Summary
Background
Living with heart failure can severely affect the physical and mental health of patients with heart failure and their caregivers. Available dyadic self-care interventions for heart failure are scarce, especially in China. We aimed to develop and test the family FOCUS programme.
Methods
This single-blind, randomised, controlled study was conducted at four hospitals in Tianjin, China. Patients with heart failure (aged at least 18 years) and their caregiver (dyads) were randomly assigned to either the intervention (n = 71) or control (n = 71) group in a 1:1 ratio. The primary outcomes of this study were patient self-care, with three specific dimensions (self-care maintenance, symptom perception, and self-care management), and caregiver contribution to self-care, mirroring these three dimensions. The outcomes were assessed at baseline (T0) and 4 (T1), 12 (T2), and 24 (T3) weeks post-discharge, respectively. This work is registered on ChiCTR, ChiCTR2100053168.
Findings
Between May 20, 2022, and September 30, 2022, 142 dyads with heart failure were enrolled. The intervention group exhibited dropout rates of 6%, 8.5%, and 18.3% at 4, 12, and 24 weeks after discharge, while the control group showed 9.9%, 12.3%, and 25.4%. Compared with the control group, patients in the intervention group reported improved self-care maintenance (β: 8.5, 95% CI: 0.7, 16.4) and management (β: 7.2, 95% CI: 0.1, 14.3) at T1, as well as improved symptom perception at both T1 (β: 9.7, 95% CI: 1.5, 17.9) and T2 (β: 9.6, 95% CI: 0.6, 18.6). Furthermore, caregiver contributions to self-care maintenance, self-care management, and symptom perception (excluding T3) exhibited significant improvements at all timepoints.
Interpretation
Although the significant improvements in patients' self-care were not long-lasting, this study suggested that the family FOCUS programme consistently enhanced caregivers' contributions to self-care. Future work could explore the effect of the family FOCUS programme on families with multiple chronic conditions.
Funding
The National Natural Science Foundation of China.
Keywords: Heart failure, Caregivers, Self-care, Mental health, Readmission, Mortality
Research in context.
Evidence before this study
We conducted a comprehensive literature review on May 12, 2023, using the following electronic databases: PubMed, EMBASE, Web of Science, CINAHL, PsycINFO, and the Cochrane Library. The search criteria included subject headings, keywords, and synonyms, containing five domains: (1) heart failure; (2) caregiver; (3) intervention; (4) self-care. We found that the available dyadic self-care interventions for heart failure are limited, especially in China. The results of the studies were inconclusive, and there was notable heterogeneity between studies. In addition, the findings paid less attention to caregiver outcomes, particularly caregiver's contribution to patient's self-care. We aimed to fill this knowledge gap.
Added value of this study
Our study provides evidence to fill the gap of dyadic self-care of heart failure dyads in China. We found that improvements in caregiver contribution to self-care were more long-lasting in family online FOCUS programme compared to self-care for patients with heart failure. Meanwhile, improvements were observed in symptom assessment, coping, relationships, and mental health of the heart failure dyads within 6 months after discharge.
Implications of all the available evidence
This study suggests that, in dyadic disease management, we should improve family communication, coping skills, and mutual interactions to promote their active involvement in self-care for heart failure. This study establishes a foundation for further exploration into the impact of family FOCUS programme on family dyads with multiple chronic conditions.
Introduction
With accelerated aging, heart failure is a rapidly growing public health problem affecting more than 64 million people worldwide.1 It is associated with increased morbidity and mortality and imposes a substantial burden on the health care system.2 It is the most prevalent cause of hospitalisation in 65-year old adults, and 50% of patients first diagnosed with heart failure are re-hospitalised within a year, with a 16.5% mortality rate.3,4 In the final phase of cardiovascular disease, patients with heart failure experience various symptoms such as fatigue, edema, dyspnea, and physical activity restrictions.2 Meanwhile, patients with heart failure are also more susceptible to anxiety and depression.5 Similar studies have shown that heart failure raises depression risk by 2–3 times.6 In turn, anxiety and depression worsen physical symptoms, poor self-care practices, and a higher probability of readmission and mortality in patients with heart failure.7
Heart failure is a shared “journey” for patients and informal caregivers. Caregivers play an important role in symptom monitoring, self-care, psychological and emotional support, and consultation navigation for patients with heart failure.8 Heart failure caregivers spend three times as much time in care as those with other cardiovascular diseases.9 Long-term living with heart failure causes caregivers to develop feelings of stress, anxiety and depression, which in turn reduces interaction with patients.10 Caregivers' physical and mental health is closely connected to patients’ health.11 Studies have demonstrated that caregivers' negative emotions might worsen patient symptoms and depression and cause clinical adverse events.12,13 Meanwhile, the dyadic relationship is also affected between patients and caregivers. Related studies have indicated that a good dyadic relationship can buffer heart failure itself and the pressure of care, reduce readmission and mortality of patients with heart failure, and improve their self-care levels and mental health.14, 15, 16 Given the adverse impact of heart failure, it is critical to develop innovative disease management toolkits that support patients and their caregivers.10
Two persons who maintain a related relationship sociologically can form a dyad. Patients with heart failure and their caregivers maintain related relationships in the family system, known as the heart failure dyad. Related review studies have shown that existing dyadic interventions for heart failure are limited, calling for more innovative forms of dyadic intervention to further discuss their effects on patients and caregivers.17,18 Furthermore, we have found that patients' mental health is never reported to be improved.19, 20, 21 One study notes that about one-third of heart failure dyads have a difference in disease management, and that dyadic inconsistencies lead to poor self-care, anxiety, and depression among patients and caregivers.9 Meanwhile, related studies have further suggested that dyadic symptom assessment inconsistency is associated with decreased dyadic mental health and increased caregiver stress.22,23 Improving the consistency of dyadic symptom perception is the key to preventing the exacerbation of heart failure.24 Therefore, patients and caregivers perceptions of heart failure symptoms need to be evaluated for effective dyadic disease management.
In China, there is a lack of reports on the dyadic disease management of patients with heart failure and their caregivers, accompanied by an absence of a relevant research framework to tackle this problem. Thus, this study presents reliable evidence to fill this knowledge gap. This study is based on the dyadic disease management theory, which consists of three elements: dyadic appraisal, dyadic management and dyadic health. The theory states that dyadic appraisal interacts with dyadic management, ultimately contributing to dyadic health.25 It has also been argued that dyadic relationships determine how heart failure dyads respond to and interpret symptom variability and the need for self-management, leading to dyadic emotional distress over time.23 Furthermore, the systems transaction model of stress and coping states that partners in a family relationship influence each other in the face of shared stressful events.26 Positive dyadic comprehension and coping can help improve their dyadic adaptation and relationship quality.27 Previous research has shown that online interventions are feasible and as effective as face-to-face interventions.28 Therefore, based on the dyadic disease management theory and the system transaction model of stress and coping, we have constructed a dyadic disease management programme (i.e., the family FOCUS programme).29 Because the theory of dyadic illness management is based largely on US studies with low recruitment of Asian population, it is important to examine its applicability within the Chinese context. Through focus group discussions and pilot experiments, we have made modest cultural modification to the theory in China's heart failure dyads and preliminarily found that the theory has favorable adaptability, but the effect still needs to be further verified in this study. We hypothesise that the family FOCUS programme will positively impact the health outcomes of patients with heart failure and their caregivers over time. The aim of this study is to assess the efficacy of the family FOCUS programme.
Methods
Study design and participants
Two-arm parallel group, a randomised controlled study with blind outcome assessment was performed at four grade III class A hospitals in Tianjin. The trial's recruitment period lasted from May 2022 to the end of September 2022, with data collection continuing until March 2023. The protocol is registered with ChiTR, registration number ChiCTR2100053168.
This study complied with the ethical principles of the Declaration of Helsinki and was approved by the Ethics Committee of Tianjin Medical University (reference number TMUHEC2019002). The participants were aware of the study's purpose, willingly volunteered to take part, and subsequently signed an informed consent form. Moreover, participants in the control group were also offered the opportunity to participate in the family FOCUS programme after the intervention ended.
The study included and excluded patients with heart failure and their caregivers in pairs from the cardiology ward. Inclusion criteria: The inpatient was diagnosed with heart failure and classified as New York Heart Association (NYHA) Class Ⅰ–Ⅲ30; aged ≥18 years; was in stable condition; was able to read and write using their smartphone and express themselves clearly. The caregiver was a family member or close relative of the patient; spent at least 24 h per week providing free care to the patient31; was able to read and write using their smartphone and express themselves clearly. Exclusion criteria: The patient or caregiver had cognitive impairment (comprehension or expression problems); took anti-anxiety or anti-depressant medication; was involved in another study for almost three months.
Sample size calculation
The sample size was calculated based on self-care management scores of patient in previous studies, where the mean scores for self-care management scores before and after intervention were 24.55 and 56.66, respectively, with standard deviations of 16.88 and 16.58.32 Assuming a power of 0.8 and α of 0.05, a minimum of 57 participants were required for each study group. Referring to attrition rates of 18%–22% in previous family-centred interventions, the attrition rate considered in this study was about 20%.33 As a result, a total of 142 heart failure dyads were recruited for this study.
Randomisation, allocation and blinding
After meeting eligibility criteria and obtaining informed consent, baseline data collection and randomisation were performed sequentially. Participants in the study were subject to random sequence set, by the Research Randomiser website (https://www.randomizer.org/), with the proportion of 1:1 randomly assigned. To ensure that the allocation was hidden, the third party sequentially numbered 142 unduplicated numbers from the random sequence set, ranging from 1 to 142, in sealed and opaque envelopes.
Interventions of the family FOCUS programme
The development process and details of the family FOCUS programme had been described in our published protocol.29 The theoretical framework of the family FOCUS programme was based on the Theory of Dyadic Illness Management and the Systemic Transactional Model of Stress and Coping (Fig. S1). The programme included discharge health education and 4 online sessions within 4 weeks after discharge, focusing on the themes of “Family Participation,” “Open Communication,” “Coping Effectiveness,” “Uncertainty Reduction,” and “Shared Dyad Life Stories” (Table S1). Individual sessions were conducted by the researcher for each heart failure dyad. The first discharge health education trained heart failure dyads on how to use the Voon Meeting mobile app and set personalised self-management goals. Heart failure dyads received the programme ‘s manual, which covers each session's topic and self-management skills like sleep, nutrition, and exercise. The smartphone app hosted the 4 online sessions. In each session, the researcher explained the material using animated videos and illustrations. Meanwhile, the researcher created a family-specific ‘Hand in Hand Plan’ following each family's traits, specifying which skills and homework they would focus on practicing and finishing during the following week. The duration of each session is approximately 60 min. Initially, in each online session, heart failure dyads devoted 5–10 min reviewing last week's “Hand in Hand Plan,” 30–40 min studying the topic's material and skills, and 10–15 min creating a new plan. In between sessions, the researcher kept in touch with patients and their family caregivers by phone or Wechat to prevent attrition.
These methods ensured intervention fidelity. First, at the end of each session, the researcher used a self-assessed fidelity checklist to confirm whether the key goals of the session had been accomplished. To verify that only intervention group dyads used the family FOCUS programme, the intervention assessment supervisor evaluated 20% of sessions in the intervention and control groups. Second, at the end of the last session, the researcher collected the satisfaction ratings from 1 (extremely dissatisfied) to 5 (very satisfied) and perceptions of patients and their family caregivers for the family FOCUS programme.
Attention control group
The control group received regular clinical nurse care, including discharge health education and follow-up. In addition, to reduce the exposure to dyads as a confounding variable, matched video interviews were provided to the control group. The interviews predominantly comprised general suggestions related to exercise, nutrition, diet, and infection prevention; they omitted any specific details from the family FOCUS programme.
Outcome measures
Primary outcomes
Self-care of heart failure index version 7.2 (SCHFI V.7.2) for patients
The Chinese version of the SCHFI V.7.2 was a 29-item 5-point Likert scale, available from http://self-care-measures.com/.34 It consisted of three subscales: self-care maintenance, symptom perception and self-care management. Each subscale used a standardised score, ranging from 0 to 100. Higher score indicated better self-care performance. In this study, the Cronbach alpha values for the self-care maintenance, symptom perception and self-care management subscales were 0.747, 0.886, and 0.867, respectively.
Caregiver contribution to self-care of heart failure index version 2 (CC-SCHFI V.2) for caregivers
The Chinese version of CC-SCHFI V.2, which evaluated the caregiver's contribution to the patient's self-care and maintained an identical number of items and scoring methods as SCHFI, was accessed at http://self-care-measures.com/.35 It contained three subscales: CC to self-care maintenance, CC to symptom perception and CC to self-care management. In this study, the Cronbach alpha values for CC to self-care maintenance, CC to symptom perception and CC to self-care management subscales were 0.791, 0.875, and 0.859, respectively.
Secondary outcomes
We provided the conceptual framework adapted from secondary outcomes (Fig. S2).
Dyadic coping inventory (DCI) for patients and family caregivers
Based on the systemic transactional model of stress and coping, the dyadic coping inventory assessed the quality of dyadic communication and coping under stress. The Chinese version of the DCI scale was a 37-item 5-point questionnaire, with high scores indicating better dyadic coping.36 The Cronbach alpha values of DCI among patients and caregivers in this study were 0.909 and 0.846.
The mutuality scale (MS) for patients and family caregivers
The Chinese version of MS, a 15-item 5-point Likert scale, was applied to evaluate the dyadic relationship between patients and their family caregivers.37 The scale included four dimensions: love, shared pleasurable activities, shared value and reciprocity. Higher scores indicated better dyadic relationships. The Cronbach alpha values of MS among patients and caregivers in this study were 0.801 and 0.842.
Heart failure somatic perception scale (HFSPS) for patients and family caregivers
The HFSPS was used to assess the awareness and distress of patients and their caregivers with heart failure symptoms over the past week, and had been translated into Chinese.38 It was an 18-item, 6-point Likert scale, with higher scores indicating higher levels of perceived distress. The Cronbach alpha values of HFSPS among patients and caregivers in this study were 0.904 and 0.907.
Self-rating anxiety scale (SAS) and self-rating depression scale (SDS) for patients and family caregivers
The Chinese version of the SAS was a 20-item, 4-point Likert scale used to assess patient and caregiver anxiety.39 Higher scores indicated higher anxiety levels. The Cronbach alpha values for the SAS were 0.890 and 0.918, respectively, in patients and caregivers.
Similarly, the Chinese version of the SDS mirrored the SAS with the same number of scale items and scoring methods to evaluate patient and caregiver depression.40 Higher scores indicated higher depression levels. This study revealed Cronbach alpha values of 0.939 and 0.880 for the SDS in patients and caregivers, respectively.
All-cause hospital admission and mortality for patients
The research assistant learned from patients with heart failure or their caregivers whether the patient had been readmitted or died from any cause within 1, 3, or 6 months after discharge. Subsequently, research assistant reviewed the cases at the hospital's cardiology or emergency department for confirmation based on information provided by patients or caregivers.
Data collection
The primary and secondary outcomes of the study were assessed prior to patient discharge and at 4, 12, and 24 weeks post-discharge. After obtaining written consent from patients with heart failure and their caregivers, data were collected by a research assistant, who was blinded to the intergroup assignment. At the baseline (T0), the heart failure dyads completed the training on how to use the Questionnaire Star (a tool for questionnaire surveys) to finish the data online filling. Participants then received sealed envelopes to complete the randomisation. The research assistant collected follow-up data of patients and their caregivers via Questionnaire Star at 4 weeks (T1), 12 weeks (T2) and 24 weeks (T3) after discharge. Two reminder messages were sent at each follow-up timepoint. If the patient or caregiver did not complete the online data filling within the specified time, the research assistant asked the reason over the phone and determined whether to proceed with the research.
Statistical analysis
IBM SPSS Statistics (version 26.0, IBM, Armonk, USA) was used for statistical analysis of all data, and the statistical significance standard was p < 0.05 (two-tailed). The data analysis was performed by a statistician who was blind to group allocation. Based on the characteristics of the variables, baseline data was compared between the two groups using Independent-samples t-test, Chi-square test, Fisher exact test, and Mann–Whitney U-test as appropriate. The inconsistencies in the dyadic symptom assessment were calculated by subtracting the caregiver's HFSPS score (caregiver appraisal of patient's symptoms) from the patient's HFSPS score (patient appraisal of own symptoms).
To prevent bias in sampling characteristics and study results, data from all randomised participants were included in the analysis (i.e., intention-to-treat analysis). We considered the limitations of repeated measurement ANOVA in evaluating datasets with missing data and the possibility of bias caused by mean matching before performing data analysis. As a result, we modified our protocol and implemented the Generalized Estimation Equation Model (GEE). GEE was used to test for interaction (Group∗Time) jointly over all times, and then also to estimate the interaction effect at each of T1, T2 and T3 relative to T0.41 In this study, the interaction effects at T1 to T3 were considered to represent the effect of the intervention at these times. The baseline measurement group (T0) and control group (group = 1) were designated as the reference categories in the GEE model. In the GEE, the parameters estimated by the built-in quasi-likelihood estimation method were unbiased if the missing data was random. The primary reasons for loss to follow-up in this study were loss of contact/interest, refusal to continue participation, and patient condition deterioration. Dropping out might cause some bias, but we noted that baseline characteristics were comparable between participants who were lost to follow-up and those who completed the study (all p > 0.05) (see Table S2). Based on the results of intergroup comparisons at baseline, variables with a p-value <0.2 were included as potential covariates in the analysis of the GEE equations to reduce the possibility of type II errors.42 Cohen's d was used to estimate the standardised effect size of the continuous outcome variable with 0.2 (small), 0.5 (medium), and 0.8 (large) as the cut-off points.43 All-cause readmission rates and mortality for heart failure patients in the intervention and control groups were assessed using Chi-square test or Fisher exact tests. For dyads with heart failure who dropped out from the study, we obtained consent from patients and their caregivers to continue tracking readmissions and deaths in patients with heart failure during the follow-up period.
Post-hoc sensitivity analysis was used to assess the robustness of the results. First, to explore potential differences in the impact of interventions, post-hoc subgroup analyses were conducted based on sociodemographic factors, including age (patients aged ≥70 or <70; caregivers aged ≥60 or <60), sex (male, female) and education attainment (middle school education or lower, high school education or above). Second, to assess the impact of the missing data on the study's results, we performed a post-hoc completed case analysis (including only those who finished the assessment at T0, T1, T2, and T3) and a post-hoc last observation carried forward analysis (input of missing data with the last observation).
Role of the funding source
The funder did not contribute to the trial design, patient recruitment, data collection, data analysis, data interpretation, or writing of the article.
Results
374 heart failure dyads were approached and screened for eligibility. Of these dyads, 185 dyads were excluded because they failed to meet the inclusion criteria and 47 declined to participate due to lack of interest, time, or for no specific reason. Finally, 142 eligible dyads were recruited and randomly assigned to either the intervention (n = 71 dyads) or control group (n = 71 dyads). All dyads completed the baseline assessment, 131 at T1, 127 at T2, and only 111 at the end of the research. The flow diagram of the research process was presented in Fig. 1.
Fig. 1.
Flow chart of the study process–adapted from CONSORT flowchart.
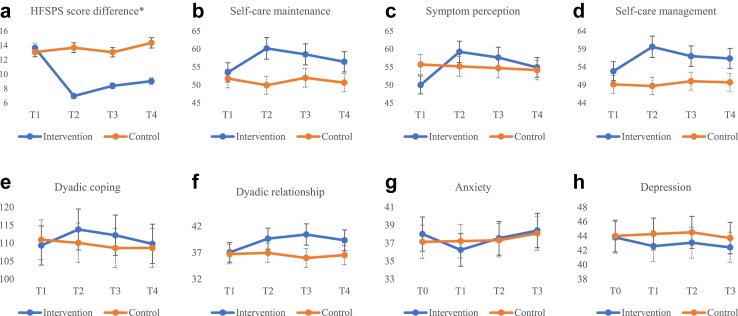
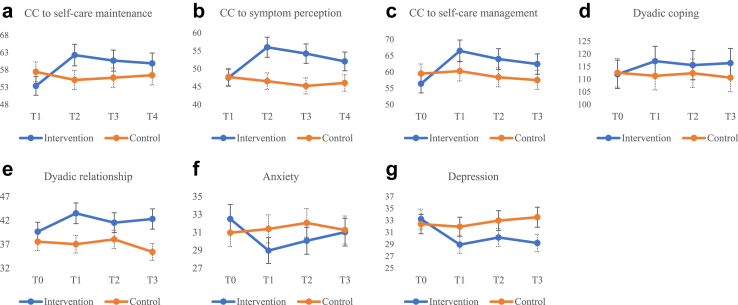
There were no significant differences in demographic and disease characteristics or outcome variables at baseline between groups (Table S3). The mean age of patients was 70.48 (12.28) years, 60.6% were male, 89.1% received medical insurance, 48.59% were NYHA group Ⅲ, and a median number of hospitalisations in the last year was 2.00 (1.00–2.00) (Table 1). Of the family caregivers, the average age was 57.51 (13.14) years, 64.1% were female, 83.8% were married, and the median time of caregiving per day was 24.00 (6.00–24.00) hours. Most family caregivers were spouses (50.7%) or children (42.3%), self-reported fair health (57.0%), lived with the patient (69.7%) and provided care alone (71.8%). To reduce type II errors in the results of the study, the following variables (p < 0.2) were included as covariates in the GEE model: patients' symptom perception and self-care management; and family caregivers’ monthly income, daily caregiving hours, and caregiver contribution to self-care maintenance (Table S3). The change in mean and standard deviation over time for the patient and caregiver variables was shown in Figs. 2 and 3.
Table 1.
Characteristics of patient-family caregiver dyads.
| Variables | Patient n (%) |
Variables | Family caregiver n (%) |
||||
|---|---|---|---|---|---|---|---|
| Total (n = 142) | Intervention (n = 71) | Control (n = 71) | Total (n = 142) | Intervention (n = 71) | Control (n = 71) | ||
| Age(years) [Mean (SD)] | 70.48 (12.28) | 70.03 (14.26) | 70.93 (10.00) | Age(years) [Mean (SD)] | 57.51 (13.14) | 57.73 (13.53) | 57.28 (12.83) |
| Sex | Sex | ||||||
| Male | 86 (60.6%) | 45 (63.4%) | 41 (57.7%) | Male | 51 (35.9%) | 25 (35.2%) | 26 (36.6%) |
| Female | 56 (39.4%) | 26 (36.6%) | 30 (42.3%) | Female | 91 (64.1%) | 46 (64.8%) | 45 (63.4%) |
| Marital status | Marital status | ||||||
| Unmarried | 4 (2.8%) | 3 (4.2%) | 1 (1.4%) | Unmarried | 12 (8.5%) | 5 (7.1%) | 7 (9.9%) |
| Married | 111 (78.2%) | 55 (77.5%) | 56 (78.9%) | Married | 119 (83.8%) | 60 (84.5%) | 59 (83.1%) |
| Divorced | 6 (4.2%) | 2 (2.8%) | 4 (5.6%) | Divorced | 7 (4.9%) | 4 (5.6%) | 3 (4.2%) |
| Widowed | 21 (14.8%) | 11 (15.5%) | 10 (14.1%) | Widowed | 4 (2.8%) | 2 (2.8%) | 2 (2.8%) |
| Educational attainment | Educational attainment | ||||||
| Primary school | 23 (16.2%) | 10 (14.1%) | 13 (18.3%) | Primary school | 12 (8.5%) | 7 (9.8%) | 5 (7.0%) |
| Middle school | 60 (42.2%) | 31 (43.6%) | 29 (40.8%) | Middle school | 45 (31.7%) | 24 (33.8%) | 21 (29.6%) |
| High school | 40 (28.2%) | 22 (31.0%) | 18 (25.4%) | High school | 62 (43.6%) | 30 (42.3%) | 32 (45.1%) |
| University degree or above | 19 (13.4%) | 8 (11.3%) | 11 (15.5%) | University degree or above | 23 (16.2%) | 10 (14.1%) | 13 (18.3%) |
| Employment | Employment | ||||||
| Employed | 18 (12.7%) | 11 (15.5%) | 7 (9.9%) | Employed | 46 (32.4%) | 24 (33.8%) | 22 (31.0%) |
| Unemployed | 124 (87.3%) | 60 (84.5%) | 64 (90.1%) | Unemployed | 96 (67.6%) | 47 (66.2%) | 49 (69.0%) |
| Monthly income (RMB) | Monthly income (RMB) | ||||||
| <2000 | 7 (4.9%) | 4 (5.6%) | 3 (4.2%) | <2000 | 7 (4.9%) | 3 (4.2%) | 4 (5.6%) |
| 2000–5000 | 75 (52.8%) | 41 (57.7%) | 34 (47.9%) | 2000–5000 | 69 (48.6%) | 36 (50.7%) | 33 (46.5%) |
| 5000–10000 | 47 (33.1%) | 19 (26.8%) | 28 (39.4%) | 5000–10000 | 51 (35.9%) | 21 (29.6%) | 30 (42.3%) |
| ≥10,000 | 13 (9.2%) | 7 (9.9%) | 6 (8.5%) | ≥10,000 | 15 (10.6%) | 11 (15.5%) | 4 (5.6%) |
| Religon | Relationship to patients | ||||||
| City | 104 (73.2%) | 55 (77.5%) | 49 (69.0%) | Spouse | 72 (50.7%) | 39 (54.9%) | 33 (46.5%) |
| County/Rural | 38 (26.8%) | 16 (22.5%) | 22 (31.0%) | Son | 25 (17.6%) | 11 (15.5%) | 14 (19.7%) |
| Payment of medical expenses | Daughter | 35 (24.7%) | 18 (25.4%) | 17 (23.9%) | |||
| Medical insurance | 115 (80.9%) | 59 (83.0%) | 56 (78.9%) | Sibling | 4 (2.8%) | 1 (1.4%) | 3 (4.2%) |
| New rural cooperative medical care | 14 (9.9%) | 6 (8.5%) | 8 (11.3%) | Grandchild | 6 (4.2%) | 2 (2.8%) | 4 (5.6%) |
| Uninsured | 13 (9.2%) | 6 (8.5%) | 7 (9.9%) | Living with patients | |||
| BMI (kg/m2) [Mean (SD)] | 22.04 (2.77) | 21.92 (2.46) | 22.16 (3.06) | Yes | 99 (69.7%) | 51 (71.8%) | 48 (67.6%) |
| age-adjusted Charlson Comorbidity Index (aCCI) | 5.26 (1.79) | 5.14 (1.83) | 5.38 (1.74) | No | 43 (30.3%) | 20 (28.2%) | 23 (32.4%) |
| NYAH classification | Self-reported health | ||||||
| Class Ⅰ or Ⅱ | 73 (51.41%) | 34 (47.9%) | 39 (54.9%) | Good | 41 (28.9%) | 17 (23.9%) | 24 (33.8%) |
| Class Ⅲ | 69 (48.59%) | 37 (52.1%) | 32 (22.5%) | Fair | 81 (57.0%) | 43 (60.6%) | 38 (53.5%) |
| Poor | 20 (14.1%) | 11 (15.5%) | 9 (12.7%) | ||||
| Left ventricular ejection fraction(LVEF%) (IQR) | 40.00 (34.00–48.00) | 39.00 (32.00–48.00) | 42.00 (35.00–47.50) | Co-caregiver | |||
| Number of hospitalizations in the past year (IQR) | 2.00 (1.00–2.00) | 2.00 (1.00–2.00) | 2.00 (1.00–2.00) | Yes | 40 (28.2%) | 18 (25.4%) | 22 (31.0%) |
| Duration of illness (years) (IQR) | 2.00 (0.75–5.00) | 2.00 (0.50–4.00) | 2.00 (1.00–5.00) | No | 102 (71.8%) | 53 (74.6%) | 49 (69.0%) |
| HFSPS score differencea | 13.37 (11.09) | 13.65 (10.06) | 13.08 (12.10) | Hours of caregiving per day, median (IQR) | 24.00 (6.00–24.00) | 24.00 (6.00–24.00) | 24.00 (6.00–24.00) |
| SCHFI V.7.2 | CC-SCHFI V.2 | ||||||
| Self-care maintenance | 52.91 (17.51) | 53.50 (16.56) | 51.80 (17.06) | CC to self-care maintenance | 55.39 (18.64) | 53.36 (18.09) | 57.42 (19.08) |
| Symptom perception | 52.90 (20.21) | 50.02 (19.18) | 55.70 (20.75) | CC to symptom perception | 47.59 (17.85) | 47.50 (16.36) | 47.68 (19.34) |
| Self-care management | 50.99 (15.57) | 52.83 (17.02) | 49.16 (13.86) | CC to self-care management | 57.93 (17.29) | 56.38 (16.99) | 59.49 (17.56) |
| Dyadic coping inventory (DCI) | 110.13 (15.71) | 109.34 (17.03) | 110.93 (14.35) | Dyadic coping inventory (DCI) | 112.26 (15.31) | 111.96 (15.99) | 112.56 (14.71) |
| Dyadic relationship (MS) | 36.88 (5.56) | 37.04 (6.08) | 36.72 (5.02) | Dyadic relationship (MS) | 38.59 (6.72) | 39.63 (7.02) | 37.55 (6.29) |
| Anxiety (SAS) | 37.58 (8.81) | 38.01 (8.73) | 37.14 (8.93) | Anxiety (SAS) | 31.73 (8.59) | 32.49 (9.59) | 30.96 (7.43) |
| Depression (SDS) | 43.89 (11.07) | 43.79 (10.72) | 43.99 (11.49) | Depression (SDS) | 32.80 (6.27) | 33.21 (5.91) | 32.38 (6.63) |
BMI: Body mass index; HFSPS: Heart Failure Somatic Perception Scale; SCHFI V.7.2: Self-Care of Heart Failure Index Version 7.2; CC-SCHFI V.2: Caregiver Contribution to Self-Care of Heart Failure Index Version 2; SAS: DCI: Dyadic coping inventory; MS: the mutuality scale; Self-Rating Anxiety Scale; SDS: Self-Rating Depression Scale.
HFSPS score difference: Patient's HFSPS score–Caregiver's HFSPS score.
Fig. 2.
Changes in mean scores of HFSPS score difference (a), patients' self-care maintenance (b), symptom perception (c), self-care management (d), dyadic coping (e), dyadic relationship (f), anxiety (g) and depression (h).
Fig. 3.
Changes in mean scores of CC to self-care maintenance (a), CC to symptom perception (b), CC to self-care management (c), dyadic coping (d), dyadic relationship (e), anxiety (f) and depression (g).
The GEE model showed that the intervention group did not significantly improve in self-care maintenance, symptom perception, or self-care management, compared to the control group over time (Table 2). Further comparison between groups showed that the self-care maintenance (β = 8.544, p = 0.032), symptom perception (β = 9.712, p = 0.021) and self-care management (β = 7.218, p = 0.046) in the intervention group exhibited significant enhancements at T1 with low-to-medium effect sizes (Cohen's d values of 0.59, 0.20, and 0.65, respectively). Patients' symptom perception (β = 9.626, p = 0.036) showed a significant improvement at T2, with a small effect size (Cohen's d = 0.15).
Table 2.
Generalized estimating equation models of the comparison of patients’ outcome between the intervention and control groups.
| Outcomes | Time | Intervention Mean (SD) | Control Mean (SD) | Group effect at T0 |
Time effect in control arm |
Group∗Time effect |
Between groups |
Cohen's d | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | Wald χ2 | p | β (95% CI) | p | |||||
| Primary outcomes | ||||||||||||
| Self-care maintenance | 1.703 (−3.789, 7.194) | 0.543 | 4.599 | 0.204 | ||||||||
| T1 | 60.18 (18.14) | 49.93 (16.53) | −1.868 (−7.693, 3.957) | 0.530 | 8.544 (0.719, 16.370) | 0.032 | 0.59 | |||||
| T2 | 58.51 (16.68) | 52.00 (18.02) | 0.246 (−5.732, 6.224) | 0.936 | 4.760 (−3.641, 13.161) | 0.267 | 0.37 | |||||
| T3 | 56.46 (17.51) | 50.63 (17.12) | −0.998 (−7.723, 5.726) | 0.771 | 4.175 (−5.058, 13.408) | 0.375 | 0.33 | |||||
| Symptom perception | −5.677 (−12.204, 0.849) | 0.088 | 6.928 | 0.074 | ||||||||
| T1 | 59.23 (20.45) | 55.18 (19.58) | −0.476 (−7.103, 6.152) | 0.888 | 9.712 (1.476, 17.947) | 0.021 | 0.20 | |||||
| T2 | 57.64 (19.61) | 54.70 (20.17) | −0.724 (−7.942, 6.493) | 0.844 | 9.626 (0.606, 18.647) | 0.036 | 0.15 | |||||
| T3 | 54.89 (18.80) | 54.15 (21.30) | −0.281 (−7.310, 6.748) | 0.937 | 6.253 (−3.126, 15.632) | 0.191 | 0.88 | |||||
| Self-care management | 3.670 (−1.398, 8.739) | 0.156 | 3.98 | 0.264 | ||||||||
| T1 | 59.61 (18.79) | 48.72 (14.46) | −0.446 (−4.824, 3.932) | 0.842 | 7.218 (0.118, 14.318) | 0.046 | 0.65 | |||||
| T2 | 57.01 (19.10) | 50.04 (14.97) | 0.881 (−4.141, 5.902) | 0.731 | 3.297 (−4.174, 10.767) | 0.387 | 0.41 | |||||
| T3 | 56.37 (18.09) | 49.73 (15.77) | 0.575 (−4.929, 6.078) | 0.838 | 2.960 (−5.319, 11.239) | 0.483 | 0.39 | |||||
| Secondary outcomes | ||||||||||||
| HFSPS score differencea | 0.563 (−3.070, 4.197) | 0.761 | 36.337 | <0.001 | ||||||||
| T1 | 6.96 (6.92) | 13.69 (12.80) | 0.603 (−0.257, 1.463) | 0.170 | −7.296 (−9.876, −4.716) | <0.001 | −0.65 | |||||
| T2 | 8.38 (7.92) | 13.05 (11.76) | −0.036 (−1.408, 1.336) | 0.959 | −5.227 (−8.673, −1.781) | 0.003 | −0.47 | |||||
| T3 | 9.03 (8.25) | 14.36 (13.11) | 1.274 (−0.259, 2.807) | 0.103 | −5.887 (−9.438, −2.337) | 0.001 | −0.49 | |||||
| Dyadic coping inventory (DCI) | −1.592 (−6.735, 3.552) | 0.544 | 29.779 | <0.001 | ||||||||
| T1 | 113.79 (15.53) | 110.06 (15.17) | −0.461 (−1.908, 0.986) | 0.533 | 5.396 (3.312, 7.480) | <0.001 | 0.31 | |||||
| T2 | 112.18 (17.10) | 108.63 (14.22) | −1.049 (−2.732, 0.635) | 0.222 | 4.643 (2.258, 7.027) | <0.001 | 0.23 | |||||
| T3 | 109.78 (16.15) | 108.64 (17.19) | −1.916 (−0.163, 4.590) | 0.032 | 3.543 (1.128, 5.957) | 0.004 | 0.07 | |||||
| Dyadic relationship (MS) | 0.324 (−1.497, 2.144) | 0.727 | 10.795 | 0.013 | ||||||||
| T1 | 39.64 (7.86) | 36.95 (6.11) | 0.235 (−1.650, 2.119) | 0.807 | 2.365 (−0.484, 5.214) | 0.104 | 0.38 | |||||
| T2 | 40.42 (5.91) | 35.98 (4.98) | −0.734 (−2.253, 0.784) | 0.343 | 4.108 (1.646, 6.569) | 0.001 | 0.81 | |||||
| T3 | 39.33 (4.97) | 36.53 (5.69) | −0.190 (−2.158, 1.778) | 0.850 | 2.475 (−0.293, 5.244) | 0.080 | 0.52 | |||||
| Anxiety (SAS) | 0.873 (−2.011, 3.757) | 0.553 | 38.528 | <0.001 | ||||||||
| T1 | 36.24 (9.07) | 37.22 (8.99) | −0.203 (−0.507.0.101) | 0.190 | −1.886 (−2.504, −1.269) | <0.001 | −0.11 | |||||
| T2 | 37.54 (8.83) | 37.32 (9.40) | 0.042 (−0.279, 0.363) | 0.797 | −0.609 (−1.040, −0.178) | 0.006 | 0.02 | |||||
| T3 | 38.40 (9.02) | 38.09 (9.87) | 0.271 (−0.045, 0.587) | 0.093 | −0.524 (−1.002, −0.046) | 0.032 | 0.03 | |||||
| Depression (SDS) | −0.197 (−3.826, 3.432) | 0.915 | 10.704 | 0.013 | ||||||||
| T1 | 42.58 (10.58) | 44.27 (11.44) | 0.296 (−0.036, 0.628) | 0.142 | −1.279 (−1.816, −0.742) | <0.001 | −0.15 | |||||
| T2 | 43.06 (11.12) | 44.50 (11.15) | 0.097 (−0.266, 0.460) | 0.601 | 0.628 (−1.184, −0.072) | 0.027 | −0.14 | |||||
| T3 | 42.40 (11.07) | 43.70 (10.48) | −0.206 (−0.595, 0.182) | 0.298 | −0.408 (−0.830, 0.013) | 0.054 | −0.12 | |||||
MS: the mutuality scale; SAS: Self-Rating Anxiety Scale; SDS: Self-Rating Depression Scale.
HFSPS score difference: Patient's HFSPS score–Caregiver's HFSPS score; T0: Baseline, T1: 4 weeks, T2: 12 weeks, T3: 24 weeks; β: regression coefficient; HFSPS: Heart Failure Somatic Perception Scale; DCI: Dyadic coping inventory.
The GEE model indicated that over time, the intervention group significantly improved in caregiver contributions to self-care maintenance and self-care management compared to the control group, but symptom perception did not (Table 3). However, the comparison between groups revealed that there was no significant enhancement in caregiver contribution to self-care management (β = 8.077, p = 0.060) within the intervention group at T3. Instead, Caregiver contribution to symptom perception had a significant improvement at T1 (β = 9.663, p = 0.019) and T2 (β = 9.229, p = 0.040), with low-to-medium effect sizes (Cohen's d values: 0.54 and 0.49).
Table 3.
Generalized estimating equation models of the comparison of caregivers’ outcome between the intervention and control groups.
| Outcomes | Time | Intervention Mean (SD) | Control Mean (SD) | Group effect at T0 |
Time effect in control arm |
Group∗Time effect |
Between groups |
Cohen's d | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | Wald χ2 | p | β (95% CI) | p | |||||
| Primary outcomes | ||||||||||||
| CC to self-care maintenance | −4.067 (−10.139, 2.005) | 0.189 | 10.196 | 0.017 | ||||||||
| T1 | 62.26 (19.51) | 55.09 (18.46) | −2.357 (−7.769, 3.054) | 0.393 | 11.260 (3.060, 19.459) | 0.007 | 0.38 | |||||
| T2 | 60.67 (17.67) | 55.73 (19.22) | −2.113 (−8.355, 4.128) | 0.507 | 9.408 (0.980, 17.836) | 0.029 | 0.27 | |||||
| T3 | 59.87 (16.57) | 56.46 (20.08) | −0.364 (−2.612, 1.885) | 0.751 | 6.352 (0.138, 12.565) | 0.045 | 0.19 | |||||
| CC to symptom perception | −0.183 (−6.035, 5.668) | 0.951 | 7.213 | 0.065 | ||||||||
| T1 | 55.99 (15.25) | 46.52 (19.84) | −1.160 (−7.728, 5.409) | 0.729 | 9.663 (1.619, 17.707) | 0.019 | 0.54 | |||||
| T2 | 54.22 (16.29) | 45.19 (20.36) | −2.500 (−9.456, 4.457) | 0.481 | 9.229 (0.439, 18.019) | 0.040 | 0.49 | |||||
| T3 | 52.04 (17.51) | 46.02 (19.74) | −1.461 (−8.609, 5.688) | 0.689 | 6.154 (−2.616, 14.924) | 0.169 | 0.32 | |||||
| CC to self-care management | −3.115 (−8.758, 2.528) | 0.279 | 7.136 | 0.004 | ||||||||
| T1 | 66.48 (19.41) | 60.27 (17.37) | 0.778 (−4.990, 6.546) | 0.791 | 9.326 (1.087, 17.564) | 0.027 | 0.34 | |||||
| T2 | 63.96 (18.50) | 58.35 (16.78) | −1.133 (−6.927, 4.660) | 0.701 | 8.716 (0.850, 16.582) | 0.030 | 0.32 | |||||
| T3 | 62.43 (18.20) | 57.51 (16.04) | −2.142 (−7.424, 3.141) | 0.427 | 8.077 (−0.346, 16.500) | 0.060 | 0.29 | |||||
| Secondary outcomes | ||||||||||||
| Dyadic coping inventory (DCI) | −0.606 (−5.624, 4.413) | 0.813 | 12.369 | 0.006 | ||||||||
| T1 | 117.15 (14.77) | 111.33 (14.92) | −0.767 (−2.039, 0.504) | 0.237 | 5.968 (0.786, 11.149) | 0.024 | 0.39 | |||||
| T2 | 115.58 (16.09) | 112.37 (13.89) | −0.247 (−1.310, 0.816) | 0.649 | 3.967 (1.300, 6.633) | 0.004 | 0.21 | |||||
| T3 | 116.38 (13.67) | 110.64 (13.94) | −1.758 (−4.169, 0.654) | 0.153 | 6.003 (0.176, 11.829) | 0.043 | 0.42 | |||||
| Dyadic relationship (MS) | 2.085 (−0.093, 4.262) | 0.061 | 17.043 | 0.001 | ||||||||
| T1 | 43.48 (6.40) | 37.02 (5.86) | −0.534 (−1.883, 0.816) | 0.438 | 4.377 (1.997, 6.758) | <0.001 | 1.05 | |||||
| T2 | 41.52 (7.47) | 38.03 (6.80) | 0.483 (−0.634, 1.600) | 0.397 | 1.406 (−1.612, 4.425) | 0.361 | 0.49 | |||||
| T3 | 42.29 (5.98) | 35.40 (5.40) | −2.153 (−3.860, −0.446) | 0.013 | 4.812 (1.914, 7.711) | 0.001 | 1.21 | |||||
| Anxiety (SAS) | 1.535 (−1.268, 4.338) | 0.283 | 14.007 | 0.003 | ||||||||
| T1 | 28.96 (8.35) | 31.38 (7.08) | 0.166 (−0.355, 0.687) | 0.532 | −3.947 (−4.880, −3.014) | <0.001 | −0.31 | |||||
| T2 | 30.06 (8.90) | 32.05 (6.84) | 0.709 (−0.239, 1.658) | 0.143 | −3.510 (−4.714, −2.307) | <0.001 | −0.25 | |||||
| T3 | 31.02 (9.07) | 31.26 (7.29) | −0.389 (−1.129, 0.351) | 0.303 | −1.648 (−3.062, −0.234) | 0.022 | −0.03 | |||||
| Depression (SDS) | 0.831 (−1.220, 2.882) | 0.427 | 20.998 | <0.001 | ||||||||
| T1 | 28.94 (6.57) | 31.94 (7.03) | −0.434 (−2.123, 1.254) | 0.614 | −3.836 (−5.760, −1.912) | <0.001 | −0.44 | |||||
| T2 | 30.14 (8.33) | 32.97 (6.88) | 0.684 (−0.873, 2.240) | 0.389 | −3.727 (−6.123, −1.332) | 0.020 | −0.37 | |||||
| T3 | 29.21 (9.27) | 33.53 (7.11) | 1.072 (−0.347, 2.491) | 0.139 | −5.035 (−7.487, −2.583) | <0.001 | −0.52 | |||||
Note: T1: 4 weeks, T2: 12 weeks, T3: 24 weeks; β: regression coefficient; CC to self-care maintenance: Caregiver Contribution to self-care maintenance; CC to symptom perception: Caregiver Contribution to symptom perception.
CC to self-care management: Caregiver Contribution to self-care management; DCI: Dyadic coping inventory; MS: the mutuality scale; SAS: Self-Rating Anxiety Scale; SDS: Self-Rating Depression Scale.
Secondary outcomes
The GEE model revealed that the intervention group showed an improvement in the difference of HFSPS scores, as assessed separately by patients and family caregivers, compared to the control group over time (Table 2). Meanwhile, the GEE model indicated significant improvements in dyadic coping, dyadic relationship, anxiety, and depression among patients in the intervention group compared to the control group over time. However, comparisons between groups showed that the patients' depression (β = −0.408, p = 0.054) and dyadic relationship (β = 2.475, p = 0.080) did not improve in the intervention group at T3. The GEE model demonstrated significant improvements in caregiver's dyadic coping, dyadic relationship, anxiety, and depression within the intervention group compared to the control group over time (Table 3). Furthermore, comparisons between groups showed significant improvements in caregivers' dyadic coping, anxiety, and depression in the intervention group at T1, T2, and T3. Meanwhile, dyadic relationship significantly improved in the intervention group at T1 and T2.
The comparison between groups revealed a significant reduction in 30-day all-cause readmission rates (χ2 = 0.097, p = 0.048) among patients in the intervention group; however, no significant differences were observed in 3-month (χ2 = 2.738, p = 0.098) and 6-month (χ2 = 1.340, p = 0.247) (Table 4). The reasons for 30-day readmission were mainly patients' physical problems, such as chest tightness (n = 2), chest pain (n = 1), dyspnea (n = 3), edema (n = 2), and respiratory infection (n = 2). There were no significant differences in 30-day, 3-month and 6-month all-cause mortality between the groups (all p > 0.05).
Table 4.
Comparisons of all-cause readmission and mortality between the study groups.
| Outcomes | FOCUS | Control | X2 | p |
|---|---|---|---|---|
| All-cause readmission | ||||
| 4 weeks | 0.097 | 0.048a | ||
| Yes | 2 (2.8%) | 8 (11.3%) | ||
| No | 69 (97.2%) | 63 (88.7%) | ||
| 12 weeks | 2.738 | 0.098 | ||
| Yes | 7 (9.9%) | 14 (19.7%) | ||
| No | 64 (90.1%) | 57 (80.3%) | ||
| 24 weeks | 1.340 | 0.247 | ||
| Yes | 15 (21.1%) | 21 (29.6%) | ||
| No | 56 (78.9%) | 50 (70.4%) | ||
| All-cause mortality | ||||
| 12 weeks | 0.620 | 0.310a | ||
| Yes | 1 (1.4%) | 3 (4.2%) | ||
| No | 70 (98.6%) | 68 (95.8%) | ||
| 24 weeks | 0.441 | 0.221a | ||
| Yes | 2 (2.8%) | 5 (7.0%) | ||
| No | 69 (97.2%) | 66 (93.0%) |
Fisher's exact test.
When stratified by age, patients aged 70 or older demonstrated greater improvement in self-care management compared to those aged below 70, while symptom perception showed the opposite trend (Table S4). Caregivers aged 60 or older showed greater improvements in caregiver contributions to self-care maintenance compared to those aged below 60 (Table S5). When stratified by sex, the impact of intervention on self-care maintenance was stronger in male patients than in female patients (Tables S6 and S7). When stratified by educational attainment, it was found that compared to those with a middle school education or lower, individuals with a high school education or above showed greater improvement in self-care maintenance and caregivers' contribution to self-care (Tables S8 and S9).
Consistent with the results of the initial analysis, both completed case analysis and last observation carried forward analysis indicated that the intervention group did not significantly improve in patients’ self-care maintenance, self-care maintenance, and self-care management, compared to the control group over time. Likewise, there were significant improvements at specific timepoints (T1 and T2) (Tables S10 and S11). For caregiver primary outcomes, the sensitivity analysis results revealed that the intervention group exhibited a significant improvement in caregiver contribution to self-care maintenance, compared to the control group over time. Similarly, the improvements in caregiver contribution to symptom perception and self-care management were observed at different timepoints (Tables S12 and S13).
The self-reported fidelity checklist for each session showed that the heart failure dyad accomplished the key goals in the session. We collected online sessions using video or audio after the heart failure dyads provided informed consent. Among 369 online sessions, 190 were in the intervention group and 179 in the control group. The intervention supervisor reviewed 75 online sessions (39 in the intervention and 36 in the control groups) and observed that the control group failed to utilise the family FOCUS programme, while the intervention group followed the study protocol. Moreover, the majority of heart failure dyads (91.7%) rated the family FOCUS programme positively (very satisfied and relatively satisfied). Most dyads with heart failure reported that they gained knowledge and skills in self-management (77.6%), recognised changes in symptoms more quickly (82.8%), actively sought help from healthcare providers (84.5%), enhanced coping and interpersonal skills (74.1%), and alleviated emotional distress (62.1%) through the programme.
Discussion
This study was the first randomised controlled trial to investigate the family customised dyadic disease management programme of patients with heart failure and their caregivers through a rigorous study design in China. Due to the challenge of simultaneously recruiting dyads that met the inclusion criteria, the final participation rate for this study was 37.97%, comparable to other studies.33 First, most hospitalised patients with heart failure had complex physical symptoms and emotional distress, leading to decreased interest in participating in this study. Second, several older patients had speech and hearing impairments that prevented them from communicating fluently with the researcher. Third, some patients with heart failure, who had limited literacy skills, encountered difficulties when using smartphones to complete questionnaires and participate in the research. Finally, due to China's one-child policy, families often have a “4-2-1″ structure where one adult couple takes care of four elderly parents and one child.44 This may possibly explain why some caregivers were not present in the care of patients with heart failure and were unable to participate in this study. The attrition rate in this research was 21.83%, which was comparable to other family-centred dyadic research.45
We found that the family FOCUS programme had a transient effect on heart failure patients' self-care and a sustained effect on caregivers' contributions to self-care. Due to the chronic and long-term nature of heart failure, patients with heart failure faced multiple symptoms and self-care issues that could not be addressed on their own and required support from family caregivers. Research had shown that the family FOCUS programme can help patients with heart failure accurately identify changes in symptoms, improve self-care, and seek help from family members or medical staff during the transition from hospital to home. Consistent with previous randomised controlled trials,46,47 self-care of patients with heart failure relied more on caregiver contributions. The caregiver took the lead in the heart failure disease management and did most of the patient's self-care work,48 which may result in the higher the caregiver's contribution to self-care, the less noticeable the change in the patient's self-care over time.
We found that the family FOCUS programme had a positive impact on secondary outcomes in patients with heart failure and their caregivers. The family FOCUS programme interweaved dyadic positive coping and dyadic comprehension into the dyadic disease management process to help patients and caregivers cope with the challenges of heart failure. Meanwhile, it also provided an opportunity for the dyads to communicate, helping them express their love and understanding for each other, thus strengthening the dyadic relationships. Consistent with previous research, family-centred communication had a positive impact on the intimacy and mental health of patients with heart failure and their caregivers.28,49 Moreover, this study further confirmed the applicability of the dyadic disease management theory to the Chinese population. Improved dyadic relationships promoted consistency in dyadic response to and interpretation of symptom changes, enhanced patient and caregiver ability to self-care, and thus alleviated patient and caregiver mental health. Related studies had also pointed out that dyadic interventions can relieve emotional stress and improve the mental health of caregivers.50,51 However, it has not previously been demonstrated that dyadic interventions have a positive effect on the mental health of patients.17,18 Based on quantitative research,16,23,52 we first demonstrated that the family FOCUS programme could improve caregiver contributions to self-care and the mental health of patients and their family caregivers.
Meanwhile, related study had previously suggested that involving caregivers in symptom assessment of patients with heart failure may have a positive impact on reducing readmissions.53 Similarly, we found that the family FOCUS programme helped reduce the 30-day readmission of patients with heart failure, but the long-term effect was not significant. This was in line with the findings of a study of dyadic interventions in patients with stroke.45 The reason may be that as the course of heart failure lengthened, participants in the control group strengthened their ability to perceive and predict changes in heart failure symptoms and took more positive measures to control the progression of the disease.54 Moreover, the family FOCUS programme lasted only one month after patients with heart failure were discharged, and it was possible that the dose of the intervention was insufficient, resulting in no significant long-term effect. Therefore, it was worth considering further extending the duration of the family FOCUS programme to provide continuous and effective help for heart failure dyads.
This research mainly had the following limitations. First, the recruitment of participants was limited to hospitals, which limited the possibility of non-hospital heart failure dyads participating in this study. Second, the study excluded patients with heart failure who did not have a stable family caregiver. Moreover, this study was conducted in the cultural context of China, so the generalisability of this study in other cultures or countries may be limited. It is important to consider cultural modification when applying these findings in different cultural settings. Third, this research delivered interventions and data collection online, which caused some heart failure dyads with low technical literacy to refuse to participate in this research. Fourth, this research did not perform a cost-benefit analysis, which will be further explored in subsequent studies. Fifth, the dropout rate in this study was 21.83%, which poses a risk of either overestimating or underestimating the effect, although we conducted sensitivity analyses for various scenarios to ensure the robustness of our results. Finally, the outcomes of this study were largely self-reported from participants. Although the scale used in this study had been validated and proven to be reliable, relying solely on patient self-reports may introduce some bias into the results. Furthermore, a large number of statistical tests have been conducted, all at the 5% level, and therefore some false positives results are to be expected.
The family FOCUS programme was the first dyadic diseases management programme for heart failure dyads in China. We found that patient self-care and all-cause hospitalisation improved temporarily but not permanently. Significant improvements were observed in the caregiver contribution to self-care, patient and caregiver dyadic coping, relationships, anxiety, and depression within 6 months of the patient's discharge. In the future, multi-centre, large-sample randomised controlled trials will examine its efficacy in heart failure and other chronic disease populations.
Contributors
YWL: Conceptualisation, Methodology, Writing—original draft. SL: Writing- Reviewing and Editing. HLL: Investigation, Visualisation. ZXN: Conceptualisation, Data curation. LQY: Investigation, Methodology. XXY: Validation. WYQ: Software. ZLH: Reviewing and Editing ZY: Conceptualisation, Reviewing and Editing. ZXY: Conceptualisation, Methodology, Writing- Reviewing and Editing. WYG: Conceptualisation, Methodology, Supervision, Writing- Reviewing and Editing.
XXY and WYQ have accessed and verified the underlying data. All authors approved the final version of the manuscript and were responsible for the decision to submit the manuscript. All authors take responsibility for all aspects of the randomised controlled trial.
Data sharing statement
Intervention materials and datasets for this study are available upon reasonable request by contacting the corresponding author.
Declaration of interests
We declare no competing interests.
Acknowledgements
The study was funded by the National Natural Science Foundation of China (grant number: 71910107004, 72304206, 71974142). Authors were not precluded from accessing data in the study, and they accept responsibility to submit for publication. We are grateful to all the heart failure dyads who have participated in this study.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102481.
Contributor Information
Yue Zhao, Email: yuezhao35@tmu.edu.cn.
Xiaoying Zang, Email: snxiaoying@tmu.edu.cn.
Yaogang Wang, Email: YaogangWANG@tmu.edu.cn.
Appendix A. Supplementary data
References
- 1.Savarese G., Becher P.M., Lund L.H., Seferovic P., Rosano G.M.C., Coats A.J.S. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. 2023;118(17):3272–3287. doi: 10.1093/cvr/cvac013. [DOI] [PubMed] [Google Scholar]
- 2.Ziaeian B., Fonarow G.C. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13(6):368–378. doi: 10.1038/nrcardio.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blecker S., Paul M., Taksler G., Ogedegbe G., Katz S. Heart failure–associated hospitalizations in the United States. J Am Coll Cardiol. 2013;61(12):1259–1267. doi: 10.1016/j.jacc.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dokainish H., Teo K., Zhu J., et al. Global mortality variations in patients with heart failure: results from the International Congestive Heart Failure (INTER-CHF) prospective cohort study. Lancet Global Health. 2017;5(7):e665–e672. doi: 10.1016/S2214-109X(17)30196-1. [DOI] [PubMed] [Google Scholar]
- 5.Bordoni B., Marelli F., Morabito B., Sacconi B. Depression and anxiety in patients with chronic heart failure. Future Cardiol. 2018;14(2):115–119. doi: 10.2217/fca-2017-0073. [DOI] [PubMed] [Google Scholar]
- 6.DeJongh B., Birkeland K., Brenner M. Managing comorbidities in patients with chronic heart failure: first, do no harm. Am J Cardiovasc Drugs. 2015;15(3):171–184. doi: 10.1007/s40256-015-0115-6. [DOI] [PubMed] [Google Scholar]
- 7.Vongmany J., Hickman L.D., Lewis J., Newton P.J., Phillips J.L. Anxiety in chronic heart failure and the risk of increased hospitalisations and mortality: a systematic review. Eur J Cardiovasc Nurs. 2016;15(7):478–485. doi: 10.1177/1474515116635923. [DOI] [PubMed] [Google Scholar]
- 8.Kitko L., McIlvennan C.K., Bidwell J.T., et al. Family caregiving for individuals with heart failure: a scientific statement from the American Heart Association. Circulation. 2020;141(22):e864–e878. doi: 10.1161/CIR.0000000000000768. [DOI] [PubMed] [Google Scholar]
- 9.Cameron J., Thompson D.R., Szer D., Greig J., Ski C.F. Dyadic incongruence in chronic heart failure: implications for patient and carer psychological health and self-care. J Clin Nurs. 2017;26(23-24):4804–4812. doi: 10.1111/jocn.13836. [DOI] [PubMed] [Google Scholar]
- 10.McHorney C.A., Mansukhani S.G., Anatchkova M., et al. The impact of heart failure on patients and caregivers: a qualitative study. PLoS One. 2021;16(3) doi: 10.1371/journal.pone.0248240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee A.A., Aikens J.E., Janevic M.R., Rosland A.M., Piette J.D. Functional support and burden among out-of-home supporters of heart failure patients with and without depression. Health Psychol. 2020;39(1):29–36. doi: 10.1037/hea0000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulz R., Beach S.R. Caregiving as a risk factor for mortality–the caregiver health effects study. J Am Med Assoc. 1999;282(23):2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 13.Hu X., Huang W., Su Y., Qu M., Peng X. Depressive symptoms in Chinese family caregivers of patients with heart failure: a cross-sectional study. Medicine. 2017;96(13) doi: 10.1097/MD.0000000000006480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hooker S.A., Grigsby M.E., Riegel B., Bekelman D.B. The impact of relationship quality on health-related outcomes in heart failure patients and informal family caregivers: an integrative review. J Cardiovasc Nurs. 2015;30(4 Suppl 1):S52–S63. doi: 10.1097/JCN.0000000000000270. [DOI] [PubMed] [Google Scholar]
- 15.Vellone E., Chung M.L., Alvaro R., Paturzo M., Dellafiore F. The influence of mutuality on self-care in heart failure patients and caregivers: a dyadic analysis. J Fam Nurs. 2018;24(4):563–584. doi: 10.1177/1074840718809484. [DOI] [PubMed] [Google Scholar]
- 16.Lyons K.S., Sadowski T., Lee C.S. The role of concealment and relationship quality on patient hospitalizations, care strain and depressive symptoms in heart failure dyads. Eur J Cardiovasc Nurs. 2020;19(2):118–124. doi: 10.1177/1474515119863791. [DOI] [PubMed] [Google Scholar]
- 17.Bernard T.L., Hetland B., Schmaderer M., Zolty R., Pozehl B. Nurse-led heart failure educational interventions for patient and informal caregiver dyads: an integrative review. Heart Lung. 2023;59:44–51. doi: 10.1016/j.hrtlng.2023.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Buck H.G., Stromberg A., Chung M.L., et al. A systematic review of heart failure dyadic self-care interventions focusing on intervention components, contexts, and outcomes. Int J Nurs Stud. 2018;77:232–242. doi: 10.1016/j.ijnurstu.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwarz K.A., Mion L.C., Hudock D., Litman G. Telemonitoring of heart failure patients and their caregivers: a pilot randomized controlled trial. Prog Cardiovasc Nurs. 2008;23(1):18–26. doi: 10.1111/j.1751-7117.2008.06611.x. [DOI] [PubMed] [Google Scholar]
- 20.Ågren S., Berg S., Svedjeholm R., Strömberg A. Psychoeducational support to post cardiac surgery heart failure patients and their partners--a randomised pilot study. Intensive Crit Care Nurs. 2015;31(1):10–18. doi: 10.1016/j.iccn.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Vellone E., Rebora P., Ausili D., et al. Motivational interviewing to improve self-care in heart failure patients (MOTIVATE-HF): a randomized controlled trial. ESC Heart Fail. 2020;7(3):1309–1318. doi: 10.1002/ehf2.12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee C.S., Mudd J.O., Auld J., et al. Patterns, relevance and predictors of heart failure dyadic symptom appraisal. Eur J Cardiovasc Nurs. 2017;16(7):595–604. doi: 10.1177/1474515117700760. [DOI] [PubMed] [Google Scholar]
- 23.Lyons K.S., Johnson S.H., Lee C.S. The role of symptom appraisal, concealment and social support in optimizing dyadic mental health in heart failure. Aging Ment Health. 2021;25(4):734–741. doi: 10.1080/13607863.2020.1711866. [DOI] [PubMed] [Google Scholar]
- 24.Bugajski A., Buck H., Zeffiro V., et al. The influence of dyadic congruence and satisfaction with dyadic type on patient self-care in heart failure. Eur J Cardiovasc Nurs. 2021;20(3):268–275. doi: 10.1177/1474515120960002. [DOI] [PubMed] [Google Scholar]
- 25.Lyons K.S., Lee C.S. The theory of dyadic illness management. J Fam Nurs. 2018;24(1):8–28. doi: 10.1177/1074840717745669. [DOI] [PubMed] [Google Scholar]
- 26.Bodenmann G., Falconier M., Randall A. 2017. Systemic-transactional model of dyadic coping; pp. 1–7. [Google Scholar]
- 27.Falconier M.K., Jackson J.B., Hilpert P., Bodenmann G. Dyadic coping and relationship satisfaction: a meta-analysis. Clin Psychol Rev. 2015;42:28–46. doi: 10.1016/j.cpr.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Yang W., Zhang X., Li Y., et al. BMJ supportive & palliative care; 2023. Advanced heart failure patients and family caregivers health and function: randomised controlled pilot trial of online dignity therapy. [DOI] [PubMed] [Google Scholar]
- 29.Yang W., Cao Y., Li Y., et al. Effectiveness of a family customised online FOCUS programme aimed on building resiliency in dyad relationship to support dyadic illness management in persons with heart failure and their informal caregiver: a randomised clinical trial protocol. BMJ Open. 2022;12(7) doi: 10.1136/bmjopen-2022-061405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDonagh T.A., Metra M., Adamo M., et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2022;24(1):4–131. doi: 10.1002/ejhf.2333. [DOI] [PubMed] [Google Scholar]
- 31.Jackson J.D., Cotton S.E., Bruce Wirta S., et al. Burden of heart failure on caregivers in China: results from a cross-sectional survey. Drug Des Dev Ther. 2018;12:1669–1678. doi: 10.2147/DDDT.S148970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sezgin D., Mert H., Özpelit E., Akdeniz B. The effect on patient outcomes of a nursing care and follow-up program for patients with heart failure: a randomized controlled trial. Int J Nurs Stud. 2017;70:17–26. doi: 10.1016/j.ijnurstu.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Wang C., Chen J., Wang Y., et al. Effects of family participatory dignity therapy on the psychological well-being and family function of patients with haematologic malignancies and their family caregivers: a randomised controlled trial. Int J Nurs Stud. 2021;118 doi: 10.1016/j.ijnurstu.2021.103922. [DOI] [PubMed] [Google Scholar]
- 34.Li F., Lin L., Sun X., Chair S., Liu X., Cao X. Psychometric testing of the Chinese version of the self-care of heart failure index version 7.2. J Cardiovasc Nurs. 2023;38(6):528–536. doi: 10.1097/JCN.0000000000000963. [DOI] [PubMed] [Google Scholar]
- 35.Vellone E., Barbaranelli C., Pucciarelli G., Zeffiro V., Alvaro R., Riegel B. Validity and reliability of the caregiver contribution to self-care of heart failure index version 2. J Cardiovasc Nurs. 2020;35(3):280–290. doi: 10.1097/JCN.0000000000000655. [DOI] [PubMed] [Google Scholar]
- 36.Xu F., Hilpert P., Randall A.K., Li Q., Bodenmann G. Validation of the Dyadic Coping Inventory with Chinese couples: factorial structure, measurement invariance, and construct validity. Psychol Assess. 2016;28(8):e127–e140. doi: 10.1037/pas0000329. [DOI] [PubMed] [Google Scholar]
- 37.Archbold P.G., Stewart B.J., Greenlick M.R., Harvath T. Mutuality and preparedness as predictors of caregiver role strain. Res Nurs Health. 1990;13(6):375–384. doi: 10.1002/nur.4770130605. [DOI] [PubMed] [Google Scholar]
- 38.Chen C., Fang W., An Y., Wang L., Fan X. The multiple mediating effects of illness perceptions and coping strategies on the relationship between physical symptoms and depressive symptoms in patients with heart failure. Eur J Cardiovasc Nurs. 2020;19(2):125–133. doi: 10.1177/1474515119864759. [DOI] [PubMed] [Google Scholar]
- 39.Gao J., Zhang X., Su P., et al. The impact of intravaginal ejaculatory latency time and erectile function on anxiety and depression in the four types of premature ejaculation: a large cross-sectional study in a Chinese population. J Sex Med. 2014;11(2):521–528. doi: 10.1111/jsm.12383. [DOI] [PubMed] [Google Scholar]
- 40.Zung W.W., Gianturco J.A. Personality dimension and the self-rating depression scale. J Clin Psychol. 1971;27(2):247–248. doi: 10.1002/1097-4679(197104)27:2<247::aid-jclp2270270230>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 41.Moher D., Hopewell S., Schulz K.F., et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10(1):28–55. doi: 10.1016/j.ijsu.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Purnell J.Q., Selzer F., Wahed A.S., et al. Type 2 diabetes remission rates after laparoscopic gastric bypass and gastric banding: results of the longitudinal assessment of bariatric surgery study. Diabetes Care. 2016;39(7):1101–1107. doi: 10.2337/dc15-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barrows S.P. China's one-child policy. JAMA. 2016;315(21):2349–2350. doi: 10.1001/jama.2016.2192. [DOI] [PubMed] [Google Scholar]
- 45.Mou H., Lam S.K.K., Chien W.T. The effects of a family-focused dyadic psychoeducational intervention for stroke survivors and their family caregivers: a randomised controlled trial. Int J Nurs Stud. 2023;143 doi: 10.1016/j.ijnurstu.2023.104504. [DOI] [PubMed] [Google Scholar]
- 46.Shao J.H., Chang A.M., Edwards H., Shyu Y.I., Chen S.H. A randomized controlled trial of self-management programme improves health-related outcomes of older people with heart failure. J Adv Nurs. 2013;69(11):2458–2469. doi: 10.1111/jan.12121. [DOI] [PubMed] [Google Scholar]
- 47.Østergaard B., Mahrer-Imhof R., Wagner L., Barington T., Videbæk L., Lauridsen J. Effect of family nursing therapeutic conversations on health-related quality of life, self-care and depression among outpatients with heart failure: a randomized multi-centre trial. Patient Educ Counsel. 2018;101(8):1385–1393. doi: 10.1016/j.pec.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 48.Nelson K.E., Saylor M.A., Anderson A., et al. "We're all we got is each other": mixed-methods analysis of patient-caregiver dyads' management of heart failure. Heart Lung. 2022;55:24–28. doi: 10.1016/j.hrtlng.2022.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bouldin E.D., Aikens J.E., Piette J.D., Trivedi R.B. Relationship and communication characteristics associated with agreement between heart failure patients and their Carepartners on patient depressive symptoms. Aging Ment Health. 2019;23(9):1122–1129. doi: 10.1080/13607863.2018.1481923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piette J.D., Striplin D., Marinec N., Chen J., Aikens J.E. A randomized trial of mobile health support for heart failure patients and their informal caregivers: impacts on caregiver-reported outcomes. Med Care. 2015;53(8):692–699. doi: 10.1097/MLR.0000000000000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lang C.C., Smith K., Wingham J., et al. A randomised controlled trial of a facilitated home-based rehabilitation intervention in patients with heart failure with preserved ejection fraction and their caregivers: the REACH-HFpEF Pilot Study. BMJ Open. 2018;8(4) doi: 10.1136/bmjopen-2017-019649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou T., Qu J., Sun H., Xue M., Liu Y. Relationship between mutuality and depression in patients with chronic heart failure and caregivers in China: an actor-partner interdependence model analysis. Front Psychol. 2022;13 doi: 10.3389/fpsyg.2022.928311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deek H., Chang S., Newton P.J., et al. An evaluation of involving family caregivers in the self-care of heart failure patients on hospital readmission: randomised controlled trial (the FAMILY study) Int J Nurs Stud. 2017;75:101–111. doi: 10.1016/j.ijnurstu.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 54.Buck H.G., Hupcey J., Wang H.L., Fradley M., Donovan K.A., Watach A. Heart failure self-care within the context of patient and informal caregiver dyadic engagement: a mixed methods study. J Cardiovasc Nurs. 2018;33(4):384–391. doi: 10.1097/JCN.0000000000000465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.