Abstract
This experiment aimed to discuss and reveal the effect and mechanism of mannanase on intestinal inflammation in broilers triggered by a soybean meal diet. In this experiment, 384 Arbor Acres broilers at 1 d old were randomly divided into 3 treatment groups. The broilers were fed a corn-soybean meal basal diet, a low-energy diet (metabolizable energy reduced by 50 kcal/kg), and a low-energy diet supplemented with 100 mg/kg mannanase for 42 d. The low-energy diet increased feed conversion ratio from 0 to 42 d, reduced ileal villus height and villus height-to-crypt depth ratio and upregulated the expression of nuclear factor kappa B (NF-κB) in the ileum (P < 0.05). It also reduced cecal short-chain fatty acids (SCFA), such as acetic acid (P < 0.05). Compared with low-energy diets, the addition of mannanase increased body weight at 42 d, promoted the digestibility of nutrients, and maintained the morphology and integrity of the intestinal epithelium of broilers (P < 0.05). In addition, mannanase upregulated the expression of claudin-1 (CLDN1) and zonula occludens-1 (ZO-1) in the jejunum at 21 d, downregulated the expression of ileal NF-κB, and increased the content of isobutyric acid in the cecum of broilers (P < 0.05). The results for the ileal microbiota showed that a low-energy diet led to a decrease in the relative abundance of Lactobacillus reuteri in the ileum of broilers. The addition of mannanase increased the relative abundance of Lactobacillus-KC45b and Lactobacillus johnsonii in broilers. Furthermore, a low-energy diet reduced the relative abundance of Butyricicoccus in the intestine of broilers and inhibited oxidative phosphorylation and phosphoinositol metabolism. Mannanase increased the relative abundance of Odoribacter, promoted energy metabolism and N-glycan biosynthesis, and increased the activities of GH3 and GH18. It is concluded that mannanase could improve the growth performance of broilers by reducing the expression of NF-κB in the ileum, increasing the production of SCFA in the cecum, suppressing intestinal inflammation, balancing the intestinal microbiota, reducing damage to the intestinal barrier, and improving the efficiency of nutrient utilization to alleviate the adverse effects caused by the decrease in dietary energy level.
Keywords: Broiler, Low-energy diet, Mannanase, Intestinal inflammatory response, Intestinal microbiota, Metagenome
1. Introduction
The amount of energy supplied to animals in feed is used mainly to meet maintenance and production requirements, and feed intake mainly depends on the concentration of energy in the diet (Maharjan et al., 2021a). It has been shown that when the dietary energy level of broilers is reduced, the body weight (BW) (M. P. Williams, 2014) and body weight gain (Ferreira et al., 2016a) are also reduced, affecting feed conversion efficiency (Zhao and Kim, 2017).
Intestinal function is regulated by a number of factors, such as intestinal microbiota and feed composition. Intestinal health not only affects the digestion and absorption of feed nutrients in animals, but also affects the immune function of livestock and poultry. Therefore, intestinal health regulation is essential to improve the growth performance of broilers fed a low-energy diet.
Soybean meal has a high protein content, a good amino acid balance, relatively low fiber, and a higher energy availability than other vegetable protein sources, making it by far the most widely used protein source in animal feed. However, soybean meal comprises approximately 17% to 27% non-starch polysaccharides (NSP) (Vangroenweghe F, 2021). The content of mannan is approximately 1.3% to 1.6% of soybean meal, which is the second largest component of hemicellulose in leguminous plants (Hsiao et al., 2006). Mannan is a water-soluble NSP consisting of repeating mannose units linked by β-1,4-glycosidic bonds. Some of the side chains of mannan contain galactose or glucose (Dawood and Ma, 2020a), and mannan is a typical anti-nutritional factor in a corn-soybean meal diet. Mannan can increase the viscosity of chyme (Caldas et al., 2018), inhibit intestinal peristalsis, increase satiety, reduce feed intake, improve the thickness of the water layer on the surface of the intestinal mucosa (Ayoola et al., 2015), hinder digestion and absorption of nutrients in feed by animals, and cause energy loss (Hsiao et al., 2006). In addition, it can disrupt the microbial community in the digestive tract (Kim et al., 2017) and affect the growth performance and intestinal health of animals.
Mannanase is a hemicellulase that hydrolyzes the β-1,4-D-mannosidic bond with strict specificity to the substrate. Hemicellulases are mainly derived from bacteria, such as Bacillus (David A, 2018) and are mostly extracellular enzymes, which usually require purification to improve their specificity and efficiency of action (Bhaturiwala et al., 2021). The addition of mannanase to feed can degrade mannan and release mannan-oligosaccharides (MOS). As a prebiotic, MOS can indirectly regulate intestinal microecology, metabolism, and immunity by promoting the increase of Lactobacillus and Bifidobacterium and inhibiting the adhesion of harmful bacteria such as Escherichia coli to the animal intestine (Gutierrez et al., 2008). Mannanase can not only eliminate the anti-nutritional effect of mannan, but also exert the probiotic effect of MOS and regulate the structure of the intestinal microbiota (Lai et al., 2015). Several studies have shown that mannanase can maintain intestinal epithelium morphology, improve the digestibility of nutrients (Ko et al., 2021), inhibit the expression of various pathogen-associated signaling pathways (Arsenault et al., 2017a) and alleviate intestinal inflammation (Arsenault et al., 2017b; Ferreira et al., 2016b). In addition, mannanase can alter the structure of the intestinal microbiota and inhibit the adhesion of E. coli to the intestine (Zheng et al., 2020), indirectly regulating the intestinal microecology, in vivo metabolism, and immunity of animals. Poultry do not have enzymes to break down mannan. Therefore, the exogenous addition of mannanase has gradually become an effective strategy to maximize the potential growth performance of poultry, improve the efficiency of nutrient digestion, absorption, and utilization, and improve intestinal health. Therefore, this experiment aimed to evaluate the effect of mannanase on the improvement of growth performance and nutrient digestibility of broilers fed a low-energy diet, and to explore the effect and mechanism of action of mannanase on intestinal inflammation in broilers triggered by a soybean meal diet.
2. Materials and methods
2.1. Animal ethics
All animal management procedures were carried out in accordance with the Beijing Experimental Animal Management Regulations and approved by the Experimental Animal Welfare and Animal Experimentation Ethics Review Committee of China Agricultural University. Animal ethics project number is AW02403202-1-1.
2.2. Experimental animals and diets
In this experiment, 384 Arbor Acres broiler chickens at 1 d old were randomly divided into 3 groups of 8 replicates, with 16 broilers in each replicate. A one-way experimental design was used in this study. The trials were grouped as follows: (1) positive control group (PC, corn-soybean meal basal diet); (2) low-energy negative control group (NC, metabolizable energy [ME] below 50 kcal/kg of the basal diet); and (3) experimental group (NC + BM, low-energy diet with an additional 100 mg/kg of mannanase). Mannanase (Shengmei Enzyme, activity 3000 U/g, derived from deep liquid fermentation of Bacillus lentus) was purchased from Beijing Strowin Biotechnology Co., Ltd. (Beijing, China).
Formulation of a corn-soybean meal basal diet was made according to the nutritional requirements of broiler chickens recommended in NY/T 33-2004. The ME of the low-energy diet was 50 kcal/kg lower than that of the basal diet. The composition and nutritional levels of the test diets are shown in Table 1. The test diets were in pellet form. All tested broilers were reared at the Zhuozhou Poultry Breeding Base at the China Agricultural University Teaching Experimental Farm (Hebei, China). During the entire experiment, 24 h of light was provided per day. The chickens were raised at an initial temperature of 33 °C which gradually decreased to 24 °C on d 21. The feeding management and nutritional supply of the birds met the recommendations and regulations of the Feeding Management Manual.
Table 1.
Composition and proportions of experimental diets1 (%, as is basis).
| Item | Positive control group |
Negative control group |
||
|---|---|---|---|---|
| 0 to 21 d | 22 to 42 d | 0 to 21 d | 22 to 42 d | |
| Compositions | ||||
| Corn (7.8% CP) | 52.95 | 57.69 | 54.60 | 57.77 |
| Soybean meal (44% CP) | 35.33 | 29.00 | 34.34 | 30.58 |
| Corn gluten meal | 3.60 | 4.29 | 4.00 | 3.17 |
| Soybean oil | 3.70 | 5.10 | 2.60 | 4.56 |
| L-Lysine hydrochloride (98.5%) | 0.17 | 0.19 | 0.21 | 0.16 |
| Calcium hydrogen phosphate | 1.85 | 1.47 | 1.76 | 1.51 |
| Stone powder | 1.32 | 1.30 | 1.41 | 1.26 |
| NaCl | 0.40 | 0.22 | 0.40 | 0.24 |
| Trace mineral feed2 | 0.20 | 0.10 | 0.20 | 0.10 |
| Choline chloride (50%) | 0.15 | 0.10 | 0.15 | 0.10 |
| DL-Methionine (99%) | 0.21 | 0.13 | 0.21 | 0.14 |
| Antioxidants | 0.05 | 0.05 | 0.05 | 0.05 |
| Vitamin premix3 | 0.03 | 0.02 | 0.03 | 0.02 |
| Phytase | 0.02 | 0.02 | 0.02 | 0.02 |
| Zeolite | 0.02 | 0.02 | 0.02 | 0.02 |
| Cr2O3 | 0.00 | 0.30 | 0.00 | 0.30 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Calculated nutrient levels4 | ||||
| Metabolizable energy, kcal/kg | 3000.00 | 3150.00 | 2950.00 | 3100.00 |
| Crude protein | 22.18 | 20.16 | 22.16 | 20.15 |
| Ca | 1.03 | 0.92 | 1.04 | 0.92 |
| Available phosphorus | 0.45 | 0.38 | 0.44 | 0.39 |
| Lysine | 1.23 | 1.10 | 1.24 | 1.10 |
| Methionine | 0.56 | 0.46 | 0.56 | 0.46 |
The feed was in granular form.
The analytical values per kilogram of trace mineral feed ingredients were as follows: Cu 8 g, Fe 40 g, Zn 55 g, Mn 60 g, I 750 mg, Se 150 mg, Co 250 mg, moisture ≤10%.
The analytical values per kilogram of vitamin premix composition were as follows: vitamin A 50 million IU, vitamin D3 12 million IU, vitamin E 100,000 IU, vitamin K3 10 g, vitamin B1 8 g, vitamin B2 32 g, vitamin B6 12 g, vitamin B12 100 mg, nicotinamide 150 g, D-pantothenic acid 46 g, folic acid 5 g, biotin 500 mg, moisture ≤6%.
Values calculated from the analysis of the experimental diets.
2.3. Measurement of growth performance
On d 21 and 42, each cage of broilers was weighed after 12 h of fasting. Feed consumption was recorded, and feed intake and BW were measured in each replicate. The average daily feed intake (ADFI), average daily gain (ADG), and feed conversion rate (FCR) were calculated for 0 to 21 d, 22 to 42 d, and 0 to 42 d, respectively. The health and mental condition of the chickens were observed daily, during which mortality was also checked for.
2.4. Measurement of the apparent total tract digestibility (ATTD) of nutrients
The exogenous indicator method was used to determine the ATTD of nutrients. At d 18, 4 broilers in each replicate of each treatment group were randomly selected to be transferred to a separate enclosure and fed the test diet supplemented with 0.3% Cr2O3 for 4 d. From 22 to 28 d, 4 broilers were randomly selected from each replicate for fecal sample collection. After removing contaminants such as feathers and feed, the surface of the feces was sprayed with 10% hydrochloric acid to fix nitrogen. The mixture of feces was then placed in an oven at 65 °C, dried to a constant weight, crushed, and passed through a 40-mesh sieve. Dry matter (DM), crude protein (CP), and gross energy (GE) in diets and feces were determined. DM and CP were determined according to GB/T 6435-2014 and GB/T 6432-2018, respectively. GE was determined according to ISO 9831:1998. The ME of feed was calculated using the formula: ME of feed (MJ/kg) = GE of feed × ATTD of GE. The ATTD was calculated using the formula as follows:
2.5. Sample collection
On d 21 and 42, one healthy chicken was randomly selected from each replicate, weighed, and their blood were then collected from wing veins. Serum was separated at 4 °C by centrifugation at 3000 × g for 15 min. The experimental broilers were euthanized by jugular vein bleeding, and molecular samples (approximately 1 cm) and tissue samples (approximately 1.5 cm) were collected from the mid-jejunum and mid-ileum. The intestinal chyme was rinsed with normal saline, the molecular samples flash-frozen in liquid nitrogen, and the tissue samples required for morphological analysis fixed with 4% paraformaldehyde. The mucosa of the posterior jejunum and ileum was scraped, and samples of the ileum and cecum were aseptically collected from the chyme. The samples were flash-frozen in liquid nitrogen. Chyme from the posterior segment of the jejunum was collected and temporarily stored at −20 °C. All serum, molecular, and chyme samples were transferred to a −80 °C freezer for storage.
2.6. Viscosity of jejunal chyme
After thawing the chyme to room temperature, a 1.5-g sample was placed into a centrifuge tube, to which deionized water was added at a ratio of 1:9 (wt:vol). The tube was then vortexed to mix its contents thoroughly. The mixture was then centrifuged at 3000 × g for 20 min at 4 °C and the supernatant removed to determine the chyme's viscosity using an Ostwald viscosimeter (1831-2; 0.55 mm; Huanguang Glass Instruments Co., Ltd., Taizhou, Zhejiang, China).
2.7. Intestinal morphology
The jejunal and ileal tissues were embedded and fixed with paraffin wax to prepare sections. The intestinal tissues were stained with AB-PAS and then placed under a Leica microscope (ModelDMi8, Wetzlar, Germany) to determine the morphology of the intestinal epithelium. Ten intact, straight-running villi were randomly selected from each section. LIOO software was used to measure each intestinal villus height (VH) and crypt depth (CD) and to calculate the ratio of VH to CD (V:C). VH was measured from the tip of the villi to the entrance of the crypt. CD refers to the vertical distance from the base of the corresponding villi to the submucosa.
2.8. Determination of diamine oxidase (DAO), D-lactic acid (D-LA), and endotoxin (ET) in serum
Chicken DAO and D-LA ELISA kits (Shanghai Guduo Biotechnology Co., Ltd., Shanghai, China) were used to determine the DAO and D-LA contents in the serum. This test was performed using a chicken endotoxin test LAL kit (Xiamen Bioendo Technology Co., Ltd., Xiamen, China) to determine ET in serum. The above indicators were tested separately according to the instructions supplied with each kit.
2.9. Determination of gene expression related to intestinal immunity and intestinal barrier
TRIzol reagent was used to extract total RNA from the jejunum and ileum. The concentration of total RNA was determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, United States). Following the instructions provided with each separate kit, reverse transcription and fluorescence quantification were performed using PrimeScript RT and SYBR Premix Ex Taq II (Tli RNaseH Plus) (Takara Biomedical Technology Co., Ltd., Beijing, China), respectively.
With β-actin as an internal reference, the gene expression related to intestinal immunity and intestinal barrier was determined by an ABI 7500 real-time fluorescence quantitative PCR instrument. Table 2 lists the primers used for real-time fluorescent quantitative PCR in this study. The relative expression of each target gene mRNA was calculated using 2−ΔΔCt.
Table 2.
Sequences of oligonucleotide primers1 for real-time quantitative fluorescence PCR.
| Gene | Sequences of primers (5′ to 3′)2 | Serial number |
|---|---|---|
| CLDN1 | F: CATACTCCTGGGTCTGGTTGGT R: GACAGCCATCCGCATCTTCT |
NM_001013611.2 |
| β-Actin | F: CAACACAGTGCTGTCTGGTGGTAC | NM_205518.1 |
| R: CTCCTGCTTGCTGATCCACATCTG | ||
| OCLN | F: ACGGCAGCACCTACCTCAA R: GGGCGAAGAAGCAGATGAG |
NM_205128.1 |
| ZO-1 | F: CTTCAGGTGTTTCTCTTCCTCCTC R: CTGTGGTTTCATGGCTGGATC |
XM_015278981.1 |
| MUC-2 | F: TTCATGATGCCTGCTCTTGTG R: CCTGAGCCTTGGTACATTCTTGT |
XM_421035 |
| IL-1β | F: ACTGGGCATCAAGGGCTA R: GGTAGAAGATGAAGCGGGTC |
NM_204524.1 |
| IL-4 | F: GCTCTCAGTGCCGCTGATG R: GAAACCTCTCCCTGGATGTCAT |
NM-0010079.1 |
| IL-6 | F: CGCCCAGAAATCCCTCCTC R: AGGCACTGAAACTCCTGGTC |
XM_015281283.1 |
| IL-8 | F: ATGAACGGCAAGCTTGGAGCTG | XM_015301388.1 |
| R: TCCAAGCACACCTCTCTTCCATCC | ||
| IL-10 | F: GCTGCCAAGCCCTGTT R: CCTCAAACTTCACCCTCA |
NM_001004414.2 |
| IL-18 | F: TGATGAGCTGGAATGCGATG | NM_204608.3 |
| R: ACTGCCAGATTTCACCTCCTG | ||
| TGF-β3 | F: CATCGAGCTCTTCCAGATCC | NM_205454.1 |
| R: GACATCGAAGGACAGCCACT | ||
| TNF-α | F: GAGCGTTGACTTGGCTGTC R: AAGCAACAACCAGCTATGCAC |
XM_204267 |
| IFN-γ | F: AGCTGACGGTGGACCTATTATT R: GGCTTTGCGCTGGATTC |
NM_205149.1 |
| NF-κB | F: GTGTGAAGAAACGGGAACTG R: GGCACGGTTGTCATAGATGG |
NM_205129.1 |
CLDN1 = claudin-1; OCLN = occludin; ZO-1 = zonula occludens-1; MUC-2 = mucin-2; IL-1β = interleukin-1β; IL-4 = interleukin-4; IL-6 = interleukin-6; IL-8 = interleukin-8; IL-10 = interleukin-10; IL-18 = interleukin-18; TGF-β3 = transforming growth factor-β3; TNF-α = tumor necrosis factor α; IFN-γ = interferon γ; NF-κB = nuclear factor kappa B.
Primers were designed using Primer Express software (Sangon Biotech Co., Ltd., Shanghai, China).
F for forward; R for reverse.
2.10. Determination of the content of short-chain fatty acids (SCFA) in the cecum
A 0.4-g sample of chyme was placed into a centrifuge tube to which 0.5 mL of normal saline was added, and the mixture shaken well. The mixture was then centrifuged at 5400 × g for 20 min at 4 °C and 0.2 mL of the resulting supernatant placed into a centrifuge tube. Fifty microliters of 25% (wt:vol) metaphosphate deproteinized solution containing 2 g/L internal standard 2 EB was then mixed in with the supernatant and the mixture incubated overnight at 4 °C to precipitate component proteins. The proteins were removed by centrifugation at 10,000 × g at 4 °C for another 10 min. The supernatant was filtered through a 0.22-μm filter membrane. SCFA in the cecal contents were detected by gas chromatography (GC2014; Shimadzu Corporation, Kyoto, Japan) and calculated by the internal standard method. The gas chromatograph used N2 as the carrier gas for the gasification chamber, with a split ratio of 40:1 and a temperature of 220 °C. An HP-INNOWax capillary column (Agilent Technologies, Santa Clara, CA, USA) was used in constant flow mode with a flow rate of 2.0 mL/min. The parameters of the detector were as follows: H2 flow rate, 40 mL/min; air flow rate, 450 mL/min; column flow rate + make-up gas flow rate, 45 mL/min; and FID temperature, 250 °C.
2.11. 16S rRNA sequencing of microorganisms in the ileum
2.11.1. DNA extraction and high-throughput sequencing
Microbial DNA was extracted from the contents of the ileum using a fecal microbial DNA extraction kit (QIAamp Fast DNA Stool Mini Kit; QIAGEN, Germany). The concentration of DNA was determined using a NanoDrop 2000 microspectrophotometer, and the purity of DNA was checked by 1% agarose gel electrophoresis. The common primers 515F (5′-GTG CCA GCM GCC GCG GTA A-3′) and 806R (5′-GGA CTA CHV GGG TWT CTA AT-3′) of the V4 region of the 16S rRNA gene were used to amplify the bacterial DNA. After amplification, the PCR products ran on a 2% agarose gel and were purified using a QIAquick Gel Extraction Kit (Qiagen, Germany). The library was constructed using the TruSeq DNA PCR-Free Sample Preparation Kit. The constructed library was quantified using Qubit and qPCR. After the library was qualified, HiSeq2500-PE250 was used for sequencing. Sequencing analysis was performed by Novogene Biotech Co., Ltd. (Beijing, China).
2.11.2. Sequence processing and bioinformatics analysis
Flash software (version 1.2.7) was used to stitch the reads of the samples to obtain raw tag data. The raw tag data were processed by referring to the QIIME (version 1.9.1) tag quality control process, and the sequences of the processed tags were compared with the database using the UCHIME algorithm. Chimeric sequences were detected and removed to obtain effective tags which were clustered using UPARSE software (UPARSE v7.0.1001). The sequences were then clustered into OTU with 97% consistency and representative sequences were selected. Based on the principles of the algorithm, the sequence with the highest frequency of occurrence was used as the representative sequence of OTU, and the representative sequence was annotated with species. SSU rRNA databases from Mothur and SILVA were used for species annotation analysis (threshold = 0.8 to 1) to obtain taxonomic information and community composition at each taxonomic level. MUSCLE software (version 3.8.31) was used for rapid multiple sequence alignment to obtain phylogenetic relationships for all OTU representative sequences, and the data of each sample was homogenized. The sample with the least amount of data was taken as the standard, and the subsequent analysis of diversity was based on the homogenized data. QIIME software was used to calculate the UniFrac distance of each sample to construct UPGMA clustering trees. Linear discriminant analysis effect size (LEfSe) software was used to identify biomarkers that were statistically different between groups (classes of bacteria with significant differences in relative content) based on linear discriminant analysis (LDA) values. R software (version 2.15.3) was used to draw a Venn diagram, dilution curve diagram, coverage index diagram, principal component analysis (PCA) diagram, and principal coordinate analysis (PCoA) diagram, together with ANOSIM analysis. The t-tests between groups were performed for genera with a relative abundance >0.001 using R software. PICRUSt software was used to predict and analyze metagenomic function.
2.12. Metagenomics sequencing of the ileum and cecum
The contents of the ileum and cecum of healthy chickens at 42 d were mixed in equal masses, and the genomic DNA of the microbiota was extracted from the contents. Quality control and quantification of DNA were performed using agarose gel electrophoresis and Qubit. Covaris ultrasound was used to fragment tested DNA samples into lengths of approximately 350 bp. Terminal repair and addition of sequencing connectors and A-tails were then performed. After purification and PCR amplification, a sequencing library was prepared. The library was quantified and diluted using Qubit 2.0. Agilent 2100 was used to conduct inspection of the insert size of the library to ensure that the sequencing library was of suitable quality. Illumina PE150 was used to sequence the library, and analysis of the sequencing data performed by Novogene Biotech Co., Ltd. (Beijing, China). During sequencing analysis, clean data were obtained by quality control and host filtration. Metagenome assembly, gene prediction, and species annotation were then performed, and finally, the relative abundance of different species was analyzed based on OTU. Functional annotation was performed based on metabolic pathways (KEGG), homologous gene clusters (eggNOG), and carbohydrases (CAZy) databases.
2.13. Statistical analysis
SPSS 22.0 software was used for one-way analysis of variance (ANOVA) of the data from each treatment group. The differences between treatments were analyzed using Duncan's multiple comparisons test and the results expressed as mean ± standard error (SEM). Significant differences were based on P < 0.05 and P values between 0.05 and 0.10 were classified as trends.
3. Results
3.1. Growth performance
The growth performance of broilers in terms of BW, ADG, ADFI and FCR is shown in Fig. 1. The low-energy diet significantly increased the FCR of broilers between 0 and 42 d (P = 0.005) and tended to increase their ADFI between 0 and 21 d (P = 0.093). The addition of mannanase to the low-energy diet increased the BW of broilers at 42 d, the ADG of broilers from 22 to 42 d, and from 0 to 42 d (P < 0.01). In addition, mannanase reduced the FCR of broilers between 0 and 21 d (P = 0.047) and from 0 to 42 d (P = 0.005). It also tended to reduce the ADFI of broilers between 0 and 21 d (P = 0.093).
Fig. 1.
Effect of mannanase on growth performance of broilers fed a low-energy diet. The PC group was the positive control group on the basal diet. The NC group was the low-energy negative control group. The NC + BM group was the low-energy negative control group + mannanase. All graphs are expressed as mean ± SEM, and P-values are marked at the top of each graph. a, b Different lower case letters indicate significant differences (P < 0.05).
3.2. Viscosity of jejunal chyme
As shown in Fig. 2A, the reduction in the energy level of the diet had no significant effect on the viscosity of the jejunal chyme of broilers (P > 0.05). A low-energy diet supplemented with mannanase significantly reduced the viscosity of the jejunal chyme of broilers on d 21 (P = 0.005).
Fig. 2.
Effects of mannanase on the viscosity of intestinal chyme and apparent total tract digestibility of nutrients of broilers fed a low-energy diet. The PC group was the positive control group on the basal diet. The NC group was the low-energy negative control group. The NC + BM group was the low-energy negative control group + mannanase. All graphs are expressed as mean ± SEM, and P-values are marked at the top of each graph. a, b Different lower case letters indicate significant differences (P < 0.05).
3.3. ATTD of nutrients
The low-energy diet had no significant effect on the ATTD of nutrients in broilers compared to the basal diet (Fig. 2). The addition of mannanase to the low-energy diet significantly increased the ATTD of DM, GE, and ME (P < 0.01).
3.4. Morphology of intestinal mucosa
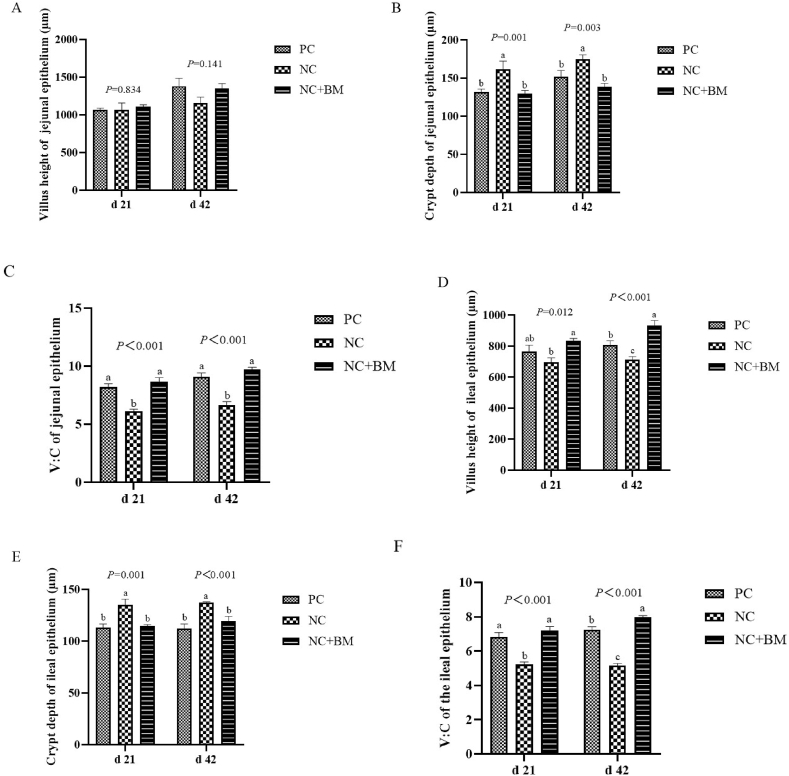
Compared to the basal diet, the low-energy negative control group showed increased CD of the jejunal epithelium at 21 and 42 d (P < 0.01) and decreased V:C of the jejunal epithelium at 21 and 42 d (P < 0.01) (Fig. 3). A low-energy diet supplemented with mannanase decreased the CD of jejunal epithelium at 21 and 42 d (P < 0.01) and increased the V:C of jejunal epithelium at 21 and 42 d (P < 0.01). In addition, the low-energy diet significantly decreased the VH of the ileal epithelium at 42 d, and the V:C at 21 d and 42 d (P < 0.01). It also significantly increased the CD of the ileal epithelium at 21 d and 42 d (P < 0.01). Compared to the low-energy diet, the addition of mannanase significantly increased the VH of ileal epithelium at 21 d (P = 0.012), the VH of ileal epithelium at 42 d, and the V:C of ileal epithelium at 21 d and 42 d (P < 0.01), and also significantly reduced the CD of the ileal epithelium at 21 d and 42 d (P < 0.01).
Fig. 3.
Effect of mannanase on the morphology of intestinal mucosa of broilers fed a low-energy diet. The PC group was the positive control group on the basal diet. The NC group was the low-energy negative control group. The NC + BM group was the low-energy negative control group + mannanase. All graphs are expressed as mean ± SEM, and P-values are marked at the top of each graph. a–c Different lower case letters indicate significant differences (P < 0.05).
3.5. Integrity of the intestinal epithelium
As shown in Fig. 4, the low-energy diet significantly increased the DAO level in the chickens’ serum at 21 d (P = 0.001). The addition of mannanase significantly reduced the DAO level in the serum at 21 d compared to the low-energy diet (P = 0.001). Additionally, a low-energy diet supplemented with mannanase could significantly reduce the content of ET in the serum of broilers at 21 d (P = 0.015) and tended to reduce ET in the serum at 42 d (P = 0.059). The difference in the effect of mannanase addition to the low-energy diet on the D-LA content in the serum of broilers was not significant (P > 0.05).
Fig. 4.
Effect of mannanase on the epithelial integrity of the intestine in broilers fed a low-energy diet. ET = endotoxin. The PC group was the positive control group on the basal diet. The NC group was the low-energy negative control group. The NC + BM group was the low-energy negative control group + mannanase. All graphs are expressed as mean ± SEM, and P-values are marked at the top of each graph. a, b Different lower case letters indicate significant differences (P < 0.05).
3.6. Expression of genes associated with the intestinal epithelial barrier
Compared with the basal diet, the low-energy diet had no significant difference in the expression of genes related to the jejunal epithelial barrier in broilers (P > 0.05) (Fig. 5). Supplementation of mannanase in the low-energy diet significantly increased the expression of claudin-1 (CLDN1) and zonula occludens-1 (ZO-1) in the jejunum at 21 d (P < 0.01), and it also tended to increase the expression of occludin (OCLN) in the jejunum of broilers at 42 d (P = 0.069). In addition, compared to the positive control group, the low-energy diet significantly decreased the expression of ZO-1 in the ileum at 21 d (P = 0.003), while there was a tendency to decrease the expression of mucin-2 (MUC-2) in the ileum at 21 d (P = 0.072). Compared to the low-energy diet, the addition of mannanase had no significant effect on the expression of genes related to the ileal epithelial barrier (P > 0.05).
Fig. 5.
Effect of mannanase on the expression of genes related to the intestinal epithelial barrier in broilers fed a low-energy diet. MUC-2 = mucin-2; ZO-1 = zonula occludens-1. The PC group was the positive control group on the basal diet. The NC group was the low-energy negative control group. The NC + BM group was the low-energy negative control group + mannanase. All graphs are expressed as mean ± SEM, and P-values are marked at the top of each graph. a, b Different lower case letters indicate significant differences (P < 0.05).
3.7. Expression of genes for cytokines and pathways associated with the intestinal inflammatory response
As shown in Fig. 6, the low-energy diet significantly increased the expression of interleukin (IL)-18 in the ileum at 21 d and interferon γ (IFN-γ) at 42 d (P < 0.05), and caused an up-regulation of the expression of nuclear factor kappa B (NF-κB) in the ileum of broilers at 21 d (P = 0.029). There was also a tendency to decrease the expression of IL-10 in the ileum at 21 d (P = 0.052). Compared to the low-energy diet, the addition of mannanase significantly increased the expression of transforming growth factor-β3 (TGF-β3) in the ileum at 21 d and the expression of IL-10 at 42 d (P < 0.05), and significantly decreased the expression of IL-18 and NF-κB in the ileum at 21 d (P < 0.05). At the same time, the low-energy diet supplemented with mannanase also decreased the expression of IL-1β (P = 0.054) and the relative expression of tumor necrosis factor α (TNF-α) in the ileum on d 21 (P = 0.067).
Fig. 6.
Effect of mannanase on the expression of genes and signaling pathways related to the intestinal inflammatory response in broilers fed a low-energy diet. IL = interleukin; TGF-β3 = transforming growth factor-β3; TNF-α = tumor necrosis factor α; IFN-γ = interferon γ; NF-κB = nuclear factor kappa B. The PC group was the positive control group on the basal diet. The NC group was the low-energy negative control group. The NC + BM group was the low-energy negative control group + mannanase. All graphs are expressed as mean ± SEM, and P-values are marked at the top of each graph. a, b Different lower case letters indicate significant differences (P < 0.05).
3.8. Contents of SCFA in the cecum
As shown in Fig. 7, compared to the positive control, the acetic acid content of the cecal contents at 21 d in the low-energy negative control group was significantly decreased (P = 0.046), and the contents of isobutyric acid and isovaleric acid were also significantly decreased (P < 0.01). Compared to the low-energy diet, the addition of mannanase significantly increased isobutyric acid content in the cecum at 21 d (P = 0.006).
Fig. 7.
Effect of mannanase on the contents of short-chain fatty acids in the intestine of broilers fed a low-energy diet. The PC group was the positive control group on the basal diet. The NC group was the low-energy negative control group. The NC + BM group was the low-energy negative control group + mannanase. All graphs are expressed as mean ± SEM, and P-values are marked at the top of each graph. a, b Different lower case letters indicate significant differences (P < 0.05).
3.9. Microorganisms of the ileum
3.9.1. Alpha and beta diversity
As shown in Fig. 8, the low-energy diet had no significant effect on the alpha diversity of the ileal chyme microorganisms of broilers in terms of the observed-species index and Shannon index compared to the positive control group (P > 0.05). Compared to the low-energy diet, the addition of mannanase had a relative tendency to increase the Shannon index of ileal chyme at 21 d (P = 0.090). It also showed a relative tendency to increase the Chao 1 index (P = 0.079) and ACE index (P = 0.091) of the ileum at 42 d. These results indicate that the addition of mannanase increased the richness and homogeneity of species of microorganisms in the ileal chyme of broilers at 21 d and 42 d. In addition, there was no significant difference in the beta diversity of the ileal microorganisms of broilers supplemented with mannanase in the low-energy diet (P > 0.05).
Fig. 8.
Effect of mannanase on the ileal microorganisms of broilers fed a low-energy diet. The alpha diversity of the ileal chyme microorganisms of broilers in terms of the Observed_species (A), Shannon index (B), Simpson index (C), Chao 1 index (D), and ACE index (E) were used to analyze the alpha diversity of ileal microorganisms of broilers. The beta-diversity (F, G) of the ileal microorganisms of broilers at 21 d and 42 d was analyzed by principal coordinate analysis (PCoA). The top 5 microbial groups at the phylum level (H, I) and the top 10 microbial groups at the genus level (J, K) of the ileal microorganisms of broilers at 21 d and 42 d were determined, respectively. In addition, the linear discriminant analysis effect size (LEfSe) test (L, M) was performed on the differential groups of the ileal microorganisms. The PC group was the positive control group on the basal diet. The NC group was the low-energy negative control group. The NC + BM group was the low-energy negative control group + mannanase. All bar charts are expressed as mean ± SEM, and P-values are marked at the top of each graph. a, b Different lower case letters indicate significant differences (P < 0.05).
3.9.2. Top 5 microorganisms in relative abundance at the phylum level in the ileum
As can be seen from Fig. 8H, in the above three treatment groups, at the phylum level, the top 5 phyla in relative abundance of the microorganisms in the ileal chyme at 21 d were Firmicutes, Cyanobacteria, Proteobacteria, Bacteroidetes, and Actinobacteria. As can be seen from Fig. 8I, in the above three treatment groups, the top 5 species in terms of relative abundance in ileal chyme at 42 d were Firmicutes, Proteobacteria, Bacteroidetes, Verrucobacteria, and Desulfobacterotamen. The addition of mannanase tended to reduce the relative abundance of Proteobacteria compared to the low-energy negative control group (P = 0.075).
3.9.3. Top 10 microorganisms in relative abundance at the genus level in the ileum
As shown in Fig. 8J, the low-energy diet significantly increased the relative abundance of Prevotella in the ileal chyme of broilers at 21 d (P < 0.05). The addition of mannanase tended to increase the relative abundance of Tyzzerella (P = 0.079). Compared to the low-energy diet, the addition of mannanase significantly increased the relative abundance of Lactobacillus in the ileal chyme microorganisms of broilers at 42 d (P < 0.05) (Fig. 8K), with a tendency to reduce the relative abundance of Enterococcus (P = 0.080).
3.9.4. LEfSe test for different microorganisms
LEfSe analysis was used to determine statistical differences in microorganisms between each group. As can be seen from Fig. 8L, the low-energy negative control group significantly increased the relative abundance of Mitochondria and Actinomycetales bacterium HO2017, while significantly decreasing the relative abundance of Prevotella. The supplementation of mannanase in the low-energy diet significantly increased the relative abundance of Prevotella and Prevotella 9 in the ileal chyme at 21 d. As shown in Fig. 8M, the low-energy diet significantly increased the relative abundance of Clostridium perfringens, Clostridium sensu stricto 1 and Rickettsiales in the ileal chyme at 42 d. The addition of mannanase significantly increased the relative abundance of probiotic bacteria, such as Lactobacillus sp. KC45b, Lactobacillus reuteri, Lactobacillus agilis, Lactobacillus johnsonii, and Lactobacillus in the ileum at 42 d.
3.10. Macro-genome of intestinal contents
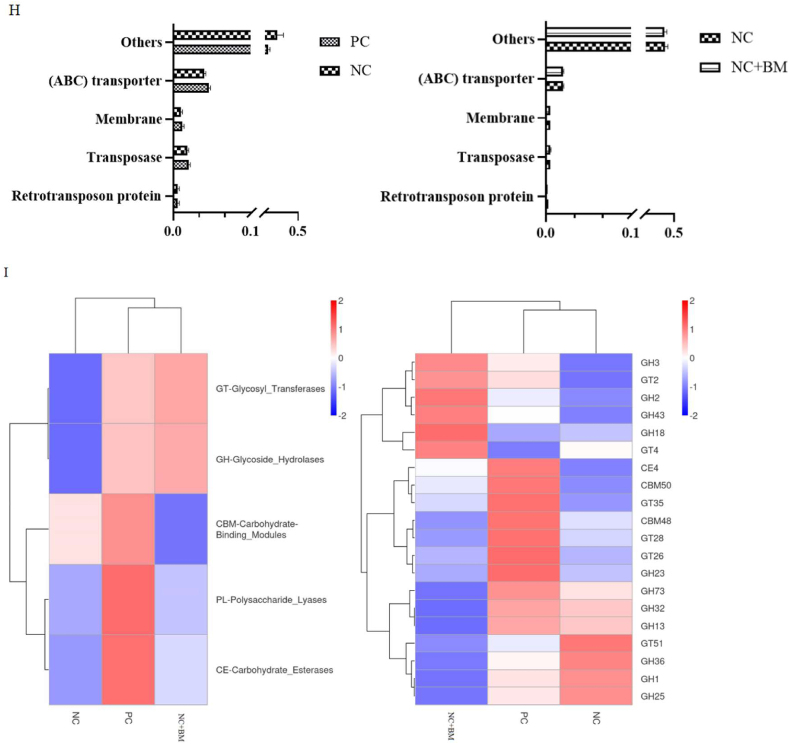
As shown in Fig. 9A, compared to the basal diet, the relative abundance of Blautia was significantly decreased in the low-energy negative control group (P < 0.05). It also tended to reduce the relative abundance of Firmicutes (P = 0.075), Lactobacillus (P = 0.082), and Butyricoccus (P = 0.063) in the intestine at 42 d. As can be seen from Fig. 9B and C, Blautia, Butyricoccus, Clostridium sp-M62-1, and Ruminococcus were dominant in the basal diet group. The low-energy negative control group had a significantly reduced relative abundance of Lachnoclostridium-sp-An131, while the relative abundance of Odoribacter was significantly increased in the low-energy diet supplemented with mannanase. As can be seen in Fig. 9D and E, the reduction in dietary energy level could inhibit pathways such as energy metabolism, metabolism of cofactors and vitamins, carbohydrate metabolism, and membrane transport. Energy metabolism and lipid metabolism could be enhanced by mannanase supplementation in a low-energy diet. As can be seen in Fig. 9F, further analysis of the above pathways revealed that the reduction in dietary energy level significantly inhibited oxidative phosphorylation (ko00190). The addition of mannanase to the low-energy diet not only enhanced oxidative phosphorylation (ko00190) and beta-alanine metabolism (ko00410), but also promoted N-glycan biosynthesis (ko00510) (P < 0.05). As can be seen from Fig. 9G and H, the low-energy diet inhibited energy production and conversion (P = 0.065), while the addition of mannanase had no significant effect on the transcriptional pathway of genes. In addition, as seen in Fig. 9I, the addition of mannanase tended to increase the activity of GH18 (P = 0.067) and GH3 (P = 0.078) compared to the low-energy diet.
Fig. 9.
Effect of mannanase on the structure and function of the intestinal microorganisms of broilers fed a low-energy diet. The relative abundance of microbiota at the phylum level, genus level and species level was analyzed based on macro-genome sequencing (A), and linear discriminant analysis effect size (LEfSe) test was performed for differential microbiota at the genus level and species level of the broilers' intestinal microorganisms (B, C). The results of Functional Annotation at three taxonomic levels were analyzed based on KEGG (D, E, F). The results of Functional Annotation at two taxonomic levels were analyzed based on eggNOG (G, H). The results of carbohydrate-active enzymes at two taxonomic levels were analyzed based on CAZy (I). The PC group was the positive control group on the basal diet. The NC group was the low-energy negative control group. The NC + BM group was the low-energy negative control group + mannanase. All bar charts are expressed as mean ± SEM, and P-values are marked on the right side of each graph, with ∗ indicating significant differences (P < 0.05).
4. Discussion
The innate immune system is the first line of host defense against pathogens and harmful substances. During long-term contact with microorganisms, animals can evolve pattern recognition receptors (PRR) to recognize the conserved components of microorganisms and rapidly initiate the innate immune response (Keestra et al., 2013; Kogut et al., 2018). In particular, Toll-like receptor 4 (TLR4), a pattern recognition receptor on the surface of immune cells, can specifically recognize lipopolysaccharide (LPS) and form the TLR4-MD-2-LPS-CD14 complex through a series of reactions. These activate the amplification reaction of the intracellular signaling cascade and ultimately activate NF-κB, leading to the synthesis and secretion of pro-inflammatory cytokines such as IL-1β and TNF-α, resulting in an inflammatory response (Fearon and Locksley, 1996; Keestra et al., 2013; Keestra and van Putten, 2008; Liu, 2006). The occurrence of an inflammatory response is helpful in protecting the body from rapid clearance of antigenic substances (Kogut et al., 2018; Medzhitov, 2008), but excessive inflammation can lead to tissue damage and increased energy loss, resulting in a severe reduction in the growth performance of animals (E. Roura, 1992; Hotamisligil, 2006; Hsiao et al., 2006). In this study, the low-energy diet resulted in a significant upregulation of the expression of NF-κB in the ileum of broiler chickens at 21 d, and the addition of mannanase to the diet downregulated the expression of NF-κB, which alleviated intestinal inflammation.
The mechanism of the inflammatory response is complex and is mainly regulated by pro-inflammatory cytokines and anti-inflammatory cytokines expressed and released by activated inflammatory cells (Kogut et al., 2018). NF-κB is a key factor in the regulation of inflammatory cytokines (Netea et al., 2004). Pro-inflammatory cytokines are mainly responsible for activating the innate and acquired immune systems and killing pathogens when they invade the body (Miller et al., 2007). After pathogens are removed, anti-inflammatory cytokines can reduce inflammation so that the body returns to a homeostatic immune and physiological state (Opal and DePalo, 2000). The major pro-inflammatory cytokines include IL-1β and TNF-α. IL-1β is mainly secreted by monocytes and tissue macrophages, which can initiate the body's inflammatory response and promote the clearance of pathogens (Corwin, 2000). As a major pro-inflammatory cytokine, TNF-α can promote the early immune response in animals, initiate the acute phase response, and activate other immune cells (Bradley, 2008). In addition, IL-10 and TGF-β3, as important anti-inflammatory cytokines, also play important roles in animal immune regulation. IL-10 can inhibit excessive activation of immune function (Koj, 1998) and maintain the intestinal barrier (Jarry et al., 2008). TGF-β3 also plays an important role in various inflammation-related diseases. In this study, lower dietary energy levels up-regulated the expression of IFN-γ and down-regulated the expression of anti-inflammatory factors, such as IL-10 in the ileum of chickens. The addition of mannanase to a low-energy diet downregulated the expression of pro-inflammatory factors such as IL-1β and TNF-α and increased the expression of anti-inflammatory factors such as IL-10 and TGF-β3 in the ileum. It was further suggested that the anti-inflammatory effect of mannanase might be related to its inhibition of the expression of the NF-κB.
The activation of signaling pathways associated with the inflammatory response and the release of inflammatory mediators can affect the intestinal barrier and absorption, ultimately affecting the growth performance of broilers by regulating their intestinal health. The function of the intestinal barrier is an ability of the intestine to provide accommodation for lumen microorganisms and molecules while maintaining the absorption of nutrients. The mechanical barrier of the intestine consists of intestinal epithelial cells and the junctional complex. The junctional complex consists of tight junctions (such as claudins and OCLN), adherens junctions, gap junctions, and desmosomes. The intestinal barrier can maintain the balance of the broiler intestinal environment, prevent the invasion of pathogenic bacteria and toxins, and protect the intestine from pathogens. CLDN1 and OCLN are tight junction proteins that form the intestinal barrier. ZO-1 is a junction protein between transmembrane proteins and the cytoskeleton, and its downregulation indicates that tight junctions are damaged (Kuo et al., 2022; Van Itallie and Anderson, 1997; Zhuang et al., 2019). In this study, lower dietary energy levels down-regulated the expression of ZO-1 and MUC-2 in the ileum of broilers, while the addition of mannanase to the low-energy diet significantly increased the expression of CLDN1 and ZO-1 in the jejunum of broilers, which could mitigate these adverse effects.
The levels of DAO (Zhang et al., 2020), D-LA (Chen et al., 2015), and ET (Assimakopoulos et al., 2021) in blood can be used as important indicators to evaluate the integrity of the intestinal mucosa and the function of the intestinal barrier in broilers, which can be released into the peripheral blood when the intestinal barrier is impaired (Li et al., 2016; Shen et al., 2020). In this study, the increase in DAO in serum indicated that the decrease in dietary energy level might have disrupted the integrity of the intestinal epithelium and damaged the intestinal barrier of broilers. However, the addition of mannanase to a low-energy diet can alleviate the increase in intestinal permeability caused by lower energy levels, reduce the levels of DAO and ET in serum, and maintain the integrity of the intestinal epithelium. Similar to these results, the addition of mannanase to the feed increased the total antioxidant capacity (Lai et al., 2015) and the activity of glutathione peroxidase in serum (Zhou et al., 2019). In this study, the reduction in DAO and ET contents in serum further explained that mannanase could maintain the intestinal integrity of broilers.
There is a close relationship between the histological morphology of the intestine and immune regulation and growth. Damage to the intestinal barrier and the function of absorption can be directly reflected by the intestinal microstructure. Intestinal VH, CD, and V:C are important indicators to evaluate the morphology, structural integrity, and functional status of the intestinal mucosa. The decrease in dietary energy level severely damaged the villous structure of the jejunum and ileum, increased the CD of the intestinal epithelium, and reduced the area for absorption of feed nutrients in broilers. The addition of mannanase to a low-energy diet could alleviate intestinal damage in broilers caused by the decrease in dietary energy level, ensure the normal development of intestinal morphology, and promote the efficient absorption of nutrients. It has been reported that the addition of mannanase to a corn-soybean meal basal diet increased intestinal VH and surface area of broilers (Ibuki et al., 2014), as well as increasing V:C (Lai et al., 2015). In addition, damage to the intestinal morphology of animals may lead to an increase in mucosal secretions. Mannanase can reduce the thickness of the ileal mucus layer (Ayoola et al., 2015), prevent the binding of intestinal epithelial cells to pathogenic bacteria, maintain the integrity of the intestinal mucosa, and establish a symbiotic intestinal ecosystem (Dawood and Ma, 2020b). The anti-inflammatory function of mannanase might explain how it maintains and improves the structure of the intestinal crypt-villus.
The intestinal microbiota mainly includes mucosal and luminal microbiota, which adhere to the intestinal mucosa and form a multi-layered microbial barrier in the intestine, participating in intestinal nutrition and defense, and ultimately regulating the immune function of the host. Under normal conditions, the number and distribution of intestinal microorganisms are relatively constant, and the microbiota is also relatively balanced. The diversity of the intestinal microbiota is beneficial for maintaining the stability of the intestinal microenvironment and resisting the invasion of pathogenic microorganisms (Oakley et al., 2014). In this study, the addition of mannanase to the low-energy diet improved the abundance and homogeneity of the ileal microbiota of broilers. This suggests that mannanase might also exert different degrees of improvement on intestinal microorganisms. Mannanase could improve intestinal health by regulating the structure of the intestinal microbiota. Studies have shown that the addition of mannanase to poultry feed could limit the binding of harmful microbiota to the intestinal epithelium, reduce the contents of intestinal E. coli and Salmonella, resist the invasion and infection of pathogenic bacteria (Gutierrez et al., 2008), and promote the proliferation of beneficial bacteria, such as Lactobacillus and Bifidobacterium (Al-Ghazzewi and Tester, 2012; Mohammadigheisar et al., 2021; Zheng et al., 2020). Proteobacteria is an important phylum of intestinal microorganisms in animals containing many pathogenic bacteria, such as Rickettsia and pathogenic E. coli, which can metabolize and produce ET to destroy the intestinal health of the host (Hiippala et al., 2018). Among the intestinal microbiota, Lactobacillus can often improve host intestinal health by producing SCFA and functional oligosaccharides through metabolism. Consistent with previous studies, the addition of mannanase to a low-energy diet reduced the relative abundance of Proteobacteria and increased the relative abundance of Lactobacillus in the ileum. LEfSe analysis showed that the low-energy diet significantly increased the relative abundance of C. perfringens and Rickettsiales in the ileal microbiota compared with the basal diet group. C. perfringens is a spore-producing and anaerobic Gram-positive bacterium that can induce necrotizing enteritis in broilers and damage the health of the host by producing toxins through metabolism (Gaucher et al., 2015). As beneficial bacteria, Lactobacillus is the genus with the largest number of bacteria in the ileum (Gong et al., 2007), and can regulate the host's intestinal health through metabolism. In this study, the addition of mannanase to a low-energy diet reduced the relative abundance of harmful bacteria, such as C. perfringens and increased the relative abundance of L. johnsonii, L. agilis, and Lactobacillus in the ileum of broiler chickens. In conclusion, the decrease in dietary energy level had negative effects on the ileal microbiota of broilers. The addition of mannanase to a low-energy diet could inhibit the adhesion of harmful bacteria, such as C. perfringens to the intestine, increase the relative abundance of Lactobacillus and improve the balance of the intestinal microbiota of broilers.
Compared with 16S amplicon sequencing, macro-genome sequencing techniques can help in understanding the impact of changes in the structure of the microbiota on the host's intestinal health (Wang et al., 2015). Therefore, to further investigate the effects of mannanase on the structure and function of the intestinal microbiota of broilers fed a low-energy soybean meal diet, macro-genome sequencing was performed for each treatment group in this study. The results showed that the low-energy negative control group reduced the relative abundance of Butyricicoccus in the intestinal microorganisms of broilers at 42 d and inhibited the pathways of energy metabolism and oxidative phosphorylation. Compared to a low-energy diet, the addition of mannanase increased the relative abundance of Odoribacter and promoted energy metabolism and N-glycan biosynthesis, while increasing the activities of GH3, etc.
SCFA are fermentation products of intestinal microorganisms, which can regulate the balance of intestinal microbiota and mediate the inflammatory signaling cascade during intestinal inflammation (McHardy et al., 2013). There are several types of SCFA, among which acetic acid can bind to coenzyme A and participate in the metabolic pathways of cellular fats and carbohydrates. Butyric acid promotes growth of animals by controlling pathogens, reducing the occurrence of oxidative stress and inflammatory responses, and regulating the development of the intestine (Bedford and Gong, 2018). Volatile fatty acids can reduce intestinal pH and inhibit the adhesion of harmful bacteria. The reduction in intestinal pH not only inhibits the proliferation of harmful bacteria but also increases the number of Bifidobacterium which can convert fermentable sugars into molecules such as lactic acid and acetic acid. These can then further reduce intestinal pH, regulate the structure of the intestinal microbiota, and maintain homeostasis of the internal environment. As reported in previous studies, in the present study, a low-energy diet reduced the contents of acetic acid and isobutyric acid in the cecal chyme of broilers, while adding mannanase promoted the synthesis of SCFA in the organism and increased the content of isobutyric acid.
It has been reported that the addition of mannanase to poultry feed can reduce the viscosity of jejunal chyme (Lee et al., 2003), promote nutrient absorption (Caldas et al., 2018) and energy utilization (Wu et al., 2005), and improve the nutritional value of soybean meal diets (Dhawan and Kaur, 2007). In this study, it was observed that the addition of mannanase to a low-energy diet reduced the viscosity of chyme in the jejunum of broilers and improved the efficiency of nutrient utilization in the feed.
Finally, growth performance is the most comprehensive indicator for evaluating the quality of commercial broilers. The energy level of the diet is a key factor affecting the growth performance of poultry, such as the conversion efficiency of feed, and plays a core role in poultry nutrition (Wen et al., 2017). The reduction in dietary energy level usually increases feed intake (Maharjan et al., 2021a, 2021b; Moraes et al., 2014; Wu et al., 2019) and decreases the BW (Zhao and Kim, 2017) and feed conversion efficiency of broilers (Cozannet et al., 2021; Maharjan et al., 2021a), which is similar to the results of this study. In addition, it has been proven that the addition of mannanase to diets can improve daily gain (Mohammadigheisar et al., 2021) and feed conversion efficiency (Lai et al., 2015), reduce FCR (Saleh et al., 2022), improve the efficiency of feed energy utilization of poultry (Ferreira et al., 2016a; Latham et al., 2018), improve growth performance, reduce production costs, and increase farming benefits. In this study, the addition of mannanase to a low-energy diet increased the BW and ADG of broilers and reduced FCR. In summary, the addition of mannanase to a low-energy diet could alleviate the decline in the growth performance of broilers caused by lower dietary energy levels. Specifically, mannanase could improve the growth performance by alleviating intestinal epithelial inflammation and improving the structure of the intestinal microbiota.
5. Conclusion
In conclusion, a low-energy diet supplemented with mannanase relieved the intestinal inflammatory response by downregulating the relative expression of NF-κB in the ileum of broilers. Mannanase could promote the proliferation of probiotic bacteria such as Lactobacillus, inhibit the colonization of harmful bacteria such as C. perfringens in the intestine, promote the energy metabolism and N-glycan synthesis of microorganisms, reduce damage to the intestinal barrier, increase the production of SCFA, promote nutrient absorption, and ultimately improve the growth performance of broiler chickens. The results obtained in this study provide a scientific basis for understanding the role of mannanase in improving intestinal function and modulating immunity of broilers fed a low-energy diet of corn-soybean meal type.
Author contributions
Xiaodan Zhang: conceptualization and data curation; Huiping Xu: methodology; Lu Gong: formal analysis; Jiao Wang: software; Jianyang Fu: resources; Zengpeng Lv: project administration; Liangjuan Zhou: funding acquisition; Xuejun Li: funding acquisition; Qiong Liu: funding acquisition; Pingyu Xia: funding acquisition; Yuming Guo: writing-review & editing.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgment
This project was financially supported by the China Agriculture Research System Program (CARS-41-G11).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Al-Ghazzewi F.H., Tester R.F. Efficacy of cellulase and mannanase hydrolysates of konjac glucomannan to promote the growth of lactic acid bacteria. J Sci Food Agric. 2012;92(11):2394–2396. doi: 10.1002/jsfa.5678. [DOI] [PubMed] [Google Scholar]
- Arsenault R.J., Lee J.T., Latham R., Carter B., Kogut M.H. Changes in immune and metabolic gut response in broilers fed beta-mannanase in beta-mannan-containing diets. Poultry Sci. 2017;96(12):4307–4316. doi: 10.3382/ps/pex246. [DOI] [PubMed] [Google Scholar]
- Arsenault R.J., Lee J.T., Latham R., Carter B., Kogut M.H. Changes in immune and metabolic gut response in broilers fed beta-mannanase in beta-mannan-containing diets. Poultry Sci. 2017;96(12):4307–4316. doi: 10.3382/ps/pex246. [DOI] [PubMed] [Google Scholar]
- Assimakopoulos S.F., Mastronikolis S., DE Lastic A.L., Aretha D., Papageorgiou D., Chalkidi T., et al. Vol. 35. 2021. Intestinal barrier biomarker ZO1 and endotoxin are increased in blood of patients with COVID-19-associated pneumonia; pp. 2483–2488. (vivo). 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoola A.A., Malheiros R.D., Grimes J.L., Ferket P.R. Effect of dietary exogenous enzyme supplementation on enteric mucosal morphological development and adherent mucin thickness in turkeys. Front Vet Sci. 2015;2:45. doi: 10.3389/fvets.2015.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford A., Gong J. Implications of butyrate and its derivatives for gut health and animal production. Anim Nutr. 2018;4(2):151–159. doi: 10.1016/j.aninu.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaturiwala R., Bagban M., Singh T.A., Modi H.A. Partial purification and application of β-mannanase for the preparation of low molecular weight galacto and glucomannan. Biocatal Agric Biotechnol. 2021;36 [Google Scholar]
- Bradley J.R. TNF-mediated inflammatory disease. J Pathol. 2008;214(2):149–160. doi: 10.1002/path.2287. [DOI] [PubMed] [Google Scholar]
- Caldas J.V., Vignale K., Boonsinchai N., Wang J., Putsakum M., England J.A., et al. The effect of beta-mannanase on nutrient utilization and blood parameters in chicks fed diets containing soybean meal and guar gum. Poultry Sci. 2018;97(8):2807–2817. doi: 10.3382/ps/pey099. [DOI] [PubMed] [Google Scholar]
- Chen Y., Miao L., Yao Y., Wu W., Wu X., Gong C., et al. Dexmedetomidine ameliorate CLP-induced rat intestinal injury via inhibition of inflammation. Mediat Inflamm. 2015;2015 doi: 10.1155/2015/918361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- China National Standard . Determination of moisture in feedstuffs (BG/T 6435-2014) Standards Press of China; Beijing: 2014. [Google Scholar]
- China National Standard . Determination of crude protein in feeds-Kjeldahl method (GB/T 6432-2018) Standards Press of China; Beijing: 2018. [Google Scholar]
- Corwin E.J. Understanding cytokines. Part I: physiology and mechanism of action. Biol Res Nurs. 2000;2(1):30–40. doi: 10.1177/109980040000200104. [DOI] [PubMed] [Google Scholar]
- Cozannet P., Davin R., Jlali M., Jachacz J., Preynat A., Molist F. Dietary metabolizable energy, digestible lysine, available phosphorus levels and exogenous enzymes affect broiler chicken performance. Animal. 2021;15(5) doi: 10.1016/j.animal.2021.100206. [DOI] [PubMed] [Google Scholar]
- David A.S.C.P.K. Coproduction of protease and mannanase from Bacillus nealsonii PN-11 in solid state fermentation and their combined application as detergent additives. Int J Biol Macromol. 2018;108:1176–1184. doi: 10.1016/j.ijbiomac.2017.09.037. [DOI] [PubMed] [Google Scholar]
- Dawood A., Ma K. Applications of microbial beta-mannanases. Front Bioeng Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.598630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawood A., Ma K. Applications of microbial beta-mannanases. Front Bioeng Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.598630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan S., Kaur J. Microbial mannanases: an overview of production and applications. Crit Rev Biotechnol. 2007;27(4):197–216. doi: 10.1080/07388550701775919. [DOI] [PubMed] [Google Scholar]
- Fearon D.T., Locksley R.M. The instructive role of innate immunity in the acquired immune response. Science. 1996;272(5258):50–53. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- Ferreira H.J., Hannas M.I., Albino L.F., Rostagno H.S., Neme R., Faria B.D., et al. Effect of the addition of beta-mannanase on the performance, metabolizable energy, amino acid digestibility coefficients, and immune functions of broilers fed different nutritional levels. Poultry Sci. 2016;95(8):1848–1857. doi: 10.3382/ps/pew076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira H.J., Hannas M.I., Albino L.F., Rostagno H.S., Neme R., Faria B.D., et al. Effect of the addition of beta-mannanase on the performance, metabolizable energy, amino acid digestibility coefficients, and immune functions of broilers fed different nutritional levels. Poultry Sci. 2016;95(8):1848–1857. doi: 10.3382/ps/pew076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaucher M.L., Quessy S., Letellier A., Arsenault J., Boulianne M. Impact of a drug-free program on broiler chicken growth performances, gut health, Clostridium perfringens and Campylobacter jejuni occurrences at the farm level. Poultry Sci. 2015;94(8):1791–1801. doi: 10.3382/ps/pev142. [DOI] [PubMed] [Google Scholar]
- Gong J., Si W., Forster R.J., Huang R., Yu H., Yin Y., et al. 16S rRNA gene-based analysis of mucosa-associated bacterial community and phylogeny in the chicken gastrointestinal tracts: from crops to ceca. FEMS Microbiol Ecol. 2007;59(1):147–157. doi: 10.1111/j.1574-6941.2006.00193.x. [DOI] [PubMed] [Google Scholar]
- Gutierrez O., Zhang C., Caldwell D.J., Carey J.B., Cartwright A.L., Bailey C.A. Guar meal diets as an alternative approach to inducing molt and improving Salmonella enteritidis resistance in late-phase laying hens. Poultry Sci. 2008;87(3):536–540. doi: 10.3382/ps.2007-00337. [DOI] [PubMed] [Google Scholar]
- Hiippala K., Jouhten H., Ronkainen A., Hartikainen A., Kainulainen V., Jalanka J., et al. The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients. 2018;10(8):988. doi: 10.3390/nu10080988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hsiao H.Y., Anderson D.M., Dale N.M. Levels of beta-mannan in soybean meal. Poultry Sci. 2006;85(8):1430–1432. doi: 10.1093/ps/85.8.1430. [DOI] [PubMed] [Google Scholar]
- Ibuki M., Fukui K., Yamauchi K. Effect of dietary mannanase-hydrolysed copra meal on growth performance and intestinal histology in broiler chickens. J Anim Physiol Anim Nutr. 2014;98(4):636–642. doi: 10.1111/jpn.12105. [DOI] [PubMed] [Google Scholar]
- Jarry A., Bossard C., Bou-Hanna C., Masson D., Espaze E., Denis M.G., et al. Mucosal IL-10 and TGF-beta play crucial roles in preventing LPS-driven, IFN-gamma-mediated epithelial damage in human colon explants. J Clin Invest. 2008;118(3):1132–1142. doi: 10.1172/JCI32140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keestra A.M., van Putten J.P. Unique properties of the chicken TLR4/MD-2 complex: selective lipopolysaccharide activation of the MyD88-dependent pathway. J Immunol. 2008;181(6):4354–4362. doi: 10.4049/jimmunol.181.6.4354. [DOI] [PubMed] [Google Scholar]
- Keestra A.M., de Zoete M.R., Bouwman L.I., Vaezirad M.M., van Putten J.P. Unique features of chicken Toll-like receptors. Dev Comp Immunol. 2013;41(3):316–323. doi: 10.1016/j.dci.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Kim M.C., Kim J.H., Pitargue F.M., Koo D.Y., Choi H.S., Kil D.Y. Effect of dietary beta-mannanase on productive performance, egg quality, and utilization of dietary energy and nutrients in aged laying hens raised under hot climatic conditions. Asian-Australas J Anim Sci. 2017;30(10):1450–1455. doi: 10.5713/ajas.17.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H., Kang H.K., Moturi J., Ingale S.L., Kim J. Supplementation of enzyme cocktail in chickens diet is an effective approach to increase the utilization of nutrient in wheat-based diets. J Anim Sci Technol. 2021;63(1):69–76. doi: 10.5187/jast.2021.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogut M.H., Genovese K.J., Swaggerty C.L., He H., Broom L. Inflammatory phenotypes in the intestine of poultry: not all inflammation is created equal. Poultry Sci. 2018;97(7):2339–2346. doi: 10.3382/ps/pey087. [DOI] [PubMed] [Google Scholar]
- Koj A. Termination of acute-phase response: role of some cytokines and anti-inflammatory drugs. Gen Pharmacol. 1998;31(1):9–18. doi: 10.1016/s0306-3623(97)00435-7. [DOI] [PubMed] [Google Scholar]
- Kuo W.T., Odenwald M.A., Turner J.R., Zuo L. Tight junction proteins occludin and ZO-1 as regulators of epithelial proliferation and survival. Ann N Y Acad Sci. 2022;1514(1):21–33. doi: 10.1111/nyas.14798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai L.P., Lee M.T., Chen C.S., Yu B., Lee T.T. Effects of co-fermented Pleurotus eryngii stalk residues and soybean hulls by Aureobasidium pullulans on performance and intestinal morphology in broiler chickens. Poultry Sci. 2015;94(12):2959–2969. doi: 10.3382/ps/pev302. [DOI] [PubMed] [Google Scholar]
- Latham R.E., Williams M.P., Walters H.G., Carter B., Lee J.T. Efficacy of beta-mannanase on broiler growth performance and energy utilization in the presence of increasing dietary galactomannan. Poultry Sci. 2018;97(2):549–556. doi: 10.3382/ps/pex309. [DOI] [PubMed] [Google Scholar]
- Lee J.T., Bailey C.A., Cartwright A.L. beta-Mannanase ameliorates viscosity-associated depression of growth in broiler chickens fed guar germ and hull fractions. Poultry Sci. 2003;82(12):1925–1931. doi: 10.1093/ps/82.12.1925. [DOI] [PubMed] [Google Scholar]
- Li H.C., Fan X.J., Chen Y.F., Tu J.M., Pan L.Y., Chen T., et al. Early prediction of intestinal mucosal barrier function impairment by elevated serum procalcitonin in rats with severe acute pancreatitis. Pancreatology. 2016;16(2):211–217. doi: 10.1016/j.pan.2015.12.177. [DOI] [PubMed] [Google Scholar]
- Liu X.S.J.J.Y. Recombinant humanery thropoietin preconditioning on nuclear factor-kappa B (NF-κB) activation & proinflammatory cytokines inducted by myocardial ischaemia-reperfusion. Indian J Med Res. 2006;3(124):343–354. [PubMed] [Google Scholar]
- Maharjan P., Hilton K.M., Mullenix G., Weil J., Beitia A., Suesuttajit N., et al. Effects of dietary energy levels on performance and carcass yield of 2 meat-type broiler lines housed in hot and cool ambient temperatures. Poultry Sci. 2021;100(3) doi: 10.1016/j.psj.2020.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharjan P., Mullenix G., Hilton K., Weil J., Beitia A., Caldas J., et al. Effects of dietary energy levels on Pectoralis major mixed muscle protein turnover and body composition in two broiler lines housed in different grow-out environments. J Anim Physiol Anim Nutr. 2021;105(3):535–548. doi: 10.1111/jpn.13467. [DOI] [PubMed] [Google Scholar]
- McHardy I.H., Goudarzi M., Tong M., Ruegger P.M., Schwager E., Weger J.R., et al. Integrative analysis of the microbiome and metabolome of the human intestinal mucosal surface reveals exquisite inter-relationships. Microbiome. 2013;1(1):17. doi: 10.1186/2049-2618-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Miller L.S., Pietras E.M., Uricchio L.H., Hirano K., Rao S., Lin H., et al. Inflammasome-mediated production of IL-1beta is required for neutrophil recruitment against Staphylococcus aureus in vivo. J Immunol. 2007;179(10):6933–6942. doi: 10.4049/jimmunol.179.10.6933. [DOI] [PubMed] [Google Scholar]
- Ministry of Agriculture of the People’s Republic of China . China National Feeding Standard of Chicken (NY/T 33-2004) China Agriculture Press; Beijing, China: 2004. [Google Scholar]
- Mohammadigheisar M., Shouldice V.L., Balasubramanian B., Kim I.H. Effect of dietary supplementation of beta-mannanase on growth performance, carcass characteristics, excreta microflora, blood constituents, and nutrient ileal digestibility in broiler chickens. Anim Biosci. 2021;34(8):1342–1349. doi: 10.5713/ab.20.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes T.G., Pishnamazi A., Mba E.T., Wenger, Renema R.A., Zuidhof M.J. Effect of maternal dietary energy and protein on live performance and yield dynamics of broiler progeny from young breeders. Poultry Sci. 2014;93(11):2818–2826. doi: 10.3382/ps.2014-03928. [DOI] [PubMed] [Google Scholar]
- Netea M.G., van der Graaf C., Van der Meer J.W., Kullberg B.J. Toll-like receptors and the host defense against microbial pathogens: bringing specificity to the innate-immune system. J Leukoc Biol. 2004;75(5):749–755. doi: 10.1189/jlb.1103543. [DOI] [PubMed] [Google Scholar]
- Oakley B.B., Lillehoj H.S., Kogut M.H., Kim W.K., Maurer J.J., Pedroso A., et al. The chicken gastrointestinal microbiome. FEMS Microbiol Lett. 2014;360(2):100–112. doi: 10.1111/1574-6968.12608. [DOI] [PubMed] [Google Scholar]
- Opal S.M., DePalo V.A. Anti-inflammatory cytokines. Chest. 2000;117(4):1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- Roura JHKC E. Prevention of immunologic stress contributes to the growth-permitting ability of dietary antibiotics in chicks. J Nutr. 1992;122:2383–2390. doi: 10.1093/jn/122.12.2383. [DOI] [PubMed] [Google Scholar]
- Saleh A.A., Nahla A., Amber K., Badawi N., Aboelenin S.M., Alzawqari M.H., et al. Effect of dietary incorporation of peanut and linseed meals with or without enzyme mixture on physiological performance of broilers. Saudi J Biol Sci. 2022;29(6) doi: 10.1016/j.sjbs.2022.103291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S., Zhao J., Dai Y., Chen F., Zhang Z., Yu J., et al. Methamphetamine-induced alterations in intestinal mucosal barrier function occur via the microRNA-181c/TNF-alpha/tight junction axis. Toxicol Lett. 2020;321:73–82. doi: 10.1016/j.toxlet.2019.12.020. [DOI] [PubMed] [Google Scholar]
- Van Itallie C.M., Anderson J.M. Occludin confers adhesiveness when expressed in fibroblasts. J Cell Sci. 1997;110(9):1113–1121. doi: 10.1242/jcs.110.9.1113. [DOI] [PubMed] [Google Scholar]
- Vangroenweghe F.P.K.T.O. Supplementation of a β-mannanase enzyme reduces post-weaning diarrhea and antibiotic use in piglets on an alternative diet with additional soybean. Porcine Health Manag. 2021;7(1):8. doi: 10.1186/s40813-021-00191-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.L., Xu S.Y., Ren Z.G., Tao L., Jiang J.W., Zheng S.S. Application of metagenomics in the human gut microbiome. World J Gastroenterol. 2015;21(3):803–814. doi: 10.3748/wjg.v21.i3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z.G., Rasolofomanana T.J., Tang J., Jiang Y., Xie M., Yang P.L., et al. Effects of dietary energy and lysine levels on growth performance and carcass yields of Pekin ducks from hatch to 21 days of age. Poultry Sci. 2017;96(9):3361–3366. doi: 10.3382/ps/pex122. [DOI] [PubMed] [Google Scholar]
- Williams BBSR M.P. Evaluation of beta-mannanase and nonstarch polysaccharide-degrading enzyme inclusion separately or intermittently in reduced energy diets fed to male broilers on performance parameters and carcass yield. J Appl Poultry Res. 2014;4(23):715–723. [Google Scholar]
- Wu G., Bryant M.M., Voitle R.A., Roland D.S. Effects of beta-mannanase in corn-soy diets on commercial leghorns in second-cycle hens. Poultry Sci. 2005;84(6):894–897. doi: 10.1093/ps/84.6.894. [DOI] [PubMed] [Google Scholar]
- Wu Y.B., Tang J., Xie M., Zhao R., Huang W., Zhang Q., et al. Effects of dietary energy and methionine on growth performance and carcass traits of growing Pekin ducks from 15 to 42 days of age. Poultry Sci. 2019;98(11):5870–5875. doi: 10.3382/ps/pez332. [DOI] [PubMed] [Google Scholar]
- Zhang B., Li G., Shahid M.S., Gan L., Fan H., Lv Z., et al. Dietary l-arginine supplementation ameliorates inflammatory response and alters gut microbiota composition in broiler chickens infected with Salmonella enterica serovar Typhimurium. Poultry Sci. 2020;99(4):1862–1874. doi: 10.1016/j.psj.2019.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P.Y., Kim I.H. Effect of diets with different energy and lysophospholipids levels on performance, nutrient metabolism, and body composition in broilers. Poultry Sci. 2017;96(5):1341–1347. doi: 10.3382/ps/pew469. [DOI] [PubMed] [Google Scholar]
- Zheng L., Cho S.H., Kang C.W., Lee K.-W., Kim K.E., An B.K. Effects of β-mannanase on egg production performance, egg quality, intestinal microbiota, viscosity, and ammonia concentration in laying hens. Rev Bras Ciência Avícola. 2020;22(1) [Google Scholar]
- Zhou Z., Zhang B., Yang X., Shang W., Ma Q., Strappe P. Regulation of hyperglycemia in diabetic mice by autolysates from beta-mannanase-treated brewer's yeast. J Sci Food Agric. 2019;99(15):6981–6988. doi: 10.1002/jsfa.9987. [DOI] [PubMed] [Google Scholar]
- Zhuang Y., Wu H., Wang X., He J., He S., Yin Y. Resveratrol attenuates oxidative stress-induced intestinal barrier injury through PI3K/Akt-Mediated Nrf 2 signaling pathway. Oxid Med Cell Longev. 2019;2019 doi: 10.1155/2019/7591840. [DOI] [PMC free article] [PubMed] [Google Scholar]