Abstract
Background.
In the northeastern United States, tick-borne diseases are a major public health concern. In controlled studies, a single springtime application of acaricide has been shown to kill 68%–100% of ticks. Although public health authorities recommend use of acaricides to control tick populations in yards, the effectiveness of these pesticides to prevent tick bites or human tick-borne diseases is unknown.
Methods.
We conducted a 2-year, randomized, double-blinded, placebo-controlled trial among 2727 households in 3 northeastern states. Households received a single springtime barrier application of bifenthrin or water according to recommended practices. Tick drags were conducted 3–4 weeks after treatment on 10% of properties. Information on human–tick encounters and tick-borne diseases was collected through monthly surveys; reports of illness were validated by medical record review.
Results.
Although the abundance of questing ticks was significantly lower (63%) on acaricide-treated properties, there was no difference between treatment groups in human-tick encounters, self-reported tick-borne diseases, or medical-record-validated tick-borne diseases.
Conclusions.
Used as recommended, acaricide barrier sprays do not significantly reduce the household risk of tick exposure or incidence of tick-borne disease. Measures for preventing tick-borne diseases should be evaluated against human outcomes to confirm effectiveness.
Keywords: Lyme disease, tick-borne diseases, ticks, prevention, pesticide, acaricide, humans
Lyme disease is the most common tick-borne disease in the United States, resulting from an estimated 300 000 infections in 2008 [1]. The etiologic agent, Borrelia burgdorferi, is transmitted in the northeastern United States through the bite of infected blacklegged ticks (Ixodes scapularis Say). Less common but potentially more-serious diseases associated with the bite of these same ticks include anaplasmosis, babesiosis, and disease due to a Powassan virus variant. In the northeastern United States, exposure to I. scapularis ticks is considered highest around the home (ie, peridomestically), owing to land-use factors and population density [2-5].
Prevention of tick-borne disease generally relies on the action of individuals or households. Personal protection strategies (eg, tick checks and repellent use) are simple measures that can reduce the incidence of tick bite but require daily vigilance [6]. Household-level measures require less frequent practice and can include landscaping and use of acaricides to control tick populations. In small studies, a single, springtime application of acaricide on residential properties has been shown to kill 68%–100% of host-seeking I. scapularis nymphs [2, 5, 7-9]. Some public health authorities recommend the use of residential acaricides to help control ticks and prevent tick-borne diseases [10-13], and in a survey of 2800 Connecticut households, 29% of homeowners reported having their property sprayed to control ticks [14].
While acaricides have been shown to reduce tick populations, their usefulness for protecting humans from Lyme or other tick-borne diseases has not been demonstrated. We conducted a double-blinded, randomized, placebo-controlled trial to evaluate the effectiveness of a single, residential, barrier acaricide application to prevent tick encounters and tick-borne diseases. This study was undertaken through TickNET [15], a collaboration between the Centers for Disease Control and Prevention (CDC) and emerging infections programs in Connecticut, Maryland, and New York.
METHODS
This study was conducted in 8 counties with a high incidence of reported Lyme disease: Fairfield, Litchfield, and New Haven counties in Connecticut; Baltimore, Harford, Howard, and Carroll counties in Maryland; and Dutchess County in New York. Households were recruited to participate for 1 season (April–December), in either 2011 or 2012, through mailings and public flyers. Mailings were targeted using a commercial marketing database to identify addresses of freestanding, single-family homes housing ≥2 people. Addresses were further screened using geographic information systems (GIS) technology to identify those meeting the following property-level eligibility criteria: (1) location ≥100 feet from bodies of water (as required by New York State law for treatment with bifenthrin) and (2) property size between 0.5 and 5 acres (used as a surrogate for risk of exposure to blacklegged ticks). All participating households were rescreened for these property eligibility criteria at enrollment. In addition, households were excluded for the following reasons: (1) having an intact deer fence (≥5 feet high) around the entire perimeter of their property or (2) use of products having acaricidal activity on their property for any reason (eg, mosquito control) during the previous summer. Last, we restricted participating households to those with ≥2 inhabitants (in 2011) and ≥3 inhabitants (in 2012) to increase the efficiency of the study with respect to the human outcomes.
One representative adult (≥18 years of age and with the authority to allow acaricide application to the property) in each household was asked to provide written consent to participate as the head of household and to respond to study surveys regarding all household members. At enrollment, the head of household completed an introductory survey with questions regarding demographic characteristics and select property characteristics (eg, property size, amount of property composed of woods, and presence of vegetable gardens, flower gardens, and compost piles). Participants received compensation of up to $40 for their time. The protocol for this study was reviewed and approved by ethics committees at the CDC, Yale University, the Connecticut Department of Public Health, the Maryland Department of Health and Mental Hygiene, and the New York State Department of Health.
Intervention
Within each state, households were randomly assigned with equal probability to receive treatment with either acaricide or placebo. Households and study coordinators were blinded to the treatment type received, until the end of the study. Pest control operators were also blinded to the treatment, when possible, under state-based pesticide regulations for labeling, storage, and transport. The households treated with acaricide received an application of Talstar Professional (FMC, Philadelphia, Pennsylvania) containing bifenthrin (2-methylbiphenyl-3-ylmethyl [Z]-[1RS,3RS]-3-[2-chloro-3,3,3-trifluoroprop-l-enyl]-2,2-dimethylcyclopropanecarboxylate), a synthetic pyrethroid shown to reduce I. scapularis populations by several orders of magnitude for up to 41 weeks [7]; the households treated with placebo received an application of water. Acaricide treatments were applied according to the manufacturer’s label for Talstar Professional (0.5–1.0 fluid ounces per 1000 square feet). All acaricide and placebo treatments were applied using the same methods by trained, licensed pest control operators (PCOs), using truck-mounted or backpack applicators or a combination of both. According to their standard practice, the pest control company selected the application method that was most appropriate and available for each yard. PCOs applied acaricide or placebo 10 feet into the lawn turf and 20 feet into the brushy or wooded edge of the property. Treatments were applied to all accessible perimeters (including front, back, and side yards). Acaricide and placebo treatments occurred on the same days, with applications taking place throughout the day, beginning in late April to early May. To precede the peak of nymphal I. scapularis activity in all states, treatments were performed 1–2 weeks earlier in Maryland than in Connecticut and New York, owing to an earlier peak in nymphal tick density in more-southern climes [16].
We aimed to enroll 1617 households, accounting for an expected attrition frequency of 10% and an in-house correlation for tick-borne disease of 1.5. This sample size enabled the study with 80% power and 95% significance to detect a 50% reduction in disease incidence (from 3% to 1.5%) in acaricide-treated households as compared to placebo-recipient households.
Entomologic Outcomes
To evaluate for an impact on nymphal tick populations, tick sampling was conducted 3–4 weeks after treatment by study staff blinded to the treatment group. Staff were trained and supervised by experienced entomologists under a common protocol. Sampling was conducted or supervised by the same study staff in both years. Ticks were collected by dragging/flagging [3, 17] on a 10% random sample of properties, with approximately 25 properties per treatment group per state sampled, for a total of 150 properties per year. A new drag was used at each location so that no carry-over of acaricide occurred. Treatment-recipient and placebo-recipient properties selected for tick sampling were stratified across days of the week, ensuring that some of both were sampled on any given day. Tick density (defined as the number of ticks collected per unit of time) was used as the outcome measure [17], with up to forty 30-second samples conducted (20 sampling minutes) on each property. Tick sampling was performed in and around areas where treatment was applied, including wooded and brushy vegetation, leaf litter, ecotones, and lawn edges [3]. Study staff specifically sampled up to three meters in each direction (into the woods and toward the lawn), which includes the areas that were treated.
Sampled ticks were identified to the species and stage levels, preserved, and sent to the CDC’s Division of Vector-Borne Diseases (Fort Collins, Colorado) for polymerase chain reaction (PCR) testing for the following tick-borne pathogens: Anaplasma phagocytophilum, Babesia microti, and B. burgdorferi. Testing results are reported elsewhere [18]. In addition, tick phenology data was collected weekly from May through July at 3 forested public areas in Connecticut and 2 areas in New York.
Human Outcomes
Heads of household were surveyed electronically monthly for 4 months after treatment to ascertain the number of ticks found attached to and crawling on household members. Study personnel administered surveys by phone to all household members who were not able to complete the survey electronically. A final telephone survey was administered to all heads of household 5–6 months after treatment to capture the number of self-reported tick-borne diseases in households and data regarding the effectiveness of blinding. Information regarding tick-borne disease was collected from the participating heads of household and the affected household members, including onset date, clinical symptoms, prescribed treatment, and diagnosing provider. Reports of illness were validated by medical record review, whenever possible, and included in the analysis when the onset date was at least 3 days after property treatment. These cases were further categorized as “verified” if, after medical record review, they met the most current national surveillance case definition for their respective disease [19], as judged by a panel of 3 study team members, including 2 physicians.
Statistical Analyses
Data were analyzed at the household level according to an intention-to-treat strategy [20]. Information regarding household and property characteristics, study outcome measures (ticks found crawling, ticks found attached, and tick-borne disease), and blinding were analyzed for differences between treatment groups using χ2 tests, t tests (for means), and the Kolmogorov–Smirnoff test (for tick count data). Logistic regression was used to evaluate all household and property characteristics as potential confounders or effect modifiers of the relationship between ticks found crawling or attached and treatment group. To evaluate the consistency of the treatment for vector control over space and time, entomologic measures (tick density comparisons) were evaluated in aggregate and by state and year, using a 1-sided Wilcoxon rank sum test. All randomizations and analyses were performed using SAS 9.3 (Cary, North Carolina).
RESULTS
Among the 4459 households assessed for study eligibility, 1397 did not meet inclusion criteria, 152 declined to participate, and 178 were not enrolled for other reasons (eg, a signed consent form was not returned). A total of 2727 households were enrolled into the study. In 2011, 1615 participating households represented 5010 household members; in 2012, 1112 participating households represented 4407 household members. About half (1362 [49.9%]) of these households were randomly assigned to the acaricide group, with the remaining 1365 households randomly assigned to the placebo group (Figure 1). There was no difference between households treated with acaricide and those treated with placebo with respect to household size, household demographic characteristics, or property characteristics (Table 1). Altogether, 2609 enrolled households (95.7%; 1312 in the acaricide group and 1297 in the placebo group) received a property treatment.
Figure 1.
Enrollment and completion of households in the study in 2011 and 2012.
Table 1.
Household Demographic and Property Characteristics, by Treatment Group
| Characteristics | Acaricide (n = 1362) |
Placebo (n = 1365) |
P Value |
|---|---|---|---|
| Residents, no. | |||
| Overall | 4704 | 4713 | NS |
| Mean | 3.45 | 3.45 | NS |
| Resident age, y, mean | 39.2 | 39.3 | NS |
| Children | 598 (44) | 597 (44) | NS |
| Pets | 778 (57) | 778 (57) | NS |
| White race, residents, no. (%) | 1268 (93) | 1266 (93) | NS |
| Income >$70 000 | 931 (68) | 893 (65) | NS |
| At least some college, residents, no. (%) | 1183 (87) | 1173 (86) | NS |
| Property size, acres | |||
| <1 | 525 (39) | 557 (41) | NS |
| 1–2 | 463 (34) | 433 (32) | |
| >2 | 369 (27) | 373 (27) | |
| Yard borders woodsa | 698 (87) | 706 (87) | NS |
| Woods on property | 1183 (87) | 1147 (84) | NS |
| Vegetable garden | 465 (34) | 450 (33) | NS |
| Flower garden | 932 (68) | 909 (67) | NS |
| Compost pile | 414 (30) | 368 (27) | NS |
| Log pile | 832 (61) | 823 (60) | NS |
| Bird feeder | 623 (46) | 603 (44) | NS |
| Fencing | 521 (38) | 491 (36) | NS |
| Stone walls | 658 (48) | 687 (50) | NS |
| Kids equipment | 497 (37) | 502 (37) | NS |
| Outside dining area | 224 (16) | 239 (18) | NS |
| Outside sitting area | 540 (40) | 533 (39) | NS |
| Lawn sport area | 400 (29) | 401(29) | NS |
Data are no. (%) of households, unless otherwise indicated.
Abbreviation: NS, not significant.
This question was only asked in 2011; the denominators are 799 for the acaricide group and 816 for the placebo group.
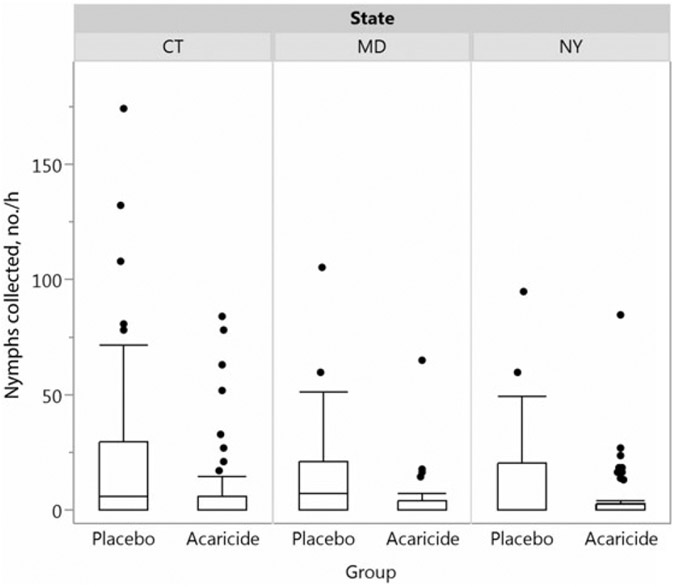
In total, 118 households (4.3%; 50 in the acaricide group and 68 in the placebo group) were withdrawn from the study before treatment. The majority of these (66.9%) were withdrawn due to the discovery of water on the property by PCOs at the scheduled time of treatment. Eleven households (6 in the acaricide group and 5 in the placebo group) were withdrawn by request in the month following treatment. Three of these households were unblinded at withdrawal. One additional household was unblinded (but not withdrawn) by request 2 months after treatment at the time of a tick-borne disease diagnosis. Acaricide and placebo treatments occurred from 9 May (in Maryland) or 16 May (in Connecticut and New York) through 29 June 2011 and from 13 April (in Maryland) or 1 May (in Connecticut and New York) through 15 June 2012. According to the phenology assessments, the peak in average nymphal tick activity occurred during the second week of June in 2011 (in Connecticut and New York) and the first week (in Connecticut) and second week (in New York) of June in 2012. Posttreatment tick sampling was conducted on 267 properties (135 in the acaricide group and 132 in the placebo group). In 2011, 688 I. scapularis ticks were collected. The average nymphal density was 68.8% lower in the acaricide group as compared to the placebo group (8.5 vs 27.4 nymphs/hour; P = .0006). In 2012, 176 ticks were collected, and the average nymphal density was 45.1% lower in the acaricide group (4.2 vs 7.6 nymphs/hour; P = .009). Overall, there was a 63.4% difference between acaricide-treated properties and placebo-treated properties over the 2 years (Figure 2), with significantly lower nymphal tick density at all study sites (Figure 3; Connecticut, P = .01; Maryland, P = .016; and New York, P = .024). Truck-mounted sprayers were used more frequently than backpack sprayers in Connecticut, whereas backpack sprayers were used more frequently in New York. Despite using varied methods of treatment, the difference in tick abundance (Figure 3) was consistently and similarly lower on acaricide-treated properties in both states, regardless of the study year. Low numbers in Maryland in 2012 did not allow for stable comparisons with the other 2 sites. The overall numbers of ticks at the phenology sites were also markedly lower in 2012 as compared to 2011 [18].
Figure 2.
Box plots of Ixodes scapularis nymphs recovered per hour across all sites 3–4 weeks after treatment, by treatment group and year.
Figure 3.
Box plots of Ixodes scapularis nymphs per hour 3–4 weeks after treatment, by treatment group and site during 2011 and 2012 combined.
In total, 98.1% of treated households completed all 4 monthly surveys (1286 in the acaricide group and 1274 in the placebo group), and 99.2% completed at least 1 monthly survey (1303 in the acaricide group and 1287 in the placebo group; Figure 1). During the 4 months following property treatment, ticks were reportedly found crawling on a household member in 26.4% of households, and a tick was found attached to a household member in 17.1%. There was no significant difference observed between treatment arms for ticks (yes vs no) found crawling on (P = .08) or attached to (P = .33) household members (Table 2). Similarly, there was no significant difference between treatment arms in the number of ticks found crawling on (P = .43) or attached to (P = .98) household members. No household or property characteristic was found to be a confounder or effect modifier of the relationship between ticks crawling or attached and treatment group. There was no change in the results when the withdrawn households were removed from analysis.
Table 2.
Proportion of Participating Households Reporting Tick Encounters or Physician-Diagnosed Tick-borne Disease, by Treatment Group, Study Year, and Study Site
| Outcome, Year(s), Site |
Acaricide | Placebo | P Value |
|---|---|---|---|
| Ticks crawling | |||
| 2011 | 185/762 (24.3) | 209/769 (27.2) | .19 |
| 2012 | 139/541 (25.7) | 150/518 (29.0) | .23 |
| Overall | 324/1303 (24.9) | 359/1287 (27.9) | .08 |
| CT | 133/474 (28.1) | 157/491 (32.0) | |
| MD | 68/319 (21.3) | 60/289 (20.8) | |
| NY | 123/510 (24.1) | 142/507 (28.0) | |
| Ticks attached | |||
| 2011 | 127/762 (16.7) | 145/769 (18.9) | .26 |
| 2012 | 86/541 (15.9) | 84/518 (16.2) | .89 |
| Overall | 213/1303 (16.3) | 229/1287 (17.8) | .33 |
| CT | 78/474 (16.5) | 103/491 (21.0) | |
| MD | 35/319 (11.0) | 30/289 (10.4) | |
| NY | 100/510 (19.6) | 96/507 (18.9) | |
| Self-reported illness | |||
| 2011 | 27/739 (3.7) | 25/755 (3.3) | .72 |
| 2012 | 14/534 (2.6) | 14/513 (2.7) | .91 |
| Overall | 41/1273 (3.2) | 39/1268 (3.0) | .78 |
| Verified illness | |||
| 2011 | 13/739 (1.8) | 14/755 (1.9) | .89 |
| 2012 | 6/534 (1.1) | 6/513 (1.2) | .94 |
| Overall | 19/1273 (1.5) | 20/1268 (1.6) | .90 |
Data are no. (%) of households with specified characteristics.
Overall, 97.4% of treated households (1273 in the acaricide group and 1268 in the placebo group) completed the final survey by November or December of the year in which they participated (Figure 1). Approximately 3.0% (80) of all households reported that ≥1 household member had a tick-borne disease diagnosed by a healthcare provider following property treatment. No statistically significant difference (P = .78) in self-reported tick-borne disease was observed for households in the acaricide group as compared to those treated with placebo (Table 2). Medical records were available for 44 of 80 tick-borne disease reports, of which 40 were verified as provider-diagnosed tick-borne disease based on record review by our panel. No statistically significant difference (P = .90) in verified tick-borne disease reports was observed between households treated with acaricide and those treated with placebo (Table 2).
A marginally significant difference (P = .054) was observed between households treated with acaricide and those treated with placebo across all sites with respect to blinding. When evaluated by study site, there was no difference in blinding between treatment groups in Connecticut (P = .88) or Maryland (P = .48). In New York, where covered treatment container labels were not permitted according to state department of transportation regulations, household respondents were more likely to correctly guess their treatment assignment (P = .005), suggesting incomplete blinding.
DISCUSSION
Results of this large, randomized, placebo-controlled intervention suggest that a springtime barrier application of acaricide to residential yards does not substantially reduce the risk of tick-borne diseases. As expected, we observed significantly lower tick abundance (63%) on acaricide-treated properties. Surprisingly, however, we observed no difference between treatment groups in human-tick encounters or tick-borne disease incidence. The discordance between tick abundance and human outcomes is consistent with a growing awareness that the effectiveness of interventions to prevent human vector-borne disease cannot be assumed based on entomologic outcomes alone [21].
There are several potential explanations for these seemingly discordant results. First, household members may have been exposed to ticks outside of their immediate property. Although some out-of-yard exposures are likely, the lack of a difference between treated and untreated households would require that essentially all ticks were acquired away from the home. This seems unlikely given the number of ticks documented in the yards of participating homeowners. Alternately, household members may have acquired ticks from areas of the yard that were not sprayed, including wooded areas and vegetable gardens, ornamental bushes, and flowers beds, for which spraying with bifenthrin is contraindicated. It is also possible that human behavior plays a greater role than absolute tick abundance in determining risk. Reducing the number of ticks in the environment could reduce the total number of ticks that exposure-prone individuals find (eg, from 10 to 4) without markedly reducing the number of individuals finding at least 1 tick. In this study, however, we showed that even the number of ticks encountered by household members was not different between treatment arms.
Much of what is believed about the effectiveness of environmental tick control to prevent human illness has been based on entomologic end points. Thus, our findings are likely to cause consternation among some entomologists. First, many will be surprised that we did not conduct pretreatment tick sampling. However, to meet the public health objective of preventing human infection, acaricide must be applied well before the majority of nymphal ticks emerge. Any pretreatment sampling would need to be done at a time when nymphal ticks are not active, and would therefore not be useful for assessing the comparability of properties, nor demonstrating a decrease in tick abundance. Second, the value of the randomized, controlled study design may not be apparent. The critical features of randomization and a large sample size provide near statistical certainty that the 2 groups will be comparable for all but the rarest of traits (Table 1). This comparability extends not just to measured variables (eg, property size and number of pets) but also unmeasured and unmeasurable risk factors and confounders (eg, outdoor activity level and care-seeking behavior) [22]. In contrast, entomologic studies are typically small-scale field trials (eg, having 5–10 test plots) with optimized application conditions (eg, using high-pressure truck-mounted sprayers) [7, 8, 23-25]. It is not possible with small sample sizes to reliably randomize test plots. While entomologic methods are clearly valuable for assessing the acaricidal efficacy of products or techniques, large-scale, randomized studies with human outcome measures are necessary to confirm their public health utility [21].
Although the acaricide used in this study is highly effective in reducing tick abundance in controlled field studies [7, 23], there are limitations to its use that may have impacted our study. Bifenthrin may not be applied before or during rain events likely to result in water runoff. In both years of the study, spring rains forced significant delays in applications, which made it difficult to time all treatments before the nymphal tick density peak. As noted, bifenthrin should not be sprayed on several types of vegetation (eg, ornamental bushes, flowers, and vegetable gardens) that are common in residential yards. The 63% difference in nymphal density we observed on treated properties is at the lower end of what has been reported in small-scale, carefully controlled research studies [9]; however, it more likely represents what can be achieved under real-world conditions. In practice, most homeowners will not have an opportunity to control neighbor treatment practices, pesticide operator equipment, or timing of treatment, and this study addresses the practical implications of this public health message. Last, this study evaluated a barrier approach to acaricide use; as such, these results may not apply to other uses of acaricide (eg, full-yard applications). However, the evidence that other acaricidal uses are effective are limited to studies with entomologic outcomes at present and can therefore not be considered effective protection against human tick-borne diseases.
There are additional study limitations that go beyond the use of bifenthrin. As mentioned, some New York households were unblinded regarding their treatment type, likely due to state department of transportation regulations regarding clear labeling and transport of hazardous materials. Nevertheless, study outcomes in New York were very similar to those reported in Connecticut and Maryland, suggesting that this unblinding did not have a major impact on study results. Another limitation may be inclusion of tick-borne disease cases occurring as soon as 3 days after treatment. There is a balance to be struck between excluding all patients who might have been exposed before yard treatment and excluding some who were likely exposed after treatment. For Lyme disease, although the incubation period can be as long as 30 days, the majority of patients having early symptoms develop them within 3 to 10 days [26]. When we reanalyzed the data by using cases with illness onset 14 days or even 21 days after treatment, there was still no difference between the acaricide and placebo groups. More importantly, the data on ticks crawling and ticks attached would not have been influenced by a lag and did not show a statistically significant difference between the acaricide and placebo groups. Finally, we relied on self-identification of ticks by homeowners, which may have led to some misclassification of outcomes. However, participants were given the opportunity to send in ticks for identification. Only 2 of 99 submissions reviewed by entomologists were identified as nonticks, suggesting that homeowners in these area of high tick endemicity are reasonably good at identifying ticks.
Future studies should collect additional information regarding the behavior of individuals in their yards and should consider interventions that can be effective throughout entire properties (such as those that can be used on vegetable gardens) and that have the potential to reduce the pathogen infection rate among ticks and animal hosts in the environment. Until an entomologic or ecologic surrogate exists for prediction of human disease at the household level, intervention studies should include human tick encounters and disease as their outcome measures.
Acknowledgments.
We thank Sarah Hook, John Jones, Mark Lamias, Joe Piesman, Christina Nelson, Meghan Brett, Kiersten Kugeler, Anna Perea, Brad Biggerstaff, Emily Zielinski-Gutierrez, Marc Dolan, and Ben Beard (Centers for Disease Control and Prevention [CDC]); Siok-Bi Wee, Heather Rutz, and Patricia Ryan (Maryland Department of Health and Mental Hygiene); Nadia Thomas, Rich Falco, and Robert S. Wills (Dutchess County; New York State Department of Health); and Ellen Stromdahl (Army Institute of Public Health).
Financial support.
This work was supported by the CDC through the Emerging Infections Program cooperative agreement (grants U01CI000307 and U50CK000195 for activities in Connecticut, U01CI000310 and U50CK000203 for activities in Maryland, and U01CI000311 and U50CK000199 for activities in New York).
Footnotes
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Presented in part: 13th International Conference on Lyme Borreliosis and Tick-borne Diseases, Boston, Massachusetts, 19 August 2013; International Conference on Emerging Infectious Diseases, Atlanta, Georgia, 26 August 2015. Abstract 201.
References
- 1.Hinckley AF, Connally NP, Meek JI, et al. Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis 2014; 59:676–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curran KL, Fish D, Piesman J. Reduction of nymphal Ixodes dammini (Acari: Ixodidae) in a residential suburban landscape by area application of insecticides. J Med Entomol 1993; 30:107–13. [DOI] [PubMed] [Google Scholar]
- 3.Maupin GO, Fish D, Zultowsky J, Campos EG, Piesman J. Landscape ecology of Lyme disease in a residential area of Westchester County, New York. Am J Epidemiol 1991; 133:1105–13. [DOI] [PubMed] [Google Scholar]
- 4.Schulze TL, Jordan RA, Vasvary LM, et al. Suppression of Ixodes scapularis (Acari: Ixodidae) nymphs in a large residential community. J Med Entomol 1994; 31:206–11. [DOI] [PubMed] [Google Scholar]
- 5.Stafford KC III. Effectiveness of carbaryl applications for the control of Ixodes dammini (Acari: Ixodidae) nymphs in an endemic residential area. J Med Entomol 1991; 28:32–6. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Notice to Readers: Final 2008 Reports of Nationally Notifiable Infectious Diseases. MMWR Morb Mortal Wkly Rep 2009; 58:859–69. [Google Scholar]
- 7.Rand PW, Lacombe EH, Elias SP, Lubelczyk CB, St Amand T, Smith RP Jr. Trial of a minimal-risk botanical compound to control the vector tick of Lyme disease. J Med Entomol 2010; 47:695–8. [DOI] [PubMed] [Google Scholar]
- 8.Schulze TL, Jordan RA, Hung RW, Taylor RC, Markowski D, Chomsky MS. Efficacy of granular deltamethrin against Ixodes scapularis and Amblyomma americanum (Acari: Ixodidade) nymphs. J Med Entomol 2001; 38:344–6. [DOI] [PubMed] [Google Scholar]
- 9.Hayes EB, Piesman J. How can we prevent Lyme disease? N Engl J Med 2003; 348:2424–30. [DOI] [PubMed] [Google Scholar]
- 10.University of Rhode Island Tick Encounter Resource Center. http://www.tickencounter.org/prevention/perimeter_spray. Accessed 21 October 2014.
- 11.Centers for Disease Control and Prevention. Preventing ticks in the yard. http://www.cdc.gov/ticks/avoid/in_the_yard.html. Accessed 21 October 2014.
- 12.Stafford KC III. Tick management handbook. http://www.ct.gov/caes/lib/caes/documents/publications/bulletins/b1010.pdf. Accessed 21 October 2014. [Google Scholar]
- 13.Town of Ridgefield C. BLAST program. http://www.ridgefieldct.org/content/46/6311/6347/8919.aspx. Accessed 2 October 2014.
- 14.Gould LH, Nelson RS, Griffith KS, et al. Knowledge, attitudes, and behaviors regarding Lyme disease prevention among Connecticut residents, 1999-2004. Vector Borne Zoonotic Dis 2008; 8:769–76. [DOI] [PubMed] [Google Scholar]
- 15.Mead P, Hinckley A, Hook S, Beard CB. TickNET—a collaborative public health approach to tickborne disease surveillance and research. Emerg Infect Dis 2015; 21:1574–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogden NH, Lindsay LR, Beauchamp G, et al. Investigation of relationships between temperature and developmental rates of tick Ixodes scapularis (Acari: Ixodidae) in the laboratory and field. J Med Entomol 2004; 41:622–33. [DOI] [PubMed] [Google Scholar]
- 17.Mather TN, Nicholson MC, Donnelly EF, Matyas BT. Entomologic index for human risk of Lyme disease. Am J Epidemiol 1996; 144:1066–9. [DOI] [PubMed] [Google Scholar]
- 18.Feldman KA, Connally NP, Hojgaard A, Jones EH, White JL, Hinckley AF. Abundance and infection rates of Ixodes scapularis nymphs collected from residential properties in Lyme disease-endemic areas of Connecticut, Maryland, and New York. J Vector Ecol 2015; 40:198–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Current and historical national notifiable conditions. http://wwwn.cdc.gov/nndss/conditions/. Accessed 12 May 2015.
- 20.Woodward M. Analysis by intention-to-treat. In: Epidemiology study design and data analysis. 2nd ed. New York: Chapman & Hall, 2005:344–5. [Google Scholar]
- 21.Wilson AL, Boelaert M, Kleinschmidt I, et al. Evidence-based vector control? Improving the quality of vector control trials. Trends Parasitol 2015; 31:380–90. [DOI] [PubMed] [Google Scholar]
- 22.Lilienfeld DE, Stolley PD. Experimental epidemiology. I. Randomized clinical trials. In: Foundations of epidemiology. New York: Oxford University Press, 1994:155–75. [Google Scholar]
- 23.Elias SP, Lubelczyk CB, Rand PW, et al. Effect of a botanical acaricide on Ixodes scapularis (Acari: Ixodidae) and nontarget arthropods. J Med Entomol 2013; 50:126–36. [DOI] [PubMed] [Google Scholar]
- 24.Schulze TL, Jordan RA, Krivenko AJ. Effects of barrier application of granular deltamethrin on subadult Ixodes scapularis (Acari: Ixodidae) and nontarget forest floor arthropods. J Econ Entomol 2005; 98:976–81. [DOI] [PubMed] [Google Scholar]
- 25.Stafford KC III, Allan SA. Field applications of entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae F52 (Hypocreales: Clavicipitaceae) for the control of Ixodes scapularis (Acari: Ixodidae). J Med Entomol 2010; 47:1107–15. [DOI] [PubMed] [Google Scholar]
- 26.Nadelman RB, Nowakowski J, Forseter G, et al. The clinical spectrum of early Lyme borreliosis in patients with culture-confirmed erythema migrans. Am J Med 1996; 100:502–8. [DOI] [PubMed] [Google Scholar]