 that can be made in biofilms (1 to 1.5 mg/ml) may contribute to induction of inflammation and facilitate its own penetration across the mucosa (52). In a prior study that showed TSST-1 did not penetrate intact porcine tissue well, the highest concentration of TSST-1 studied was 50 μg/ml, which may be insufficient to induce inflammation (13).
One of the major pathways to activation of the innate immune system by gram-positive bacteria is through TLRs, most notably TLR-2. TLR-2 is primarily activated by peptidoglycan and lipoteichoic acids (9). Our microarray studies showed modest and variable up-regulation of TLR genes, with TLR-2 being most affected with either S. aureus MNSM or MN 8. TLR-2 has been shown to be constitutively expressed by immortalized HVECs and would be expected to be activated by bacterial cells (19). Therefore, this TLR may participate in activation of HVECs by TSS S. aureus. In contrast, TSST-1 (100 μg/ml) caused variable up-regulation of the gene for TLR-3 but not other TLR genes. The role of this TLR in TSST-1 activation of HVECs is unclear.
A potentially interesting and surprising effect of TSST-1 (100 μg/ml) on the HVECs was a modest but consistent up-regulation of genes that affected MHC class I types. MHC class I molecules are responsible for endogenous processing of antigens and presentation of antigenic peptides on the cell surface (23, 24), with MHC class I gene up-regulation by epithelial cells enhanced by gamma interferon (2, 27). The significance of our data is unknown and awaits further study. MHC class II genes were not up-regulated in our studies by either TSST-1 (100 μg/ml) or TSS S. aureus strains.
In sum, our studies demonstrate that TSST-1 and TSS S. aureus interact with HVECs to cause significant innate immunity gene activation. Of particular importance is the observation that the most affected genes in HVECs were those that lead to chemokine and cytokine synthesis in response to S. aureus. Histologically, significant subepithelial lymphocytic infiltration and separation of the epithelium from the connective tissue at the basement membrane region, with concomitant desquamation of epithelial cells, were noted when intact tissue was preincubated with MN8 live cells prior to topical application of radiolabeled TSST-1. Hypothetically, the inflammatory response of vaginal epithelial cells to S. aureus triggers immune activation cascades that result in TSST-1 penetration across the vaginal mucosa.
Acknowledgments
This work was supported by National Institutes of Health grant HL36611, the Minnesota Medical Foundation, The Procter & Gamble Company (grants to P.M.S., K.A., and C.A.S.), and The University of Iowa Foundation (to K.A.). M.L.P. was supported by National Institutes of Health training grant T32-HD07381. Animal experimentation was undertaken with funding to P.M.S. from the Minnesota Medical Foundation with Institutional Animal Care and Use Committee approval.
We thank Diane Maher and Peter Southern, Department of Microbiology, University of Minnesota, for assistance with microscopy.
Editor: A. D. O'Brien
REFERENCES
- 1.Abbas, A. K., K. M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383:787-793. [DOI] [PubMed] [Google Scholar]
- 2.Albanesi, C., A. Cavani, and G. Girolomoni. 1998. Interferon-gamma-stimulated human keratinocytes express the genes necessary for the production of peptide-loaded MHC class II molecules. J. Investig. Dermatol. 110:138-142. [DOI] [PubMed] [Google Scholar]
- 3.Albanesi, C., C. Scarponi, S. Sebastiani, A. Cavani, M. Federici, S. Sozzani, and G. Girolomoni. 2001. A cytokine-to-chemokine axis between T lymphocytes and keratinocytes can favor Th1 cell accumulation in chronic inflammatory skin diseases. J. Leukoc. Biol. 70:617-623. [PubMed] [Google Scholar]
- 4.Anderson, D. J., J. A. Politch, L. D. Tucker, R. Fichorova, F. Haimovici, R. E. Tuomala, and K. H. Mayer. 1998. Quantitation of mediators of inflammation and immunity in genital tract secretions and their relevance to HIV type 1 transmission. AIDS Res. Hum. Retrovir. 14(Suppl. 1):S43-S49. [PubMed] [Google Scholar]
- 5.Arad, G., R. Levy, D. Hillman, and R. Kaempfer. 2000. Superantigen antagonist protects against lethal shock and defines a new domain for T-cell activation. Nat. Med. 6:414-421. [DOI] [PubMed] [Google Scholar]
- 6.Baggiolini, M. 1998. Chemokines and leukocyte traffic. Nature 392:565-568. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee, G., A. Damodaran, N. Devi, K. Dharmalingam, and G. Raman. 2004. Role of keratinocytes in antigen presentation and polarization of human T lymphocytes. Scand. J. Immunol. 59:385-394. [DOI] [PubMed] [Google Scholar]
- 8.Bergdoll, M. S., B. A. Crass, R. F. Reiser, R. N. Robbins, and J. P. Davis. 1981. A new staphylococcal enterotoxin, enterotoxin F, associated with toxic-shock-syndrome Staphylococcus aureus isolates. Lancet i:1017-1021. [DOI] [PubMed] [Google Scholar]
- 9.Beutler, B., K. Hoebe, X. Du, and R. J. Ulevitch. 2003. How we detect microbes and respond to them: the Toll-like receptors and their transducers. J. Leukoc. Biol. 74:479-485. [DOI] [PubMed] [Google Scholar]
- 10.Blomster-Hautamaa, D. A., and P. M. Schlievert. 1988. Preparation of toxic shock syndrome toxin-1. Methods Enzymol. 165:37-43. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention.1999. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus—Minnesota and North Dakota, 1997-1999. JAMA 282:1123-1125. [PubMed] [Google Scholar]
- 12.Charbonnier, A. S., N. Kohrgruber, E. Kriehuber, G. Stingl, A. Rot, and D. Maurer. 1999. Macrophage inflammatory protein 3alpha is involved in the constitutive trafficking of epidermal langerhans cells. J. Exp. Med. 190:1755-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis, C. C., M. J. Kremer, P. M. Schlievert, and C. A. Squier. 2003. Penetration of toxic shock syndrome toxin-1 across porcine vaginal mucosa ex vivo: permeability characteristics, toxin distribution, and tissue damage. Am. J. Obstet. Gynecol. 189:1785-1791. [DOI] [PubMed] [Google Scholar]
- 14.Davis, J. P., P. J. Chesney, P. J. Wand, and M. LaVenture. 1980. Toxic-shock syndrome: epidemiologic features, recurrence, risk factors, and prevention. N. Engl. J. Med. 303:1429-1435. [DOI] [PubMed] [Google Scholar]
- 15.Dieu-Nosjean, M. C., C. Massacrier, B. Homey, B. Vanbervliet, J. J. Pin, A. Vicari, S. Lebecque, C. Dezutter-Dambuyant, D. Schmitt, A. Zlotnik, and C. Caux. 2000. Macrophage inflammatory protein 3alpha is expressed at inflamed epithelial surfaces and is the most potent chemokine known in attracting Langerhans cell precursors. J. Exp. Med. 192:705-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinges, M. M., P. M. Orwin, and P. M. Schlievert. 2000. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 13:16-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fey, P. D., B. Said-Salim, M. E. Rupp, S. H. Hinrichs, D. J. Boxrud, C. C. Davis, B. N. Kreiswirth, and P. M. Schlievert. 2003. Comparative molecular analysis of community- or hospital-acquired methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 47:196-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fichorova, R. N., and D. J. Anderson. 1999. Differential expression of immunobiological mediators by immortalized human cervical and vaginal epithelial cells. Biol. Reprod. 60:508-514. [DOI] [PubMed] [Google Scholar]
- 19.Fichorova, R. N., A. O. Cronin, E. Lien, D. J. Anderson, and R. R. Ingalls. 2002. Response to Neisseria gonorrhoeae by cervicovaginal epithelial cells occurs in the absence of toll-like receptor 4-mediated signaling. J. Immunol. 168:2424-2432. [DOI] [PubMed] [Google Scholar]
- 20.Fichorova, R. N., P. J. Desai, F. C. Gibson III, and C. A. Genco. 2001. Distinct proinflammatory host responses to Neisseria gonorrhoeae infection in immortalized human cervical and vaginal epithelial cells. Infect. Immun. 69:5840-5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fichorova, R. N., L. D. Tucker, and D. J. Anderson. 2001. The molecular basis of nonoxynol-9-induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. J. Infect. Dis. 184:418-428. [DOI] [PubMed] [Google Scholar]
- 22.Fields, B. A., E. L. Malchiodi, H. Li, X. Ysern, C. V. Stauffacher, P. M. Schlievert, K. Karjalainen, and R. A. Mariuzza. 1996. Crystal structure of a T-cell receptor beta-chain complexed with a superantigen. Nature 384:188-192. [DOI] [PubMed] [Google Scholar]
- 23.Gromme, M., and J. Neefjes. 2002. Antigen degradation or presentation by MHC class I molecules via classical and non-classical pathways. Mol. Immunol. 39:181-202. [DOI] [PubMed] [Google Scholar]
- 24.Gromme, M., F. G. Uytdehaag, H. Janssen, J. Calafat, R. S. van Binnendijk, M. J. Kenter, A. Tulp, D. Verwoerd, and J. Neefjes. 1999. Recycling MHC class I molecules and endosomal peptide loading. Proc. Natl. Acad. Sci. USA 96:10326-10331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halbert, C. L., G. W. Demers, and D. A. Galloway. 1992. The E6 and E7 genes of human papillomavirus type 6 have weak immortalizing activity in human epithelial cells. J. Virol. 66:2125-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris, T. O., D. Grossman, J. W. Kappler, P. Marrack, R. R. Rich, and M. J. Betley. 1993. Lack of complete correlation between emetic and T-cell-stimulatory activities of staphylococcal enterotoxins. Infect. Immun. 61:3175-3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hasseus, B., M. Jontell, G. Bergenholtz, and U. I. Dahlgren. 2004. T-cell costimulatory capacity of oral and skin epithelial cells in vitro: presence of suppressive activity in supernatants from skin epithelial cell cultures. Eur. J. Oral Sci. 112:48-54. [DOI] [PubMed] [Google Scholar]
- 28.Homey, B., A. Muller, and A. Zlotnik. 2002. Chemokines: agents for the immunotherapy of cancer? Nat. Rev. Immunol. 2:175-184. [DOI] [PubMed] [Google Scholar]
- 29.Kass, E. H., M. I. Kendrick, Y. C. Tsai, and J. Parsonnet. 1987. Interaction of magnesium ion, oxygen tension, and temperature in the production of toxic-shock-syndrome toxin-1 by Staphylococcus aureus. J. Infect. Dis. 155:812-815. [DOI] [PubMed] [Google Scholar]
- 30.Kiyono, T., S. A. Foster, J. I. Koop, J. K. McDougall, D. A. Galloway, and A. J. Klingelhutz. 1998. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 396:84-88. [DOI] [PubMed] [Google Scholar]
- 31.Kushnaryov, V. M., M. S. Bergdoll, H. S. MacDonald, J. Vellinga, and R. Reiser. 1984. Study of staphylococcal toxic shock syndrome toxin in human epithelial cell culture. J. Infect. Dis. 150:535-545. [DOI] [PubMed] [Google Scholar]
- 32.Kushnaryov, V. M., H. S. MacDonald, R. Reiser, and M. S. Bergdoll. 1984. Staphylococcal toxic shock toxin specifically binds to cultured human epithelial cells and is rapidly internalized. Infect. Immun. 45:566-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 34.Larkin, S. M., D. N. Williams, M. T. Osterholm, R. W. Tofte, and Z. Posalaky. 1982. Toxic shock syndrome: clinical, laboratory, and pathologic findings in nine fatal cases. Ann. Intern. Med. 96:858-864. [DOI] [PubMed] [Google Scholar]
- 35.Li, H., A. Llera, E. L. Malchiodi, and R. A. Mariuzza. 1999. The structural basis of T cell activation by superantigens. Annu. Rev. Immunol. 17:435-466. [DOI] [PubMed] [Google Scholar]
- 36.Li, H., A. Llera, D. Tsuchiya, L. Leder, X. Ysern, P. M. Schlievert, K. Karjalainen, and R. A. Mariuzza. 1998. Three-dimensional structure of the complex between a T cell receptor beta chain and the superantigen staphylococcal enterotoxin B. Immunity 9:807-816. [DOI] [PubMed] [Google Scholar]
- 37.Li, Y., H. Li, N. Dimasi, J. K. McCormick, R. Martin, P. Schuck, P. M. Schlievert, and R. A. Mariuzza. 2001. Crystal structure of a superantigen bound to the high-affinity, zinc-dependent site on MHC class II. Immunity 14:93-104. [DOI] [PubMed] [Google Scholar]
- 38.Luther, S. A., and J. G. Cyster. 2001. Chemokines as regulators of T cell differentiation. Nat. Immunol. 2:102-107. [DOI] [PubMed] [Google Scholar]
- 39.Marrack, P., and J. Kappler. 1990. The staphylococcal enterotoxins and their relatives. Science 248:705-711. [DOI] [PubMed] [Google Scholar]
- 40.McCormick, J. K., and P. M. Schlievert. 2000. Toxins and superantigens of group A streptococci, p. 43-52. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, D.C.
- 41.McCormick, J. K., T. J. Tripp, A. S. Llera, E. J. Sundberg, M. M. Dinges, R. A. Mariuzza, and P. M. Schlievert. 2003. Functional analysis of the TCR binding domain of toxic shock syndrome toxin-1 predicts further diversity in MHC class II/superantigen/TCR ternary complexes. J. Immunol. 171:1385-1392. [DOI] [PubMed] [Google Scholar]
- 42.McCormick, J. K., J. M. Yarwood, and P. M. Schlievert. 2001. Toxic shock syndrome and bacterial superantigens: an update. Annu. Rev. Microbiol. 55:77-104. [DOI] [PubMed] [Google Scholar]
- 43.Murray, D. L., C. A. Earhart, D. T. Mitchell, D. H. Ohlendorf, R. P. Novick, and P. M. Schlievert. 1996. Localization of biologically important regions on toxic shock syndrome toxin 1. Infect. Immun. 64:371-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murray, D. L., G. S. Prasad, C. A. Earhart, B. A. Leonard, B. N. Kreiswirth, R. P. Novick, D. H. Ohlendorf, and P. M. Schlievert. 1994. Immunobiologic and biochemical properties of mutants of toxic shock syndrome toxin-1. J. Immunol. 152:87-95. [PubMed] [Google Scholar]
- 45.Musser, J. M., P. M. Schlievert, A. W. Chow, P. Ewan, B. N. Kreiswirth, V. T. Rosdahl, A. S. Naidu, W. Witte, and R. K. Selander. 1990. A single clone of Staphylococcus aureus causes the majority of cases of toxic shock syndrome. Proc. Natl. Acad. Sci. USA 87:225-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Osterholm, M. T., J. P. Davis, R. W. Gibson, J. S. Mandel, L. A. Wintermeyer, C. M. Helms, J. C. Forfang, J. Rondeau, and J. M. Vergeront. 1982. Tri-state toxic-state syndrome study. I. Epidemiologic findings. J. Infect. Dis. 145:431-440. [DOI] [PubMed] [Google Scholar]
- 47.Rajan, N., D. L. Pruden, H. Kaznari, Q. Cao, B. E. Anderson, J. L. Duncan, and A. J. Schaeffer. 2000. Characterization of an immortalized human vaginal epithelial cell line. J. Urol. 163:616-622. [PubMed] [Google Scholar]
- 48.Reingold, A. L., N. T. Hargrett, B. B. Dan, K. N. Shands, B. Y. Strickland, and C. V. Broome. 1982. Nonmenstrual toxic shock syndrome: a review of 130 cases. Ann. Intern. Med. 96:871-874. [DOI] [PubMed] [Google Scholar]
- 49.Rogge, L. 2002. A genomic view of helper T cell subsets. Ann. N. Y. Acad. Sci. 975:57-67. [DOI] [PubMed] [Google Scholar]
- 50.Rollins, B. J. 1997. Chemokines. Blood 90:909-928. [PubMed] [Google Scholar]
- 51.Romagnani, S. 1994. Lymphokine production by human T cells in disease states. Annu. Rev. Immunol. 12:227-257. [DOI] [PubMed] [Google Scholar]
- 52.Schlievert, P. M. 1996. Effect of Merocel vaginal sponge on growth of Staphylococcus aureus and production of toxic shock syndrome-associated toxins. J. Am. Coll. Surg. 183:19-24. [PubMed] [Google Scholar]
- 53.Schlievert, P. M., and D. A. Blomster. 1983. Production of staphylococcal pyrogenic exotoxin type C: influence of physical and chemical factors. J. Infect. Dis. 147:236-242. [DOI] [PubMed] [Google Scholar]
- 54.Schlievert, P. M., L. M. Jablonski, M. Roggiani, I. Sadler, S. Callantine, D. T. Mitchell, D. H. Ohlendorf, and G. A. Bohach. 2000. Pyrogenic toxin superantigen site specificity in toxic shock syndrome and food poisoning in animals. Infect. Immun. 68:3630-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schlievert, P. M., M. T. Osterholm, J. A. Kelly, and R. D. Nishimura. 1982. Toxin and enzyme characterization of Staphylococcus aureus isolates from patients with and without toxic shock syndrome. Ann. Intern. Med. 96:937-940. [DOI] [PubMed] [Google Scholar]
- 56.Schlievert, P. M., K. N. Shands, B. B. Dan, G. P. Schmid, and R. D. Nishimura. 1981. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J. Infect. Dis. 143:509-516. [DOI] [PubMed] [Google Scholar]
- 57.Schmuth, M., S. Neyer, C. Rainer, A. Grassegger, P. Fritsch, N. Romani, and C. Heufler. 2002. Expression of the C-C chemokine MIP-3 alpha/CCL20 in human epidermis with impaired permeability barrier function. Exp. Dermatol. 11:135-142. [DOI] [PubMed] [Google Scholar]
- 58.Shands, K. N., G. P. Schmid, B. B. Dan, D. Blum, R. J. Guidotti, N. T. Hargrett, R. L. Anderson, D. L. Hill, C. V. Broome, J. D. Band, and D. W. Fraser. 1980. Toxic-shock syndrome in menstruating women: association with tampon use and Staphylococcus aureus and clinical features in 52 cases. N. Engl. J. Med. 303:1436-1442. [DOI] [PubMed] [Google Scholar]
- 59.Shehin, S. E., M. B. Jones, A. E. Hochwalt, F. C. Sarbaugh, and S. Nunn. 2003. Clinical safety-in-use study of a new tampon design. Infect. Dis. Obstet. Gynecol. 11:89-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shupp, J. W., M. Jett, and C. H. Pontzer. 2002. Identification of a transcytosis epitope on staphylococcal enterotoxins. Infect. Immun. 70:2178-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thompson, I. O., P. van der Bijl, C. W. van Wyk, and A. D. van Eyk. 2001. A comparative light-microscopic, electron-microscopic and chemical study of human vaginal and buccal epithelium. Arch. Oral Biol. 46:1091-1098. [DOI] [PubMed] [Google Scholar]
- 62.von Aulock, S., S. Morath, L. Hareng, S. Knapp, K. P. van Kessel, J. A. van Strijp, and T. Hartung. 2003. Lipoteichoic acid from Staphylococcus aureus is a potent stimulus for neutrophil recruitment. Immunobiology 208:413-422. [DOI] [PubMed] [Google Scholar]
- 63.Yarwood, J. M., J. K. McCormick, M. L. Paustian, V. Kapur, and P. M. Schlievert. 2002. Repression of the Staphylococcus aureus accessory gene regulator in serum and in vivo. J. Bacteriol. 184:1095-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yarwood, J. M., and P. M. Schlievert. 2000. Oxygen and carbon dioxide regulation of toxic shock syndrome toxin 1 production by Staphylococcus aureus MN8. J. Clin. Microbiol. 38:1797-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]