Abstract
The present study was to evaluate the effect of trace minerals (Zn, Mn, and Cu) from complexed amino acid minerals (ZMCAA) and bis-glycinate chelated minerals (ZMCGly) in laying hen diets on performance, internal and external egg quality, yolk mineral deposition, intestinal morphometry, and bone characteristics. From 78 to 98 weeks of age, 400 White LSL-Lite strain laying hens were distributed in a randomized design with 4 treatments with 10 replicates per treatment. Treatments were distributed in a 2 × 2 factorial arrangement using either Zn, Mn, and Cu of ZMCAA or ZMCGly source at 2 levels: low (20, 20, and 3.5 mg/kg of Zn, Mn, and Cu, respectively) or high (40, 40, and 7 mg/kg of Zn, Mn, and Cu, respectively). The analysis of variance was performed, and in cases where differences were observed, the means were compared using Tukey's test (P < 0.05). The source and level of trace mineral supplementation had a significant impact on the performance of laying hens. Hens fed ZMCAA had higher egg production (P = 0.01), egg weight (P = 0.02), egg mass (P = 0.01), and lower feed conversion ratio (P = 0.05) compared to those fed ZMCGly. The ZMCAA supplementation showed higher albumen height (P = 0.01), albumen weight (P = 0.01), and eggshell thickness (P < 0.01). The deposition of Zn (P < 0.01), Mn (P < 0.01), and Cu (P < 0.01) in the egg yolk was greater for hens received ZMCAA. Tibia weight (P = 0.04) and bone densitometry (P < 0.01) in the tibia were higher with ZMCAA supplementation. In the small intestine, ZMCAA resulted in longer villi (P = 0.02) and shorter crypt depth (P = 0.01) in the duodenum. Jejunum and ileum measurements were influenced by the level and source of trace minerals (P < 0.05). Laying hens fed ZMCAA exhibited superior performance, egg quality, deposition of trace minerals in the egg yolk, and bone density compared to hens fed ZMCGly. In this study, older laying hens supplemented with ZMCAA at lower levels demonstrated adequate levels of supplementation.
Keywords: Bis-glycinates, Metal-amino acids, Radiodensity, Trace minerals
1. Introduction
Trace minerals are essential for poultry development and production. Zinc (Zn), copper (Cu), iron (Fe), manganese (Mn), and selenium (Se) are essential elements involved in various digestive processes (Huang et al., 2019), physiological functions (Vohra and Kratzer, 1957), immunological responses (Smith et al., 2018), and biological synthesis (Pasternak et al., 2012). Deficiencies in trace minerals can lead to reduced feed intake, impaired development, and death (Suttle, 2010).
To prevent poultry deficiencies, minerals are generally supplemented through inorganic salts such as oxides or sulfates as the feed ingredients commonly utilized in diets do not supply the birds' full mineral requirements. However, the digestibility of inorganic salt ranges from 5% to 22% (M'Sadeq et al., 2018), and an excess of these minerals does not allow the animal to express its maximum genetic potential; additionally, excessive inorganic salt supplementation leads to environmental pollution through excretion. Trace minerals bound to organic molecules have been employed in the poultry industry as an alternative to inorganic salt sources to enhance bird performance and health status (Favero et al., 2013; Noetzold et al., 2022; Pereira et al., 2020; Silva et al., 2020).
Due to minerals bound to different organic molecules having distinct characteristics and bioavailabilities, the AAFCO (2002) classified them as chelates, bis-glycinates, proteinates, and amino acid complexed minerals (AACM) based on the organic molecule used. The AACM refers to a metallic ion linked to an amino acid, which creates a more stable organic mineral, less susceptible to interactions with other organic molecules. Consequently, AACM is more available to the organism compared to inorganic salt (Goff, 2018). This is because these molecules do not participate in the process of ionic competition, which involves antagonistic interactions that inhibit the absorption process (Ashmead, 1993). These attributes will positively influence egg production, decrease stress and mortality, as well as reduce mineral excretion (Byrne and Murph, 2022).
Research has shown that trace minerals bound to nonspecific essential amino acids in a 1:1 ratio can enhance the development of reproductive organs in laying hens (Pereira et al., 2020), increase bone mineralization, and decrease phosphorus (P) excretion in layer-type chickens (Medeiros-Ventura et al., 2023; Ventura et al., 2020). They have also been found to improve egg production in laying hens and broiler breeders (Carvalho et al., 2015; Favero et al., 2013). Noetzold et al. (2022) found that there were 5 more chicks per hen housed when broiler breeders were fed AACM compared to inorganic salt sources.
Increasing inorganic salt supplementation in poultry diets reaches an obvious point of diminishing returns where more mineral no longer translates into increased performance benefits. Furthermore, excessive levels of minerals in the body can lead to unwanted metabolic reactions and physiological stress, which can compromise bird performance and health (Suganya et al., 2016; Surai et al., 2019). In contrast, some studies have shown that AACM can be supplemented at lower levels than inorganic salts and still lead to performance benefits (Noetzold et al., 2022), while decreasing the amount of minerals excreted by the animals (Medeiros et al., 1997). Therefore, the use of these molecules requires careful adjustments in supplementation levels, as inadequate levels may not elicit the desired effects of organic supplementation. Other aspects are that trace minerals bound to an organic molecule have higher bioavailability, and their inclusion in birds' diets and subsequent excretion into the environment is lower than that of inorganic salt sources (Pereira et al., 2020). This aspect allows for greater efficiency in the poultry industry and promotes sustainability within the system.
Bis-glycinate chelated minerals Zn, Mn and Cu (ZMCGly) are another organic mineral source used by the poultry industry as an alternative to inorganic salt in broiler breeders, broilers, laying hens, and quails (Ibatullin and Holubiev, 2017; Kwiatkowska et al., 2018; Sun et al., 2020; Xie et al., 2019). The ZMCGly has been included in poultry diets due to its flexible industrial manufacturing process and competitive pricing compared to other organic sources. It is believed that a metal bound to 2 molecules of glycine (Gly) has higher absorption than inorganic salt sources, which could bring benefits to the birds. However, the effects of ZMCGly on poultry remains controversial.
For instance, Zhang et al. (2017) reported that replacing inorganic salt with Zn-Gly increased feed intake, egg weight, fertility, and hatchability in broiler breeders. Conversely, Irani et al. (2019) investigated the intestinal absorption and bioavailability of Mn and found that Mn-Gly and MnSO4 had the same rate of absorption. Buckiuniene et al. (2016), studying the supplementation of FeSO4 and Fe-Gly in 4 different concentrations, concluded that supplementation of these Fe sources did not affect performance or meat quality attributes. Furthermore, Yeung et al. (2005) stated that Fe-Gly and FeSO4 have similar absorption kinetics, and their intestinal absorptions are significantly inhibited by divalent metal ions. Zhu et al. (2022) supplemented broilers with Zn-Gly and ZnSO4 and found no significant difference in body weight, average body weight gain, feed intake, and feed conversion ratio (FCR) at 40 and 50 d of age. However, it was observed that broilers fed Zn-Gly excreted more Zn than those fed the inorganic salt source. As such, further research is needed to fully understand the effects of ZMCGly in poultry diets.
Given that amino acids have unique chemical structures, kinetics (Gardner, 1976), and channels of absorption (Kan, 1975), it was hypothesized that the utilization of complexed amino acid minerals (ZMCAA), at low and high levels would lead to improved zootechnical performance and physiological processes compared to the use of the 2 molecules of a single non-essential amino acid, Gly, in a 2:1 ratio bound to metals. This experiment was conducted to evaluate the effects of Zn, Mn, and Cu derived from ZMCAA or ZMCGly in laying hen diets, supplemented at low and high levels, on performance, internal and external egg quality, yolk mineral deposition, intestinal morphometry, and bone characteristics.
2. Materials and methods
2.1. Animal ethics statement
The research was approved by the Animal Use Ethics Committee - CEUA of the Federal Rural University of Pernambuco (CEUA, N° 041/2018) and all animal experiments complied with the ARRIVE guidelines.
2.2. Animals and husbandry
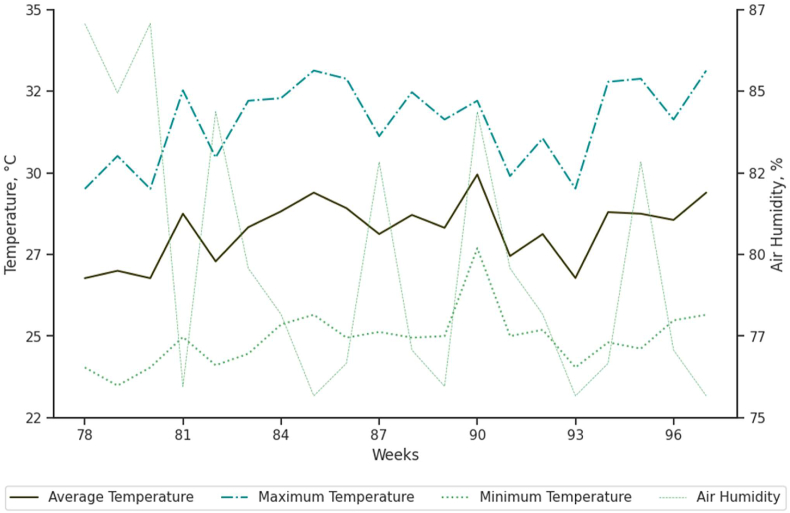
A total of 400 laying hens of the Lohmann White LSL-Lite strain, aged 78 to 98 weeks, were housed in cages measuring 100 cm × 40 cm × 45 cm (10 birds per cage) at the experimental poultry facilities. Feed and water were offered ad libitum throughout the experimental phase. The light program consisted of 16 h of (natural + artificial) light. The temperature and relative humidity of the air inside the house (Fig. 1) were recorded using digital thermohydrometers (Incoterm) and a datalogger (HOBOware; U12-012, Onset Computer Corporation, Bourne, MA, USA).
Fig. 1.
Average, maximum, and minimum temperature and average air humidity of total experimental period. The laying hens (78 to 98 weeks of age) were supplemented with trace minerals complexed to amino acids or glycine chelated to trace minerals.
2.3. Experimental design
The experiment was divided into 5 periods of 28 d, totaling 140 d for data collection. The birds were distributed in a completely randomized experimental design with a factorial arrangement consisting of 4 treatments, 10 replicates, and 10 birds per experimental unit.
The factorial arrangement comprised 2 sources of Zn, Mn, and Cu, either ZMCAA or ZMCGly, and both organic sources were supplemented as premixes in 2 levels: low (20, 20, and 3.5 mg/kg of Zn, Mn, and Cu, respectively) or high (40, 40, and 7 mg/kg of Zn, Mn, and Cu, respectively). The low levels corresponded to 34%, 31%, and 38% (Zn, Mn, and Cu, respectively) of the recommended inorganic salt levels (Rostagno et al., 2017), while the higher levels corresponded to 67%, 62%, and 67% (Zn, Mn, and Cu, respectively) of the inorganic salt recommendations. The AACM source consisted of trace minerals (Zn, Mn, and Cu) complexed to essential amino acids in a 1:1 ratio.
2.4. Dietary treatments
The ingredients and calculated nutrient concentrations are presented in Table 1. The diets were formulated based on the nutritional requirements recommended in the Lohmann LSL-Lite guideline manual. However, the chemical composition and energy values of the feed ingredients were obtained from Rostagno et al. (2017). The composition of mineral premixes and diet analysis are detailed in Table 2. Dry matter, crude protein, crude fat, calcium (Ca), and P were analyzed in a laboratory of Federal Rural University of Pernambuco. Prior to analysis, the experimental diets were ground to a particle size that allowed them to pass through a 0.5-mm screen. The content of dry matter (method 934.01), crude protein (calculated as nitrogen × 6.25, method 990.03), crude fat (method 954.02), and crude fiber (method 991.43) in the diets were determined using the AOAC (2012) procedures. The quantification of Ca and P in the diets was determined utilizing optical emission spectrophotometry with an inductively coupled plasma-optical emission spectrometry (ICP-OES).
Table 1.
Ingredients and calculated nutrient content of the basal diet.1
| Item | Content |
|---|---|
| Ingredients, g/kg as-fed basis | |
| Corn | 597.40 |
| Soybean meal | 250.00 |
| Soybean oil | 17.00 |
| Limestone | 108.60 |
| Dicalcium phosphate | 9.20 |
| Sodium bicarbonate | 1.50 |
| Salt | 2.90 |
| DL-Met, 99% | 2.90 |
| L-Thr, 98.5% | 0.50 |
| Phytase (AB Vista) 2 | 0.06 |
| Vitamin premix3 | 1.00 |
| Mineral premix4 | 1.50 |
| Inert | 6.50 |
| Aluminosilicates | 1.00 |
| Total | 1000 |
| Nutritional levels, g/kg as-fed basis | |
| Metabolizable energy, MJ/kg | 11.50 |
| Dry matter | 910.00 |
| Crude protein5 | 159.00 |
| Crude fiber5 | 30.10 |
| Crude fat5 | 40.10 |
| Digestible Lys | 7.60 |
| Digestible Met | 5.30 |
| Digestible Met + Cys | 7.40 |
| Digestible Thr | 5.90 |
| Calcium5 | 45.00 |
| Phosphorus5 | 6.80 |
| Available phosphorus | 4.50 |
| Sodium | 1.80 |
| Chlorine | 2.30 |
| Potassium | 6.50 |
The feeds were formulated based on the recommendation of Rostagno et al. (2017).
Supplementation per kilogram of the product: phytase, ≥10,000 FTU/kg.
Supplementation per kilogram of the product: vitamin A, ≥8,000,000 IU; vitamin D3, ≥2,500,000 IU; vitamin E, ≥6,000 IU; vitamin K3, ≥1,000 mg; vitamin B1, ≥1,000 mg; vitamin B2, ≥4,500 mg; vitamin B6, ≥2,000 mg; vitamin B12, ≥12 mg; niacin, ≥15,000 mg; calcium pantothenate, ≥6,000 mg; folic acid, ≥400 mg; biotin, ≥25 mg.
Supplementation per kilogram of the product: iodine, 1 mg; selenium, 0.2 mg; iron, 20 mg.
Analysed values.
Table 2.
The mineral compositions of ZMCGly and ZMCAA in experimental diets and water.
| Item | Level | Zn, mg/kg | Mn, mg/kg | Cu, mg/kg | Ca, g/kg | P, g/kg |
|---|---|---|---|---|---|---|
| Calculated values | ||||||
| ZMCGly | Low | 20 | 20 | 3.5 | 45 | 6.8 |
| ZMCGly | High | 40 | 40 | 7.0 | 45 | 6.8 |
| ZMCAA | Low | 20 | 20 | 3.5 | 45 | 6.8 |
| ZMCAA | High | 40 | 40 | 7.0 | 45 | 6.8 |
| Analyzed values1 | ||||||
| ZMCGly | Low | 37.1 | 27.0 | 4.5 | 46.6 | 6.8 |
| ZMCGly | High | 60.9 | 51.0 | 8.5 | 46.0 | 7.0 |
| ZMCAA | Low | 35.3 | 30.1 | 4.5 | 46.5 | 6.8 |
| ZMCAA | High | 66.3 | 51.3 | 8.3 | 46.0 | 6.8 |
| Water content | <0.010 | 0.005 | <0.010 | 9.9 | <0.05 | |
ZMCGly = Zn, Mn, and Cu bis-glycinates.
ZMCAA = Zn, Mn, and Cu amino acid complexed minerals. The amino acid complex sources of Zn, Mn, and Cu were Zinpro Availa Zn, Zinpro Availa Mn, and Zinpro Availa Cu (Zinpro Corp., Eden Prairie, MN, United States), respectively. Other mineral supplementation per kilogram of diet: Iron sulfate 20 mg, sodium selenite 0.2 mg, and calcium iodate 1 mg were offered per kilogram of diet in inorganic form for all treatments.
Obtained by inductively coupled plasma source, dry matter basis.
2.5. Performance of laying hens from 78 to 98 weeks of age
The following variables were evaluated: egg production (%), feed intake (g/bird per day), egg weight (g), egg mass (g/bird per day), and FCR (g:g and kg/dozen eggs). Eggs were collected once a day in the afternoon. All produced eggs were counted and weighed. To calculate FCR, dead birds and feed leftovers were weighed following the methodology described by Sakomura and Rostagno (2007).
2.6. Egg quality
In the last 3 d of each study period, 3 eggs per experimental unit were collected, totaling 30 eggs per treatment, to evaluate egg quality variables: egg weight (g), albumen height (mm), albumen weight (g), eggshell thickness (mm), Haugh unit, and percentages of albumen, eggshell, and yolk. To determine the albumen height, eggs were broken, and their contents (albumen + yolk) were placed on a flat surface.
The albumen height was then measured using a digital caliper (precision of 0.01 mm, model Absolute Digital AOS; Mitutoyo, SP, Brazil). The Haugh unit was calculated by the equation described by Card and Nesheim (1966): Haugh unit = 100 × log (AH + 57 – 1.7 × EW0.37), where AH = albumen height (mm), and EW = egg weight (g).
Subsequently, the yolk was separated from the albumen and weighed on a precision balance. The eggshells were air-dried for 48 h before weighing and their thickness measurements were performed using a digital caliper. Yolk, albumen, and eggshell percentages were calculated by considering their weights in relation to the egg weight. To measure yolk color, a colorimeter (YolkFan, DSM) with a numerical range from 1 to 15 was used.
2.7. Mineral concentration in the yolk
After the end of each period (28 d), eggs were collected for mineral quantification. The yolks were packed in plastic bags, and then a pool of 2 yolks per experimental unit was created for each cycle. The material was placed in a Petri dish and dried in an oven with forced air circulation at a temperature of 55 °C for 72 h.
After drying, the samples were crushed and approximately 0.5 g of each dried sample was aliquoted for analysis. Each sample was combined with 6 mL of concentrated nitric acid and placed in a microwave vial. Digestion was performed in a microwave (model MarsXpress-CEM Technology) for 35 min at a temperature of 160 °C. At the end of digestion, the tubes were removed, the extracts were weighed on an analytical balance, and then deionized water was added to the samples to produce a total volume of 25 mL. The samples were filtered using quantitative filter paper, and their volume was subsequently adjusted, up to a total of 25 mL (Ramos et al., 2010). The quantification of minerals in the yolk was determined utilizing optical emission spectrophotometry with an ICP-OES.
2.8. Bone strength analysis and Seedor index
At the end of the trial, 1 bird was randomly selected from each replicate and euthanized through cervical dislocation after 12 h of fasting. The tibias were then separated and preserved at −20 °C for further analysis. Subsequently, the surrounding muscles, ligaments, and tendons were removed. All the tissues surrounding the tibia were removed without causing damage to the bone structure.
Later, the bones were weighed on a semi-analytical balance (±0.01 g) and their lengths were measured using a digital caliper. The Seedor index (Seedor et al., 1991) was then calculated by dividing the ash weight (mg) by the bone length (mm). This index was used as an indicator of bone density, with a higher index indicating superior density. Bone strength analysis was performed using a universal tester (model TA-XT Plus; Stable Micro Systems, Surrey, UK) with a 50-kg load cell at a speed of 30 mm/min at the Animal Products Evaluation Laboratory of the Federal University of Paraiba, Brazil (LAPOA, UFPB).
2.9. Mineral concentration in the tibia
For mineral composition analysis, the tibia previously used for bone strength analysis was utilized. The bones were dried in an oven at 105 °C (model SL100; Solab, SP, Brazil) for 24 h and then calcined in a muffle furnace (model 2000F; Zezimaq, Minas Gerais, Brazil) for 4 h at 600 °C. Subsequently, approximately 0.5 g of the sample was weighed to be digested with 6 mL of nitric acid (65% analytical purity) in an open system for 30 min. Finally, deionized water was added to a final volume of 50 mL. The quantification of minerals in the sample was performed using ICP-OES. The percentage of ash, Ca, and P was calculated by multiplying the content (mg) by 100 and dividing it by the weight of the tibia.
2.10. Bone densitometry
The procedure was conducted on 5 tibias per treatment using the Hi-Speed FXI CT scanner equipment (General Electric, Fairfield, CT 06824, USA). To capture the images, the tibias were removed from the formaldehyde solution and placed side by side on the examination table with separation between treatments. Cross-sectional images with a thickness of 2 mm and a reconstruction interval of 1 mm were acquired.
These images were then analyzed using the Dicom software (version 1.1.7, Horos, Purview, Annapolis, MD 21401, USA) to estimate the individual values of bone radiodensity at three levels of the diaphysis: proximal, medial, and distal. Each region was divided into quadrants, and a circular region of interest was selected for densitometric assessment of the cortical bone (Oliveira et al., 2012). Bone mass densitometry (BMD) results were obtained in Hounsfield units (HU) and subsequently converted to Ca hydroxyapatite (mg/cm³) using the equation described by Park et al. (2015).
where HUt is the tibia radiodensity measured, HUb is the radiodensity of the tibia phantom containing 200 mg of Ca hydroxyapatite/cm³, and HUw is the radiodensity of the water phantom without Ca hydroxyapatite.
2.11. Histomorphometric analysis
At the end of the experimental period, one bird was selected per experimental unit for histomorphometric analysis. The birds were euthanized and the small intestine sections (duodenum, jejunum, and ileum) were collected. The tissue obtained was weighed, packed in airtight containers with 10% formaldehyde solution, identified, and stored at room temperature.
For histological analysis, the intestines were cut into 0.5-cm sections and embedded in paraffin. Subsequently, they were cross-sectioned into 5-μm slides, stained with hematoxylin-eosin, and examined under optical microscopy (Junqueira and Carneiro, 2008). The analysis of villus length was performed using a 4-fold magnification objective, while the measurement of crypt depth (CD) was conducted with a 10-fold magnification objective.
To capture the images, a microscope coupled with a computer was used, utilizing image analysis software (Leica Qwin D-1000, version 4.1). For functional structures such as villus height (VH) and villus width (VW), objective lenses with 4-fold magnification were employed, and for CD and crypt width (CW), objective lenses with 10-fold magnification were used. The measurements were performed using the computer program Image J (Broeke et al., 2015).
The variables analyzed in the segments of the duodenum, jejunum, and ileum were VH, VW, CD, CW, absorption area (AREA), and villus height to crypt depth (V:C) ratio. Based on the measurements of VH, VW, and CW, and by employing the formula proposed by Kisielinski et al. (2002), it was possible to calculate the characteristics of the absorptive surfaces of the duodenal, jejunal, and ileal segments using the following formula.
2.12. Statistical analysis
The assumptions of normality and homoscedasticity were tested for the analysis of variance. The data were analyzed using the PROC GLM procedure of the Statistical Analysis System software, version 9.2 (SAS userR8S2Q1M7s guide: statistics. Cary, NC, 2008). In cases where differences were observed, the means were compared using Tukey's test (P < 0.05).
The statistical model was the following.
where Yijk is the response variable for bird i in treatment j at level k; μ is the overall mean; αi is the effect of source i (ZMCAA or ZMCGly); βj is the effect of level j (high or low); αβij is the interaction effect of the source i and level j; and εijk is the random error.
3. Results
3.1. Performance
The laying hen's performance was significantly influenced by the source and level of supplementation. Birds fed ZMCAA had higher egg production (P = 0.014), egg weight (P = 0.024), egg mass (P = 0.007), but lower FCR (P = 0.049) at 5.5%, 1.3%, 6.1%, and 4%, respectively, than those of birds fed ZMCGly diets (Table 3). Birds supplemented in low levels showed higher FCR (P = 0.05) and lower feed conversion for dozen eggs (FCD; P = 0.03) than those supplemented in high levels.
Table 3.
Performance of white laying hens fed different sources and levels of Zn, Mn, and Cu from 78 to 98 weeks of age.
| Item | ADFI, g/hen | Egg production, % | Egg weight, g | Egg mass, g/hen per day | FCR, g:g | FCD, kg/dozen eggs |
|---|---|---|---|---|---|---|
| ZMCGly source | 106.6 | 75.9B | 68.6B | 52.1B | 2.017A | 1.656 |
| ZMCAA source | 107.0 | 80.4A | 69.5A | 55.4A | 1.935B | 1.601 |
| Low level | 107.6 | 78.1 | 69.0 | 53.8 | 2.007a | 1.586b |
| High level | 106.0 | 78.2 | 69.0 | 53.5 | 1.931b | 1.657a |
| P-value | ||||||
| Source | 0.578 | 0.014 | 0.024 | 0.007 | 0.049 | 0.145 |
| Level | 0.082 | 0.963 | 0.861 | 0.993 | 0.050 | 0.032 |
| Source × Level | 0.701 | 0.286 | 0.338 | 0.497 | 0.439 | 0.584 |
| SEM | 0.46 | 0.92 | 0.20 | 0.63 | 0.0192 | 0.0161 |
ADFI = average daily feed intake; FCR = feed conversion ratio for egg mass; FCD = feed conversion for dozen eggs; SEM = standard error of the mean.
ZMCGly = Zn, Mn, and Cu bis-glycinates.
ZMCAA = Zn, Mn, and Cu amino acid complexed minerals.
Low level = 20, 20, and 3.5 mg of Zn, Mn, and Cu per kilogram of diet, respectively.
High level = 40, 40, and 7 mg of Zn, Mn, and Cu per kilogram of diet, respectively.
A,B Within a column, values with different letters differ significantly from the source by Tukey’s test (P < 0.05). a,b Within a column, values with different letters differ significantly from the level by Tukey's test (P < 0.05).
3.2. Egg quality
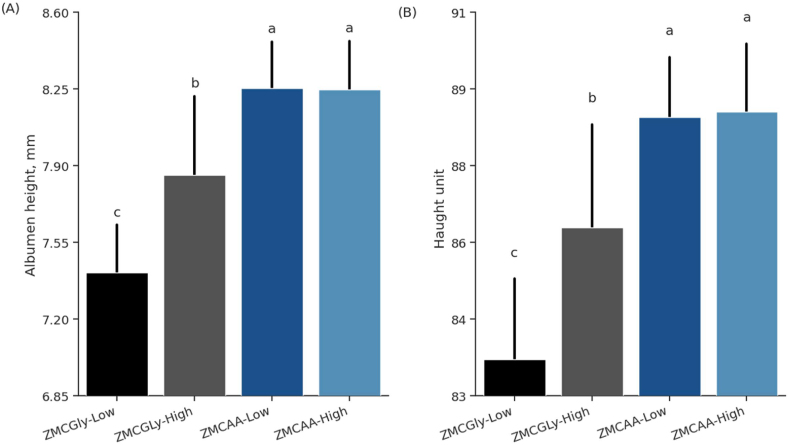
The egg quality variables are shown in Table 4. The variables yolk color (P = 0.50), eggshell weight (P = 0.37), yolk weight (P = 0.61), percentages of albumen (P = 0.46), eggshell (P = 0.44), and yolk (P = 0.614) were not affected by the source or level of trace minerals. However, birds supplemented with ZMCAA showed significantly higher albumen weight (P = 0.014) and eggshell thickness (P < 0.001), at 1.8% and 12.8% greater, respectively, than those fed ZMCGly. An interaction between factors was observed for albumen height (P = 0.001) and Haugh unit (P = 0.016), in which the use of ZMCGly, independent of the levels, reduced the mean values of these variables (Fig. 2).
Table 4.
Egg quality from white laying hens fed different sources and levels of Zn, Mn, and Cu from 78 to 98 weeks of age.
| Item | Egg quality |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yolk color | Albumen height, mm | Albumen weight, g | Eggshell weight, g | Yolk weight, g | Eggshell thickness, mm | Albumen1, % | Eggshell1, % | Yolk1, % | Haugh unit | |
| ZMCGly source | 5.22 | 7.64 | 44.2B | 6.18 | 18.2 | 0.41B | 64.4 | 9.02 | 26.6 | 85.0 |
| ZMCAA source | 5.22 | 8.25 | 45.0A | 6.28 | 18.3 | 0.47A | 64.5 | 9.08 | 26.4 | 88.5 |
| Low level | 5.20 | 7.83 | 44.6 | 6.25 | 18.2 | 0.44 | 64.5 | 9.09 | 26.5 | 86.1 |
| High level | 5.25 | 8.05 | 44.6 | 6.21 | 18.3 | 0.44 | 64.5 | 9.01 | 26.5 | 87.4 |
| P-value | ||||||||||
| Source | 0.921 | <0.001 | 0.014 | 0.087 | 0.667 | <0.001 | 0.779 | 0.439 | 0.666 | <0.001 |
| Level | 0.267 | 0.011 | 0.994 | 0.537 | 0.908 | 0.365 | 0.888 | 0.271 | 0.908 | 0.010 |
| Source × Level | 0.500 | 0.001 | 0.825 | 0.372 | 0.614 | 0.177 | 0.458 | 0.429 | 0.614 | 0.016 |
| SEM | 0.020 | 0.068 | 0.17 | 0.027 | 0.09 | 0.004 | 0.14 | 0.034 | 0.15 | 0.19 |
SEM = standard error of the mean.
ZMCGly = Zn, Mn, and Cu bis-glycinates.
ZMCAA = Zn, Mn, and Cu amino acid complexed minerals.
Low level = 20, 20, and 3.5 mg of Zn, Mn, and Cu, respectively.
High level = 40, 40, and 7 mg of Zn, Mn, and Cu, respectively.
A,B Within a column, values with different letters differ significantly from source by Tukey’s test (P < 0.05).
Values were calculated based on the egg weight.
Fig. 2.
Albumen height (A), and Haught unit (B) in old laying hens (78 to 98 weeks) supplemented with bis-glycinate chelated Zn, Mn, and Cu at low (ZMCGly-Low) and high (ZMCGly-High) levels, and amino acid-complexed minerals at low (ZMCAA-Low) and high (ZMCAA-High) levels. Data are presented as means ± SD. Bars with different letters differ at P < 0.05. The Tukey test (P < 0.05) was used to analyze variations between the groups. ZMCAA = Zn, Mn, and Cu amino acid complexed minerals. ZMCGly = Zn, Mn, and Cu bis-glycinates. Low level = 20, 20, and 3.5 mg/kg of Zn, Mn, and Cu, respectively. High level = 40, 40, and 7 mg/kg of Zn, Mn, and Cu, respectively.
3.3. Mineral deposition in egg yolk
The variables for mineral deposition in egg yolk are shown in Table 5. The levels and sources of trace minerals in the laying hen diets statistically influenced the yolk mineral content of Zn, Mn, Cu, (P < 0.01), and Fe (P = 0.01). The deposition of these metals was 13.6%, 17.3%, 19.7%, and 17% greater, respectively, when the birds received ZMCAA in their diets compared to ZMCGly. Independent of source, low levels of trace minerals were able to provide greater depositions of Zn, Mn, Cu (P < 0.01), and Fe (P = 0.02) in the egg yolk than higher levels.
Table 5.
Egg yolk mineral deposition of zinc, manganese, copper, and iron of eggs from white laying hens fed different sources and levels of Zn, Mn, and Cu from 78 to 98 weeks of age (mg/kg of dry yolk).
| Item | Egg yolk mineral deposition |
|||
|---|---|---|---|---|
| Zn | Mn | Cu | Fe | |
| ZMCGly source | 51.4B | 0.91B | 1.39B | 66.6B |
| ZMCAA source | 59.5A | 1.10A | 1.73A | 77.4A |
| Low level | 60.7a | 1.26a | 1.88a | 77.5a |
| High level | 49.9b | 0.78b | 1.34b | 65.9b |
| P-value | ||||
| Source | 0.009 | 0.004 | 0.001 | 0.015 |
| Level | 0.001 | <0.001 | <0.001 | 0.013 |
| Source × Level | 0.281 | 0.154 | 0.421 | 0.088 |
| SEM | 1.89 | 0.053 | 0.062 | 2.51 |
Zn = zinc; Mn = manganese; Cu = copper; Fe = Iron; SEM = standard error of the mean.
ZMCGly = Zn, Mn, and Cu bis-glycinates.
ZMCAA = Zn, Mn, and Cu amino acid complexed minerals.
Low level = 20, 20, and 3.5 mg of Zn, Mn, and Cu, respectively.
High level = 40, 40, and 7 mg of Zn, Mn, and Cu, respectively.
A,B Within a column, values with different letters differ significantly from the source by Tukey's test (P < 0.05). a,b Within a column, values with different letters differ significantly from the level by Tukey's test (P < 0.05).
3.4. Bone characteristics
The tibia weight, tibia length, Seedor index, bone strength, and tibial bone densitometry are shown in Table 6. Tibia weight was influenced by the source of trace minerals in laying hen diets, with ZMCAA resulting in heavier tibia (P = 0.038) when compared to ZMCGly. The Seedor index showed a significant tendency (P = 0.057) towards higher means for ZMCAA when compared to ZMCGly. No significant differences were observed in tibia length (P = 0.49) or breaking strength (P = 0.99).
Table 6.
Tibia weight, tibia length, Seedor Index, breaking strength, and tibial bone densitometry of white laying hens fed different sources and levels of trace minerals from 78 to 98 weeks of age.
| Item | Bone characteristics |
Tibial bone densitometry |
||||||
|---|---|---|---|---|---|---|---|---|
| Tibia weight, mg | Tibia length, mm | Seedor index | Bone strength, N | Proximal, mg/cm3 | Medial, mg/cm3 | Distal, mg/cm3 | Average, mg/cm3 | |
| ZMCGly source | 7601B | 113.6 | 21.9 | 278.9 | 720.0 | 694 .9B | 705.3 | 703.0 |
| ZMCAA source | 7882A | 113.7 | 22.9 | 287.6 | 732.8 | 820.2A | 729.5 | 760.8 |
| Low level | 7759 | 113.8 | 22.3 | 271.5 | 727.9 | 744.0 | 670.9 | 714.3 |
| High level | 7722 | 113.5 | 22.6 | 294.2 | 724.9 | 779.5 | 770.4 | 754.8 |
| P-value | ||||||||
| Source | 0.038 | 0.889 | 0.057 | 0.174 | 0.720 | 0.003 | 0.505 | 0.114 |
| Level | 0.781 | 0.691 | 0.573 | 0.615 | 0.933 | 0.460 | 0.030 | 0.310 |
| Source × Level | 0.856 | 0.488 | 0.345 | 0.990 | 0.406 | 0.439 | 0.006 | 0.449 |
| SEM | 66.1 | 0.30 | 1.46 | 7.98 | 18.34 | 22.37 | 25.12 | 17.75 |
TW = tibia weight; TL = tibia length; SI = Seedor index; BS = breaking strength; SEM = standard error of the mean.
ZMCGly = Zn, Mn, and Cu bis-glycinates.
ZMCAA = Zn, Mn, and Cu amino acid complexed minerals.
Low level = 20, 20, and 3.5 mg of Zn, Mn, and Cu, respectively.
High level = 40, 40, and 7 mg of Zn, Mn, and Cu, respectively.
A,B Within a column, values with different letters differ significantly from the source by Tukey's test (P < 0.05).
Regarding bone densitometry in laying hens, the supplementation of ZMCAA led to a significant increase (P = 0.003) in radiodensity in the medial segment of the tibia. A significant interaction was noted between the source and level of mineral supplementation in the distal segment (P = 0.006). Hens that received diets with high levels of ZMCAA exhibited denser tibias, whereas those fed diets with low levels of ZMCAA displayed lower values for this variable (Fig. 3).
Fig. 3.
Distal tibia densitometry of old laying hens (78 to 98 weeks) supplemented with chelated bis-glycinate Zn, Mn, and Cu at low (ZMCGly-Low) and high (ZMCGly-High) levels, and amino acid-complexed Zn, Mn, and Cu at low (ZMCAA-Low) and high (ZMCAA-High) levels. Data are presented as means ± SD. Bars with different letters differ at P < 0.05. The Tukey test (P < 0.05) was used to analyze variations between the groups. ZMCAA = Zn, Mn, and Cu amino acid complexed minerals. ZMCGly = Zn, Mn, and Cu bis-glycinates. Low level = 20, 20, and 3.5 mg/kg of Zn, Mn, and Cu, respectively. High level = 40, 40, and 7 mg/kg of Zn, Mn, and Cu, respectively.
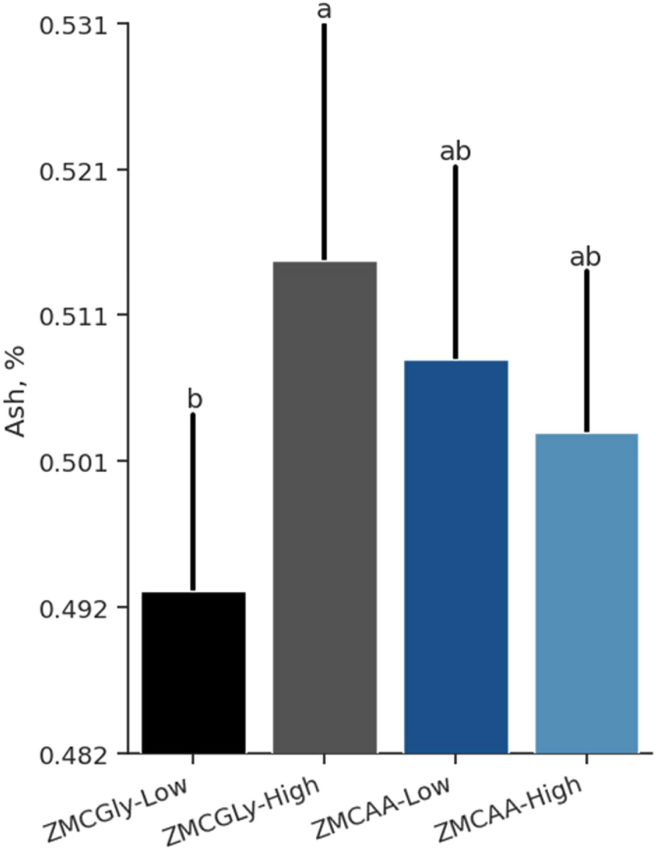
The content of ash (g) in the laying hen bones was significantly higher (P = 0.040) when they were fed ZMCAA diets (Table 7). The minerals Ca (P = 0.688), P (P = 0.966), and Ca:P ratio (P = 0.121) were not influenced by level or source of supplemental Zn, Mn, and Cu. Level and source of supplemented minerals influenced ash (%) in the laying hens’ tibias, as birds fed low levels of ZMCGly had a lower ash concentration (P < 0.05) than birds fed high levels of ZMCGly (Fig. 4).
Table 7.
Ash, calcium, phosphorus, and calcium to phosphorus ratio of white laying hens fed different sources and levels of trace minerals from 78 to 98 weeks of age.
| Item | Ash, g | Bone mineral content |
|||||
|---|---|---|---|---|---|---|---|
| Ca, mg | P, mg | Ash1, % | Ca1, % | P1, % | Ca:P | ||
| ZMCGly source | 2.49B | 159.2 | 73.2 | 0.51 | 15.9 | 7.32 | 2.18 |
| ZMCAA source | 2.62A | 164.2 | 75.2 | 0.51 | 16.3 | 7.45 | 2.18 |
| Low level | 2.53 | 160.0 | 73.5 | 0.50 | 15.8 | 7.28 | 2.18 |
| High level | 2.58 | 163.7 | 74.9 | 0.51 | 16.4 | 7.49 | 2.19 |
| P-value | |||||||
| Source | 0.040 | 0.288 | 0.316 | 0.633 | 0.489 | 0.519 | 0.603 |
| Level | 0.449 | 0.448 | 0.532 | 0.062 | 0.217 | 0.288 | 0.484 |
| Source × Level | 0.579 | 0.688 | 0.966 | 0.004 | 0.985 | 0.712 | 0.121 |
| SEM | 0.031 | 2.15 | 0.91 | 0.023 | 1.21 | 7.387 | 0.006 |
Ca = calcium; P = phosphorus; Ca:P = calcium to phosphorus ratio; SEM = standard error of the mean.
ZMCGly = Zn, Mn, and Cu bis-glycinates.
ZMCAA = Zn, Mn, and Cu amino acid complexed minerals.
Low level = 20, 20, and 3.5 mg of Zn, Mn, and Cu, respectively.
High level = 40, 40, and 7 mg of Zn, Mn, and Cu, respectively.
A,B Within a column, values with different letters differ significantly from the source by Tukey's test (P < 0.05).
Values based in tibia weight.
Fig. 4.
Ash percentage in tibias of old laying hens (78 to 98 weeks) supplemented with bis-glycinate chelated Zn, Mn, and Cu at low (ZMCGly-Low) and high (ZMCGly-High) levels, and amino acid-complexed Zn, Mn, and Cu at low (ZMCAA-Low) and high (ZMCAA-High) levels. Data are presented as means ± SD. Bars with different letters differ at P < 0.05. The Tukey test (P < 0.05) was used to analyze variations between the groups. ZMCAA = Zn, Mn, and Cu amino acid complexed minerals. ZMCGly = Zn, Mn, and Cu bis-glycinates. Low = 20, 20, and 3.5 mg/kg of Zn, Mn, and Cu, respectively. High = 40, 40, and 7 mg/kg of Zn, Mn, and Cu, respectively.
3.5. Histology of the small intestine
3.5.1. Duodenum
The morphometry of the duodenum, jejunum, and ileum in laying hens fed different sources and levels of trace minerals are shown in Table 8. The source of trace mineral had a significant impact on the VH of laying hens (P = 0.022). Hens that were fed diets containing ZMCAA exhibited longer villi compared to those fed diets containing ZMCGly. The CW was reduced by supplementing ZMCAA and increased by supplementing ZMCGly (P < 0.001). Independent of source, a higher level of supplementation reduced CW (P = 0.001). Furthermore, birds that received ZMCAA supplementation demonstrated a greater absorptive area compared to those supplemented with ZMCGly (P < 0.001).
Table 8.
Duodenum, jejunum, and ileum morphometry variables of white laying hens fed different sources and levels of trace minerals from 78 to 98 weeks of age.
| Item | VH, μm | VW, μm | CD, μm | CW, μm | AREA, μm2 | V:C ratio |
|---|---|---|---|---|---|---|
| Duodenum | ||||||
| ZMCGly source | 1771B | 217.7 | 259.2 | 84.0A | 17.7B | 8.3 |
| ZMCAA source | 1909A | 213.3 | 266.9 | 66.5B | 21.5A | 8.5 |
| Low level | 1855 | 223.0 | 245.0 | 78.7a | 19.2 | 8.7 |
| High level | 1826 | 207.9 | 281.7 | 71.6b | 20.1 | 8.1 |
| P-value | ||||||
| Source | 0.022 | 0.642 | 0.606 | <0.001 | <0.001 | 0.581 |
| Level | 0.633 | 0.092 | 0.010 | 0.001 | 0.130 | 0.191 |
| Source × Level | 0.216 | <0.001 | 0.011 | 0.857 | 0.798 | <0.001 |
| SEM | 30.3 | 4.63 | 7.28 | 1.20 | 0.33 | 0.26 |
| Jejunum | ||||||
| ZMCGly source | 1342 | 154.6 | 148.8 | 70.4 | 9.6 | 17.1 |
| ZMCAA source | 1399 | 147.4 | 145.1 | 62.8 | 10.1 | 18.5 |
| Low level | 1444a | 156.6 | 154.9 | 70.7 | 10.4 | 17.5 |
| High level | 1296b | 145.3 | 138.3 | 62.3 | 9.3 | 18.1 |
| P-value | ||||||
| Source | 0.355 | 0.174 | 0.72 | 0.003 | 0.239 | 0.045 |
| Level | 0.001 | 0.005 | 0.025 | 0.001 | 0.001 | 0.001 |
| Source × Level | 0.146 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| SEM | 24.9 | 0.13 | 0.16 | 1.39 | 0.24 | 0.01 |
| Ileum | ||||||
| ZMCGly source | 1383 | 145.9 | 166.2 | 62.1 | 19.0 | 9.3A |
| ZMCAA source | 1285 | 140.4 | 166.9 | 60.6 | 17.4 | 8.4B |
| Low level | 1374 | 142.0 | 181.1 | 64.6 | 17.8 | 8.2b |
| High level | 1296 | 144.4 | 151.8 | 58.0 | 18.5 | 9.5a |
| P-value | ||||||
| Source | 0.006 | 0.345 | 0.911 | 0.438 | 0.003 | 0.041 |
| Level | 0.049 | 0.649 | 0.003 | 0.001 | 0.203 | 0.003 |
| Source × Level | <0.001 | <0.001 | <0.001 | 0.0 01 | <0.001 | 0.164 |
| SEM | 18.3 | 2.45 | 4.45 | 0.90 | 0.21 | 0.24 |
VH = villus height; VW = villus width; CD = crypt depth; CW = crypt width; AREA = absorption area; V:C ratio = villus height to crypt depth ratio; SEM = standard error of the mean.
ZMCGly = Zn, Mn, and Cu bis-glycinates.
ZMCAA = Zn, Mn, and Cu amino acid complexed minerals.
Low level = 20, 20, and 3.5 mg of Zn, Mn, and Cu, respectively.
High level = 40, 40, and 7 mg of Zn, Mn, and Cu, respectively.
A,B Within a column, values with different letters differ significantly from the source by Tukey's test (P < 0.05). a,b Within a column, values with different letters differ significantly from the level by Tukey's test (P < 0.05).
The interaction effects of the source and level for the variables VW (P < 0.01), CD (P = 0.01), and V:C ratio (P < 0.05) were observed (Fig. 5). Birds supplemented with ZMCGly at low levels and ZMCAA at high levels exhibited greater VW compared to those fed ZMCGly at high levels and ZMCAA at low levels, respectively (Fig. 5A). A lower CD was observed in birds supplemented with ZMCGly at low levels than those supplemented with ZMCGly at high levels (Fig. 5B). The lowest V:C ratio was found in birds supplemented with ZMCGly at high levels (Fig. 5C).
Fig. 5.
Villus width (A), crypt depth (B), and villus height to crypt depth ratio (C) of the duodenum in old laying hens (78 to 98 weeks) supplemented with bis-glycinate chelated Zn, Mn, and Cu at low (ZMCGly-Low) and high (ZMCGly-High) levels, and amino acid-complexed Zn, Mn, and Cu at low (ZMCAA-Low) and high (ZMCAA-High) levels. Data are presented as means ± SD. Bars with different letters differ at P < 0.05. The Tukey test (P < 0.05) was used to analyze variations between the groups. ZMCAA = Zn, Mn, and Cu amino acid complexed minerals. ZMCGly = Zn, Mn, and Cu bis-glycinates. Low = 20, 20, and 3.5 mg/kg of Zn, Mn, and Cu, respectively. High = 40, 40, and 7 mg/kg of Zn, Mn, and Cu, respectively.
3.5.2. Jejunum
High levels of trace minerals supplementation negatively influenced the jejunal VH (P < 0.01), and the level and source of trace minerals significantly interacted (P < 0.05) for VW, CD, CW, AREA, and V:C ratio (Table 8) in the jejunum.
According to Fig. 6A, birds that were supplemented with ZMCGly at low levels and ZMCAA at high levels demonstrated greater VW compared to those fed the high ZMCGly and low ZMCAA diets, respectively. The CD was higher for the treatments that received the low ZMCGly and high ZMCAA diets (Fig. 6B). Birds fed ZMCGly at low level showed the highest CW (Fig. 6C); this group showed lowest AREA, differing from the ZMCGLy-high and ZMCAA-low groups (Fig. 6D).
Fig. 6.
Villus width (A), crypt depth (B), crypt width (C), area (D) and villus height to crypt depth ratio (E) of the jejunum in old laying hens (78 to 98 weeks) supplemented with bis-glycinate chelated Zn, Mn, and Cu at low (ZMCGly-Low) and high (ZMCGly-High) levels, and amino acid-complexed Zn, Mn, and Cu at low (ZMCAA-Low) and high (ZMCAA-High) levels. Data are presented as means ± SD. Bars with different letters differ at P < 0.05. The Tukey test (P < 0.05) was used to analyze variations between the groups. ZMCAA = Zn, Mn, and Cu amino acid complexed minerals. ZMCGly = Zn, Mn, and Cu bis-glycinates. AREA absorption area. Low = 20, 20, and 3.5 mg/kg of Zn, Mn, and Cu, respectively. High = 40, 40, and 7 mg/kg of Zn, Mn, and Cu, respectively.
The interaction effect revealed that hens fed diets with low level supplementation of ZMCAA had the highest V:C ratios compared to the other groups (Fig. 6E). The groups of hens fed low ZMCGly and high ZMCAA diets showed the lowest average for this variable.
3.5.3. Ileum
Ileal variables were influenced by the levels and sources of trace minerals supplemented in laying hen diets (P < 0.001; Table 8). The V:C ratio was significantly higher when high levels (P < 0.01) or ZMCGly (P = 0.04) were supplemented. A significant interaction (P < 0.01) of the source and level was observed for VH, VW, CD, CW, and AREA (Fig. 7). The longest villus was observed in birds supplemented with ZMCGly at low levels (Fig. 7A). Birds receiving low levels of ZMCGly and high levels of ZMCAA exhibited wider villi (Fig. 7B). Birds receiving high levels of ZMCGly showed the lowest CD, which is not different from bird receiving low levels of ZMCAA (Fig. 7C). Birds receiving low levels of ZMCGly showed higher CW (Fig. 7D); however, the lowest AREA was observed in birds fed low levels of ZMCAA.
Fig. 7.
Villus height (A), villus width (B), crypt depth (C), crypt width (D), and AREA (E) of the ileum in old laying hens (78 to 98 weeks) supplemented with bis-glycinate chelated Zn, Mn, and Cu at low (ZMCGly-Low) and high (ZMCGly-High) levels, and amino acid-complexed Zn, Mn, and Cu at low (ZMCAA-Low) and high (ZMCAA-High) levels. Data are presented as means ± SD. Bars with different letters differ at P < 0.05. The Tukey test (P < 0.05) was used to analyze variations between the groups. ZMCAA = Zn, Mn, and Cu amino acid complexed minerals. ZMCGly = Zn, Mn, and Cu bis-glycinates. AREA = absorption area. Low = 20, 20, and 3.5 mg/kg of Zn, Mn, and Cu, respectively. High = 40, 40, and 7 mg/kg of Zn, Mn, and Cu, respectively.
4. Discussion
The present study provides evidence supporting the hypothesis that the inclusion of ZMCAA in diets for aged laying hens can result in superior zootechnical performance, egg quality, trace mineral deposition in the yolk, and bone characteristics when compared to the use of ZMCGly. In this study, supplementation with ZMCAA at either low or higher levels improved the performance, egg quality, and bone strength of hens after production peak, compared to ZMCGly. The ZMCAA has been shown to be absorbed by amino acid transporter sites in the intestine (Gao et al., 2014; Sauer et al., 2017), following the same amino acid absorption efficiency, which ranges from 68% to 81% (Ghanima et al., 2023; Reis et al., 2018). This mechanism ensures a better absorption of these trace minerals at both high and low levels in comparison to ZMCGly, where the organic ligand is the amino acid with the smallest absorption rate and tissue accumulation for several species (Gardner, 1976).
For efficient absorption, the trace mineral bound to an organic molecule should remain stable at low pH (1.2 to 2.1; Lee et al., 2017), un-ionized in the gastrointestinal tract, and be absorbed by different transporter than the inorganic salt source in the intestine. Trace minerals in the ZMCAA source are complexed with various essential amino acids in a 1:1 M ratio. This complex maintains stability under physiological pH variations and, upon reaching the small intestine, encounters a significant number of amino acid transmembrane transporters, enabling rapid absorption without competition from antagonistic minerals (Sauer et al., 2017). On the other hand, Gly is poorly absorbed in the intestine compared to other amino acids. Research conducted by Gardner (1976) and Friedman (2018) has demonstrated that Gly, Pro, and Ala exhibit significantly lower absorption and tissue accumulation rates, as measured through in vitro or in vivo methodologies in various animal species, including humans. Scharrer and Brüggermann (1971) reported that small hydrophilic neutral amino acids are taken up less efficiently compared to larger hydrophobic amino acids. In a classic study, Fleshler et al. (1966) demonstrated that Gly displayed reduced absorption due to competitive inhibition, a finding later supported by Scharrer (1971), who reported Gly as having the slowest absorption rate when equimolar concentrations of amino acids were used. Additionally, a study by Sepúlveda and Smith (1978) showed that the presence of glucose and galactose leads to a 50% decrease in Gly absorption, and short-chain amino acids also inhibit the preferential uptake of Gly. These observations may account for the decreased response seen in this study.
The supplementation of ZMCAA allowed for a better action of Zn, Cu, and Mn in intestinal morphology, influencing the VH in the duodenum and the V:C ratio in the jejunum when birds were supplemented at low levels. Additionally, this mineral source promotes favorable changes in intestinal morphology, stimulating increased cell proliferation and reduced apoptosis, thereby enhancing the absorptive function of the gastrointestinal tract, and subsequently improving animal performance (Levkut et al., 2017; Shao et al., 2014). The higher V:C ratio in the ileum for ZMCGly suggests that the absorption of these mineral elements was higher in this segment of the intestine. Bai et al. (2008) found higher absorption of Mn in the ileum of birds fed MnSO4. As glycinate minerals are unlikely to be absorbed efficiently using the amino acid transport system, perhaps ZMCGly used the inorganic salt transporters to be absorbed. Yu et al. (2019) found that Fe bis-glycinates were likely transported into enterocytes using the same pathway as FeSO4, which was the divalent metal ion transporter (DMT1). However, more research is needed to confirm this hypothesis.
Growth factors control cellular intestinal villus proliferation, differentiation, and migration through enzymes such as epidermal growth factor (Fukada et al., 2011; Miguel et al., 2016) and insulin-like growth factor (Freake et al., 2001; Sun et al., 2015). Zinc acts in this process as a cofactor in DNA replication and cell division enzymes, as well as DNA and RNA polymerases (Costa et al., 2023). Additionally, Zn influences the maintenance of tight junction integrity, intestinal permeability, and the absorptive surface area (Wan and Zhang, 2022). Angiogenesis regulated by Cu-dependent enzymes is also important for the supply of nutrients in the intestine, in which lysyl oxidase controls the synthesis of collagen and elastin (Khandia et al., 2016). Likewise, Mn is involved in the synthesis of glycosaminoglycans (Huang et al., 2021), constituents of the extracellular matrix and glycocalyx. This structure regulates the development and maintenance of villi (Xiao et al., 2014).
In the present study, improvements in laying hen performance with higher egg production, egg weight, and egg mass were observed in birds fed ZMCAA compared to ZMCGly. This response may be attributed to the poor availability of ZMCGly, as Gly makes a poor ligand for mineral delivery to the animal. Pereira et al. (2020) reported early body maturation and enhanced egg production as the reasons for a heavier oviduct when layer hens were fed ZMCAA during the early stages of life.
A higher concentration of Zn circulating in the laying hens fed AACM influenced the egg weight. This mineral is involved in the regulation of hormones such as estrogen and progesterone (Assersohn et al., 2021; Park et al., 2004). Studies by Cao and Chen, (1987) demonstrated that Mn is involved in ovarian steroid synthesis in layers, as Mn serves as one of the cofactors in the biosynthesis of cholesterol compounds from enzymes, mevalonate kinase, and geranyl pyrophosphate synthetase (Klimis Tavantzis et al., 1983; Studer et al., 2022). Leach and Gross (1983) reported that dietary Mn deficiency decreased egg production and eggshell thickness. The regulation of steroid synthesis by Mn may justify part of the response observed in the present study, which can be attributed to the higher bioavailability of Mn from ZMCAA, leading to increased growth hormone release and circulating insulin (Xie et al., 2014).
Regarding egg quality, the birds fed ZMCAA minerals produced thicker eggshells, which may be related to improved structural traits of the shell. In fact, it has been reported that the ultrastructure of the eggshell is affected by Zn, Mn, and Cu supplementation (Xiao et al., 2014). Additionally, these trace minerals are directly related to the activation of enzymes involved in eggshell synthesis (Mabe et al., 2003). The density of nucleation sites deposited in the outer membrane of the eggshell is modulated by Mn, which acts on the shell structure, increasing its thickness and reducing the width of mammillary knobs (Zhang et al., 2017b). Furthermore, Venglovská et al. (2014) reported that Mn participates in the activation of glycosyltransferase, an enzyme that acts in the synthesis of mucopolysaccharides that control the structure and texture of the eggshell. Eggshell thickness is known to be directly related to egg fracture resistance (Sun et al., 2012). Thicker eggshells promote a reduction in water and carbon dioxide losses and confer superior freshness in the egg's internal contents through the conservation of albumen proteins (Williams, 1992).
Regarding internal quality, our findings demonstrate that regardless of the level included in the laying hen diets, ZMCGly does not influence the content of these trace minerals in the egg yolk. However, ZMCAA leads to a greater deposition of trace minerals in the yolk. The increased deposition of Zn, Mn, Cu, and Fe in the egg yolk for hens fed ZMCAA indicates a higher metabolic availability of these metal amino acid complexes. The peculiar characteristics of Gly may have hindered its uptake by enterocytes. As reported by Yu et al. (2019), glycinate is absorbed in a similar manner to conventional sources such as oxides and sulfates. Therefore, when considering strategies to enrich eggs for human consumption, glycinate minerals may not be an effective option.
The results obtained in this study, particularly regarding egg yolk deposition, suggest an enhanced absorptive capacity of minerals in the intestine with reduced inclusion levels. However, it is probable that the excess minerals in the hen's body are excreted, resulting in a lower deposition of minerals in the egg yolk. Considering the inclusion of phytase in the diets, which releases substantial amounts of cations (Mn, Zn, and Cu) from the macro ingredients (Liu and Ru, 2010), there is likely a synergistic effect between phytase and amino acid-complexed minerals. The lower antagonism of Zn, Cu, Mn, and Fe in the intestines of birds supplemented with AACM may lead to increased Fe deposition in the egg yolk.
Distinct patterns of bone characteristics between the 2 diets of mineral supplementation sources were noted in the present study. The use of ZMCAA shows an increase in medial density, and at higher concentrations, it promotes denser distal tibias bones in older laying hens, implying better bone structure and laying welfare. Additionally, higher bone densitometry confirmed the greater bioavailability of ZMCAA compared to the ZMCGly source. Considering the bone quality variables studied, a difference was observed in tibia weight and Seedor index between sources, and interaction of the source and mineral level for percentage of ash. Hens fed ZMCAA had heavier bones than those fed ZMCGly. The numerical differences obtained in tibia length and tibia breaking strength were not statistically significant between mineral levels or sources. Although these characteristics have been widely utilized (Brito et al., 2023; Farias et al., 2019; Seedor et al., 1991) as measures of bone quality and health, these variables did not correspond to the results of bone densitometry in our study.
Bone densitometry, as shown by computer tomographic images, provides detailed information about the bone structure and may be a promising method for measuring bone quality in laying hens. The collagenous matrix of bone is formed through the actions of Mn and Cu-containing enzymes (glucuronyltransferases and lysyl oxidase, respectively), as shown by several studies (Richards et al., 2010; Xiao et al., 2014; Zhang et al., 2017). Additionally, bone deposition and resorption take place through enzymes activated by Zn, such as carbonic anhydrase and phosphatases (Silva et al., 2015). Furthermore, longitudinal bone growth is dependent on the synthesis and release of insulin-like growth factor-I, which stimulates growth hormone. Lower levels of dietary Zn can cause a reduced concentration of insulin-like growth factor-I (Freake et al., 2001; Roughead and Lukaski, 2003). However, serum insulin-like growth factor-I concentration is also related to Cu intake (Roughead and Lukaski, 2003). Even when diets contain adequate levels of Ca and P, deficiencies of Cu and Fe inhibit bone growth and decrease bone strength (Medeiros et al., 1997). Moreover, Mn deficiency can lead to thickened long bones and osteochondrosis, the latter being characterized by gross enlargement and malformation of the tibiometatarsal joint (Olgun and Aygun, 2016; Scott et al., 1969).
Bone quality may change throughout the lifespan of the bird. At the end of the production period, the bone quality dependents on 2 factors: the capacity for bone formation in the growth phase and lower intensity of bone resorption during the laying phase (Whitehead and Fleming, 2000). Thus, the current results demonstrate that the birds did not present negative responses to bone ash, Ca, P, and Ca:P ratio, even with the use of different sources and levels of trace minerals. This implies that there was no increase in the activity of osteoblastic and osteoclasts cells. These finds are consistent with those of M'Sadeq et al. (2018) and Saldanha et al. (2009), who found that sources and levels of trace elements did not influence the content of Ca and P in the tibia of birds. These authors argued that even the smallest amount of organic trace minerals was able to balance the minerals in the tibia.
The bioavailability of trace minerals is influenced by changes in pH values throughout the digestive tract of birds, which ultimately impacts their absorption. This can result in the occurrence of antagonistic interactions among metals, as well as interactions with other compounds that insoluble complexes that are not absorbed by birds (Świątkiewicz et al., 2014). Furthermore, losses in absorption can occur due to competition for absorption sites between mineral elements (Goff, 2018). For example, elevated levels of dietary Ca in birds can diminish the absorption of Zn, Mn, and Cu, therefore disrupting normal bone development (Waldroup, 1996). However, if the trace minerals complexed to amino acids remain stable during the pH changes of the gastrointestinal tract, they can be absorbed without competition for absorption sites from other minerals. This may explain the greater deposition of ash in the bones for the ZMCAA fed birds in this study, as there was none antagonism between minerals.
5. Conclusions
Laying hens fed ZMCAA exhibited superior performance, egg quality, deposition of trace minerals in the egg yolk, and bone density compared to hens fed diets containing ZMCGly. In this study, the levels of ZMCAA at 20, 20, and 3.5 mg/kg of Zn, Mn, and Cu, respectively, supplemented to older laying hens, demonstrated to be adequate, based on presented results.
Author contributions
Carlos B. V. Rabello and Mércia R. Barros: conceptualization, and supervision. Marcos J. B. Santos: software development, curated data, and writing—original draft preparation. Leandro M. Silva, Clariana S. Santos, and Jamille S. S. Wanderley: formal analysis and investigation. Marcos J. B. Santos and Carlos B. V. Rabello: writing—review and editing. Carlos B. V. Rabello and Maria C. M. M. Ludke and Fabiano S. Costa: animals and equipment.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
The authors would like to thank the Coordination for the Improvement of Higher Education Personnel (CAPES) for the fellowship grant and to the National Council for Scientific and Technological Development (Grant No. 308168/2018-6) for the financial support for this study.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- AAFCO (Association of American Feed Control Officials) AAFCO manual. AAFCO Inc.; West Lafayette (IN): 2002. [Google Scholar]
- AOAC . 19th ed. Association of Official Agricultural Chemists; Arlington, VA, USA: 2012. Official methods of analysis. [Google Scholar]
- Ashmead H.D. 1st ed. Noyes Publications; 1993. The roles of amino acid chelates in animal nutrition. [Google Scholar]
- Assersohn K., Brekke P., Hemmings N. Physiological factors influencing female fertility in birds. R Soc Open Sci. 2021;8 doi: 10.1098/rsos.202274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai S.P., Lu L., Luo X.G., Liu B. Kinetics of manganese absorption in ligated small intestinal segments of broilers. Poultry Sci. 2008;87:2596–2604. doi: 10.3382/ps.2008-00117. [DOI] [PubMed] [Google Scholar]
- Brito A.N.E.F., Kaneko I.N., Cavalcante D.T., Cardoso A.S., Fagundes N.S., Fontinhas-Netto G., et al. Hydroxy-selenomethionine enhances the productivity and egg quality of 50- to 70-week-old semi-heavy laying hens under heat stress. Poultry Sci. 2023 doi: 10.1016/j.psj.2022.102320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeke J., Mateos Pérez J.M., Pascau J. Image processing with ImageJ. Biophot Int. 2015:231. [Google Scholar]
- Buckiuniene V., Gruzauskas R., Kliseviciute V., Stupeliene A.R., Svirmickas G., Bliznikas S., et al. Effect of organic and inorganic iron on iron content, fatty acid profile, content of malondialdehyde, texture and sensory properties of broiler meat. Verl Eug Ulm. 2016 doi: 10.1399/eps.2016.141. [DOI] [Google Scholar]
- Byrne L., Murphy R.A. Relative bioavailability of trace minerals in production animal nutrition: a review. Animals. 2022 doi: 10.3390/ANI12151981/S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S.F., Chen L.J. Effects of manganese of the concentrations of plasma, LH, estrogen and progesterone in white ear hens. J Shanghai Jiaot Univ Sci. 1987;5:109–116. [Google Scholar]
- Card L.E., Nesheim M.C. 2rd ed. Lea & Febiger; Philadelphia: 1966. Print. [Google Scholar]
- Carvalho L.S.S., Rosa D.R.V., Litz F.H., Fagundes N.S., Fernandes E.A. Effect of the inclusion of organic copper, manganese, and zinc in the diet of layers on mineral excretion, egg production, and eggshell quality. Rev Bras Ciência Avícola. 2015 doi: 10.1590/1516-635XSPECIALISSUENutrition-PoultryFeedingAdditives087-092. [DOI] [Google Scholar]
- Costa M.I., Ribeiro A.B.S., Gonçalves A.C. Zinc: from biological functions to therapeutic potential. Int J Mol Sci. 2023 doi: 10.3390/ijms24054822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias de M.R.S., Leite S.C.B., Moura C.P., Costa A.C., Abreu C.G., Sena T.L., et al. Rev Bras de Zootec; 2019. Organic minerals with different chemical characteristics in diets for Hy-Line White laying hens: performance, biometry of digestive organs, and bone quality. [DOI] [Google Scholar]
- Favero A., Vieira S.L., Angel C.R., Bess F., Cemin H.S., Ward T.L. Reproductive performance of Cobb 500 breeder hens fed diets supplemented with Zn, Mn, and Cu from inorganic and amino acid-complexed sources. J Appl Poultry Res. 2013 doi: 10.3382/japr.2012-00607. [DOI] [PubMed] [Google Scholar]
- Fleshler B., Butt J.H., Wismar J.D. Absorption of glycine and L-alanine by the human jejunum. J Clin Invest. 1966 doi: 10.1172/JCI105451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freake H.C., Govoni K.E., Guda K., Huang C., Zinn S.A. Actions and interactions of thyroid hormone and zinc status in growing rats. J Nutr. 2001 doi: 10.1093/jn/131.4.1135. [DOI] [PubMed] [Google Scholar]
- Friedman M. 1rd ed. CRC Press; 2018. Absorption and utilization of amino acids. [Google Scholar]
- Fukada T., Yamasaki S., Nishida K., Murakami M., Hirano T. Zinc homeostasis and signaling in health and diseases. J Biol Inorg Chem. 2011 doi: 10.1007/s00775-011-0797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S., Yin T., Xu B., Ma Y., Hu M. Amino acid facilitates absorption of copper in the Caco-2 cell culture model. Life Sci. 2014 doi: 10.1016/j.lfs.2014.05.021. [DOI] [PubMed] [Google Scholar]
- Gardner M.L. Absorption from a mixture of seventeen free amino acids by the isolated small intestine of the rat. J Physiol. 1976 doi: 10.1113/jphysiol.1976.sp011295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanima M.M.A., El-Hack M.E.A., Al-Otaibi A.M., Nasr S., Almohmadi N.H., Taha A.E., et al. Growth performance, liver and kidney functions, blood hormonal profile and economic efficiency of broilers fed different levels of threonine supplementation during feed restriction. Poultry Sci. 2023 doi: 10.1016/j.psj.2023.102796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff J.P. Invited review: mineral absorption mechanisms, mineral interactions that affect acid–base and antioxidant status, and diet considerations to improve mineral status. J Dairy Sci. 2018 doi: 10.3168/jds.2017-13112. [DOI] [PubMed] [Google Scholar]
- Huang L., Li X., Wang W., Yang L., Zhu Y. The role of zinc in poultry breeder and hen nutrition: an update. Biol Trace Elem Res. 2019 doi: 10.1007/s12011-019-1659-0. [DOI] [PubMed] [Google Scholar]
- Huang Y.-F., Mizumoto S., Fujita M. Novel insight into glycosaminoglycan biosynthesis based on gene expression profiles. Front Cell Dev Biol. 2021 doi: 10.3389/fcell.2021.709018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibatullin, Holubiev M.I. Scientific Messenger of LNU of Veterinary Medicine and Biotechnologies; 2017. Effect of feeds containing different sources of manganese on certain carcass parameters of quail. [DOI] [Google Scholar]
- Irani F.K., Janmohammadi H., Kianfar R., Sahraei M. Evaluation of chemical characteristics and effects of different manganese sources on kinetics of manganese absorption and performance of broiler chickens. Iran J Appl Anim Sci. 2019;9:463–471. [Google Scholar]
- Junqueira L.C.U., Carneiro P.J.C. 11rd ed. 2008. Histologia básica. Rio de Janeiro. [Google Scholar]
- Kan C.A. The intestinal absorption of amino acids and peptides with special reference to the domestic fowl: a literature review. World’s Poult Sci J. 1975 doi: 10.1079/WPS19750006. [DOI] [Google Scholar]
- Khandia R., Vishwakarma P., Dwivedi A., Mehra R., Kujur A., Dhama K., et al. Evaluation of the modulatory effects of copper salts on the process of angiogenesis (neovascularization) with therapeutic perspectives. Adv Anim Vet Sci. 2016 doi: 10.14737/journal.aavs/2016/4.8.405.410. [DOI] [Google Scholar]
- Kisielinski K., Willis S., Prescher A., Klosterhalfen B., Schumpelick V. A simple new method to calculate small intestine absorptive surface in the rat. Clin Exp Med. 2002 doi: 10.1007/s102380200018. [DOI] [PubMed] [Google Scholar]
- Klimis Tavantzis D.J., Kris Etherton P.M., Leach R.M. The effect of dietary manganese deficiency on cholesterol and lipid metabolism in the estrogen-treated chicken and the laying hen. J Nutr. 1983 doi: 10.1093/JN/113.2.320. [DOI] [PubMed] [Google Scholar]
- Kwiatkowska K., Winiarska-Mieczan A., Kwiecień M. Effect of application of fe-glycinate chelate in diet for broiler chickens in an amount covering 50 or 25% of the requirement on physical, morphometric and strength parameters of tibia bones. Biol Trace Elem Res. 2018 doi: 10.1007/s12011-017-1171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach R.M., Gross J.R. The effect of manganese deficiency upon the ultrastructure of the eggshell. Poultry Sci. 1983 doi: 10.3382/ps.0620499. [DOI] [PubMed] [Google Scholar]
- Lee S.A., Dunne J., Mottram T., Bedford M.R. Effect of diet phase change, dietary Ca and P level and phytase on bird performance and real-time gizzard pH measurements. Br Poultry Sci. 2017 doi: 10.1080/00071668.2017.1293799. [DOI] [PubMed] [Google Scholar]
- Liu N., Ru Y.J. Effect of phytate and phytase on the ileal flows of endogenous minerals and amino acids for growing broiler chickens fed purified diets. Anim Feed Sci Technol. 2010;156(3–4):126–130. doi: 10.1016/j.anifeedsci.2010.01.008. [DOI] [Google Scholar]
- Levkut M., Fukasová M., Bobíková K., Levkutová M., Čobanová K., Levkut M. The effect of inorganic or organic zinc on the morphology of the intestine in broiler chickens. Folia Vet. 2017 doi: 10.1515/fv-2017-0027. [DOI] [Google Scholar]
- M'Sadeq S.A., Wu S.B., Choct M., Swick R.A. Influence of trace mineral sources on broiler performance, lymphoid organ weights, apparent digestibility, and bone mineralization. Poultry Sci. 2018 doi: 10.3382/PS/PEY197. [DOI] [PubMed] [Google Scholar]
- Mabe I., Rapp C., Bain M.M., Nys Y. Supplementation of a corn-soybean meal diet with manganese, copper, and zinc from organic or inorganic sources improves eggshell quality in aged laying hens. Poultry Sci. 2003 doi: 10.1093/ps/82.12.1903. [DOI] [PubMed] [Google Scholar]
- Medeiros D.M., Ilich J., Ireton J., Matkovic V., Shiry L., Wildman R. Femurs from rats fed diets deficient in copper or iron have decreased mechanical strength and altered mineral composition. J Trace Elem Exp Med. 1997:2–8. doi: 10.1002/(SICI)1520-670X(1997)10:3<197::AID-JTRA7>3.0.CO. [DOI] [Google Scholar]
- Medeiros-Ventura W.R.L., Rabello C.B.V., Santos M.J.B., Barros M.R., Silva Junior R.V., Oliveira H.B., et al. The impact of phytase and different levels of supplemental amino acid complexed minerals in diets of older laying hens. Animals. 2023;13:3709. doi: 10.3390/ani13233709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel J.C., Maxwell A.A., Hsieh J.J., Harnisch L.C., Al Alam D., Polk D.B., et al. Epidermal growth factor suppresses intestinal epithelial cell shedding via a MAPK dependent pathway. J Cell Sci. 2016 doi: 10.1242/jcs.182584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noetzold T.L., Vieira S.L., Xavier B.B., Olabarriaga Y.J., Fireman A.K. Supplemental effects of amino acid-complexed trace minerals on broiler breeder hen performance. Anim Feed Sci Technol. 2022 doi: 10.1016/j.anifeedsci.2022.115371. [DOI] [Google Scholar]
- Olgun O., Aygun A. Nutritional factors affecting the breaking strength of bone in laying hens. World’s Poult Sci J. 2016 doi: 10.1017/S0043933916000696. [DOI] [Google Scholar]
- Oliveira J.F., Ross J.L., Leite F.L.G., Oliveira D.C., Costa L.A.V.S., Silva I.C.C., et al. Densitometria da vértebra dorsal, osso pleural e osso neural em tartarugas verdes hígidas por tomografia computadorizada quantitativa. Ciência Rural. 2012 doi: 10.1590/S0103-84782012000800018. [DOI] [Google Scholar]
- Park A.J., Choi J.-H., Kang H., Park K.J., Kim H.Y., Kim S.H., et al. Result of proficiency test and comparison of accuracy using a european spine phantom among the three bone densitometries. J Bone Metab. 2015 doi: 10.11005/jbm.2015.22.2.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.Y., Birkhold S.G., Kubena L.F., Nisbet D.J., Ricke S.C. Review on the role of dietary zinc in poultry nutrition, immunity, and reproduction. Biol Trace Elem Res. 2004 doi: 10.1385/BTER:101:2:147. [DOI] [PubMed] [Google Scholar]
- Pasternak K., Kocot J., Horecka A. Biochemistry of magnesium. J Elem. 2012 doi: 10.5601/jelem.2010.15.3.601-616. [DOI] [Google Scholar]
- Pereira C.G., Rabello C.B.V., Barros M.R., Manso H.E.C.C.C., Santos dos M.J.B., Faria A.G., et al. Zinc, manganese and copper amino acid complexed in laying hens' diets affect performance, blood parameters and reproductive organs development. PLoS One. 2020 doi: 10.1371/journal.pone.0239229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos S.J., Faquin V., Guilherme L.R.G., Castro E.M., Ávila F.W., Carvalho G.S., et al. Selenium biofortification and antioxidant activity in lettuce plants fed with selenate and selenite. Plant Soil Environ. 2010 doi: 10.17221/113/2010-PSE. [DOI] [Google Scholar]
- Reis M.D.P., Sakomura N.K., Teixeira I.A.M.A., da Silva E.P., Kebreab E. Partitioning the efficiency of utilization of amino acids in growing broilers: multiple linear regression and multivariate approaches. PLoS One. 2018 doi: 10.1371/journal.pone.0208488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J.D., Zhao J., Harreil R.J., Atwell C.A., Dibner J.J. Trace mineral nutrition in poultry and swine. Asian-Australas J Anim Sci. 2010 doi: 10.5713/ajas.2010.r.07. [DOI] [Google Scholar]
- Rostagno H.S., Albino L.F.T., Hannas M.I., Donzele J.L., Sakomura N.S., Perazzo F.G., et al. Viçosa - Mg. 4rd ed. Departamento de Zootecnia; UFV: 2017. Tabelas brasileiras para aves e suínos. [Google Scholar]
- Roughead Z.K., Lukaski H.C. Inadequate copper intake reduces serum insulin-like growth factor-I and bone strength in growing rats fed graded amounts of copper and zinc. J Nutr. 2003 doi: 10.1093/jn/133.2.442. [DOI] [PubMed] [Google Scholar]
- Sakomura N.K., Rostagno H.S. Métodos de pesquisa em nutrição de monogástricos. vol. 1. FUNEP; Jaboticabal: 2007. [Google Scholar]
- Saldanha E., Garcia E., Pizzolante C., Faittarone A., Sechinato da A., Molino A., et al. Effect of organic mineral supplementation on the egg quality of semi-heavy layers in their second cycle of lay. Rev Bras Ciência Avícola. 2009 doi: 10.1590/S1516-635X2009000400005. [DOI] [Google Scholar]
- SAS Institute Inc . 2008. SAS user’s guide: statistics. Cary, NC. [Google Scholar]
- Sauer A.K., Pfaender S., Hagmeyer S., Tarana L., Mattes A.K., Briel F., et al. Characterization of zinc amino acid complexes for zinc delivery in vitro using Caco-2 cells and enterocytes from hiPSC. Biometals. 2017 doi: 10.1007/s10534-017-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharrer E.M. Active intestinal transport of amino acids in chickens. Archiv Fur Geflugelkunde. 1971;35:21–35. [Google Scholar]
- Scharrer V.E., Brüggermann J.Z. In-vitro-Studien zur Aminosäurenresorption. Tierphysol Tierernahr Futtermittelkd. 1971;27(6):327–337. [PubMed] [Google Scholar]
- Scott M.L., Nesheim M.C., Young R.J. 1 rd ed. Cornell Univ; Ner York: 1969. Nutrition of the chicken. [Google Scholar]
- Seedor J.G., Quartuccio H.A., Thompson D.D. The bisphosphonate alendronate (MK-217) inhibits bone loss due to ovariectomy in rats. J Bone Miner Res. 1991 doi: 10.1002/JBMR.5650060405. [DOI] [PubMed] [Google Scholar]
- Sepúlveda F.V., Smith M.W. Discrimination between different entry mechanisms for neutral amino acids in rabbit ileal mucosa. J Physiol. 1978 doi: 10.1113/jphysiol.1978.sp012449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y., Lei Z., Yuan J., Yang Y., Guo Y., Zhang B. Effect of zinc on growth performance, gut morphometry, and cecal microbial community in broilers challenged with Salmonella enterica serovar typhimurium. J Microbiol. 2014 doi: 10.1007/s12275-014-4347-y. [DOI] [PubMed] [Google Scholar]
- Silva A.P., Rebollo M.A., Gallardo R.A. Effects of amino acid–bound zinc and manganese feed additives on mhc haplotype chickens challenged with infectious bronchitis coronavirus. Avian Dis. 2020 doi: 10.1637/aviandiseases-D-20-00031. [DOI] [PubMed] [Google Scholar]
- Silva R.F., Sasso G.R.S., Cerri E.S., Simões M.J., Cerri P.S. Biology of bone tissue: structure, function, and factors that influence bone cells. BioMed Res Int. 2015 doi: 10.1155/2015/421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.D., Panickar K.S., Urban J.F., Dawson H.D. Impact of micronutrients on the immune response of animals. Annu Rev Anim Biosci. 2018 doi: 10.1146/annurev-animal-022516-022914. [DOI] [PubMed] [Google Scholar]
- Studer J.M., Schweer W.P., Gabler N.K., Ross J.W. Functions of manganese in reproduction. Anim Reprod Sci. 2022 doi: 10.1016/j.anireprosci.2022.106924. [DOI] [PubMed] [Google Scholar]
- Suganya T., Senthilkumar S., Deepa K., Muralidharan J., Sasikumar P., Muthusamy N. Metal toxicosis in poultry – a review. Int J Sci Environ Technol. 2016;5:515–524. [Google Scholar]
- Sun C.J., Chen S.R., Xu G.Y., Liu X.M., Yang N. Global variation and uniformity of eggshell thickness for chicken eggs. Poultry Sci. 2012 doi: 10.3382/PS.2012-02220. [DOI] [PubMed] [Google Scholar]
- Sun R.C., Diaz-Miron J.L., Choi P.M., Sommovilla J., Guo J., Erwin C.R., et al. Both epidermal growth factor and insulin-like growth factor receptors are dispensable for structural intestinal adaptation. J Pediatr Surg. 2015 doi: 10.1016/j.jpedsurg.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W., Wang G., Pei X., Liu L., Xiao Z., Tao W., et al. Effects of replacing inorganic with respective complexed glycinate minerals on apparent mineral bioavailability and deposition rate in tissues of broiler breeders. Biol Trace Elem Res. 2020;198:654–660. doi: 10.1007/s12011-020-02102-1. [DOI] [PubMed] [Google Scholar]
- Surai Kochish, Fisinin Kidd. Antioxidant defence systems and oxidative stress in poultry biology: an update. Antioxidants. 2019;8:235. doi: 10.3390/antiox8070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle N.F. 4rd ed. Cabi; 2010. Mineral nutrition of livestock. [Google Scholar]
- Swiatkiewicz S., Swiatkiewicz M., Arczewska-Wlosek A., Jozefiak D. Chitosan and its oligosaccharide derivatives (chito-oligosaccharides) as feed supplements in poultry and swine nutrition. J Anim Physiol Anim Nutr. 2014 doi: 10.1111/jpn.12222. [DOI] [PubMed] [Google Scholar]
- Venglovská K., Grešáková L., Plachá I., Ryzner M., Čobanová K. Effects of feed supplementation with manganese from its different sources on performance and egg parameters of laying hens. Czech J Anim Sci. 2014 doi: 10.17221/7338-cjas. [DOI] [Google Scholar]
- Ventura W.R.L.M., Rabello C.B.V., Barros M.R., Silva Junior R.V., Oliveira H.B., Faria A.G., et al. Zinc, manganese, and copper amino acid complexes improve performance and bone characteristics of layer-type chicks under thermoneutral and cold stress conditions. Poultry Sci. 2020 doi: 10.1016/j.psj.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohra P., Kratzer F.H. The effect of dietary copper and molybdenum on Turkey poults. Poultry Sci. 1957 doi: 10.3382/ps.0361096. [DOI] [Google Scholar]
- Waldroup P.W. Bioassays remain necessary to estimate phosphorus, calcium bioavailability. Feedstuffs. 1996;68:13–20. [Google Scholar]
- Wan Y., Zhang B. The impact of zinc and zinc homeostasis on the intestinal mucosal barrier and intestinal diseases. Biomolecules. 2022 doi: 10.3390/biom12070900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead C.C., Fleming R.H. Osteoporosis in cage layers. Poultry Sci. 2000;79(7):1033–1041. doi: 10.1093/ps/79.7.1033. [DOI] [PubMed] [Google Scholar]
- Williams K.C. Some factors affecting albumen quality with particular reference to Haugh unit score. World’s Poult Sci J. 1992 doi: 10.1079/WPS19920002. [DOI] [Google Scholar]
- Xiao J.F., Zhang Y.N., Wu S.G., Zhang H.J., Yue H.Y., Qi G.H. Manganese supplementation enhances the synthesis of glycosaminoglycan in eggshell membrane: a strategy to improve eggshell quality in laying hens. Poultry Sci. 2014 doi: 10.3382/ps.2013-03354. [DOI] [PubMed] [Google Scholar]
- Xie C., Elwan H.A.M., Elnesr S.S., Dong X.Y., Zou X.T. Effect of iron glycine chelate supplementation on egg quality and egg iron enrichment in laying hens. Poultry Sci. 2019 doi: 10.3382/PS/PEZ421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Tian C., Zhu Y., Zhang L., Lu L., Luo X. Physiology, endocrinology, and reproduction:effects of inorganic and organic manganese supplementation on gonadotropin-releasing hormone-i and follicle-stimulating hormone expression and reproductive performance of broiler breeder hens. Poultry Sci. 2014 doi: 10.3382/ps.2013-03598. [DOI] [PubMed] [Google Scholar]
- Yeung C.K., Glahn R.P., Miller D.D. Inhibition of iron uptake from iron salts and chelates by divalent metal cations in intestinal epithelial cells. J Agric Food Chem. 2005 doi: 10.1021/jf049255c. [DOI] [PubMed] [Google Scholar]
- Yu X., Chen L., Ding H., Zhao Y., Feng J. Iron transport from ferrous bisglycinate and ferrous sulfate in dmt1-knockout human intestinal caco-2 cells. Nutrients. 2019;11:485. doi: 10.3390/nu11030485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Wang Y.-X., Xiao X., Wang J.-S., Wang Q., Li K.-X., et al. Effects of zinc glycinate on productive and reproductive performance, zinc concentration and antioxidant status in broiler breeders. Biol Trace Elem Res. 2017 doi: 10.1007/s12011-016-0928-4. [DOI] [PubMed] [Google Scholar]
- Zhang Y.N., Zhang H.J., Wu S.G., Wang J., Qi G.H. Dietary manganese supplementation modulated mechanical and ultrastructural changes during eggshell formation in laying hens. Poultry Sci. 2017 doi: 10.3382/ps/pex042. [DOI] [PubMed] [Google Scholar]
- Zhu X., Shang X., Lin G., Li H., Feng X., Zhang H. Effects of zinc glycinate on growth performance, serum biochemical indexes, and intestinal morphology of yellow feather broilers. Biol Trace Elem Res. 2022 doi: 10.1007/s12011-021-02990-x. [DOI] [PMC free article] [PubMed] [Google Scholar]