Abstract
Objective
This study is designed to investigate the mode of action of the synergistic effect of 5-fluorouracil (5-FU) and magnolol against cervical cancer.
Methods
Network pharmacological approach was applied to predict the molecular mechanism of 5-FU combined with magnolol against cervical cancer. CCK-8 assay, colony formation assay, immunofluorescence staining, adhesion assay, wound healing mobility assay, cell migration and invasion assay and Western blot analysis were conducted to validate the results of in silico study.
Results
Phosphatidylinositol 3 kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) signaling pathway was identified as the key pathway in silico study. The experimental results showed that 5-FU combined with magnolol strongly inhibited cervical cancer cell proliferation, induced the morphological change of HeLa cells by down-regulating the expression of α-actinin, tensin-2 and vinculin. Moreover, magnolol enhanced inhibitory effect of 5-FU on the cell adhesion, migration and invasion. The phosphorylation of AKT and PI3K and the expression of mTOR were strongly inhibited by the combination of 5-FU and magnolol. Moreover, the expression of E-cadherin and β-catenin was upregulated and the expression of Snail, Slug and vimentin was down-regulated by the 5-FU together with magnolol.
Conclusion
Taken together, this study suggests that 5-FU combined with magnolol exerts a synergistic anti-cervical cancer effect by regulating the PI3K/AKT/mTOR and epithelial-mesenchymal transition (EMT) signaling pathways.
Keywords: cervical cancer, 5-fluorouracil, EMT pathway, magnolol, network pharmacology, PI3K/AKT/mTOR pathway
1. Introduction
Cervical cancer is the fourth most common malignant tumor in women, which is the main cause of female death (Revathidevi et al., 2021). Although the incidence and mortality of cervical cancer have been significantly decreased with the popularity of human papilloma virus (HPV) vaccine and cancer screening, patients with advanced cervical cancer and the metastasis of cervical cancer after operation and chemotherapy are still suffering due to lack of effective treatment (Olusola et al., 2019). Moreover, up to 26% of early stage patients experience relapse after primary surgery (Cibula et al., 2021). Therefore, it is urgent to find a new treatment strategy with low toxicity and high efficiency for cancer treatment.
5-Fluorouracil (5-FU) is an anti-metabolic antitumor drug, which can inhibit thymine nucleotide synthetase and interfere with DNA synthesis (Taflin et al., 2021). It exerts significant antitumor effect in a variety of tumors (Akalovich et al., 2021, Proietti et al., 2021). However, the clinical application of 5-FU is facing serious challenges due to the increased drug resistance and the high incidence of adverse reactions (Vodenkova et al., 2020). 5-FU resistance in cancer treatment is regulated by a variety of signaling pathways, such as Wnt/β-catenin signaling pathway, PI3K/AKT signaling pathway and epithelial-mesenchymal transition (EMT) (Hou et al., 2020, Liang et al., 2020, Sun et al., 2021). Therefore, targeting those signaling pathways will be a promising direction for relieving drug resistance. Application of traditional Chinese medicine is known to enhance the effect of 5-FU based chemotherapy and reduces adverse reactions for colorectal cancer (Chen et al., 2019, Chen et al., 2019). For example, honokiol, one of active components of the bark of Magnolia officinalis Rehd. et Wils. (Houpo in Chinese), has a potential synergistic effect with 5-FU in human urothelial cell carcinoma cells (Lee et al., 2019).
Magnolol is an anti-inflammatory, antibacterial and anti-tumor compound extracted from M. officinalis. Magnolol could regulate cell proliferation, activate inflammatory response and affect tumor migration and invasion by regulating a variety of biological signaling pathways, such as nuclear factor-κB/mitogen-activated protein kinase and PI3K/AKT/mTOR signaling pathways (Woo et al., 2020, Zhang et al., 2019, Zhang et al., 2019). However, the synergistic effect of 5-FU and magnolol against cervical cancer has not been reported.
Network pharmacology is an emerging discipline based on the theory of systems biology. By analyzing the interaction between drugs and diseases from multiple components, multiple targets and multiple pathways, it could reveal the synergistic effect and mechanisms of drugs (Zhou et al., 2020). Network pharmacology is considered as a new trend to understand traditional Chinese medicine.
Cell adhesion plays an important role in the process of cell migration, which is the basis for pathological processes such as immune response and tumor metastasis (Laubli & Borsig, 2019). Cancer cells can promote the formation of protuberance structure to increase the ability of migration and invasion by remodeling cytoskeleton and contracting actin (Dart & Gordon-Weeks, 2017). So cytoskeletal related proteins such as α-actinin, vinculin and tensin-2 are closely related to tumor progression (Dirican and Akkiprik, 2017, Yoshii et al., 2013, Zhang et al., 2019, Zhang et al., 2019). PI3K/AKT/mTOR signaling pathway is proved to be involved in the process of cell adhesion, migration and invasion (Miricescu et al., 2020, Si et al., 2020). It can regulate the expression of hypoxia-inducible factor 1, inhibit the activity of proapoptotic members and activate antiapoptotic factors through phosphorylation, thereby accelerating tumor angiogenesis, inhibiting apoptosis and promoting tumor cell proliferation (Yu et al., 2018, Zhu et al., 2020). Moreover, 5-FU resistance can be reversed by inhibiting PI3K/AKT signaling pathway (Lan et al., 2021). Therefore, regulating PI3K/AKT/mTOR signaling pathway to inhibit tumor cell migration and invasion and reduce 5-FU resistance of cervical cancer cell will be an effective strategy for cancer treatment.
Epithelial-mesenchymal transition (EMT) refers to the transformation of epithelial cells to mesenchymal cells, which is closely related to the migration and invasion of tumors (Yang et al., 2021). EMT is closely related to 5-FU resistance (Xie et al., 2021). E-cadherin, which is a major factor in the progression of cancer, is crucial in the occurrence of EMT. And the lack of E-cadherin is a key step in EMT (Wong et al., 2018). Snail and Slug are inducible genes of EMT and the inhibitors of E-cadherin, they can down-regulate the expression level of E-cadherin by binding to the promoter of E-cadherin. Moreover, the decreased expression of epithelial markers such as E-cadherin and β-catenin and the increased expression of mesenchymal markers such as vimentin and N-cadherin are the main characteristics of EMT (Pastushenko & Blanpain, 2019).
Therefore, the aim of this study is to assess whether magnolol plays a synergistic role with 5-FU and further investigate the underlying synergic molecular mechanisms by using network pharmacological approach in silico and cell experiment in vitro. Our results suggested that magnolol significantly enhances the anticancer effect of 5-FU by regulation of PI3K/AKT/mTOR and EMT pathways.
2. Materials and methods
2.1. Materials
Dulbecco’s modified Eagle’s Medium (DMEM), Transwell nesting (with polycarbonate film 605 mm 8.0 μm), and Matrigel™ were purchased from Corning (NY, USA). Fetal bovine serum (FBS) was purchased from Tianhang (Huzhou, China). Dimethyl sulfoxide (DMSO) and 0.25% trypsin-EDTA (1X) were purchased from Sigma-Aldrich (Shanghai, China). The penicillin and streptomycin (100×) was purchased from Solarbio (Beijing, China). Magnolol and 5-FU were purchased from Selleck (Shanghai, China). Cell counting kit-8 (CCK-8), Phosphate buffer solution (PBS), Western transfer solution, SDS-PAGE electrophoresis solution and Crystal violet stain solution were purchased from Beyotime (Shanghai, China). iFluor 488-conjugated phalloidin was purchased from Abcam (Shanghai, China). First and second antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA).
2.2. Cell culture
Cervical cancer HeLa and SiHa cell lines were purchased from Shanghai Institutes for Biological Sciences of Chinese Academy of Sciences (SIBS, CAS). The cells were grown in DMEM with 10% FBS and 1% penicillin and streptomycin at 37 °C in a 5% CO2 air atmosphere.
2.3. Network pharmacology
The procedure of network pharmacology study was designed following these guidelines (Li, 2021) and previous literature (Guo et al., 2019, Zhang et al., 2015).
2.3.1. Disease targets
The disease targets of cervical cancer were searched in GeneCards database (https://www.genecards.org/) and CTD database (https://ctdbase.org/) with the keyword of “Cervical cancer”. Next, the obtained disease targets were merged by overlapping the two databases, and the final targets were the disease targets of cervical cancer.
2.3.2. Targets of 5-FU and magnolol
The compound targets of 5-FU and magnolol were searched in GeneCards database, CTD database and PubMed database (https://www.ncbi.nlm.nih.gov/pubmed/) respectively by using “5-Fluorouracil” and “Magnolol” as keywords. The obtained compound targets were merged and the final targets were considered to be the compound targets of 5-FU and magnolol.
2.3.3. Compound-disease targets
Venny2.1.0 software (http://bioinfogp.cnb.csic.es/tools/venny/) was used to draw the Venn diagram of the compound targets of 5-FU and magnolol and the disease targets of cervical cancer. The overlapped targets may be the potential targets of 5-FU combined with magnolol against cervical cancer.
2.3.4. PPI network construction
The potential targets of 5-FU and magnolol against cervical cancer obtained above were imported into the Protein-Protein Interaction NetworksFunctional Enrichment Analysis (STRING) platform (https://string-db.org) to construct the protein–protein interaction (PPI) network. The species was set as “Homo sapiens” and the criteria of confidence >0.9 was selected. Then the results were imported into Cytoscape3.7.0 and the size and color of nodes were set to reflect the degree of freedom. Maximal Clique Centrality (MCC) method was used to rank the top 10 key targets.
2.3.5. GO enrichment and KEGG pathway analysis
Database for Annotation, Visualization and Integrated Discovery (DAVID) database (https://www.david.ncifcrf.gov/) was used to conduct Gene Ontology (GO) biological process enrichment analysis on the screened targets. The biological process with significant difference was screened by setting the threshold P < 0.05 and false discovery rate (FDR) < 0.05. The biological process (BP), cellular component (CC) and molecular function (MF) of the targets were extracted to analyze the gene function. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of the top 20 pathways was carried out by using R package.
2.4. CCK-8 assay
HeLa/SiHa cells were seeded into a 96-well plate at a concentration of (1 × 105) cells/mL and incubated for 24 or 48 h with different concentrations of 5-FU alone or different compounds (5-FU, magnolol, 5-FU & magnolol). DMSO group was used as the control group. Subsequently, 10 μL of CCK-8 was added to each well. After incubation in a 37 °C, 5% CO2 incubator for 1 h, the optical density (OD) value at 450 nm was measured by a microplate plate reader (BioRad, Hercules, CA, USA). The experiment was repeated three times.
2.5. Colony formation assay
HeLa/SiHa cells were seeded into a 6-well plate at a concentration of (1 × 103) cells/mL and incubated with different compounds (5-FU, magnolol, 5-FU & magnolol). DMSO group was used as the control group. After 7 d, cells were fixed and then stained with crystal violet. The images were taken under an inverted microscope (Nikon, Japan).
2.6. Immunofluorescence staining
HeLa cells were seeded into a 24-well plate at a concentration of (5 × 104) cells/ mL and incubated for 24 h with different compounds (5-FU, magnolol, 5-FU & magnolol). DMSO group was used as the control group. Cells were fixed in 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. Next, the cells were incubated with iFluor 488-conjugated phalloidin (1:1 000) for 1 h. Subsequently, the nuclei were stained by adding 10 μL 4′,6-diamidino-2-phenylindole (DAPI) solution. Then the images were photographed by a laser confocal microscope (Leica, Wetzlar, Germany).
2.7. Adhesion assay
HeLa/SiHa cells were seeded into a 6-well plate at a concentration of (1 × 105) cells/mL and incubated for 24 h with different compounds (5-FU, magnolol, 5-FU & magnolol). DMSO group was used as the control group. Then the cells were harvested and re-seeded into a 24-well plate for 3 h. Next, the cells were fixed with 4% paraformaldehyde and stained with crystal violet. Photographs were taken under a microscope (Nikon, Japan).
2.8. Wound healing mobility assay
HeLa/SiHa cells were seeded into a 6-well plate at a concentration of (1 × 105) cells/mL and grown to approximately 90% confluence after 24 h. DMSO group was used as the control group. The medium was removed and three straight lines were drawn at the bottom of the cell plate to cause cell monolayer damage. Then the cells were washed with PBS to remove cellular debris. Next, the cells were cultured in serum-free medium containing different compounds (5-FU, magnolol, 5-FU & magnolol) for 24 h. The wound areas were photographed at 0 h, 8 h, and 24 h by a microscope (Nikon). The wound area was measured to calculate the percentage of migration distance relative to the control. Percent of control (%) = each dosing group area/control group area × 100%.
2.9. Cell migration and invasion assay
HeLa/SiHa cells in serum-free DMEM were seeded into the upper layer of Transwell® cell culture chamber for invasion assay, the chamber which was pre-coated with Matrigel™, and together incubated with different compounds (5-FU, magnolol, 5-FU & magnolol). DMSO group was used as the control group. DMEM containing 20% FBS was added to the lower chamber. After 24 h, the cells that migrated or invaded into the lower surface of the membrane were fixed and stained. At least five random fields per chamber were photographed in an inverted microscope (Nikon, Japan).
2.10. Western blot analysis
HeLa cells were incubated with different compounds (5-FU, magnolol, 5-FU & magnolol) for 24 h. DMSO group was used as the control group. Cells were lysed with lysate buffer on ice (cell lysates: protease inhibitor: phosphatase inhibitor A: phosphatase inhibitor B = 100:1:1:1) and then the proteins were extracted. Proteins were separated by gel electrophoresis on 10% Sodium dodecyl sulfate - polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to PVDF membrane by electroblotting. The membrane was probed with primary antibody (1:1 000) and secondary antibody (1:3 000) and then detected by Enhanced chemiluminescence (ECL) chemiluminescence (NCM Biotech, Suzhou, China).
2.11. Statistical analysis
The data were expressed as the mean ± standard deviation. Student's-t test was used to determine the statistical significance, and P < 0.05 was considered significant difference.
3. Results
3.1. Network pharmacological results
3.1.1. Integrating disease-compound targets
A total of 32 096 cervical cancer disease targets, 1633 5-FU compound targets and 85 magnolol compound targets were identified in GeneCards, CTD and PubMed databases, respectively. The disease targets and compound targets were imported into Venny2.1 to draw the Venn diagram. Finally, 66 potential therapeutic targets of 5-FU combined with magnolol against cervical cancer were obtained, such as tumor protein P53 (TP53), phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (PIK3CG), RAC-alpha serine/threonine-protein kinase (AKT1), signal transducer and activator of transcription 3 (STAT3), mitogen-activated protein kinase 1 (MAPK1), mitogen-activated protein kinase 3 (MAPK3), nuclear transcription factor 1 (NFjB1), nitric oxide synthase 2 (NOS2), etc. (Fig. 1).
Fig. 1.
Venn diagram of the targets of 5-FU combined with magnolol against cervical cancer. Three different color circles represent magnolol targets (purple), 5-FU targets (yellow) and cervical cancer targets (green), respectively. The overlapped 66 targets were the potential targets of 5-FU combined with magnolol against cervical cancer.
3.1.2. Constructing PPI network
The STRING database was used to analyze 66 potential targets online. The PPI network was constructed according to the condition of confidence >0.9 and then the results were visualized by Cytoscape3.7.0. As shown in Fig. 2A, the network consisted of a total of 64 nodes (remove two free nodes). The degrees of freedom of the middle circle, the right circle and the left circle increased gradually, which were 0–14, 15–19 and 20–52 respectively. The top 10 Hub targets were screened out by MCC algorithm: MAPK1, MAPK3, AKT1, STAT3, Proto-oncogene tyrosineprotein kinase Src (SRC), transcription factor p6 (RELA), Janus kinase 2 (JAK2), NFKB1, Myc proto-oncogene protein (MYC), and TP53 (Fig. 2B).
Fig. 2.
PPI network diagram (A, node represents target and size and color of nodes reflect degree value); Analysis of top 10 Hub targets of 5-FU combined with Magnolol in the treatment of cervical cancer ranked by MCC method (B, node size from big to small and the color from deep to shallow represent the decreasing importance).
3.1.3. GO enrichment analysis
In order to systematically understand the related physiological functions of 5-FU and magnolol combined targets, GO enrichment analysis was performed on 66 predicted potential targets of 5-FU combined with magnolol against cervical cancer by using DAVID database. The screening conditions were P < 0.05 and FDR < 0.05. The results showed the top 10 biological processes, cell components and molecular functions. Biological processes mainly included negative regulation of apoptosis, positive regulation of drug response and cell proliferation, etc. Cell components were mainly nucleus, nucleoplasm and cytoplasm, etc. Molecular functions involve enzyme binding, identical protein binding, and transcription factor binding, etc (Fig. 3).
Fig. 3.
GO enrichment analysis of anti-cervical cancer targets of 5-FU combined with magnolol.
3.1.4. KEGG pathway analysis
KEGG pathway analysis of 66 potential targets was performed by using DAVID 6.7 database. The results showed that 102 pathways were involved. The top 20 pathways were visualized by R language (Fig. 4). Top 20 pathways mainly include apoptosis, PI3K/AKT signaling pathway, tumor necrosis factor signaling pathway, forkhead box protein O (FoxO) signaling pathway and cancer signaling pathway, etc.
Fig. 4.
KEGG pathway analysis of 5-FU combined with magnolol against cervical cancer.
3.2. Combination of 5-FU and magnolol inhibits proliferation of cervical cancer cells
As shown in Fig. 5A, in HeLa cells, 25–100 μmol/L concentration of magnolol inhibited cell proliferation for 24–48 h treatment. Combined with the experimental results and related literatures (Su et al., 2020), 50 μmol/L of magnolol was selected for subsequent experiment. In HeLa cells, 5-FU (50 μmol/L) slightly influenced the cell proliferation at 24 h and 48 h (P > 0.05), magnolol (50 μmol/L) significantly inhibited cell proliferation at 24 h and 48 h (P < 0.01), while 5-FU combined with magnolol showed a stronger inhibitory effect on cell proliferation compared with 5-FU or magnolol alone (Fig. 5B). We further used SiHa cells to confirm the synergy effect. In SiHa cells, 5-FU showed inhibitory effect at 48 h (P < 0.01). Magnolol exerts the same behavior as in HeLa cells. The inhibitory effect of 5-FU combined with magnolol on SiHa cell proliferation at 24 h and 48 h was weaker than that of magnolol, but still stronger than that of 5-FU (Fig. 5C). In addition, 5-FU combined with magnolol can inhibit cervical cancer cell colony formation more significantly than 5-FU or magnolol alone (Fig. 5D–E). The results showed that 5-FU combined with magnolol exerts a stronger inhibitory effect on the proliferation of cervical cancer cells than 5-FU alone.
Fig. 5.
Effects of 5-FU combined with magnolol on the activity of cervical cancer cells. (A) CCK-8 assay of different concentrations of magnolol on HeLa cells. (B) CCK-8 assay of 5-FU combined with magnolol on HeLa cells. (C) CCK-8 assay of 5-FU combined with magnolol on SiHa cells. (D) The colony formation assay of 5-FU combined with magnolol on HeLa cells. (E) The colony formation assay of 5-FU combined with magnolol on SiHa cells. The data displayed is the average of three repeated experiments. *P < 0.05, **P < 0.01 vs DMSO control group. The scale bar is 200 μm.
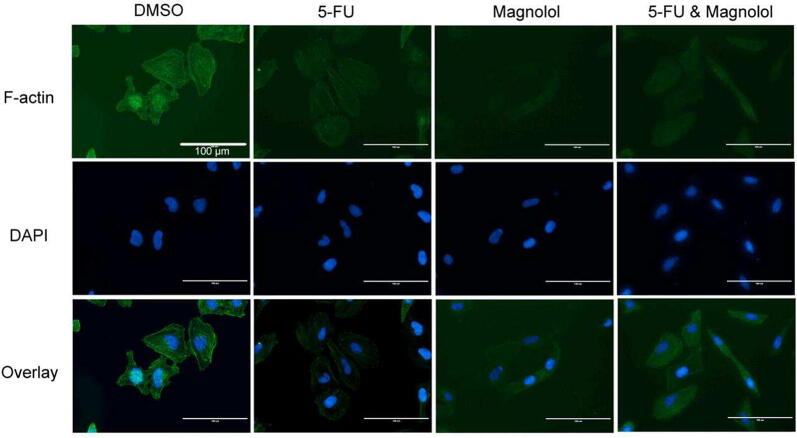
3.3. Combination of 5-FU and magnolol affects morphology and cytoskeleton of cervical cancer cells
As the morphology of cervical cancer cells was significantly changed after treatment with 5-FU (50 μmol/L) and magnolol (50 μmol/L) by using light microscope, immunofluorescence staining of cytoskeleton structure of F-actin was further performed to detect the effect of drug treatment. In the control group, the structures of F-actin filaments were bright and observable. 5-FU had no significant effect on cell morphology, magnolol and 5-FU combined with magnolol, respectively, induced the cell morphology to an enlonged and slender form, and disrupted the cell F-actin structure (Fig. 6). Therefore, 5-FU combined with magnolol exerted stronger effects on the cytoskeleton F-actin structure and the morphology of cervical cancer cells than 5-FU alone.
Fig. 6.
Effects of 5-FU combined with magnolol on morphology and F-actin cytoskeleton structure of cervical cancer cells.
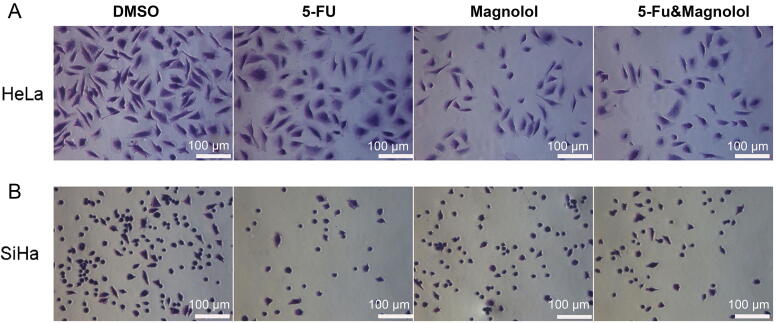
3.4. Combination of 5-FU and magnolol affects adhesion of cervical cancer cells
Cell adhesion is indispensable for tumor cell migration and invasion, so we observed the effect of 5-FU (50 μmol/L) combined with magnolol (50 μmol/L) on the adhesion of cervical cancer cells by adhesion assay. Our results showed that 5-FU combined with magnolol could inhibit the adhesion of both HeLa and SiHa cells. The inhibitory ability in HeLa cells was stronger than 5-FU alone, while the inhibitory effect in SiHa cells was weaker than 5-FU alone (Fig. 7), which suggested that SiHa cells were not so sensitive to the combination of 5-FU and magnolol.
Fig. 7.
5-FU combined with magnolol inhibited adhesion of HeLa (A) and SiHa cells (B) for 24 h.
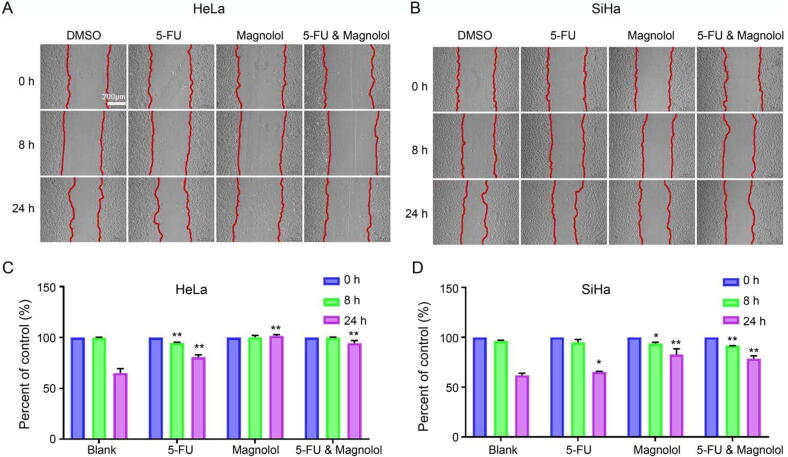
3.5. Combination of 5-FU and magnolol affects migration of cervical cancer cells
We further conducted the wound healing mobility assay to study whether 5-FU (50 μmol/L) combined with magnolol (50 μmol/L) can inhibit the migration of cervical cancer cells. As shown from Fig. 8, 5-FU promoted HeLa cell migration at 8 h compared with the control group (P < 0.01). However, after 24 h, 5-FU, magnolol and 5-FU & magnolol, respectively, significantly inhibited cell migration (P < 0.01). The inhibitory effect of 5-FU & magnolol group on migration was stronger than that of 5-FU group; In SiHa cells, 5-FU group had no significant effect on cell migration compared with the control group at 8 h (P > 0.05), but 5-FU, magnolol and 5-FU & magnolol, respectively, significantly inhibited cell migration at 24 h. Moreover, magnolol had strongest inhibitory effect. The results indicated that the combination of 5-FU and magnolol showed stronger inhibitory effect on the migration of cervical cancer cells than that of 5-FU alone.
Fig. 8.
5-FU combined with magnolol inhibited migration of HeLa and SiHa cells. The wound area was photographed at specified time (0, 8 and 24 h). Results of HeLa (A) and SiHa (B) cellswound healing mobility assay. The mobility of HeLa (C) and SiHa (D) cells at 0, 8 and 24 h (mean ± SD, n = 3). *P < 0.05, **P < 0.01 vs DMSO group.
3.6. Combination of 5-FU and magnolol affects migration and invasion of cervical cancer cells
We further measured the effect of 5-FU (50 μmol/L) combined with magnolol (50 μmol/L) on the migration and invasion of cervical cancer cells by cell migration assay and invasion assay. The results were consistent in HeLa and SiHa cells: After 24 h, the number of migrated cells in 5-FU, magnolol and 5-FU & magnolol significantly decreased compared with the control group, and the inhibitory effect of the combination of 5-FU and magnolol was significantly stronger than that of 5-FU alone (Fig. 9A and B), which was consistent with the wound healing mobility assay. Therefore, the combination of 5-FU and magnolol showed a stronger effect in inhibiting the migration of cervical cancer cells than 5-FU alone.
Fig. 9.
5-FU combined with magnolol inhibited migration and invasion of HeLa and SiHa cells in vitro. (A) HeLa cell migration assay. (B) SiHa cell migration assay. (C) HeLa cell invasion assay. (D) SiHa cell migration assay.
The effect of 5-FU (50 μmol/L) and magnolol (50 μmol/L) alone or in combination on the invasion of cervical cancer cells was detected in Transwell chamber coated with Matrigel gel. In HeLa cells, 5-FU and magnolol alone or in combination significantly reduced the number of invasive cells, and 5-FU combined with magnolol had the strongest inhibitory effect; In SiHa cells, 5-FU had no significant effect on cell invasion, magnolol and 5-FU combined with magnolol inhibited cell invasion (Fig. 9C and D). So the combination of 5-FU and magnolol exerted stronger inhibitory effect on the invasion of cervical cancer cells than that of 5-FU alone.
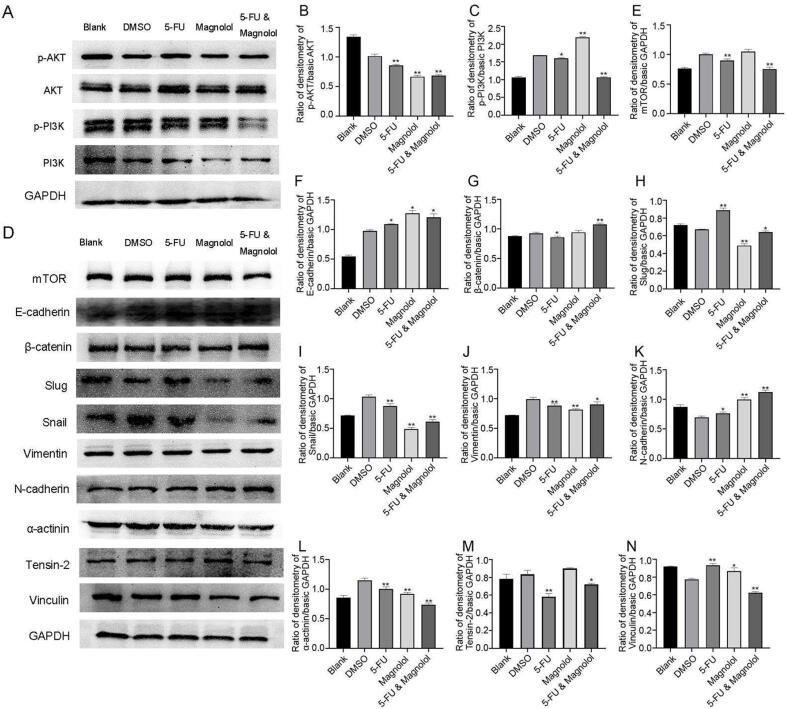
3.7. Combination of 5-FU and magnolol regulates PI3K/AKT/mTOR signaling and EMT pathway
Network pharmacological study showed that the combination use of 5-FU and magnolol may target the apoptosis, PI3K/AKT signaling pathway, tumor necrosis factor signaling pathway, FoxO signaling pathway and cancer signaling pathway. We found that 5-FU (50 μmol/L) combined with magnolol (50 μmol/L) can inhibit the cell viability, the migration and invasion of cervical cancer cells. PI3K/AKT/mTOR signaling pathway has been widely proved to regulate tumor migration and invasion to promote tumor progression (Marquard & Jucker, 2020). Therefore, we further investigated the effect of 5-FU combined with magnolol on the expression of PI3K/AKT signaling pathway-related proteins. The results showed that 5-FU alone could inhibit the phosphorylation of AKT and PI3K and the expression level of mTOR. Magnolol alone showed the strongest inhibitory effect on the phosphorylation of AKT, while it promoted PI3K phosphorylation and had no significant influence on the expression of mTOR. 5-FU combined with magnolol could strongly inhibit the phosphorylation of AKT and PI3K and the expression level of mTOR, and its synergistic effect was much stronger than that of 5-FU or magnolol alone (Fig. 10A–E). These results indicated that 5-FU combined with magnolol may affect cell migration and invasion by inhibiting PI3K/AKT/mTOR pathway, which well confirmed the predicted results in network pharmacological approach.
Fig. 10.
Effects of 5-FU combined with magnolol on PI3K/AKT/mTOR pathway and EMT related proteins in HeLa cells. (A) After blotting, the PVDF membranes were treated with p-AKT, AKT, p-PI3K, PI3K and GAPDH antibodies. (B − C) Comprehensive band intensity was determined by Image J software. (D) After blotting, the PVDF membranes were treated with EMT pathway antibodies and mTOR, α-actinin, tensin-2, vinculin and GAPDH antibodies. (E − N) Comprehensive band intensity was determined by Image J software. *P < 0.05, **P < 0.01 vs DMSO group.
EMT signaling pathway has been shown to regulate the proliferation, migration and invasion of tumor cells through multiple mechanisms in various types of tumors and involve in the 5-FU resistance (Kong et al., 2021, Xie et al., 2021). The results showed that 5-FU combined with magnolol inhibited the expression of Vimentin, Snail and Slug and up-regulated the expression of E-cadherin, β-catenin and N-cadherin after 24 h treatment (Fig. 10D and F–K). The results indicated that the combination of 5-FU and magnolol could inhibit cell migration and invasion by regulating EMT-related processes.
Since the combination of 5-FU and magnolol can change the cell morphology, we detected the expression levels of proteins related to cytoskeleton such as tensin-2, α-actinin and vinculin. The results showed that the combination of 5-FU and magnolol could down-regulate the expression of α-actinin, tensin-2 and vinculin. Moreover, the inhibitory effects on α-actinin and Vinculin were more significant than those of 5-FU alone (Fig. 10D and L–N).
4. Discussion
5-FU, a thymidylate synthase inhibitor, has a significant effect on the treatment of malignant tumor diseases. 5-FU can be transformed into active metabolite fluorouracil deoxyribonucleic acid in tumor cells to interfere with the function of normal nucleic acid and protein synthesis, thereby promoting tumor cell death (Sun et al., 2020). However, the drug resistance and cytotoxicity of 5-FU have greatly reduced its clinical efficacy (Blondy et al., 2020). Traditional Chinese medicine has been proved to reduce the drug resistance of 5-FU and improve the anti-tumor efficacy of 5-FU on colorectal cancer cells (Yin et al., 2019), so the combination of traditional Chinese medicine with chemotherapy would be a promising strategy. Magnolol is an effective component of M. officinalis. It exerts anticancer activity and can inhibit many kinds of cancers such as bladder cancer, lung cancer and esophageal cancer (Chen et al., 2019). But the synergistic effect of 5-FU and magnolol against cervical cancer has not been reported. Therefore, this study was carried out to systematically clarify whether the combination of 5-FU and magnolol can enhance the anti-cervical cancer effect of 5-FU and its effects on the proliferation, morphology, adhesion, migration and invasion of cervical cancer cells and 5-FU drug resistance.
We firstly screened 66 potential therapeutic targets of 5-FU combined with magnolol against cervical cancer by network pharmacology, including TP53, PIK3CG, AKT1, STAT3, MAPK1, MAPK3, NFKB1, NOS2, etc. Top 10 Hub targets mainly regulated by the combination of the two compounds were identified: MAPK1, MAPK3, AKT1, STAT3, SRC, RELA, JAK2, NFKB1, MYC, TP53. Furthermore, the key pathway PI3K/AKT signaling pathway was screened. These targets and pathways have been widely proved to play an important role in tumors and they are involved in 5-FU resistance and various physiological processes such as tumor proliferation, migration and invasion (Bi et al., 2021, Ma and Wang, 2021, Wei et al., 2021).
By the experimental validation, we observed that magnolol played a synergistic effect, which was helpful for 5-FU to strongly inhibit the proliferation and colony formation of cervical cancer cells. This observation result was consistent with the inhibitory effect of magnolol on tumor proliferation in other different types of tumors (Cheng et al., 2020).
Cell migration and invasion are accompanied by changes in cell morphology and cytoskeleton, such as the formation of filamentous or lamellar feet and the remodeling of actin filaments (Chiu et al., 2016). Therefore, to further understand the effect of 5-FU combined with magnolol on cell morphology and cytoskeleton, we observed the HeLa cells under the microscope and carried out immunofluorescence staining. The results showed that after treatment with 5-FU and magnolol, the fluorescence intensity of F-actin decreased and the morphology of cells became slender. Furthermore, the effect of 5-FU combined with magnolol on the cytoskeleton was more significant than that of 5-FU alone. Western blotting analysis also showed that the combination of 5-FU and magnolol could down-regulate the expression levels of α-actinin, tensin-2 and vinculin, thereby changing the cytoskeleton structure. These results proved that 5-FU combined with magnolol can change the cell morphology and reshape the cytoskeleton.
In order to clarify whether the change of cell morphology can affect the migration and invasion of tumor cells, we evaluated the effect of 5-FU combined with magnolol on cell adhesion. The results suggested that 5-FU and magnolol significantly reduced the number of adherent cells. Although the effect of 5-FU combined with magnolol in SiHa cells was not as obvious as that of 5-FU alone, it still significantly inhibited cell adhesion. Moreover, the inhibitory ability in HeLa cells was stronger than that of 5-FU alone. This may be related to the different sensitivity of HeLa and SiHa cells to 5-FU. Then we carried out wound healing mobility assay, cell migration and invasion assay to observe the effect of 5-FU combined with magnolol on the migration and invasion of cervical cancer cells. These results further proved the adhesion assay results, that is, the combined action of 5-FU and magnolol can significantly inhibit the migration and invasion of cervical cancer compared with 5-FU alone. The results indicated that the combination of 5-FU and magnolol can indeed affect the migration and invasion of cervical cancer by changing cell morphology and cytoskeleton. This result is consistent with the existing results, that is, targeting cytoskeleton can regulate tumor metastasis and proliferation (Ruggiero & Lalli, 2021).
PI3K/AKT/mTOR signaling pathway is highly expressed in various tumors (Li et al., 2021, Roudsari et al., 2021) and mediates various physiological processes such as cell growth, migration and invasion (Dong et al., 2021). In this study, 5-FU combined with magnolol was observed a stronger inhibitory effect on the phosphorylation of PI3K and AKT and down regulated the expression of mTOR than 5-FU alone. This result suggested 5-FU combined with magnolol can regulate PI3K/AKT/mTOR signaling pathway to inhibit the growth and metastasis of cervical cancer, which could be used as a chemotherapy adjuvant.
In addition, since PI3K/AKT/mTOR signaling pathway is closely related to the occurrence and development of EMT (Chen et al., 2021, Zhao et al., 2021), we also studied the effect of 5-FU combined with magnolol on the expression of EMT-related proteins. We found that the combination of 5-FU and magnolol down-regulated the expression of Snail, Slug and vimentin and promoted the high expression of E-cadherin and β-catenin. PI3K/AKT/mTOR signaling pathway can inhibit the transcription of heterogeneous nuclear ribonucleoprotein E1 by activating AKT, and then activate EMT (Wei et al., 2019). Therefore, when PI3K/AKT/mTOR signaling pathway is blocked, it will also have adverse effects on the occurrence of EMT, which is consistent with our experimental results. Furthermore, PI3K/AKT/mTOR signaling pathway and EMT are involved in 5-FU resistance (Escalante et al., 2021, Jin et al., 2021). Down-regulation of E-cadherin and up-regulation of N-cadherin was observed in 5-FU-resistant tumor cells (Harada et al., 2014). Our results showed that 5-FU combined with magnolol can not only down-regulate the expression level of PI3K/AKT/mTOR signaling pathway-related proteins, but also inhibit the occurrence of EMT to inhibit the proliferation, migration and invasion of cervical cancer cells and reverse 5-FU resistance.
5. Conclusion
In conclusion, the combination of 5-FU and magnolol plays a synergistic anti-cervical cancer effect by inhibiting cell proliferation, changing cell morphology and cytoskeletal structure, hindering cell adhesion, migration and invasion, down-regulating the expression of PI3K/AKT/mTOR signaling pathway-related proteins and reducing the occurrence of EMT. These results provide a strong evidence for the study of the combination use of traditional Chinese medicine and western medicine in cancer treatment, and lay a foundation for the development of new clinical treatment strategy of cervical cancer with high efficiency and low toxicity.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was supported by the Scientific Research Foundation of Jiangsu Province, China (No. BK20211327) and Jiangsu Province Traditional Chinese Medicine Science and Technology Project (No. 2020ZX21), Jiangsu Qinglian Project (2022), and Yangzhou University Top Talent Project (2020).
References
- Akalovich S., Portyanko A., Pundik A., Mezheyeuski A., Doroshenko T. 5-FU resistant colorectal cancer cells possess improved invasiveness and betaIII-tubulin expression. Experimental Oncology. 2021;43(2):111–117. doi: 10.32471/exp-oncology.2312-8852.vol-43-no-2.16314. [DOI] [PubMed] [Google Scholar]
- Bi X., Lv X., Liu D., Guo H., Yao G., Wang L.…Yang Y. METTL3-mediated maturation of miR-126-5p promotes ovarian cancer progression via PTEN-mediated PI3K/Akt/mTOR pathway. Cancer Gene Therapy. 2021;28(3–4):335–349. doi: 10.1038/s41417-020-00222-3. [DOI] [PubMed] [Google Scholar]
- Blondy S., David V., Verdier M., Mathonnet M., Perraud A., Christou N. 5-Fluorouracil resistance mechanisms in colorectal cancer: From classical pathways to promising processes. Cancer Science. 2020;111(9):3142–3154. doi: 10.1111/cas.14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Huang K., Ding X., Tang H., Xu Z. Magnolol inhibits growth and induces apoptosis in esophagus cancer KYSE-150 cell lines via the MAP kinase pathway. Journal of Thoracic Disease. 2019;11(7):3030–3038. doi: 10.21037/jtd.2019.07.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Ni W., Xie T., Sui X. Meta-analysis of 5-fluorouracil-based chemotherapy combined with traditional Chinese medicines for colorectal cancer treatment. Integrative Cancer Therapies. 2019;18 doi: 10.1177/1534735419828824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Tian F., Lun P., Feng Y. Curcumin Inhibits HGF-Induced EMT by Regulating c-MET-Dependent PI3K/Akt/mTOR Signaling Pathways in Meningioma. Evidence-based Complementary and Alternative Medicine. 2021;2021:5574555. doi: 10.1155/2021/5574555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G., Hardy M., Zielonka J., Weh K., Zielonka M., Boyle K.A.…Kalyanaraman B. Mitochondria-targeted magnolol inhibits OXPHOS, proliferation, and tumor growth via modulation of energetics and autophagy in melanoma cells. Cancer Treatment and Research Communications. 2020;25 doi: 10.1016/j.ctarc.2020.100210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu K.Y., Wu C.C., Chia C.H., Hsu S.L., Tzeng Y.M. Inhibition of growth, migration and invasion of human bladder cancer cells by antrocin, a sesquiterpene lactone isolated from Antrodia cinnamomea, and its molecular mechanisms. Cancer Letters. 2016;373(2):174–184. doi: 10.1016/j.canlet.2015.11.046. [DOI] [PubMed] [Google Scholar]
- Cibula D., Dostalek L., Jarkovsky J., Mom C.H., Lopez A., Falconer H.…Lonkhuijzen L.R.C.W.V. Post-recurrence survival in patients with cervical cancer. Gynecologic Oncology. 2021;164(2):362–369. doi: 10.1016/j.ygyno.2021.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart A.E., Gordon-Weeks P.R. The Role of Drebrin in Cancer Cell Invasion. Advances in Experimental Medicine and Biology. 2017;1006:375–389. doi: 10.1007/978-4-431-56550-5_23. [DOI] [PubMed] [Google Scholar]
- Dirican E., Akkiprik M. Phosphatidylinositol 3-kinase regulatory subunit 1 and phosphatase and tensin homolog as therapeutic targets in breast cancer. Tumour Biology. 2017;39(3):1393394135. doi: 10.1177/1010428317695529. [DOI] [PubMed] [Google Scholar]
- Dong C., Wu J., Chen Y., Nie J., Chen C. Activation of PI3K/AKT/mTOR pathway causes drug resistance in breast cancer. Frontiers in Pharmacology. 2021;12 doi: 10.3389/fphar.2021.628690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante P.I., Quinones L.A., Contreras H.R. Epithelial-mesenchymal transition and microRNAs in colorectal cancer chemoresistance to FOLFOX. Pharmaceutics. 2021;13(1):75. doi: 10.3390/pharmaceutics13010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y.C., Bao C., Ma D.C., Cao Y.B., Li Y.D., Xie Z., Li S. Network-Based Combinatorial CRISPR-Cas9 Screens identify synergistic modules in human cells. ACS Synthetic Biology. 2019;8(3):482–490. doi: 10.1021/acssynbio.8b00237. [DOI] [PubMed] [Google Scholar]
- Harada K., Ferdous T., Ueyama Y. Establishment of 5-fluorouracil-resistant oral squamous cell carcinoma cell lines with epithelial to mesenchymal transition changes. International Journal of Oncology. 2014;44(4):1302–1308. doi: 10.3892/ijo.2014.2270. [DOI] [PubMed] [Google Scholar]
- Hou G., Yuan X., Li Y., Hou G., Liu X. Cardamonin, a natural chalcone, reduces 5-fluorouracil resistance of gastric cancer cells through targeting Wnt/beta-catenin signal pathway. Investigational New Drugs. 2020;38(2):329–339. doi: 10.1007/s10637-019-00781-9. [DOI] [PubMed] [Google Scholar]
- Jin M., Kong L., Han Y., Zhang S. Gut microbiota enhances the chemosensitivity of hepatocellular carcinoma to 5-fluorouracil in vivo by increasing curcumin bioavailability. Phytotherapy Research. 2021;35(10):5823–5837. doi: 10.1002/ptr.7240. [DOI] [PubMed] [Google Scholar]
- Kong D., Zhou H., Neelakantan D., Hughes C.J., Hsu J.Y., Srinivasan R.R.…Ford H.L. VEGF-C mediates tumor growth and metastasis through promoting EMT-epithelial breast cancer cell crosstalk. Oncogene. 2021;40(5):964–979. doi: 10.1038/s41388-020-01539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan W., Zhao J., Chen W., Shang H., Peng J., Lin J. Anlotinib overcomes multiple drug resistant colorectal cancer cells via inactivating PI3K/AKT pathway. Anti-Cancer Agents in Medicinal Chemistry. 2021;21(15):1987–1995. doi: 10.2174/1871520621666210112113852. [DOI] [PubMed] [Google Scholar]
- Laubli H., Borsig L. Altered cell adhesion and glycosylation promote cancer immune suppression and metastasis. Frontiers in Immunology. 2019;10:2120. doi: 10.3389/fimmu.2019.02120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.Y., Shi C.S., Hsu Y.C., Huang K.J., Chen S.H., Zhao P.W.…Lee Y.R. Honokiol is a potential therapeutic agent and has a synergistic effect with 5-FU in human urothelial cell carcinoma cells. Anticancer Research. 2019;39(12):6555–6565. doi: 10.21873/anticanres.13871. [DOI] [PubMed] [Google Scholar]
- Li S. Network pharmacology evaluation method guidance-draft. World Journal of Traditional Chinese Medicine. 2021;7(1) doi: 10.19540/j.cnki.cjcmm.20210914.702. 146–154+165-166. [DOI] [PubMed] [Google Scholar]
- Li G., Zhang C., Liang W., Zhang Y., Shen Y., Tian X. Berberine regulates the Notch1/PTEN/PI3K/AKT/mTOR pathway and acts synergistically with 17-AAG and SAHA in SW480 colon cancer cells. Pharmaceutical Biology. 2021;59(1):21–30. doi: 10.1080/13880209.2020.1865407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L., Wu J., Luo J., Wang L., Chen Z.X., Han C.L.…Cai Z.W. Oxymatrine reverses 5-fluorouracil resistance by inhibition of colon cancer cell epithelial-mesenchymal transition and NF-kappaB signaling in vitro. Oncology Letters. 2020;19(1):519–526. doi: 10.3892/ol.2019.11090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Wang Y. JAK2/STAT3 inhibitor reduced 5-FU resistance and autophagy through ATF6-mediated ER stress. Journal of Receptors and Signal Transduction. 2021;42(2):206–213. doi: 10.1080/10799893.2021.1887219. [DOI] [PubMed] [Google Scholar]
- Marquard F.E., Jucker M. PI3K/AKT/mTOR signaling as a molecular target in head and neck cancer. Biochemical Pharmacology. 2020;172 doi: 10.1016/j.bcp.2019.113729. [DOI] [PubMed] [Google Scholar]
- Miricescu D., Totan A., Stanescu-Spinu I.I., Badoiu S.C., Stefani C., Greabu M. PI3K/AKT/mTOR signaling pathway in breast cancer: From molecular landscape to clinical aspects. International Journal of Molecular Sciences. 2020;22(1):173. doi: 10.3390/ijms22010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olusola P., Banerjee H.N., Philley J.V., Dasgupta S. Human papilloma virus-associated cervical cancer and health disparities. Cells. 2019;8(6):622. doi: 10.3390/cells8060622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastushenko I., Blanpain C. EMT Transition states during tumor progression and metastasis. Trends in Cell Biology. 2019;29(3):212–226. doi: 10.1016/j.tcb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- Proietti S., Cucina A., Catizone A., Ricci G., Pensotti A., Bizzarri M. Zebrafish embryo extracts enhance 5-FU anti-cancer effects upon breast cancer cells. European Review for Medical and Pharmacological Sciences. 2021;25(8):3235–3245. doi: 10.26355/eurrev_202104_25732. [DOI] [PubMed] [Google Scholar]
- Revathidevi S., Murugan A.K., Nakaoka H., Inoue I., Munirajan A.K. APOBEC: A molecular driver in cervical cancer pathogenesis. Cancer Letters. 2021;496:104–116. doi: 10.1016/j.canlet.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roudsari N.M., Lashgari N.A., Momtaz S., Abaft S., Jamali F., Safaiepour P.…Bishayee A. Inhibitors of the PI3K/Akt/mTOR pathway in prostate cancer chemoprevention and intervention. Pharmaceutics. 2021;13(8):1195. doi: 10.3390/pharmaceutics13081195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero C., Lalli E. Targeting the cytoskeleton against metastatic dissemination. Cancer and Metastasis Reviews. 2021;40(1):89–140. doi: 10.1007/s10555-020-09936-0. [DOI] [PubMed] [Google Scholar]
- Si X., Xu F., Xu F., Wei M., Ge Y., Chenge S. CADM1 inhibits ovarian cancer cell proliferation and migration by potentially regulating the PI3K/Akt/mTOR pathway. Biomedicine & Pharmacotherapy. 2020;123 doi: 10.1016/j.biopha.2019.109717. [DOI] [PubMed] [Google Scholar]
- Su C.M., Weng Y.S., Kuan L.Y., Chen J.H., Hsu F.T. Suppression of PKCδ/NF-κB signaling and apoptosis induction through extrinsic/intrinsic pathways are associated magnolol-inhibited tumor progression in colorectal cancer in vitro and in vivo. International Journal of Molecular Sciences. 2020;21(10):3527. doi: 10.3390/ijms21103527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Hou W., Liu X., Chai J., Guo H., Yu J. Targeting REV7 effectively reverses 5-FU and oxaliplatin resistance in colorectal cancer. Cancer Cell International. 2020;20(1):580. doi: 10.1186/s12935-020-01668-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L.T., Zhang L.Y., Shan F.Y., Shen M.H., Ruan S.M. Jiedu Sangen decoction inhibits chemoresistance to 5-fluorouracil of colorectal cancer cells by suppressing glycolysis via PI3K/AKT/HIF-1alpha signaling pathway. Chinese Journal of Natural Medicines. 2021;19(2):143–152. doi: 10.1016/S1875-5364(21)60015-8. [DOI] [PubMed] [Google Scholar]
- Taflin H., Odin E., Carlsson G., Tell R., Gustavsson B., Wettergren Y. Plasma deoxyuridine as a surrogate marker for toxicity and early clinical response in patients with metastatic colorectal cancer after 5-FU-based therapy in combination with arfolitixorin. Cancer Chemother Pharmacol. 2021;87(1):31–41. doi: 10.1007/s00280-020-04173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodenkova S., Buchler T., Cervena K., Veskrnova V., Vodicka P., Vymetalkova V. 5-Fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacology & Therapeutics. 2020;206 doi: 10.1016/j.pharmthera.2019.107447. [DOI] [PubMed] [Google Scholar]
- Wei J., Liu R., Hu X., Liang T., Zhou Z., Huang Z. MAPK signaling pathway-targeted marine compounds in cancer therapy. Journal of Cancer Research and Clinical Oncology. 2021;147(1):3–22. doi: 10.1007/s00432-020-03460-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei R., Xiao Y., Song Y., Yuan H., Luo J., Xu W. FAT4 regulates the EMT and autophagy in colorectal cancer cells in part via the PI3K-AKT signaling axis. Journal of Experimental & Clinical Cancer Research. 2019;38(1):112. doi: 10.1186/s13046-019-1043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S., Fang C.M., Chuah L.H., Leong C.O., Ngai S.C. E-cadherin: Its dysregulation in carcinogenesis and clinical implications. Critical Reviews in Oncology/Hematologyl. 2018;121:11–22. doi: 10.1016/j.critrevonc.2017.11.010. [DOI] [PubMed] [Google Scholar]
- Woo S.M., Min K.J., Kwon T.K. Magnolol Enhances the therapeutic effects of TRAIL through DR5 upregulation and downregulation of c-FLIP and Mcl-1 proteins in cancer cells. Molecules. 2020;25(19):4591. doi: 10.3390/molecules25194591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie P., Yuan F.Q., Huang M.S., Zhang W., Zhou H.H., Li X., Liu Z.Q. DCBLD2 affects the development of colorectal cancer via EMT and angiogenesis and modulates 5-FU drug resistance. Frontiers in Cell and Developmental Biology. 2021;9 doi: 10.3389/fcell.2021.669285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Liu Y., Wang Y., Wang X., Ci H., Song C., Wu S. Low PRRX1 expression and high ZEB1 expression are significantly correlated with epithelial-mesenchymal transition and tumor angiogenesis in non-small cell lung cancer. Medicine (Baltimore) 2021;100(4):e24472. doi: 10.1097/MD.0000000000024472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W., Zhong G., Fan H., Xia H. The effect of compound sophora on fluorouracil and oxaliplatin resistance in colorectal cancer cells. Evidence-based Complementary and Alternative Medicine. 2019;2019:7564232. doi: 10.1155/2019/7564232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii H., Ito K., Asano T., Horiguchi A., Hayakawa M., Asano T. Increased expression of alpha-actinin-4 is associated with unfavorable pathological features and invasiveness of bladder cancer. Oncology Reports. 2013;30(3):1073–1080. doi: 10.3892/or.2013.2577. [DOI] [PubMed] [Google Scholar]
- Yu Y., Wu X., Pu J., Luo P., Ma W., Wang J.…Fei Z. Lycium barbarum polysaccharide protects against oxygen glucose deprivation/reoxygenation-induced apoptosis and autophagic cell death via the PI3K/Akt/mTOR signaling pathway in primary cultured hippocampal neurons. Biochemical and Biophysical Research Communications. 2018;495(1):1187–1194. doi: 10.1016/j.bbrc.2017.11.165. [DOI] [PubMed] [Google Scholar]
- Zhang J., Chen Z., Huang X., Shi W., Zhang R., Chen M.…Wu L. Insights on the multifunctional activities of magnolol. Biomed Research International. 2019;2019:1847130. doi: 10.1155/2019/1847130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Liu P., Xu F., He Y., Xie X., Jiang X. Vinculin promotes gastric cancer proliferation and migration and predicts poor prognosis in patients with gastric cancer. Journal of Cellular Biochemistry. 2019;120(8):14107–14115. doi: 10.1002/jcb.28686. [DOI] [PubMed] [Google Scholar]
- Zhang B., Lu C., Bai M., He X.J., Tan Y., Bian Y.Q.…Li S. Tetramethylpyrazine identified by a network pharmacology approach ameliorates methotrexate-induced oxidative organ injury. Journal of Ethnopharmacology. 2015;175:638–647. doi: 10.1016/j.jep.2015.09.034. [DOI] [PubMed] [Google Scholar]
- Zhao Y.Y., Jia J., Zhang J.J., Xun Y.P., Xie S.J., Liang J.F.…Zhang S.R. Inhibition of histamine receptor H3 suppresses the growth and metastasis of human non-small cell lung cancer cells via inhibiting PI3K/Akt/mTOR and MEK/ERK signaling pathways and blocking EMT. Acta Pharmacologica Sinica. 2021;42(8):1288–1297. doi: 10.1038/s41401-020-00548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Chen B., Chen S., Lin M., Chen Y., Jin S.…Zhang Y. Applications of network pharmacology in traditional Chinese medicine research. Evidence-based Complementary and Alternative Medicine. 2020;2020 doi: 10.1155/2020/1646905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Liu X., Zhao P., Zhao H., Gao W., Wang L. Celastrol suppresses glioma vasculogenic mimicry formation and angiogenesis by blocking the PI3K/Akt/mTOR signaling pathway. Frontiers in Pharmacology. 2020;11:25. doi: 10.3389/fphar.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]