Abstract
Introduction:
Cannabis use is common in people with psychotic disorders and is associated with the exacerbation of symptoms, poor treatment adherence, and an increased risk of relapse. Accurate assessment of cannabis use is thus critical to the clinical management of psychosis.
Discussion:
Cannabis use is usually assessed with self-report questionnaires that were originally developed for healthy individuals or people with a cannabis use disorder. Compared to these groups, the pattern of cannabis use and the associated harms in patients with psychosis are quite different. Moreover, in people with psychosis, the accuracy of self-reported use may be impaired by psychotic symptoms, cognitive deficits, and a desire to conceal use when clinicians have advised against it. Although urinary screening for delta-9-tetrahydrocannabinol is sometimes used in the assessment of acute psychotic episodes, it is not used in routinely. Cannabis use could be assessed by measuring the concentration of cannabinoids in urine and blood, but this is rarely done in either clinical settings or research.
Conclusion:
Using quantitative biological measures could provide a more accurate guide to the effects of use on the disorder than asking patients or using questionnaires.
Keywords: cannabis, cannabis use disorder, psychosis, schizophrenia, assessment, urinalysis, toxicology
Introduction
The prevalence of cannabis use in patients with psychosis is very high,1,2 with as many as 36% of patients with first-episode psychosis and 21% of those with established schizophrenia meeting diagnostic criteria for a cannabis use disorder.3 Moreover, in people with psychosis, cannabis use can have a major effect on the course of the disorder: it is associated with more severe symptoms, an increased risk of relapse and violence, longer hospital admissions, and a lower quality of life.4–7 These effects appear to be dose-dependent, with worse outcomes in frequent users and users of high-potency strains.8,9 A recent study from Denmark found that almost half of the harm associated with cannabis use across the entire population was observed in patients with schizophrenia.10
The harms associated with cannabis in psychosis populations appear to be increasing. Between 2000 and 2016, the incidence of “cannabis-induced psychosis” increased by 67% in Norway, 115% in Denmark, and 238% in Sweden.11 In Canada, the number of patients presenting to emergency departments with a “cannabis-induced psychoses” doubled between 2015 and 2019.12 This is a major issue, as many of these individuals subsequently develop a psychotic disorder.13 In a survey conducted in the United States, the proportion of people with a self-reported diagnosis of a psychotic disorder who also reported daily cannabis use increased from 3% in 2001 to 8% in 2012.14 These trends may be explained by softening societal attitudes to cannabis use, alongside decriminalization and legalization in several jurisdictions.15–17 Another factor may be an increase in the potency of illicit cannabis: since the 1990s, the average concentration of delta-9-tetrahydrocannabinol (THC) quadrupled in the United States, from 4% to 15%, and doubled in Europe, from 6% to 11%.18
Patients with psychosis who stop using cannabis have better outcomes than those who continue to use the drug.5 However, at present, there are no evidence-based pharmacological or psychological treatments to reduce or stop cannabis use,19 an important unmet clinical need. Progress in developing new interventions may have been hampered by the lack of standardized assessments for cannabis use.20 Clinical guidelines for assessing drug use are vague, simply suggesting that clinicians should assess patterns of drug use and that biological tests “may be useful.”21
Quantifying Cannabis Exposure via Self-Report
Several aspects of cannabis use can be assessed: frequency of use, total amount of cannabis used, time spent intoxicated, the subjective effects of intoxication, withdrawal symptoms, motivation to use, desire to quit, functional impairment, and the presence of cannabis use disorder or dependence. A summary of some of the most established self-rating scales is provided in Table 1. From a clinical perspective, assessing total cannabis exposure is important, as it has a dose–response relationship with key clinical outcomes.8,9
Table 1.
Selected Scales for Structured Assessment of Cannabis Use, Cannabis Use Disorder, Cannabis Withdrawal, and Other Harms
| Measure | Aim | Items | Example questions | Validation/psychometric assessment in a psychosis population |
|---|---|---|---|---|
| Timeline Followback Method (TLFB)23 | Quantify recent exposure | From 7 days to 3 months | Can you remember what you did last Saturday? How many joints did you smoke that day? | Hjorthøj et al.24 |
| Daily Sessions, Frequency, Age of Onset, and Quantity of Cannabis Use Inventory (DFAQ-CU)25 | Quantify recent exposure and age of onset | 41 | On a typical day you use marijuana, how many sessions do you have? How many times a day, on a typical weekend, do you use cannabis? |
No |
| Severity of Dependence Scale (SDS)26 | Assess severity of dependence | 5 | Did you think your use of cannabis was out of control? How difficult did you find it to stop, or go without cannabis? |
Hides et al.27 |
| Cannabis Abuse Screening Test (CAST)28 | Screen for cannabis use disorders | 6 | Have you smoked cannabis before midday? Have friends or members of your family told you that you ought to reduce your cannabis use? |
No |
| Cannabis Use Disorders Identification Test–Revised (CUDIT-R)29 | Screen for cannabis use disorders | 8 | How often do you use cannabis? How often during the past 6 months did you fail to do what was normally expected from you because of cannabis? |
No |
| The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST)30 | Screen for substance use disorders | 7 | In the past three months, how often have you… … used cannabis? … had a strong desire or urge to use cannabis? … failed to do what was normally expected of you because of your use of cannabis? |
Hides et al.31 |
| Marijuana Withdrawal Checklist (MWC)32 | Assess withdrawal symptoms | 22 | Indicate how much you are feeling each symptom right now: “Irritability,” “Craving,” “Headaches,” “Sleep problems” |
No |
| Cannabis Withdrawal Scale (CWS)33 | Assess withdrawal symptoms | 19 | I had some angry outbursts Nightmares or strange dreams Trouble getting to sleep |
No |
| Cannabis Experiences Questionnaire (CEQ)34 | Assess symptoms of intoxication | 42 | How often do you have these experiences when smoking cannabis? “Enhanced perceptual awareness,” “Ecstatic,” “Paranoid,” “Depressed” |
Birnbaum et al.35 |
| 13 (Short version) | ||||
| Marijuana Craving Questionnaire (MCQ)36 | Assess craving | 47 | I would do almost anything for a joint Smoking marijuana would help me sleep at night |
No |
| 12 (Short version) | ||||
| Obsessive Compulsive Drug Use Scale for Cannabis (OCDUS-CAN)37 | Assess craving | 12 | If you don't use, how often do you feel the urge or drive to use cannabis? How much control do you have over your cannabis use? |
Dekker et al.38 |
Accurately quantifying cannabis use is difficult as there is no standardized unit of cannabis.22 Users of cannabis may differ in their frequency of use, the number of joints they use each day, joint size or amount per session, formulation (flower, resin, edible, concentrate), potency (i.e., % concentration of THC), and method of administration (smoked, vaporized, or oral) (Fig. 1).
FIG. 1.
The increased diversity of cannabis formulations and methods of administration has complicated the assessment of cannabis exposure.
Perhaps the single most important variable is the frequency of use, normally recorded in days of use per week or month. Frequency is relatively easy to measure, reliable, and has strong associations with cannabis dependence.39 Its main limitation as a metric is that it does not differentiate between a user who smokes a single small joint each evening and another who uses several grams of cannabis throughout the day. This is particularly important for populations with psychosis, as daily use is relatively common. In a recent European study, 45% of all patients with psychosis who had ever used cannabis reported that they were or had been daily smokers, compared to just 15% of controls.1 Estimates of joint size and potency can be inaccurate, an issue which has likely worsened with the arrival of novel formulations such as cannabis concentrates (Fig. 1).40,41 Other aspects, such as route of administration and sharing, make estimating total cannabis exposure even more complicated.42 Even if a self-report assessment was able to measure the exact amount of cannabis used, it would still not account for the large intra- and inter-subject variation in bioavailability. When cannabis is smoked, for example, these estimates range from 2% to 56%.42
The Gold-standard method for collecting self-reported cannabis use data is the Timeline Followback (TLFB).23,43 To complete an assessment, the participant records whether or not they used cannabis on each day over the past week, month, or longer. Most studies use the TLFB to record the number of joints per day, although additional information regarding joint size, formulation, potency, and method of administration can also be collected.44 However, the TLFB is yet to be comprehensively empirically tested as an assessment for cannabis exposure. Its incremental validity, the extent to which a measure provides unique information when used alongside existing tests, should be further examined. In one study, the average grams per cannabis administration, assessed using the TLFB method, had stronger associations with urine cannabinoid levels and cannabis-related harms than simply assessing frequency or quantity of use.45 However, in other studies results have been less encouraging.46,47 A further limitation of the TLFB is that it is time-consuming, complex, and its accuracy depends on the expertise of the assessor and engagement of the user. In research studies, there may be time to complete thorough assessments, but in clinical settings professionals will rarely have the time to collect such detailed data. The majority of clinical trials in populations of patients with psychosis and comorbid cannabis use disorder have used the TLFB method to assess frequency of use and/or quantity of cannabis consumed (Table 2). However, major clinical trials and epidemiological studies which recruit general psychosis patients have used a much more heterogenous range of assessments (Table 3).
Table 2.
Cannabis-Related Outcome Measures Used in Clinical Trials of Comorbid Psychosis and Cannabis Use Disorder
| Study | Population | Intervention | Self-report measures of cannabis exposure | Scales | Biochemical measures |
|---|---|---|---|---|---|
| CapOpus48 | Psychosis and cannabis use disorder, age 18–35, and managed by an early intervention team (n=103) | Motivational interviewing and cognitive behavioral therapy vs. treatment as usual | Number of days cannabis use in the past month, assessed using TLFB “Standard” joints per month. Defined as 0.17 g high-potency cannabis or 0.5 g of cannabis resin, assessed using TLFB |
None | Plasma THC, THC-OH, and THC-COOH concentration |
| Rabin et al.49 | Schizophrenia or schizoaffective disorder and cannabis dependence (n=19) | Single-arm trial of contingency management and individual supportive therapy | Cannabis in grams/day over 4 weeks, assessed using TLFB | Marijuana Withdrawal Checklist | Qualitative urinalysis Creatinine-normalized urine THC-COOH concentration |
| CIRCLE50 | Psychosis and cannabis use disorder, age 18–36, and managed by an early intervention team (n=551) | Contingency management (up to £240)+psychoeducation vs. psychoeducation alone | Number of days cannabis use in the past 3 months, assessed using TLFB | None | Qualitative urinalysis |
| Smeerdijk et al.51 | Recent-onset schizophrenia (n=75) and their parents | Family motivational intervention vs. routine family support | Number of days cannabis use in the past 3 months, assessed using TLFB Cannabis in grams/day assessed using TLFB |
Obsessive Compulsive Drug Use Scale | Qualitative urinalysis |
| Schnell et al.52 | Schizophrenia or schizoaffective disorder and cannabis abuse or dependence (n=30) | Clozapine vs. ziprasidone | Joints per month, assessed using a detailed interview | Stages of Change Readiness and Treatment Eagerness Scale | Qualitative urinalysis and toxicological hair analysis |
THC, delta-9-tetrahydrocannabinol; THC-COOH, 11-nor-9-carboxy-THC; THC-OH, 11-hydroxy-THC; TLFB, Timeline Followback.
Table 3.
Cannabis Exposure-Related Outcome Measures Reported in Major Clinical Trials and Epidemiological Studies in Psychosis Populations
| Study | Design | Population | Self-report measures of cannabis exposure | Other Assessments | Biochemical measures |
|---|---|---|---|---|---|
| CATIE53 | Clinical trial of antipsychotic medications | Schizophrenia (n=1493) | Current use (Y/N) | None | Qualitative urinalysis Hair radioimmunoassay |
| EUFEST54 | Clinical trial of antipsychotic medications | First-episode schizophrenia (n=323) | Frequency of use (days/month) | DSM-IV criteria | None |
| OPTIMISE55 | Clinical trial of antipsychotic medications | Schizophrenia and schizophreniform disorder (n=446) | Frequency of use, amount used, and route of administration | DSM-IV criteria | None |
| PAFIP56 | Clinical trial of antipsychotic medications | First-episode psychosis (n=376) | Baseline: current vs. non-users Follow-up: persistent users, ex-users, and never-users |
Excluded if met DSM-IV criteria for drug dependence | None |
| AESOP57 | Case–control study | First-episode psychosis (n=511) and controls (n=412) | Ever use (verified using case-notes review and collateral history) | None | None |
| EUGEI58,59 | Case–control study | First-episode psychosis (n=901), clinical high risk (n=316), and controls (n=1237) | Cannabis Experiences Questionnaire (includes questions on frequency of use, cannabis potency, and amount per use) | None | Quantitative analysis of plasma |
| NAPLS-260 | Case–control study | Clinical high risk (n=764) and controls (n=280) | Current use (Y/N) Lifetime use (Y/N) Frequency of use |
DSM-IV criteria | None |
DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, fourth edition.
Additional Limitations of Self-Report Measures in Psychosis Populations
The reliability and validity of self-report measures depend on the individual collecting the data, the individual being assessed, and the rationale for and context of testing. A fundamental issue with studies comparing self-reported drug use with an objective test is that the expectation of testing itself may increase the likelihood of honest disclosure and therefore artificially enhance the supposed validity of the self-report measure. Despite this, a significant proportion of people are still hesitant to disclose their illicit drug use in such studies. In a large sample of healthy young people from the United States, only 61% of those with a THC positive urine sample reported that they had used cannabis in the past month.61 Perhaps the only exception to this rule are patients who volunteer for treatment programs or clinical trials for substance use disorders, as they recognize that their drug use is causing harm and are seeking support to reduce it.62 This is a major issue, as most of the data supporting the validity self-reported cannabis use are from studies which recruited this type of patient.63 The effect of this issue on the reliability of self-report measures is demonstrated by comparing two clinical trials in patients with psychosis: CapOpus and CATIE.
CapOpus randomized 103 patients with psychosis who used cannabis to either motivational interviewing and cognitive behavioral therapy or treatment as usual with the aim of reducing their cannabis use.24 It found moderately strong correlations between self-reported number of days of cannabis use (r=0.49) and number of joints smoked per month (r=0.49) with the plasma concentration of THC, which increased after exclusion of extreme outliers (r=0.75 and r=0.83, respectively). The CATIE trial recruited patients with psychosis, with or without current cannabis use, and randomized them to different oral antipsychotics. The study was designed to compare their effectiveness at treating psychotic symptoms, not to reduce problematic substance use.53 Of the 168 participants who had a positive urine or hair test for cannabis in CATIE, almost half (38%) denied that they had used cannabis in the past 90 days. Thus, even when participants knew that they were going to be tested, self-report was unreliable in patients who hadn't actively volunteered to reduce their cannabis use.
In the CapOpus trial, correlations between self-report and plasma THC levels were weaker as symptom severity increased (either total or negative symptoms), but impaired cognition (as measured by a verbal learning task) did not impact the correlation between self-reported cannabis use and plasma THC levels. In the CATIE trial, older age, non-White race, and criminal proceedings were all associated with under-reporting, as were positive psychotic symptoms and impaired cognition.53
Further evidence demonstrating the unreliability of self-report in people with psychosis comes from a study of 203 patients with schizophrenia.64 Just 33 (16%) participants reported illicit substance use within the past 3 months despite 67 (33%) returning a positive hair or urine sample. In another study of forensic patients, under close supervision and with requirements to abstain from drug use, the accuracy of self-report was even worse.65 Of 37 patients with a positive urine drug screen, the majority (70%) denied recent drug use. Tampering of samples was also an issue: 10% of samples were suspiciously dilute and three patients returned consecutive samples with remarkably similar creatinine levels. Together these studies demonstrate how self-report measures are unreliable in psychosis populations. Despite this, few studies in psychosis populations report objective measures of cannabis use (Table 3).
Quantitative Biochemical Assessment of Cannabis Exposure
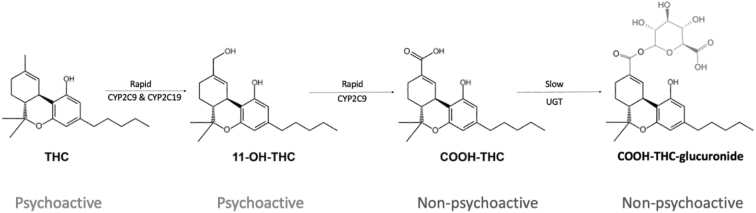
The limitations of self-reported cannabis use in psychosis populations suggest that objective analyses of biological samples may be necessary to obtain an accurate assessment. In this study, we consider which analyses provide the best assessment of overall cannabis exposure, as well as recent use. The main psychoactive constituent of cannabis is THC which is rapidly metabolized to an active metabolite 11-hydroxy-THC (Fig. 2). The concentration of THC is highest during smoking, while 11-hydroxy-THC concentration peaks soon after. 11-hydroxy-THC is converted to carboxy-THC, which is nonpsychoactive and is the most prevalent metabolite in plasma. THC and its metabolites can be measured in blood, urine, saliva, hair, breath, and sweat (Table 4).43,66,67
FIG. 2.
Simplified diagram of THC metabolism. THC, delta-9-tetrahydrocannabinol.
Table 4.
The Advantages and Disadvantages of Testing Different Biological Matrices for THC and Its Metabolites in Cannabis Smokers
| Biological Matrix | Acceptability | Standard analytes | Maximum detection window | Advantages | Limitations |
|---|---|---|---|---|---|
| Urine | High | Carboxy-THC | Infrequent users: several days Heavy users: weeks–months |
Immunoassays can provide an immediate qualitative result Quantitative measurement of carboxy-THC will provide a reasonable estimate of total cannabis exposure if creatinine corrected |
Not a reliable biomarker for recent use in frequent users |
| Blood | Medium | THC Hydroxy-THC Carboxy-THC |
THC and hydroxy-THC: several hours Carboxy-THC and carboxy-THC-glucoronide: several weeks |
THC and hydroxy-THC can be used as biomarkers of recent use in occasional users Quantitative measurement of carboxy-THC will provide a reasonable estimate of total cannabis exposure |
Not a reliable biomarker for recent use in frequent users |
| Hair | Low | Carboxy-THC | Months | A long detection window (up to 3 months) means that it can be used to identify historic use | It is questionable whether quantitative measures are valid biomarkers as exposure to environmental factors such as sunlight can affect results considerably |
| Saliva | High | THC | Several hours | Immunoassays can be used to exclude recent use | Short detection window. Results may be affected by recent consumption of food or drinks. |
| Sweat | Low | THC | 1 week | Cumulative exposure can be measured for up to 7 days. | Dose-response relationship is unreliable |
| Breath | High | THC | Several hours | May be a useful biomarker for identifying recent use (i.e., <4 h) | Not established or validated |
Cannabinoids are highly lipophilic; they build up in fatty tissues and as a result have a very long terminal elimination half-life (>5 days).42 As a result, many heavy cannabis users will have positive plasma and urine samples even after a month of abstinence, and simple immunoassay tests cannot be used to confirm recent abstinence.68,69 Furthermore, for many patients, abstinence may be an unreasonably ambitious objective and a harm reduction approach may be more realistic. In this group, immunoassay tests are also not useful as they do not provide a quantitative result which could demonstrate changes in the amount of cannabis use over time.
The biomarker used in most clinical trials is creatinine-corrected urine carboxy-THC. Carboxy-THC has an initial urinary excretion half-life of about 1.4 days (range=1.0–2.3) in frequent smokers, making it a reasonable biomarker for cannabis exposure.70 To our knowledge, only one study has measured creatinine-corrected carboxy-THC in patients with psychosis (Table 2). Rabin et al. performed gas chromatography-mass spectrometry on the urine of 13 cannabis-dependent patients with schizophrenia and 13 controls with cannabis dependence and no other psychiatric comorbidities.49 They found that the creatinine corrected-carboxy-THC (THC-COOH) was 431±421 ng/mg in patients compared to 882±917 ng/mg in controls (p=0.12), in keeping with the amount of cannabis that each group reported that they used (1.22 ± 0.8 grams per day vs. 1.63 ± 1.2 grams per day [p=0.21], respectively). In another recent study, Barguil et al. collected hair samples from four groups of patients: acute cannabis-induced psychosis, schizophrenia and other chronic psychoses, personality and mood disorders, and a control group of cannabis users hospitalized for a nonpsychiatric illness.71 Perhaps counterintuitively, the lowest mean THC concentration was found in the acute cannabis-induced psychosis group, 0.16 ng/mg (95% confidence interval [CI]=0.016–0.30). The schizophrenia group had a concentration eight times higher, 1.3 ng/mg (95% CI=0.78–1.73). The personality and mood disorder group and the control group had concentrations in between: 0.29 ng/mg (95% CI=0.16–0.43) and 0.44 ng/mg (95% CI=0.23–0.65), respectively. It is unclear whether these observations are solely due to differences in the total cannabis exposure between the groups or may also be due to differences in exposure to environmental factors that can reduce cannabinoid concentrations, such as sunlight and the use of cosmetic hair treatments72,73: patients with severe mental illness may be less frequently exposed to these factors.74
The Potential Advantages of Obtaining Quantitative Biological Assessment of Cannabis Exposure in Psychosis Populations
Quantitative biochemical measurement of cannabinoids could be used to track treatment progress. While the data from people with psychosis are limited, their potential has been demonstrated in several clinical trials of cannabis use disorder.75–77 Each of these trials demonstrated significant differences between treatment groups using urine carboxy-THC as an outcome, despite relatively small sample sizes. Further research is needed to establish whether collecting serial urine samples is valuable at the individual level, particularly as it may not be as informative in users with inconsistent or binge patterns of use. The choice of biomarker will depend on the nature of the treatment program, clinical trial, or epidemiological study in question, and it is important to carefully consider the metabolite (or metabolites) to analyze, as well as which biological medium to sample (Table 4) in each case.
Offering quantitative tests to patients may also promote therapeutic alliance and engagement with mental health treatment.78 It may demonstrate that mental health services are in tune with patients' needs and interests. Many cannabis users are interested in cannabis science and are aware of the range of cannabinoids found in cannabis. This might provide a rationale for measuring a broader profile of compounds, such as cannabidiol, delta-8-THC, and terpenes. However, whether demonstrating to a heavy user that the concentration of cannabis in their body is several times over a limit for safe use would encourage them to moderate their cannabis consumption remains unclear.79
Standardized objective assessments will enable accurate comparisons between research studies across populations and time. Other benefits include the simplicity of data collection, particularly for clinical services who can collect urine and plasma samples without having to train staff to use complex and time-consuming self-report measures. Recently, novel point of care technologies have been developed to measure medication levels using finger-prick samples of blood, a method which could also be used to test for concentrations of cannabinoids.80 Quantitative assessments will also address tampering of urine samples as the samples are creatinine-corrected, a feature which may be particularly useful in high-risk settings, such as forensic services. The main limitation of quantitative analysis is the cost, but in comparison to the costs associated with other medical investigations, hospital admission, or even the cost of a clinician's time to complete an in-depth assessment of substance use, these are small.
Conclusions
There is no gold-standard assessment for assessing cannabis use in people with psychosis. Self-report methods are not accurate, partly because patients are dis-incentivized to disclose their drug use, and because the psychotic and cognitive symptoms that are part of the disorder can impair accurate recall. Quantifying cannabis exposure by measuring cannabinoids in biological samples may prove to be particularly useful both clinically and in research (Table 5). Studies should establish whether creatinine-corrected urine THC-COOH concentration could serve as the gold-standard objective measure of cannabis exposure. Its validity should be further scrutinized in both healthy and psychosis populations, particularly in terms of temporal reliability in consistent users. Further investigation of its value in aiding psychiatric diagnosis and formulation, determining thresholds for risky use, monitoring treatment progress in individual patients, and promoting engagement and therapeutic alliance is worthwhile.
Table 5.
Suggested Approaches to the Assessment of Cannabis Exposure According to Population and Setting
| Setting | Population | Recommendation |
|---|---|---|
| Clinical | Psychosis, engaged with treatment | Further research is required to investigate the validity of different self-report measures, particularly for patients with severe cognitive or psychosis symptoms Qualitative immunoassays may aid honest disclosure of recent drug use |
| Clinical | Psychosis, not engaged with treatment | Self-report measures should be interpreted with caution Quantitative biochemical tests |
| Clinical | Psychosis, high-risk/forensic | Qualitative immunoassay tests may be subject to tampering; quantitative biochemical tests (plasma>urine) are indicated |
| RCT | Psychosis with cannabis use disorder Cannabis use as a primary outcome |
In depth self-report measures such as TLFB or ecological momentary assessment may be worthwhile Quantitative biochemical tests |
| RCT | Psychosis with cannabis use disorder Cannabis use as a secondary outcome |
Concise self-report measures may be sufficient Quantitative biochemical tests |
| Epidemiological study | General population | Concise self-report measures alongside quantitative biochemical tests |
| Epidemiological study | Psychosis populations | Concise self-report measures alongside quantitative biochemical tests |
RCT, randomised controlled trial.
Abbreviations Used
- CI
confidence interval
- THC
delta-9-tetrahydrocannabinol
- THC-COOH
11-nor-9-carboxy-THC
- THC-OH
11-hydroxy-THC
- TLFB
Timeline Followback
Authors' Contributions
E.C.: Conceptualization (lead); writing—original draft (lead); writing—review and editing (equal). W.L.: Writing—review and editing (equal). P.M.: Writing—review and editing (equal).
Author Disclosure Statement
The authors have nothing to disclose.
Funding Information
E.C. is funded by a National Institute for Health and Care Research doctoral research fellowship (NIHR300372).
Cite this article as: Chesney E, Lawn W, McGuire P (2024) Assessing cannabis use in people with psychosis, Cannabis and Cannabinoid Research 9:1, 49–58, DOI: 10.1089/can.2023.0032.
References
- 1. Di Forti M, Quattrone D, Freeman TP, et al. The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): A multicentre case-control study. Lancet Psychiatry 2019;6(5):427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Myles H, Myles N, Large M. Cannabis use in first episode psychosis: Meta-analysis of prevalence, and the time course of initiation and continued use. Aust N Z J Psychiatry 2016;50(3):208–219. [DOI] [PubMed] [Google Scholar]
- 3. Hunt GE, Large MM, Cleary M, et al. Prevalence of comorbid substance use in schizophrenia spectrum disorders in community and clinical settings, 1990–2017: Systematic review and meta-analysis. Drug Alcohol Depend 2018;191:234–258. [DOI] [PubMed] [Google Scholar]
- 4. Henquet C, Van Os J, Kuepper R, et al. Psychosis reactivity to cannabis use in daily life: An experience sampling study. Br J Psychiatry 2010;196(6):447–453. [DOI] [PubMed] [Google Scholar]
- 5. Schoeler T, Monk A, Sami MB, et al. Continued versus discontinued cannabis use in patients with psychosis: A systematic review and meta-analysis. Lancet Psychiatry 2016;3(3):215–225. [DOI] [PubMed] [Google Scholar]
- 6. Bruins J, Pijnenborg GHM, Wunderink L, et al. The association of cannabis use with quality of life and psychosocial functioning in psychosis. Schizophr Res 2021;228:229–234. [DOI] [PubMed] [Google Scholar]
- 7. Lamsma J, Cahn W, Fazel S. Use of illicit substances and violent behaviour in psychotic disorders: Two nationwide case-control studies and meta-analyses. Psychol Med 2020;50(12):2028–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quattrone D, Ferraro L, Tripoli G, et al. Daily use of high-potency cannabis is associated with more positive symptoms in first-episode psychosis patients: The EU-GEI case-control study. Psychol Med 2021;51(8):1329–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schoeler T, Petros N, Di Forti M, et al. Effects of continuation, frequency, and type of cannabis use on relapse in the first 2 years after onset of psychosis: An observational study. Lancet Psychiatry 2016;3(10):947–953. [DOI] [PubMed] [Google Scholar]
- 10. Weye N, Santomauro DF, Agerbo E, et al. Register-based metrics of years lived with disability associated with mental and substance use disorders: A register-based cohort study in Denmark. Lancet Psychiatry 2021;8(4):310–319. [DOI] [PubMed] [Google Scholar]
- 11. Rognli EB, Taipale H, Hjorthøj C, et al. Annual incidence of substance-induced psychoses in Scandinavia from 2000 to 2016. Psychol Med 2023;53(11):5246–5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Callaghan RC, Sanches M, Murray RM, et al. Associations between Canada's cannabis legalization and emergency department presentations for transient cannabis-induced psychosis and schizophrenia conditions: Ontario and Alberta, 2015–2019. Can J Psychiatry 2022;67(8):616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Murrie B, Lappin J, Large M, et al. Transition of substance-induced, brief, and atypical psychoses to schizophrenia: A systematic review and meta-analysis. Schizophr Bull 2020;46(3):505–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Livne O, Shmulewitz D, Sarvet AL, et al. Association of cannabis use-related predictor variables and self-reported psychotic disorders: U.S. adults, 2001–2002 and 2012–2013. Am J Psychiatry 2022;179(1):36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cerdá M, Mauro C, Hamilton A, et al. Association between recreational marijuana legalization in the United States and changes in marijuana use and cannabis use disorder from 2008 to 2016. JAMA Psychiatry 2020;77(2):165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaur N, Keyes KM, Hamilton AD, et al. Trends in cannabis use and attitudes toward legalization and use among Australians from 2001–2016: An age-period-cohort analysis. Addiction 2021;116(5):1152–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Murray RM, Hall W. Will legalization and commercialization of cannabis use increase the incidence and prevalence of psychosis? JAMA Psychiatry 2020;77:777–778. [DOI] [PubMed] [Google Scholar]
- 18. United Nations Office on Drugs and Crime. Drug Market Trends: Cannabis, Opioids. In: World Drug Report 2021: Vienna, Austria; 2021. [Google Scholar]
- 19. Lees R, Hines LA, D'Souza DC, et al. Psychosocial and pharmacological treatments for cannabis use disorder and mental health comorbidities: A narrative review. Psychol Med 2021;51(3):353–364. [DOI] [PubMed] [Google Scholar]
- 20. Lee DC, Schlienz NJ, Peters EN, et al. Systematic review of outcome domains and measures used in psychosocial and pharmacological treatment trials for cannabis use disorder. Drug Alcohol Depend 2019;194:500–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kendall T, Tyrer P, Whittington C, et al. Assessment and management of psychosis with coexisting substance misuse: Summary of NICE guidance. BMJ 2011;342(7800):760–761. [DOI] [PubMed] [Google Scholar]
- 22. Freeman TP, Lorenzetti V. “Standard THC units”: A proposal to standardize dose across all cannabis products and methods of administration. Addiction 2020;115(7):1207–1216. [DOI] [PubMed] [Google Scholar]
- 23. Sobell LC, Sobell MB. Timeline follow-back. In: Measuring alcohol consumption. Humana Press: Totowa, NJ; 1992; pp. 41–72. [Google Scholar]
- 24. Hjorthøj CR, Fohlmann A, Larsen AM, et al. Correlations and agreement between delta-9-tetrahydrocannabinol (THC) in blood plasma and timeline follow-back (TLFB)-assisted self-reported use of cannabis of patients with cannabis use disorder and psychotic illness attending the CapOpus randomized clin. Addiction 2012;107(6):1123–1131. [DOI] [PubMed] [Google Scholar]
- 25. Cuttler C, Spradlin A. Measuring cannabis consumption: Psychometric properties of the Daily Sessions, Frequency, Age of Onset, and Quantity of Cannabis Use Inventory (DFAQ-CU). PLoS One 2017;12(5):e0178194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gossop M, Darke S, Griffiths P, et al. The Severity of Dependence Scale (SDS): Psychometric properties of the SDS in English and Australian samples of heroin, cocaine and amphetamine users. Addiction 1995;90(5):607–614. [DOI] [PubMed] [Google Scholar]
- 27. Hides L, Dawe S, Young RMD, et al. The reliability and validity of the severity of dependence scale for detecting cannabis dependence in psychosis. Addiction 2007;102(1):35–40. [DOI] [PubMed] [Google Scholar]
- 28. Legleye S, Karila L, Beck F, et al. Validation of the CAST, a general population Cannabis Abuse Screening Test. J Subst Use 2007;12(4):233–242. [Google Scholar]
- 29. Adamson SJ, Kay-Lambkin FJ, Baker AL, et al. An improved brief measure of cannabis misuse: The Cannabis Use Disorders Identification Test-Revised (CUDIT-R). Drug Alcohol Depend 2010;110(1–2):137–143. [DOI] [PubMed] [Google Scholar]
- 30. Ali R, Awwad E, Babor TF, et al. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): Development, reliability and feasibility. Addiction 2002;97(9):1183–1194. [DOI] [PubMed] [Google Scholar]
- 31. Hides L, Cotton SM, Berger G, et al. The reliability and validity of the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) in first-episode psychosis. Addict Behav 2009;34(10):821–825. [DOI] [PubMed] [Google Scholar]
- 32. Budney AJ, Novy PL, Hughes JR. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction 1999;94(9):1311–1322. [DOI] [PubMed] [Google Scholar]
- 33. Allsop DJ, Norberg MM, Copeland J, et al. The Cannabis Withdrawal Scale development: Patterns and predictors of cannabis withdrawal and distress. Drug Alcohol Depend 2011;119(1–2):123–129. [DOI] [PubMed] [Google Scholar]
- 34. Quinn CA, Wilson H, Cockshaw W, et al. Development and validation of the cannabis experiences questionnaire—Intoxication effects checklist (CEQ-I) short form. Schizophr Res 2017;189:91–96. [DOI] [PubMed] [Google Scholar]
- 35. Birnbaum ML, Cleary SD, Wan CR, et al. Factor structure of the Cannabis experiences questionnaire in a first-episode psychosis sample. Early Interv Psychiatry 2019;13(3):495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heishman SJ, Singleton EG, Liguori A. Marijuana Craving Questionnaire: Development and initial validation of a self-report instrument. Addiction 2001;96(7):1023–1034. [DOI] [PubMed] [Google Scholar]
- 37. Van Nimwegen LJ, De Haan L, Van Beveren NJM, et al. Effect of olanzapine and risperidone on subjective well-being and craving for cannabis in patients with schizophrenia or related disorders: A double-blind randomized controlled trial. Canadian J Psychiatr 2008;53(6):400–405. [DOI] [PubMed] [Google Scholar]
- 38. Dekker N, Koeter M, Van Den Brink W, et al. Craving for cannabis in patients with psychotic disorder, their non-affected siblings and healthy controls: Psychometric analysis of the obsessive compulsive drug use scale. Int J Methods Psychiatr Res 2012;21(4):286–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Curran HV, Hindocha C, Morgan CJA, et al. Which biological and self-report measures of cannabis use predict cannabis dependency and acute psychotic-like effects? Psychol Med 2019;49(9):1574–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hindocha C, Freeman TP, Curran HV. Anatomy of a Joint: Comparing self-reported and actual dose of cannabis and tobacco in a joint, and how these are influenced by controlled acute administration. Cannabis Cannabinoid Res 2017;2(1):217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Freeman TP, Morgan CJA, Hindocha C, et al. Just say “know”: How do cannabinoid concentrations influence users’ estimates of cannabis potency and the amount they roll in joints? Addiction (Abingdon, England) 2014;109(10):1686–1694. [DOI] [PubMed] [Google Scholar]
- 42. Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers 2007;4(8):1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lorenzetti V, Hindocha C, Petrilli K, et al. The International Cannabis Toolkit (iCannToolkit): A multidisciplinary expert consensus on minimum standards for measuring cannabis use. Addiction 2021;117(6):1510–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lawn W, Mokrysz C, Lees R, et al. The CannTeen Study: Cannabis use disorder, depression, anxiety, and psychotic-like symptoms in adolescent and adult cannabis users and age-matched controls. J Psychopharmacol 2022;36(12):1350–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tomko RL, Baker NL, McClure EA, et al. Incremental validity of estimated cannabis grams as a predictor of problems and cannabinoid biomarkers: Evidence from a clinical trial. Drug Alcohol Depend 2018;182:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Norberg MM, Mackenzie J, Copeland J. Quantifying cannabis use with the Timeline Followback approach: A psychometric evaluation. Drug Alcohol Depend 2012;121(3):247–252. [DOI] [PubMed] [Google Scholar]
- 47. Smith MJ, Alden EC, Herrold AA, et al. Recent self-reported cannabis use is associated with the biometrics of delta-9-tetrahydrocannabinol. J Stud Alcohol Drugs 2018;79(3):441–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hjorthoj CR, Fohlmann A, Larsen AM, et al. Specialized psychosocial treatment plus treatment as usual (TAU) versus TAU for patients with cannabis use disorder and psychosis: The CapOpus randomized trial. Psychol Med 2013;43(7):1499–1510. [DOI] [PubMed] [Google Scholar]
- 49. Rabin RA, Kozak K, Zakzanis KK, et al. A method to achieve extended cannabis abstinence in cannabis dependent patients with schizophrenia and non-psychiatric controls. Schizophr Res 2018;194:47–54. [DOI] [PubMed] [Google Scholar]
- 50. Sheridan Rains L, Marston L, Hinton M, et al. Clinical and cost-effectiveness of contingency management for cannabis use in early psychosis: The CIRCLE randomised clinical trial. BMC Med 2019;17(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Smeerdijk M, Keet R, Van Raaij B, et al. Motivational interviewing and interaction skills training for parents of young adults with recent-onset schizophrenia and co-occurring cannabis use: 15-month follow-up. Psychol Med 2015;45(13):2839–2848. [DOI] [PubMed] [Google Scholar]
- 52. Schnell T, Koethe D, Krasnianski A, et al. Ziprasidone versus clozapine in the treatment of dually diagnosed (DD) patients with schizophrenia and cannabis use disorders: A randomized study. American J Addict 2014;23(3):308–312. [DOI] [PubMed] [Google Scholar]
- 53. Bahorik AL, Newhill CE, Queen CC, et al. Under-reporting of drug use among individuals with schizophrenia: Prevalence and predictors. Psychol Med 2014;44(1):61–69. [DOI] [PubMed] [Google Scholar]
- 54. Wobrock T, Falkai P, Schneider-Axmann T, et al. Comorbid substance abuse in first-episode schizophrenia: Effects on cognition and psychopathology in the EUFEST study. Schizophr Res 2013;147(1):132–139. [DOI] [PubMed] [Google Scholar]
- 55. Kahn RS, Winter van Rossum I, Leucht S, et al. Amisulpride and olanzapine followed by open-label treatment with clozapine in first-episode schizophrenia and schizophreniform disorder (OPTiMiSE): a three-phase switching study. Lancet Psychiatr 2018;5(10):797–807. [DOI] [PubMed] [Google Scholar]
- 56. Gómez-Revuelta M, Pelayo-Terán JM, Juncal-Ruiz M, et al. Antipsychotic treatment effectiveness in first episode of psychosis: PAFIP 3-Year follow-up randomized clinical trials comparing haloperidol, olanzapine, risperidone, aripiprazole, quetiapine, and ziprasidone. Int J Neuropsychopharmacol 2020;23(4):217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Donoghue K, Doody GA, Murray RM, et al. Cannabis use, gender and age of onset of schizophrenia: Data from the ÆSOP study. Psychiatry Res 2014;215(3):528–532. [DOI] [PubMed] [Google Scholar]
- 58. Di Forti M, Quattrone D, Freeman TP, et al. The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): A multicentre case-control study. Lancet Psychiatry 2019;6(5):427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hedges EP, Dickson H, Tognin S, et al. Verbal memory performance predicts remission and functional outcome in people at clinical high-risk for psychosis. Schizophr Res Cogn 2022;28:100222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Buchy L, Seidman LJ, Cadenhead KS, et al. Evaluating the relationship between cannabis use and IQ in youth and young adults at clinical high risk of psychosis. Psychiatry Res 2015;230(3):878–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Harrison LD, Martin SS, Enev T, et al. Comparing drug testing and self-report of drug use among youths and young adults in the general population. Substance Abuse and Mental Health Services Administration, Office of Applied Studies: Rockville, MD; 2007. [Google Scholar]
- 62. Darke S. Self-report among injecting drug users: A review. Drug Alcohol Depend 1998;51(3):253–263. [DOI] [PubMed] [Google Scholar]
- 63. Hjorthøj CR, Hjorthøj AR, Nordentoft M. Validity of Timeline Follow-Back for self-reported use of cannabis and other illicit substances: Systematic review and meta-analysis. Addict Behav 2012;37(3):225–233. [DOI] [PubMed] [Google Scholar]
- 64. Swartz MS, Swanson JW, Hannon MJ. Detection of illicit substance use among persons with schizophrenia by radioimmunoassay of hair. Psychiat Servi 2003;54(6):891–895. [DOI] [PubMed] [Google Scholar]
- 65. Penney SR, McLaren S, Wilkie T. Urine drug screening in a forensic mental health population: frequency and clinical utility in risk management. J Forensic Psychiatry Psychol 2021;32(4):431–448. [Google Scholar]
- 66. Loflin MJE, Kiluk BD, Huestis MA, et al. The state of clinical outcome assessments for cannabis use disorder clinical trials: A review and research agenda. Drug Alcohol Depend 2020;212:107993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hubbard JA, Hoffman MA, Ellis SE, et al. Biomarkers of recent cannabis use in blood, oral fluid and breath. J Anal Toxicol 2021;45(8):820–828. [DOI] [PubMed] [Google Scholar]
- 68. Bergamaschi MM, Karschner EL, Goodwin RS, et al. Impact of prolonged cannabinoid excretion in chronic daily cannabis smokers’ blood on per se drugged driving laws. Clin Chem 2013;59(3):519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Goodwin RS, Darwin WD, Chiang CN, et al. Urinary elimination of 11-nor-9-carboxy-Δ9-tetrahydrocannnabinol in cannabis users during continuously monitored abstinence. J Anal Toxicol 2008;32(8):562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Smith-Kielland A, Skuterud B, Mørland J. Urinary excretion of 11-nor-9-carboxy-Δ9-tetrahydrocannabinol and cannabinoids in frequent and infrequent drug users. J Anal Toxicol 1999;23(5):323–332. [DOI] [PubMed] [Google Scholar]
- 71. Barguil Y, Chiaradia L, Southwell G, et al. Hair concentrations of Δ-9-tetrahydrocannabinol and cannabidiol in cannabis consumers psychiatric patients. Toxicol Anal Ciln 2022;34:247–254. [Google Scholar]
- 72. Van Elsué N, Yegles M. Influence of cosmetic hair treatments on cannabinoids in hair: Bleaching, perming and permanent coloring. Forensic Sci Int 2019;297:270–276. [DOI] [PubMed] [Google Scholar]
- 73. Skopp G, Potsch L, Mauden M. Stability of cannabinoids in hair samples exposed to sunlight. Clin Chem 2000;46(11):1846–1848. [PubMed] [Google Scholar]
- 74. Yee JY, See YM, Abdul Rashid NA, et al. Association between serum levels of bioavailable vitamin D and negative symptoms in first-episode psychosis. Psychiatry Res 2016;243:390–394. [DOI] [PubMed] [Google Scholar]
- 75. D’Souza DC, Cortes-Briones J, Creatura G, et al. Efficacy and safety of a fatty acid amide hydrolase inhibitor (PF-04457845) in the treatment of cannabis withdrawal and dependence in men: a double-blind, placebo-controlled, parallel group, phase 2a single-site randomised controlled trial. Lancet Psychiatry 2019;6(1):35–45. [DOI] [PubMed] [Google Scholar]
- 76. Freeman TP, Hindocha C, Baio G, et al. Cannabidiol for the treatment of cannabis use disorder: A phase 2a, double-blind, placebo-controlled, randomised, adaptive Bayesian trial. Lancet Psychiatry 2020;7(10):865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. McRae-Clark AL, Gray KM, Baker NL, et al. Varenicline as a treatment for cannabis use disorder: A placebo-controlled pilot trial. Drug Alcohol Depend 2021;229:109111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Harris BA, Panozzo G. Therapeutic alliance, relationship building, and communication strategies-for the schizophrenia population: An integrative review. Arch Psychiatr Nurs 2019;33(1):104–111. [DOI] [PubMed] [Google Scholar]
- 79. Fischer B, Robinson T, Bullen C, et al. Lower-Risk Cannabis Use Guidelines (LRCUG) for reducing health harms from non-medical cannabis use: A comprehensive evidence and recommendations update. Int J Drug Policy 2021;103381. [DOI] [PubMed] [Google Scholar]
- 80. Atkins M, Taylor D, Harland R, et al. Acceptability of point of care testing for antipsychotic medication levels in schizophrenia. Psychiatry Res Commun 2022;2(4):100070. [Google Scholar]