Abstract
After binding, Clostridium perfringens enterotoxin (CPE) initially localizes in a small (∼90-kDa) complex in plasma membranes. This event is followed by formation of a second membrane complex, referred to as large (160-kDa) complex. Contrary to a previous hypothesis proposing that CPE inserts into intestinal brush border membranes (BBMs) when this toxin is localized in the small complex, this study shows that BBMs do not offer CPE localized in the small complex protection from pronase. However, our experiments indicate that BBMs do substantially protect CPE from pronase when this toxin is localized in large complex. Since the onset of CPE-induced permeability alterations closely coincides with large-complex formation, these new results suggest that CPE-induced alterations in permeability may result from pore formation due to the partial membrane insertion of CPE when this toxin is present in large complex.
Clostridium perfringens enterotoxin (CPE) is a 35-kDa single polypeptide that contributes to the pathogenesis of C. perfringens type A food poisoning, as well as several nonfoodborne human diarrheas (e.g., antibiotic-associated diarrhea) and gastrointestinal illnesses of domestic animals (1–3, 6, 13, 14, 22). The enteropathogenic effects of CPE are primarily mediated through a multistep cytotoxic action (13), which initiates when CPE binds to a proteinaceous receptor(s) (5, 7, 8, 16, 18, 24, 25). While the full repertoire of membrane proteins capable of serving as functional CPE receptors remains undetermined, recent expression cloning experiments (7, 8) have demonstrated that several homologues of the rat androgen withdrawal apoptosis protein RVP1 are functional CPE receptors. Further, a biochemical study (24) has suggested that an ∼50-kDa CPE-binding membrane protein may also be a CPE receptor. Upon binding, CPE rapidly becomes localized in a small (∼90-kDa) complex present in plasma membranes (24); at temperatures above 4°C, this small CPE complex then apparently associates with at least one additional membrane protein to form a large (160-kDa) complex (7, 15, 24, 26). Formation of this large complex coincides with the onset of CPE-induced small-molecule membrane permeability alterations (15), which (in turn) result in cell death from overt lysis or metabolic disruption (13).
Even though CPE apparently possesses a relatively modest initial binding affinity for cells or isolated membranes (16), reports indicate that, after binding, this toxin does not appreciably dissociate from either isolated membranes or intact cells (4, 15–17, 25). Further, agents (e.g., chaotropic salts or divalent cation chelators) known to release peripherally bound membrane proteins do not promote dissociation of bound CPE (16, 17). The irreversible nature of CPE binding cannot be explained by the internalization of CPE into the cytoplasm, since a subcellular localization study (23) indicated that CPE remains plasma membrane associated throughout its action. Further, this essentially irreversible CPE binding does not correspond to large-complex formation, since CPE reportedly also does not dissociate appreciably at 4°C (16), a temperature where large-complex formation is sharply inhibited (15).
Nearly 20 years ago, McDonel reported that specifically bound CPE exhibits resistance to pronase-induced release from membranes (17), even though both CPE and the CPE receptor are highly sensitive to pronase (16, 17). McDonel hypothesized that this resistance of bound CPE to pronase-induced release from membranes reflects protection of CPE due to the insertion of this toxin into the lipid bilayers of plasma membranes and proposed that this insertion event explains why CPE does not dissociate after binding. Since CPE bound at 4°C also reportedly develops resistance to pronase-induced release from membranes (15), McDonel’s hypothesis implies that membrane insertion occurs when CPE is sequestered in small complex. This hypothesis has enjoyed wide acceptance because insertion is known to contribute to the action of several other bacterial toxins that disrupt plasma membrane permeability properties (20).
However, an antibody probe study (9) of CPE-containing membranes has recently shown that, whether CPE is localized in small or large complex, at least a portion of the enterotoxin molecule remains exposed on the external surfaces of mammalian plasma membranes. Since at least a portion of CPE remains exposed on the membrane surface even under conditions where CPE is reportedly resistant to pronase-induced release from membranes, these recent antibody probe findings appear to be in some conflict with the hypothesis that, when it is sequestered in the small complex, CPE becomes resistant to pronase-induced release from membranes due to the insertion of this toxin into lipid bilayers. To address the apparent conflict between these antibody probe results and the CPE small-complex insertion hypothesis, the present study has carefully characterized the effects of pronase treatment of rabbit intestinal brush border membranes (BBMs) containing CPE localized in either small complex or large complex.
MATERIALS AND METHODS
Materials.
CPE was prepared and purified, and its biologic activity was assayed as described previously (19). Purified CPE was radiolabeled as described previously (16) with lactoperoxidase-glucose oxidase (Bio-Rad) and 2 mCi of Na125I (17 mCi/mg; ICN Radiochemicals). The specific radioactivity of 125I-CPE was 1 to 3 mCi/mg of protein. Rabbit intestinal BBMs were prepared from the small intestines of female New Zealand White rabbits (2 to 4 kg each) by the method of Sigrist et al. (21). Pronase was purchased from Boehringer Mannheim; antipain, chymostatin, leupeptin, pepstatin, phenylmethylsulfonyl fluoride, dimethyl sulfoxide, EDTA, and Azocoll (Azo dye-impregnated collagen) were purchased from Sigma; and AEBSF [4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride] was obtained from ICN Biomedicals.
Binding of 125I-CPE to intact BBMs.
BBMs (100 μg) were gently shaken for 5 min at 4°C or room temperature (RT) in 100 μl of DPBS (140 mM NaCl, 2.7 mM KCl, 9 mM Na2HPO4, 1.5 mM KH2PO4 [pH 7.4]) containing 1.5 μg of 125I-CPE in the presence or absence of a 50-fold excess of unlabeled CPE. As described previously (15, 16, 24), specific binding of 125I-CPE was calculated by subtracting the radioactive counts associated with BBMs that had been cotreated with excess unlabeled CPE (nonspecific binding) from the radioactive counts associated with BBMs that had been treated only with 125I-CPE (total binding). Specific binding was ∼105 cpm/sample and represented ∼80% of the total bound radioactivity.
Optimization of pronase inhibition conditions.
Prior to characterization of the 125I-CPE-containing complexes remaining in pronase-treated BBMs (see below), it was first necessary to develop conditions that would stop proteolysis at the termination of the desired pronase treatment period, i.e., to stop proteolysis when the 125I-CPE-containing complexes were still present in pronase-treated BBMs, so that no further degradation of these complexes would occur during detergent extraction or electrophoresis. To optimize conditions for stopping pronase activity, 100 μg of BBMs was treated at 4°C with 300 μg of freshly prepared pronase and these BBMs were then washed with DPBS, with or without various concentrations of protease inhibitors, zero to three times before being extracted with cold DPBS containing 1% Triton X-100, with or without various concentrations of protease inhibitors. Free 125I-CPE was then added to each sample during extraction, and the extracted samples were boiled, electrophoresed by denaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiographed. These experiments indicated that, when they were present during detergent extraction and three washes, 500 μg of antipain per ml, 20 μg of aprotinin per ml, 600 μg of chymostatin per ml, 5 mg of EDTA per ml, 5 μg of leupeptin per ml, 10 mg of AEBSF per ml, 7 μg of pepstatin per ml, and 2 mM phenylmethylsulfonyl fluoride prevented any significant degradation of free 125I-CPE (data not shown). Consequently, this combination of protease inhibitors was employed throughout this study to stop proteolysis after the conclusion of the desired pronase treatment period.
Pronase treatment of 125I-CPE-containing BBMs.
BBMs containing 125I-CPE bound at 4°C or RT were prepared as described above, washed once with cold DPBS, and resuspended in 50 μl of cold DPBS containing 300 μg of freshly prepared pronase. These BBMs were treated with pronase for 5 min or 1 h (as specified in the figures) at 4°C and then microcentrifuged. The radioactivity of the pellets was then either directly counted in a gamma counter (unwashed samples) or counted after the pellets were washed three times with 100 μl of cold DPBS containing the optimal concentrations of protease inhibitors (as stated above) (washed samples).
Pronase treatment of control BBMs.
In some experiments, BBMs (100 μg) that had never been exposed to 125I-CPE were washed with cold DPBS and treated for 5 min or 1 h at 4°C in 50 μl of cold DPBS containing 300 μg of freshly prepared pronase. After this pronase treatment, the BBMs were washed three times with 100 μl of cold DPBS (containing protease inhibitors) before being extracted with 100 μl of cold DPBS containing both 1% Triton X-100 and our standard combination of protease inhibitors. These extracts were analyzed by denaturing SDS-PAGE.
Analytical PAGE of samples.
Some samples of washed pronase-treated BBMs containing 125I-CPE bound at either 4°C or RT were analyzed by PAGE (as described below). These BBMs were prepared, pronase treated, and washed as described above and then extracted at 4°C with 100 μl of cold DPBS containing both 1% Triton X-100 and the same concentrations of protease inhibitors that were used for washing. The radioactivity of these membrane extracts was then counted in a gamma counter prior to electrophoresis (as specified below). Wash samples were also collected and their radioactivity was counted in a gamma counter, and then the samples were frozen and lyophilized for later electrophoretic analysis.
To compare the pronase sensitivity of membrane-associated extracts with that of extracted small (or large) complex, some BBMs containing 125I-CPE bound at 4°C or RT were extracted at 4°C with DPBS containing 1% Triton X-100 (but no protease inhibitors) prior to a 0- to 60-min treatment with 300 μg of pronase at 4°C. After completion of this pronase digestion, our standard combination of protease inhibitors was added and these samples were electrophoresed as specified below.
To help evaluate the protective effects of BBMs against the pronase digestion of 125I-CPE (see Results), 0.15 μg of free (i.e., no BBMs present) 125I-CPE was dissolved in DPBS in the presence of 100 μg of bovine serum albumin. This mixture was then treated with 300 μg of pronase for 5 min or 1 h (as specified in the figures) at 4°C, before the digestion was stopped with our standard combination of protease inhibitors and samples were boiled and electrophoresed (see below).
As specified in the figure legends, the samples prepared as described above were analyzed by one of the following electrophoretic systems. (i) In denaturing SDS-PAGE (with sample boiling and treatment with β-mercaptoethanol), samples containing Triton X-100 received 5× SDS sample buffer (resulting in final sample concentrations of 2% SDS and 5% β-mercaptoethanol) before being boiled for 3 min in a boiling-water bath. These samples were then electrophoresed by conventional SDS-PAGE on 10, 12, or 15% acrylamide gels (as specified below) by using the buffer system of Laemmli (11). In some cases, as noted in the figure legends, samples were treated with urea (6 M final concentration) for 20 min at 95°C before receiving SDS sample buffer and being boiled. After electrophoresis, gels were stained with Coomassie brilliant blue R-250, dried, and autoradiographed at −80°C. (ii) In SDS-PAGE (without sample boiling) with 6% acrylamide gels, migration of the 160-kDa large complex was analyzed with 6% acrylamide gels, without boiling of samples or β-mercaptoethanol treatment, as described previously (9, 10, 15, 24). Following electrophoresis, the gels were dried and autoradiographed at −80°C. (iii) In Triton X-100 PAGE, migration of the 90-kDa small complex was analyzed with 6% native acrylamide gels containing 0.1% Triton X-100, as described previously (9, 24). Note that these samples were not boiled before electrophoresis. After electrophoresis, gels were dried and autoradiographed at −80°C.
Preparative electrophoresis of small- and large-complex samples.
BBMs (5 mg) were incubated (with gentle shaking) for 5 min, at either 4°C or RT, in 5 ml of DPBS with 15 μg of 125I-CPE. These BBMs were then washed with cold DPBS, resuspended in cold DPBS, and incubated at 4°C for 5 min or 1 h in 2.5 ml of cold DPBS containing 3 mg of freshly prepared pronase. After three washes at 4°C with cold DPBS (containing our standard combination of protease inhibitors), the washed BBMs were extracted with 2.5 ml of cold DPBS containing both 1% Triton X-100 and our standard combination of protease inhibitors. Some BBMs containing bound 125I-CPE were extracted with 1% Triton X-100 in cold DPBS (but without protease inhibitors) prior to the pronase treatment described above. After completion of this pronase treatment, protease inhibitors were added to these samples at the same final concentrations used in other experiments.
Samples containing small complex (i.e., samples that were 125I-CPE treated at 4°C) received 2.5 ml of native PAGE sample buffer and were then electrophoresed on preparative (1.5-mm-thick) native 6% acrylamide gels containing 0.1% Triton X-100. Samples containing large complex (samples that had been treated with 125I-CPE at RT) received 2.5 ml of SDS sample buffer without β-mercaptoethanol, before being electrophoresed on preparative SDS–6% acrylamide gels.
After electrophoresis, these gels were exposed to X-ray films overnight at −80°C. Bands corresponding to small or large complex were then excised from native or SDS gels, respectively. Proteins in the excised gel slices were electroeluted at 50 mA in SDS buffer (25 mM Tris, 0.192 M glycine, 0.1% [wt/vol] SDS [pH 8.3]) containing protease inhibitors (as specified above) for 3 h at 4°C with a SpectaPor 3 (Spectrum) membrane (molecular weight cutoff, 3,500). Eluted samples (usually 4 to 5 ml) were then concentrated with Centricon 3 microconcentrators (Amicon) to a final volume of 500 μl, before storage at −20°C and denaturing SDS-PAGE.
Azocoll assay.
The Azocoll colorimetric assay was used to compare the proteolytic activity of freshly prepared pronase solution (300 μg/50 μl) with that of the same pronase solution after it had been used to treat BBMs. In this assay, 50 mg of Azocoll was incubated with an aliquot of pronase solution for 15 min at 37°C in 5 ml of DPBS. The nondigested collagen was then pelleted, and the remaining supernatant was used to measure A520.
Other methods.
Protein determinations were by the method of Lowry et al. (12), with bovine serum albumin as the protein standard. Radioactivity in samples containing 125I was quantitated with a Packard gamma counter. Densitometric scans of autoradiographs and gels were performed with a ScanJet Plus (Hewlett-Packard) with the DeskScan II, version 2.3, program, and peak-area integrations were determined with the 1-D Process & Report program (Zeineh Biomedical Instruments).
RESULTS
Washing increases pronase-induced release of specifically bound 125I-CPE from BBMs.
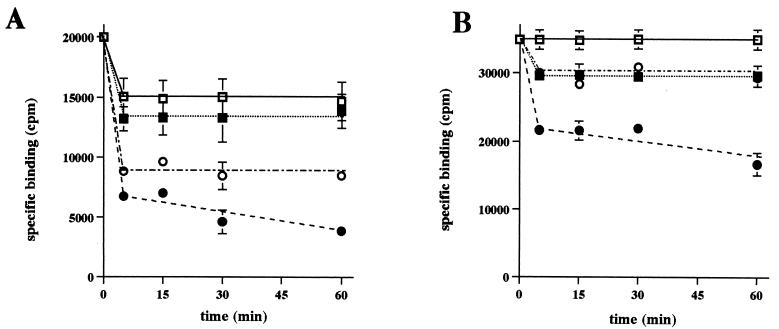
This study initially reevaluated previous reports (4, 15–17) indicating that, at either 4°C or warmer temperatures, CPE binding is essentially irreversible. Surprisingly, our experiments revealed (Fig. 1A) that about 25% of specifically bound radioactivity does spontaneously dissociate (i.e., dissociate in the absence of washing) in the first five minutes after 125I-CPE binding at 4°C. However, the remaining 75% of specifically bound radioactivity present in these samples was still membrane associated after 60 min of incubation at 4°C. Further, extensively washing BBMs containing 125I-CPE bound at 4°C only slightly (∼10%) increased the release of specifically bound radioactivity throughout the 1-h incubation period of this experiment (Fig. 1A).
FIG. 1.
Effects of washing BBMs on pronase-induced release of specifically bound 125I-CPE. 125I-CPE was bound to BBMs at 4°C (A) or at RT (B) in the presence or absence of excess unlabeled CPE. After unbound 125I-CPE was removed, the BBMs were incubated at 4°C with or without pronase (300 μg) for 5 min to 1 h. Circles, specifically bound radioactivity remaining in these pronase-treated BBMs; squares, specifically bound radioactivity remaining in the non-pronase-treated BBMs. After this pronase treatment, BBMs were pelleted and then their radioactivity was either directly counted in a gamma counter (nonwashed samples [open symbols]) or counted after the pellets were washed three times with cold DPBS containing protease inhibitors (see the text) (washed samples [filled symbols]). Values are means ± standard errors of the means. Error bars not shown indicate that the values were too small to depict. Each experiment was repeated three times with triplicate samples.
When the same experiment was repeated with 125I-CPE bound at RT, no appreciable spontaneous dissociation of specifically bound radioactivity was detected at any time during the 1-h experimental period (Fig. 1B). Further, extensively washing BBMs containing 125I-CPE bound at RT caused only a slight (∼10 to ∼15%) increase in dissociation of specifically bound radioactivity, with essentially all of this limited, washing-induced dissociation occurring in the initial 5-min period after binding (Fig. 1B). Therefore, the results shown in Fig. 1 collectively confirm that, except for a brief, initial period of limited CPE dissociation that is particularly apparent for samples containing 125I-CPE bound at 4°C, 125I-CPE specifically bound at either 4°C or RT does not readily dissociate from control (i.e., non-pronase-treated) BBMs incubated at 4°C, even if these BBMs are extensively washed after 125I-CPE binding.
A series of experiments was then performed (Fig. 1) to reassess previous conclusions (4, 15–17) indicating that, regardless of whether 125I-CPE binding is performed at 4°C or RT, pronase treatment releases only limited (∼20 to ∼40%) amounts of specifically bound radioactivity from these 125I-CPE-containing BBMs. Our results confirmed that substantial amounts of radioactivity specifically bound at either 4°C or RT remain present in unwashed, pronase-treated BBMs. We found that, relative to the level of radioactivity of similarly prepared, non-pronase-treated, and unwashed BBMs that had been incubated at 4°C for either 5 or 60 min after 125I-CPE binding at 4°C, ∼60% of the radioactivity specifically bound at 4°C remained membrane associated in BBMs treated with pronase at 4°C for 5 to 60 min without subsequent washing. Additionally, relative to the level of radioactivity of similarly prepared, non-pronase-treated and unwashed BBMs that had been incubated at 4°C for either 5 or 60 min after 125I-CPE binding at RT, ∼85% of the radioactivity specifically bound at RT remained membrane associated in BBMs treated with pronase at 4°C for 5 or 60 min without subsequent washing.
However, modifying these experiments so that the BBMs were washed three times with cold DPBS (containing our standard combination of protease inhibitors) after pronase treatment was found to substantially increase the release of specifically bound radioactivity, irrespective of whether these BBMs contained 125I-CPE bound at 4°C or RT. As shown in Fig. 1A, relative to the levels of radioactivity of similarly prepared, but unwashed and non-pronase-treated, BBMs incubated for 5 or 60 min after 125I-CPE binding at 4°C, only ∼45 or ∼30% of the radioactivity that was specifically bound at 4°C remained membrane associated when these BBMs were pronase treated at 4°C for 5 min or 60 min, respectively, and subsequently washed. Washing also increased the release of specifically bound radioactivity from pronase-treated membranes containing 125I-CPE bound at RT (Fig. 1B), i.e., relative to the levels of radioactivity of similarly prepared, but unwashed and non-pronase-treated, BBMs that were incubated for 5 or 60 min after 125I-CPE binding at RT, only ∼70 or 52% of radioactivity specifically bound at RT remained membrane associated when these BBMs were pronase treated at 4°C for 5 or 60 min, respectively, and subsequently washed.
Effects of washing on the recovery of BBM proteins.
The ability of washing to increase the release of specifically bound radioactivity from pronase-treated BBMs containing 125I-CPE, as shown in Fig. 1, could indicate that either (i) specifically bound radioactivity becomes more loosely membrane associated after pronase treatment (suggesting that this radioactivity may be affected by pronase) or that (ii) extensive washing sharply decreases the recovery of pronase-treated BBMs, which would cause a nonspecific decrease in the recovery of all proteins (including 125I-CPE) present in BBMs before pronase treatment. To discriminate between these two possibilities, we performed an experiment in which pronase-treated BBMs, with or without bound CPE, were washed three times with DPBS (containing our standard combination of protease inhibitors), extracted with 1% Triton X-100, electrophoresed by denaturing SDS-PAGE with sample boiling, and stained with Coomassie brilliant blue (Fig. 2). Densitometric scanning of gels from seven independent repetitions of this experiment (a representative gel is shown in Fig. 2) indicated that, relative to the recovery of washed BBMs processed similarly except for the omission of pronase treatment, 77% ± 6% of total BBM proteins were recovered from BBMs treated for 5 min with pronase before being washed and 71% ± 6% of the total BBM proteins were recovered from membranes treated for 1 h with pronase before being washed. Recovery of total BBM proteins from either pronase-treated BBMs or non-pronase-treated BBMs, whether washed or unwashed, was unaffected (data not shown) by the presence of CPE, whether this toxin had been bound at 4°C or RT. These results indicate that loss of total BBM proteins during washing cannot account for all of the washing-induced increase in the release of specifically bound CPE from pronase-treated BBMs that was observed in the experiments whose results are shown in Fig. 1.
FIG. 2.
Effect of washing on recovery of BBM proteins. BBMs were treated at 4°C with pronase (300 μg) and washed with cold DPBS (containing protease inhibitors; see Materials and Methods). These washed BBMs were extracted at 4°C with 1% Triton X-100, boiled, and electrophoresed on an SDS–10% polyacrylamide gel. The gel was then stained with Coomassie brilliant blue R-250. First lane, third molecular mass (Mr) markers; second lane, washed BBMs not treated with pronase (control); third lane, washed BBMs treated for 5 min with pronase; fourth lane, washed BBMs treated for 1 h with pronase. The gel shown in this figure is representative of gels used in seven repetitions of this experiment.
Electrophoretic analysis of pronase-treated 125I-CPE bound to BBMs at 4°C.
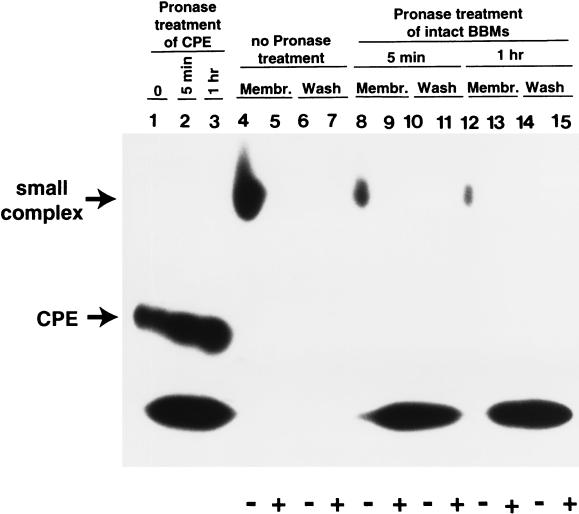
Before we analyzed whether CPE bound to BBMs at 4°C is affected by pronase, it was important to first confirm that the CPE present in these BBMs is localized in small complex. When BBMs containing 125I-CPE bound at 4°C were washed three times with DPBS, extracted with 1% Triton X-100, and analyzed (Fig. 3, lane 4) by native PAGE (24), virtually all of the specifically bound 125I-CPE in these samples was clearly localized in small complex. This result is fully consistent with previous reports (9, 15) indicating that little or no large complex forms at this low temperature. Further, native PAGE analysis of the washes collected from these non-pronase-treated BBMs showed (Fig. 3, lane 6) that there is little or no spontaneous dissociation of small complex from these BBMs. This observation is fully consistent with the Fig. 1A results indicating that, 5 min after binding at 4°C, there is only limited spontaneous dissociation of 125I-CPE from BBMs.
FIG. 3.
Native PAGE analysis of pronase-treated 125I-CPE bound to BBMs at 4°C. 125I-CPE was bound at 4°C to BBMs in the presence (+ lanes, i.e., lanes 5, 7, 9, 11, 13, and 15) or absence (− lanes, i.e., lanes 4, 6, 8, 10, 12, and 14) of a 50-fold excess of unlabeled CPE. Samples were then treated at 4°C with pronase (300 μg) for 5 min (lanes 8 to 11) or 1 h (lanes 12 to 15) or were kept at 4°C without pronase treatment (lanes 4 to 7). All BBMs were then washed with cold DPBS (containing protease inhibitors; see Materials and Methods), extracted with cold DPBS containing 1% Triton X-100 and our standard combination of protease inhibitors, and electrophoresed on a 6% native gel (membrane [Membr.] lanes, i.e., lanes 4, 5, 8, 9, 12, and 13), as described previously (24). Washes from the BBMs were also collected, lyophilized, and electrophoresed on the same gel (Wash lanes, i.e., lanes 6, 7, 10, 11, 14, and 15). Lanes 1 to 3 show free 125I-CPE that was or was not treated with pronase and electrophoresed on the same native gel. Lane 1, 125I-CPE without pronase treatment; lane 2, 125I-CPE treated for 5 min with pronase; lane 3, 125I-CPE treated for 1 h with pronase. Migration of small complex and free 125I-CPE are noted at the left. The gel shown is representative of gels used in six independent repetitions of the experiment. Note that some nonspecific binding samples show significant levels of dye front radioactivity; this result is an artifact due to diffusion of low-Mr radioactive material from gel lanes containing pronase-treated specifically bound radioactivity during the native PAGE used in this experiment (data not shown).
When similarly prepared membranes containing only 125I-CPE sequestered in small complex were treated with pronase (300 μg), washed three times with cold DPBS (containing our standard combination of protease inhibitors), subjected to extraction with cold 1% Triton X-100, and analyzed by native PAGE (Fig. 3, lanes 8 and 12), some of the specifically bound radioactivity that remained membrane associated was still observed to comigrate with the small complex extracted from washed, non-pronase-treated BBMs. However, compared to amounts of membrane-associated radioactivity migrating like small complex in non-pronase-treated BBMs, there were substantially smaller amounts of such radioactivity migrating like small complex present in the pronase-treated BBMs. Six independent repetitions of the procedures used with the representative gel shown in Fig. 3 indicated that, relative to the level of specifically bound radioactivity migrating like small complex in washed, but not pronase-treated, BBMs incubated at 4°C for either 5 or 60 min after 125I-CPE binding at 4°C, only 42% ± 5% of the specifically bound radioactivity migrating like small complex remained in BBMs treated for 5 min with pronase before being washed but that only 28% ± 6% of the specifically bound radioactivity migrating like small complex remained in BBMs treated with pronase for 1 h before being washed. If small complex was extracted from BBMs prior to pronase treatment, native PAGE analysis (Fig. 4, third and fifth lanes) showed that some of this pronase-treated, specifically bound radioactivity still also comigrated with the small complex extracted from the control (i.e, non-pronase-treated) BBMs. After 5 min or 1 h of pronase treatment at 4°C, the pronase-treated extract samples contained 48% ± 3% or 30 ± 4%, respectively, of the specifically bound radioactivity migrating as small complex that was present in similarly prepared BBM extracts not treated with pronase.
FIG. 4.
Native PAGE analysis of pronase-treated Triton X-100 extracts from BBMs containing 125I-CPE bound at 4°C. 125I-CPE was bound to BBMs at 4°C in the absence (− lanes) or the presence (+ lanes) of a 50-fold excess of unlabeled CPE. After the unbound 125I-CPE was removed, BBMs were extracted with cold DPBS containing 1% Triton X-100 and then treated at 4°C with pronase (300 μg) for 5 min (third and fourth lanes) or 1 h (fifth and sixth lanes); the first and second lanes contain samples that were not pronase treated. After the conclusion of pronase treatment, protease inhibitors were added (see Materials and Methods) and all samples were electrophoresed on a native 6% polyacrylamide gel. The migration of small complex is noted at the left. The gel shown is representative of gels used in six independent repetitions of the experiment.
The radioactive material released from washed, pronase-treated BBMs containing small complex was also examined by native PAGE. In some experiments, a small amount of the specifically bound radioactivity released from pronase-treated BBMs still migrated like intact small complex. However, in most experiments (such as with the samples shown in Fig. 3, lanes 10 and 14), the specifically bound radioactivity released from these BBMS was highly degraded and ran at the dye front.
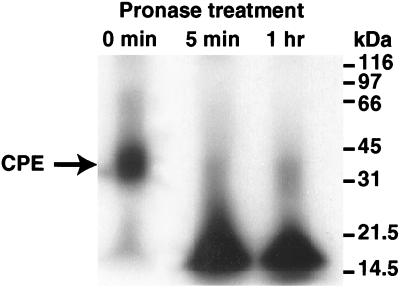
The apparent stability of the small complex remaining in washed, pronase-treated BBMs (as shown in Fig. 3) may indicate that either (i) membranes offer some small complex present in membranes protection from pronase (which would offer support for the hypothesis that CPE inserts into membranes when this toxin is localized in small complex) or (ii) the small complex present in washed, pronase-treated BBMs is affected by pronase but that the resultant proteolytic fragments remain associated together under the native electrophoresis conditions used to obtain the results shown in Fig. 3 and 4. Before we discriminated between these two possibilities, an experiment was performed where free 125I-CPE was treated at 4°C with pronase, followed by analysis of the resulting sample by denaturing SDS-PAGE with sample boiling. This experiment, which was performed to ensure that the pronase treatment conditions being used in our studies were sufficient to degrade the 125I-CPE present in our BBM samples containing small complex, became necessary when native PAGE (Fig. 3, lanes 2 and 3) analysis suggested that significant amounts of free CPE might remain unaffected by our standard pronase treatment. However, SDS-PAGE analysis indicated that, despite appearing to be unaffected by pronase on native PAGE, the 125I-CPE samples shown in Fig. 3 were, in fact, strongly degraded by pronase. Ten repetitions of the procedures used with the representative denaturing SDS-polyacrylamide gel shown in Fig. 5 demonstrated that even 5 min of treatment with the same pronase preparations used in our Fig. 3 and 4 experiments is sufficient to substantially degrade >95% of free 125I-CPE (note that this degradation of free 125I-CPE occurred despite the presence in these samples of 100 μg of bovine serum albumin, which was added so that these samples would contain the same total protein levels that were present in the pronase-treated BBM samples used in the Fig. 3 and 4 experiments).
FIG. 5.
SDS-PAGE analysis of free 125I-CPE treated with pronase. 125I-CPE (0.15 μg), in the presence of 100 μg of bovine serum albumin, was treated at 4°C with pronase (300 μg) for either 5 min (second lane) or 1 h (third lane) before receiving cold DPBS containing protease inhibitors (see Materials and Methods). These samples were then boiled and electrophoresed on an SDS–10% polyacrylamide gel. For comparison, the first lane shows 125I-CPE that had not been pronase treated. Migrations of molecular mass markers are shown at the right. The gel shown is representative of gels used in 10 independent repetitions of the experiment.
In order to discern conclusively whether the apparently full-size small complex remaining in our pronase-treated BBMs had, or had not, been affected by our pronase treatment conditions, large-scale preparations of washed, pronase-treated BBMs containing small complex were prepared, extracted with cold DPBS–1% Triton X-100, and electrophoresed by preparative native PAGE. When gel slices corresponding to the radioactivity migrating like small complex were excised from these preparative gels and the proteins in these excised gel slices were electroeluted, concentrated, treated with 6 M urea, boiled, and electrophoresed by denaturing SDS-PAGE with sample boiling, it was found (Fig. 6, lane 1) that pronase-treated BBMs containing 125I-CPE bound at 4°C had <10% of the levels of intact (i.e., 35-kDa) CPE that are present in similarly prepared and washed, but non-pronase-treated, BBMs (Fig. 6, lane 2). Similarly, when this experiment was repeated with small complex extracted from BBMs prior to pronase treatment, almost no radioactivity in this sample still ran as intact CPE after denaturing SDS-PAGE with sample boiling (Fig. 6, lane 3). Collectively, results shown in Fig. 6 conclusively demonstrate that CPE localized in small complex is highly susceptible to pronase treatment, regardless of whether the small complex is present in membranes during the pronase treatment or is extracted from BBMs prior to the pronase treatment.
FIG. 6.
SDS-PAGE analysis of pronase-treated 125I-CPE bound to BBMs at 4°C. 125I-CPE was bound to BBMs at 4°C, and after removal of unbound 125I-CPE, these samples were either treated for 60 min at 4°C with 300 μg of pronase (lane 1), kept for 60 min at 4°C as an untreated control (lane 2), or subjected to extraction with cold DPBS containing 1% Triton X-100 before treatment for 60 min at 4°C with 300 μg of pronase (lane 3). The lane 1 and 2 samples were washed with cold DPBS containing protease inhibitors (see Materials and Methods) before being subjected to extraction with 1% Triton X-100, while cold DPBS containing similar protease inhibitors were added to the lane 3 sample at the completion of pronase treatment. Each sample was then electrophoresed on preparative native 6% polyacrylamide gel, and samples containing small complex were excised from these gels. Proteins in the excised gel slices were electroeluted, concentrated, treated with urea, boiled, and analyzed on an SDS–12% polyacrylamide gel containing urea. Note that in lane 2 only half the volume of sample was loaded compared to that loaded in lanes 1 and 3. Migrations of molecular mass markers are shown at the right, while the migration of free 125I-CPE is shown at the left. The gel shown is representative of gels used in five independent repetitions of the experiment. Note that the presence of small amounts of intact 125I-CPE in lanes 1 and 3 is not due to spillover, since similar results were observed when these samples were each run on individual gels.
Electrophoretic analysis of pronase-treated 125I-CPE bound to BBMs at RT.
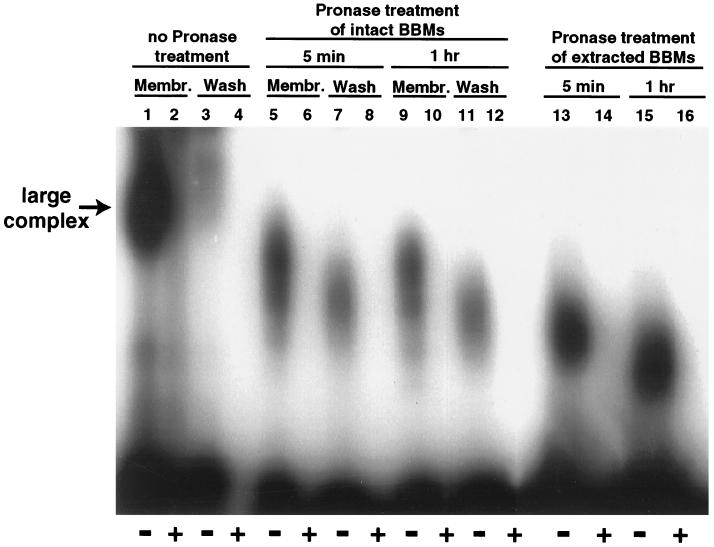
To confirm that most of the 125I-CPE bound to our BBM samples at RT had become localized in large complex, BBMs containing 125I-CPE bound at RT were extracted with 1% Triton X-100, and the Triton X-100 extracts were then analyzed with SDS–6% acrylamide gels without β-mercaptoethanol treatment or boiling of samples. As expected from previous studies (15, 24), most specifically bound radioactivity in these samples was observed to migrate as a large (∼160-kDa) complex (Fig. 7, lane 1). Even washing caused little or no release of this large complex from control (non-pronase-treated) BBMs (Fig. 7, lane 3), which is consistent with Fig. 1B results indicating that 125I-CPE that specifically bound at RT shows little or no dissociation.
FIG. 7.
SDS-PAGE analysis of pronase-treated 125I-CPE bound to BBMs at RT. 125I-CPE was bound to BBMs at RT in the absence (− lanes, i.e., odd-numbered lanes) or the presence (+ lanes, i.e., even-numbered lanes) of a 50-fold excess of unlabeled CPE. BBMs containing bound 125I-CPE were then treated at 4°C with pronase (300 μg) for 5 min (membrane [Membr.] lanes 5 and 6) or 1 h (Membr. lanes 9 and 10) or were extracted with 1% Triton X-100 before being treated at 4°C with 300 μg of pronase for 5 min (lanes 13 and 14) or 1 h (lanes 15 and 16). Membr. lanes 1 and 2 represent similarly prepared samples that were not treated with pronase. Samples shown in lanes 1, 2, 5, 6, 9, and 10 were washed with cold DPBS containing protease inhibitors (see Materials and Methods) before extraction. Washes (Wash lanes) were collected from all samples and then lyophilized. Lanes 7 and 8 and 11 and 12 contain wash samples collected after 5 min or 1 h of pronase treatment of BBMs, respectively. All samples were then electrophoresed on an SDS–6% polyacrylamide gel without sample boiling or β-mercaptoethanol treatment. The migration of intact large complex is noted at the left. The gel shown is representative of gels used in six independent repetitions of the experiment.
When similarly prepared BBMs containing large complex were pronase treated at 4°C, washed with cold DPBS (containing our standard combination of protease inhibitors), extracted with Triton X-100, and analyzed by SDS-PAGE with 6% acrylamide gels without either boiling or β-mercaptoethanol treatment of samples, significant amounts of membrane-associated large complex could still be detected (Fig. 7, lanes 5 and 9). Densitometric scanning of gels from six repetitions of this experiment (a representative gel is shown in Fig. 7) indicated that, relative to the amount of large complex present in similarly prepared and washed, but not pronase-treated, BBMs incubated for either 5 or 60 min at 4°C, 61% ± 2% of the amount of large complex remained in BBMs treated with pronase for 5 min before being washed but that 46% ± 3% of the levels of large complex remained in BBMs treated with pronase for 1 h before being washed. The results shown in Fig. 7 also indicate that pronase treatment decreases the size of the large complex remaining in these BBMs. This size decrease in large complex present in washed, pronase-treated BBMs progressed through the first hour of pronase treatment (Fig. 7, lanes 5 and 9) but then stabilized (data not shown), even though Azocoll digestion experiments indicated that the pronase used in this experiment remained fully active beyond an hour (data not shown).
When washes collected from the pronase-treated BBMs containing large complex were similarly analyzed by SDS-PAGE with 6% acrylamide gels without either boiling or β-mercaptoethanol treatment of samples, the released specifically bound radioactivity was also found to be sequestered in the large complex (Fig. 7, lanes 7 and 11). However, results shown in lanes 5 and 9 of Fig. 7 indicate that, at equivalent pronase treatment times, the large complex present in these washes was, on average, even smaller in size than the large complex remaining in washed, pronase-treated BBMs. Similarly, when BBMs containing radioactive large complex were extracted with Triton X-100 before pronase treatment at 4°C, most specifically bound radioactivity present in these samples still migrated as large complex (Fig. 7, lane 13 and 15), although this species was again of smaller size than the large complex remaining in washed, pronase-treated BBMs (Fig. 7, lanes 5 and 9).
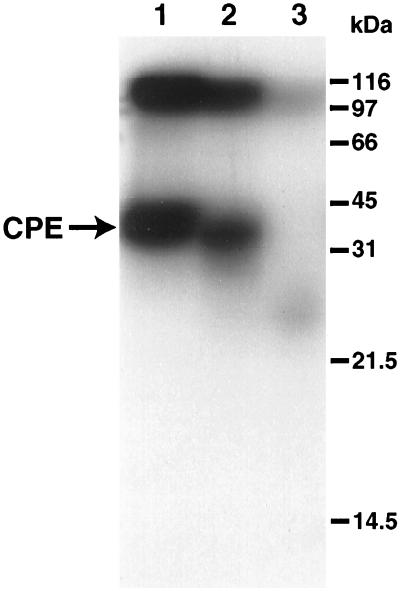
To characterize the size of the 125I-CPE molecule present in these large-complex species, samples containing large complex extracted from washed BBMs that had, or had not, been treated with pronase were run on preparative SDS–6% acrylamide gels, without either boiling or β-mercaptoethanol treatment of samples. When proteins corresponding to large-complex-associated radioactivity were electroeluted from each gel, concentrated, treated with 6 M urea, boiled, and electrophoresed on SDS–15% acrylamide gels with boiling and β-mercaptoethanol treatment of samples, it was found that the large complex that electroeluted from washed, non-pronase-treated BBMs still contained substantial amounts of specifically bound, full-size CPE (Fig. 8, lane 1). Interestingly, much of the specifically bound radioactivity remaining in washed, pronase-treated BBMs was also found to be nearly full size (i.e., >30-kDa) 125I-CPE (Fig. 8, lane 2). However, when this experiment was repeated with samples containing the large complex present in pronase-treated membrane extracts, all specifically bound radioactivity in this sample was observed to run at the gel dye front (Fig. 8, lane 3), as did the specifically bound radioactivity present in washes from pronase-treated BBMs (data not shown).
FIG. 8.
SDS-PAGE analysis (with sample boiling and urea) of pronase-treated 125I-CPE bound to BBMs at RT. 125I-CPE was bound to BBMs at RT, and after removal of unbound 125I-CPE, samples were treated for 60 min at 4°C with 300 μg of pronase (lane 2) or subjected to extraction with 1% Triton X-100 and then treated for 60 min at 4°C with 300 μg of pronase (lane 3). Lane 1 shows a similarly prepared control sample that was kept at 4°C for 60 min without pronase treatment. The samples shown in lanes 1 and 2 were washed with cold DPBS containing protease inhibitors (see Materials and Methods) after pronase treatment, but before Triton X-100 extraction, while cold DPBS containing similar protease inhibitors was added to the lane 3 sample after the conclusion of the pronase treatment. Each sample was then electrophoresed on preparative SDS–6% polyacrylamide gels without sample boiling or β-mercaptoethanol treatment. Slices of these preparative gels corresponding to large complex were then excised, and proteins in these gel slices were electroeluted, concentrated, treated with urea, boiled, and electrophoresed on an SDS–15% polyacrylamide gel containing urea. Migrations of molecular mass markers are shown at the right. Migration of 125I-CPE is shown at the left. The gel shown is representative of gels used in five independent repetitions of the experiment.
Precise quantitation of the amounts of nearly full-size 125I-CPE remaining in the large complex that electroeluted from washed, pronase-treated BBMs was difficult, since (consistent with results in previous reports [24, 26]) this species was resistant to full dissociation, even in the presence of with urea and boiling before SDS-PAGE. However, the gel shown in Fig. 8 (and in several other repetitions of this experiment) indicates that this nearly full-size CPE species accounts for at least 50% of the specifically bound radioactivity associated with the large complex of washed, pronase-treated BBMs. Further, when the species in the top band of the sample shown in lane 2 of Fig. 8 was electroeluted, treated with 6 M urea and reelectrophoresed, additional amounts of the nearly full-size CPE species became detectable (data not shown).
DISCUSSION
Collectively, results presented in this study indicate that, even when it is membrane-associated, CPE localized in small complex is fully susceptible to pronase; however, membranes do offer CPE sequestered in large complex substantial protection from pronase. Although there are other possible explanations for the ability of membranes to protect CPE from pronase when the toxin is localized in large complex, this protective effect is fully consistent with the possibility that a significant portion of the CPE molecule inserts into lipid bilayers after the toxin becomes localized in the large complex. If membrane insertion occurs when CPE is present in large complex and if this inserted CPE can no longer dissociate from BBMs, these factors could help explain the lower rate of spontaneous dissociation of CPE at RT than at 4°C as shown in Fig. 1, since large-complex formation occurs very quickly at RT (15). Further, the new hypothesis proposing that CPE insertion coincides with large-complex formation makes considerably more biologic sense than previous models of CPE action (for a review, see reference 13) that had envisioned an insertion event occurring when CPE is localized in the small complex, given (i) the close association (20) between insertion and the onset of membrane permeability alterations for many other membrane-active bacterial toxins and (ii) observations that CPE-induced membrane permeability alterations develop simultaneously with formation of large complex, not small complex (15, 24).
This study also demonstrates that pronase treatment causes a significant decrease in the size of large complex remaining in washed BBMs. However, our results also indicate that the large complex present in these washed, pronase-treated BBMs still contains substantial amounts of nearly full-sized (i.e., >30-kDa) CPE. Collectively, these observations strongly suggest that much of the decreased size noted for large complex in washed, pronase-treated BBMs is a result of the degradation of one or more of the eucaryotic membrane protein constituents of large complex. Further, the slightly decreased size of CPE present in large complex of pronase-treated BBMs is fully consistent with previous antibody probe results (9) suggesting that at least a portion of the CPE molecule remains surface exposed when this toxin is sequestered in large complex.
While our present results demonstrating that membranes fail to offer CPE localized in small complex any protection from pronase do not support previous hypotheses proposing that insertion occurs when CPE is localized in small complex, some explanation is still needed to explain the limited CPE dissociation that occurs at 4°C. Previous observations (16) indicating that initial interactions between CPE and its receptor are relatively weak suggest that the irreversible membrane association of CPE noted at 4°C does not simply result from a strong receptor-ligand binding affinity. Further, since our observations indicate that the irreversible association of CPE with membranes develops progressively with time after binding at 4°C, this irreversible binding must involve, at least in part, some postbinding change or changes.
One hypothesis involving postbinding changes that may explain the irreversible association of CPE with BBMs at 4°C can be drawn from recent studies (7, 8, 24) that collectively suggest that CPE bound at 4°C may be associated with at least two eucaryotic membrane proteins, i.e., an RVP1 homologue and an ∼40- to ∼50-kDa protein. Based upon this information, it can be hypothesized that irreversible CPE binding at 4°C might result from the trapping of receptor-bound CPE on the membrane surface, possibly due to one or both of the following postbinding changes: (i) small-complex formation (e.g., perhaps the initial binding of CPE to a receptor is followed by an interaction with a second membrane protein to form small complex; this interaction could effectively trap CPE on the membrane surface) or (ii) a conformational change that occurs soon after small-complex formation (e.g., perhaps CPE simultaneously binds to coreceptors, such as RVP-1 and the 40- to 50-kDa CPE-binding protein [7, 8, 24], to form small complex; this binding could trigger a subsequent conformational change to small complex that traps CPE on the membrane surface). Notably, the possibility that CPE in small complex might be trapped on the membrane surface appears fully consistent with results from this and previous (9) studies indicating that CPE sequestered in small complex remains accessible to both externally applied antibodies and pronase, i.e., at least a portion of CPE remains surface exposed.
Finally, the new information provided by this study can be incorporated into a revised model for CPE action, which involves (i) the binding of CPE to one or more types of receptors; (ii) a postbinding change or changes, possibly involving small-complex formation or a conformational change to small complex, that result in the trapping of CPE on the membrane surface; (iii) a subsequent interaction (which apparently requires membrane diffusion, since it occurs only at warmer temperatures [15]) between this small complex and, possibly, a 70-kDa membrane protein (26) to form large complex; (iv) the large-complex-mediated onset of membrane permeability alterations, which could result from pore formation due to the partial insertion of the CPE molecule (possibly along with one or more eucaryotic proteins present in large complex) when the toxin becomes localized in the large complex; and (v) the breakdown of the cellular osmotic equilibrium, which leads to cell death from lysis or metabolic disturbances. Studies to evaluate this revised model are under way in our laboratory.
ACKNOWLEDGMENT
This work was generously supported by Public Health Service grant AI 19844-15 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Borriello, S. P. 1995. Clostridial diseases of the gut. Clin. Infect. Dis. 20(Suppl. 2):S242–S250. [DOI] [PubMed]
- 2.Carman, R. J. 1997. Clostridium perfringens in spontaneous and antibiotic-associated diarrhea of man and other animals. Rev. Med. Microbiol. 8(Suppl. 1):S43–S45.
- 3.Collie R E, McClane B A. Evidence that the enterotoxin gene can be episomal in Clostridium perfringens isolates associated with nonfoodborne human gastrointestinal diseases. J Clin Microbiol. 1998;36:30–36. doi: 10.1128/jcm.36.1.30-36.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanna P C, McClane B A. A recombinant C-terminal toxin fragment provides evidence that membrane insertion is important for Clostridium perfringens enterotoxin cytotoxicity. Mol Microbiol. 1991;5:225–230. doi: 10.1111/j.1365-2958.1991.tb01843.x. [DOI] [PubMed] [Google Scholar]
- 5.Horiguchi Y, Uemura T, Kozaki S, Sakaguchi G. The relationship between cytotoxic effects and binding to mammalian cultured cells of Clostridium perfringens enterotoxin. FEMS Microbiol Lett. 1985;28:131–135. [Google Scholar]
- 6.Johnson S, Gerding D N. Enterotoxemic infections. In: Rood J I, McClane B A, Songer J G, Titball R W, editors. The clostridia: molecular biology and pathogenesis. London, United Kingdom: Academic Press; 1997. pp. 117–140. [Google Scholar]
- 7.Katahira J, Inoue N, Horiguchi Y, Matsuda M, Sugimoto N. Molecular cloning and functional characterization of the receptor for Clostridium perfringens enterotoxin. J Cell Biol. 1997;136:1239–1247. doi: 10.1083/jcb.136.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katahira J, Sugiyama H, Inoue N, Horiguchi Y, Matsuda M, Sugimoto N. Clostridium perfringens enterotoxin utilizes two structurally related membrane proteins as functional receptors in vivo. J Biol Chem. 1997;272:26652–26658. doi: 10.1074/jbc.272.42.26652. [DOI] [PubMed] [Google Scholar]
- 9.Kokai-Kun J F, McClane B A. Evidence that a region(s) of the Clostridium perfringens enterotoxin molecule remains exposed on the external surface of the mammalian plasma membrane when the toxin is sequestered in small or large complexes. Infect Immun. 1996;64:1020–1025. doi: 10.1128/iai.64.3.1020-1025.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kokai-Kun J F, McClane B A. Deletion analysis of the Clostridium perfringens enterotoxin. Infect Immun. 1997;65:1014–1022. doi: 10.1128/iai.65.3.1014-1022.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 12.Lowry O H, Rosebrough N J, Farr A L, Randal R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 13.McClane B A. Clostridium perfringens. In: Doyle M P, Beuchat L R, Montville T J, editors. Food microbiology: fundamentals and frontiers. Washington, D.C: ASM Press; 1997. pp. 305–326. [Google Scholar]
- 14.McClane, B. A. The action, genetics, and synthesis of Clostridium perfringens enterotoxin. In J. W. Cary, J. E. Linz, M. A. Stein, and D. Bhatnager (ed.), Microbial foodborne diseases: mechanisms of pathogenesis and toxin synthesis, in press. Technomics Press, Lancaster, Pa.
- 15.McClane B A, Wnek A P. Studies of Clostridium perfringens enterotoxin action at different temperatures demonstrate a correlation between complex formation and cytotoxicity. Infect Immun. 1990;58:3109–3115. doi: 10.1128/iai.58.9.3109-3115.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McClane B A, Wnek A P, Hulkower K I, Hanna P C. Divalent cation involvement in the action of Clostridium perfringens type A enterotoxin. J Biol Chem. 1988;263:2423–2435. [PubMed] [Google Scholar]
- 17.McDonel J L. Binding of Clostridium perfringens125I-enterotoxin to rabbit intestinal cells. Biochemistry. 1980;21:4801–4807. doi: 10.1021/bi00562a014. [DOI] [PubMed] [Google Scholar]
- 18.McDonel J L, McClane B A. Binding vs. biological activity of Clostridium perfringens enterotoxin in Vero cells. Biochem Biophys Res Commun. 1979;87:497–504. doi: 10.1016/0006-291x(79)91823-0. [DOI] [PubMed] [Google Scholar]
- 19.McDonel J L, McClane B A. Production, purification and assay of Clostridium perfringens enterotoxin. Methods Enzymol. 1988;165:94–103. doi: 10.1016/s0076-6879(88)65018-x. [DOI] [PubMed] [Google Scholar]
- 20.Menestrina G, Schiavo G, Montecucco C. Molecular mechanisms of action of bacterial protein toxins. Mol Asp Med. 1994;15:79–193. doi: 10.1016/0098-2997(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 21.Sigrist H, Ronner P, Semenza G. A hydrophobic form of the small-intestinal sucrase-isomaltase complex. Biochim Biophys Acta. 1975;406:433–446. doi: 10.1016/0005-2736(75)90022-x. [DOI] [PubMed] [Google Scholar]
- 22.Songer J G. Clostridial disease of animals. In: Rood J I, McClane B A, Songer J G, Titball R W, editors. The clostridia: molecular biology and pathogenesis. London, United Kingdom: Academic Press; 1997. pp. 153–184. [Google Scholar]
- 23.Tolleshaug H, Skjelkvale R, Berg T. Quantitation of binding and subcellular distribution of Clostridium perfringens enterotoxin in rat liver cells. Infect Immun. 1982;37:486–491. doi: 10.1128/iai.37.2.486-491.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wieckowski E U, Wnek A P, McClane B A. Evidence that an ∼50kDa mammalian plasma membrane protein with receptor-like properties mediates the amphiphilicity of specifically-bound Clostridium perfringens enterotoxin. J Biol Chem. 1994;269:10838–10848. [PubMed] [Google Scholar]
- 25.Wnek A P, McClane B A. Comparison of receptors for Clostridium perfringens type A and cholera enterotoxins in isolated rabbit intestinal brush border membranes. Microb Pathog. 1986;1:89–100. doi: 10.1016/0882-4010(86)90035-5. [DOI] [PubMed] [Google Scholar]
- 26.Wnek A P, McClane B A. Preliminary evidence that Clostridium perfringens type A enterotoxin is present in a 160,000-Mr complex in mammalian membranes. Infect Immun. 1989;57:574–581. doi: 10.1128/iai.57.2.574-581.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]