Abstract
By mimicking hemostatic structural domains of collagen, Streptococcus sanguis (aggregation-positive phenotype; Agg+) induces platelets to aggregate in vitro. To test the hypothesis that aggregation occurs in vivo, S. sanguis (Agg+ or Agg− suspension) was infused intravenously into rabbits. The extent of hemodynamic and cardiopulmonary changes and the fate of circulating platelets were Agg+ strain dose dependent. Within 45 to 50 s of the start of infusion, 40 × 108 CFU of the Agg+ strain caused increased blood pressure. Thirty seconds after infusion, other changes occurred. Intermittent electrocardiographic abnormalities (13 of 15 rabbits), ST-segment depression (10 of 15 rabbits), and preventricular contractions (7 of 15 rabbits) manifested at 3 to 7 min, with frequencies dose dependent. Respiratory rate and cardiac contractility increased during this phase. Blood catecholamine concentration, thrombocytopenia, accumulation of 111Indium-labeled platelets in the lungs, and ventricular axis deviation also showed dose dependency. Rabbits were unaffected by inoculation of an Agg− strain. Therefore, Agg+ S. sanguis induced platelet aggregation in vitro. Platelet clots caused hemodynamic changes, acute pulmonary hypertension, and cardiac abnormalities, including ischemia.
Bacteria are exogenous factors that may be viewed as thrombotic agonists when introduced into the circulation (37, 38, 53). Certain strains of Streptococcus sanguis, a microorganism most commonly found in human dental plaque, induce human platelets in plasma to aggregate in vitro (17, 23–25, 52). Platelet aggregation in vitro occurs after a lag time of 1.5 to 15 min depending on the platelet donor and the strain of S. sanguis (24, 25, 50). When induced by the strains we have studied, aggregation is dependent upon expression of the platelet-aggregation associated protein (PAAP) on the streptococcal cell surface (14, 16). This protein contains a collagen-like structural motif which is essentially indistinguishable from the known platelet-interactive domains on types I and III collagen (13, 15, 16). Collagen and S. sanguis induce in vitro platelet responses similarly (13).
Collagen also induces platelet aggregation in vivo. When collagen was infused into the circulation of rabbits, platelet clotting occurred causing coronary vasospasm, myocardial ischemia and sudden death (40). S. sanguis bacteremias frequently cause an often fatal, disseminated intravascular coagulation shock-like syndrome in immunocompromised patients (12, 43, 51, 54, 55). In the immunocompromised, S. sanguis apparently enters the circulation through ulcerated mucous membranes secondary to cytotoxic immunosuppressive therapy and associated neutropenia. S. sanguis bacteremias also occur through life in apparently healthy people (11). Consequently, people with prior heart valve disease are at risk for infective endocarditis associated most frequently with S. sanguis bacteremias (11). S. sanguis apparently causes thrombotic infection of damaged heart valves involving the accumulation of platelets and fibrin (2, 10, 26) and expression of tissue factor (3, 4, 7). Yet it is unclear whether S. sanguis induces platelet aggregation in vivo.
Given the ability to induce aggregation in vitro and perhaps cause coagulation-associated diseases, we hypothesized that a platelet aggregating strain of S. sanguis could induce platelet aggregation or clots in the circulation. To test this hypothesis, we first identified strains of S. sanguis that differed in their ability to induce rabbit platelets to aggregate in vitro (26). After intravenous infusion of an in vitro aggregation-inducing strain (Agg+), platelets were analyzed for signs of aggregation in vivo. Platelet clots would be expected to cause changes in hemodynamic and cardiopulmonary variables. Preliminary findings about some variables in vivo suggested that these expectations were reasonable (30, 31). Therefore, arterial blood pressure, heart and breathing rates, blood catecholamines, and electrocardiograms (ECGs) were monitored and analyzed with sufficient numbers of rabbits to permit comprehensive analysis of the dose-response relationships. To ensure that platelet aggregation caused the hemodynamic and cardiopulmonary changes, parallel control studies in rabbits were conducted with pyrogen-free saline and a strain of S. sanguis which did not cause rabbit platelets to aggregate in vitro (Agg−).
MATERIALS AND METHODS
Experimental design.
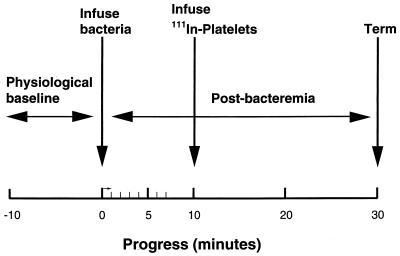
To test our hypothesis, apparently healthy New Zealand White rabbits were studied before and after t = 0, when viable streptococci (expressed in CFU) were infused for 1 min (Fig. 1). In each rabbit, a baseline set of hemodynamic and cardiovascular variables was obtained (t = −10 to 0 min), including heart rate, blood pressure, standard three leads of the ECG, respiratory rate, platelet count, and blood catecholamine level. The blood pressure, heart rate, and ECG were then monitored continuously from t = 0 to 7 and at 10, 20, and 30 min. Intermittently, blood was sampled (3 ml each sample) for platelet counts and catecholamine analysis at baseline, t = 7 to 8 (catecholamines only), and t = 30 min. At t = 10 min, 111Indium-labeled platelets were infused into the venous circulation of some rabbits. At t 30 min, the rabbits were euthanized.
FIG. 1.
Experimental protocol. To study the consequences of S. sanguis infusion in rabbits, physiological variables were measured or monitored for pre- (10 min) and postbacteremia (30 min) periods. Ten minutes after induction of the bacteremia, 111Indium-labeled donor platelets were infused to assess circulating platelets. Figure reproduced with modifications (31) with permission of the publisher.
Animal protocol.
All protocols, including euthanization of rabbits, were consistent with the Guiding Principles in Care and Use of Animals of the American Physiological Society and approved by the University of Minnesota Animal Care and Use Committee. Before entering the experimental protocol, rabbits were conditioned by daily gentle handling for 2 to 3 weeks.
Rabbits (diet, Purina Rabbit Chow; age, 5 to 6 months; weight, 3.2 to 4.2 kg; gender, males and females) were anesthetized with intramuscular (i.m.) xylazine hydrochloride (Rompum, 5 to 10 mg/kg of body weight and ketamine, 30 to 40 mg/kg). Supplements of ketamine or nembutal were given to maintain anesthesia, and xylocaine was injected intradermally to prevent discomfort during surgical procedures. The femoral vein was cannulated for the infusion of streptococci, 111Indium-labeled donor platelets, and the euthanizing agent. Arterial blood pressure was recorded through the carotid artery cannula. Viable S. sanguis cells suspended in sterile, pyrogen-free saline, or pyrogen-free saline alone were infused. Rabbits were euthanized by lethal intravenous injection of nembutal, and the lungs were removed.
Bacterial strains.
Two representative strains of S. sanguis from our culture collection were selected for study and were described in earlier reports (25, 26). Strain 133-79 was originally isolated from blood culture of a confirmed case of subacute bacterial endocarditis and was obtained from R. Facklam, Centers for Disease Control and Prevention, Atlanta, Ga. This was a biotype I strain according to the phenotyping scheme of R. R. Facklam as described previously (24, 25) and fails to bind amylase (20). Strain L50 was a biotype I isolate from human dental plaque provided by William F. Liljemark, School of Dentistry, University of Minnesota, Minneapolis. The original isolates were purified, aliquoted, and stored at −20°C in skim milk. Aliquots of pure cultures were thawed only once and used to inoculate Todd-Hewitt broth for the experiments on a given day. Subculturing was minimized by preparation and storage of large numbers of aliquots at one time. Periodically the platelet aggregation phenotype of stored aliquots was confirmed by in vitro aggregometry experiments. Strain 133-79 (Agg+) induced aggregation of human plasma (PRP) within 3 to 15 min in the platelet aggregometer (24, 25, 50). Strain L50 (Agg−) elicited no measurable response of platelets by in vitro aggregometry (24, 25).
Liquid cultures were grown 18 h at 37°C in 10% CO2, harvested by centrifugation and washed five times in 0.9% (wt/vol) nonpyrogenic saline. To disperse clumps and chains of streptococci without affecting viability, cells were sonicated for 8 s at 4°C by using 50 W output from a microprobe sonifier. After sonication, microscopic examination of both strains showed primarily single cells and relatively few small chains generally not exceeding four cells in length. Biomass was estimated spectrophotometrically (24) based upon a standard curve of dilutions of 4 × 109 cells of strain 133-79/ml as determined by counting in a Petroff-Hausser chamber. The Petroff-Hausser counts in cells per milliliter varied with counts of S. sanguis CFU by a factor of 10−1, reflecting viability and differences in counting sensitivity and accuracy of the techniques. Given the limitations of the techniques, both strains were infused as equivalent numbers of streptococcal cells expressed as CFU.
Platelet aggregometry.
Platelet aggregation in vitro models the annular vortex of flowing blood and physiological temperature by study in the aggregometer (24). Strains 133-79 and L50 were harvested, washed, sonicated briefly, and resuspended to 2 × 109 cells per ml as previously reported (24, 25). Platelet-rich plasma was isolated from the blood of donor rabbits as previously reported for human samples, adjusted to 4 × 108 per ml with rabbit platelet-poor plasma, warmed to 37°C, and challenged with an equal number of S. sanguis cells in the aggregometer.
Platelets: labeling and in vivo analysis.
Blood (27 to 36 ml in acid citrate-dextrose) was obtained from donor rabbits (sedated with ketamine and Rompum or nembutal) by sterile cannulation of deep branch of the femoral artery. To prepare 111Indium-labeled platelets, platelet-rich plasma was isolated and washed platelets were prepared and then incubated with indium-111-oxine (Amersham, Arlington Heights, Ill.) as described previously (26). The total radioactivity infused, A (in counts/minute), was determined from aliquots of the injectate as described previously (26, 46). More than 95% of radioactivity in the blood was determined to be platelet associated. The lungs were harvested, and samples were placed in preweighed vials and reweighed. The uptake of radioactivity, Ai, in the organ samples was counted with a Packard Gamma Counter, and the fractional uptake per gram was calculated as FU/gram = [Ai/mass of organ]/A, as described previously (26, 46).
Heart rate, blood pressure, and ECG.
Heart rate, blood pressure, and ECG were recorded on a two-channel strip chart recorder and on analogue tape. Data on tape was transferred directly to a computer for subsequent evaluation. To facilitate analysis, data was reviewed using an expanded scale and rerecorded on the strip-chart recorder. The first derivative of the blood pressure record was determined with custom software. Differences in ECG and electrical axis were determined from both the strip chart recording and the computer display at selected time periods. ECG abnormalities were identified and verified by comparison to reference tracings. The deviation in electrical axis was determined from the vector geometry of the QRS complexes from the standard limb leads.
dP/dt.
A continuous computer-generated display of arterial pulses and associated first derivative time curves were used to assess changes in cardiac contractility. A custom computer program determined the maximum change in pressure with respect to time (dP/dt) of the arterial pulse. The change in maximum dP/dt was calculated at baseline and postbacteremia times. Following a perturbation by intravenous nembutal in preliminary experiments (data not shown), the percentage change in the maximum ventricular and carotid pulse dP/dt were found to be directly related. Consequently, change in left ventricular contractility was indexed by determination of the maximum dP/dt in the carotid arterial pulse as also validated by others (1, 33).
Respiratory rate.
The respiratory rate was measured by two methods. The respiratory fluctuations on the blood pressure recording were noted and counted. A fiberoptic probe detected movements of the nose, which were recorded on a strip-chart recorder. The two rates generally coincided, but the fiberoptic recording was given preference.
Catecholamine determination.
Catecholamine concentrations (norepinephrine and epinephrine combined) in arterial blood were determined in triplicate by radioenzymatic assay (CAT-A-KIT; Amersham International). Replicate determinations showed a coefficient of variation of up to 30%. Dose dependency was evaluated by the frequency of rabbits showing change between baseline and t = 7 to 8 min. The dose-response relationship in those rabbits with known change in [CAT] was determined as a function of the frequency of the change in dP/dt (Fisher exact test, two-tailed tests).
Platelet counts.
Platelet counts were determined with a hemocytometer by standard methods. Blood was taken for platelet counts at baseline, t = 7 to 8 min and at t = 30 min. At least two samples were counted at each bleed.
Calculations, data analysis, and statistics.
To estimate heart work, the average carotid blood pressure multiplied by the heart rate was calculated as described earlier (26). The heart rate-blood pressure product is proportional to the pressure-volume work of the left ventricle or an estimate of the hemodynamic status of the rabbit.
Differences between doses, and Agg+ and Agg− phenotypes and sham saline groups were ascertained. Percentage change (magnitude) and frequency of change were calculated to facilitate evaluation of dose dependency. The frequency of occurrence was evaluated by linear regression analysis and Fisher exact test (two-tailed), while percentage change (magnitude) was evaluated only by linear regression analysis. Differences in magnitude between baseline and postbacteremia values were evaluated by the Student t test.
RESULTS
Platelet aggregation in vitro.
In response to strain 133-79, the mean lag time to onset of rabbit platelet aggregation in vitro was 2.0 min (range, 1.8 to 2.5 min; n = 6 rabbits). Strain L50 failed to induce aggregation of platelets from any rabbit tested.
Hemodynamic consequences.
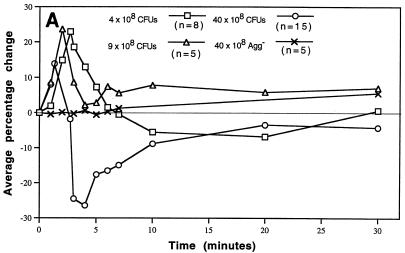
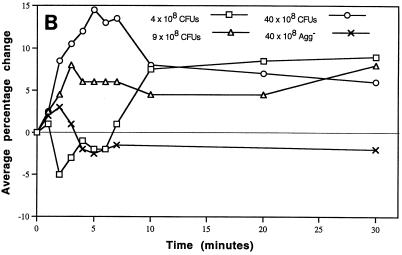
Infusion of doses ranging from 4 × 108 to 40 × 108 CFU of the Agg+ strain altered blood pressure and heart rate primarily during the first 7 min, which changed the index of cardiac work (Fig. 2A to C). Initial preliminary studies in rabbits suggested that hemodynamic changes would occur as a function of dose (30, 31). Compared to the Agg+ strain, infusion of 40 × 108 CFU of the Agg− strain (or physiological saline vehicle) caused virtually no change in any measured parameter and the blood pressure and heart rate remained at baseline values (Fig. 2A and B). The comparatively low Agg+ dose of 0.1 × 108 CFU was infused into two rabbits. Hemodynamics did not change from baseline after infusion. In contrast, three rabbits infused with 100 × 108 CFU of the Agg+ strain became hypotensive within 40 s and the blood pressure approached zero within 7 min. During the first 7 min after infusion of 4 or 9 × 108 CFU of the Agg+ strain, the blood pressure response was hypertensive (Fig. 2A). The time of maximal increase in blood pressure was inversely related to dose (r = 0.78; slope different from 0, P < 0.001 by linear regression analysis). Similarly the average maximal percentage increase in blood pressure decreased with increasing doses of the Agg+ strain (r = 0.52; slope different from 0, P = 0.004). After infusion of 40 × 108 CFU (n = 15 rabbits), the blood pressure response was predominantly diphasic, first hyper- and then suddenly hypotensive. During the hypotensive phase, the heart rate was markedly elevated (Fig. 2B).
FIG. 2.
Hemodynamic consequences of S. sanguis bacteremia. The hemodynamic status over time was monitored. Blood pressure and heart rate were normalized as percentage changes over time as a function of dose of S. sanguis. The heart rate × blood pressure product (an index of cardiac energy and hemodynamic status) was normalized and plotted. The number of rabbits (n) used to establish the mean for each dose is indicated in panel A. For each dose, significant differences from the baseline were determined by the Student t test. (A) Arterial blood pressure. Average percentage change with the Agg+ strain was plotted for each minute of the first 7 min, and at 10, 20, and 30 min. The time and value of the maximal increase was plotted for each dose. Changes ≥± 8% were significantly different from baseline or zero. All values calculated for the Agg− strain were not different from zero. (B) Heart rate. Average percentage change was plotted at the same minute intervals as blood pressure. Changes ≥± 4 to 5% were significantly different from zero. The Agg− strain, even at 40 × 108 CFU, did not alter the heart rate during the period of bacteremia. (C) Heart rate × blood pressure. The average percentage change was plotted over time as above. Changes ≥± 8% were significantly different from zero. Panels A and B have been reproduced with modifications (30) with permission of the publisher.
Cardiopulmonary consequences.
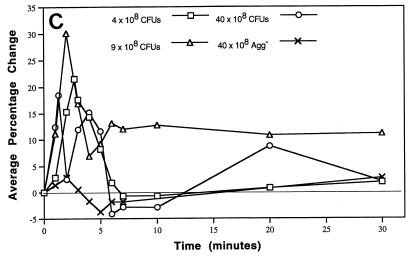
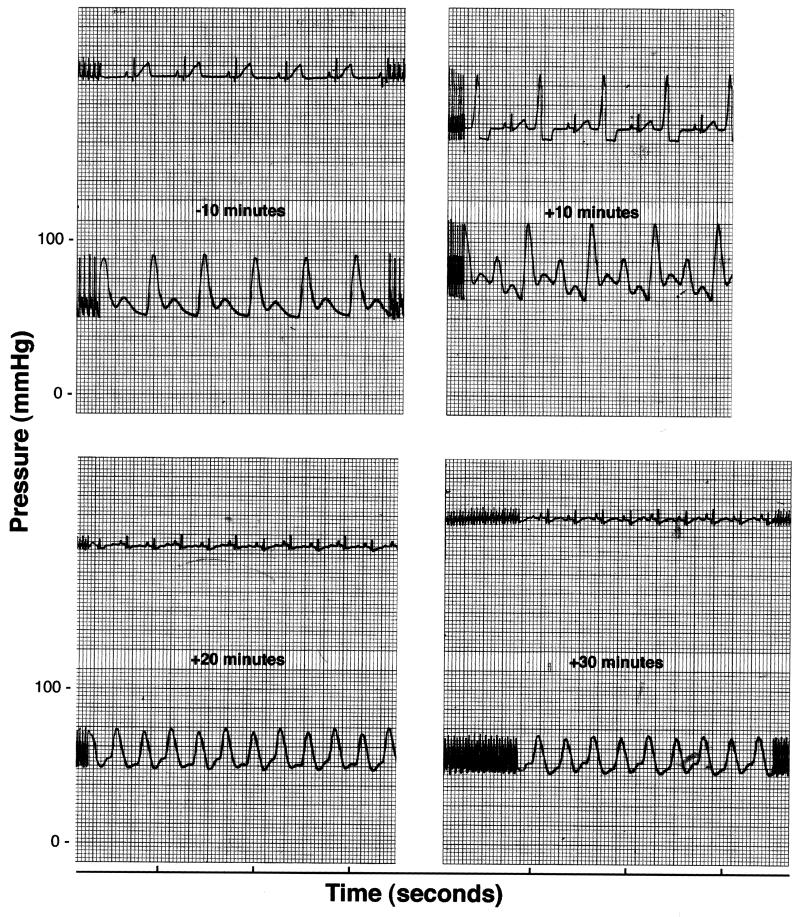
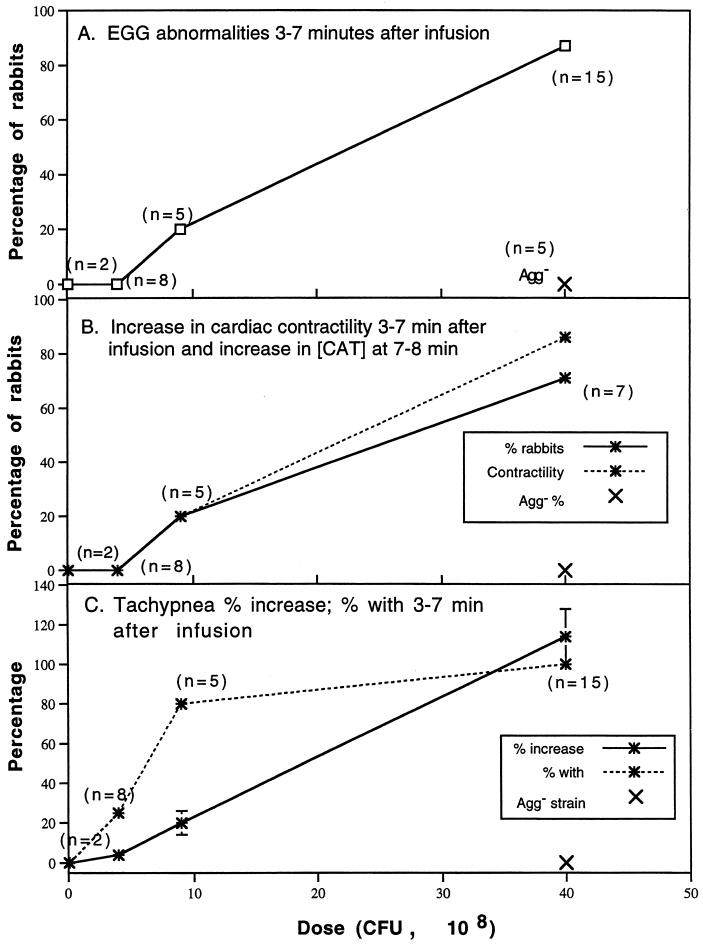
Within 3 min of initiation of infusion (t = 3 min), rabbits showed electrocardiographic abnormalities that changed over time, often reverting to normal and then abnormal in a single beat. For example, one rabbit showed abnormal electrocardiographic ST-segment depression and alternating premature ventricular contractions (PVCs) in Lead I through t = 10 min (Fig. 3). At t = 20 and 30 min, the heart rate remained elevated and ST-segment depression persisted. In comparison to baseline, the peak systolic pressure is delayed relative to the QRS complex (excitation of the ventricles) and the dichrotic notch in the pressure recording disappears. When seen during the first 7 min postinfusion, ST-segment depression usually continued through t = 30 min (data not shown for all rabbits). The dose of Agg+ S. sanguis was directly related to the frequency of ECG abnormalities at t = 3 to 7 min, with the regression line significantly different from zero (P < 0.01) (Fig. 4A). After infusion of 40 × 108 CFU of the Agg+ strain, 13 of 15 rabbits showed ECG abnormalities (Table 1). The most frequent abnormalities were ST-segment depression observed in 10 of 15 rabbits and PVCs in 7 of 15. With this dose, changes in the ventricular electrical axis at t = 30 min were seen in 11 of 15 rabbits (six showed right axis deviation and five left axis deviation). During the 30-min postbacteremia period, rabbits often developed more than one type of abnormal ECG pattern.
FIG. 3.
Representative electrocardiographic tracing. Selected segments of an ECG and blood pressure recording for a rabbit given 40 × 108 CFU of the Agg+ strain is shown. Note ST-segment depression and alternating preventricular contractions (PVCs) by 10 min. The ST-segment depression persists through t = 30 min, the heart rate remains elevated, and the pulse pressure is greatly reduced.
FIG. 4.
Cardiopulmonary consequences of S. sanguis bacteremia. For selected variables, the Agg+ dose-dependent changes in frequency or magnitude are presented. Two rabbits infused with 0.1 × 108 CFU of the Agg+ strain and five with 40 × 108 CFU of the Agg− strain are also included for comparison. Note that the Agg− strain had no effect upon any of the cardiopulmonary variables studied. The regression line for each variable shown was significantly different from zero (linear regression analysis). (A) ECG abnormalities at t = 3 to 7 min. During this period after infusion, the ECG was monitored and changes were evaluated for the numbers of rabbits shown. (B) Increases in cardiac contractility (t = 3 to 7 min) and catacholamine concentration (t = 7 to 8 min). Cardiac contractility was computed as dP/dt as described in Materials and Methods. In the same rabbits, catacholamine concentrations were determined by radioenzymatic assay as described in Materials and Methods. (C) Frequency and percent increase in tachypnea at t = 3 to 7 min. Note the high frequency but comparatively low magnitude with an Agg+ dose of 9 × 108 CFU. Catecholamines did not vary from baseline in rabbits given Agg+ doses of 0.1 and 4 × 108 CFU. Since the cardiopulmonary responses were similar to rabbits given lower doses of the Agg+ strain, rabbits given 40 × 108 CFU of the Agg− strain were not sampled for catecholamines.
TABLE 1.
Frequency of ECG abnormalities 3 to 7 min after infusion with S. sanguis (Agg+)a
| Dose (108 CFU) | Total no. of rabbits | No. of rabbits with:
|
||||||
|---|---|---|---|---|---|---|---|---|
| Abnormal ECGs | ST segment depression | PVCs | Alternans | Bigemia | Axis deviation at 30 min
|
|||
| Right | Left | |||||||
| 40 | 15 | 13 | 10 | 7 | 4 | 2 | 6 | 5 |
| 9 | 5 | 1 | 1 | 1 | 0 | 0 | 0 | 1 |
| 4 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
No abnormalities observed after infusion of 40 × 108 Agg− strain.
In 11 of the 15 rabbits given 40 × 108 CFU of the Agg+ strain, the arterial dP/dt increased significantly during t = 3 to 7 min. In contrast, only one of five rabbits showed an increase in cardiac contractility after infusion of 9 × 108 CFU. Since first rabbits studied showed an increase in dP/dt, blood was sampled for catecholamine concentration [CAT] in subsequent rabbits. When both were determined in the same rabbits, the frequency of elevated dP/dt was directly related to [CAT] in blood sampled at 7 to 8 min (P = 0.0002, two-tailed Fisher exact test) (Fig. 4B). The percentage increase in tachypnea was also related directly to dose of Agg+ S. sanguis (r = 0.79; slope different from 0, P < 0.01) (Fig. 4C). In rabbits infused with 100 × 108 CFU of the Agg+ strain, tachypnea developed within 90 s (data not shown). All of rabbits given 40 × 108 CFU of the Agg+ strain developed tachypnea during the interval from t = 3 min to t = 7 min. Tachypnea also developed in 80% of rabbits given 9 × 108 CFU and 25% of those given 4 × 108 CFU of the Agg+ strain. As noted in Fig. 4, cardiopulmonary variables were not influenced by the infusion of the Agg− strain.
Platelet fate.
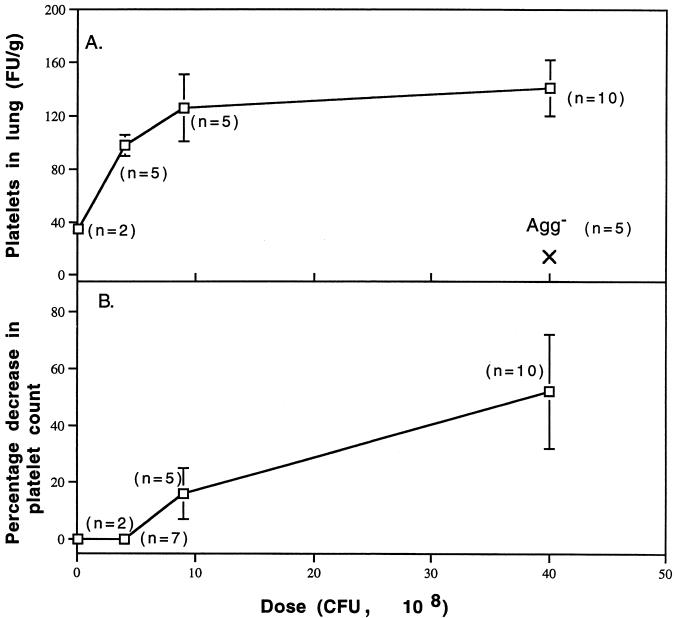
If they formed in vivo, then platelet clots should be observed in the lungs (Fig. 5A) and the fate of circulating platelets should be reflected in thrombocytopenia (Fig. 5B). Both of these measures of platelet clotting in vivo were shown to occur as a function of infused dose of Agg+ S. sanguis. Platelet clots in the lungs occurred in response to all doses when analyzed at t = 30 min, but appeared to maximize upon infusion of 9 × 108 CFU (Fig. 5A). The FU of 111Indium-labeled platelets/g was not significantly different at doses of 9 × 108 and 40 × 108 CFU. After infusion of 40 × 108 CFU of the Agg− strain, the FU/gram was 3 standard deviations lower than after infusion of 0.1 × 108 CFU of the Agg+ strain. Platelets cleared from the circulation to the spleen, but the FU/gram of spleen decreased as a function of dose of Agg+ S. sanguis (r = 0.82; slope different than 0, P = 0.003 by linear regression analysis) (data not shown). The platelet count at t = 7 to 8 minutes was generally unaffected by infusion of up to 4 × 108 CFU of the Agg+ strain (thrombocytopenia in one of seven rabbits) (Fig. 5B). The frequency of thrombocytopenia was 80% after infusion of 9 × 108 CFU and 100% after 40 × 108 CFU (data not shown). The percentage decrease in platelet counts was significantly related to dose of Agg+ S. sanguis (r = 0.83; slope different than 0, P < 0.001 by linear regression analysis). Since Agg+ strain doses of ≤4 × 108 CFU did not cause detectable thrombocytopenia, platelet counts were not completed for rabbits given the Agg− strain.
FIG. 5.
Platelet fate. Dose-dependent signs of platelet aggregation in vivo were quantified. (A) Fractional uptake (FU/g) of 111Indium-labeled platelets in the lungs at t = 30 min. The FU/gram of lung becomes saturated at doses larger than 9 × 108 CFU of the Agg+ strain (no difference between doses; Student’s t test). In those rabbits given 40 × 108 CFU of the Agg− strain, the FU/gram of lung was nearly three-fold less than in the two rabbits given 0.1 × 108 CFU of the Agg+ strain. (B) Percentage decrease in platelet count. Platelets were counted at baseline and at t = 30 min as described in Materials and Methods.
DISCUSSION
The Agg+ strain of S. sanguis induces platelet aggregation or clotting in vivo as predicted by the in vitro data. Aggregation in vitro is all-or-none with a dose-dependent delay (23). In vitro, rabbit platelets aggregate in response to the Agg+ strain in about two minutes. The experiments in vivo had been designed to record hemodynamic and cardiopulmonary responses upon a 1-min infusion of S. sanguis. Direct evidence for platelet aggregation or clotting in vivo include thrombocytopenia and, when compared to the Agg− strain, accumulation of 111Indium-labeled platelets in the lungs (Fig. 5), but not in the spleen. Platelets normally clear from the circulation to the spleen, but the 111Indium-labeled platelets used as tracers do not. The frequency and extent of manifestations of platelet clotting in vivo increase directly with the dose of inoculated Agg+ S. sanguis.
Changes in heart rate and blood pressure occur more rapidly than would be predicted by the two minute lag time for in vitro platelet aggregation. The blood pressure and heart rate change 10 to 15 s before completing the 1-min infusion of doses of Agg+ S. sanguis ≥9 × 108 CFU and reach maxima shortly thereafter (Fig. 2). In vivo clots may incorporate red blood cells to increase the rate of accumulating volume and mass when compared to aggregation of only platelet-rich plasma. An early dose-dependent increase in circulating catecholamines would also be expected to potentiate clotting in response to S. sanguis. In vitro, physiological concentrations of epinephrine potentiate human platelet aggregation in response to S. sanguis (27, 37a). The rapid increase in circulating catecholamines may originate from post-sympathetic discharge (19) or endogenous platelet stores (27, 56). Upon release from platelets, thromboxane A2 and serotonin will also potentiate aggregation. Therefore, the changing hemodynamics suggest that platelet clots form earlier and are probably larger in vivo than would have been predicted by in vitro experiments.
Regulated by a negative feedback system, cardiac output is equal to the stroke volume times the heart rate and is dependent upon the blood pressure gradient divided by the total peripheral resistance of the vascular bed. A clot-inducing material like S. sanguis in the vascular bed alters the resistance to flow. In response to Agg+ S. sanguis, platelet clotting caused increased pulmonary vascular resistance. The reduced venous return promoted the hypotensive phase (Fig. 2A). Ventricular contractility (dP/dt) and heart rate (Fig. 2B) increased to regulate stroke volume and restore cardiac output.
Consistent with the accumulation of platelet clots in the lungs, tachypnea developed rapidly, maximizing within 90 seconds of infusion of 100 × 108 CFU of the Agg+ strain. In response to 40 × 108 CFU, tachypnea maximized at about 3.2 min. Platelet clot-associated occlusion persisted in some rabbits, causing pulmonary hypertension. Pulmonary hypertension resulted in right axis deviation of the heart as observed in 6 of 15 rabbits. Even the dose of 9 × 108 CFUs of the Agg+ strain caused tachypnea frequently, but with low severity (Fig. 4C). The magnitude of tachypnea in experimental animals is dependent upon the number of injected glass microspheres and independent of the anatomic region of the lungs to which the beads embolized (32, 34). The responses to experimental embolism appear to be determined primarily by the magnitude of the pulmonary obstruction (45). Hence, tachypnea is best explained by persistent dose-dependent platelet clots in the pulmonary circulation as shown by the association between accumulation of 111Indium-labeled platelets in the lungs with thrombocytopenia (Fig. 5A and B).
In our model, the pulmonary changes and accumulation of 111Indium-labeled platelets in the lungs may underestimate the extent of platelet clots formed or trapped in the pulmonary circulation. Ketamine used in our protocol has been shown to cause ex vivo pulmonary vasodilation in rabbits (39), which would antagonize the accumulation of platelet clots. Hence the potential for S. sanguis to induce in vivo platelet clotting may be even greater than suggested by our data.
An important consequence of S. sanguis-induced platelet clotting is ST-segment depression, which indicates heart ischemia (9). Evidence of ST-segment depression first appeared during the hypotensive phase, occurred as early as three minutes after infusion, and generally persisted until term (Fig. 3). All rabbits with 40 × 108 CFU showed increased respiratory rate, diphasic blood pressure and thrombocytopenia, but only 13 of 15 rabbits showed abnormal ECGs and only 2 of 3 showed ST-segment depression. The frequency of abnormal electrocardiographic responses may reflect inter-rabbit variations in fibrinogen levels, plasminogen activator activity, platelet reactivity, displacement or disaggregation of platelet clots, or the anatomy and function of the coronary circulation, which might provide restoration of blood flow by reflexive vasodilation. Coronary vasospasm accompanied in vivo platelet clotting in response to intravenous collagen (40). It is unclear if coronary artery vasospasm was reflected in the response to the Agg+ strain, but a spectrum of vasoactive mediators may be released in response to S. sanguis-induced platelet clotting. Based on the hemodynamic and cardiopulmonary changes, platelet clotting occurred upon infusion of only the Agg+ and not the Agg− strain of S. sanguis.
During the 30 min of our protocol, platelet clots appear to persist and may become thrombotic. Thrombi, however, require the generation of thrombin and fibrin. In humans, platelet clots can occur in the absence of initiation of coagulation and the production of fibrin (49). In our model, initiation of coagulation through tissue injury and the cytokine-mediated expression of tissue-factor is unlikely to occur in only 30 min (18, 41). Based on in vitro data, the platelet clots would be expected to contain activated platelets in proximity to the endothelium. Thromboxane A2 released from activated platelets, for example, can “activate” endothelium (48, 57). Coagulation can be initiated on the surface of activated endothelium and platelets, generating thrombin and fibrin (22). The contact factor (intrinsic) system of coagulation can be assembled and activated within minutes on endothelial cells (or platelets). Therefore, the platelet clots may become more thrombus-like over time.
Activation of coagulation would also rapidly generate vasoactive bradykinin and plasminogen activators (47). These products of coagulation may act synergistically with thromboxane A2 and serotonin from activated platelets, and the elevated circulating catecholamines seen after infusion of Agg+ S. sanguis, to promote an arteriolar pressor response. Clearly there are several available mechanisms to cause rapid vasoconstriction. In contrast, the norepinephrine pressor response was probably not inhibited by nitric oxide during the hypotensive phase of the diphasic blood pressure response caused by 40 × 108 CFU of Agg+ S. sanguis. Nitric oxide production requires several hours for cytokine-mediated gene expression in response to related streptococci or gram-negative lipopolysaccharides (42). S. sanguis caused changes far too rapidly to be explained based on events requiring the translation of genes and synthesis of new proteins. The rapid expression of a set of vasoconstrictors and platelet clotting appear to be sufficient to promote vascular occlusion and contribute to persistent ST-segment depression and heart ischemia in our model. Hence, S. sanguis-induced platelet aggregation in vivo may trigger several complimentary mechanisms to promote thrombosis rapidly in the apparent absence of endothelial or atherosclerotic disease. Thrombocytopenia in response to S. sanguis may suggest the occurrence of disseminated intravascular coagulation (DIC). DIC can occur with gram-negative or gram-positive sepsis (6, 41) and is marked by thrombocytopenia and the deposition of fibrin in the microvasculature. The gram-positive Staphylococcus aureus induces DIC through activation of the coagulation cascade in vivo (36) and correlates with the ability of the microbe to induce platelet aggregation ex vivo (35). In our experiments, fibrin deposition was unlikely to have occurred coincidentally with the sudden hemodynamic and cardiopulmonary changes, or the onset of platelet clotting. Hence, platelet clotting in response to S. sanguis appears to occur in the absence of DIC.
Agg+ S. sanguis bacteremias also cause the formation of thrombotic platelet vegetations in the rabbit model of endocarditis (26, 46). The Agg+ strain induced the formation of significantly larger vegetations than the Agg− strain. One day after infusion of Agg+ S. sanguis, the vegetation was a dense mass of aggregated platelets and fibrin, with few isolated and trapped bacterial cells comprising less than 1/100th of the cross-sectional area (29). The development of the vegetation could be inhibited by infusion of specific anti-fibrin antibody (58) or reversed by administration of tissue plasminogen activator (46). Comprised of fibrin and aggregated platelets, the vegetation formed in response to the Agg+ strain of S. sanguis was a thrombus. Since aggregation of human and rabbit platelets also occurs in vitro (24, 25, 50) and in vivo, as shown in this study, there are now three lines of evidence that Agg+ S. sanguis can be thrombogenic. That S. sanguis experimental bacteremia can induce signs of thrombosis and heart ischemia is also of interest because of the possible epidemiological relationship between dental infections and myocardial infarction (5, 8, 44). Individuals in several different populations who have suffered recent myocardial infarction show a greater prevalence of periodontitis, pulpal abscesses, and other oral infections than control subjects. Similar findings have also been reported for stroke as an outcome variable (21). The underlying biological basis for these epidemiological findings is unclear. S. sanguis comprises about 30% of the population of bacteria in the complex microbial community of the dental plaque biofilm. Fragments of this biofilm containing S. sanguis and other potentially thrombogenic microorganisms such as Porphyromonas gingivalis (28) are detected as bacteremias often through life (11), although the character and amount of the inoculum is unknown. Since the frequency and magnitude of these bacteremias may increase with the occurrence of chronic infections such as periodontitis, it is tempting to speculate that S. sanguis bacteremias may cause similar hemodynamic and cardiopulmonary changes in humans as shown in rabbits. While the data showed that comparatively low doses of pure cultures of S. sanguis caused in vivo responses, environmental regulation in the biofilm may result in expression of bacteria that are more or less thrombogenic. Platelet clotting in vivo suggests a new and novel paradigm of cardiac dysfunction caused by a nonpathogenic commensal bacteria from the oral cavity.
ACKNOWLEDGMENTS
We thank Dorothy Aeppli and James Hodges for excellent statistical assistance, Urve Daigle for computer-assisted graphics, and Angie Witt, Gordon MacFarlane, and Nick Armstrong for technical support.
This work was supported by NIH/NIDR R01DE05501, R03DE07303, P30DE09737, and K16DE00270.
REFERENCES
- 1.Adler D, Nikolic S D, Sonnenbick E H, Yellin E L. Time to dP/dtmax, a preload-independent index of contractility: open-chest dog study. Basic Res Cardiol. 1996;91:94–100. doi: 10.1007/BF00788870. [DOI] [PubMed] [Google Scholar]
- 2.Angrist A A, Oka M, Nakao K. Vegetative endocarditis. Pathol Annu. 1967;2:155–212. [Google Scholar]
- 3.Bancsi M J L M F, Thompson J, Bertina R M. Stimulation of monocyte tissue factor expression in an in vitro model of bacterial endocarditis. Infect Immun. 1994;62:5669–5672. doi: 10.1128/iai.62.12.5669-5672.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bancsi M J, Veltrop M H, Bertina R M, Thompson J. Influence of monocytes and antibiotic treatment on tissue factor activity of endocardial vegetation in rabbits infected with Streptococcus sanguis. Infect Immun. 1996;64:448–451. doi: 10.1128/iai.64.2.448-451.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck, J., R. Garcia, G. Heiss, P. S. Vokonas, and S. Offenbacher. 1996. Periodontal disease and cardiovascular disease. J. Periodontol. 67(Suppl.; symposium proceedings):1123–1137. [DOI] [PubMed]
- 6.Bick R L. Disseminated intravascular coagulation: pathophysiological mechanisms and manifestations. Semin Thromb Hemost. 1998;24:3–18. doi: 10.1055/s-2007-994971. [DOI] [PubMed] [Google Scholar]
- 7.Buiting A G M, Thompson J, van der Keur D, Schmal-Bauer W C, Bertina R M. Procoagulant activity of experimental vegetations and blood monocytes in rabbits with Streptococcus sanguis endocarditis. Thromb Haemostasis. 1989;62:1029–1033. [PubMed] [Google Scholar]
- 8.DeStefano F, Anda R F, Kahn H S, Williamson D F, Russell C M. Dental disease and risk of coronary heart disease and mortality. Br Med J. 1993;306:688–691. doi: 10.1136/bmj.306.6879.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Downey J M. The electrocardiogram. In: Johnson L R, editor. Essential medical physiology. 2nd ed. Philadelphia, Pa: Lippincott-Raven; 1998. pp. 175–198. [Google Scholar]
- 10.Durack D T. Infective and non-infective endocarditis. In: Hurst J W, Schlant R C, Rackley C R, Sonnenblick E H, Wenger N K, editors. The heart, arteries, and veins. 7th ed. Vol. 2. New York, N.Y: McGraw-Hill; 1990. pp. 1230–1255. [Google Scholar]
- 11.Durack D T. Prevention of infective endocarditis. N Engl J Med. 1995;332:38–44. doi: 10.1056/NEJM199501053320107. [DOI] [PubMed] [Google Scholar]
- 12.Elting L S, Rubenstein E B, Rolston K V, Bodey G P. Outcomes of bacteremia in patients with cancer and neutropenia: observations from two decades of epidemiological and clinical trials. Clin Infect Dis. 1997;25:247–259. doi: 10.1086/514550. [DOI] [PubMed] [Google Scholar]
- 13.Erickson P R, Herzberg M C. A collagen-like immunodeterminant on the surface of Streptococcus sanguis induces platelet aggregation. J Immunol. 1987;138:3360–3366. [PubMed] [Google Scholar]
- 14.Erickson P R, Herzberg M C. Purification and partial characterization of a 65-kDa platelet aggregation-associated antigen from the surface of Streptococcus sanguis. J Biol Chem. 1990;265:14080–14087. [PubMed] [Google Scholar]
- 15.Erickson P R, Herzberg M C, Tierney G. Crossreactive immunodeterminants on Streptococcus sanguis and collagen: predicting the structure of platelet-interactive domains. J Biol Chem. 1992;267:10018–10023. [PubMed] [Google Scholar]
- 16.Erickson P R, Herzberg M C. The Streptococcus sanguis platelet aggregation-associated protein: identification and characterization of the minimal platelet-interactive domain. J Biol Chem. 1993;268:1646–1649. [PubMed] [Google Scholar]
- 17.Ford I, Douglas C W I, Preston F E, Lawless A, Hampton K K. Mechanisms of platelet aggregation by Streptococcus sanguis, a causative organism in infective endocarditis. Br J Haematol. 1993;84:95–100. doi: 10.1111/j.1365-2141.1993.tb03030.x. [DOI] [PubMed] [Google Scholar]
- 18.Geelen S, Bhattacharyya C, Tuomanen E. Induction of procoagulant activity on human endothelial cells by Streptococcus pneumoniae. Infect Immun. 1992;60:4179–4183. doi: 10.1128/iai.60.10.4179-4183.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldstein D S. Stress, catecholamines, and cardiovascular disease. New York, N.Y: Oxford University Press; 1995. pp. 103–163. [Google Scholar]
- 20.Gong K, Ouyang T, Herzberg M C. A streptococcal adhesion system for salivary pellicle and platelets. Infect Immun. 1998;66:5388–5392. doi: 10.1128/iai.66.11.5388-5392.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grau A J, Buggle F, Ziegler C, Schwarz W, Meuser J, Tasman A-J, Buhler A, Benesch C, Becher H, Hacke W. Association between acute cerebrovascular ischemia and chronic and recurrent infection. Stroke. 1997;28:1724–1729. doi: 10.1161/01.str.28.9.1724. [DOI] [PubMed] [Google Scholar]
- 22.Harker L A, Mann K G. Thrombosis and fibrinolysis. In: Verstraete M, Fuster V, Topol E J, editors. Cardiovascular thrombosis: thrombocardiology and thromboneurology. 2nd ed. Philadelphia, Pa: Lippincott-Raven; 1998. pp. 3–22. [Google Scholar]
- 23.Herzberg M C, Brintzenhofe K L. ADP-like platelet aggregation activity generated by viridans streptococci incubated with exogenous ATP. Infect Immun. 1983;40:120–125. doi: 10.1128/iai.40.1.120-125.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herzberg M C, Brintzenhofe K L, Clawson C C. Aggregation of human platelets and adhesion of Streptococcus sanguis. Infect Immun. 1983;39:1457–1469. doi: 10.1128/iai.39.3.1457-1469.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herzberg M C, Gong K, MacFarlane G D, Erickson P R, Soberay A H, Krebsbach P H, Manjula G, Schilling K, Bowen W H. Phenotypic characterization of Streptococcus sanguis virulence factors associated with bacterial endocarditis. Infect Immun. 1990;58:515–522. doi: 10.1128/iai.58.2.515-522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herzberg M C, MacFarlane G D, Gong K, Armstrong N N, Witt A R, Erickson P R, Meyer M W. The platelet interactivity phenotype of Streptococcus sanguis influences the course of experimental endocarditis. Infect Immun. 1992;60:4809–4818. doi: 10.1128/iai.60.11.4809-4818.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herzberg M C, Krishnan L K, MacFarlane G D. Involvement of α2-adrenoreceptors and G-proteins in the modulation of platelet secretion in response to Streptococcus sanguis. Crit Rev Oral Biol Med. 1993;4:435–442. doi: 10.1177/10454411930040032501. [DOI] [PubMed] [Google Scholar]
- 28.Herzberg M C, MacFarlane G D, Liu P-X, Erickson P R. The platelet as an inflammatory cell in periodontal diseases: interactions with Porphyromonas gingivalis. In: Genco R J, Mergenhagen S, McGhee J, Lehner T, Hamada S, editors. Molecular basis for pathogenesis and molecular targeting in periodontal diseases. Washington, D.C: American Society for Microbiology; 1994. pp. 247–255. [Google Scholar]
- 29.Herzberg M C. Platelet-streptococcal interactions in endocarditis. Crit Rev Oral Biol Med. 1996;7:222–236. doi: 10.1177/10454411960070030201. [DOI] [PubMed] [Google Scholar]
- 30.Herzberg, M. C., and M. W. Meyer. 1996. Effects of oral flora on platelets: possible consequences in cardiovascular disease. J. Periodontol. 67(Suppl.; symposium proceedings):1138–1142. [DOI] [PubMed]
- 31.Herzberg M C, Meyer M W. Dental plaque, platelets and cardiovascular diseases. Ann Periodontol. 1998;3(symposium proceedings):151–160. doi: 10.1902/annals.1998.3.1.151. [DOI] [PubMed] [Google Scholar]
- 32.Horres A D, Bernthal T. Localized multiple minute pulmonary embolism and breathing. J Appl Physiol. 1961;16:842–846. doi: 10.1152/jappl.1961.16.5.842. [DOI] [PubMed] [Google Scholar]
- 33.Ishise H, Asanoi H, Ishikawa S, Joho S, Kameyama T, Umeno K, Inoue H. Time course of sympathovagal imbalance and left ventricular dysfunction in conscious dogs with heart failure. J Appl Physiol. 1998;84:1234–1241. doi: 10.1152/jappl.1998.84.4.1234. [DOI] [PubMed] [Google Scholar]
- 34.Katz S, Horres A D. Medullary respiratory neuron response to pulmonary emboli and pneumothorax. J Appl Physiol. 1972;33:390–396. doi: 10.1152/jappl.1972.33.3.390. [DOI] [PubMed] [Google Scholar]
- 35.Kessler C M, Nussbaum E, Tuazon C U. Disseminated intravascular coagulation associated with Staphylococcus aureus septicemia is mediated by peptidoglycan-induced platelet aggregation. J Infect Dis. 1991;164:101–107. doi: 10.1093/infdis/164.1.101. [DOI] [PubMed] [Google Scholar]
- 36.Kessler C M, Tang Z, Jacobs H M, Szymanski L M. The suprapharmacologic dosing of antithrombin concentrate for Staphylococcus aureus-induced disseminated intravascular coagulation in guinea pigs: substantial reduction in morbidity and mortality. Blood. 1997;89:4393–4401. [PubMed] [Google Scholar]
- 37.Kitchens C S. Disseminated intravascular coagulation. Curr Opin Hematol. 1995;2:402–406. doi: 10.1097/00062752-199502050-00012. [DOI] [PubMed] [Google Scholar]
- 37a.Krishnan, L. K., et al. Unpublished data.
- 38.Kruithof E K, Agay D, Mestries J C, Gascon M P, Ythier A. The effect of factor Xa/phospholipid infusion on the acute phase response in baboons. Thromb Haemostasis. 1997;77:308–311. [PubMed] [Google Scholar]
- 39.Lee T S, Hou X. Vasoactive effects of ketamine on isolated rabbit pulmonary arteries. Chest. 1995;107:1152–1155. doi: 10.1378/chest.107.4.1152. [DOI] [PubMed] [Google Scholar]
- 40.Mallarkey G, Smith G M. The involvement of platelets and the coronary vasculature in collagen-induced sudden death in rabbits. Thromb Haemostasis. 1985;53:70–74. [PubMed] [Google Scholar]
- 41.Mammen, E. F. 1998. The haematological manifestations of sepsis. J. Antimicrob. Chemother. 41(Suppl. A):17–24. [DOI] [PubMed]
- 42.Martin V, Kleschyov A L, Klein J-P, Beretz A. Induction of nitric oxide production by polyosides from the cell walls of Streptococcus mutans OMZ 175, a gram-positive bacterium, in the rat aorta. Infect Immun. 1997;65:2074–2079. doi: 10.1128/iai.65.6.2074-2079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martino R, Manteiga R, Sanchez I, Brunet S, Sureda A, Badell I, Argiles B, Subira M, Bordes R, Dominog-Albos A. Viridans streptococcal shock syndrome during bone marrow transplantation. Acta Haematol. 1995;94:69–73. doi: 10.1159/000203976. [DOI] [PubMed] [Google Scholar]
- 44.Mattila K J, Nieminen M S, Valtonen V V, Rasi V P, Kesaniemi Y A, Syrjala S L, et al. Association between dental health and myocardial infarction. Br Med J. 1989;298:779–782. doi: 10.1136/bmj.298.6676.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McIntyre K M, Sasahara A A. Hemodynamic and ventricular responses to pulmonary embolism. Prog Cardiovasc Dis. 1974;XVII:175–190. doi: 10.1016/0033-0620(74)90042-5. [DOI] [PubMed] [Google Scholar]
- 46.Meyer M W, Witt A R, Krishnan L K, Yokota M, Roszkowski M J, Rudney J D, Herzberg M C. Therapeutic advantage of recombinant human plasminogen activator in endocarditis: evidence from experiments in rabbits. Thromb Haemostasis. 1995;73:680–682. [PubMed] [Google Scholar]
- 47.Motta G, Rojkjaer R, Hasan A A K, Cines D B, Schmaier A H. High molecular weight kininogen regulates prekallekrein assembly and activation on endothelial cells: a novel mechanism for contact activation. Blood. 1998;91:516–528. [PubMed] [Google Scholar]
- 48.Pfister, S. L., D. D. Deinhart, and W. B. Campbell. 1998. Methacholine-induced contraction of rabbit pulmonary artery: role of platelet-endothelial transcellular thromboxane synthesis. Hypertension 31(Suppl. 1, Pt. 2):206–212. [DOI] [PubMed]
- 49.Ridker P M, Hennekens C H, Lindpaintner K, Stampfer M J, Eisenberg P R, Miletich J P. Mutation in the gene encoding for coagulation factor V and the risk of myocardial infarction, stroke, and venous thrombosis in apparently healthy men. N Engl J Med. 1995;332:912–917. doi: 10.1056/NEJM199504063321403. [DOI] [PubMed] [Google Scholar]
- 50.Soberay A H, Herzberg M C, Rudney J D, Sixma J J, Nieuwenhuis H K, Seligsohn U. Response of platelets to strains of Streptococcus sanguis: findings in healthy subjects, Bernard-Soulier, Glanzmann’s, and collagen-unresponsive patients. Thromb Haemostasis. 1987;57:222–225. [PubMed] [Google Scholar]
- 51.Steiner M, Villablanca J, Kersey J, Ramsey N, Haake R, Ferrieri P, Weisdorf D. Viridans streptococcal shock in bone marrow transplant patients. Am J Hematol. 1993;42:354–358. doi: 10.1002/ajh.2830420405. [DOI] [PubMed] [Google Scholar]
- 52.Sullam P M, Valone F H, Mills J. Mechanisms of platelet aggregation by viridans group streptococci. Infect Immun. 1987;55:1743–1750. doi: 10.1128/iai.55.8.1743-1750.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van der Poll T, Levi M, Bulle H R, van Derventer S J, de Boer J P, Hack C E, ten Cate J W. Fibrinolytic response to tumor necrosis factor in healthy subjects. J Exp Med. 1991;174:729–732. doi: 10.1084/jem.174.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Villablanca J G, Steiner M, Kersey J, Ramsey N K, Ferrieri P, Haake R, Weisdorf D. The clinical spectrum of infections with viridans streptococci in bone marrow transplant patients. Bone Marrow Transplant. 1990;5:387–393. [PubMed] [Google Scholar]
- 55.Weisman S J, Scoopo F J, Johnson G M, Altman A J, Quinn J J. Septicemia in pediatric oncology patients: the significance of viridans streptococcal infections. J Clin Oncol. 1990;8:453–459. doi: 10.1200/JCO.1990.8.3.453. [DOI] [PubMed] [Google Scholar]
- 56.Wilson A P, Smith C C, Prichard B N, Betteridge D J. Platelet catecholamines and platelet function in normal human subjects. Clin Sci. 1987;73:99–103. doi: 10.1042/cs0730099. [DOI] [PubMed] [Google Scholar]
- 57.Yang Z, Arnet U, Bauer E, von Segesser L, Siebenmann R, Turina M, Luscher T F. Thrombin-induced endothelium-dependent inhibition and direct activation of platelet-vessel wall interaction. Role of prostacyclin, nitric oxide, and thromboxane A2. Circulation. 1994;89:2266–2272. doi: 10.1161/01.cir.89.5.2266. [DOI] [PubMed] [Google Scholar]
- 58.Yokota, M., et al. Unpublished data.