Abstract
Introduction
In Chinese patients with NSCLC, prevalence of EGFR-mutated (EGFRm) disease is high. In the global phase 3 ADAURA study (NCT02511106), adjuvant osimertinib was found to have a statistically significant and clinically meaningful improvement in disease-free survival (DFS) versus placebo in resected stage IB to IIIA EGFRm NSCLC. We present efficacy and safety data from a subgroup analysis of 159 Chinese patients enrolled in the People’s Republic of China from ADAURA.
Methods
In ADAURA, patients with completely resected stage IB to IIIA EGFRm (exon 19 deletion/exon 21 L858R) NSCLC were randomized 1:1 to receive osimertinib (80 mg once daily) or placebo for 3 years or until disease recurrence/discontinuation. Adjuvant chemotherapy was permitted before randomization, per physician/patient choice. Primary end point was investigator-assessed DFS in stage II to IIIA disease; secondary end points included DFS in stage IB to IIIA (overall population), overall survival, health-related quality of life (HRQoL), and safety.
Results
Of 682 patients enrolled globally, 159 patients in the People’s Republic of China were included in this subgroup analysis (osimertinib n = 77; placebo n = 82). Baseline characteristics were balanced across the treatment arms. At data cutoff, stage II to IIIA DFS hazard ratio (HR) was 0.23 (95% confidence interval [CI]: 0.13–0.42; maturity 59%); stage IB to IIIA DFS HR was 0.29 (95% CI: 0.17–0.48; maturity 42%). At 13% maturity (21 deaths), HR for overall survival in the stage IB to IIIA population was 0.51 (95% CI: 0.21–1.20). HRQoL was maintained from baseline, and safety was consistent with the global population.
Conclusions
In this population of Chinese patients from ADAURA, adjuvant osimertinib was found to have a clinically meaningful improvement in DFS versus placebo, with maintained HRQoL and a safety profile consistent with the global study population.
Keywords: Adjuvant, Osimertinib, EGFR, NSCLC, China
Introduction
Approximately 34% of patients with NSCLC in the People’s Republic of China present with resectable disease at diagnosis.1 Surgery followed by adjuvant chemotherapy is the primary treatment option for patients with resectable stage II to III NSCLC and select patients with resectable stage IB disease, subject to postoperative evaluation of the benefits and risks.2, 3, 4, 5, 6 Despite surgery with curative intent, recurrence rates remain high and only a 5% overall survival (OS) benefit with adjuvant chemotherapy has been reported globally.7
EGFR mutations are common oncogenic drivers in NSCLC.8,9 In Chinese patients with NSCLC, the prevalence of EGFR-mutated (EGFRm) disease is high (38%–58%), compared with other parts of the world, such as Europe (14%) and the United States of America (24%).10, 11, 12, 13, 14 Osimertinib is a third-generation, irreversible, oral EGFR tyrosine kinase inhibitor (TKI) that potently and selectively inhibits both EGFR TKI sensitizing and EGFR T790M resistance mutations, with efficacy in patients with EGFRm NSCLC including central nervous system (CNS) metastases.15, 16, 17, 18, 19
In the 2020 primary analysis of the global phase 3 ADAURA study, adjuvant osimertinib was found to have a statistically significant and clinically meaningful improvement in disease-free survival (DFS) versus placebo in patients with completely resected stage IB to IIIA EGFRm NSCLC (hazard ratio [HR] = 0.20, 99.12% confidence interval [CI]: 0.14–0.30; p < 0.001).19 On the basis of these results from ADAURA,19 osimertinib was the first targeted agent to be approved and recommended for use as an adjuvant treatment in patients with resected EGFRm (exon 19 deletion [Ex19del] or exon 21 L858R [L858R] mutation) NSCLC.3,20, 21, 22, 23 After 2 years of additional follow-up in the global ADAURA study, a sustained DFS benefit was observed with adjuvant osimertinib (HR = 0.27, 95% CI: 0.21–0.34 in patients with stage IB–IIIA disease), along with an improvement in CNS DFS versus placebo (CNS DFS HR 0.36, 95% CI: 0.23–0.57),21 in line with the primary analysis.19 A DFS benefit favoring osimertinib treatment was observed consistently across all predefined global subgroups, with and without adjuvant chemotherapy,21 as in the primary analysis.19 This DFS benefit translated to a significant OS benefit for adjuvant osimertinib versus placebo (OS HR 0.49, 95% CI: 0.34–0.70, p < 0.001) in patients with stage IB to IIIA disease.24
Here, we report an exploratory subgroup analysis of 159 Chinese patients enrolled at 30 study centers across the People’s Republic of China from ADAURA.
Materials and Methods
Patients
Full details of the ADAURA study methodology have been published previously.19,25 In brief, eligible patients were aged 18 years or above with completely resected stage IB to IIIA NSCLC (American Joint Committee on Cancer cancer staging manual seventh edition; tumor size for stage IB was not limited to <4 cm), with centrally confirmed EGFR mutation (Ex19del or L858R) and a WHO performance status of 0 to 1. Adjuvant chemotherapy was permitted before randomization, per patient and physician choice. Preoperative, postoperative, or planned radiation therapy was not permitted.
Study Design and Treatment
ADAURA (NCT02511106) is an ongoing phase 3, double-blind, placebo-controlled, randomized, global study conducted in 26 different countries across Europe, the Asia-Pacific, North America, and South America. Patients were stratified according to disease stage (IB, II, or IIIA), EGFR mutation status (Ex19del or L858R), and race (Asian or non-Asian) and randomly assigned in a 1:1 ratio to receive osimertinib 80 mg orally once daily or placebo for 3 years (until treatment completion), or until disease recurrence or fulfillment of a discontinuation criterion. After disease recurrence, patients could be made aware of the treatment allocation at the request of the investigator so that the next step of their treatment could be chosen; this could include open-label osimertinib if patients were eligible according to the local product label and protocol requirements.
The ADAURA study was conducted in accordance with the provisions of the Declaration of Helsinki, Good Clinical Practice guidelines (as defined by the International Conference for Harmonisation), applicable regulatory requirements, and the policy of the trial sponsor (AstraZeneca) on bioethics and human biological samples. All the patients provided written informed consent before participation.
End Points and Assessments
The primary end point was investigator-assessed DFS in patients with stage II to IIIA disease. Secondary end points included DFS in the overall population (stage IB–IIIA disease), OS, health-related quality of life (HRQoL), and safety.
DFS was defined as the time from randomization to disease recurrence or death from any cause in the absence of recurrence. Baseline assessments were performed within 28 days before administration of osimertinib or placebo, with follow-up assessments at weeks 12 and 24, then every 24 weeks until 5 years (and annually thereafter). After disease recurrence, follow-up assessments for survival took place every 24 weeks until 5 years (and annually thereafter). Subsequent anticancer treatments and radiotherapy received by the patients were recorded.
HRQoL was assessed using the short form-36 health survey (SF-36) version 2 (third edition), which includes eight health domains summarized into two summary scores: the physical component summary (PCS) and mental component summary (MCS).23 SF-36 data were collected at randomization, weeks 12 and 24, and then every 24 weeks until disease recurrence, treatment completion at 3 years, or treatment discontinuation. Time to deterioration (TTD) of PCS and MCS was assessed in patients with stage II to IIIA disease and was defined as the time from randomization to the first clinically important worsening confirmed at the subsequent assessment, or death (by any cause) in the absence of a clinically important worsening. Clinically important worsening was assigned using values for minimal clinically important differences defined in the third edition of the SF-36 scoring manual: PCS ±3.8 points and MCS ±4.6 points.
Safety was assessed at randomization, weeks 2, 4, and 12, and then every 12 weeks until treatment completion or study discontinuation. Adverse events (AEs) were graded using the Common Terminology Criteria for Adverse Events (version 4.03) and included AEs with onset date on or after the date of first dose up to and including 28 days after discontinuation of study treatment and before starting subsequent cancer therapy.
Statistical Methods
The ADAURA China population included all Chinese patients who were randomized in China (across 30 study centers). The full analysis set comprised all randomized patients. The sample size of the China population was calculated to reveal consistency with the results of the global population (to provide at least approximately 75% probability of a 50% retention of global effect size, in terms of the primary end point). Briefly, DFS was analyzed using a log-rank test stratified by stage (II, IIIA or IB, II, IIIA) and mutation type (Ex19del, L858R) in both the stage II to IIIA and overall populations. The Kaplan-Meier method was used to summarize the DFS and OS by treatment group. Subgroup analyses in the China population were conducted by comparing DFS between treatments in the following planned subgroups: stage (IB, II, IIIA), EGFR mutation type (Ex19del, L858R), adjuvant chemotherapy use (yes, no), sex (male, female), age at screening (<65 y, ≥65 y), and smoking history (yes, no). For each subgroup level, HRs and 95% CIs were calculated using a Cox proportional hazards model including a term for treatment, the subgroup covariate of interest, and the treatment-by-subgroup interaction term. Nevertheless, subgroup categories with less than 20 events were excluded from the analysis to allow for a meaningful analysis. The final OS analysis of the global population was planned to be conducted when approximately 94 deaths have been reported in patients with stage II to IIIA disease (approximately 20% maturity). OS for the China population was analyzed using similar methods to DFS, provided there were more than 20 deaths to allow for a meaningful analysis. All statistical analyses of the China population were exploratory.
DFS and OS were analyzed using a log-rank test stratified according to disease stage and mutation type, but not race. The Kaplan-Meier method was used to estimate the median DFS and OS by treatment group. The TTD of PCS and MCS evaluated by the SF-36 was analyzed as for DFS. In the placebo group, earlier discontinuation in completing SF-36 forms was observed owing to earlier disease recurrence. SF-36 data were therefore interpreted descriptively until week 156. The data cutoff (DCO) for DFS and SF-36 analyses was the same as the updated DFS analysis in the global population: April 11, 2022. The DCO for OS and subsequent treatment analyses for the China population was as for the global OS analysis: January 27, 2023.
Results
Patients and Treatment
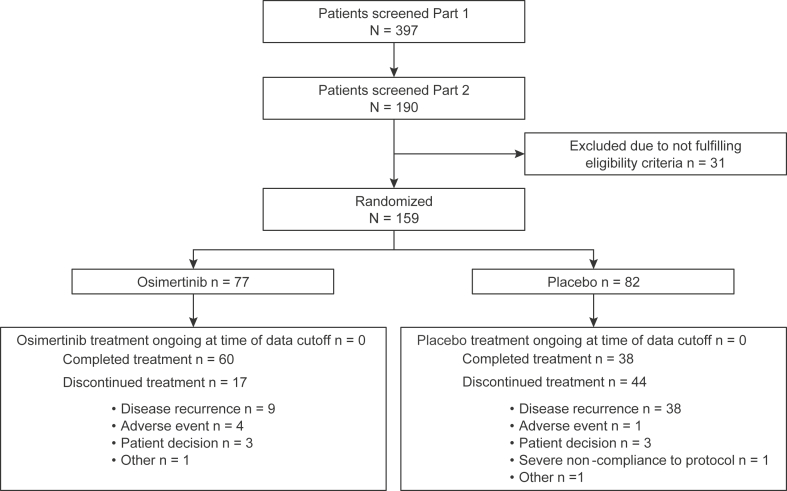
Of 682 patients enrolled globally, 159 Chinese patients in the People’s Republic of China were randomized between August 2016 and February 2019 to osimertinib (n = 77) or placebo (n = 82). The full analysis set of this subgroup analysis comprised 159 patients, all of whom had received at least one dose of the study drug. At DCO for the final DFS analysis, the median duration of total treatment exposure was 35.8 months (range: 0–37 mo) in the osimertinib group and 31.8 months (range: 0–37 mo) in the placebo group. A total of 64 patients (83%) in the osimertinib group and 42 patients (51%) in the placebo group reached exposure of 30 months or more. At DCO, all patients had completed the planned treatment duration or prematurely discontinued treatment. A total of 60 patients (78%) in the osimertinib group and 38 patients (46%) in the placebo group (62% total) had completed the maximum 3 years of the study treatment (Fig. 1).
Figure 1.
Patient disposition for the overall China population (stage IB–IIIA disease). Part 1 of screening comprised central EGFR mutation testing of tumor tissue for Ex19del or L858R mutations; part 2 of screening comprised confirmation of other eligibility criteria. Ex19del, exon 19 deletion.
Baseline demographics were generally well balanced between the two treatment groups (Table 1 and Supplementary Table 1). Compared with placebo, a higher proportion of patients in the osimertinib group were aged 65 years or above (30% versus 23%), had WHO performance status of 1 (44% versus 38%), had stage IB disease (45% versus 40%), and had and Ex19del mutation (47% versus 38%).
Table 1.
Baseline Patient Demographics and Disease Characteristics in the Overall China Population (Stage IB–IIIA Disease)
| Characteristics | Osimertinib (n = 77) | Placebo (n = 82) |
|---|---|---|
| Sex, n (%) | ||
| Female | 46 (60) | 49 (60) |
| Male | 31 (40) | 33 (40) |
| Age, y | ||
| Median (range) | 61 (32–78) | 60 (31–76) |
| <65 y, n (%) | 54 (70) | 63 (77) |
| ≥65 y, n (%) | 23 (30) | 19 (23) |
| Smoking history, n (%) | ||
| Never | 55 (71) | 62 (76) |
| Current | 1 (1) | 0 |
| Former | 21 (27) | 20 (24) |
| WHO performance status, n (%) | ||
| 0 | 43 (56) | 51 (62) |
| 1 | 34 (44) | 31 (38) |
| AJCC stage, n (%)a | ||
| IB | 35 (45) | 33 (40) |
| II | 16 (21) | 23 (28) |
| IIIA | 26 (34) | 26 (32) |
| Histologic type, n (%) | ||
| Adenocarcinoma | 75 (97) | 82 (100) |
| Malignant | 46 (60) | 56 (68) |
| Acinar adenocarcinoma | 28 (36) | 19 (23) |
| Papillary, malignant | 1 (1) | 6 (7) |
| Bronchioloalveolar | 0 | 1 (1) |
| Carcinoma, adenosquamous, malignant | 1 (1) | 0 |
| Other | 1 (1) | 0 |
| Lung cancer resection, n (%) | ||
| Lobectomy | 77 (100) | 76 (93) |
| Bilobectomy | 0 | 1 (1) |
| Pneumonectomy | 0 | 5 (6) |
| Regional lymph nodes, n (%) | ||
| N0 | 36 (47) | 37 (45) |
| N1 | 16 (21) | 23 (28) |
| N2 | 25 (32) | 22 (27) |
| EGFR mutation type at randomization,b n (%) | ||
| Ex19del | 36 (47) | 31 (38) |
| L858R | 41 (53) | 51 (62) |
| T790M | 1 (1) | 1 (1) |
| S768I | 0 | 1 (1) |
| Adjuvant chemotherapy, n (%) | ||
| Yes | 48 (62) | 58 (71) |
| No | 29 (38) | 24 (29) |
Note: Percentages may not add up to 100% owing to rounding. Data cutoff was January 17, 2020.
AJCC, American Joint Committee on Cancer; Ex19del, exon 19 deletion.
Staging was determined according to the seventh edition of the cancer staging manual of the AJCC.
EGFR mutational status at randomization was centrally tested. Patients may have had more than one EGFR mutation.
Overall, adjuvant platinum-based chemotherapy was received by a slightly smaller proportion of patients in the osimertinib group (61%) compared with the placebo group (71%; Table 1 and Supplementary Table 2). Of these, adjuvant platinum-based chemotherapy was received by 10 (29%) and 14 (42%) of patients with stage IB disease, 14 (88%) and 20 (87%) of patients with stage II disease, and 23 (88%) and 24 (92%) of patients with stage IIIA disease in the osimertinib and placebo groups, respectively.
Efficacy
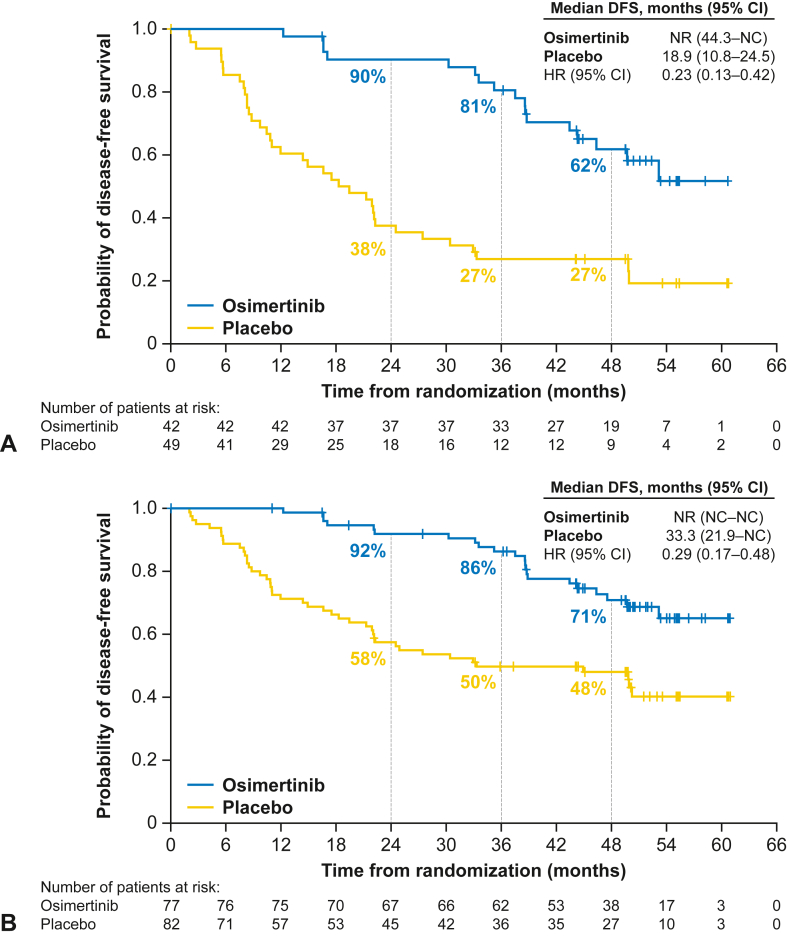
For patients with stage II to IIIA disease from the China population (n = 91), disease recurrence or death occurred in 17 (40%) and 37 (76%) patients in the osimertinib and placebo groups, respectively (59% maturity for osimertinib and placebo combined). Median follow-up for DFS was 44.7 months and 18.3 months in the osimertinib and placebo groups, respectively. No DFS events occurred in the first 12 months in the osimertinib group, whereas the DFS rate was 60% (95% CI: 45–73) in the placebo group. The DFS rates at 24, 36, and 48 months were 90% (95% CI: 76–96), 81% (95% CI: 65–90), and 62% (95% CI: 45–75), respectively, in the osimertinib group. The corresponding DFS rates in the placebo group were 38% (95% CI: 24–51), 27% (95% CI: 15–40), and 27% (95% CI: 15–40), respectively (Supplementary Table 3). The estimated HR for DFS was 0.23 (95% CI: 0.13–0.42) corresponding to a 77% reduction in the risk of disease recurrence or death for patients receiving osimertinib versus placebo (Fig. 2A). Median DFS was not reached (95% CI: 44.3–not calculable [NC]) in the osimertinib group and was 18.9 months (95% CI: 10.8–24.5) in the placebo group.
Figure 2.
Kaplan-Meier plot of DFS according to investigator assessment in (A) the China population of patients with stage II to IIIA disease and (B) the overall China population (stage IB–IIIA disease). DFS events are type of disease recorded as local/regional or distant or death. DFS events that do not occur within two scheduled visits (plus visit window) of the last assessable time point (or randomization) are censored and therefore excluded in the number of events. CI, confidence interval; DFS, disease-free survival; HR, hazard ratio; NC, not calculable; NR, not reached.
In the overall China population (stage IB–IIIA disease; n = 159), disease recurrence or death occurred in 22 (29%) and 44 (54%) patients in the osimertinib and placebo groups, respectively (42% maturity for osimertinib and placebo combined). Median follow-up for DFS was 47.5 months and 31.7 months in the osimertinib and placebo groups, respectively. No DFS events occurred in the first 12 months in the osimertinib group, whereas the DFS rate was 71% (95% CI: 60–80) in the placebo group. The DFS rates at 24, 36, and 48 months were 92% (95% CI: 83–96), 86% (95% CI: 76–92), and 71% (95% CI: 58–80), respectively, in the osimertinib group. The corresponding DFS rates in the placebo group were 58% (95% CI: 46–67), 50% (95% CI: 38–60), and 48% (95% CI: 37–59), respectively, in the placebo group (Supplementary Table 3). The estimated HR for DFS was 0.29 (95% CI: 0.17–0.48), which corresponds to a 71% reduction in the risk of disease recurrence or death in the osimertinib versus placebo group (Fig. 2B). The median DFS was not reached in the osimertinib group (95% CI: NC–NC) and was 33.3 months (95% CI: 21.9–NC) in the placebo group.
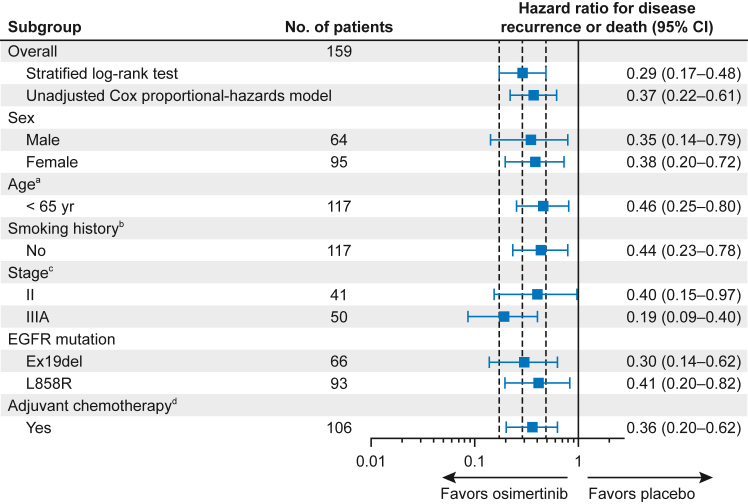
A DFS benefit with osimertinib versus placebo was observed in all prespecified subgroups with sufficient events (≥20 DFS events) for meaningful analysis (stage II, stage IIIA, Ex19del and L858R mutations, male and female sex, age [<65 y], smoking history [no], and adjuvant chemotherapy use) with estimated HRs at or below 0.46 for these subgroups (Fig. 3).
Figure 3.
Subgroup analysis of DFS according to investigator assessment in the overall China population (stage IB–IIIA disease). The subgroup analysis was performed using a Cox proportional hazards model including treatment, subgroup, and a treatment-by-subgroup interaction term. Subgroup categories with less than 20 events were excluded from the analysis; this included the following subgroups: aage (≥65 y); bsmoking history (yes); cstage (IB); and dadjuvant chemotherapy (no). A hazard ratio less than 1 implies a lower risk of disease recurrence or death with osimertinib versus placebo. CI, confidence interval; Ex19del, exon 19 deletion.
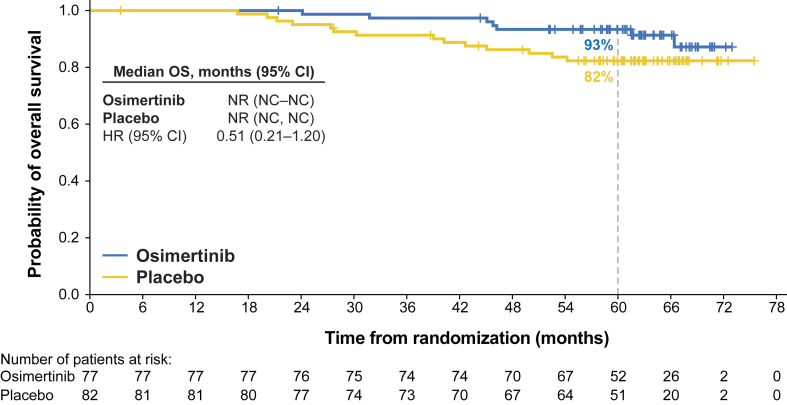
At the DCO for OS (January 27, 2023), the number of deaths in the stage II–IIIA China population was five in the osimertinib group and 12 in the placebo group (19% maturity). The number of deaths in the overall China population was seven in the osimertinib group and 14 in the placebo group (13% maturity). The median duration of follow-up for OS in all patients in the overall China population (stage IB–IIIA) was 63 months in the osimertinib group and 62 months in the placebo group. The 60-month OS rate was 93% (95% CI: 85–97) in the osimertinib group and 82% (95% CI: 72–89) in the placebo group. The overall HR for OS was 0.51 (95% CI: 0.21–1.20). The Kaplan-Meier curves are found in Figure 4. Median OS was not reached in either treatment group.
Figure 4.
Kaplan-Meier plot of OS in the overall China population (stage IB–IIIA disease). Data for patients not known to have died at the time of the analysis were censored at the last recorded date on which the patient was known to be alive. Median duration of follow-up for OS in patients whose data were censored was 63 months in both the osimertinib group and the placebo group. CI, confidence interval; HR, hazard ratio; NC, not calculable; NR, not reached; OS, overall survival.
Subsequent Treatments
At the January 27, 2023, DCO, among the randomized patients, 15 patients (19%) in the osimertinib group and 38 patients (46%) in the placebo group had received a subsequent anticancer treatment. The recurrence status of patients who received subsequent anticancer treatment after the final DFS analysis was not collected per the protocol. The most frequently received type of subsequent treatment was EGFR TKIs (Supplementary Table 4).
Health-Related Quality of Life
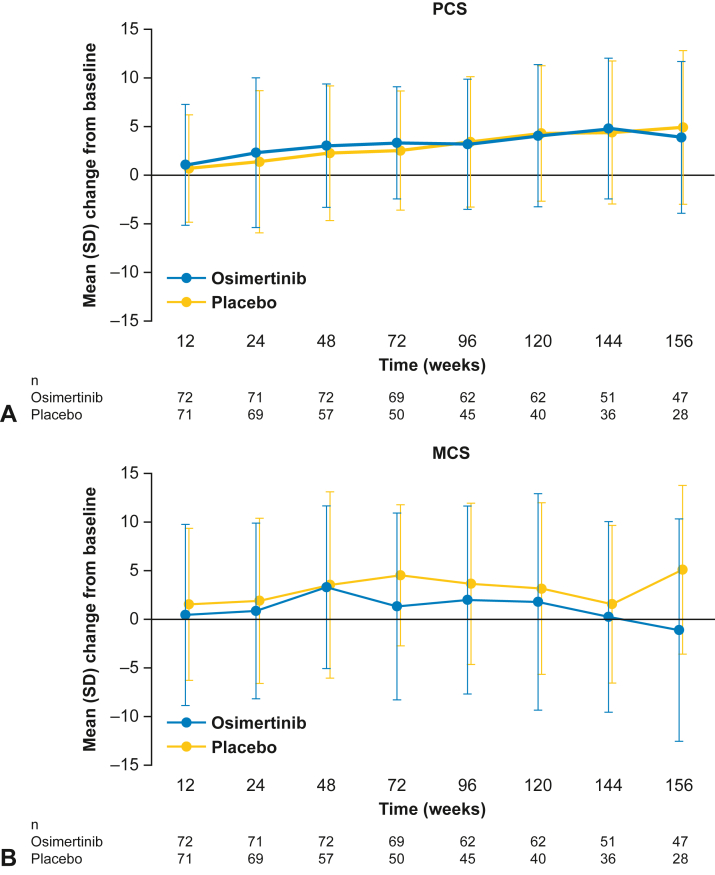
In the overall China population, from baseline to week 156, compliance rates with the SF-36 survey were high, ranging from 80% to 100% in the osimertinib group and 74% to 100% in the placebo group. SF-36 PCS and MCS scores were maintained from baseline to week 156 (Fig. 5A and B). Small improvements in PCS from baseline were observed in both treatment groups, and at week 156, most SF-36 health domains contributing to the PCS had improvements from baseline in both treatment groups. In the MCS, small improvements from baseline were also found for both treatment groups, except for the osimertinib group at week 156, where a small decrease from baseline was observed.
Figure 5.
Change in SF-36 (A) PCS and (B) MCS scores from baseline to week 156 in the overall China population (stage IB–IIIA disease). Error bars represent SDs. MCS, mental component summary; PCS, physical component summary; SF-36, short form-36 health survey.
In the primary China population (patients with stage II–IIIA disease) TTD analyses, for the PCS, 83% of the patients in the osimertinib group did not experience a clinically meaningful deterioration or death at DCO compared with 71% in the placebo group. Improvement of TTD of PCS was observed in the osimertinib group versus placebo (HR = 0.37, 95% CI: 0.15–0.91; Supplementary Table 5). It should be noted that the number of PCS deterioration or death events observed by DCO was small (n = 21; osimertinib n = 7; placebo n =14) and the median TTD of PCS was not reached in either treatment group. For the MCS, 71% of patients in the osimertinib group and 67% in the placebo group did not experience a clinically meaningful deterioration or death by DCO. There was a trend of improvement in TTD of MCS in the osimertinib group versus placebo (HR = 0.62, 95% CI: 0.29–1.31). Again, the number of MCS deterioration or death events was small (n = 28; osimertinib n = 12; placebo n = 16). Median TTD of MCS was not reached in the osimertinib treatment group and was 33 months (95% CI: 22.1–NC) in the placebo group.
Safety
AEs were reported in 77 (100%) and 81 patients (99%) in the osimertinib and placebo groups, respectively. The most common AEs reported with osimertinib treatment were diarrhea (49%), upper respiratory tract infection (39%), and mouth ulceration (34%; Table 2). No individual grade 3 or higher AEs were reported in more than two patients, except for diarrhea (n = 3; 4%) in the osimertinib group and pneumonia (n = 3; 4%) in the placebo group. AEs that were considered by the investigator to be causally related to osimertinib or placebo are presented in Supplementary Table 6. Serious AEs were reported in 18 patients (23%) in the osimertinib group and 14 patients (17%) in the placebo group; no AEs with an outcome of death were reported in either group. The incidence of AEs of special interest was low in both treatment groups. AEs of cardiac effects (grouped term) occurred in three (4%) and two patients (2%) in the osimertinib and placebo groups, respectively. Interstitial lung disease (grouped term) occurred in one patient (1%) in the osimertinib group and no patient in the placebo group (Supplementary Table 7). Electrocardiogram QT prolongation was reported in six patients (8%) in the osimertinib group and four patients (5%) in the placebo group.
Table 2.
Safety Summary in the Overall China Population (Stage IB–IIIA Disease)
| AE Category, n (%) | Osimertinib (n = 77) | Placebo (n = 82) |
|---|---|---|
| Any AE | 77 (100) | 81 (99) |
| Causally related to treatmenta | 74 (96) | 66 (80) |
| Most common AEs (all causality)b | ||
| Diarrhea | 38 (49) | 19 (23) |
| Upper respiratory tract infection | 30 (39) | 21 (26) |
| Mouth ulceration | 26 (34) | 7 (9) |
| Cough | 23 (30) | 20 (24) |
| Pruritus | 19 (25) | 10 (12) |
| Paronychia | 15 (19) | 0 |
| Neutrophil count decreased | 17 (22) | 3 (4) |
| Nasopharyngitis | 15 (19) | 12 (15) |
| Weight decreased | 16 (21) | 6 (7) |
| White blood cell count decreased | 15 (19) | 1 (1) |
| Dry skin | 13 (17) | 3 (4) |
| Influenza | 12 (16) | 4 (5) |
| AST increased | 9 (12) | 18 (22) |
| Toothache | 6 (8) | 12 (15) |
| ALT increased | 4 (5) | 20 (24) |
| AE grade ≥3 | ||
| Any | 21 (27) | 17 (21) |
| Causally related to treatmenta | 6 (8) | 1 (1) |
| Most common grade ≥3 AEs (all causality)c | ||
| Diarrhea | 3 (4) | 0 |
| Hypertension | 2 (3) | 1 (1) |
| Hyperuricemia | 2 (3) | 1 (1) |
| Gastroenteritis | 2 (3) | 0 |
| Viral upper respiratory tract infection | 2 (3) | 0 |
| Stomatitis | 2 (3) | 0 |
| Pneumonia | 1 (1) | 3 (4) |
| AE with an outcome of death | ||
| Any | 0 | 0 |
| Causally related to treatmenta | 0 | 0 |
| SAE | ||
| Any | 18 (23) | 14 (17) |
| Causally related to treatmenta | 3 (4) | 1 (1) |
| Most common SAEsc | ||
| Hyperuricemia | 2 (3) | 1 (1) |
| Pneumonia | 1 (1) | 3 (4) |
| AE leading to discontinuation of treatment | ||
| Any | 4 (5) | 0 |
| Causally related to treatmenta | 3 (4) | 0 |
| Any AE leading to dose reduction | 6 (8) | 0 |
| Any AE leading to dose interruption | 16 (21) | 6 (7) |
Note: Patients with multiple events in the same category are counted only once in that category. Patients with events in more than one category are counted once in each of those categories.
AE, adverse events; ALT, alanine aminotransferase; AST, aspartate aminotransferase; SAE, serious adverse event.
As assessed by the investigator.
Reported in 15% or more of patients in either treatment group.
Reported in two patients or more in either treatment group.
Dose interruptions, dose reductions, and study discontinuations due to AEs occurred in 21%, 8%, and 5% of patients in the osimertinib group, respectively, compared with 7%, 0%, and 0% in the placebo group (Table 2).
Discussion
In this exploratory analysis of Chinese patients from ADAURA, where all patients had the opportunity to receive 3 years of study treatment, adjuvant osimertinib was found to have a clinically meaningful improvement in DFS versus placebo, with maintained HRQoL and a safety profile consistent with the global study population. In the ADAURA China population, a 77% reduction in the risk of disease recurrence or death was found with osimertinib versus placebo in the primary population of patients with stage II to IIIA disease. Similarly, a 71% reduction in the risk of disease recurrence or death with osimertinib versus placebo was observed in the overall China population of patients with stage IB to IIIA disease. In the overall China population (stage IB–IIIA), there were seven deaths in the osimertinib group and 14 deaths in the placebo group (overall maturity 13%), with a HR for OS of 0.51 (95% CI: 0.21–1.20), similar to the global study population.24 Nevertheless, data should be interpreted with caution due to the small number of deaths observed in the China population. Subsequent anticancer treatments were received by 19% of patients in the osimertinib group and 46% in the placebo group, with EGFR TKIs being the most frequently received type of subsequent treatment. No clinically meaningful differences in HRQoL between osimertinib and placebo were observed, and most patients with stage II to IIIA disease in both groups did not experience any clinically meaningful deterioration by week 156. No new safety signals were observed despite the prolonged treatment period, and the known benefit-risk profile of osimertinib remains the same.
The patient demographics and disease characteristics were representative of the intended population of Chinese patients with stage IB to IIIA EGFRm NSCLC who had undergone complete tumor resection. Compared with the global population,19 a higher percentage of patients in the China population were aged below 65 years, had stage IB disease, and had L858R mutation. The higher proportion of younger patients (aged <65 y) in the ADAURA China population (74%), compared with the global population (56%), was consistent with the higher proportion of patients with stage IB disease in the China population (43%) compared with the global population (32%), as younger patients are more likely to have stage I disease at diagnosis.26 The prevalence of L858R mutation was slightly higher in the ADAURA China population (58%) versus the global population (45%).19
In the ADAURA global population, 60% of patients received postoperative adjuvant chemotherapy.19,21 Consistent with this, 67% of patients in the ADAURA China population received adjuvant chemotherapy, which was in line with Chinese treatment guidelines for NSCLC.2,6 Nevertheless, the uptake rate of adjuvant chemotherapy was higher in the China placebo group (71%), compared with the osimertinib group (62%) and global population placebo group (60%).19 This difference may be explained by the smaller number of patients in this China subgroup analysis, compared with the global population. Nevertheless, the differences in patient characteristics between the China population and global population were not considered to affect the overall efficacy and safety conclusions for this analysis.
Median duration of treatment exposure was slightly longer in the ADAURA China placebo group (osimertinib, 35.8 mo; placebo, 31.8 mo) versus the global population (osimertinib, 35.8 mo; placebo, 25.1 mo), and discontinuations due to AEs were slightly lower in the China population (osimertinib, 5%; placebo, 0%) compared with the global population (osimertinib, 13%; placebo 3%).21 Despite this variability, in both the primary population of patients with stage II to IIIA disease and overall population of patients with stage IB to IIIA disease, the DFS results in the China population were consistent with that of the global population.21 The Kaplan-Meier curves of the osimertinib and placebo groups separated early, after the first assessment at 12 weeks, and remained separated for the duration of follow-up until the DCO. In patients with stage II to IIIA disease, reduction in risk of disease recurrence or death was similar in the China population (HR = 0.23, 95% CI: 0.13–0.42) and the global population (HR = 0.23, 95% CI: 0.18–0.30).21 Furthermore, in the overall population of patients with stage IB to IIIA disease, reduction in risk of disease recurrence or death was also similar in the China population (HR = 0.29, 95% CI: 0.17–0.48) and global population (HR = 0.27, 95% CI: 0.21–0.34).21 Four-year DFS rates were also similar with osimertinib in the China (71%) and global (73%) populations.19 An improvement in DFS with osimertinib treatment was found across all predefined subgroups with greater than or equal to 20 DFS events in the China population; however, these data should be interpreted with a level of caution due to smaller patient numbers across all subgroups.
A key consideration for long-term adjuvant treatment following surgical resection is the effect on HRQoL.5 In the ADAURA China population, HRQoL measured by SF-36 was maintained during osimertinib treatment.
The safety profile of osimertinib in the ADAURA China population was consistent with the global population, and with the known safety profile for osimertinib.19,21 The most common AEs reported with osimertinib treatment in the China population were diarrhea (49%), upper respiratory tract infection (39%), and mouth ulceration (34%). The number of casually related grade 3 or higher AEs was low (osimertinib, 8%; placebo, 1%).
In conclusion, the efficacy and safety results from the ADAURA China population were consistent with those for the global population.21 Adjuvant osimertinib was found to have a clinically meaningful improvement in DFS versus placebo in Chinese patients with stage IB to IIIA EGFRm NSCLC. The DFS benefit with osimertinib translated to an OS benefit, though the small number of deaths warrants cautious interpretation. HRQoL was maintained and a tolerable safety profile was observed. These results therefore support adjuvant osimertinib as a highly effective treatment for Chinese patients with resected stage IB to IIIA EGFRm NSCLC.
CRediT Authorship Contribution Statement
Jie Wang: Investigation, Resources, Writing—review and editing.
Yi-Long Wu: Conceptualization, Investigation, Resources, Writing—review and editing.
Shun Lu: Formal analysis, Investigation, Data curation, Writing—review and editing.
Qun Wang: Resources, Data curation, Writing—review and editing.
Shanqing Li: Investigation, Resources, Writing—review and editing.
Wen-Zhao Zhong: Investigation, Resources, Writing—review and editing.
Qiming Wang: Investigation, Resources, Writing—review and editing
Wei Li: Investigation, Resources, Writing—review and editing.
Buhai Wang: Investigation, Resources, Writing—review and editing.
Jun Chen: Investigation, Resources, Writing—review and editing.
Ying Cheng: Investigation, Resources, Writing—review and editing.
Hongbing Duan: Investigation, Resources, Writing—review and editing.
Gaofeng Li: Investigation, Resources, Writing—review and editing.
Li Shan: Investigation, Resources, Writing—review and editing.
Yangbo Liu: Formal analysis, Writing—review and editing.
Jing Liu: Formal analysis, Data curation, Writing—review and editing.
Xiangning Huang: Methodology, Validation, Formal analysis, Investigation, Data curation, Writing—reviewing and editing.
Ana Bolanos: Validation, Formal analysis, Writing—review and editing.
Jie He: Investigation, Resources, Writing—review and editing.
Jie Wang, Yi-Long Wu, Shun Lu, Qun Wang, Shanqing Li, Wen-Zhao Zhong, Qiming Wang, Wei Li, Buhai Wang, Jun Chen, Ying Cheng, Hongbing Duan, Gaofeng Li, Li Shan, Yangbo Liu, Jing Liu, Xiangning Huang, Ana Bolanos, Jie He: Final approval of the manuscript.
Acknowledgments
The authors thank all patients and their families and the staff and investigators at all study sites. The ADAURA study (NCT02511106) was funded by AstraZeneca, Cambridge, UK. The sponsor was involved in the study design; in the collection, analysis, and interpretation of data; and in the writing of the report. Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Sally Cotterill, PhD, CMPP, of Ashfield MedComms, an Inizio company, and Clare McCleverty, PhD, contracted to Ashfield MedComms, and was funded by AstraZeneca in accordance with Good Publications Practice guidelines (http://www.ismpp.org/gpp-2022).
Data Sharing Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli could be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. AstraZeneca Vivli member page is also available outlining further details: https://vivli.org/ourmember/astrazeneca/.
Footnotes
Disclosure Dr. J. Wang reports receiving honoraria from AstraZeneca, BeiGene, Boehringer Ingelheim, and Merck Sharp & Dohme. Dr. Wu reports receiving honoraria from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Merck Sharp & Dohme, Pfizer, Sanofi, and Roche; research funding for AstraZeneca, Bristol-Myers Squibb, Pfizer, and Roche. Dr. Lu reports having membership on an advisory council or committee for AstraZeneca, Boehringer Ingelheim, GenomiCare Biotechnology, Hutchison MediPharma, Menarini, prIME Oncology, Roche, Simcere, Yuhan Corporation, and Zai Lab; receiving consulting fees from AstraZeneca, Hansoh, Hengrui Therapeutics, and Roche; having corporate-sponsored research at AstraZeneca, Bristol-Myers Squibb, Hengrui Therapeutics, Hutchison MediPharma, Roche, and Rui; and serving on the speakers bureau for AstraZeneca, Hansoh, and Roche. Dr. Zhong reports receiving honoraria from AstraZeneca and Roche. Drs. Y. Liu, J. Liu, and Bolanos report having employment with AstraZeneca. Dr. Huang reports having employment and stock or other ownership with AstraZeneca. The remaining authors declare no conflict of interest.
Cite this article as: Wang J, Wu YL, Lu S, et al. Adjuvant osimertinib in patients with stage IB to IIIA EGFR mutation-positive NSCLC after complete tumor resection: ADAURA China subgroup analysis. JTO Clin Res Rep. 2024;5:100621.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2023.100621.
Supplementary Data
References
- 1.Fan H., Shao Z.Y., Xiao Y.Y., et al. Incidence and survival of non-small cell lung cancer in Shanghai: a population-based cohort study. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2015-009419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Health Commission of the People’s Republic of China Chinese guidelines for diagnosis and treatment of primary lung cancer 2018. Chin J Cancer Res. 2019;31:1–28. doi: 10.21147/j.issn.1000-9604.2019.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Remon J., Soria J.C., Peters S., ESMO Guidelines Committee Early and locally advanced non-small-cell lung cancer: an update of the ESMO Clinical Practice Guidelines focusing on diagnosis, staging, systemic and local therapy. Ann Oncol. 2021;32:1637–1642. doi: 10.1016/j.annonc.2021.08.1994. [DOI] [PubMed] [Google Scholar]
- 4.Kris M.G., Gaspar L.E., Chaft J.E., et al. Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non-small-cell lung cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline Update. J Clin Oncol. 2017;35:2960–2974. doi: 10.1200/JCO.2017.72.4401. [DOI] [PubMed] [Google Scholar]
- 5.Postmus P.E., Kerr K.M., Oudkerk M., et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv1–iv21. doi: 10.1093/annonc/mdx222. [DOI] [PubMed] [Google Scholar]
- 6.Zhi X.Y., Yu J.M., Shi Y.K. Chinese guidelines on the diagnosis and treatment of primary lung cancer (2015 version) Cancer. 2015;121(suppl 17):3165–3181. doi: 10.1002/cncr.29550. [DOI] [PubMed] [Google Scholar]
- 7.Pignon J.P., Tribodet H., Scagliotti G.V., et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 8.Sharma S.V., Bell D.W., Settleman J., Haber D.A. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 9.Sholl L.M., Aisner D.L., Varella-Garcia M., et al. Multi-institutional oncogenic driver mutation analysis in lung adenocarcinoma: the lung cancer mutation consortium experience. J Thorac Oncol. 2015;10:768–777. doi: 10.1097/JTO.0000000000000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li D., Ding L., Ran W., et al. Status of 10 targeted genes of non-small cell lung cancer in eastern China: a study of 884 patients based on NGS in a single institution. Thorac Cancer. 2020;11:2580–2589. doi: 10.1111/1759-7714.13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y.-L., Yuan J.-Q., Wang K.-F., et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget. 2016;7:78985–78993. doi: 10.18632/oncotarget.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X.N., Yan H.H., Wang J., et al. Real-world survival outcomes based on EGFR mutation status in Chinese patients with lung adenocarcinoma after complete resection: results from the ICAN study. JTO Clin Res Rep. 2022;3 doi: 10.1016/j.jtocrr.2021.100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang H., Song X., Zhang Y., et al. Real-world data on EGFR/ALK gene status and first-line targeted therapy rate in newly diagnosed advanced non-small cell lung cancer patients in Northern China: a prospective observational study. Thorac Cancer. 2019;10:1521–1532. doi: 10.1111/1759-7714.13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen S., Dai L., Wang L., et al. Genomic signature of driver genes identified by target next-generation sequencing in Chinese non-small cell lung cancer. Oncologist. 2019;24:e1070–e1081. doi: 10.1634/theoncologist.2018-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cross D.A., Ashton S.E., Ghiorghiu S., et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046–1061. doi: 10.1158/2159-8290.CD-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mok T.S., Wu Y.L., Ahn M.J., et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer [e-pub ahead of print]. J Clin Oncol. https://doi.org/10.1200/JCO.2018.78.3118. Accessed January 31, 2024. [DOI] [PubMed]
- 18.Soria J.C., Ohe Y., Vansteenkiste J., et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 19.Wu Y.L., Tsuboi M., He J., et al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2020;383:1711–1723. doi: 10.1056/NEJMoa2027071. [DOI] [PubMed] [Google Scholar]
- 20.European Medicines Agency Summary of product characteristics. TAGRISSO (osimertinib) https://www.ema.europa.eu/en/documents/product-information/tagrisso-epar-product-information_en.pdf Accessed December 2022.
- 21.Herbst R.S., Wu Y.L., John T., et al. Adjuvant osimertinib for resected EGFR-mutated stage IB-IIIA non-small-cell lung cancer: updated results from the phase III randomized ADAURA trial. J Clin Oncol. 2023;41:1830–1840. doi: 10.1200/JCO.22.02186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.AstraZeneca Tagrisso approved in China for the adjuvant treatment of patients with early-stage EGFR-mutated lung cancer. https://www.astrazeneca.com/media-centre/press-releases/2021/tagrisso-approved-in-china-in-early-lung-cancer.html Accessed November 2022.
- 23.Pisters K., Kris M.G., Gaspar L.E., Ismaila N. Adjuvant Systemic Therapy and Adjuvant Radiation Therapy for Stage I to IIIA NSCLC Guideline Expert Panel. Adjuvant systemic therapy and adjuvant radiation therapy for stage I-IIIA completely resected non-small-cell lung cancer: ASCO guideline rapid recommendation update. J Clin Oncol. 2022;40:1127–1129. doi: 10.1200/JCO.22.00051. [DOI] [PubMed] [Google Scholar]
- 24.Tsuboi M., Herbst R.S., John T., et al. Overall survival with osimertinib in resected EGFR-mutated NSCLC. N Engl J Med. 2023;389:137–147. doi: 10.1056/NEJMoa2304594. [DOI] [PubMed] [Google Scholar]
- 25.Wu Y.L., Herbst R.S., Mann H., Rukazenkov Y., Marotti M., Tsuboi M. ADAURA: phase III, double-blind, randomized study of osimertinib versus placebo in EGFR mutation-positive early-stage NSCLC after complete surgical resection. Clin Lung Cancer. 2018;19:e533–e536. doi: 10.1016/j.cllc.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Liu M., Cai X., Yu W., Lv C., Fu X. Clinical significance of age at diagnosis among young non-small cell lung cancer patients under 40 years old: a population-based study. Oncotarget. 2015;6:44963–44970. doi: 10.18632/oncotarget.5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.