Highlights
-
•
Mass of Hb variants obtained from Triple Quadrupole Mass Spectrometer suggest whether a mutation is in the α or β globin chains.
-
•
Suspected globin chain can then be sequenced for definite hemoglobinopathy identification.
-
•
Pathophysiology of hemoglobinopathies can be correlated to rare Hb variants.
Keywords: Hemoglobinopathies, Diagnosis, Diagnostic techniques and procedures, Blood proteins
Abstract
Background
The presumptive diagnosis of hemoglobinopathies relies on routine tests such as Complete Blood Count (CBC), peripheral blood smear, Liquid Chromatography (LC), and Capillary Electrophoresis (CE), along with clinical findings. Pathologists suggest molecular sequencing of HBA and HBB genes to correlate blood picture with clinical findings in order to identify unknown rare haemoglobin (Hb) variants or variants that coelute with Hb. This paper presents a low-resolution mass spectrometry (MS)-based method for presumptive identification of variants that eluted in zone 12 of CE, followed by molecular sequencing of the HBB gene for a definitive diagnosis of hemoglobinopathies.
Methods
Eight patient samples with a variant peak in zone 12 of CE (Sebia) were analyzed using MS. The mass-to-charge ratio (m/z) observed was deconvoluted to determine the mass of Hb variants. The β variants were subsequently confirmed through molecular sequencing.
Results
Based on the intact mass of the variants, there were two samples of the α variant (α + 58 Da and α + 44 Da), and six samples of the β variant. Out of these six β variant samples, three were the β + 58 Da variant, and three were the β + 30 Da variant. By correlating the intact mass information with the CE pattern and considering the ethnicity of the patients, it was presumed that the α variants were HbJ Meerut (α + 58 Da, x-axis 102) and HbJ Paris-I (α + 44 Da, x-axis 80). Molecular analysis confirmed the identity of β variants as Hb Rambam/HbJ Cambridge, HbJ Bangkok (+58 Da), and Hb Hofu (+30 Da).
Conclusion
The mass information of Hb variants obtained using Electrospray triple quadrupole MS assists pathologists in recommending the appropriate molecular sequencing for identifying unknown variants.
Introduction
HbVar, a database of haemoglobin (Hb) disorders known as hemoglobinopathies, has documented 1422 Hb variants to date [1]. These variants occur due to point mutations on the α, β, δ, and γ globin chains. In addition to the clinically significant Hb variants such as HbS, C, E, D-Punjab, and O-Arab, other Hb variants can also be observed in diagnostic laboratories using cation exchange-high performance liquid chromatography (CX-HPLC) [2] or capillary electrophoresis (CE) [3]. Fast-moving Hbs on HPLC and CE are rare variants that are accidentally detected during antenatal or family screening [4]. One example of such a variant is the HbJ family, which encompasses around 50 different forms [5], [6]. Some examples of α variants include HbJ Meerut, HbJ Birmingham, and HbJ Cape Town; while β variants include HbJ Baltimore/Bangkok/Kaohsiung/Cape Town, which are observed in various ethnicities globally [7].
The clinical presentation of patients with HbJ variants range from asymptomatic cases to mild effects on hematologic indices [8], [9]. Presumptive identification of these variants has been reported based on migration zone, migration axis and percentage of the variant using CE [7]. Confirmation is typically performed through molecular sequencing [9] of the α and β genes of Hb. However, when the involvement of the globin chain is not known, it becomes cost-prohibitive to sequence both α genes (HBA1 and HBA2) along with the β globin gene (HBB) to confirm the presence of an Hb variant. Instead, a cost-effective method, known as Amplification Refractory Mutation System (ARMS) PCR, is utilized for detecting rare mutations [10], as well as studying genotype-phenotype correlation in prospective research studies.
In recent years, mass spectrometry (MS), a powerful analytical technique, has been used in research laboratories to identify hemoglobin variants based on the intact mass information of the variant globin chain [11], [12], [13]. However, the use of MS in clinical diagnostic laboratories has been mainly limited to low-resolution MS for quantification of small molecules [14], [15] and peptides [16], [17], while high-resolution MS and Matrix Assisted Laser Desorption Ionization - Time-of-Flight (MALDI-ToF) MS have been explored for analyzing intact protein diagnostic markers [18], [19].
Unlike CX-HPLC and CE which measure the Hb tetrameric complex, MS measures the mass-to-charge ratio (m/z) of expressed monomeric globin proteins. Therefore, this study aims to highlight the value of intact mass information in determining the affected globin chain type in a clinical laboratory setting. The analysis of proteins using low-resolution Triple Quadrupole MS in a diagnostic laboratory is demonstrated. This report presents the first correlation between mass information and CE pattern to identify HbJ variants that migrated in zone 12 of CE.
Materials and methods
All the K2 EDTA blood samples analyzed for variants were routine clinical samples. These samples were originally sent to the laboratory requesting a complete blood picture and the detection of hemoglobinopathies using CE. Any extra samples that remained after testing were then used to record mass information using Liquid Chromatography (LC) Electrospray Ionisation TQMS and to perform molecular analysis in order to confirm any β variants detected.
Reagents and instruments
HPLC grade acetonitrile, 98 % formic acid (Ranbaxy Fine Chemicals Limited, New Delhi, India), and HPLC grade water (Thermo Fisher Scientific, USA) were utilized. For molecular studies, DNA was extracted from blood samples using the QIAamp DNA Mini Kit (Qiagen). Complete blood count analysis was performed using the Sysmex XN1000 automatic analyzer (Sysmex Corporation, Kobe, Japan). Identification and quantification of normal Hb and Hb variants were conducted using the CAPILLARYS Hemoglobin (E) kit on a CAPILLARYS 2 Flex Piercing Instrument (Sebia, Lisses, France).
Sample preparation
To release Hb from whole blood, 5 μl of the blood sample was diluted with 995 µl of water and vortexed. The resulting mixture was then centrifuged at 10,000 rpm for 5 min. From the supernatant, 40 µl was taken and further diluted with 360 µl of MS-compatible solvent consisting of 40 % acetonitrile acidified with 0.2 % formic acid.
LCMS procedure
For the separation of Hb proteins from impurities, an ultra-performance liquid chromatography (UPLC) system (Acquity H-Class Plus, Waters, India Pvt Ltd) was used. A BEH C18 column with dimensions of 2.1 mm x 50 mm, a particle size of 1.7 µm, and a pore size of 130 Å was used for the elution of globin chains. Solvent A consisted of HPLC-grade water with 0.2 % formic acid, while solvent B consisted of acetonitrile with 0.2 % formic acid. The globin chains were eluted isocratically at 60 % solvent B for one minute with a flow rate of 300 µl.
The mass measurement of intact Hb and its variants was performed using Xevo TQD (Waters, India Pvt Ltd) in positive ion mode with a capillary voltage of 3.2 kV, cone voltage of 40v, source temperature of 150OC, desolvation temperature of 550 OC with a mass range of 650–1200 m/z. All the instruments were calibrated according to the manufacturer’s instructions. LCMS data was acquired using MassLynx v4.1.
To obtain better mass accuracy, spectral peaks (m/z) were internally calibrated for each sample using α globin chain peaks as a reference file provided by the vendor. The internally calibrated m/z data were then deconvoluted using Max Ent1 software (Waters, India Pvt Ltd) to obtain the mass information for intact Hb and its variants. The parameters used were uniform Gaussian as a damage model, minimum intensity ratio set to 80 %, and iterations until convergence.
For molecular identification of β variants, Sanger sequencing was performed on PCR products obtained from the β globin chain using a BigDye terminator sequencing kit on an Applied Biosystems 3730xI DNA Analyzer. The sequence data were analyzed using Geneious software v2022.0.1, and the reference sequence used was NG_059281.1 for the HBB gene.
The study was approved by the Institutional Human Ethics Committee (IHEC) according to DCGI guidelines (Reg. No: Ec/NEW/INST/2022/2627) and the study approval no. is NAALM/EC/1.1/02-2022.
Results
Eight samples, with a variant peak in zone 12 of CE were subjected to intact mass measurement. Except for one sample, all were from female patients within the age group of 21–39 years. All patients were asymptomatic and had normal red blood cell (RBC) indices, except for cases 3, 4, and 8, which exhibited a microcytic hypochromic blood picture. The CE patterns of all variants are depicted in Fig. 1.
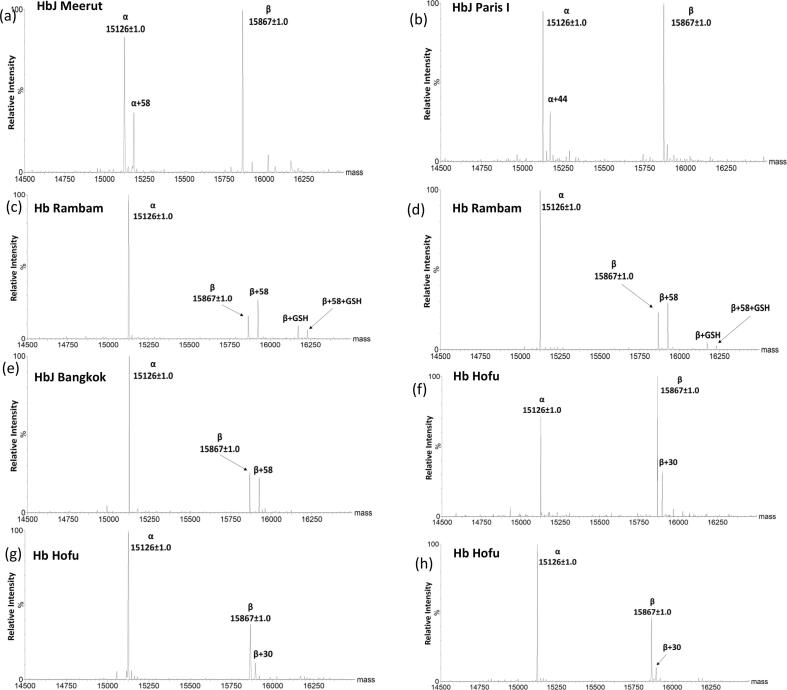
Fig. 1.
Capillary electrophoresis migration pattern of presumptive Hb variants in zone 12 along with variant %. 1a- HbJ Meerut; 1b-HbJ Paris I; 1c, d-HbJ Cambridge/Rambam; 1e-HbJ Bangkok; 1f-h-Hb Hofu.
Cases one and two (Fig. 1a, b) were presumed to be α variants based on the presence of minor peaks in zone D or zone D/S. Case one displayed a different migration position on the x-axis (1 0 2) compared to case two (x-axis 80), indicating the presence of two distinct α variants [7]. However, the variant percentage was similar in both cases at 28 %.
In contrast, cases three to eight (Fig. 1c–h) did not exhibit minor peaks in zones D/S and thus were designated as β variants. The x-axis positions ranged from 90 to 96 for these samples and displayed two different CE patterns with respect to variant levels. Cases three to five (Fig. 1c–e) demonstrated variant levels of 50–54 %, while cases six to eight (Fig. 1f–h) exhibited variant levels ranging from 21 % to 37 %. Detailed information regarding complete blood count (CBC) and CE parameters can be found in Table 1.
Table 1.
Summary of sample details, CBC, red cell morphology, CE parameters, the mass difference from the normal globin chains, mutation location and the presumptive identification of the variants. The presumptive identification of β variants was confirmed by molecular sequencing. Hb-Hemoglobin, RBC- Red Blood cell Count, MCV-Mean Corpuscular Volume, MCH-Mean Corpuscular Hemoglobin, RDW-Red blood cell Distribution Width, RCM-Red Cell Morphology, NN-Normocytic normochromic, MH microcytic hypochromic.
| Case No. | Hb (g/dl) | RBC(x106/µl) | MCV (fl) | MCH (pg) | RDW (%) | HbA0 (%) | HbA2 (%) | F (%) | Variant (%) | CE (x Axis) | RCM | Mass diff (Da) | Mutation | Variant |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12.4 | 4.61 | 82.9 | 32.6 | 13.8 | 69.7 | 1.5 | 1 | 28.8 | 102 | NN | α + 58 | α120Ala → Glu | HbJ Meerut |
| 2 | 15.4 | 5.43 | 83.9 | 28.5 | 13.9 | 70.6 | 1.8 | 0 | 27.6 | 80 | NN | α + 44 | α12Ala → Asp | HbJ Paris I |
| 3 | 12.5 | 5.27 | 74.4 | 23.7 | 14 | 44.1 | 2.0 | 0 | 53.9 | 91 | MH | β + 58 | β69Gly → Asp | Hb Rambam |
| 4 | 10.5 | 5.17 | 67 | 20.3 | 14.4 | 44.3 | 1.8 | 0 | 53.9 | 90 | MH | β + 58 | β69Gly → Asp | Hb Rambam |
| 5 | 13.5 | 5.07 | 80.4 | 33.1 | 12.5 | 47 | 2.4 | 0 | 50.6 | 94/95 | NN | β + 58 | β56Gly → Asp | HbJ Bangkok |
| 6 | 11.5 | 4.49 | 83.3 | 25.6 | 15.4 | 59.7 | 3.3 | 0 | 37 | 93 | NN | β + 30 | β126Val → Glu | Hb Hofu |
| 7 | 11.8 | 4.56 | 82.2 | 25.9 | 13.9 | 67.8 | 4.0 | 0 | 28.2 | 96 | NN | β + 30 | β126Val → Glu | Hb Hofu |
| 8 | 6.6 | 4.44 | 62.2 | 14.9 | 20.7 | 75.8 | 3.0 | 0 | 21.2 | 96 | MH | β + 30 | β126Val → Glu | Hb Hofu |
Intact mass information for all the variant samples was obtained through LC ESI TQMS after deconvoluting the raw data (m/z). Fig. 2 illustrates the deconvoluted mass of Hb and its variants that migrated in zone 12 on CE. Fig. 2a shows a variant mass of α + 58 Da, while Fig. 2b displays a variant mass of α + 44 Da. Fig. 2c–e demonstrate the presence of a β variant with + 58 Da, which has an intensity equivalent to that of the β chain but different from that of the α chain. Lastly, Fig. 2f–h exhibit the mass of variants as + 30 Da from the β chain. The table provided in Table 1 presents details regarding the molecular masses, variations compared to normal Hb, and identification of the analyzed variants within this study.
Fig. 2.
Deconvoluted mass spectra of presumptive Hb variants using Max Ent software. 2a and 2b are presumed to be α variants with a mass difference of + 58 Da (HbJ Meerut) and + 44 Da (HbJ Paris I). 2c and 2d are presumed to be β variants (HbJ Cambridge/Rambam) with a mass difference of + 58 Da and + 305 Da (glutathionylation). 2e with a mass difference of + 58 Da from β globin is presumed to be HbJ Bangkok. 2f-h are β variants with a mass difference of + 30 Da presumed to be Hb Hofu. All β variants are confirmed using molecular sequencing.
Discussion
Cation exchange HPLC (CX-HPLC) is considered the gold standard for identifying structural variants or defects in globin chain expression [20], [21]. However, CE has also been documented as an alternative technique by Keren et al. [3]. While both CX-HPLC and CE can identify variants based on elution profile or migration pattern, there have been reports highlighting the overlap of certain variants [22], [23], leading to potential misdiagnosis of Hb disorders [24].
To achieve a definitive diagnosis of variants, molecular techniques such as gene sequencing [25] or protein sequencing using high-resolution MS [26], [27] are necessary. However, due to their cost and limited availability in diagnostic laboratories, low-resolution TQMS, which is commonly used for quantifying metabolites and peptides, is employed for protein analysis.
In this study, the intact mass information of eight variants that eluted in zone 12 of CE is provided. This information assists in the identification of Hb variants when higher resolution techniques are not accessible.
The presumptive identification of Hb variants, based on the intact mass information obtained, was compared to the presumptive identification derived from the CE pattern, considering parameters such as migration zone, migration position, and variant percentage. To supplement this analysis, information from relevant literature sources [28], the Hemoglobin Atlas from Sebia (Sebia customer extranet: https://extranet.sebia.com) and patient ethnicity was considered.
Based on these considerations and information sources, case one was presumed to be HbJ-Meerut and case two presumed to be HbJ-Paris I. This study represents the first report of correlating intact mass information with CE patterns for providing presumptive identification of α variants. However, molecular confirmation is still necessary for definitive identification of these variants.
Two masses of β variants were observed in this study: β + 58 Da (cases three-five) and β + 30 Da (cases six-eight). In addition to the β + 58 Da variant, glutathionylation (+305 Da) was also detected for both the β chain and its variant. Such modifications are not evident in CE and are concealed within the HbA peak.
To identify the possible variants associated with a mass increase of β + 58 Da, the HbVar database [1] was consulted, which indicated that this mass change could result from amino acid substitutions such as Gly > Asp or Ala > Glu. By comparing this information with the probable variants that could migrate to zone 12 on CE according to Sebia's data, potential candidates were deduced from the UniProt sequence of HBB (P69905). Presumptive identification included J-Bangkok, J-Calabria, Rambam (J-Cambridge), and Pyrgos.
In cases three and four, similar parameters were observed in terms of variant percentage, migration position on CE, intact mass, and glutathionylation and the clinical picture of microcytic hypochromic, while it was different for case five in all the above parameters (Table 1). These factors indicate that there may be two different β variants with a similar mass increase (+58 Da). Molecular analysis confirmed cases three and four as Rambam (Hb J-Cambridge). Rambam is a rare Hb variant with normal RBC function and stability [29]. Bisse et al. [30] in 1998 using MS reported Hb Rambam along with β glycation (+162 Da) of both the variant and the wild type. In the present study, we observed glutathionylation of both variant (Hb Rambam) and wild type (+305 Da). Glutathionylation of hemoglobin at βCys93 residue may result from an oxidative imbalance in erythrocytes [31]. Case five was confirmed by molecular sequencing as Hb J Bangkok, with similar CE patterns as observed by Beverley [7]. In the absence of mass information, definitive identification of variants requires molecular sequencing of HBA1, HBA2 and HBB genes.
Cases six and seven exhibited similar intact mass of β + 30 Da and all CE parameters, except for the migration position. The red blood cells (RBCs) of both cases displayed normocytic normochromic characteristics, with a slightly higher percentage of A2, although its significance is not known.
For case eight, severe anemia was observed with a Hb level of 6.6 g/dl. The microcytic hypochromic blood picture in this case could be attributed to iron deficiency.
Considering the point mutations that could result in a mass increase of + 30 Da, possibilities include Val > Glu; Gly > Ser; Ala > Thr; Arg > Trp; and Thr > Met substitutions. Among the identified variants in zone 12 according to Sebia data, only two variants (Hb Hofu and Hb Trollhattan) were associated with the Val > Glu mutation.
Molecular sequencing of the β chain revealed negative results for thalassemia, but positive results for heterozygous Hb Hofu in all three cases. These findings correlate with a previous study by Purohit et al. [32], which characterized Hb Hofu in four families from eastern India using HPLC techniques.
Riou et al. [23] conducted a study demonstrating the highly reproducible migration position of Hb variants on CE. In our present study, a CE migration position of 93 indicated the presence of Hb Hofu (+30 Da), consistent with the findings reported by Riou et al. A migration position of 96 with a + 30 Da mass was also observed, further supporting the identification of Hb Hofu in cases 7 and 8. On the other hand, a migration position of 94/95 corresponded to a mass increase of + 58 Da in case 5.
The co-migration of variants poses challenges for screening techniques like CX-HPLC and CE, making it difficult to achieve unambiguous identification of specific variants. Intact mass information obtained from ESI TQMS is valuable as it provides insights into which globin chain is affected by the variant. Low-resolution TQMS has been demonstrated by Rai et al. [11] to successfully identify Hb Lepore BW through intact mass measurement in clinical samples.
Intact mass information can be routinely obtained in high-throughput manner within clinical diagnostic laboratories. This information aids pathologists in determining or advising on which globin chain should be sequenced for definitive diagnosis of hemoglobinopathies using molecular techniques.
Many of the clinically significant Hb variants arise due to mutations in the β chain, resulting in a lack of detailed studies on α variants in the literature. The clinical presentation of these variants can vary depending on coexisting abnormalities in other globin chains [33], [34]. Pitfalls can occur in diagnosing HbS due to additional mutations in the α chain that contribute to clinical symptoms, making it challenging to reach a conclusive diagnosis [35].
The intact mass information presented in this study could serve as a valuable tool for pathologists and clinicians by providing guidance on appropriate molecular tests to be conducted. Additionally, it offers insights into modifications occurring within the globin chain, such as β glutathionylation as observed in cases three and four.
Newborn screening for clinically significant variants like HbS, E, C, D-Punjab, and O-Arab using TQMSMS based on analysis of variant-specific peptides has gained wide acceptance and is routinely employed in diagnostic laboratories [36], [37]. The intact mass information provided for these variants adds another dimension to the complex process of diagnosing hemoglobinopathies.
Overall, intact mass information is a valuable addition that complements existing diagnostic approaches and enhances our understanding of hemoglobinopathies.
In this study, the potential of ESI TQMS as a complementary technique to CX-HPLC and CE in a clinical setting was demonstrated. This technique provides intact mass information on unknown variants and post-translational modifications of globin chains in a clinical setting. The intact mass information of the clinically significant variants such as HbE, C, D-Punjab, and O-Arab overlap with the wild type β chain on low-resolution instruments.
To resolve overlapping peaks and improve accuracy, high resolution MS (HRMS) can be employed for specific cases [18], [19]. The quality of spectral data and the selection of appropriate regions for deconvolution contribute to the precision of mass measurements.
Nevertheless, low-resolution MS remains highly valuable due to its cost-effectiveness in qualitative analyses of Hb variants within diagnostic laboratories. It serves an important role in routine clinical practice.
Conclusion
ESI TQMS is an alternative technique that can effectively differentiate between α and β variants of Hb. It offers valuable guidance to pathologists in determining the appropriate globin chain to be sequenced for a better understanding of the underlying pathophysiology in patients with Hb disorders. The integration of intact mass information as an additional parameter, aids in the interpretation and diagnosis of these conditions.
The feasibility of utilizing low-resolution MS for measuring the intact mass of Hb and its variants in a diagnostic laboratory setting is demonstrated in this study. This highlights the potential for implementing this technique as a routine tool within clinical practice, providing valuable insights into Hb disorders.
Financial support and sponsorship
No funds or grants were received for this work.
CRediT authorship contribution statement
Deepalakshmi Dakshinamoorthy Putchen: Conceptualization, Writing – original draft, Writing – review & editing, Investigation, Formal analysis, Supervision, Project administration. Athira Nambiar: Data curation, Writing – original draft, Visualization, Formal analysis, Methodology. Aswathy Ashok Menon: Investigation, Formal analysis. Ananthvikas Jayaram: Data curation, Writing – review & editing, Validation, Formal analysis, Methodology. Sujay Ramaprasad: Writing – review & editing, Resources.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: RS is a shareholder and director of both the companies, Neuberg Anand Academy of Laboratory Medicine Pvt Ltd and Neuberg Anand Reference Laboratory, a Unit of Neuberg Diagnostics Pvt Ltd. The other authors declare they have no known competing financial interests or personal relationships that could affect the work described in this article.
Acknowledgements
The authors thank pathologists, Dr Pradeep K and Dr Swathi K, for the interpretation of routine samples. The work of technicians and logistics personnel is greatly appreciated. The deconvolution software provided by WATERS India Pvt Ltd. is acknowledged. The authors thank Grammarly-AI tool, for writing assistance.
Ethics statements
Patient consent for publication: Since the data is anonymised, patient consent is waived for the study.
Ethics approval
The study protocol was approved by the Institutional Human Ethics Committee (Reg. No: EC/NEW/INST/2022/2627) prior to the commencement of the study. The Ethics approval number for this study is NAALM/EC/1.1/01-2022.
References
- 1.Giardine B., Borg J., Viennas E., Pavlidis C., Moradkhani K., Joly P., et al. Updates of the HbVar database of human hemoglobin variants and thalassemia mutations. Nucleic Acids Res. 2014;42(D1):1063–1069. doi: 10.1093/nar/gkt911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wajcman H., Prehu C., Bardakdjian-Michau J., Promé D., Riou J., Godart C., Mathis M., Hurtrel D., Galactéros F. Abnormal hemoglobins:laboratory methods. Hemoglobin. 2001;25:169–181. doi: 10.1081/hem-100104026. [DOI] [PubMed] [Google Scholar]
- 3.Keren D.F., Hedstrom D., Gulbranson R., Ou C.N., Bak R. Comparison of Sebia Capillarys capillary electrophoresis with the Primus high-pressure liquid chromatography in the evaluation of hemoglobinopathies. Am. J. Clin. Pathol. 2008;130:824–831. doi: 10.1309/AJCPQY80HZWHHGZF. [DOI] [PubMed] [Google Scholar]
- 4.Srinivas U., Mahapatra M., Pati H. Hb J Meerut, A fast moving Hemoglobin-A study of seven cases from India and a review of litterature. Am. J. Hemat. 2007;82:666–667. doi: 10.1002/ajh.20826. [DOI] [PubMed] [Google Scholar]
- 5.Giardine B., Borg J., Viennas E., Pavlidis C., Moradkhani K., Joly P., Bartsakoulia M., Riemer C., Miller W., Tzimas G., Wajcman H., Hardison R.C.P.G. Updates of the HbVar database of human hemoglobin variants and thalassemia mutations [Internet] Nucleic Acids Res. 2014 doi: 10.1093/nar/gkt911. https://globin.bx.psu.edu/hbvar/menu.html [cited 2022 Apr 8]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kountouris P., Lederer C.W., Fanis P., et al. Itha genes:an interactive database for hemoglobin variations and epidemiology. PLoS One. 2014;9:2103020. doi: 10.1371/journal.pone.0103020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pullon B.M. Chromatogram characteristics of seven Hb J variants on capillary zone electrophoresis. N. Z. J. Med. Sci. 2021;75:06–10. [Google Scholar]
- 8.Pootrakul S., Wasi P., Na-Nakorn S. Hemoglobin J-Bangkok:a clinical, hematological and genetic study. Br. J. Hematol. 1967;13:303–309. doi: 10.1111/j.1365-2141.1967.tb08744.x. [DOI] [PubMed] [Google Scholar]
- 9.Charache S., Jenkins T. Oxygen equilibrium of Hemoglobin J Cape Town. J. Clin. Invest. 1971;50:1554–1555. doi: 10.1172/JCI106642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinha S., Dutta A.K., Bhattacharya P., et al. Spectrum of Rare and Novel Indel Mutations Responsible for β Thalassemia in Eastern India. Ind. J. Clin. Biochem. 2023 doi: 10.1007/s12291-022-01098-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rai D.K., Green B.N., Landin B., Alvelius G., Griffiths W.J. Accurate mass measurement and tandem mass spectrometry of intact globin chains identify the low proportion variant hemoglobin Lepore-Boston-Washington from the blood of a heterozygote. J. Mass Spectrom. 2004;39(3):289–294. doi: 10.1002/jms.581. [DOI] [PubMed] [Google Scholar]
- 12.Aebersold R. Mass spectronetry-based proteomics. Nature. 2003;422:198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- 13.Bruno D., Aebersold R. Mass spectrometry and protein analysis. Science. 2006;312:212–217. doi: 10.1126/science.1124619. [DOI] [PubMed] [Google Scholar]
- 14.Dooley K.C. Tandem mass spectrometry in the clinical chemistry laboratory. Clin. Biochem. 2003;36(6):471–481. doi: 10.1016/s0009-9120(03)00105-x. [DOI] [PubMed] [Google Scholar]
- 15.Vogeser M., Seger C. A decade of HPLC-MS/MS in the routine clinical laboratory-goals for further developments. Clin. Biochem. 2008;41:649–662. doi: 10.1016/j.clinbiochem.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 16.MacNeill R., Stromeyer R., Urbanowicz B., Acharya V., Moussallie M. LC MS/MS quantification of parathyroid hormone fragment 1-34 in human plasma. Bioanalysis. 2013;5(4):415–422. doi: 10.4155/bio.12.334. [DOI] [PubMed] [Google Scholar]
- 17.Wild B.J., Green B.N., Cooper E.K., Lalloz M.R.A., Erten S., Stephens A.D., et al. Rapid identification of hemoglobin variants by electrospray ionization mass spectrometry. Blood Cell Mol. Dis. 2001;27(3):691–704. doi: 10.1006/bcmd.2001.0430. [DOI] [PubMed] [Google Scholar]
- 18.Luo R.Y., Wong C., Xia J.Q., Glader B.E., Shi R.Z., Zehnder J. Neutral-Coating Capillary Electrophoresis Coupled with High-Resolution Mass Spectrometry for Top-Down Identification of Hemoglobin Variants. Clin. Chem. 2023;69:56–67. doi: 10.1093/clinchem/hvac171. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Q., Wang G., Sun D., Lin W., Yan T., Wu Y., et al. MALDI–TOF–MS for Rapid Screening and Typing of β-Globin Variant and β-Thalassemia through Direct Measurements of Intact Globin Chains. Clin. Chem. 2022;68:1541–1551. doi: 10.1093/clinchem/hvac151. [DOI] [PubMed] [Google Scholar]
- 20.Riou J., Godart C., Hurtrel D., et al. Cation-exchange HPLC evaluated for presumptive identification of hemoglobin variants. Clin. Chem. 1997;43:34–39. [PubMed] [Google Scholar]
- 21.Ou C.N., Rognerud C. Diagnosis of hemoglobinopathies:electrophoresis vs HPLC. Clin Chem Acta. 2001;313:187–194. doi: 10.1016/s0009-8981(01)00672-6. [DOI] [PubMed] [Google Scholar]
- 22.Joutovsky A., Hadzi-Nesic J., Nardi M.A. HPLC retention time as a diagnostic tool for hemoglobin variants and hemoglobinopathies: a study of60000 samples in a clinical diagnostic laboratory. Clin. Chem. 2004;50:1736–1747. doi: 10.1373/clinchem.2004.034991. [DOI] [PubMed] [Google Scholar]
- 23.Riou J., Szuberski J., Godart C., Wajcman H., Oliveira J.L., Hoyer J.D., et al. Precision of CAPILLARYS 2 for the detection of hemoglobin variants based on their migration positions. Am. J. Clin. Pathol. 2018;149(2):172–180. doi: 10.1093/ajcp/aqx148. [DOI] [PubMed] [Google Scholar]
- 24.Fucharoen S., Singsanan S., Hama A., Fucharoen G., Kanokwan S. Rapid molecular characterisation of Hb Queens and Hb Siam: Two variants easily misidentified as sickle Hb. Clin. Biochem. 2007;40:137–140. doi: 10.1016/j.clinbiochem.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Harteveld C.L. State of the art and new developments in molecular diagnostics for hemoglobinopathies in multiethnic societies. Int. J. Lab. Hematol. 2014;36(1):1–12. doi: 10.1111/ijlh.12108. [DOI] [PubMed] [Google Scholar]
- 26.Pais R.J., Jardine C., Zmuidinaite R., Lacey J., Butler S., Iles R. Rapid, affordable and efficient screening of multiple blood abnormalities made possible using an automated tool for MALDI-ToF spectrometry analysis. Appl. Sci. 2019;9(23) [Google Scholar]
- 27.Lassout O., Hartmer R., Jabs W., Clerici L., Tsybin Y.O., Samii K., et al. Clinical method evaluation of hemoglobin S and C identification by top-down selected reaction monitoring and electron transfer dissociation. Clin. Proteomics. 2019;16(1):1–9. doi: 10.1186/s12014-019-9261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fucharoen G., Srivorakun H., Singsanan S., Fucharoen S. Presumptive diagnosis of common hemoglobinopathies in Southeast Asia using a capillary electrophoresis system. Int. J. Lab. Hematol. 2011;33:424–433. doi: 10.1111/j.1751-553X.2011.01301.x. [DOI] [PubMed] [Google Scholar]
- 29.Plaseska-Karanfilska D., de Weinstein B.I., Efremov G.D. Hb Rambam [β69(E13)Gly+Asp]/βo-Thalassemia [Codon 5 (-CT)] in a Family From Argentina. Hemoglobin. 2000;24(2):157–161. doi: 10.3109/03630260009003437. [DOI] [PubMed] [Google Scholar]
- 30.Bissé E., Zorn N., Eigel A., Lizama M., Huaman-Guillen P., März W., et al. Hemoglobin Rambam (β69[E13]Gly → Asp), a pitfall in the assessment of diabetic control: Characterization by electrospray mass spectrometry and HPLC. Clin. Chem. 1998;44(10):2172–2177. [PubMed] [Google Scholar]
- 31.Mandal A.K., Woodi M., Sood V., Krishnaswamy P.R., Rao A., Ballal S., Balaram P. Quantitation and characterization of glutathionyl haemoglobin as an oxidative stress marker in chronic renal failure by mass spectrometry. Clin. Biochem. 2007;40:986–994. doi: 10.1016/j.clinbiochem.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Purohit P., Mashon R.S., Patel S., Dehury S., Pattanayak C., Das K., et al. Clinical and molecular characterization of Hb Hofu in eastern India. Int. J. Lab. Hematol. 2014;36(1):71–76. doi: 10.1111/ijlh.12128. [DOI] [PubMed] [Google Scholar]
- 33.Henni T., Belhani M., Morle F., Bachir D., Tabone P., Colonna P., Godet J. Alpha globin gene triplication in severe heterozygous beta thalassemia. Acta Haematol. 1985;74(4):236–239. doi: 10.1159/000206230. [DOI] [PubMed] [Google Scholar]
- 34.Pandey S.K., Pandey S., Ranjan R., Shah V., Mishra R.M., Sharma M., et al. Phenotypic Effect of α-Globin Gene Numbers on Indian Sickle β-Thalassemia Patients. J. Clin. Lab. Anal. 2014;28(2):110–113. doi: 10.1002/jcla.21652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moradkhani K., Riou J., Wajcman H. Pitfalls in the genetic diagnosis of Hb S. Clin. Biochem. 2013;46(4–5):291–299. doi: 10.1016/j.clinbiochem.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 36.Daniel Y., Elion J., Allaf B., Badens C., Bouva M.J., Brincat I., et al. Newborn screening for sickle cell disease in Europe. Int. J. Neonatal Screen. 2019;5(1):1–12. doi: 10.3390/ijns5010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lobitz S., Klein J., Brose A., Blankenstein O., Frömmel C. Newborn screening by tandem mass spectrometry confirms the high prevalence of sickle cell disease among German newborns. Ann. Hematol. 2019 Jan 30;98(1):47–53. doi: 10.1007/s00277-018-3477-4. [DOI] [PubMed] [Google Scholar]