Abstract
Background:
The popularity of edible cannabis products continues to grow in states with legal cannabis access, but few studies have investigated the acute effects of these commercially available products. The present study sought to explore the effects of three commercially available edible products with different levels of delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD).
Methods:
A sample of regular cannabis users (N=99) were evaluated. Fifty participants completed the study procedures in-person, whereas 49 participants completed the study procedures remotely via Zoom. Subjective effects and plasma cannabinoid levels (in-person participants only) were assessed before and 2 h after participants self-administered one of three products ad libitum: a THC-dominant edible product, a CBD-dominant edible product, or a THC+CBD edible product.
Results:
At the 2-h post-use assessment, among in-person participants, plasma THC and CBD levels were robustly correlated with self-reported milligrams of THC and CBD consumed, respectively. Across all three conditions, in-person and remote participants experienced (1) an increase in subjective intoxication and elation, (2) a decrease in tension, and (3) no change in paranoia from pre-use to post-use. At post-use, participants who used a CBD product reported less intoxication relative to participants who used a THC+CBD or THC-only product. Participants who used a THC+CBD product reported consuming less THC—and displayed lower plasma THC levels (in-person participants)—relative to participants who used a THC-only product, despite reporting similar levels of positive (intoxication, elation, liking) and psychotomimetic (paranoia, tension) effects. Psychotomimetic effects were very low among both in-person and remote participants across all three conditions, and there were no post-use differences across conditions.
Conclusions:
Findings suggest that experienced users who consumed a THC+CBD product reported similar levels of positive and psychotomimetic effects relative to those who consumed a THC-only product, despite consuming less THC and displaying lower plasma THC concentrations. Given the potential harms associated with acute cannabis reward and long-term THC exposure, further research is needed to establish whether edible cannabis products with CBD pose less risk to users. Future studies should examine whether these effects generalize to samples of infrequent users, who may have less experience with edible cannabis use. ClinicalTrials.gov ID: NCT03522103.
Keywords: oral administration, tetrahydrocannabinol, cannabidiol, subjective effects, harm reduction, edibles
Introduction
The legalization of cannabis has led to an expansive retail marketplace with increased production and sale of diverse cannabis products and administration methods.1,2 Although the prevalence of edible use continues to increase, the effects of legal market edible products have not been rigorously evaluated in biopsychological research studies due to federal regulations.3 Importantly, prior research findings may not generalize due to differences in potency and composition between cannabis forms used for research versus what is available on the legal market.4,5 Hence, there is a need to investigate the effects of legal market edible products, particularly those of differing cannabinoid concentrations,6,7 as such studies can provide relevant and important public health information.
Edible cannabis is a common consumption method for medical cannabis users8,9 and older adults,10,11 suggesting that it may satisfy the demands of a broader range of adult buyers who do not want to inhale cannabis smoke or who want to avoid respiratory risks associated with combustion.3,12–14 Although peak plasma delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) concentrations are observed rapidly after smoking (∼5–10 min), cannabinoids in oral formulations undergo first-pass metabolism, resulting in delayed peak concentrations (∼2 h)15–17 and prolonged psychotropic effects that can last between 6 and 8 h.14 The interindividual differences in metabolism, absorption rates, and delayed onset of effects make it more challenging to study oral (vs. inhaled) forms of cannabis.
In laboratory studies where the administered dose is controlled, oral synthetic THC formulations mimic some of the behavioral (e.g., anxiety, dysphoria) and physiological effects (e.g., increased heart rate) typically observed after smoking flower.18–20 Specifically, among naive and infrequent users, the effects of THC are dose-dependent, exerting positive effects on mood and reward at lower doses21 but increased anxiety, dissociation, and paranoia as the THC dose increases.22 Two clinical studies have shown that a single oral CBD dose of 300 mg has anxiolytic effects with a dose-dependent inverted U-shaped curve in healthy subjects who were not observed at doses of 100, 150, 600, or 900 mg.23,24
Notably, however, although these dose-dependent anxiogenic effects are often observed in naive and infrequent cannabis users, they are rarely observed in regular users.25,26 Furthermore, there is some debate as to whether CBD might influence some of the acute effects of THC. For instance, several studies show that co-administering CBD with THC (via oral and inhaled routes) diminishes the effects of THC on subjective mood,27–31 whereas other studies have not supported this assertion,32–34 with one study suggesting that CBD enhances the effects of THC on positive mood.35
Since prior studies often utilize forms and doses that are not representative of the products sold on the legal market, it has yet to be determined how edible products commonly sold and used by the public may confer distinct subjective effects. Therefore, externally valid research designs will allow a better assessment of the real-world effects users experience. Hence, the purpose of the present study was to determine the subjective effects of edible cannabis products and the extent to which these effects might vary based on their relative concentrations of THC and CBD.
In a sample of both in-person and remote participants, we tested three edible cannabis products that are commonly available on the legal cannabis market: a THC-dominant product (e.g., 10 mg THC, 0 mg CBD), a CBD-dominant product (e.g., 1.65 mg THC, 25 mg CBD), and a product with equivalent THC and CBD amounts (e.g., 5 mg THC, 5 mg CBD). Participants' plasma cannabinoid levels (in-person participants only) and self-reported subjective effects were assessed before and after ad libitum self-administration. Given (1) that we conducted separate analyses for in-person and remote participants, thus reducing our statistical power, and (2) the dearth of research on legal market edible cannabis products in general, these analyses were considered exploratory in nature.
Materials and Methods
Participants
This study was approved by the University of Colorado Boulder Institutional Review Board and was carried out in accordance with the Declaration of Helsinki. Participants were recruited using social media advertisements and mailed flyers. Participants were compensated $150 for their time and effort.
As a result of the COVID-19 pandemic, the University of Colorado suspended all in-person research in March 2020. Consequently, the research team made the decision to finish the study remotely via Zoom (zoom.us). The revised study protocol was also approved by the university's institutional review board. A total of 50 participants completed the study in-person, and a total of 49 participants completed the study remotely. The primary difference was that participants in the remote version of the study were unable to provide biological samples (e.g., blood). Importantly, there is precedent for conducting acute cannabis administration studies remotely.36
Eligibility criteria
Eligibility screening was conducted by trained research staff. Participants were eligible for participation if they met the following inclusion criteria: (1) between 21 and 70 years of age; (2) cannabis use (any form) at least four times in the past month; (3) endorse prior use of oral cannabis products; (4) no nonprescription drug use (other than cannabis) in the past 60 days; (5) no daily tobacco use; (6) three or fewer drinking occasions per week, and <5 (men)/<4 (women) drinks per drinking occasion; (7) not pregnant or trying to become pregnant; and (8) no current/history of psychotic disorder, bipolar disorder, or schizophrenia.
Baseline appointment
After providing informed consent, in-person participants were screened via urinalysis for pregnancy (if female) and the presence of recreational drugs such as amphetamines, benzodiazepines, cocaine, and opiates. A research staff member also breathalyzed in-person participants to ensure that their blood alcohol content was at 0%. Remote participants were asked to verbally confirm that they were not pregnant (if female) and had not used cannabis, alcohol, or any other recreational drugs that day before their appointment. Next, participants completed self-report measures of demographics, substance use, medical history, subjective drug effects, and mood. In-person participants then provided a baseline blood draw.
At the conclusion of their baseline appointment, participants were randomly assigned to one of the three oral cannabis products: (1) a THC-dominant tablet (e.g., 10 mg THC, 0 mg CBD), (2) a CBD-dominant tablet (e.g., 1.65 mg THC, 25 mg CBD), or (3) a THC+CBD tablet (e.g., 5 mg THC, 5 mg CBD). Participants were instructed to purchase their assigned product at a local study-partnered dispensary and were told that they should use the products as they normally would (i.e., no specific instructions regarding how many tablets should be taken were given to participants). If the dispensary did not have the assigned product in stock, participants were instructed to purchase a tablet with a similar ratio (e.g., participants in the THC+CBD condition could purchase other 1:1 products). Consistent with State of Colorado requirements, the THC and CBD potencies of all study products were labeled according to testing in an International Organization of Standards 17025 accredited laboratory.
Experimental appointment
Between the baseline and experimental appointment, participants were free to familiarize themselves with their assigned oral cannabis product during a 5-day ad libitum use period. On the fifth day, participants completed the experimental appointment. In-person participants completed the experimental appointment in our mobile pharmacology laboratory (which was driven to the residence of each participant), and remote participants completed the appointment over Zoom. Participants were instructed to refrain from cannabis use the day of their experimental appointment. The experimental appointment included two assessment time points: (1) pre-use and (2) 2 h post-use. Before using their oral cannabis product (pre-use), participants completed a 5-day timeline followback (TLFB) and the primary outcome measures (see below).
Then, in-person participants returned to their residence to use as much of their oral cannabis product as they wished. For remote participants, research staff members ended the Zoom call during this ad libitum administration period. Participants recorded the time they consumed their product as well as the total milligrams of THC/CBD. Two hours after using their product, in-person participants returned to the mobile laboratory and remote participants were called back on Zoom to complete the primary outcome measures a second time (2 h post-use). The 2-h post-use time point was selected given findings that the psychotropic effects of orally ingested cannabis reach their maximum after 2 to 3 h.14,37
Measures
Remote and in-person participants completed self-report measures via REDCap (redcap.ucdenver.edu).
Subjective drug effects
Subjective drug effects were intoxication, elation, tension, paranoia, drug liking, and “want more.” Intoxication was assessed with three items, asking participants to indicate how “high” (10-point scale), “mentally stoned” (5-point scale), and “physically stoned” (5-point scale) they felt. Items were averaged to create a composite intoxication score (α=0.76).
Items from the Profile of Mood States (POMS) questionnaire38 were averaged to create subscales of Elation (“joyful,” “euphoric,” “elated,” “cheerful”; α=0.85) and Tension (“nervous,” “anxious,” “unable to relax,” “shaky/jittery”; α=0.73), while a single POMS item assessed paranoia (“paranoid”). POMS items were assessed on a 5-point Likert-type scale ranging from (1) not at all to (5) extremely.
Drug liking (“do you like any of the effects you're feeling?”) and “want more” (“would you like more of the drug you took, right now?”) were each assessed with single items from the Drug Effects Questionnaire39 ranging from (1) not at all to (5) a lot. (Note: Drug liking and “want more” were only assessed at the 2-h post-use time point.)
Plasma cannabinoids
At baseline, pre-use, and 2 h post-use, in-person participants had 32 mL of venous blood drawn at each time point by a certified phlebotomist through venipuncture of a peripheral arm using standard, sterile phlebotomy techniques and plasma was separated from erythrocytes. Quantification of THC, 11-OH-THC, THC-COOH, and CBD was conducted using validated high-performance liquid chromatography/mass spectroscopy (API5500) in Multiple Reaction Monitoring mode.40 All blood draws were conducted at the start of each time point (baseline, pre-use, post-use), before self-report measures.
Timeline followback
Participants completed two calendar-assisted researcher-administered TLFBs,41 once at baseline and once at the beginning of the experimental appointment. The TLFB queried their drug use over (1) the 30-day retrospective time frame before baseline, and (2) the 5-day ad libitum time frame before the experimental appointment.
Cannabis Use Disorder Scale
Cannabis use disorder symptoms were assessed using the Cannabis Use Disorder Scale (CUDS).42 The CUDS is an 11-item measure and was developed based on the Diagnostic and Statistical Manual of Mental Disorders IV's criteria for cannabis dependence.43
Alcohol Use Disorders Identification Test
To further characterize the sample in terms of substance use, participants completed the Alcohol Use Disorders Identification Test (AUDIT),44 a 10-item measure designed to assess participants' alcohol consumption, drinking behaviors, and alcohol-related problems. AUDIT scores range from 0 to 40, with a score of 8 or more indicating harmful or hazardous drinking.
Planned analyses
Primary analyses of plasma cannabinoid concentrations and subjective effects (across the two experimental time points: pre-use and 2 h post-use) were conducted separately using a series of mixed-effects models estimating random intercepts to determine whether conditions were different in terms of their plasma THC levels, plasma THC-COOH levels, plasma 11-OH-THC levels, plasma CBD levels, subjective intoxication, elation, tension, and paranoia. In each model, as fixed effects, we included (1) linear change over time from pre-use to post-use, and (2) a set of two orthogonal contrast codes to test for condition differences.
For each outcome of interest (e.g., plasma THC), we ran three models varying the set of orthogonal contrast codes to test for relevant condition differences in cannabinoid concentrations and subjective effects: THC versus CBD, THC versus THC+CBD, and CBD versus THC+CBD. Interaction effects tested whether the linear change over time varied by condition, and simple effects tests were conducted to determine condition differences at the 2-h post-use assessment time point. (Note: As drug liking and “want more” were not assessed at the pre-use assessment time point, we conducted a series of linear regression models to determine whether these outcomes differed by condition at the 2-h post-use assessment time point.)
All analyses were conducted in R version 3.6.1 (www.rstudio.com). Mixed-effects models were conducted using the lme4 package version 1.1–25, which implements maximum likelihood estimation, and linear regression models were conducted in the stats package version 3.6.2.
Results
One hundred twenty-four participants were recruited for the study, of whom 110 completed both the baseline and experimental session. Of those 110, 11 participants were excluded from the analysis due to reported THC/CBD mg that indicated that they did not use the assigned product (e.g., a participant in the CBD condition reporting no CBD or high levels of THC in the product they consumed during the experimental appointment). Thus, the final sample consisted of 99 participants (men=41, women=55, nonbinary=3), of whom 50 participants completed the study appointments in-person and 49 participants completed the study components remotely. Given the absence of biological verification for remote participants, we present results for in-person and remote participants separately below.
Combined analyses are presented in the Supplementary Materials. (Note: No significant differences in demographics, cannabis use history, other substance use factors, or milligrams of THC and CBD used during the experimental appointment emerged between in-person and remote participants.) Table 1 provides demographics and baseline characteristics of participants across the three edible conditions as a function of group (i.e., in-person or remote). Among in-person participants, no significant differences in demographics, cannabis use history, current cannabis use, or other substance use factors emerged between edible conditions, indicating successful random assignment. Among remote participants, although participants in the CBD condition reported using cannabis concentrates more frequently at baseline and over the 5-day ad libitum use period relative to participants in the THC condition, no other significant differences emerged.
Table 1.
Demographics and Baseline Characteristics by Condition Among In-Person and Remote Participants
| In-person participants (N=50) |
Remote participants (N=49) |
|||||
|---|---|---|---|---|---|---|
| THC |
THC+CBD |
CBD |
THC |
THC+CBD |
CBD |
|
| n=15 | n=19 | n=16 | n=19 | n=15 | n=15 | |
| Demographics | ||||||
| Age | 39 (14.26) | 39.21 (16.60) | 35.06 (13.39) | 40.37 (13.96) | 38.13 (10.29) | 32.87 (16.45) |
| Gender (% female) | 9 (60.0%) | 11 (57.9%) | 9 (37.5%) | 11 (57.9%) | 8 (53.3%) | 7 (46.7%) |
| Education (% bachelors or higher) | 6 (40.0%) | 9 (47.4%) | 8 (56.3%) | 11 (57.9%) | 7 (46.7%) | 7 (46.7%) |
| Employment (% full-time employed) | 7 (46.7%) | 6 (31.6%) | 7 (43.8%) | 9 (47.4%) | 8 (53.3%) | 5 (33.3%) |
| Race (% non-Hispanic White) | 13 (86.7%) | 18 (94.7%) | 14 (87.5%) | 13 (68.4%) | 10 (66.7%) | 15 (100.0%) |
| Beck Depression Inventory scores | 12.47 (10.88) | 13.21 (14.11) | 7.44 (7.62) | 9.89 (9.18) | 13.20 (13.44) | 8.67 (8.86) |
| Beck Anxiety Inventory scores | 9.33 (6.26) | 9.63 (10.67) | 7.44 (11.15) | 9.37 (8.15) | 9.80 (8.15) | 6.13 (4.88) |
| Cannabis history and current use | ||||||
| Cannabis use disorder score | 2.40 (2.29) | 1.47 (1.47) | 1.75 (2.67) | 1.74 (2.18) | 2.60 (2.72) | 2.07 (2.34) |
| Days of flower usea (past 30 days) | 14.60 (12.44) | 9.84 (10.54) | 14.69 (10.70) | 13.16 (12.50) | 17.79 (10.84) | 14.87 (11.45) |
| Days of concentrate use† (past 30 days) | 9.47 (12.23)a,b | 5.95 (10.59)a,b | 6.88 (10.57)a,b | 5.26 (9.64)a | 5.79 (9.99)a,b | 16.07 (10.54)b |
| Days of edible use† (past 30 days) | 7.67 (10.43) | 7.79 (8.77) | 6.81 (9.03) | 5.74 (8.43) | 5.64 (8.31) | 5.80 (8.25) |
| Baseline plasma THC‡ (ng/mL) | 3.43 (5.18) | 1.78 (2.64) | 3.09 (3.76) | N/A | N/A | N/A |
| Baseline plasma CBD‡ (ng/mL) | 1.27 (3.79) | 1.09 (2.42) | 0.57 (0.97) | N/A | N/A | N/A |
| Baseline plasma THC-COOH‡ (ng/mL) | 39.90 (53.79) | 36.09 (81.63) | 53.16 (63.37) | N/A | N/A | N/A |
| Baseline plasma 11-OH-THC‡ (ng/mL) | 1.64 (2.43) | 0.80 (1.53) | 1.59 (1.93) | N/A | N/A | N/A |
| Other substance use factors | ||||||
| Days of alcohol use† (past 30 days) | 6.33 (7.77) | 5.05 (5.10) | 4.56 (7.88) | 6.42 (5.96) | 4.29 (4.78) | 2.67 (4.27) |
| Days of tobacco use† (past 30 days) | 6.07 (12.39) | 3.16 (9.46) | 1.88 (7.50) | 6.37 (12.04) | 4.21 (10.51) | 4.40 (10.11) |
| AUDIT | 2.60 (2.23) | 2.74 (2.62) | 3.38 (3.63) | 4.74 (3.48) | 3.80 (2.57) | 3.33 (3.79) |
| Cannabis use during 5-day ad libitum use period | ||||||
| Days of flower use§ | 2.27 (1.87) | 1.53 (1.50) | 2.75 (1.53) | 1.28 (1.81) | 2.20 (1.78) | 1.73 (1.44) |
| Days of concentrate use§ | 1.67 (1.88)a,b | 1.0 (1.63)a,b | 1.06 (1.65)a,b | 0.50 (1.10)a | 0.80 (1.42)a,b | 2.33 (1.68)b |
| Days of edible use§ (study product) | 1.07 (0.80)a | 1.79 (1.18)a | 1.19 (1.11)a | 3.72 (1.23)b | 4.07 (1.58)b | 3.33 (1.59)b |
| Total mg used§ (study product) | 37.17 (56.46) | 39.44 (26.00) | 64.68 (43.07) | 112.03 (145.70) | 109.07 (66.89) | 122.89 (52.29) |
| Days of edible use§ (non-study product) | 1.47 (1.92) | 1.32 (1.38) | 1.44 (1.67) | 0.06 (0.24) | 0.53 (1.46) | 0.53 (1.25) |
| Total mg used§ (non-study product) | 154.17 (201.46) | 63.08 (82.17) | 82.62 (94.97) | 15.00 (NA) | 112.50 (38.89) | 57.33 (35.23) |
Means and proportions with different superscripts are significantly different from one another, p<0.05. Standard deviations or percentages are included in parentheses.
Using a 30-day timeline followback.
Given restrictions on in-person research during the COVID-19 pandemic, plasma levels were only available for a subset of participants (n=50).
Using a 5-day timeline followback.
AUDIT, Alcohol Use Disorders Identification Test; CBD, cannabidiol; THC, delta-9-tetrahydrocannabinol.
In-person participants
Milligrams of THC versus CBD used by condition
First, to characterize milligrams consumed during ad libitum use and validate product compliance during the experimental appointment among in-person participants, we report milligrams of THC and CBD consumed during the experimental appointment by condition. As presented in Table 2, in-person participants appeared to adhere to their product assignment.
Table 2.
Milligrams of Delta-9-Tetrahydrocannabinol and Cannabidiol Consumed During the Experimental Appointment by Condition (In-Person Participants)
| In-person participants (N=50) |
||||||
|---|---|---|---|---|---|---|
| THC (n=15) |
THC+CBD (n=19) |
CBD (n=16) |
||||
| Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | |
| THC (mg) | 20.00 (11.95) | 10.00–50.00 | 13.29 (9.21) | 5.00–35.00 | 5.61 (3.49) | 0.30–11.55 |
| CBD (mg) | 0.00 (0.00) | 0.00–0.00 | 13.29 (9.21) | 5.00–35.00 | 92.09 (59.77) | 4.90–200.00 |
Plasma cannabinoid concentrations by condition
See Figure 1 for plasma THC and CBD levels across the two experimental time points (pre-use, 2 h post-use) by condition. Plasma THC levels at the 2-h post-use assessment were strongly correlated with self-reported milligrams of THC consumed (r=0.74, p<0.001), and plasma CBD levels at the 2-h post-use assessment were significantly correlated with self-reported milligrams of CBD consumed (r=0.87, p<0.001).
FIG. 1.
Mean plasma cannabinoid levels (ng/mL) across the two assessment time points: pre-use and 2 h post-use. (A) At the post-use assessment, participants in the THC (n=15) condition displayed higher plasma THC levels relative to participants in the CBD (n=16) condition and marginally higher plasma THC levels relative to participants in the THC+CBD (n=19) condition. THC levels did not differ between participants in the CBD and THC+CBD conditions. (B) At the post-use assessment, participants in the CBD condition displayed higher plasma CBD levels relative to participants in the THC and THC+CBD conditions. CBD levels did not differ between participants in the THC and THC+CBD conditions. Error bars are standard errors. CBD, cannabidiol; THC, delta-9-tetrahydrocannabinol.
Plasma THC
Plasma THC levels increased from pre-use to 2 h post-use across all three conditions (B=4.32, standard error [SE]=1.11, p<0.001). The CBD versus THC condition×time interaction was significant (B=3.56, SE=1.36, p=0.01), such that participants in the THC condition displayed a steeper increase relative to participants in the CBD condition. No other condition×time interactions were significant (p>0.17). Simple effects tests indicated that plasma THC levels did not differ by condition at the pre-use assessment (ps>0.33). At the 2-h post-use assessment, participants in the THC condition had significantly higher plasma THC levels compared with participants in the CBD condition (p=0.002) and marginally higher plasma THC levels compared with participants in the THC+CBD condition (p=0.07). THC levels did not significantly differ between participants in the CBD and THC+CBD conditions (p=0.20).
Plasma CBD
Plasma CBD levels increased from pre-use to 2 h post-use across the three conditions (B=12.48, SE=2.04, p<0.001). The CBD versus THC condition×time interaction (B=−14.63, SE=2.52, p<0.001) and the CBD versus THC+CBD condition×time interaction (B=−12.87, SE=2.43, p<0.001) were significant, such that participants in the CBD condition displayed a steeper increase relative to participants in the THC and THC+CBD conditions. Simple effects tests indicated that plasma CBD levels did not differ by condition at the pre-use assessment (ps>0.66). At the 2-h post-use assessment, participants in the CBD condition displayed significantly higher CBD levels compared with participants in the THC (p<0.001) and THC+CBD (p<0.001) conditions. CBD levels did not significantly differ between participants in the THC and THC+CBD conditions (p=0.35).
Plasma 11-OH-THC
Plasma 11-OH-THC levels increased from pre-use to 2 h post-use across the three conditions (B=2.70, SE=0.60, p<0.001). There were no significant condition×time interactions (ps>0.45). Simple effects tests indicated that plasma 11-OH-THC levels did not differ by condition at the pre- or post-use assessments (ps>0.35).
Plasma THC-COOH
Plasma THC-COOH levels increased from pre-use to 2 h post-use across the three conditions (B=11.22, SE=4.25, p=0.01). There were no significant condition×time interactions (ps>0.76). Simple effects tests indicated that plasma THC-COOH levels did not differ by condition at the pre- or post-use assessments (ps>0.10).
Subjective effects during the experimental appointment
Zero-order correlations between 2-h post-use plasma cannabinoid levels and subjective effects are presented in Supplementary Table S1.
Subjective intoxication
There was a significant increase in intoxication from pre-use to 2 h post-use (B=2.00, SE=0.18, p<0.001) across all three conditions (Fig. 2A). The CBD versus THC+CBD condition×time interaction was significant (B=0.58, SE=0.21, p=0.01), and the CBD versus THC condition×time interaction was marginally significant (B=0.37, SE=0.22, p=0.10), such that participants in the THC and THC+CBD conditions displayed a steeper increase in subjective intoxication relative to participants in the CBD condition. Simple effects tests indicated that at the 2-h post-use assessment, participants in the THC condition (p=0.01) and THC+CBD conditions (p<0.001) reported greater intoxication than participants in the CBD condition. Participants reported similar levels of intoxication in the THC and THC+CBD conditions (p=0.40).
FIG. 2.
Mean subjective effects among in-person participants across the two assessment time points: pre-use and 2 h post-use. (A) At the post-use assessment, in-person participants in the THC (n=15) and THC+CBD (n=19) conditions reported greater subjective intoxication relative to in-person participants in the CBD condition (n=16). Levels of subjective intoxication did not differ between in-person participants in the THC and THC+CBD conditions. (B–D) No significant differences in elation, tension, or paranoia emerged at the post-use assessment. Error bars are standard errors. All subjective effects were assessed on a 1 to 5 scale, with the exception of subjective intoxication ratings, which ranged from 1 to 6.67.
Elation
There was a significant increase in elation from pre-use to 2 h post-use (B=0.23, SE=0.10, p=0.03) across all three conditions (Fig. 2B). There were no significant condition×time interactions (ps>0.20). No condition differences in elation emerged at the post-use assessment (ps>0.11).
Tension
There was a marginal decrease in tension from pre-use to 2 h post-use (B=−0.09, SE=0.06, p=0.11) across all three conditions (Fig. 2C). There were no significant condition×time interactions (ps>0.36). No condition differences in tension emerged at the post-use assessment (ps>0.27).
Paranoia
There was not a significant change in paranoia from pre-use to 2 h post-use (B=−0.04, SE=0.06, p=0.55) across conditions (Fig. 2D). There were no significant condition×time interactions (ps>0.23). No condition differences in paranoia emerged at the post-use assessment (ps>0.60).
Drug liking
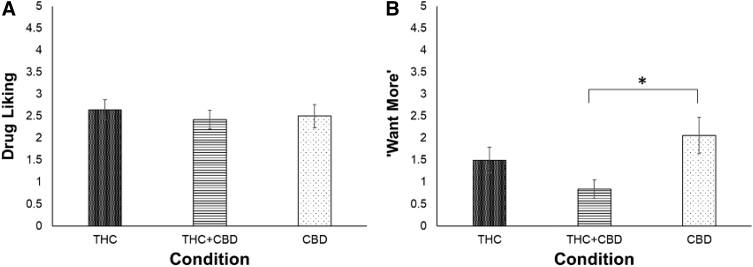
At 2 h post-use, drug liking was similar between participants in the THC and THC+CBD conditions (B=0.11, SE=0.16, p=0.50), THC and CBD conditions (B=0.07, SE=0.17, p=0.68), and THC+CBD and CBD conditions (B=−0.04, SE=0.16, p=0.80; Fig. 3A).
FIG. 3.
Mean drug liking (A) and “want more” (B) reported by in-person participants at the 2-h post-use assessment time point by condition. (A) At the post-use assessment, drug liking did not differ by condition. (B) At the post-use assessment, participants in the CBD condition (n=16) reported wanting to use more of their product relative to participants in the THC+CBD condition (n=19) but not the THC condition (n=15). Ratings of “want more” did not differ between participants in the THC and THC+CBD conditions. Error bars are standard errors. Drug liking and “want more” were assessed on a 1 to 5 scale. *p<0.05.
“Want More”
At 2 h post-use, participants in the CBD condition reported wanting to use significantly more of their product compared with participants in the THC+CBD condition (B=−0.61, SE=0.21, p=0.01). Ratings of “want more” were similar between participants in the THC and CBD conditions (B=−0.28, SE=0.23, p=0.23) and in the THC and THC+CBD conditions (B=0.33, SE=0.22, p=0.14; Fig. 3B).
Remote participants
Milligrams of THC versus CBD used by condition
To characterize milligrams consumed during ad libitum use and validate product compliance during the experimental appointment among remote participants, we report milligrams of THC and CBD consumed during the experimental appointment by condition. As presented in Table 3, remote participants appeared to adhere to their product assignment.
Table 3.
Milligrams of Delta-9-Tetrahydrocannabinol and Cannabidiol Consumed During the Experimental Appointment by Condition (Remote Participants)
| Remote participants (N=49) |
||||||
|---|---|---|---|---|---|---|
| THC (n=19) |
THC+CBD (n=15) |
CBD (n=15) |
||||
| Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | |
| THC (mg) | 34.68 (36.88) | 5.00–150.00 | 17.00 (11.15) | 5.00–50.00 | 5.52 (12.58) | 0.75–50.00 |
| CBD (mg) | 0.00 (0.00) | 0.00–0.00 | 17.00 (11.15) | 5.00–50.00 | 39.75 (39.66) | 1.25–175.00 |
Subjective effects during the experimental appointment
Subjective intoxication
There was a significant increase in intoxication from pre-use to 2 h post-use (B=2.35, SE=0.21, p<0.001) across all three conditions (Fig. 4A). The CBD versus THC+CBD condition×time interaction (B=1.20, SE=0.27, p<0.001) and the CBD versus THC condition×time interaction (B=1.23, SE=0.25, p<0.001) were significant, such that participants in the THC and THC+CBD conditions displayed a steeper increase in subjective intoxication relative to participants in the CBD condition. Simple effects tests indicated that at the 2-h post-use assessment, participants in the THC condition (p<0.001) and THC+CBD conditions (p<0.001) reported greater intoxication than participants in the CBD condition. Participants reported similar levels of intoxication in the THC and THC+CBD conditions (p=0.83).
FIG. 4.
Mean subjective effects among remote participants across the two assessment time points: pre-use and 2 h post-use. (A) At the post-use assessment, remote participants in the THC (n=19) and THC+CBD (n=15) conditions reported greater subjective intoxication relative to remote participants in the CBD condition (n=15). Levels of subjective intoxication did not differ between remote participants in the THC and THC+CBD conditions. (B–D) No significant differences in elation, tension, or paranoia emerged at the post-use assessment. Error bars are standard errors. All subjective effects were assessed on a 1 to 5 scale, with the exception of subjective intoxication ratings, which ranged from 1 to 6.67.
Elation
There was a significant increase in elation from pre-use to 2 h post-use (B=0.42, SE=0.11, p<0.001) across all three conditions (Fig. 4B). There were no significant condition×time interactions (ps>0.46). Participants in the THC condition reported marginally more elation than participants in the CBD condition at the post-use assessment (p=0.07). No other condition differences emerged (ps>0.35).
Tension
There was a significant decrease in tension from pre-use to 2 h post-use (B=−0.34, SE=0.09, p<0.001) across all three conditions (Fig. 4C). There were no significant condition×time interactions (ps>0.21). No condition differences in tension emerged at the post-use assessment (ps>0.56).
Paranoia
There was not a significant change in paranoia from pre-use to 2 h post-use (B=−0.07, SE=0.05, p=0.15) across conditions (Fig. 4D). There were no significant condition×time interactions (ps>0.25). No condition differences in paranoia emerged at the post-use assessment (ps>0.36).
Drug liking
At 2 h post-use, participants in the THC (B=0.36, SE=0.17, p=0.04) and THC+CBD (B=0.37, SE=0.18, p=0.05) conditions reported more drug liking compared with participants in the CBD condition. Drug liking was similar among participants in the THC and THC+CBD conditions (B=−0.01, SE=0.17, p=0.98; Fig. 5A).
FIG. 5.
Mean drug liking (A) and “want more” (B) reported by remote participants at the 2-h post-use assessment time point by condition. (A) At the post-use assessment, participants in the CBD condition (n=15) reported less drug liking relative to participants in the THC (n=19) and THC+CBD conditions (n=15). (B) At the post-use assessment, ratings of “want more” did not differ by condition. Error bars are standard errors. Drug liking and “want more” were assessed on a 1 to 5 scale. *p<0.05.
“Want More”
At 2 h post-use, ratings of “want more” were similar between participants in the THC and THC+CBD conditions (B=−0.03, SE=0.21, p=0.89), THC and CBD conditions (B=−0.33, SE=0.21, p=0.13), and THC+CBD and CBD conditions (B=−0.30, SE=0.23, p=0.19; Fig. 5B).
Discussion
The growing popularity of edible cannabis has ignited a public health need for current and externally valid research data on their acute psychological effects. To understand how edible forms of cannabis may confer distinct subjective effects, the present study utilized an observational design with high external validity to determine the extent to which the subjective effects of edibles vary based on their relative THC and CBD concentrations. As expected, among both in-person and remote participants, participants in the CBD condition reported consuming less THC and more CBD relative to participants in the THC and THC+CBD conditions. In addition, individuals in the THC+CBD condition used less THC than those in the THC condition. Furthermore, among in-person participants, plasma THC and CBD concentrations at the post-use time point were strongly correlated with milligrams of THC and CBD consumed during ad libitum administration, respectively. The strength of these correlations is in line with previous controlled oral administration studies,14 as well as a previous naturalistic study that compared plasma cannabinoid concentrations among flower and edible users,45 supporting the validity of self-reported cannabinoid use based on information contained on Colorado state product labels.
We also examined how subjective effects differed across the conditions. As expected, among both in-person and remote participants, there were differences in subjective intoxication, with participants in the THC and THC+CBD conditions reporting greater intoxication relative to participants in the CBD condition. Interestingly, individuals in the THC+CBD condition reported similar levels of positive effects (intoxication, elation, liking) as those in the THC condition, despite consuming ∼50% less THC, which replicates our previous finding using inhaled cannabis.46 Moreover, this finding may be due to the habitual use of cannabis in our sample as controlled administration studies have shown that the positive subjective effects of THC (e.g., “high,” “liking”) were not dose-dependent in habitual/frequent cannabis users,47–49 but THC can dose-dependently alter subjective effects in non-habitual/infrequent cannabis users.48,50
Interestingly, among in-person participants, participants in the CBD condition expressed a greater desire to use more of their product relative to participants in both THC conditions, although the difference between the CBD and THC condition was not statistically significant. This finding contradicts previous research suggesting that THC produces greater drug craving effects after acute use.48 However, this may reflect participants in the CBD condition needing to use more cannabis to achieve their desired high. Although the same pattern of drug craving was observed among remote participants, none of the condition differences were significant. Additionally, among remote participants, participants in the CBD condition reported significantly less drug liking than participants in the THC and THC+CBD conditions.
The same pattern was not observed among in-person participants. As remote participants in the CBD condition reported using cannabis concentrates more frequently at baseline and during the 5-day ad libitum use period relative to remote participants in the THC condition, this may reflect a preference for higher potency products among these participants. Among both in-person and remote participants, other mood outcomes were similar across all three conditions; this finding supports previous naturalistic and controlled administration studies, which have found that co-administering CBD with THC does not diminish THCs positive subjective effects (e.g., Haney et al33 and Gibson et al46), a finding that has also been observed in both light and heavy cannabis users, as well as a previous report suggesting that CBD may actually enhance the effects of THC on positive mood.35
Previous research suggests that oral formulations of cannabis are associated with an increased risk of adverse experiences relative to combustible cannabis products due to difficulties associated with precise dosing and an increased likelihood of overconsumption.12–14 Interestingly, however, across all conditions, in-person and remote participants reported low levels of psychotomimetic effects (i.e., tension, paranoia) at the post-use time point, and tension actually decreased after cannabis consumption, although marginally among in-person participants. This finding is surprising, given that THC has been associated with increased anxiety and paranoia at higher doses,22 and many participants in the THC and THC+CBD conditions reported consuming significantly more than 10 mg of THC, which is considered a “standard dose” in several states with legal cannabis access.12
Notably, although these results contradict previous findings from controlled administration studies showing an increase in anxiety after consumption of oral THC,22 they are in line with a previous study in which frequent cannabis users did not experience a change in paranoia after acute use of vaporized cannabis as was observed in non-using healthy controls.51 Due to the well-documented effects of long-term cannabis use on CB1 receptor density, it is possible that our participants, who are experienced cannabis users, have developed a tolerance to THCs psychotomimetic effects.49 However, as the psychotropic effects of orally ingested cannabis reach their maximum after 2 to 3 h,14 it is also possible that participants could have experienced greater psychotomimetic effects at a later time point (e.g., 3 h), after the study had concluded.
Indeed, as the full pharmacokinetic curve was not assessed in this naturalistic study, it is possible that the full range of group differences in drug effects may not have been captured by our design. The single post-use time point also limits our ability to capture group differences in plasma cannabinoid concentrations among in-person participants, as peak concentrations of cannabinoids in the blood may have varied due to differences in within-subject physiological factors, such as absorption, metabolic rates, and excretion. However, prior studies have shown that the Tmax of orally consumed cannabinoids is not dose-dependent or affected by the frequency of current or prior cannabis use. Future studies should assess the full pharmacokinetic curve so that the full range of group differences in drug effects and plasma cannabinoid concentrations are captured.
Strengths of the present study include its federally compatible, naturalistic design, which emphasizes external validity and allows us to study cannabis products that are widely available in state-regulated markets. Although tightly controlled laboratory studies are important for establishing internal validity, it is also critical to study the cannabis forms and doses that are widely available in the legal market, in a setting that reflects the conditions in which individuals consume cannabis in real life. This study also provides new data on the amounts of THC and CBD people choose to use when they are free to consume edible cannabis products as they would normally. Additionally, participants were randomized to conditions that differed in cannabinoid composition (i.e., THC, THC+CBD, CBD), making this the first study, to the best of our knowledge, to examine the effects of legal market edible cannabis products with varying ratios of THC and CBD.
Despite these strengths, several important limitations should be noted. First, as this was a naturalistic study, there was no placebo-control condition and participants administered their assigned product ad libitum rather than consuming controlled doses using a standardized administration protocol, which limits our ability to pinpoint causal effects. Indeed, the milligrams consumed varied widely within and between conditions. For instance, among both in-person and remote participants, milligrams of THC consumed in the CBD condition ranged widely (i.e., from 0.30 to 11.55 mg among in-person participants and from 0.75 to 50.0 mg among remote participants), meaning that some participants in the CBD condition were consuming more THC than participants in the THC and THC+CBD conditions.
Additionally, although participants were instructed to use ad libitum (i.e., as they would when using cannabis in their daily life) and could thus use as few or as many tablets as they liked, it is possible that the lower THC content per tablet in the THC+CBD condition (5 mg vs. 10 mg)—rather than the inclusion of CBD—led participants to consume fewer tablets relative to participants in the THC condition. As such, future naturalistic studies should match THC and THC+CBD tablets on THC potency. Furthermore, although most participants in the THC+CBD condition were able to purchase the assigned study product (5 mg THC, 5 mg CBD), a small number of remote participants (n=6) had to purchase a different 1:1 product containing 10 mg THC and 10 mg CBD. As such, it is possible that this led to additional variability in dosing among remote participants in the THC+CBD condition. However, as all participants were experienced edible users who were instructed to use according to their typical use patterns, any variability is likely the result of individual differences in typical use. Future studies should investigate whether edible packaging influences the amount of cannabis consumed by both regular and novice users.
It is also important to note that due to restrictions surrounding in-person research during the COVID-19 pandemic, not all participants completed the study in the same setting; approximately half completed the experimental session in a mobile laboratory, whereas the other half completed the experimental session via Zoom in their own residences. Due to the change to a remote modality halfway through the study, we were unable to conduct breathalyzer, blood, and urine tests for 49 participants; for these participants, we had to rely on self-report data and were unable to biologically confirm that participants had adhered to their product assignment and abstained from other substance use.
Given that were unable to biologically confirm product compliance for these participants, along with research suggesting that participants behave differently when they know there will be biological verification,52 we elected to analyze the in-person and remote groups separately. As such, we were not fully powered to detect the effects of interest and findings should be interpreted with caution. It is important to note, however, that findings in each group—as well as the combined sample presented in the Supplementary Materials—were largely similar. Nevertheless, it is still possible that differences in study settings could have impacted unmeasured aspects of participants' subjective experience of acute cannabis use. Future, fully powered research designs that compare findings across in-person and remote modalities are needed to assess whether remote modalities are a viable alternative to in-person methods when it comes to conducting acute administration studies of legal market cannabis products.
Additionally, although researchers were blind to condition, participants were not, as state legislation requires the THC and CBD potency of all legal market products to be labeled. Thus, it is possible that participants' expectancies could have influenced their self-reports of subjective drug and mood effects. Notably, although a recent report found the cannabinoid content on edible packing in the State of Colorado to be reasonably accurate,53 other studies have found that cannabinoid content is often mislabeled,54 raising the possibility that participants were consuming their products at higher or lower doses than intended. Furthermore, among remote participants, participants in the CBD condition reported using cannabis concentrates more frequently than participants in the THC condition, raising the possibility that findings may be driven, in part, by baseline differences in cannabis use patterns.
Although the number of days participants used study and non-study cannabis products during the 5-day ad libitum use period did not differ by condition among in-person participants, it is possible that unmeasured differences in use patterns could have impacted their responses during the experimental session. Finally, participants were also regular cannabis users, and reported using cannabis edibles an average of 6.60 days per month (in-person participants=7.44, remote participants=5.73) and cannabis flower an average of 13.91 days per month (in-person participants=12.82, remote participants=15.04). As such, these findings may not generalize to infrequent or naive users.
Conclusions
The popularity of edible cannabis products is increasing in states with legal cannabis access;6–8 however, few studies have examined ad libitum use and the acute effects of commercially available cannabis edibles.45 This is the first study, to the best of our knowledge, that has explored whether the acute effects of these products vary as a function of their cannabinoid content, notably the inclusion of CBD. Results suggest that, across both study modalities, participants using a THC+CBD product reported similar levels of positive (e.g., intoxication, elation, liking) and negative (e.g., tension, paranoia) effects relative to participants using a THC-only product. Notably, despite reporting similar levels of subjective effects after acute use, participants in the THC+CBD condition consumed less THC relative to participants in the THC condition.
Furthermore, in-person participants in the THC+CBD condition displayed lower levels of THC exposure relative to participants in the THC condition. Given the potential harms associated with short- and long-term THC exposure (e.g., cognitive impairment),55 the ability to achieve similar levels of desirable effects—such as elation, high, and reductions in tension—at a lower THC dose has important harm reduction potential. At the same time, given possible risks associated with increased acute cannabis reward, to the extent that CBD enhances THCs effects,35 these products could be associated with more risk. Further research, which compares THC and THC+CBD products matched on THC potency, is needed to establish whether THC+CBD products may pose less risks to users relative to THC-only products.
Supplementary Material
Abbreviations Used
- AUDIT
Alcohol Use Disorders Identification Test
- CBD
cannabidiol
- CUDS
Cannabis Use Disorder Scale
- POMS
Profile of Mood States
- SE
standard error
- THC
delta-9-tetrahydrocannabinol
- TLFB
timeline followback
Authors' Contributions
K.E.H. was responsible for study design and for overseeing all aspects of study execution and article preparation. L.P.G. was responsible for conceptualizing and performing the data analysis. L.P.G. and R.L.M. led the interpretation and write-up of findings. E.A.W. and S.W. assisted with data analysis and supported the write-up of findings. C.S. and J.K. validated the laboratory methods and performed the cannabinoid blood analysis for the study. A.D.B. and L.C.B. contributed to the conception and design, analysis and interpretation, drafting and final approval. All authors critically reviewed content and approved the final version for publication.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the National Institute on Drug Abuse (DA039707 to K.E.H.). L.P.G. is supported by the National Science Foundation Graduate Research Fellowship (DGE 1650115). E.A.W. is supported by the National Institute on Mental Health Institutional Research Training Grant (T32MH015442).
Supplementary Material
Cite this article as: Gibson LP, Mueller RL, Winiger EA, Klawitter J, Sempio C, Williams S, Bryan AD, Bidwell LC, Hutchison KE (2024) Cannabinoid exposure and subjective effects of THC and CBD in edible cannabis products, Cannabis and Cannabinoid Research 9:1, 320–334, DOI: 10.1089/can.2022.0020.
References
- 1. Hutchison KE, Bidwell LC, Ellingson JM, et al. Cannabis and health research: Rapid progress requires innovative research designs. Value Health 2019;22(11):1289–1294; doi: 10.1016/j.jval.2019.05.005 [DOI] [PubMed] [Google Scholar]
- 2. Spindle TR, Bonn-Miller MO, Vandrey R. Changing landscape of cannabis: Novel products, formulations, and methods of administration. Curr Opin Psychol 2019;30:98–102; doi: 10.1016/j.copsyc.2019.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Russell C, Rueda S, Room R, et al. Routes of administration for cannabis use—basic prevalence and related health outcomes: A scoping review and synthesis. Int J Drug Policy 2018;52:87–96; doi: 10.1016/j.drugpo.2017.11.008 [DOI] [PubMed] [Google Scholar]
- 4. Freeman TP, Craft S, Wilson J, et al. Changes in delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) concentrations in cannabis over time: Systematic review and meta-analysis. Addiction 2021;116(5):1000–1010; doi: 10.1111/add.15253 [DOI] [PubMed] [Google Scholar]
- 5. Matheson J, Le Foll B. Cannabis legalization and acute harm from high potency cannabis products: A narrative review and recommendations for public health. Front Psychiatry 2020;11:591979; doi: 10.3389/fpsyt.2020.591979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Borodovsky JT, Budney AJ. Legal cannabis laws, home cultivation, and use of edible cannabis products: A growing relationship? Int J Drug Policy 2017;50:102–110; doi: 10.1016/j.drugpo.2017.09.014.Legal [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Borodovsky JT, Crosier BS, Lee DC, et al. Smoking, vaping, eating: Is legalization impacting the way people use cannabis? Int J Drug Policy 2016;36:141–147; doi: 10.1016/j.drugpo.2016.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pacula RL, Jacobson M, Maksabedian EJ. In the weeds: A baseline view of cannabis use among legalizing states and their neighbours. Addiction 2016;111(6):973–980; doi: 10.1111/add.13282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weinkle L, Domen CH, Shelton I, et al. Exploring cannabis use by patients with multiple sclerosis in a state where cannabis is legal. Mult Scler Relat Disord 2019;27:383–390; doi: 10.1016/j.msard.2018.11.022 [DOI] [PubMed] [Google Scholar]
- 10. Bobitt J, Qualls SH, Schuchman M, et al. Qualitative analysis of cannabis use among older adults in Colorado. Drugs Aging 2019;36:655–666; doi: 10.1007/s40266-019-00665-w [DOI] [PubMed] [Google Scholar]
- 11. Murphy F, Sales P, Murphy S, et al. Baby boomers and cannabis delivery systems. J Drug Issues 2015;45(3):293–313; doi: 10.1177/0022042615580991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barrus DG, Capogrossi KL, Cates SC, et al. Tasty THC: Promises and challenges of cannabis edibles. Methods Rep RTI Press 2016;176:139–148; doi: 10.3768/rtipress.2016.op.0035.1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giombi KC, Kosa KM, Rains C, et al. Consumers' perceptions of edible marijuana products for recreational use: Likes, dislikes, and reasons for use. Subst Use Misuse 2018;53:541–547; doi: 10.1080/10826084.2017.1343353 [DOI] [PubMed] [Google Scholar]
- 14. Poyatos L, Pérez-Acevedo AP, Papaseit E, et al. Oral administration of cannabis and Δ-9-tetrahydrocannabinol (THC) preparations: A systematic review. Medicina 2020;56(6):309; doi: 10.3390/medicina56060309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chayasirisobhon S. Mechanisms of action and pharmacokinetics of cannabis. Perm J 2020;25:1–3; doi: 10.7812/TPP/19.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J Anal Toxicol 1992;16(5):276–282; doi: 10.1093/jat/16.5.283 [DOI] [PubMed] [Google Scholar]
- 17. Newmeyer MN, Swortwood MJ, Barnes AJ, et al. Free and glucuronide whole blood cannabinoids' pharmacokinetics after controlled smoked, vaporized, and oral cannabis administration in frequent and occasional cannabis users: Identification of recent cannabis intake. Clin Chem 2016;62(12):1579–1592; doi: 10.1373/clinchem.2016.263475 [DOI] [PubMed] [Google Scholar]
- 18. Chait LD, Zacny JP. Reinforcing and subjective effects of oral Δ9-THC and smoked marijuana in humans. Psychopharmacology (Berl) 1992;107:255–262; doi: 10.1007/BF02245145 [DOI] [PubMed] [Google Scholar]
- 19. Hart C, Ward A, Haney M, et al. Comparison of smoked marijuana and oral delta 9-tetrahydrocannabinol in humans. Psychopharmacology 2002;164:407–415; doi: 10.1007/s00213-002-1231-y [DOI] [PubMed] [Google Scholar]
- 20. Martin-Santos R, Crippa JA, Batalla A, et al. Acute effects of a single, oral dose of d9-tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Curr Pharm Des 2012;18(32):4966–4979; doi: 10.2174/138161212802884780 [DOI] [PubMed] [Google Scholar]
- 21. Hunault CC, Böcker KBE, Stellato RK, et al. Acute subjective effects after smoking joints containing up to 69 mg Δ9-tetrahydrocannabinol in recreational users: A randomized, crossover clinical trial. Psychopharmacology 2014;231:4723–4733; doi: 10.1007/s00213-014-3630-2 [DOI] [PubMed] [Google Scholar]
- 22. Crippa JA, Zuardi AW, Martín-Santos R, et al. Cannabis and anxiety: A critical review of the evidence. Hum Psychopharmacol 2009;24(7):515–523; doi: 10.1002/HUP.1048 [DOI] [PubMed] [Google Scholar]
- 23. Linares IM, Zuardi AW, Pereira LC, et al. Cannabidiol presents an inverted U-shaped dose-response curve in a simulated public speaking test. Rev Bras Psiquiatr 2019;41:9–14; doi: 10.1590/1516-4446-2017-0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zuardi AW, Rodrigues NP, Silva AL, et al. Inverted U-shaped dose-response curve of the anxiolytic effect of cannabidiol during public speaking in real life. Front Pharmacol 2017;8:259–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haney M, Hart CL, Vosburg SK, et al. Marijuana withdrawal in humans: Effects of oral THC or divalproex. Neuropsychopharmacology 2004;29:158–170. [DOI] [PubMed] [Google Scholar]
- 26. Schlienz NJ, Lee DC, Stitzer ML, et al. The effect of high-dose dronabinol (oral THC) maintenance on cannabis self-administration. Drug Alcohol Depend 2018;187:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Freeman AM, Petrilli K, Lees R, et al. How does cannabidiol (CBD) influence the acute effects of delta-9-tetrahydrocannabinol (THC) in humans? A systematic review. Neurosci Biobehav Rev 2019;107:696–712; doi: 10.1016/j.neubiorev.2019.09.036 [DOI] [PubMed] [Google Scholar]
- 28. Bhattacharyya S, Morrison PD, Fusar-Poli P, et al. Opposite effects of Δ-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology 2010;35:764–774; doi: 10.1038/npp.2009.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Englund A, Morrison PD, Nottage J, et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol 2013;27(1):19–27; doi: 10.1177/0269881112460109 [DOI] [PubMed] [Google Scholar]
- 30. Morgan CJ, Freeman TP, Schafer GL, et al. Cannabidiol attenuates the appetitive effects of Δ9-tetrahydrocannabinol in humans smoking their chosen cannabis. Neuropsychopharmacology 2010;35:1879–1885; doi: 10.1038/npp.2010.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Niesink RJM, van Laar MW. Does cannabidiol protect against adverse psychological effects of THC? Front Psychiatry 2013;4:130–130; doi: 10.3389/fpsyt.2013.00130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arkell TR, Lintzeris N, Kevin RC, et al. Cannabidiol (CBD) content in vaporized cannabis does not prevent tetrahydrocannabinol (THC)-induced impairment of driving and cognition. Psychopharmacology 2019;236:2713–2724; doi: 10.1007/s00213-019-05246-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Haney M, Malcolm RJ, Babalonis S, et al. Oral cannabidiol does not alter the subjective, reinforcing or cardiovascular effects of smoked cannabis. Neuropsychopharmacology 2016;41:1974–1982; doi: 10.1038/npp.2015.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Woelfl T, Rohleder C, Mueller JK, et al. Effects of cannabidiol and delta-9-tetrahydrocannabinol on emotion, cognition, and attention: A double-blind, placebo-controlled, randomized experimental trial in healthy volunteers. Front Psychiatry 2020;11:576877; doi: 10.3389/fpsyt.2020.576877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Solowij N, Broyd S, Greenwood LM, et al. A randomised controlled trial of vaporised Δ9-tetrahydrocannabinol and cannabidiol alone and in combination in frequent and infrequent cannabis users: Acute intoxication effects. Eur Arch Psychiatry Clin Neurosci 2019;269(1):17–35; doi: 10.1007/s00406-019-00978-2 [DOI] [PubMed] [Google Scholar]
- 36. Cuttler C, LaFrance EM, Stueber A. Acute effects of high-potency cannabis flower and cannabis concentrates on everyday life memory and decision making. Sci Rep 2021;11:1–13; doi: 10.1038/s41598-021-93198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet 2003;42(4):327–360; doi: 10.2165/00003088-200342040-00003 [DOI] [PubMed] [Google Scholar]
- 38. Shacham S, Reinhardt LC, Raubertas RF, et al. Emotional states and pain: Intraindividual and interindividual measures of association. J Behav Med 1983;6:405–419; doi: 10.1007/BF00846327 [DOI] [PubMed] [Google Scholar]
- 39. Morean ME, de Wit H, King AC, et al. The drug effects questionnaire: Psychometric support across three drug types. Psychopharmacology 2013;227:177–192; doi: 10.1007/s00213-012-2954-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Klawitter J, Sempio C, Mörlein S, et al. An atmospheric pressure chemical ionization MS/MS assay using online extraction for the analysis of 11 cannabinoids and metabolites in human plasma and urine. Ther Drug Monit 2017;39:556–564; doi: 10.1097/FTD.0000000000000427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sobell LC, Sobell MB. Timeline Follow-Back: A Technique for Assessing Self-Reported Alcohol Consumption. In: Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. (Litten RZ, Allen, JP. eds.) Humana Press: Totowa, NJ, USA; 1992; pp. 41–72. [Google Scholar]
- 42. Stephens RS, Roffman RA, Curtin L. Comparison of extended versus brief treatments for marijuana use. J Consult Clin Psychol 2000;68(5):898–908; doi: 10.1037/0022-006X.68.5.898 [DOI] [PubMed] [Google Scholar]
- 43. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. APA: Washington, D.C.,1994. [Google Scholar]
- 44. Saunders JB, Aasland OG, Babor TF, et al. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction 1993;88(6):791–804; doi: 10.1111/j.1360-0443.1993.tb02093.x [DOI] [PubMed] [Google Scholar]
- 45. Bidwell LC, Karoly HC, Torres MO, et al. A naturalistic study of orally administered vs. inhaled legal market cannabis: Cannabinoids exposure, intoxication, and impairment. Psychopharmacology 2021;239:385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gibson LP, Karoly HC, Ellingson JM, et al. Effects of cannabidiol in cannabis flower: Implications for harm reduction. Addict Biol 2021;27(1):e13092; doi: 10.1111/adb.13092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ramesh D, Haney M, Cooper ZD. Marijuana's dose-dependent effects in daily marijuana smokers. Exp Clin Psychopharmacol 2013;21(4):287–293; doi: 10.1037/a0033661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Curran VH, Brignell C, Fletcher S, et al. Cognitive and subjective dose-response effects of acute oral Δ9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology 2002;164:61–70. [DOI] [PubMed] [Google Scholar]
- 49. D'souza DC, Ranganathan M, Braley G, et al. Blunted psychotomimetic and amnestic effects of Δ-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology 2008;33:2505–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schlienz NJ, Spindle TR, Cone EJ, et al. Pharmacodynamic dose effects of oral cannabis ingestion in healthy adults who infrequently use cannabis. Drug Alcohol Depend 2020;211:107969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Morgan CJA, Freeman TP, Hindocha C, et al. Individual and combined effects of acute delta-9-tetrahydrocannabinol and cannabidiol on psychotomimetic symptoms and memory function. Transl Psychiatry 2018;8:181; doi: 10.1038/s41398-018-0191-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Patrick DL, Cheadle A, Thompson DC, et al. The validity of self-reported smoking: A review and meta-analysis. Am J Public Health 1994;84:1086–1093; doi: 10.2105/ajph.84.7.1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Córdova L, Humphreys H, Amend C, et al. MED 2019 Annual Update. Colorado, USA: Colorado Department of Revenue, 2020;1–16. [Google Scholar]
- 54. Vandrey R, Raber JC, Raber ME, et al. Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA 2015;313(24):2491–2493; doi: 10.1001/jama.2015.6613 [DOI] [PubMed] [Google Scholar]
- 55. Morgan CJA, Gardener C, Schafer G, et al. Sub-chronic impact of cannabinoids in street cannabis on cognition, psychotic-like symptoms and psychological well-being. Psychol Med 2012;42:391–400; doi: 10.1017/S0033291711001322 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.