Abstract
Gasdermins are effectors of pyroptosis downstream of diverse signaling pathways. Emerging evidence suggests that a number of post-translational modifications regulate the function of gasdermins in pyroptosis, a highly inflammatory form of cell death, and lytic or non-lytic secretion of intracellular contents. These include processing by different caspases and other proteases that may activate or suppress pyroptosis, ubiquitination by a bacterial E3 ligase that suppresses pyroptosis as an immune evasion mechanism, modifications at Cys residues in mammalian or microbial gasdermins that promote or inhibit pyroptosis, and potential phosphorylation that represses pyroptosis. Such diverse regulatory mechanisms by host and microbial proteases, ubiquitin ligases, acyltransferases, kinases and phosphatases may underlie the divergent physiological and pathological functions of gasdermins, and furnish opportunities for therapeutic targeting of gasdermins in infectious diseases and inflammatory disorders.
1. Introduction
The gasdermin family was initially identified as proteins highly expressed in the epithelia of the skin and gastrointestinal tract (Saeki, Kuwahara, Sasaki, Satoh, & Shiroishi, 2000; Sato et al., 1998; Van Laer et al., 1998). There are six paralogs in human or murine genome: gasdermin A (GSDMA), GSDMB, GSDMC, GSDMD, GSDME (deafness autosomal dominant 5, DFNA5), and deafness autosomal recessive 59 (DFNB59, Pejvakin or PJVK) (Aglietti & Dueber, 2017; Broz, Pelegrín, & Shao, 2020; Kovacs & Miao, 2017; Liu, Xia, Zhang, Wu, & Lieberman, 2021; Schutter et al., 2021; Shi, Gao, & Shao, 2017). Renewed enthusiasm in the gasdermin family stems from the discovery that GSDMD is the long sought-after effector of pyroptosis, a highly inflammatory form of cell death (Cookson & Brennan, 2001; Galluzzi et al., 2018), downstream of the infammasome signaling pathways (He et al., 2015; Kayagaki, Stowe, Lee, & O’Rourke, 2015; Shi, Zhao, et al., 2015) (Fig. 1).
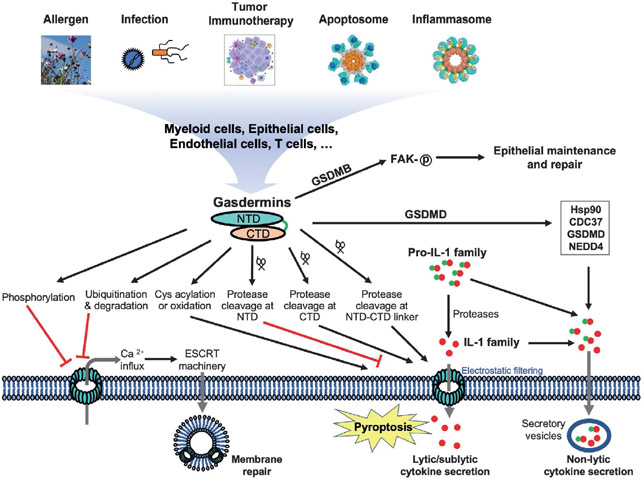
Fig. 1.
Diverse mechanisms regulate gasdermin function in pyroptosis and cytokine release. Gasdermins in myeloid cells, epithelial cells, endothelial cells, T cells and others may be stimulated by the inflammasome pathways, apoptotic pathways, microbial infections, allergens, or immune response to tumors. These may result in protease cleavage, ubiquitination, Cys acylation or oxidation, or phosphorylation of gasdermins that promote or suppress pyroptosis. Calcium influx triggered by gasdermin pores may stimulate membrane repair through the ESCRT machinery. Another membrane repair mechanism not depicted here is mediated by caspase-7 that cleaves acid sphingomyelinase (ASM) to induce endocytosis. Gasdermins may mediate lytic/sublytic cytokine release through membrane pores, or may mediate non-lytic cytokine secretion through secretory vesicles. GSDMB has been shown to harbor non-pyroptosis function in epithelial maintenance and repair.
The inflammasomes are crucial innate immune signaling platforms implicated in immune defense against infections and autoimmune/autoinflammatory disorders such as diabetes, multiple sclerosis and Alzheimer’s disease (Guo, Callaway, & Ting, 2015; Latz, Xiao, & Stutz, 2013; Schroder & Tschopp, 2010, p. 2010). Activation of the inflammasomes may lead to the maturation and secretion of proinflammatory cytokines IL-1β and IL-18, as well as pyroptosis mediated by GSDMD upon cleavage by inflammatory caspases-1, −4, −5, and −11 at the linker between its N- and C-terminal domains (NTDs and CTDs) (He et al., 2015; Kayagaki et al., 2015; Shi, Zhao, et al., 2015). Pyroptosis is a crucial immune defense mechanism against infections through the exposure of intracellular pathogens to extracellular environment conducive to microbial killing (Aachoui, Sagulenko, Miao, & Stacey, 2013; Ding et al., 2016; Jorgensen, Zhang, Krantz, & Miao, 2016; Liu et al., 2016; Miao et al., 2010). On the other hand, uncontrolled pyroptosis plays a major role in septic shock (Hagar, Powell, Aachoui, Ernst, & Miao, 2013; Kayagaki et al., 2013; Liu & Lieberman, 2017; Rathinam, Zhao, & Shao, 2019; Shi et al., 2014) and other inflammatory disorders (Ma et al., 2018; Van Opdenbosch & Lamkanfi, 2019).
Except for PJVK, all mammalian gasdermin family members adopt a two-domain structure with their positively charged and conserved NTDs capable of binding phospholipids and assembling oligomeric membrane pores (Hansen et al., 2021; Ruan, Xia, Liu, Lieberman, & Wu, 2018; Xia et al., 2021) (Fig. 1). The CTDs bind the NTDs to prevent pore formation in the resting states. The only exception seems to be that PJVK possesses a shortened CTD and its NTD harbors minimal capacity to induce cell death when expressed in mammalian cells (de Beeck et al., 2011). In addition, the GSDMB-CTD did not suppress its NTD from lipid-binding (Chao, Kulakova, & Herzberg, 2017). The CTDs of most gasdermins are thus viewed as autoinhibitory domains that maintain homeostasis. Furthermore, a new function for the GSDMD-CTD was reported as a recruitment module for the inflammatory caspases that cleave GSDMD (Liu, Wang, et al., 2020; Wang, Sun, et al., 2020). Protease processing of the linker regions between the NTDs and CTDs of gasdermins facilitates the release of the NTD-CTD autoinhibition to allow the NTDs to form oligomeric membrane pores (Fig. 2). There is little sequence conservation at the linker regions of gasdermins, which harbor the majority of the known cleavage sites in gasdermins by various proteases such as the inflammatory caspases, apoptotic caspases, neutrophil elastase, and granzymes implicated in the activation and regulation of different gasdermin family members (Burgener et al., 2019; Chen, Demarco, et al., 2019; He et al., 2015; Kambara et al., 2018; Kayagaki et al., 2015; Orning et al., 2018; Rogers et al., 2017; Sarhan et al., 2018; Shi, Zhao, et al., 2015; Taabazuing, Okondo, & Bachovchin, 2017; Wang et al., 2017; Zhou et al., 2020). Clearly protease processing has been the most intensively studied mechanism of gasdermin regulation.
Fig. 2.
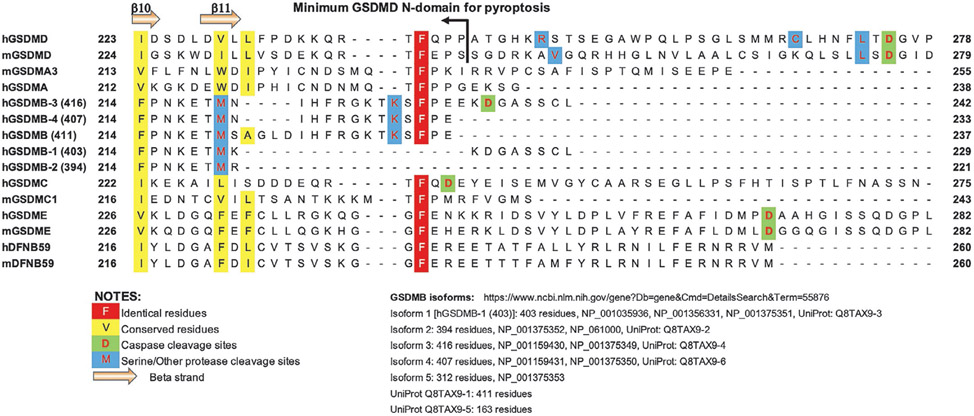
Sequence alignment of the gasdermin NTD-CTD linker region. Selected gasdermin family members from human and mouse are shown with identical and conserved residues, cleavage sites by caspases and other proteases marked as noted. The F240 residue in hGSDMD was shown to be crucial for oligomerization and pore formation. Among the hGSDMB isoforms, only three harbor the conserved Phe residue.
Besides protease processing, emerging evidence suggests that gasdermins are regulated by several other post-translational modifications such as ubiquitination, palmitoylation, and phosphorylation. Ubiquitination is long known to be a crucial mechanism targeting several components of the inflammasome signaling pathways including the sensor/receptor molecules, the adaptor ASC and inflammatory cytokines (Bednash & Mallampalli, 2016; Cockram et al., 2021; Zangiabadi & Abdul-Sater, 2022). Recent literature suggests that GSDMB and GSDMD are ubiquitinated by a bacterial E3 ligase invasion plasmid antigen H 7.8 (IpaH7.8), which employs its N-terminal leucine-rich repeats (LRRs) to recognize the gasdermin NTDs, and its novel E3 ligase (NEL) domain to ubiquitinate and mark gasdermins for proteasome degradation (Hansen et al., 2021; Luchetti et al., 2021). This serves as an immune evasion strategy for pathogens such as Shigella flexneri, since human GSDMB (hGSDMB) and hGSDMD can directly induce lysis of the invading bacteria as well as the infected host cells. In fact, IpaH7.8 is conserved among disease-causing clinical isolates of Shigella, which suggests that targeting of gasdermins by IpaH7.8 is an important virulence mechanism. The molecular mechanisms of such gasdermin ubiquitination are starting to emerge with the recent publication of the structure of hGSDMB in complex with the LRR of IpaH7.8 (Yin et al., 2023).
Yet another mechanism of gasdermin regulation was suggested by several reports on cysteine modifications in multiple gasdermin family members including GSDMD, GSDME and microbial gasdermin homologs that regulate their pyroptotic function. Shortly after the discovery of GSDMD as a pyroptosis effector, a cysteine residue conserved among GSDMDs from different species (Cys191 in hGSDMD and Cys192 in murine GSDMD, mGSDMD) was reported to be important for pyroptosis activities (Liu et al., 2016). A recent study demonstrates that these cysteines are S-palmitoylated, which facilitates GSDMD translocation to the plasma membrane (Balasubramanian et al., 2023). Similarly, evidence for S-palmitoylation of mammalian GSDME (Hu, Chen, et al., 2020) and bacterial and fungal gasdermin homologs has been reported (Johnson et al., 2022). Protein S-palmitoylation is a common acylation of proteins that regulates membrane association, localization, stability, or interaction with partner proteins (Aicart-Ramos, Valero, & Rodriguez-Crespo, 2011; Linder & Deschenes, 2007), all of which may be relevant to the function of different gasdermins.
The evidence for gasdermin phosphorylation is starting to emerge for GSDMA (Rogers et al., 2019; Santamaria et al., 2011), GSDMD (Li, Mo, et al., 2022) and GSDME (Rogers et al., 2019), though the mechanistic details remain to be elucidated. It appears that gasdermin phosphorylation prevents assembly of oligomeric pores, thus suppressing pyroptotic activities. The kinases and phosphatases that regulate such phosphorylation events remain to be identified or validated.
This review summarizes emerging themes on gasdermin regulation by post-translational modifications including protease cleavage, ubiquitination, lipidation, and phosphorylation. Since the main functions of gasdermins seems to be execution of cytolysis and/or release of inflammatory cytokines, this review will discuss how gasdermins mediate the release of intracellular cytokines with or without pyroptosis. The fact that multiple post-translational modifications of gasdermins regulate their function suggests that diverse mechanisms have evolved to modulate cell death and the release of intracellular contents, which in turn elicit strong inflammatory responses that may have beneficial or deleterious consequences. For discussion of pore formation by gasdermins, the roles of gasdermins in inflammatory disorders or tumor immunology, or therapeutic targeting of gasdermins, the readers are referred to a number of excellent reviews published recently (Broz et al., 2020; Hou, Hsu, & Hung, 2021; Liu et al., 2021; Magnani, Colantuoni, & Mortellaro, 2022; Ryder, Kondolf, O’Keefe, Zhou, & Abbott, 2022; Schutter et al., 2021; Wang & Ruan, 2022).
2. Processing of gasdermins by host proteases
The discovery of GSDMD and other gasdermins as executors of pyroptosis has sparked intense interests in the molecular mechanisms of gasdermin and pyroptosis regulation under physiological and pathological conditions. To maintain cellular homeostasis, most gasdermin family members reside in autoinhibited conformations through NTD–CTD interactions in the resting states. Protease processing has been shown to be the primary mechanisms of promoting or suppressing the gasdermin NTD-mediated membrane pore-formation and pyroptosis. The effects of protease processing is in large part dependent on the location of the cleavage sites within gasdermins, and the conditions for the stimulation of the relevant proteases. The diverse signaling pathways that impact proteolytic activities towards different gasdermins reveal distinct mechanisms for regulation of pyroptosis and cytokine release (Liu, Busscher, Storl-Desmond, & Xiao, 2022).
2.1. Protease processing and function of GSDMB
Genes encoding GSDMB are found in primates and ruminants, but not rodents or carnivores (Tamura et al., 2007). There are six different isoforms of human GSDMB in UniProt (The UniProt Consortium, 2023): Q8TAX9-1 to −6, with Q8TAX9-4 being the “canonical” isoform and the longest at 416 residues, and Q8TAX9-5 only at 163 residues that has a short C-terminal domain (CTD). Except for Q8TAX9-5, the other five variants listed in UniProt have identical NTDs and CTDs but different NTD-CTD linker regions (Fig. 2). Nonetheless, the five variants all harbor the same caspase cleavage sites DNVD (Panganiban et al., 2018) (protease cleaves at the C-terminus of the underlined Asp residue) and neutrophil elastase cleavage sites KETM (Oltra et al., 2022) within the NTDs. Cleavage at these sites within NTDs are expected to diminish pore-forming activities. The variants also harbor the granzyme A cleavage sites KSLG that likely facilitate liberation of NTDs from CTDs. Note that the mRNAs coding the longest GSDMB isoform Q8TAX9-4 (416 residues) is referred to as transcript variant 3 (NM_001165958) and transcript variant 6 (NM_001388420) that code “isoform 3” in the nucleotide database of NCBI. This is the isoform commonly used in hGSDMB studies. Care must be taken when a specific GSDMB isoform from a specific database is referred to, as the different isoforms are of various lengths and possible different function (Carl-McGrath, Schneider-Stock, Ebert, & Röcken, 2008; Sun et al., 2008).
The longest isoform of hGSDMB (Q8TAX9-4) was reported to be cleaved by caspase-1 at residue 233-EEKD-236, which led to pyroptosis by the NTD (Panganiban et al., 2018). In agreement, over-expression of the N-terminal 232 residues of this hGSDMB isoform in HEK293T cells led to cytolysis, whereas a shorter fragment of 220 residues did not (Panganiban et al., 2018). Notably, the 220 residue fragment in the Panganiban study is identical among all of the hGSDMB isoforms except for the Q8TAX9-5 isoform that harbors no NTD. The pyroptosis-deficient fragment of 220 residues was also observed in a study of hGSDMB cleaved at residue M220 by the neutrophil elastase, a serine protease localized in the azurophilic granules in neutrophils, which blocks the pyroptotic activity of hGSDMB (Oltra et al., 2022). Again the minimal pyroptosis-competent fragment of hGSDMB was shown to be residues 1–233 in this report (Oltra et al., 2022), in agreement with the Panganiban study. It remains to be determined what important roles the extra 12 residues NIHFRGKTKSFP play in mediating pore-formation or pyroptosis. These are located at the C-terminus of the hGSDMB NTD (isoform Q8TAX9-4) encoded by exon 6. Coincidently, another isoform Q8TAX9-3 of 403 residues, which does not contain the above 12 residues, failed to induce pyroptosis with or without processing by caspase-1 (Panganiban et al., 2018). Notably, among the 12 residues mentioned above is a highly conserved Phe residue in all gasdermin variants known to induce pyroptosis (Liu et al., 2018) (Fig. 2). This conserved F240 residue plays an important role in hGSDMD oligomerization and pore formation, as it anchors the α3 helix of the hGSDMD NTD, which mediates a crucial interface between adjacent NTD subunits in the pore form (Rathkey, Xiao, & Abbott, 2020).
Besides caspase-1 and neutrophil elastase, caspases-3, −6 and −7 cleave hGSDMB after residue D91 and produce a pyroptosis-deficient form (Chao et al., 2017). On the other hand, lymphocyte-derived granzyme A can cleave GSDMB to induce pyroptosis and contributes to antitumor immunity. The cleavage sites are located predominantly at K244 and secondarily at K229 within the interdomain linker (Zhou et al., 2020).
Curiously, before the discovery of gasdermins as executors of pyroptosis, a study focused on skin cell death revealed that the hGSDMB NTD, unlike the NTDs from other gasdermins, did not induce pyroptosis in HEK 293T cells, even though the specific hGSDMB variant or the boundary of the hGSDMB NTD under study was not specified (Shi, Tang, et al., 2015). The same group later published that for the longest hGSDMB isoform, the N-terminal 275-residue but not 236-residue fragment induced cell death (Chen, Shi, et al., 2019). Even though the same 275-residue fragment of hGSDMB was previously shown to induce cell death in HEK293T cells (Ding et al., 2016), the discrepancy between the failure of the 236-residue fragment to induce pyroptosis here vs the 232/233-residue fragment that was pyroptosis competent in the Panganiban study (Panganiban et al., 2018) remain to be resolved. Both of these studies focused on the longest hGSDMB isoform. Interestingly, co-expression of GSDMB, GSDMD, and caspase-4 revealed that the full-length hGSDMB promoted caspase-4 cleavage of hGSDMD, perhaps through binding of the hGSDMB N-terminal region (residues 1–83) to the CARD of caspase-4 (Chen, Shi, et al., 2019). Another unusual feature of GSDMB is that, unlike other gasdermins, both the full-length and NTD of GSDMB can bind phosphoinositides and sulfatides, suggesting a weak NTD–CTD interaction that allows lipid binding within the context of the full-length protein, even though the full-length GSDMB does not induce pyroptosis (Chao et al., 2017).
As is evident from the above, the contribution of GSDMB variants in pyroptosis and subsequent cell lysis remains to be clarified (Li, Li, & Bai, 2020; Oltra et al., 2022; Rana et al., 2022; Zheng, Yuan, Zhang, & Tang, 2022). For example, GSDMB cleaved by granzyme A from NK cells mediates pyroptosis of target cells (Zhou et al., 2020), including esophageal carcinoma cell lines. In addition, analysis of the Cancer Genome Atlas (TCGA) database suggested a strong positive correlation between GSDMB expression and patient survival for bladder carcinoma and skin cutaneous melanoma (Zhou et al., 2020). In contrast to these observations, elevated GSDMB expression levels have been observed in patients suffering from other cancers such as cervical, breast, hepatic, colon, and gastric cancers (Carl-McGrath et al., 2008; Edgren et al., 2011; Hergueta-Redondo et al., 2016; Komiyama et al., 2010; Lutkowska et al., 2017; Saeki, Komatsuzaki, Chiwaki, Yanagihara, & Sasaki, 2015). As such, GSDMB expression is linked to poor prognosis and therapeutic responses, and the development of distant metastasis of these cancers. Besides various cancers, variants of GSDMB have been implicated in asthma (Das et al., 2016; Li et al., 2021; Moffatt et al., 2007; Panganiban et al., 2018; Stein et al., 2018), type 1 diabetes (Saleh et al., 2011), and inflammatory bowel disease (Christodoulou et al., 2013; Jostins et al., 2012; Rana et al., 2022; Söderman, Berglind, & Almer, 2015). Recent studies of inflammatory bowel disease (IBD) demonstrated that hGSDMB was highly expressed in intestinal epithelial cells and regulated focal adhesion kinase phosphorylation to promote epithelial maintenance and repair, instead of participating in pyroptosis (Rana et al., 2022). In this work, the hGSDMB being studied is the longest isoform encoded by transcript 3. Nevertheless, pyroptosis could not be ruled out as a potential pathogenic mechanism, and cleavage of hGSDMB was observed in IBD intestinal biopsies. Future studies may clarify how the pyroptosis and non-pyroptosis function of hGSDMB may be dependent on multiple factors including the expression of different isoforms, susceptibility to different protease cleavage, and different tissue environments.
2.2. Activation of hGSDMC by apoptotic caspases
In PD-L1-expressing breast cancer cells, expression of GSDMC but no other gasdermins was enhanced, and hGSDMC cleavage was observed under hypoxia conditions upon TNFα and cycloheximide (CHX) treatment (Hou et al., 2020). In this case, both caspase-8 and caspase-6 can cleave hGSDMC, though the latter is not induced by TNFα and CHX treatment. The cleavage site is at residue 362-LELD-365 of GSDMC, which allows the resulting NTD to form pores and inducing pyroptosis (Hou et al., 2020). In another study, an essential metabolite in the tricarboxylic acid (TCA) cycle α-ketoglutarate (α-KG) induces pyroptosis through caspase-8-mediated cleavage of hGSDMC at residue 237-TFQD-240 (Zhang et al., 2021), which is not conserved in mGSDMC. The different cleavage site from the previous study suggests that different stimuli may impact caspase-8 cleavage of hGSDMC. Mechanistically, α-KG elevates ROS levels that leads to oxidation of the plasma membrane-localized death receptor 6 (DR6). Oxidation of DR6 triggers its endocytosis and recruitment of both procaspase-8 and hGSDMC through protein–protein interactions, thus leading to cleavage of hGSDMC and pyroptosis. This α-KG-induced pyroptosis was shown to inhibit tumor growth and metastasis in mouse models. Both of the above studies demonstrate the potential of apoptotic caspase-activated GSDMC in tumor immunotherapy.
2.3. Processing of GSDMD by host proteases
GSDMD has been the most intensively studied member of the gasdermin family and was initially reported to be cleaved by inflammatory caspases-1, −4, −5, and −11 at the linker between its NTD and CTD (He et al., 2015; Kayagaki et al., 2015; Shi, Zhao, et al., 2015). The cleavage site is located at residue 272-FLTD-275 in hGSDMD and 273-LLSD-276 in mGSDMD. In addition to inflammatory caspases, GSDMD is cleaved at the same site by apoptotic caspase-8, though with less efficiency compared with the inflammatory caspases (Chen, Demarco, et al., 2019; Orning et al., 2018; Sarhan et al., 2018). Besides caspases, GSDMD was reported to be cleaved and activated by serine proteases such as neutrophil elastase (ELANE) (Kambara et al., 2018; Sollberger et al., 2018) or cathepsin G (CatG) (Burgener et al., 2019). The multiple serine protease cleavage sites are located at the NTD-CTD linker region, thus facilitating the NTD fragment to be released from the CTD to assemble membrane pores. Protease processing can also inactivate GSDMD if the cleavage sites are located within the NTD. For example, both caspase-3 and caspase-7 cleave GSDMD at residue D87 to generate an NTD that is deficient in pore formation (Chen, Demarco, et al., 2019; Sarhan et al., 2018; Taabazuing et al., 2017).
Investigation of GSDMD recognition by inflammatory caspases have revealed a dual site recognition mechanism, in which the GSDMD NTD-CTD linker binds the caspase active site, while a hydrophobic pocket at the GSDMD-CTD engages the caspase exosite near its L2-L2′ strands (Liu, Wang, et al., 2020; Wang, Sun, et al., 2020). The exosite is sufficient to mediate GSDMD-caspase interaction, and is likely the initial enzyme-substrate contact, referred to as priming interaction. This initial interaction brings the NTD-CTD linker within the vicinity of the caspase active site for protease cleavage. Similar exosites have been identified in caspase-7 that engages poly(ADP ribose) polymerase 1 (PARP) (Boucher, Blais, & Denault, 2012), and in caspase-8 that binds the inhibitor baculovirus p35 protein (Lu, Min, Eliezer, & Wu, 2006, p. 8). Clearly, the recognition of caspase substrates may be influenced by their tertiary structures that provide crucial binding surface for exosites, which contributes to the more stringent substrate selectivity for some caspases. However, it is unclear if this also applies to the more promiscuous caspases such as caspase-3 that also cleaves GSDMD (Wang et al., 2017). It would thus be important to clarify and characterize diverse modes of gasdermin recognition by different proteases. A recent study by Devant and colleagues (Devant, Cao, & Kagan, 2021) revealed three classes of caspase-4 homologs in carnivorans. Among these, canine caspase-1/4 is a hybrid of both human caspase-4 and caspase-1 due to its shared sequence features with both, and cleaves both pro-IL-1β and GSDMD. It is important to understand the molecular determinants of substrate selectivity by different inflammatory caspases through evolution, with a focus on how GSDMD is recognized by caspases from different species.
Because the GSDMD-NTD is able to induce strong phenotype of pyroptosis and inflammatory responses, multiple studies have utilized GSDMD in tumor immunotherapy. An extracellular vesicles-based GSDMD-NTD mRNA delivery system was devised to induce pyroptosis of breast cancer cells and elicit an immunogenic tumor microenvironment for tumor immunotherapy (Xing et al., 2023). As the endosomal sorting complexes required for transport (ESCRT) machinery-mediated membrane repair was reported to significantly dampen GSDMD-mediated pyroptosis (Rühl et al., 2018), blocking the calcium influx-triggered ESCRT III-dependent membrane repair was investigated as a strategy to enhance pyroptosis of primary and metastatic tumors (Li, Mo, et al., 2022). Another membrane repair mechanism triggered by caspase-7 cleavage of acid sphingomyelinase (ASM) may also be targeted to enhance cytolysis (Nozaki et al., 2022). Nanoparticle-mediated release of calcium chelator BAPTA-AM was shown to strongly enhance tumor pyroptosis from hydrogel-delivered GSDMD (Li, Mo, et al., 2022). Combination of this strategy with immune checkpoint blockade may improve the immunotherapy efficacy against immunogenic and non-immunogenic tumors.
2.4. Cleavage of GSDME by caspases and granzyme B
Caspases-3 and −7 were reported to cleave human or mouse GSDME at D270 to generate a pore-forming NTD, which causes secondary necrosis in apoptotic cells (Rogers et al., 2017; Sarhan et al., 2018; Taabazuing et al., 2017; Wang et al., 2017). Caspase-3 plays a dominant role in GSDME activation, while caspase-7 makes minor contributions, as caspase-3 deficiency in macrophages nearly abolished GSDME cleavage (Sarhan et al., 2018; Taabazuing et al., 2017). In addition to caspases, GSDME may be cleaved by granzyme B, a serine protease expressed in NK, CD8+, or chimeric antigen receptor (CAR) T cells (Liu, Fang, et al., 2020; Zhang et al., 2020). Granzyme B can be delivered through perforin pores to tumor cells such as leukemia, melanoma, breast cancer and colorectal cancer cells. Granzyme B can directly cleave GSDME, while at the same time activates caspase-3 to further enhance GSDME cleavage. In agreement, expression of GSDME led to suppression of tumor growth, which was only partially dependent on caspase-3. On the other hand, GSDME-mediated pyroptosis was shown to stimulate caspase-1 activation and GSDMD cleavage in macrophages, which resulted in excessive cytokine release that contributed to severe cytokine release syndrome (CSR) during CAR T cell therapy (Liu, Fang, et al., 2020; Obstfeld et al., 2017). Depletion of macrophages or inhibition of caspase-1 and GSDMD activation may circumvent CRS without diminishing the efficacy of tumor clearance through pyroptosis (Liu, Fang, et al., 2020).
2.5. Cleavage of GSDMA and GSDMD by microbial and allergen proteases
Besides proteases from host cells, microbial and allergen proteases have been reported to either activate or suppress pyroptosis by gasdermins. Grampositive group A Streptococci (GAS) expresses a cysteine protease SpeB as a virulence factor. It was shown recently that SpeB triggers pyroptosis of skin cells by cleaving human GSDMA at residue Q246, generating an active N-terminal fragment that induce cell death (Deng et al., 2022; LaRock et al., 2022). As such, GSDMA functions as a sensor and effector against intracellular bacterial infection by triggering the elimination of the infected skin cell, thus depriving the pathogen of its protective niche. Pyroptosis of the infected cell ultimately prevents systemic dissemination of bacteria from the epithelial barrier. Notably, extracellular SpeB does not efficiently activate GSDMA. Cytosolic translocation of SpeB through either electroporation, packaging in liposomes, or infection by GAS is required for activation of GSDMA-mediated pyroptosis.
In addition to proteases from bacteria, viral proteases are known to counteract gasdermin-mediated pyroptosis. Enterovirus 71 (EV71) is a major causative agent of hand-foot-and-mouth disease (HFMD) in young children and has caused many large-scale epidemics (Chang, Chen, & Chen, 2016). Protease 3C from EV71 cleaves human GSDMD at residue Q193 within its NTD to inhibit its pyroptosis activity (Lei et al., 2017, p. 71). Such suppression of GSDMD-mediated pyroptosis promotes EV71 replication and survival in vivo. Presumably other microbial proteases with similar substrate specificity may subvert GSDMD-mediated pyroptosis using similar mechanism. On the other hand, viral proteases may also activate pyroptosis mediated by gasdermins. Oncolytic viruses such as Zika virus, which is neurotropic, was reported to induce pyroptosis of glioblastoma cells through cleavage and activation of GSDMD by the viral protease NS2B3 (Kao et al., 2023). Its cleavage site in hGSDMD is at 246-GHKR-249, and R249H mutation led to resistance to viral protease cleavage and pyroptosis. It remains to be determined whether such GSDMD-activating viral proteases may be utilized in tumor therapy.
In a study focusing on airway inflammation in response to allergens, it was revealed that lung epithelial cells harbor unique mechanisms to sense allergen proteases such as papain (Chen et al., 2022). Papain treatment led to the cleavage of hGSDMD and mGSDMD into p35 and p40 fragments, respectively, which assemble membrane pores and promote the secretion of IL-33, an IL-1 family of cytokines implicated in allergic inflammation. Residues 289-GLRAE-293 in hGSDMD and 309-ELRQQ-313 in mGSDMD are necessary for cleavage by papain, revealing novel pore formation-competent GSDMD fragments. The endogenous protease responsible for generating the p40/p35 GSDMD fragment has yet to be identified.
3. Regulation of GSDMB and GSDMD through ubiquitination
Human GSDMB, which is absent in rodents, and hGSDMD are ubiquitinated by a bacterial E3 ligase IpaH7.8 as an immune evasion strategy for pathogens such as S. flexneri, since hGSDMB and hGSDMD can directly induce lysis of the invading bacteria as well as infected host cells (Hansen et al., 2021; Luchetti et al., 2021). The molecular mechanisms of such gasdermin ubiquitination are starting to emerge with the recent publication of the structure of hGSDMB in complex with the LRR of IpaH7.8 (Yin et al., 2023), which reveals that the hGSDMB-NTD contacts the IpaH7.8 LRR through charged and hydrophobic residues. Of interest, the GSDMB residues (E15, D21, L96D, R124 and R208) essential for interacting with IpaH7.8 are also crucial for pore formation. Surprisingly, among the 13 hGSDMB residues that bind IpaH7.8, only 4 are conserved in hGSDMD, suggesting that the modes of hGSDMB and hGSDMD recognition by IpaH7.8 are likely to be distinct. Perhaps more importantly, the mechanisms of gasdermin ubiquitination by IpaH7.8 remain poorly understood, as the IpaH7.8 NEL domain that catalyzes ubiquitination is absent in the published structure. Moreover, binding of gasdermins by IpaH7.8 does not necessarily lead to ubiquitination or degradation. For example, IpaH7.8 binds both hGSDMD and mGSDMD, but only ubiquitinates and degrades the former but not the latter (Luchetti et al., 2021; Yin et al., 2023). This escape from IpaH7.8 catalyzed ubiquitination by mGSDMD may allow mice to employ mGSDMD-mediated pyroptosis to protect against Shigella infection. By contrast, Shigella infection causes severe hemorrhagic gastroenteritis in primates. Residues within the poorly conserved α1-α2 loops in hGSDMB and hGSDMD were suggested to influence their ubiquitination, even though no lysine residues are located at this loop and multiple lysine residues outside this region may be ubiquitinated (Luchetti et al., 2021; Yin et al., 2023). It is clear that we are only beginning to understand the antagonism of gasdermins by the microbial E3 ligase, and mechanisms for the recognition and ubiquitination of hGSDMD, as well as ubiquitination of hGSDMB remain to be elucidated.
4. Cys modifications in gasdermins that regulate pyroptosis
Since the initial report that the conserved Cys191 in hGSDMD and Cys192 in mGSDMD were important for their pyroptosis activities (Liu et al., 2016), structural studies have revealed that Cys191 is not in close proximity to other cysteines or each other in the resting state or the oligomeric pore structure, thus unlikely to form disulfides (Liu et al., 2019; Xia et al., 2021). Subsequently, it was reported that mutation of hGSDMD Cys191 led to ~50% reduction of pyroptosis (Hu, Liu, et al., 2020; Rathkey et al., 2018). Intriguingly, small molecule compounds such as necrosulfonamide (Rathkey et al., 2018) or disulfiram (Hu, Liu, et al., 2020) covalently modify Cys191 to inhibit hGSDMD-mediated pyroptosis. Accordingly, C191A mutation diminishes the efficacies of these inhibitors. Furthermore, the same Cys191 was shown to be covalently modified by fumarate, a tricarboxylic acid (TCA) cycle intermediate, which suppresses hGSDMD processing, oligomerization and its capacity to induce pyroptosis (Humphries et al., 2020). A recent study shed light on the mysterious Cys191/192 residues. Balasubramanian and colleagues demonstrated that Cys191 in hGSDMD and Cys192 in mGSDMD are S-palmitoylated, which is essential for GSDMD NTD translocation to the plasma membrane thus facilitating pyroptosis (Balasubramanian et al., 2023). Such GSDMD palmitoylation is mediated by palmitoyl acyltransferases zinc finger aspartate-histidine-histidine-cysteine (ZDHHC)-5/9, and facilitated by LPS-induced reactive oxygen species (ROS). Suppression of GSDMD palmitoylation mitigated the pathology of sepsis in mouse models.
Besides GSDMD, GSDME cysteine residues C407 and C408 are reported to be palmitoylated during chemotherapy-induced pyroptosis, which is suppressed by an acyl transferase inhibitor 2-bromopalmitate (Hu, Chen, et al., 2020). Such palmitoylation does not affect GSDME cleavage by caspase-3, but may facilitate the dissociation of GSDME-NTD from CTD thus promoting pyroptosis. Among the 23 ZDHHCs that catalyze protein palmitoylation (Aicart-Ramos et al., 2011; Jin, Zhi, Wang, & Meng, 2021; Linder & Deschenes, 2007), ZDHHC-2/7/11/15 were identified as enzymes that palmitoylate GSDME (Hu, Chen, et al., 2020). In addition to Cys modifications in mammalian gasdermins, a cysteine residue conserved in bacterial and fungal gasdermin homologs was shown to be palmitoylated, as shown in a bacterial gasdermin structure (Johnson et al., 2022). This stabilizes the structure and contribute to the pore-forming activities of the gasdermin in antiphage defense. Protein S-palmitoylation is the most common form of acylation that provides an important regulatory mechanism for membrane association, localization, stability, or interaction with partner proteins (Aicart-Ramos et al., 2011; Linder & Deschenes, 2007), all of which may be relevant to the function of gasdermins. The potentially widespread S-palmitoylation of gasdermins in bacteria and fungi, as well as evidence on S-palmitoylation in mammalian GSDMD and GSDME indicate that cysteine modifications are conserved and important mechanisms for regulating gasdermin function. Understanding the mechanisms for such regulation may facilitate the development of small molecule tool compounds or potential therapeutics targeting cysteines in gasdermins and pyroptosis.
5. Phosphorylation of gasdermins
Phosphorylation is the most common form of protein modification and is present in most signal transduction pathways controlling nearly every aspect of cellular physiology (Hunter, 2012). Emerging evidence suggests that some gasdermin family members may be regulated by phosphorylation though the mechanistic details remain to be elucidated. A search of the high-throughput mass spectrometry database PhosphoSitePlus (Hornbeck et al., 2015) revealed that GSDME can be phosphorylated at multiple Ser and Thr residues including T6, S69, S113, S114, T117, and S252 (Rogers et al., 2019). Mutagenesis studies revealed that phosphomimetic T6E mutation significantly inhibited the pyroptotic activity of GSDME, likely through preventing GSDME dimerization/oligomerization in membranes (Rogers et al., 2019). The kinase that phosphorylates GSDME T6 residue has not been identified. The corresponding T8 residue in GSDMA was phosphorylated in response to activation of Polo-like kinase 1 (Plk1) (Santamaria et al., 2011), and phosphomimetic T8E mutation abolished the pyroptotic activity of GSDMA (Rogers et al., 2019). Both T6 and T8 residues are located at the α1 helix of the corresponding GSDMA or GSDME, and their phosphorylation may result in electrostatic repulsion that prevents assembly of oligomeric pores.
The same phosphomimetic strategy was applied to GSDMD, which led to reduced pyroptosis by GSDMD when multiple Ser and Thr residues were mutated to Glu residues (Li, Pu, Huang, Zhang, & Yin, 2022). Phosphate-binding tag gel electrophoresis revealed that Thr213 is a major site of phosphorylation, which suppresses GSDMD oligomerization. This phosphorylation is negatively regulated by protein phosphatase 1 (PP1), in particular two catalytic subunits PP1α and PP1γ. It remains to be determined which kinases phosphorylate GSDMD, whether other GSDMD residues are phosphorylated and whether such phosphorylation impacts GSDMD-mediated pyroptosis.
6. The roles of gasdermins in cytokine release
Pore formation by gasdermins allows the release of intracellular contents such as danger signals or cytokines to the extracellular environment. These intracellular contents in turn orchestrate multiple aspects of innate and adaptive immunity (Declercq, De Leeuw, & Lambrecht, 2022; Dinarello, 2009; Evavold & Kagan, 2022; Migliorini, Italiani, Pratesi, Puxeddu, & Boraschi, 2020; Vora, Lieberman, & Wu, 2021). Some of the most intensively studied cytokines released through the gasdermin pores are the IL-1 family of leaderless cytokines. Due to the lack of signal peptides, the IL-1 family of cytokines do not exit the cell via the conventional ER-Golgi secretory pathway (Dinarello, 2009; Rubartelli, Cozzolino, Talio, & Sitia, 1990). Recent literature suggests that lytic or non-lytic release of cytokines is mediated by various gasdermins.
6.1. Cytolytic release of cytokines from myeloid cells
In the inflammasome signaling pathway, the pro-forms of IL-1β and IL-18 are processed by inflammatory caspases, followed by the secretion of mature cytokines facilitated by the GSDMD pores (Evavold et al., 2018; He et al., 2015; Karmakar et al., 2020; Kayagaki et al., 2015; Shi, Zhao, et al., 2015; Xia et al., 2021). Investigation of IL-1β/IL-18 maturation and trafficking revealed that mature IL-1β/IL-18 harbors a polybasic motif that directs its relocation to PIP2-rich plasma membrane ruffles through electrostatic attraction (Monteleone et al., 2018). The pro-forms of the cytokines are unable to localize to the plasma membrane due to the acidic nature of the pro-domain. Such localization of the mature cytokines facilitates their slow secretion from non-pyroptotic macrophages that is independent of GSDMD. In the presence of GSDMD activated by caspase-1 cleavage, the speed of cytokine secretion is significantly boosted. Xia and colleagues elucidated the molecular details of the predominantly negatively charged GSDMD pore conduit that exhibits a preference for neutral or positively charged cargo, such as mature IL-1β or IL-18 (Xia et al., 2021). This mechanism of cargo preference is referred to as electrostatic filtering.
GSDMD-mediated cytokine secretion may be further enhanced by lysosomal cathepsins in macrophages. While studying NAIP/NLRC4-dependent IL-1β secretion, Branco and colleagues discovered that the most abundant lysosomal proteases cathepsins act cooperatively with GSDMD to increase IL-1β secretion (Branco et al., 2022). IL-1β secretion in response to cytosolic flagellin was partially blocked in GSDMD-deficient cells but was fully abrogated upon cathepsin inhibition. The cathepsins did not affect the priming or assembly of the NAIP/NLRC4 inflammasome, nor did they impact GSDMD cleavage, which is in direct contrast to the roles of cathepsins in the priming and assembly of the NLRP3-inflammasome (Campden & Zhang, 2019). How GSDMD and cathepsins act in concert to optimize IL-1β secretion remain to be determined.
6.2. Gasdermin-mediated cytokine release in non-lytic myeloid cells
Since the discovery of GSDMD as a pore-forming effector protein mediating pyroptosis (Broz et al., 2020; He et al., 2015; Kayagaki et al., 2015; Shi et al., 2017; Shi, Zhao, et al., 2015), various studies have identified GSDMD pores and lytic cell death as the primary method of IL-1β secretion from macrophages. Other studies demonstrate that cytokine secretion, more often than not, is observed from myeloid cells that are considered “hyperactivated” but remain viable. The pattern of non-lytic cytokine secretion after inflammasome activation is unique to specific triggers. Several examples include LPS-stimulated human monocytes that secret IL-1β in the absence of pyroptosis (Gaidt et al., 2016); bacterial peptidoglycan-derived N-acetyl glucosamine that stimulates IL-1β secretion in macrophages (Wolf et al., 2016); oxidized phospholipid (oxPAPC)-stimulated dendritic cells secret IL-1β in the absence of cell death (Zanoni et al., 2016); Salmonella-stimulated neutrophils, a major source of IL-1β during acute Salmonella infection, maintain viability to sustain IL-1β release and clear infection (Chen et al., 2014). It should be noted that in neutrophils, the GSDMD NTD forms membrane pores not in the plasma membrane, but mostly in the membranes of intracellular organelles, particularly azurophilic granules (Karmakar et al., 2020). Nevertheless, IL-1β secretion from neutrophils is GSDMD-dependent, despite the absence of GSDMD pore-formation in the plasma membrane.
Multiple mechanisms have been proposed for the above hyperactivated states of myeloid cells. For example, upon inflammasome stimulation, only a small amount of GSDMD is expressed and/or activated such that the number of pores formed is insufficient to induce pyroptosis but just enough to allow for transport of small proteins such as mature cytokines (Chen et al., 2014; Evavold et al., 2018; Monteleone et al., 2018). Others suggest that membrane repair by the ESCRT machinery is efficient enough to prevent cell rupture but not cytokine secretion (Rühl et al., 2018). The use of cytoprotectant, such as glycine has demonstrated that macrophages, dendritic cells, and neutrophils are all capable of IL-1β secretion in a GSDMD-dependent, pyroptosis-independent manner (Heilig et al., 2018).
GSDMD is not the only gasdermin capable of mediating IL-1β release after inflammasome activation. Zhou and Abbott report two distinct phases of IL-1β release in macrophages, in which GSDME facilitated sublytic IL-1β release in the absence of GSDMD (Zhou & Abbott, 2021). In the presence of GSDMD, these two gasdermins work together in a cooperative fashion to facilitate IL-1β release upon activation of the NLRP1, NLRP3, or NLRC4 inflammasome. Interestingly, the expression levels of GSDME determine whether IL-1β is released rapidly through cytolysis upon overexpression, or slowly and continuously in living macrophages at endogenous expression levels. It is likely that membrane repair mechanisms such as ESCRT may be triggered to counter pore formation by gasdermins until the former is overwhelmed upon excessive gasdermin activation.
6.3. Gasdermin-mediated cytokine release in non-myeloid cells
Even though the gasdermin family was initially identified as proteins highly expressed in the gastrointestinal tract and skin (Saeki et al., 2000; Sato et al., 1998; Van Laer et al., 1998), the majority of research following the discovery of GSDMD as an effector for pyroptosis has been focused on myeloid cells. As the field of inflammasome activation and gasdermin function evolves, there are increasing efforts on epithelial and other non-myeloid cells. A recent report by Bulek et al. revealed a previously undocumented role of GSDMD in guiding the release of polyubiquitinated IL-1β via extracellular vesicles in non-pyroptotic intestinal epithelial cells (IECs) (Bulek et al., 2020). LPS + ATP stimulation of colonic IECs led to caspase-8-mediated NLRP3 inflammasome activation, which, instead of cleaving GSDMD and pro-IL-1β, facilitated the release of LC3+ secretory vesicles containing Hsp90/Cdc37-bound full-length GSDMD, active caspase-8, polyubiquitinated IL-1β, and E3 ubiquitin ligase NEDD4. This study highlights an interesting alternative role of GSDMD in secretory vesicles that facilitates cytokine release, as opposed to the typical role of membrane pore conduit for cytokine secretion.
Another investigation into the role of GSDMD in epithelial cells involves the release of IL-1 family IL-33 (Chen et al., 2022). IL-33 is constitutively expressed in many cell types, with particular importance in structural/stromal or barrier epithelial cells. Previously, it was thought that IL-33 was released from cells passively via cellular injury, physical stress, or necrosis (Martin & Martin, 2016). Treatment of lung epithelial cell lines MLE-12 and A549 cells with an allergen protease, papain, resulted in translocation of IL-33 from the nucleus to the cytoplasm via stress granule assembly, as well as cleavage of mGSDMD or hGSDMD to generate p40 or p35 N-terminal fragments capable of forming membrane pores (Chen et al., 2022). IL-33 was secreted through such pores in the absence of cytolysis and independent of caspase-1 and −11. It is notable that GSDMD may switch among various cleavage products depending on different environmental triggers and cell types, which in turn facilitates the release of particular cytokines responsible for specific inflammatory response. Importantly, almost all tested allergen proteases from microbial or environmental sources stimulated the generation of the p40 GSDMD NTD and induced IL-33 secretion in a dose-dependent manner. By contrast, no GSDMD fragmentation or IL-33 release was observed after stimulation with nonenzymatic allergens. This suggests that a common protease stress-sensing pathway may be operating in lung epithelial cells in response to allergen proteases, which leads to GSDMD p40 generation and IL-33 release, but not pyroptosis. The endogenous protease responsible for generating the p40/p35 GSDMD fragment, perhaps downstream of the allergen protease-sensing pathway, has yet to be identified.
Besides epithelial cells, human coronary artery endothelial cells and smooth muscle cells have been reported to secret IL-1β in response to oxidized low-density lipoprotein (oxLDL) (Almansouri, Patel, Chamberlain, & Francis, 2022). OxLDL is known to promote inflammatory cytokine secretion and contribute to both initiation and progression of atherosclerosis (Itabe, Obama, & Kato, 2011; Yang et al., 2014). Treatment of these endothelial and smooth muscle cells with oxLDL led to release of IL-1β, which was dependent on NLRP3, caspase-1 and GSDMD for endothelial cells, and was caspase-1-dependent for smooth muscle cells. Even though elevated LDH release was detected for the endothelial cells, they remain alive for at least 24 h (Almansouri et al., 2022), reminiscent of the IL-33 secreting epithelial cells without cytolysis. This study further suggests that caspase-1 or gasdermin D inhibition may be an effective strategy for targeting atherosclerosis.
Similar to the role of GSDME in cytokine release from macrophages, TH17 cells (but not TH1, TH2, or Treg) were reported to utilize GSDME pores to release IL-1α, which was dependent on T cell-intrinsic NLRP3 infammasome and caspase-8/3 activation (Chao et al., 2023). The level of IL-1α secretion from TH17 cells stimulated with anti-CD3 and anti-CD28 was comparable to that from human monocytes primed with LPS and stimulated with nigericin, suggesting that TH17 cells are a major source of IL-1α. Processing of pro-IL-1α by calcium-dependent protease calpain was a prerequisite for IL-1α secretion. Even though both GSDME and GSDMD were induced upon T cell stimulation, only GSDME was shown to be cleaved, likely by caspase-3. Nonetheless, the IL-1α-secreting TH17 cells retained viability and full proliferation potential compared with the non-IL-1α-secreting cells. This is yet another example of GSDME-mediated cytokine release independent of cytolysis. This study further demonstrates that the ability of TH17 cells to secret IL-1α is an important anti-fungal host defense mechanism, in addition to their ability to produce IL-17.
7. Conclusions and future perspectives
The diverse regulatory mechanisms of gasdermins by host and microbial enzymes such as proteases, ubiquitin ligases, acyltransferases, kinases and phosphatases underlie the divergent physiological and pathological functions of gasdermins as effectors of pyroptosis and cytokine secretion. The molecular mechanisms for the recognition of gasdermins by this wide variety of enzymes are poorly understood except for the recent elucidation of GSDMD recognition by inflammatory caspases (Liu, Wang, et al., 2020; Wang, Sun, et al., 2020). Even though specific gasdermin residues targeted by some proteases (Liu, Wang, et al., 2020) and Cys modifications have been identified, it is clear that the tertiary structures of gasdermins play important roles in their specific recognition by the various host and microbial enzymes. Future studies may reveal additional gasdermin-enzyme interfaces outside of the enzyme active site, as in the “exosite” interface between GSDMD and inflammatory caspases. This mode of recognition may contribute to higher affinity gasdermin binding and more stringent substrate selectivity, which is crucial to differentiate various gasdermins family members or different gasdermin isoforms. This may be of particular interest for GSDMB, which has multiple isoforms that may be susceptible to different enzymatic modifications, and participate in pyroptosis or non-pyroptosis function depending on different stimulation conditions and tissue environments.
Our understanding of gasdermin ubiquitination, Cys modification, and phosphorylation is much limited compared with protease processing of gasdermins. Despite recent progress on GSDMB recognition by IpaH7.8, the mechanisms of GSDMB/GSDMD ubiquitination by IpaH7.8 remain poorly understood, especially due to the fact that binding of gasdermins by IpaH7.8 does not necessarily lead to ubiquitination or degradation (Luchetti et al., 2021; Yin et al., 2023). The potentially widespread S-palmitoylation of gasdermins in bacteria and fungi, as well as evidence on S-palmitoylation in mammalian GSDMD and GSDME indicate that cysteine modifications are conserved and important mechanisms for regulating gasdermin function. GSDMD-mediated pyroptosis was reported to be regulated by reactive oxygen species (ROS) that directly oxidizes Cys192 in murine GSDMD (Devant et al., 2023). Mutation of Cys192 or absence of oxidation reduces pyroptosis in macrophages. ROS production in mitochondria from Ragulator-Rag-mTOR Complex 1 (mTORC1) signaling act either upstream or downstream of GSDMD processing by proteases (Evavold et al., 2021; Zheng et al., 2021). Whether Cys oxidation occurs in other gasdermins, impacts their pyroptosis activities, or cross-regulates S-palmitoylation remain to be determined.
Multiple gasdermins have been utilized in anti-tumor inflammatory responses including GSDMA3 (Wang, Wang, et al., 2020), GSDMB (Zhou et al., 2020), GSDMC (Hou et al., 2020), GSDMD (Li, Mo, et al., 2022; Xing et al., 2023), and GSDME (Liu, Fang, et al., 2020; Zhang et al., 2020). Combination of this strategy with immune checkpoint blockade may improve the immunotherapy efficacy against multiple immunogenic and non-immunogenic tumors. On the other hand, GSDME-mediated pyroptosis led to activation of caspase-1 and GSDMD in macropahges, which in turn resulted in cytokine release syndrome that is deleterious to cancer treatment (Liu, Fang, et al., 2020). In this case, the excessive cytokine release may be partially triggered by cellular contents including ATP that stimulate inflammasomes in macrophages. As a result, depletion of macropahges or inhibition of caspase-1/GSDMD may block the occurrence of cytokine release syndrome. Mechanistical understanding of the gasdermin-induced inflammatory environment and proper reduction of excessive cytokine release may be crucial in harnessing the full potential of gasdermins in tumor immunotherapy.
Emerging evidence suggests that gasdermins are important mediators of cytokine release with or without pyroptosis. The types of cytokine release through lytic or non-lytic routes may be dependent on the levels of expression of specific gasdermins, which has been shown elegantly for GSDME (Zhou & Abbott, 2021). Besides myeloid cells, TH17 cells, epithelial cells, endothelial cells, smooth muscle cells or other are all capable of cytokine secretion in a gasdermin-dependent yet pyroptosis-independent manner. Recent studies of IL-33 release from lung epithelial cells suggest a common protease stress-sensing pathway in response to allergen proteases, which leads to GSDMD p40 fragment generation and IL-33 release, but not pyroptosis. Intriguingly, almost all tested allergen proteases from microbial or environmental sources stimulated the generation of the p40 fragment, while nonenzymatic allergens did not. It is of great interest to characterize the endogenous protease(s) responsible for generating the p40/p35 GSDMD fragment that facilitate IL-33 secretion without cytolysis.
Acknowledgments
This work is supported by NIH grants R01GM127609 and P01141360 to T.S.X, and R03AI173549 to Z.L.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests.
References
- Aachoui Y, Sagulenko V, Miao EA, & Stacey KJ (2013). Inflammasome-mediated pyroptotic and apoptotic cell death, and defense against infection. Current Opinion in Microbiology, 16(3), 319–326. 10.1016/j.mib.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aglietti RA, & Dueber EC (2017). Recent insights into the molecular mechanisms underlying pyroptosis and gasdermin family functions. Trends in Immunology, 38(4), 261–271. 10.1016/j.it.2017.01.003. [DOI] [PubMed] [Google Scholar]
- Aicart-Ramos C, Valero RA, & Rodriguez-Crespo I (2011). Protein palmitoylation and subcellular trafficking. Biochimica et Biophysica Acta (BBA) - Biomembranes, 1808(12), 2981–2994. 10.1016/j.bbamem.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Almansouri M, Patel P, Chamberlain J, & Francis S (2022). OxLDL induces the release of IL-1β from primed human endothelial and smooth muscle cells via different caspase −1-dependent mechanisms. Vascular Biology, 4(1), 11–18. 10.1530/VB-22-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian A, Ghimire L, Hsu AY, Kambara H, Liu X, Hasegawa T, et al. (2023). Palmitoylation of gasdermin D directs its membrane translocation and pore formation in pyroptosis. bioRxiv. 10.1101/2023.02.21.529402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednash JS, & Mallampalli RK (2016). Regulation of inflammasomes by ubiquitination. Cellular & Molecular Immunology, 13(6), Article 6. 10.1038/cmi.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher D, Blais V, & Denault J-B (2012). Caspase-7 uses an exosite to promote poly(ADP ribose) polymerase 1 proteolysis. Proceedings of the National Academy of Sciences of the United States of America, 109(15), 5669–5674. 10.1073/pnas.1200934109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco LM, Amaral MP, Boekhoff H, de Lima ABF, Farias IS, Lage SL, et al. (2022). Lysosomal cathepsins act in concert with Gasdermin-D during NAIP/NLRC4-dependent IL-1β secretion. Cell Death & Disease, 13(12). Article 12 10.1038/s41419-022-05476-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broz P, Pelegrín P, & Shao F (2020). The gasdermins, a protein family executing cell death and inflammation. Nature Reviews. Immunology, 20(3), 143–157. 10.1038/s41577-019-0228-2. [DOI] [PubMed] [Google Scholar]
- Bulek K, Zhao J, Liao Y, Rana N, Corridoni D, Antanaviciute A, et al. (2020). Epithelial-derived gasdermin D mediates nonlytic IL-1β release during experimental colitis. The Journal of Clinical Investigation, 130(8). 10.1172/JCI138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgener SS, Leborgne NGF, Snipas SJ, Salvesen GS, Bird PI, & Benarafa C (2019). Cathepsin G inhibition by Serpinb1 and Serpinb6 prevents programmed necrosis in neutrophils and monocytes and reduces GSDMD-driven inflammation. Cell Reports, 27(12), 3646–3656. 10.1016/j.celrep.2019.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campden RI, & Zhang Y (2019). The role of lysosomal cysteine cathepsins in NLRP3 inflammasome activation. Archives of Biochemistry and Biophysics, 670, 32–42. 10.1016/j.abb.2019.02.015. [DOI] [PubMed] [Google Scholar]
- Carl-McGrath S, Schneider-Stock R, Ebert M, & Röcken C (2008). Differential expression and localisation of gasdermin-like (GSDML), a novel member of the cancer-associated GSDMDC protein family, in neoplastic and non-neoplastic gastric, hepatic, and colon tissues. Pathology,40(1), 13–24. 10.1080/00313020701716250. [DOI] [PubMed] [Google Scholar]
- Chang P-C, Chen S-C, & Chen K-T (2016). The current status of the disease caused by enterovirus 71 infections: Epidemiology, pathogenesis, molecular epidemiology, and vaccine development. International Journal of Environmental Research and Public Health, 13(9), Article 9. 10.3390/ijerph13090890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao KL, Kulakova L, & Herzberg O (2017). Gene polymorphism linked to increased asthma and IBD risk alters gasdermin-B structure, a sulfatide and phosphoinositide binding protein. Proceedings of the National Academy of Sciences of the United States of America, 114(7), E1128–E1137. 10.1073/pnas.1616783114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Y-Y, Puhach A, Frieser D, Arunkumar M, Lehner L, Seeholzer T, et al. (2023). Human TH17 cells engage gasdermin E pores to release IL-1α on NLRP3 inflammasome activation. Nature Immunology, 24(2), Article 2. 10.1038/s41590-022-01386-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Chen S, Yan C, Zhang Y, Zhang R, Chen M, et al. (2022). Allergen protease-activated stress granule assembly and gasdermin D fragmentation control interleukin-33 secretion. Nature Immunology, 23(7), 1021–1030. 10.1038/s41590-022-01255-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KW, Demarco B, Heilig R, Shkarina K, Boettcher A, Farady CJ, et al. (2019). Extrinsic and intrinsic apoptosis activate pannexin-1 to drive NLRP3 inflammasome assembly. The EMBO Journal, 38(10), e101638. 10.15252/embj.2019101638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KW, Groβ CJ, Sotomayor FV, Stacey KJ, Tschopp J, Sweet MJ, et al. (2014). The neutrophil NLRC4 inflammasome selectively promotes IL-1β maturation without pyroptosis during acute Salmonella challenge. CellReports, 8(2), 570–582. 10.1016/j.celrep.2014.06.028. [DOI] [PubMed] [Google Scholar]
- Chen Q, Shi P, Wang Y, Zou D, Wu X, Wang D, et al. (2019). GSDMB promotes non-canonical pyroptosis by enhancing caspase-4 activity. Journal of Molecular Cell Biology, 11(6), 496–508. 10.1093/jmcb/mjy056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulou K, Wiskin AE, Gibson J, Tapper W, Willis C, Afzal NA, et al. (2013). Next generation exome sequencing of paediatric inflammatory bowel disease patients identifies rare and novel variants in candidate genes. Gut, 62(7), 977–984. 10.1136/gutjnl-2011-301833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockram PE, Kist M, Prakash S, Chen S-H, Wertz IE, & Vucic D (2021). Ubiquitination in the regulation of inflammatory cell death and cancer. Cell Death and Differentiation, 28(2). Article 2 10.1038/s41418-020-00708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson BT, & Brennan MA (2001). Pro-inflammatory programmed cell death. Trends in Microbiology, 9(3), 113–114. [DOI] [PubMed] [Google Scholar]
- Das S, Miller M, Beppu AK, Mueller J, McGeough MD, Vuong C, et al. (2016). GSDMB induces an asthma phenotype characterized by increased airway responsiveness and remodeling without lung inflammation. Proceedings of the National Academy of Sciences of the United States of America. 10.1073/pnas.1610433113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beeck KO, Van Camp G, Thys S, Cools N, Callebaut I, Vrijens K, et al. (2011). The DFNA5 gene, responsible for hearing loss and involved in cancer, encodes a novel apoptosis-inducing protein. European Journal of Human Genetics, 19(9), 965–973. 10.1038/ejhg.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declercq J, De Leeuw E, & Lambrecht BN (2022). Inflammasomes and IL-1 family cytokines in SARS-CoV-2 infection: From prognostic marker to therapeutic agent. Cytokine, 157, 155934. 10.1016/j.cyto.2022.155934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Bai Y, Deng F, Pan Y, Mei S, Zheng Z, et al. (2022). Streptococcal pyrogenic exotoxin B cleaves GSDMA and triggers pyroptosis. Nature, 602(7897), 496–502. 10.1038/s41586-021-04384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devant P, BorŠić E, Ngwa EM, Xiao H, Chouchani ET, Thiagarajah JR, et al. (2023). Gasdermin D pore-forming activity is redox-sensitive. Cell Reports, 42(1). 10.1016/j.celrep.2023.112008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devant P, Cao A, & Kagan JC (2021). Evolution-inspired redesign of the LPS receptor caspase-4 into an interleukin-1β–converting enzyme. Science Immunology, 6(62). 10.1126/sciimmunol.abh3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA (2009). Immunological and inflammatory functions of the interleukin-1 family. Annual Review of Immunology, 27, 519–550. 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, et al. (2016). Pore-forming activity and structural autoinhibition of the gasdermin family. Nature, 535, 111–116. 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- Edgren H, Murumagi A, Kangaspeska S, Nicorici D, Hongisto V, Kleivi K, et al. (2011). Identification of fusion genes in breast cancer by paired-end RNA-sequencing. Genome Biology, 12(1), R6. 10.1186/gb-2011-12-1-r6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evavold CL, Hafner-Bratkovič I, Devant P, D’Andrea JM, Ngwa EM, Boršić E, et al. (2021). Control of gasdermin D oligomerization and pyroptosis by the Ragulator-Rag-mTORC1 pathway. Cell, 184(17), 4495–4511.e19. 10.1016/j.cell.2021.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evavold CL, & Kagan JC (2022). Diverse control mechanisms of the interleukin-1 cytokine family. Frontiers in Cell and Development Biology, 10. https://www.frontiersin.org/articles/10.3389/fcell.2022.910983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evavold CL, Ruan J, Tan Y, Xia S,Wu H, & Kagan JC (2018). The pore-forming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity, 48(1), 35–44. 10.1016/j.immuni.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidt MM, Ebert TS, Chauhan D, Schmidt T, Schmid-Burgk JL, Rapino F, et al. (2016). Human monocytes engage an alternative inflammasome pathway. Immunity, 44(4), 833–846. 10.1016/j.immuni.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. (2018). Molecular mechanisms of cell death: Recommendations of the nomenclature committee on cell death 2018. Cell Death and Differentiation, 25(3), 486–541. 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Callaway JB, & Ting JPY (2015). Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nature Medicine, 21(7), 677–687. 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagar JA, Powell DA, Aachoui Y, Ernst RK, & Miao EA (2013). Cytoplasmic LPS activates caspase-11: Implications in TLR4-independent endotoxic shock. Science, 341(6151), 1250–1253. 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JM, de Jong MF, Wu Q, Zhang L-S, Heisler DB, Alto LT, et al. (2021). Pathogenic ubiquitination of GSDMB inhibits NK cell bactericidal functions. Cell, 184(12), 3178–3191. 10.1016/j.cell.2021.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W-T, Wan H, Hu L, Chen P, Wang X, Huang Z, et al. (2015). Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Research, 25, 1285–1298. 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig R, Dick MS, Sborgi L, Meunier E, Hiller S, & Broz P (2018). The Gasdermin-D pore acts as a conduit for IL-1β secretion in mice. European Journal of Immunology, 48(4), 584–592. 10.1002/eji.201747404. [DOI] [PubMed] [Google Scholar]
- Hergueta-Redondo M, Sarrio D, Molina-Crespo Á, Vicario R, Bernadó-Morales C, Martínez L, et al. (2016). Gasdermin B expression predicts poor clinical outcome in HER2-positive breast cancer. Oncotarget, 7(35), 56295–56308. 10.18632/oncotarget.10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornbeck PV, Zhang B, Murray B, Kornhauser JM, Latham V, & Skrzypek E (2015). PhosphoSitePlus, 2014: Mutations, PTMs and recalibrations. Nucleic Acids Research, 43(Database issue), D512–D520. 10.1093/nar/gku1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J, Hsu J-M, & Hung M-C (2021). Molecular mechanisms and functions of pyroptosis in inflammation and antitumor immunity. Molecular Cell, 81(22), 4579–4590. 10.1016/j.molcel.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J, Zhao R, Xia W, Chang C-W, You Y, Hsu J-M, et al. (2020). PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nature Cell Biology, 22(10), 1264–1275. 10.1038/s41556-020-0575-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Chen M, Chen X, Zhao C, Fang Z, Wang H, et al. (2020). Chemotherapy-induced pyroptosis is mediated by BAK/BAX-caspase-3-GSDME pathway and inhibited by 2-bromopalmitate. Cell Death & Disease, 11(4), Article 4. 10.1038/s41419-020-2476-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JJ, Liu X, Xia S, Zhang Z, Zhang Y, Zhao J, et al. (2020). FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nature Immunology, 21(7), Article 7. 10.1038/s41590-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries F, Shmuel-Galia L, Ketelut-Carneiro N, Li S, Wang B, Nemmara VV, et al. (2020). Succination inactivates gasdermin D and blocks pyroptosis. Science, 369, 1633–1637. 10.1126/science.abb9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. (2012). Why nature chose phosphate to modify proteins. Philosophical Transactions of the Royal Society, B: Biological Sciences, 367(1602), 2513–2516. 10.1098/rstb.2012.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itabe H, Obama T, & Kato R (2011). The dynamics of oxidized LDL during atherogenesis. Journal of Lipids, 2011, e418313. 10.1155/2011/418313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Zhi X, Wang X, & Meng D (2021). Protein palmitoylation and its pathophysiological relevance. Journal of Cellular Physiology, 236(5), 3220–3233. 10.1002/jcp.30122. [DOI] [PubMed] [Google Scholar]
- Johnson AG, Wein T, Mayer ML, Duncan-Lowey B, Yirmiya E, Oppenheimer-Shaanan Y, et al. (2022). Bacterial gasdermins reveal an ancient mechanism of cell death. Science, 375(6577), 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen I, Zhang Y, Krantz BA, & Miao EA (2016). Pyroptosis triggers pore-induced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis. Journal of Experimental Medicine, 213(10), 2113–2128. 10.1084/jem.20151613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. (2012). Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature, 491(7422), 119–124. 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambara H, Liu F, Zhang X, Liu P, Bajrami B, Teng Y, et al. (2018). Gasdermin D exerts anti-inflammatory effects by promoting neutrophil death. Cell Reports, 22(11), 2924–2936. 10.1016/j.celrep.2018.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao Y-T, Wang H-I, Shie C-T, Lin C-F, Lai MMC, & Yu C-Y (2023). Zika virus cleaves GSDMD to disseminate prognosticable and controllable oncolysis in a human glioblastoma cell model. Molecular Therapy - Oncolytics, 28, 104–117. 10.1016/j.omto.2022.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmakar M, Minns M, Greenberg EN, Diaz-Aponte J, Pestonjamasp K, Johnson JL, et al. (2020). N-GSDMD trafficking to neutrophil organelles facilitates IL-1β release independently of plasma membrane pores and pyroptosis. Nature Communications, 11(1), 2212. 10.1038/s41467-020-16043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayagaki N, Stowe IB, Lee BL, & O’Rourke K (2015). Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature, 526, 666–671. [DOI] [PubMed] [Google Scholar]
- Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, et al. (2013). Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science, 341, 1246–1249. 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- Komiyama H, Aoki A, Tanaka S, Maekawa H, Kato Y, Wada R, et al. (2010). Alu-derived cis-element regulates tumorigenesis-dependent gastric expression of GASDERMIN B (GSDMB). Genes & Genetic Systems, 85(1), 75–83. [DOI] [PubMed] [Google Scholar]
- Kovacs SB, & Miao EA (2017). Gasdermins: Effectors of pyroptosis. Trends in Cell Biology, 27(9), 673–684. 10.1016/j.tcb.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRock DL, Johnson AF, Wilde S, Sands JS, Monteiro MP, & LaRock CN (2022). Group A Streptococcus induces GSDMA-dependent pyroptosis in keratinocytes. Nature, 605(7910), Article 7910. 10.1038/s41586-022-04717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz E, Xiao TS, & Stutz A (2013). Activation and regulation of the inflammasomes. Nature Reviews Immunology, 13(6), 397–411. 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X, Zhang Z, Xiao X, Qi J, He B, & Wang J (2017). Enterovirus 71 inhibits pyroptosis through cleavage of Gasdermin D. Journal of Virology, 91(18). e01069–17 10.1128/JVI.01069-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Christenson SA, Modena B, Li H, Busse WW, Castro M, et al. (2021). Genetic analyses identify GSDMB associated with asthma severity, exacerbations, and antiviral pathways. Journal of Allergy and Clinical Immunology, 147(3), 894–909. 10.1016/j.jaci.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Li Y, & Bai Y (2020). Role of GSDMB in pyroptosis and cancer. Cancer Management and Research, 12, 3033–3043. 10.2147/CMAR.S246948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Mo F, Wang Y, Li W, Chen Y, Liu J, et al. (2022). Enhancing Gasdermin-induced tumor pyroptosis through preventing ESCRT-dependent cell membrane repair augments antitumor immune response. Nature Communications, 13(1), Article 1. 10.1038/s41467-022-34036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Pu D, Huang J, Zhang Y, & Yin H (2022). Protein phosphatase 1 regulates phosphorylation of gasdermin D and pyroptosis. Chemical Communications, 58(85), 11965–11968. 10.1039/D2CC03590A. [DOI] [PubMed] [Google Scholar]
- Linder ME, & Deschenes RJ (2007). Palmitoylation: Policing protein stability and traffic. Nature Reviews Molecular Cell Biology, 8(1), Article 1. 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]
- Liu Z, Busscher BM, Storl-Desmond M, & Xiao TS (2022). Mechanisms of Gasdermin recognition by proteases. Journal of Molecular Biology, 434(4), 167274. 10.1016/j.jmb.2021.167274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Fang Y, Chen X, Wang Z, Liang X, Zhang T, et al. (2020). Gasdermin E-mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome. Science Immunology, 5(43). 10.1126/sciimmunol.aax7969. [DOI] [PubMed] [Google Scholar]
- Liu X, & Lieberman J (2017). A mechanistic understanding of pyroptosis: The fiery death triggered by invasive infection. Advances in Immunology, 135, 81–117. 10.1016/bs.ai.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wang C, Rathkey JK, Yang J, Dubyak GR, Abbott DW, et al. (2018). Structures of the gasdermin D C-terminal domains reveal mechanisms of autoinhibition. Structure, 26(5), 778–784. 10.1016/j.str.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wang C, Yang J, Chen Y, Zhou B, Abbott DW, et al. (2020). Caspase-1 engages full-length gasdermin D through two distinct interfaces that mediate caspase recruitment and substrate cleavage. Immunity, 53(1), 106–114. 10.1016/j.immuni.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Wang C, Yang J, Zhou B, Yang R, Ramachandran R, et al. (2019). Crystal structures of the full-length murine and human gasdermin D reveal mechanisms of auto-inhibition, lipid binding, and oligomerization. Immunity, 51(1), 43–49. 10.1016/j.immuni.2019.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Xia S, Zhang Z, Wu H, & Lieberman J (2021). Channelling inflammation: Gasdermins in physiology and disease. Nature Reviews Drug Discovery, 20(5), 384–405. 10.1038/s41573-021-00154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, et al. (2016). Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature, 535(7610), 153–158. 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Min T, Eliezer D, & Wu H (2006). Native chemical ligation in covalent caspase inhibition by p35. Chemistry & Biology, 13(2), 117–122. 10.1016/j.chembiol.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Luchetti G, Roncaioli JL, Chavez RA, Schubert AF, Kofoed EM, Reja R, et al. (2021). Shigella ubiquitin ligase IpaH7.8 targets gasdermin D for degradation to prevent pyroptosis and enable infection. Cell Host & Microbe, 29(10), 1521–1530. 10.1016/j.chom.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkowska A, Roszak A, Lianeri M, Sowińska A, Sotiri E, & Jagodziński PP (2017). Analysis of rs8067378 polymorphism in the risk of uterine cervical cancer from a polish population and its impact on gasdermin B expression. Molecular Diagnosis & Therapy, 21(2), 199–207. 10.1007/s40291-017-0256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y,Jiang J, Gao Y, Shi T, Zhu X, Zhang K, et al. (2018). Research progress of the relationship between pyroptosis and disease. American Journal of Translational Research, 10(7), 2213–2219. [PMC free article] [PubMed] [Google Scholar]
- Magnani L, Colantuoni M, & Mortellaro A (2022). Gasdermins: New therapeutic targets in host defense, inflammatory diseases, and cancer. Frontiers in Immunology, 13. https://www.frontiersin.org/articles/10.3389/fimmu.2022.898298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin NT, & Martin MU (2016). Interleukin 33 is a guardian of barriers and a local alarmin. Nature Immunology, 17(2), Article 2. 10.1038/ni.3370. [DOI] [PubMed] [Google Scholar]
- Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, et al. (2010). Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nature Immunology, 11(12), 1136–1142. 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliorini P, Italiani P, Pratesi F, Puxeddu I, & Boraschi D (2020). The IL-1 family cytokines and receptors in autoimmune diseases. Autoimmunity Reviews, 19(9), 102617. 10.1016/j.autrev.2020.102617. [DOI] [PubMed] [Google Scholar]
- Moffatt MF, Kabesch M, Liang L, Dixon AL, Strachan D, Heath S, et al. (2007). Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature, 448(7152), Article 7152. 10.1038/nature06014. [DOI] [PubMed] [Google Scholar]
- Monteleone M, Stanley AC, Chen KW, Brown DL, Bezbradica JS, von Pein JB, et al. (2018). Interleukin-1β maturation triggers its relocation to the plasma membrane for gasdermin-D-dependent and -independent secretion. Cell Reports, 24(6), 1425–1433. 10.1016/j.celrep.2018.07.027. [DOI] [PubMed] [Google Scholar]
- Nozaki K, Maltez VI, Rayamajhi M, Tubbs AL, Mitchell JE, Lacey CA, et al. (2022). Caspase-7 activates ASM to repair gasdermin and perforin pores. Nature, 606(7916), Article 7916. 10.1038/s41586-022-04825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obstfeld AE, Frey NV, Mansfield K, Lacey SF, June CH, Porter DL, et al. (2017). Cytokine release syndrome associated with chimeric-antigen receptor T-cell therapy: Clinicopathological insights. Blood, 130(23), 2569–2572. 10.1182/blood-2017-08-802413. [DOI] [PubMed] [Google Scholar]
- Oltra SS, Sin L, Colomo S, Pérez-López M, Molina-Crespo A, Choi K-H, et al. (2022). Distinct GSDMB protein isoforms and protease cleavage processes differentially control pyroptotic cell death and mitochondrial damage in cancer cells. bioRxiv. 10.1101/2022.07.24.501218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orning P, Weng D, Starheim K, Ratner D, Best Z, Lee B, et al. (2018). Pathogen blockade of TAK1 triggers caspase-8–dependent cleavage of gasdermin D and cell death. Science, 362(6418), 1064–1069. 10.1126/science.aau2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panganiban RA, Sun M, Dahlin A, Park H-R, Kan M, Himes BE, et al. (2018). A functional splice variant associated with decreased asthma risk abolishes the ability of gasdermin B to induce epithelial cell pyroptosis. Journal of Allergy and Clinical Immunology, 142(5), 1469–1478.e2. 10.1016/j.jaci.2017.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana N, Privitera G, Kondolf HC, Bulek K, Lechuga S, De Salvo C, et al. (2022). GSDMB is increased in IBD and regulates epithelial restitution/repair independent of pyroptosis. Cell, 185(2), 283–298.e17. 10.1016/j.cell.2021.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam VAK, Zhao Y, & Shao F (2019). Innate immunity to intracellular LPS. Nature Immunology, 20(5), 527–533. 10.1038/s41590-019-0368-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathkey JK, Xiao TS, & Abbott DW (2020). Human polymorphisms in GSDMD alter the inflammatory response. Journal of Biological Chemistry, 295(10), 3228–3238. 10.1074/jbc.RA119.010604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathkey JK, Zhao J, Liu Z, Chen Y, Yang J, Kondolf HC, et al. (2018). Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Science Immunology, 3(26), eaat2738. 10.1126/sciimmunol.aat2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C, Erkes DA, Nardone A, Aplin AE, Fernandes-Alnemri T, & Alnemri ES (2019). Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nature Communications, 10(1), 1–17. 10.1038/s41467-019-09397-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, & Alnemri ES (2017). Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nature Communications, 8, 14128. 10.1038/ncomms14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan J, Xia S, Liu X, Lieberman J, & Wu H (2018). Cryo-EM structure of the gasdermin A3 membrane pore. Nature, 557(7703), 62–67. 10.1038/s41586-018-0058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubartelli A, Cozzolino F, Talio M, & Sitia R (1990). A novel secretory pathway for interleukin-1 beta, a protein lacking a signal sequence. The EMBO Journal, 9(5), 1503–1510. 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rühl S, Shkarina K, Demarco B, Heilig R, Santos JC, & Broz P (2018). ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science, 362(6417), 956–960. 10.1126/science.aar7607. [DOI] [PubMed] [Google Scholar]
- Ryder CB, Kondolf HC, O’Keefe ME, Zhou B, & Abbott DW (2022). Chemical modulation of gasdermin-mediated pyroptosis and therapeutic potential. Journal of Molecular Biology, 434(4), 167183. 10.1016/j.jmb.2021.167183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki N, Komatsuzaki R, Chiwaki F, Yanagihara K, & Sasaki H (2015). A GSDMB enhancer-driven HSV thymidine kinase-expressing vector for controlling occult peritoneal dissemination of gastric cancer cells. BMC Cancer, 15(1), 439. 10.1186/s12885-015-1436-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki N, Kuwahara Y, Sasaki H, Satoh H, & Shiroishi T (2000). Gasdermin (Gsdm) localizing to mouse chromosome 11 is predominantly expressed in upper gastrointestinal tract but significantly suppressed in human gastric cancer cells. Mammalian Genome, 11(9), 718–724. [DOI] [PubMed] [Google Scholar]
- Saleh NM, Raj SM, Smyth DJ, Wallace C, Howson JMM, Bell L, et al. (2011). Genetic association analyses of atopic illness and proinflammatory cytokine genes with type 1 diabetes. Diabetes/Metabolism Research and Reviews, 27(8), 838–843. 10.1002/dmrr.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria A, Wang B, Elowe S, Malik R, Zhang F, Bauer M, et al. (2011). The Plk1-dependent phosphoproteome of the early mitotic spindle. Molecular & Cellular Proteomics, 10(1). 10.1074/mcp.M110.004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarhan J, Liu BC, Muendlein HI, Li P, Nilson R, Tang AY, et al. (2018). Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proceedings of the National Academy of Sciences of the United States of America, 115(46), E10888–E10897. 10.1073/pnas.1809548115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Koide T, Masuya H, Wakana S, Sagai T, Umezawa A, et al. (1998). A new mutation Rim3 resembling Re(den) is mapped close to retinoic acid receptor alpha (Rara) gene on mouse chromosome 11. Mammalian Genome, 9(1), 20–25. [DOI] [PubMed] [Google Scholar]
- Schroder K, & Tschopp J (2010). The inflammasomes. Cell, 140(6), 821–832. 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Schutter ED, Roelandt R, Riquet FB, Camp GV, Wullaert A, & Vandenabeele P (2021). Punching holes in cellular membranes: Biology and evolution of gasdermins. Trends in Cell Biology, 31(6), 500–513. 10.1016/j.tcb.2021.03.004. [DOI] [PubMed] [Google Scholar]
- Shi J, Gao W, & Shao F (2017). Pyroptosis: Gasdermin-mediated programmed necrotic cell death. Trends in Biochemical Sciences, 42(4), 245–254. 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- Shi P, Tang A, Xian L, Hou S, Zou D, Lv Y, et al. (2015). Loss of conserved Gsdma3 self-regulation causes autophagy and cell death. Biochemical Journal, 468(2), 325–336. 10.1042/BJ20150204. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, et al. (2014). Inflammatory caspases are innate immune receptors for intracellular LPS. Nature, 514, 187–192. 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. (2015). Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature, 526, 660–665. 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- Söderman J, Berglind L, & Almer S (2015). Gene expression-genotype analysis implicates GSDMA, GSDMB, and LRRC3C as contributors to inflammatory bowel disease susceptibility. BioMed Research International, 2015, e834805. 10.1155/2015/834805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollberger G, Choidas A, Burn GL, Habenberger P, Di Lucrezia R, Kordes S, et al. (2018). Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Science Immunology, 3(26), eaar6689. 10.1126/sciimmunol.aar6689. [DOI] [PubMed] [Google Scholar]
- Stein MM, Thompson EE, Schoettler N, Helling BA, Magnaye KM, Stanhope C, et al. (2018). A decade of research on the 17q12-21 asthma locus: Piecing together the puzzle. Journal of Allergy and Clinical Immunology, 142(3), 749–764.e3. 10.1016/j.jaci.2017.12.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Yang J, Xing G, Sun Q, Zhang L, & He F (2008). Expression of GSDML associates with tumor progression in uterine cervix cancer. Translational Oncology, 1(2), 73–IN1. 10.1593/tlo.08112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taabazuing CY, Okondo MC, & Bachovchin DA (2017). Pyroptosis and apoptosis pathways engage in bidirectional crosstalk in monocytes and macrophages. Cell Chemical Biology, 24(4), 507–514. 10.1016/j.chembiol.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M, Tanaka S, Fujii T, Aoki A, Komiyama H, Ezawa K, et al. (2007). Members of a novel gene family, Gsdm, are expressed exclusively in the epithelium of the skin and gastrointestinal tract in a highly tissue-specific manner. Genomics, 89(5), 618–629. 10.1016/j.ygeno.2007.01.003. [DOI] [PubMed] [Google Scholar]
- The UniProt Consortium. (2023). UniProt: The universal protein knowledgebase in 2023. Nucleic Acids Research, 51(D1), D523–D531. 10.1093/nar/gkac1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Laer L, Huizing EH, Verstreken M, van Zuijlen D, Wauters JG, Bossuyt PJ, et al. (1998). Nonsyndromic hearing impairment is associated with a mutation in DFNA5. Nature Genetics, 20(2), 194–197. 10.1038/2503. [DOI] [PubMed] [Google Scholar]
- Van Opdenbosch N, & Lamkanfi M (2019). Caspases in cell death, inflammation, and disease. Immunity, 50(6), 1352–1364. 10.1016/j.immuni.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vora SM, Lieberman J, & Wu H (2021). Inflammasome activation at the crux of severe COVID-19. Nature Reviews Immunology, 21(11), Article 11. 10.1038/s41577-021-00588-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Gao W, Shi X, Ding J, Liu W, He H, et al. (2017). Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature, 547(7661), 99–103. 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- Wang C, & Ruan J (2022). Mechanistic insights into gasdermin pore formation and regulation in pyroptosis. Journal of Molecular Biology, 434(4), 167297. 10.1016/j.jmb.2021.167297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Sun Q, Zhong X, Zeng M, Zeng H, Shi X, et al. (2020). Structural mechanism for GSDMD targeting by autoprocessed caspases in pyroptosis. Cell, 180(5), 941–955. 10.1016/j.cell.2020.02.002. [DOI] [PubMed] [Google Scholar]
- Wang Q, Wang Y, Ding J, Wang C, Zhou X, Gao W, et al. (2020). A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature, 579(7799), Article 7799. 10.1038/s41586-020-2079-1. [DOI] [PubMed] [Google Scholar]
- Wolf AJ, Reyes CN, Liang W, Becker C, Shimada K, Wheeler ML, et al. (2016). Hexokinase is an innate immune receptor for the detection of bacterial peptidoglycan. Cell, 166(3), 624–636. 10.1016/j.cell.2016.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S, Zhang Z, Magupalli VG, Pablo JL, Dong Y, Vora SM, et al. (2021). Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature, 593(7860), 607–611. 10.1038/s41586-021-03478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Zhang F, Ji P, Wei M, Yin C, Yang A, et al. (2023). Efficient delivery of GSDMD-N mRNA by engineered extracellular vesicles induces pyroptosis for enhanced immunotherapy. Small. 10.1002/smll.202204031. [DOI] [PubMed] [Google Scholar]
- Yang K, Zhang XJ, Cao LJ, Liu XH, Liu ZH, Wang XQ, et al. (2014). Toll-like receptor 4 mediates inflammatory cytokine secretion in smooth muscle cells induced by oxidized low-density lipoprotein. PLoS One, 9(4), e95935. 10.1371/journal.pone.0095935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Zheng J, He Q, Zhang X, Li X, Ma Y, et al. (2023). Insights into the GSDMB-mediated cellular lysis and its targeting by IpaH7.8. Nature Communications, 14(1), Article 1. 10.1038/s41467-022-35725-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangiabadi S, & Abdul-Sater AA (2022). Regulation of the NLRP3 inflammasome by posttranslational modifications. The Journal of Immunology, 208(2), 286–292. 10.4049/jimmunol.2100734. [DOI] [PubMed] [Google Scholar]
- Zanoni I, Tan Y, Gioia MD, Broggi A, Ruan J, Shi J, et al. (2016). An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science, 352(6290), 1232–1236. 10.1126/science.aaf3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhang Y, Xia S, Kong Q, Li S, Liu X, et al. (2020). Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature, 579(7799), 415–420. 10.1038/s41586-020-2071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhou B, Sun R, Ai Y, Cheng K, Li F, et al. (2021). The metabolite α-KG induces GSDMC-dependent pyroptosis through death receptor 6-activated caspase-8. Cell Research, 31(9), Article 9. 10.1038/s41422-021-00506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Deng W, Bai Y, Miao R, Mei S, Zhang Z, et al. (2021). The lysosomal rag-Ragulator complex licenses RIPK1- and caspase-8-mediated pyroptosis by yersinia. Science, 372(6549). 10.1126/science.abg0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Yuan D, Zhang F, & Tang R (2022). A systematic pan-cancer analysis of the gasdermin (GSDM) family of genes and their correlation with prognosis, the tumor microenvironment, and drug sensitivity. Frontiers in Genetics, 13, 926796. 10.3389/fgene.2022.926796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, & Abbott DW (2021). Gasdermin E permits interleukin-1 beta release in distinct sublytic and pyroptotic phases. Cell Reports, 35(2), 108998. 10.1016/j.celrep.2021.108998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, He H, Wang K, Shi X, Su Y, Wang Y, et al. (2020). Granzyme a from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science, 368(6494), eaaz7548. 10.1126/science.aaz7548. [DOI] [PubMed] [Google Scholar]