Abstract
Background:
Due to their close relationship with the environment, Alaskans are at risk for zoonotic pathogen infection. One way to assess a population’s disease burden is to determine the seroprevalence of pathogens of interest. The objective of this study was to determine the seroprevalence of 11 zoonotic pathogens in people living in Alaska.
Methods:
In a 2007 avian influenza exposure study, we recruited persons with varying wild bird exposures. Using sera from this study, we tested for antibodies to Cryptosporidium spp., Echinococcus spp., Giardia intestinalis, Toxoplasma gondii, Trichinella spp., Brucella spp., Coxiella burnetii, Francisella tularensis, California serogroup bunyaviruses, and hepatitis E virus (HEV).
Results:
Eight hundred eighty-seven persons had sera tested, including 454 subsistence bird hunters and family members, 160 sport bird hunters, 77 avian wildlife biologists, and 196 persons with no wild bird exposure. A subset (n=481) of sera was tested for California serogroup bunyaviruses. We detected antibodies to 10/11 pathogens. Seropositivity to Cryptosporidium spp. (29%), California serotype bunyaviruses (27%), and G. intestinalis (19%) was the most common; 63% (301/481) of sera had antibodies to at least one pathogen. Using a multivariable logistic regression model, Cryptosporidium spp. seropositivity was higher in females (35.7% vs. 25.0%; p=0.01) and G. intestinalis seropositivity was higher in males (21.8% vs. 15.5%; p=0.02). Alaska Native persons were more likely than non-Native persons to be seropositive to C. burnetii (11.7% vs. 3.8%; p=0.005) and less likely to be seropositive to HEV (0.4% vs. 4.1%; p=0.01). Seropositivity to Cryptosporidium spp., C. burnetii, HEV, and Echinococcus granulosus was associated with increasing age (p ≤ 0.01 for all) as was seropositivity to ≥1 pathogen (p<0.0001).
Conclusion:
Seropositivity to zoonotic pathogens is common among Alaskans with the highest to Cryptosporidium spp., California serogroup bunyaviruses, and G. intestinalis. This study provides a baseline for use in assessing seroprevalence changes over time.
Keywords: seroprevalence, antibodies, Alaska
Introduction
Zoonotic diseases are those that can be transmitted from animals to humans or from humans to animals. Worldwide, the majority of pathogens that cause human disease are zoonotic, and zoonotic species are twice as likely to be associated with emerging diseases compared with nonzoonotic species (Taylor et al. 2001, Woolhouse and Gowtage-Sequeria 2005). Between 1940 and 2004, 60.3% of emerging infectious disease events worldwide were caused by zoonotic pathogens (Jones et al. 2008).
People who live and work in Alaska are at risk for infection with zoonotic pathogens. Alaska Native people (the indigenous peoples of Alaska) have a close relationship with the land and depend upon its resources for food and cultural identity (Hess et al. 2008, Parkinson and Berner 2009, Hotez 2010, State of Alaska Section of Epidemiology 2018). Alaskan hunters and trappers, in particular, are at risk as surveys have shown that zoonotic pathogens are present in the environment and in wildlife populations found in Alaska, some of which are taken for sport or subsistence food (Huntley et al. 1963, Walters et al. 1999, Hansen et al. 2011, Jenkins et al. 2013, Minor et al. 2013, Weger et al. 2017). Those working in the Alaskan wilderness may have prolonged exposure to the environment and to wildlife, which increases the likelihood of a zoonotic infection (Pike et al. 2010). Farmers, such as those involved in a 2008 outbreak of Campylobacter, are also at risk (Kwan et al. 2014).
Despite evidence of human zoonotic disease in Alaska, the interactions between hosts, vectors, pathogens, and the environment in Alaska are poorly understood (Hueffer et al. 2011). Environmental and land use changes are occurring in Alaska and affecting its wildlife populations (Parkinson and Butler 2005, Bruce et al. 2016, State of Alaska Section of Epidemiology 2018). This could result in increases in illness or shifts in the geographical range of zoonotic pathogens as has been documented for an outbreak of gastroenteritis in Alaska caused by consumption of raw farmed oysters containing Vibrio parahaemolyticus (McLaughlin et al. 2005). This bacterium is a well-recognized threat in warmer coastal waters of North America, but was previously unreported in Alaska. Surface water temperature above the implicated shellfish beds had warmed enough to support growth of V. parahaemolyticus and allowed the pathogen to extend its range 1000 km north.
Although some zoonotic diseases are reportable to public health authorities, many of these illnesses are rare and likely underreported. This makes detecting changes in disease prevalence difficult and is even harder in Alaska, which has a small widely dispersed population and relatively limited access to laboratory diagnostic services. Therefore, statewide, notifiable disease data likely underestimate the disease burden of zoonoses. As an alternative, studies of antibodies to zoonotic pathogens can be used as a surrogate of exposure among human populations and provide another view of the risk in those populations. Such seroprevalence studies, if repeated at intervals, can help us understand changes in risk and allow correlation with changes in animal and vector populations or environmental conditions. The objective of this study was to determine the seroprevalence of 11 zoonotic pathogens in Alaska Native and non-Native people living and working in rural and urban Alaskan communities.
Materials and Methods
Study participants
This study is a secondary analysis of sera collected for an investigation of exposure to highly pathogenic avian influenza virus H5N1 as described by Reed et al. (2014). During 2007–2008, we enrolled a convenience sample of persons with wild bird contact: Alaska Native bird hunters and their family members, sport bird hunters, and avian wildlife biologists, as well as persons with no reported wild bird contact. Alaska Native family members were defined as anyone living in the same house as a bird hunter at the time of recruitment regardless of their relationship to the hunter. Enrolled hunters had to have hunted for wild birds at least once in the past 2 years; avian wildlife biologists had to have participated in field activity related to wild birds at least once in the past 5 years. Study participants were ≥ 5 years old and resided in 1 of 15 communities throughout Alaska representing both coastal and inland areas and containing a variety of flora and fauna (Fig. 1).
FIG. 1.
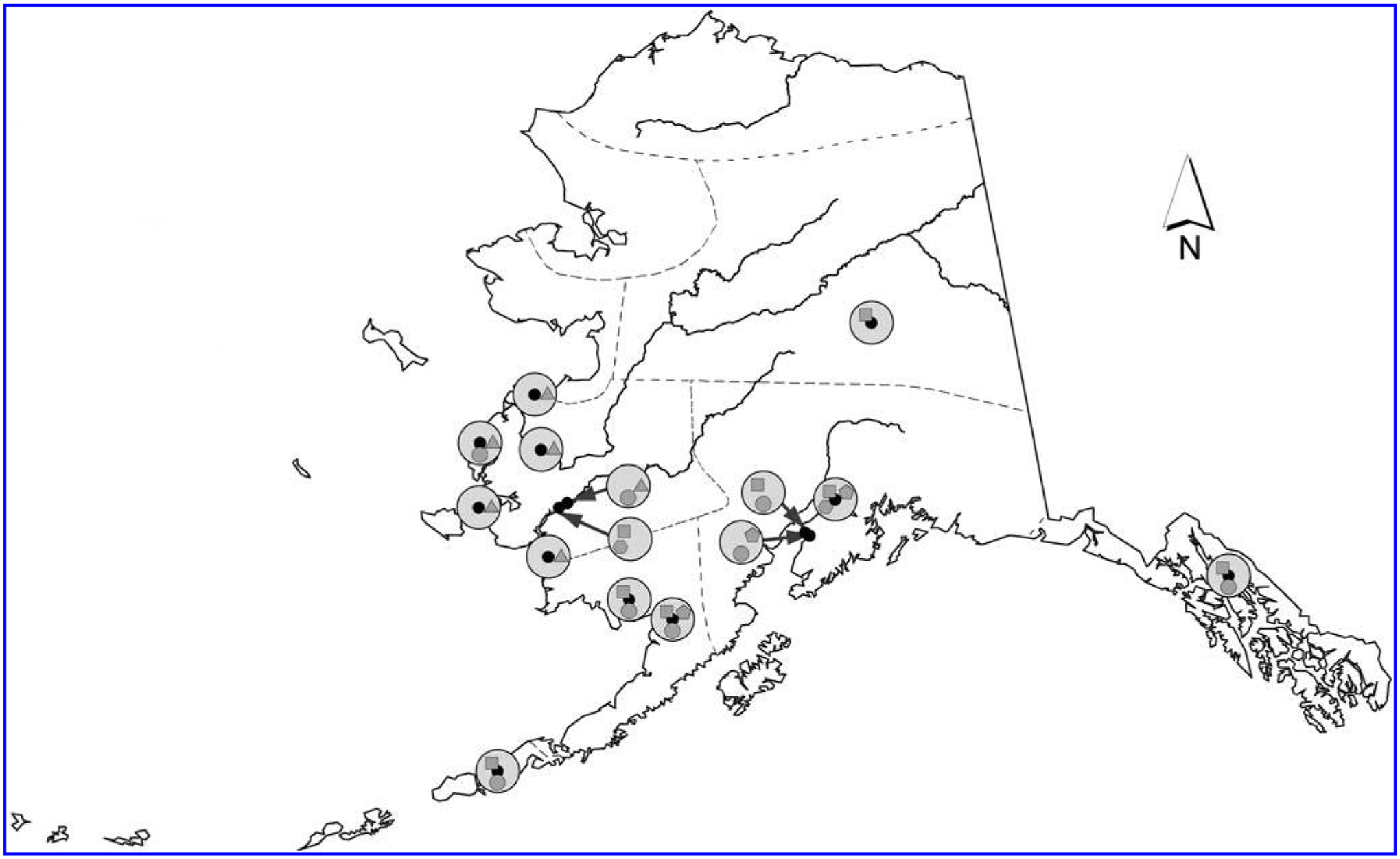
Map of Alaska with study groups identified; 2007–2008.  , Sport Hunters;
, Sport Hunters;  , Subsistence Bird Hunters and Family Members;
, Subsistence Bird Hunters and Family Members;  , No Wild Bird Exposure;
, No Wild Bird Exposure;  , Wildlife Biologists;
, Wildlife Biologists;  , Not Tested for California Serogroup Bunyaviruses; Other Features:
, Not Tested for California Serogroup Bunyaviruses; Other Features:  , Community Location;
, Community Location;  , Community Study Groups;
, Community Study Groups;  , Rivers;
, Rivers;  , Service Units.
, Service Units.
At recruitment, we asked participants questions about risk factors that could be related to avian influenza exposure. Most of these questions were not applicable to the zoonotic diseases we are reporting on here; however, we did retain data regarding cat ownership and how it relates to Toxoplasma gondii. Participants ≥ 18 years of age provided written informed consent that included broad consent for specimen use. Participants <18 years old provided written informed assent in addition to their parent or legal guardian’s written consent. During the consent process, participants were informed that they would not receive individual study results. Aggregate results were disseminated through the Yukon-Kuskokwim Health Corporation and yearly Alaska Native Health Research Conferences. The study was approved by the Institutional Review Boards of the Centers for Disease Control and Prevention and the Alaska Area Indian Health Service, as well as all appropriate regional Alaska Native Health Boards.
Serological assays
From each participant we collected a blood specimen in a standard serum separator tube. Tubes were centrifuged and refrigerated in the field and then transported using cold packs to the Centers for Disease Control and Prevention’s (CDC’s) Arctic Investigations Program in Anchorage where the serum was frozen at −30°C until shipped for testing.
Serum samples were tested for antibodies to Cryptosporidium spp., Echinococcus granulosus, Echinococcus multilocularis, Giardia intestinalis, T. gondii, Trichinella spp., Brucella spp., Coxiella burnetii, Francisella tularensis, California serogroup bunyaviruses, and hepatitis E virus (HEV) (Table 1). Cutoff values indicating seropositivity for each assay were previously determined.
Table 1.
Testing Methodology
| Pathogen | Laboratory methodology | Criteria for positivity | Refs. |
|---|---|---|---|
| Brucella spp. (Brucella abortus, Brucella melitensis, Brucella suis) | Total antibody titers were detected with a microagglutination test using the B. abortus strain 1119-3 as antigen with minor modifications from the reference method (discontinuation of safranin and use of U-bottom plates). This strain reacts with antibodies to naturally occurring strains of B. abortus, B. melitensis, and B. suis. It can also detect the Brucella marine strains. | A total antibody titer of ≥ 160 is a positive result. | Brown et al. (1981) |
| California serogroup bunyaviruses (JC, SSH) | Antibodies were detected using a plaque reduction neutralization test. Serum samples inhibiting at least 90% of possible plaque formation relative to virus controls were deemed positive for viral antibodies. | A titer of ≥ 1:20 is a positive result. | Beaty et al. (1995); Lowell et al. (2011); Rocheleau et al. (2017) |
| Coxiella burnetii | The commercial IgG ELISA (Virion Serion, Wurzburg, Germany) was used according to the manufacturer’s instructions with the exception that samples were diluted 1:100. Positive and borderline samples were further analyzed for both Phase I and Phase II IgG by IFA. The IFA has a sensitivity of 81% and a specificity of 90%. | A positive or equivocal ELISA and a Phase I and/or Phase II IFA titer ≥ 1:64 is a positive result. | Anderson et al. (2009); Kersh et al. (2013) |
| Cryptosporidium spp. (Cryptosporidium parvum, Cryptosporidium felis, Cryptosporidium hominis) | IgG antibodies were detected with a multiplex bead assay using Cp17 and Cp23 as antigen. The Cp17 assay has a sensitivity of 91% and a specificity of 87% compared with the gold standard western blot. The Cp23 assay is 95% sensitive and 100% specific. | Antibody response to ≥ 2 antigens is a positive result. | Priest et al. (2010) |
| Echinococcus granulosus | IgG antibodies were detected with Luminex bead technology using hydatid cyst fluid. This test has a sensitivity of 82% and a specificity of 99%. | A titer of >373.35 MFI is a positive result. | Zhang et al. (2012) |
| Echinococcus multilocularis | IgG antibodies were detected using an ELISA with Em18 antigen. This test has a sensitivity of 86% and a specificity of 99%. | A titer of >8.5U/μL is a positive result. | Ito et al. (2002) |
| Francisella tularensis (F. tularensis tularensis, F. tularensis holarctica) | Total antibodies were detected using a microagglutination test where the titer is determined by counting the last well of full agglutination. | A titer of ≥ 1:128 is a positive result. | Sato et al. (1990) |
| Giardia intestinalis | IgG antibodies were detected with a multiplex bead assay using VSP3 and VSP5 as antigen. This assay has a sensitivity of 93% for stool positive giardiasis patients.a | Antibody response to ≥ 2 antigens is a positive result. | Priest et al. (2010) |
| Hepatitis E Virus | IgG antibodies were detected using the DS-EIA-ANTI-HEV-G Kit (DSI, Italy). This assay uses the ORF2 region of the HEV genome as antigen. | OD ≥ (negative control +0.2) is a positive result. | Kodani et al. (2017) |
| Toxoplasma gondii | The commercial IgG enzyme immunoassay Platelia Toxo-G (Bio-Rad, Hercules, CA) was used according to the manufacturer’s instructions. This test has a sensitivity of 97% and a specificity of 100%. | A titer of >10 IU is a positive result. | Jones et al. (2007); Villard et al. (2016) |
| Trichinella spp. | IgG antibodies were detected using two ELISA kits, the Trichinella antibody detection test kit (Tn-96; Scimedx Corporation) with a sensitivity of 84% and a sensitivity of 94% and the Trichinella spiralis ELISA IgG Test kit (GSD01-3002; Gold Standard Diagnostics) with a sensitivity of >95% and a specificity of 95%.b | A titer of >43.15 is a positive result for the Scimedx kit. A titer of >11U is a positive result for the Gold Standard Diagnostics Kit. |
Gold Standard Diagnostics (2014); Ivanoska et al. (1989) |
n=14 for this comparison.
Due to logistical issues, 719 samples were tested using the Scimedx Kit, and 168 samples were tested using the Gold Standard Diagnostics Kit.
ELISA, enzyme-linked immunosorbent assay; IFA, immunofluorescence assay; JC, Jamestown Canyon; MFI, median fluorescence intensity; SSH, snowshoe hare.
Briefly, cutoffs for T. gondii (>10 IU), Trichinella spp. (>43.15, Scimedx and >11 U, Gold Standard Diagnostics), and HEV (negative control +0.2) followed the kit’s manufacturer guidelines that were determined by testing positive, negative, and potentially cross-reactive sera. Those for E. granulosus (>373.35 median fluorescence intensity [MFI]) and E. multilocularis (>8.5 U/μL) were determined using receiver operator characteristic (ROC) curves that gave the best assay performance using positive, negative, and potentially cross-reactive sera (Ito et al. 2002, Zhang et al. 2012). The C. burnetii immunofluorescence assay cutoff titer (≥ 1:64) was determined by testing sera from vaccinated individuals and unvaccinated blood donors at low risk for exposure (Kersh et al. 2013). Cutoff values for C. parvum Cp17 and Cp23 antigens were determined using sera previously characterized with the large format Western blot IgG assay (Priest et al. 2001, 2010) and for G. intestinalis VSP3 and VSP5 antigens using sera from U.S. citizens with no history of foreign travel (Moss et al. 2014); values in MFI minus background units were 933, 1870, 262, and 206, respectively (Mosites et al. 2018). The titer for F. tularensis (≥ 1:128) follows the World Health Organization guidelines on Tularemia (World Health Organization 2007), and the one for Brucella spp. (≥ 160) is standard for nonendemic regions (Brown et al. 1981). For California serogroup bunyaviruses, the cutoff of ≥ 1/20 has previously been used in diagnostic and research studies and been shown to be a consistent benchmark for identifying current or past exposure to these agents (Blitvich et al. 2012, Rocheleau et al. 2017).
Due to inconsistencies in serum volumes, some samples were not tested for all pathogens. In addition, due to funding constraints, only a subset of available sera was tested for antibodies to California serogroup bunyaviruses. As we have no reason to believe that our wild bird exposure study groups are related to risk of exposure to bunyaviruses, we used geographic location as the basis for this sampling scheme; the sample subset was chosen to have a wide geographic coverage and included persons living in both coastal and inland communities.
Data analysis
Because the demographics (age and gender) varied to a large degree by study group, residence (rural/urban), and race (Alaska Native persons vs. all others), all prevalence estimates were adjusted by age and gender before presentation. For the same reason, p value presentation is restricted to results from multivariate analysis. Variables in the multivariate models were age (as a continuous variable), gender, residence, race, and study group. For the logistic regression model, study groups were combined into a bird exposure group (sport bird hunters, subsistence bird hunters, subsistence family members, and avian wildlife biologists) and a no exposure group (no wild bird exposure). A separate logistic regression model was run for each pathogen with >1 positive result. p Values are two sided and are exact when sample size necessitated. All analyses were run using SAS version 9.4 (Cary, NC).
Results
Study population
Of the 916 people enrolled in the H5N1 avian influenza exposure study, 887 (97%) had sera available for zoonotic disease pathogen seroprevalence testing (Table 2). Ages of people tested for seroprevalence to zoonotic pathogens ranged from 5 to 85 years; 504 (57%) were male; 523 (59%) were Alaska Native.
Table 2.
Demographics of Study Groups; Alaska 2007–2008
| Demographic factor | No wild bird exposure | Sport bird hunters | Avian wildlife biologists | Subsistence bird hunters | Subsistence family members | Total |
|---|---|---|---|---|---|---|
| n (Original study) (Reed et al. 2014) | 204 | 164 | 82 | 237 | 229 | 916 |
| n (Zoonosis pathogen study) | 196 (95%) | 160 (98%) | 77 (94%) | 233 (98%) | 221 (97%) | 887 (97%) |
| Age (years) | ||||||
| <15 | 6% (n = 11) | 3% (n = 5) | 0% (n = 0) | 16% (n = 38) | 33% (n = 72) | 14% (n = 126) |
| 15–24 | 24% (n = 47) | 9% (n = 15) | 4% (n = 3) | 37% (n = 87) | 24% (n = 52) | 23% (n = 204) |
| 25–34 | 19% (n = 38) | 11% (n = 17) | 29% (n = 22) | 16% (n = 37) | 13% (n = 28) | 16% (n = 142) |
| 35–49 | 27% (n = 52) | 36% (n = 58) | 35% (n = 27) | 21% (n = 49) | 17% (n = 38) | 25% (n = 224) |
| ≥ 50 | 24% (n = 48) | 41% (n = 65) | 32% (n = 25) | 9% (n = 22) | 14% (n = 31) | 22% (n = 191) |
| Gender | ||||||
| % Female | 71% (n = 139) | 7% (n = 11) | 32% (n = 25) | 9% (n = 21) | 85% (n = 187) | 43% (n = 383) |
| Residence | ||||||
| % Rural | 24% (n = 47) | 4% (n = 7) | 21% (n = 16) | 100% (n = 233) | 100% (n = 221) | 59% (n = 524) |
| Ethnicity | ||||||
| % Alaska Native Persons | 31% (n = 60) | 6% (n = 9) | 0% (n = 0) | 100% (n = 233) | 100% (n = 221) | 59% (n = 523) |
| n (California serogroup bunyaviruses subgroup) | 84 (43%) | 60 (38%) | 44 (57%) | 130 (56%) | 163 (74%) | 481 (54%) |
There were 233 subsistence bird hunters and 221 subsistence family members. The majority (91%) of subsistence bird hunters were male, while the majority (85%) of their family members were female. All subsistence bird hunters and their family members were Alaska Native and lived in rural communities. We also tested sera from 160 sport bird hunters, 77 avian wildlife biologists, and 196 people with no wild bird exposure. The majority of people in these three groups were non-Native (≥ 69% for all three) and lived in an urban community (≥ 76% for all three). Avian wildlife biologists and sport bird hunters were predominantly male (68% and 93%, respectively), and persons with no wild bird exposure were predominantly female (71%). Participant ages differed between the study groups with 53% of subsistence bird hunters and their family members under the age of 25 years and only 4%, 12%, and 30%, respectively, of avian wildlife biologists, sport bird hunters, and persons with no wild bird contact younger than 25.
Adult subsistence bird hunters had spent an average of 21 years hunting wild birds, with an average of 24 days each year in the 2 years before study enrollment. Adult sport bird hunters had spent an average of 30 years hunting wild birds, with an average of 18 days each year in the 2 years before study enrollment. Avian wildlife biologists had spent an average of 16 years doing fieldwork, with an average of 47 days each year in the 2 years before study enrollment.
We tested for antibodies to California serogroup bunyaviruses in 481/887 (54%) people from seven Alaskan communities (Fig. 1). Of these 481 people, 249 (52%) were male, 330 (69%) were Alaska Native, and 341 (71%) resided in a rural community. Compared with persons whose sample was not tested for antibodies to California serogroup bunyaviruses, the subgroup tested for antibodies to the viruses was more likely to be Alaska Native (69% vs. 48%; p < 0.0001), female (48% vs. 37%; p = 0.0002), and live in a rural community (71% vs. 45%; p < 0.0001). In addition, there is more representation in the subgroup by persons <15 years old and by subsistence family members and less representation by sport bird hunters and persons with no wild bird exposure.
Seroprevalence
We detected antibodies to 10 of the 11 pathogens investigated (Fig. 2); no specimen was positive for antibodies to F. tularensis (0/887). Of those with antibodies detected, seropositivity ranged from 0.1% (1/887) for both Brucella spp. and E. multilocularis to 29% (256/887) for Cryptosporidium spp. Among the 481 persons who had a specimen tested for antibodies to all 11 pathogens, 301 (63%) had antibodies to at least one pathogen.
FIG. 2.
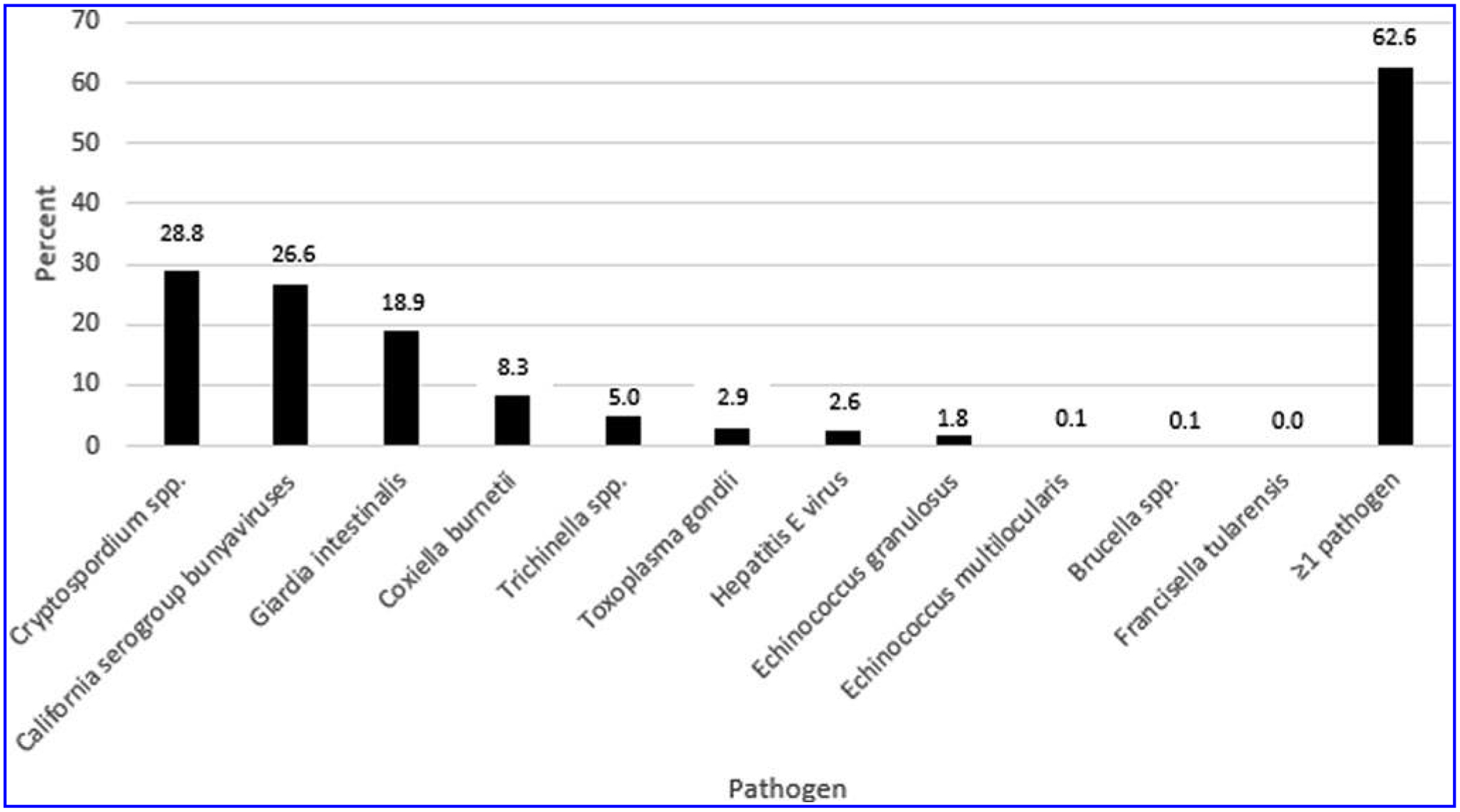
Unadjusted seropositivity to zoonotic pathogens; Alaska 2007–2008.
A separate multivariable model was run for each of the eight pathogens with adequate sample sizes (Table 3). Females were more likely than males to be seropositive to Cryptosporidium spp. (35.7% vs. 25.0%; p = 0.01), while males were more likely than females to be seropositive to G. intestinalis (21.8% vs. 15.5%; p = 0.02). Compared to non-Native persons, Alaska Native persons had a seroprevalence that was threefold higher to C. burnetii (3.8% vs. 11.7%; p = 0.005), and they were significantly less likely to be seropositive to HEV (4.1% vs. 0.4%; p = 0.01). Seropositivity to Cryptosporidium spp., C. burnetii, HEV, and E. granulosus was associated with increasing age (p ≤ 0.01 for all); seropositivity to ≥ 1 pathogen was also associated with increasing age (p < 0.0001). Rural residents were more likely than urban residents to be seropositive to Cryptosporidium spp. (36.2% vs. 21.3%; p = 0.01) and less likely to be seropositive to Trichinella spp. (3.3% vs. 7.7%; p = 0.04). Reporting exposure to wild birds was associated with seropositivity to California serotype bunyaviruses and G. intestinalis (p ≤ 0.01 for both). Finally, cat ownership was related to seropositivity to T. gondii with 7% (7/104) of cat owners exhibiting seropositivity compared with 2% (18/778) among those not owning cats (p = 0.04). However, when we control for residence, cat ownership was no longer significant (p = 0.29) because most of the cat ownership occurred in the urban areas where T. gondii seropositivity was also higher.
Table 3.
Age and Gender Adjusted Seropositivity to Zoonotic Pathogens by Demographic Data; Alaska 2007–2008
| Characteristic | Level | Cryptosporidium spp. (n = 887) | California serogroup bunyavirusesa (n = 481) | Giardia intestinalis (n = 887) | Coxiella burnetii (n = 883) | Trichinella spp. (n = 887) | Toxoplasma gondii (n = 887) | HEV (n = 837) | Echinococcus granulosus (n = 887) | ≥ 1 Pathogen (n = 481) |
|---|---|---|---|---|---|---|---|---|---|---|
| Gender (%) | Female | 35.7 | 24.3 | 15.5 | 8.8 | 5.1 | 2.9 | 3.2 | 1.1 | 66.0 |
| Male | 25.0 | 28.2 | 21.8 | 8.2 | 4.7 | 2.8 | 2.0 | 2.2 | 59.9 | |
| Race (%) | Alaska Native | 35.7 | 25.0 | 27.1 | 11.7 | 4.2 | 1.1 | 0.4 | 2.1 | 65.8 |
| Non-Native | 23.2 | 26.8 | 7.4 | 3.8 | 6.9 | 4.2 | 4.1 | 1.1 | 54.2 | |
| Age class (years, %) | <15 | 4.0 | 17.5 | 35.8 | 4.0 | 0.0 | 3.2 | 0.0 | 1.6 | 50.0 |
| 15–24 | 13.1 | 22.7 | 15.1 | 6.4 | 5.8 | 4.5 | 0.4 | 0.5 | 41.9 | |
| 25–34 | 25.2 | 28.4 | 13.2 | 9.8 | 5.4 | 3.2 | 0.0 | 0.8 | 61.4 | |
| 35–49 | 41.7 | 31.6 | 19.6 | 10.0 | 5.0 | 3.3 | 2.7 | 1.2 | 77.1 | |
| ≥50 | 51.3 | 31.7 | 16.1 | 10.8 | 6.6 | 4.4 | 8.0 | 4.7 | 85.3 | |
| Residence (%) | Rural | 36.2 | 24.8 | 26.4 | 10.7 | 3.3 | 1.5 | 1.0 | 1.6 | 65.4 |
| Urban | 21.3 | 27.1 | 6.8 | 4.6 | 7.7 | 4.1 | 3.8 | 1.4 | 51.7 | |
| Study group (%) | Subsistence bird hunters | 35.1 | 20.2 | 27.0 | 10.0 | 2.5 | 0.7 | 0.0 | 1.3 | 60.8 |
| Subsistence family members | 34.9 | 27.3 | 30.7 | 16.1 | 1.0 | 0.6 | 0.5 | 1.4 | 66.6 | |
| Avian wildlife biologists | 31.0 | 53.8 | 6.9 | 0.9 | 0.7 | 5.8 | 5.0 | 2.2 | 67.9 | |
| Sport bird hunters | 14.0 | 35.7 | 13.3 | 2.0 | 5.1 | 5.3 | 7.3 | 0.7 | 53.2 | |
| No wild bird exposure | 20.3 | 16.2 | 5.2 | 7.9 | 6.5 | 1.6 | 3.5 | 1.6 | 48.9 | |
| Multivariate p valuesb | ||||||||||
| Gender | 0.01 | 0.93 | 0.02 | 0.32 | 0.94 | 0.33 | 0.07 | 0.19 | ||
| Race | 0.21 | 0.25 | 0.11 | 0.005 | 0.48 | 0.12 | 0.01 | 1.00 | ||
| Age | <0.0001 | 0.06 | 0.60 | <0.0001 | 0.29 | 0.76 | <0.0001 | 0.01 | <0.0001 | |
| Residence | 0.01 | 0.85 | 0.05 | 0.34 | 0.04 | 0.45 | 0.90 | 0.74 | 0.28 | |
| Bird exposurec | 0.39 | 0.01 | 0.003 | 0.11 | 0.13 | 0.25 | 0.37 | 0.28 | 0.07 | |
JC, SSH.
p Values are from a multivariable logistic regression model. Due to sample size limitations, the Echinococcus granulosus model only included age, residence, and bird exposure. Significant p values are bolded.
Subsistence bird hunters and family members, sport bird hunters, and avian wildlife biologists versus no wild bird exposure.
JC, Jamestown Canyon; SSH, snowshoe hare.
Discussion
This report provides new information on seroprevalence among Alaskans to a group of zoonotic pathogens that have potential for emergence. Previous seroprevalence reports from Alaska exist for some of these pathogens but they are decades old and are focused on single pathogens (Table 4). This study included five groups of Alaskans who may be at increased risk for exposure to wildlife infected with or carrying zoonotic pathogens. Among participants with serum tested for all 11 pathogens, nearly two-thirds were seropositive to at least one pathogen. Seropositivity to Cryptosporidium spp., California serogroup bunyaviruses, and G. intestinalis was the most common. Seropositivity to at least one pathogen increased with increasing age with 85% of people 50 or older seropositive. We observed a greater proportion of people seropositive to G. intestinalis among our bird exposure groups, to California serogroup bunyaviruses among avian wildlife biologists, and to C. burnetii among Alaska Native people. This suggests that exposure to these pathogens may be related to time spent in close contact with wildlife and the environment.
Table 4.
Human Zoonotic Disease Prevalence Studies Conducted in Alaska, 1967–2008
| Description of study | Refs. | Feltz et al. (1972) | Zarnke et al. (1983) | Stansfield et al. (1988) | Walters et al. (1999) | Murphy (1981) | State of Alaska Section of Epidemiology (1986) | State of Alaska Section of Epidemiology (2014) | Peterson et al. (1974) | Miller (1974) | Current study |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | 1968–1969 | 1979 | 1981 | 1983–1986 | 1969–1979 | 1986 | 1980–2000 | 1970–1971 | 1968–1969 | 2007–2008 | |
| Location | East central | Western | Statewide | Statewide | Statewide | Southcentral | Southwest | Northwest, Southeast, Westcentral | Southwest, East central | Western, Southcentral, Interior, Southeast | |
| Population sampled | 325 Alaska Native people | 121 Alaska Native people | 435 USFS and USGS employees | 1635 Alaska Native people | 10,816 Alaskans | 59 Child care attendees £5 years old | 621 Alaska Native people | 1056 Alaska Native people | 1212 Alaska Native people | 887 Alaskan bird hunters and families, avian wildlife biologists, persons with no wild bird exposure | |
| Test method | Plaque reduction neutralizationa | Plaque reduction neutralizationa | Plaque reduction neutralizationa | Antibody capture ELISA | Fecal ether formalin | Fecal immunofluorescent assay | Immunofluorescent assaya | Indirect fluorescent antibody | Tube agglutination testa | Various | |
| Pathogenb | Prevalence | ||||||||||
| California serogroup bunyavirusesc | 72% | 54% (JC); 42% (SSH) | 22% | 17.6% (JC); 6.8% (SSH) | 27% | ||||||
| Giardia intestinalis | 3% | 14% | 19% | ||||||||
| Coxiella burnetii | 12% | 8% | |||||||||
| Toxoplasma gondii | 31% | 3% | |||||||||
| Francisella tularensis | 5%d | 0% |
Similar method as the one used in this study.
We know of no previous population-based prevalence studies from Alaska for Cryptosporidium spp., Trichinella spp., HEV, Echinococcus granulosus, Brucella spp., and Echinococcus multilocularis.
JC and SSH seropositivity data given, when available.
A cutoff of ≥ 1:20 was used in this study.
HEV, hepatitis E virus; USFS, United States Forest Service; USGS, United States Geological Survey; JC, Jamestown Canyon; SSH, snowshoe hare.
Almost one-third of participants in our study had antibodies to Cryptosporidium spp. This is slightly higher than the U.S. seroprevalence of 21.2% found from sera collected as part of the 1999–2000 National Health and Nutrition Examination Survey (NHANES) (Becker et al. 2015). In our study, women were more likely than men to be antibody positive.
Transmission of Cryptosporidium oocysts occurs directly from person-to-person by means of the fecal–oral route or indirectly through contaminated drinking or recreational water (Jenkins et al. 2013). Direct transmission is often related to caring for children or elders (Ryan et al. 2014). Because women are more often the primary caregivers for ill household members, our data are suggestive of this transmission mechanism in our study.
Despite the high proportion of seropositivity found in this study, cryptosporidiosis among Alaskans is uncommonly reported to health officials. For 2007–2008, only seven cases of cryptosporidiosis were reported to the Alaska Department of Health and Social Services (AK DHSS) with the 2008 Alaska incidence one-eighth of the U.S. average (0.4 vs. 3.5 cases/100,000 people) (Yoder et al. 2010a). The Alaska incidence rate was consistently lower than the U.S. rate over a 12-year period, 1999–2010 (Jenkins et al. 2013).
There are a number of possible explanations for the discrepancy between the proportion of seropositivity found in this study and the incidence rate for the same time period. Antibodies to Cryptosporidium spp. are relatively long lasting and do not necessarily represent current infection; thus, we would expect seropositivity to be higher. In addition, current infection is identified by visualization of oocysts in stool, which can be difficult, often requiring a special acid-fast test which a physician may not request or which may not be available. This means that illness may be underdiagnosed and thus underreported. Finally, asymptomatic infections are known to occur, especially in adults who have had multiple previous exposures.
Despite reports describing outbreaks of cryptosporidiosis in Arctic regions outside of Alaska, we can find no other reports of seroprevalence of the organism in humans living in the Arctic. Additional studies from other Arctic countries would be of value for comparison. For a more detailed review of our data on Cryptosporidium spp. and G. intestinalis, please refer to a recent publication by Mosites et al. (2018) that was able to include data on access to different water sources and their association with seropositivity.
In the contiguous United States, California serogroup bunyaviruses are considered emerging threats, potentially causing meningitis or encephalitis (Walters et al. 1999). Their transmission cycle to humans involves mosquitoes, which are abundant in Alaska in the summer months. In this study, 27% of participants were seropositive to Jamestown Canyon (JC) and/or snowshoe hare (SSH) bunyaviruses. Seropositivity was nearly 18% in persons <15 years old, so exposure to these viruses appears to be ongoing.
Four studies in Alaska conducted since 1967 (Table 4) report a range of JC and/or SSH seroprevalence from 22% in a study of persons with potential occupational exposure to the pathogens to 72% in a 1968–1969 study of Alaska Native people living in the east central part of the state (Feltz et al. 1972, Zarnke et al. 1983, Stansfield et al. 1988, Walters et al. 1999). In our study, avian wildlife biologists had the highest exposure at 54%. The fieldwork conducted by Alaskan avian wildlife biologists consists of days to months spent continuously in remote portions of Alaska, generally living in tent-type structures or small cabins. In the study by Stansfield et al. (1988), where 22% of U.S. Geological Survey and U.S. Forest Service employees showed exposure to JC and/or SSH, only 59.4% of participants reported having done any field work; the amount of time spent in the field was positively associated with seroprevalence. Avian wildlife biologists in our study had spent an average of 47 days in the field in each of the 2 years before study enrollment; thus, their mosquito exposure was likely higher compared with participants in the Stansfield et al. study and is suggestive of extensive time outdoors being associated with exposure to these viruses.
G. intestinalis seropositivity was the third highest in our study with 19% of persons having antibody. A previous statewide all ages study from 1969 to 1979 found a much lower prevalence of 3% (Table 4) (Murphy 1981). However, that study used a stool ova and parasite assay that detects active cyst shedding and is indicative of current infection. Antibodies to G. intestinalis are relatively long lasting so will reflect both current and historic exposure. In the current study, seropositivity was associated with male gender and was higher in our bird exposure groups. Seropositivity was high in persons <15 years old (36%) leading us to conclude that transmission continues.
Giardiasis is a commonly reported disease to the AK DHSS, and annual rates are normally higher for Alaska compared with the rest of the United States. Alaska rates in 2006–2008 ranged from 11.6–16.5/100,000; this compares with the U.S. average of 7.4–7.6/100,000 for those same years (Yoder et al. 2010b). Similar to our seroprevalence data, the percentage of cases of giardiasis in the U.S. is higher for males (55.5–56.4% for 2006–2008) than for females (Yoder et al. 2010b).
The higher seropositivity in our bird exposure groups is suggestive that time spent in the Alaskan wilderness may be a risk factor. A known risk factor for giardiasis is consuming unfiltered or untreated water or failing to practice good hygiene behaviors when participating in outdoor activities such as hunting, backpacking, or camping (Birkhead and Vogt 1989, Welch 2000, Yoder et al. 2010b, Reses et al. 2018). Unfortunately, we did not collect extensive data on wilderness water use or hygiene practices so cannot comment on this as a risk factor in our study.
Seropositivity to C. burnetii in this study was 8%. In a 2014 report, sera collected from 1980 to 2000 were tested for antibodies to C. burnetii (Table 4) (State of Alaska Section of Epidemiology 2014). The sera were from Alaska Native persons <1 to 91 years old living on the Pribilof Islands. In that study, 12% of sera were antibody positive, and persons ≥ 20 years old were nearly twice as likely to have a positive result compared with persons aged <20 years (13% vs. 7%). In the current study, we also found a positive association with increasing age as did Sampasa-Kanyinga et al. in their 2008 study conducted in a Canadian Cree community (Sampasa-Kanyinga et al. 2012).
Seroprevalence in the current study was higher in Alaska Native persons compared with non-Native persons. This may be due to their close relationship with the land and its resources, which may put them in contact with animal hosts more frequently. Adult Alaska Native subsistence bird hunters in this study had spent an average of 21 years hunting wild birds. In addition, C. burnetii antibodies have been detected in wildlife such as northern fur seal, Steller sea lion, caribou, and Dall’s sheep (Zarnke 1983, Minor et al. 2013), all of which are taken for subsistence use. From sera collected during the 2003–2004 NHANES cycle, the overall U.S. adult C. burnetii seroprevalence was 3.1% and from the 2004 Nunavik Health Survey (NHS) of Nunavik Inuit in Northern Quebec, Canada, the seroprevalence was <1% (Anderson et al. 2009, Messier et al. 2012). These are both lower than what has been found in the two surveys from Alaska.
Q fever, the disease associated with C. burnetii infection, has been a reportable condition to the AK DHSS since 2007, but through 2014 no locally-acquired case had been identified (State of Alaska Section of Epidemiology 2011b, 2014). Q fever infections are often asymptomatic or have nonspecific symptoms so diagnosis can be difficult (Anderson et al. 2013). Infection is likely underdiagnosed and underreported making seroprevalence studies such as this one important to understanding the prevalence of exposure in Alaska.
In this study Trichinella spp. seropositivity was 5% which is higher than that reported from the Canadian NHS (<1%) yet lower than the 19% reported from the 2007–2008 International Polar Year Inuit Health Survey for Adults (IPY IHSA) (Messier et al. 2012, Goyette et al. 2014). Trichinellosis has been a notifiable disease to the AK DHSS since 1968 and is a current public health threat in Alaska. The Alaska incidence in 2008–2012 was more than 40 times higher than the overall U.S. incidence (4.1 vs. 0.1 cases/1,000,000) (Wilson et al. 2015). Historically, ~¾ of trichinellosis infections in the United States were associated with T. spiralis from undercooked pork; however, more recently a greater proportion are associated with game meat that harbors T. nativa or T6 (Wilson et al. 2015). In Alaska, disease is often due to consumption of undercooked bear or walrus meat; a 2017 trichinellosis outbreak in Northwest Alaska was associated with eating walrus infected with T. nativa (Springer et al. 2017).
Despite the high incidence, cases of trichinellosis in Alaska have been declining in recent years. No cases were reported in 12 of the 20 years from 1991–2010, and 1992 was the last year there were more than 10 cases (Jenkins et al. 2013). Our seroprevalence data seem to support the incidence data. No one under the age of 15 had detectable antibodies to Trichinella spp. Prevalence in the other age groups likely represents pre-1992 exposure when incidence in Alaska was higher.
Toxoplasmosis is not a reportable condition in Alaska; thus, seroprevalence studies such as this one are important to understanding prevalence of infection with T. gondii. A 1970–1971 T. gondii study conducted in 11 rural Alaska communities reported a seroprevalence of 31% (Table 4), and in 14 Northern Canadian study sites, seroprevalence ranged from 3% to 65%, with most of the sites (10/14) reporting a seroprevalence at or slightly higher (3–14%) than the 3% we are reporting in this study (Peterson et al. 1974, Jenkins et al. 2013, Goyette et al. 2014).
Human infection with T. gondii can occur from ingesting water or food contaminated with oocysts from cat feces or by ingesting tissue cysts in undercooked contaminated meat. In this study, as well as the earlier study conducted in Alaska, cat ownership was associated with having antibodies to T. gondii. However, in our study, only 12% of people reported owning cats, so other transmission routes may be of more importance.
Although the earlier Alaska study used an IFA testing methodology instead of the IgG enzyme-linked immunosorbent assay (ELISA) used in the current study, the performance of the two tests is similar so the results are comparable (Pearce et al. 2013). Thus, it seems that the seroprevalence of T. gondii may be decreasing in Alaska. This is similar to what has been reported from the United States as a whole. Using sera collected from three NHANES surveys, age-adjusted seroprevalence in U.S.-born persons 12–49 years old has steadily decreased from 14.1% in 1988–1994 to 9.0% in 1999–2004 and finally to 6.7% in 2009–2010 (Jones et al. 2014).
As far as we know, there are no reports of either HEV or Brucella spp. seroprevalence in Alaska. In this study, overall seroprevalence to HEV was 3% and was very low in Alaska Native persons (0.4%). Seroprevalence was also low (0.4%) among younger persons (<35 years), but increased to 8% in persons ≥ 50 years. HEV antibody durability is largely unknown, but if antibodies to HEV are long lasting, this is suggestive of reduced exposure in recent years. In many parts of the world HEV is a pork-related zoonosis (Christou and Kosmidou 2013); however, deer and caribou in Canada have also exhibited seropositivity to the virus (Weger et al. 2017). As these animals are found in Alaska, it is possible that the virus is also present in wildlife there. No studies have been done looking for HEV in Alaskan wildlife; however, even if the virus is present in the state’s wildlife population, our study suggests that the risk of zoonotic transmission is currently small.
Brucellosis is a reportable disease to the AK DHSS. Over the 23 years from 1958 to 1981, there were 24 reported cases for an average of one case per year; however, only seven cases were reported over the next 28 years (1982–2010) (State of Alaska Section of Epidemiology 2011a). Antibodies to Brucella spp. decline rapidly in the first year after treatment for the pathogen. In one brucellosis outbreak investigation, sera were collected from 92 patients at intervals ranging from biweekly to monthly for up to 18 months. In that study, at 1 year post-treatment, only 9% (8/92) of persons remained seropositive (Buchanan and Faber 1980). The one seropositive sample in our study had a titer of 160 with an IgG titer of <20 indicating that most of the antibody was IgM. This is suggestive of cross-reactivity with other bacteria or an infection from years earlier when disease was more prevalent. Data from the Canadian NHS also show a low (<1%) Brucella spp. seropositivity (Messier et al. 2012).
Reports of disease from E. granulosus and E. multilocularis in Alaska date back to the 1940 s and in some years reached 10 cases per year (Jenkins et al. 2013). Humans in the Arctic are exposed to Echinococcus spp. mainly through accidental ingestion of eggs in the feces of wolves, foxes, and domesticated dogs. From 1990–2010, likely due to efforts to control the disease in dogs, as well as the decreased use of dogs as a major means of transportation, AK DHSS received no reports of E. multilocularis cases and only 12 reports of E. granulosus cases (State of Alaska Section of Epidemiology 2003, Jenkins et al. 2013).
In this study, we found seroprevalence was 2% for E. granulosus, which is slightly lower than the 6% and 8% found, respectively, in the Canadian IPY IHSA and NHS (Messier et al. 2012, Goyette et al. 2014). Seroprevalence was highest in persons ≥ 50 years old, which likely reflects antibody persistence in persons who were exposed many years ago. Only one serum was positive for E. multilocularis in our study. Because alveolar hydatid disease from E. multilocularis can be life threatening, we sent that sera for confirmatory testing by EgHF-ELISA, Em2-ELISA, Em18-ELISA, and two Western blots (Poretti et al. 1999, Muller et al. 2007). That testing was negative leading us to conclude that the participant was not currently infected and that follow-up was not necessary.
F. tularensis occurs throughout the Northern hemisphere, including the Arctic areas. Human cases are rare and typically sporadic. From 1946 to 2010, 23 laboratory confirmed cases of F. tularensis were reported to public health authorities in Alaska. Of these, 19 had known exposure sources, with musk-rats or hares being the most common (74%), followed by dogs (11%), cats (5%), beavers (5%), or bears (5%) (Hansen et al. 2011).
An extensive review of tularemia in Alaska from 1938 to 2010 describes three prevalence surveys with results of 4% (29/810) to 18% (139/793) (Hansen et al. 2011); the Canadian NHS reports a seroprevalence of 19% (Messier et al. 2012). The most recent Alaska serosurvey, from 1968 to 1969, found a prevalence of 5% in Alaska Native people from the southwest and east central parts of the state, when a titer of ≥ 1:20 was utilized (Table 4) (Miller 1974). In the same study, prevalence was only 0.5% using a cutoff of ≥ 1:160. The Canadian NHS also utilized the ≥ 1:20 cutoff; however, data were not presented in such a way that other cutoff points could be investigated. The 0% seropositivity rate in this study, based on a titer ≥ 1:128, is therefore perhaps not remarkable. Because of possible cross-reactions at lower titers, the higher cutoff is typically considered diagnostically significant (World Health Organization 2007).
Results from this study should be interpreted cautiously as there are several limitations associated with these data. First, this was a cross-sectional seroprevalence study among persons who, as far as we know, were not reporting symptoms of infection at the time of recruitment. A positive antibody test on a single blood sample drawn for this study does not mean that the individual is currently infected or ever had a symptomatic infection with the pathogen. Serologic evidence of current infection is typically based on sequential blood samples that show an increase in antibody concentration in an individual who had compatible signs and symptoms of infection. We did not collect any illness history from participants nor did we do medical chart reviews on persons testing positive for any of the pathogens to determine if there was a history of infection or a compatible illness.
Furthermore, assay cutoff values are determined to give the best overall test performance; thus, the assays are not 100% sensitive and specific. There is the potential for false positive or false negative results for these assays, and we did not collect specific individual exposure histories. In addition, because the duration of detectable antibody is unknown for some of these pathogens, we may have underestimated exposure risk in this study from loss of antibody due to past exposure.
Another limitation of this study is that it was a convenience sample designed to investigate avian influenza. The study groups were recruited to represent varying exposures to wild birds in specific regions of Alaska, and the participants were asked questions related to the handling of those birds. Thus, we did not ask questions about, or investigate factors that may be associated with, exposure to the specific animals that carry the pathogens described in this study. Despite this limitation, the study population likely represents persons with exposure to Alaska wildlife in general and therefore may be considered representative of persons living and working in the southwest, southcentral, and interior regions of Alaska.
In conclusion, over 60% of Alaskans in this study were seropositive to at least one of the 11 zoonotic pathogens investigated. This study sets a baseline for future human seroprevalence studies in Alaska and highlights trends that could be explored further in studies designed specifically for the pathogens of interest. Such studies could include data on specific animal exposures, illness history, and a medical record review, if appropriate. In addition, retrospective studies using stored human sera could provide information on changes in zoonotic pathogen seropositivity over time and could be correlated with factors such as the changing frequency of contact between animal hosts and humans, changes in land use, human and animal migration, habitat change, and weather. Because changes in these factors are occurring throughout the Arctic, we also propose comparative seroprevalence studies of zoonotic pathogens be done across the circumpolar north using the same or similar methods to give a more comprehensive picture of zoonosis risk.
Acknowledgments
The authors acknowledge Kathy Byrd, Helen Peters, Kim Boyd Hummel, and Justin Ortiz for their help recruiting study participants, Kristina Dimitrova and Maya Andonova for technical assistance in the performance of the California serogroup bunyavirus serological testing, and Steve Bentley for data management. The authors also thank Bruno Gottstein and Norbert Müller of the Institute of Parasitology at the University of Bern, Switzerland for performing the E. multilocularis confirmatory testing.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names and commercial sources is for identification only and does not constitute endorsement by the U.S. Department of Health and Human Services or the U.S. Centers for Disease Control and Prevention.
Author Disclosure Statement
No conflicting financial interests exist.
References
- Anderson A, Bijlmer H, Fournier PE, Graves S, et al. Diagnosis and management of Q fever—United States, 2013: Recommendations from CDC and the Q Fever Working Group. MMWR Recomm Rep 2013; 62:1–30. [PubMed] [Google Scholar]
- Anderson AD, Kruszon-Moran D, Loftis AD, McQuillan G, et al. Seroprevalence of Q fever in the United States, 2003–2004. Am J Trop Med Hyg 2009; 81:691–694. [DOI] [PubMed] [Google Scholar]
- Beaty BJ, Calisher CH, Shope RE. Arboviruses. In: Lennette E, ed. Diagnostic Procedures for Viral and Rickettsial Diseases. Washington, DC: American Public Health Association, 1995: 189–212. [Google Scholar]
- Becker DJ, Oloya J, Ezeamama AE. Household socioeconomic and demographic correlates of Cryptosporidium seropositivity in the United States. PLoS Negl Trop Dis 2015; 9: e0004080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkhead G, Vogt RL. Epidemiologic surveillance for endemic Giardia lamblia infection in Vermont. The roles of waterborne and person-to-person transmission. Am J Epidemiol 1989; 129:762–768. [DOI] [PubMed] [Google Scholar]
- Blitvich BJ, Saiyasombat R, Travassos da Rosa A, Tesh RB, et al. Orthobunyaviruses, a common cause of infection of livestock in the Yucatan peninsula of Mexico. Am J Trop Med Hyg 2012; 87:1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SL, Klein GC, McKinney FT, Jones WL. Safranin O-stained antigen microagglutination test for detection of brucella antibodies. J Clin Microbiol 1981; 13:398–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce M, Zulz T, Koch A. Surveillance of infectious diseases in the Arctic. Public Health 2016; 137:5–12. [DOI] [PubMed] [Google Scholar]
- Buchanan TM, Faber LC. 2-mercaptoethanol Brucella agglutination test: Usefulness for predicting recovery from brucellosis. J Clin Microbiol 1980; 11:691–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou L, Kosmidou M. Hepatitis E virus in the Western world—A pork-related zoonosis. Clin Microbiol Infect 2013; 19:600–604. [DOI] [PubMed] [Google Scholar]
- Feltz ET, List-Young B, Ritter DG, Holden P, et al. California encephalitis virus: Serological evidence of human infections in Alaska. Can J Microbiol 1972; 18:757–762. [DOI] [PubMed] [Google Scholar]
- Gold Standard Diagnostics. 2014. Trichinella spiralis ELISA IgG Test kit. Davis, CA, 2014:1–8. [Google Scholar]
- Goyette S, Cao Z, Libman M, Ndao M, et al. Seroprevalence of parasitic zoonoses and their relationship with social factors among the Canadian Inuit in Arctic regions. Diagn Microbiol Infect Dis 2014; 78:404–410. [DOI] [PubMed] [Google Scholar]
- Hansen CM, Vogler AJ, Keim P, Wagner DM, et al. Tularemia in Alaska, 1938–2010. Acta Vet Scand 2011; 53:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess JJ, Malilay JN, Parkinson AJ. Climate change: The importance of place. Am J Prev Med 2008; 35:468–478. [DOI] [PubMed] [Google Scholar]
- Hotez PJ. Neglected infections of poverty among the indigenous peoples of the arctic. PLoS Negl Trop Dis 2010; 4:e606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueffer K, O’Hara TM, Follmann EH. Adaptation of mammalian host-pathogen interactions in a changing arctic environment. Acta Vet Scand 2011; 53:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley BE, Philip RN, Maynard JE. Survey of brucellosis in Alaska. J Infect Dis 1963; 112:100–106. [DOI] [PubMed] [Google Scholar]
- Ito A, Xiao N, Liance M, Sato MO, et al. Evaluation of an enzyme-linked immunosorbent assay (ELISA) with affinity-purified Em18 and an ELISA with recombinant Em18 for differential diagnosis of alveolar echinococcosis: Results of a blind test. J Clin Microbiol 2002; 40:4161–4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanoska D, Cuperlovic K, Gamble HR, Murrell KD. Comparative efficacy of antigen and antibody detection tests for human trichinellosis. J Parasitol 1989; 75:38–41. [PubMed] [Google Scholar]
- Jenkins EJ, Castrodale LJ, de Rosemond SJ, Dixon BR, et al. Tradition and transition: Parasitic zoonoses of people and animals in Alaska, northern Canada, and Greenland. Adv Parasitol 2013; 82:33–204. [DOI] [PubMed] [Google Scholar]
- Jones JL, Kruszon-Moran D, Rivera HN, Price C, et al. Toxoplasma gondii seroprevalence in the United States 2009–2010 and comparison with the past two decades. Am J Trop Med Hyg 2014; 90:1135–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JL, Kruszon-Moran D, Sanders-Lewis K, Wilson M. Toxoplasma gondii infection in the United States, 1999 2004, decline from the prior decade. Am J Trop Med Hyg 2007; 77: 405–410. [PubMed] [Google Scholar]
- Jones KE, Patel NG, Levy MA, Storeygard A, et al. Global trends in emerging infectious diseases. Nature 2008; 451:990–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersh GJ, Fitzpatrick KA, Self JS, Biggerstaff BJ, et al. Long-Term immune responses to Coxiella burnetii after vaccination. Clin Vaccine Immunol 2013; 20:129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodani M, Kamili NA, Tejada-Strop A, Poe A, et al. Variability in the performance characteristics of IgG anti-HEV assays and its impact on reliability of seroprevalence rates of hepatitis E. J Med Virol 2017; 89:1055–1061. [DOI] [PubMed] [Google Scholar]
- Kwan PS, Xavier C, Santovenia M, Pruckler J, et al. Multilocus sequence typing confirms wild birds as the source of a Campylobacter outbreak associated with the consumption of raw peas. Appl Environ Microbiol 2014; 80:4540–4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell J, Higgins DP, Drebot M, Makowski K, et al. Human Jamestown canyon virus infection—Montana, 2009. Morb Mortal Wkly Rep 2011; 60:652–655. [PubMed] [Google Scholar]
- McLaughlin JB, DePaola A, Bopp CA, Martinek KA, et al. Outbreak of Vibrio parahaemolyticus gastroenteritis associated with Alaskan oysters. N Engl J Med 2005; 353:1463–1470. [DOI] [PubMed] [Google Scholar]
- Messier V, Levesque B, Proulx JF, Rochette L, et al. Seroprevalence of seven zoonotic infections in Nunavik, Quebec (Canada). Zoonoses Public Health 2012; 59:107–117. [DOI] [PubMed] [Google Scholar]
- Miller LG. Further studies on tularemia in Alaska: Human tularemia. Can J Microbiol 1974; 20:1539–1544. [DOI] [PubMed] [Google Scholar]
- Minor C, Kersh GJ, Gelatt T, Kondas AV, et al. Coxiella burnetii in northern fur seals and Steller sea lions of Alaska. J Wildl Dis 2013; 49:441–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosites E, Miernyk K, Priest JW, Bruden D, et al. Giardia and Cryptosporidium antibody prevalence and correlates of exposure among Alaska residents, 2007–2008. Epidemiol Infect 2018; 146:888–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss DM, Priest JW, Hamlin K, Derado G, et al. Longitudinal evaluation of enteric protozoa in Haitian children by stool exam and multiplex serologic assay. Am J Trop Med Hyg 2014; 90:653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller N, Frei E, Nunez S, Gottstein B. Improved serodiagnosis of alveolar echinococcosis of humans using an in vitro-produced Echinococcus multilocularis antigen. Parasitology 2007; 134:879–888. [DOI] [PubMed] [Google Scholar]
- Murphy NJ. Giardiasis in Alaska 1969–1979: A descriptive epidemiological study. Alaska Med 1981; 23:22–27. [PubMed] [Google Scholar]
- Parkinson AJ, Berner J. Climate change and impacts on human health in the Arctic: An international workshop on emerging threats and the response of Arctic communities to climate change. Int J Circumpolar Health 2009; 68:84–91. [DOI] [PubMed] [Google Scholar]
- Parkinson AJ, Butler JC. Potential impacts of climate change on infectious diseases in the Arctic. Int J Circumpolar Health 2005; 64:478–486. [DOI] [PubMed] [Google Scholar]
- Pearce BD, Hubbard S, Rivera HN, Wilkins PP, et al. Toxoplasma gondii exposure affects neural processing speed as measured by acoustic startle latency in schizophrenia and controls. Schizophrenia Res 2013; 150:258–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson DR, Cooney MK, Beasley RP. Prevalence of antibody to Toxoplasma among Alaskan natives: Relation to exposure to the felidae. J Infect Dis 1974; 130:557–563. [DOI] [PubMed] [Google Scholar]
- Pike BL, Saylors KE, Fair JN, Lebreton M, et al. The origin and prevention of pandemics. Clin Infect Dis 2010; 50:1636–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poretti D, Felleisen E, Grimm F, Pfister M, et al. Differential immunodiagnosis between cystic hydatid disease and other cross-reactive pathologies. Am J Trop Med Hyg 1999; 60:193–198. [DOI] [PubMed] [Google Scholar]
- Priest JW, Li A, Khan M, Arrowood MJ, et al. Enzyme immunoassay detection of antigen-specific immunoglobulin g antibodies in longitudinal serum samples from patients with cryptosporidiosis. Clin Diagn Lab Immunol 2001; 8:415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest JW, Moss DM, Visvesvara GS, Jones CC, et al. Multiplex assay detection of immunoglobulin G antibodies that recognize Giardia intestinalis and Cryptosporidium parvum antigens. Clin Vaccine Immunol 2010; 17:1695–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed C, Bruden D, Byrd KK, Veguilla V, et al. Characterizing wild bird contact and seropositivity to highly pathogenic avian influenza A (H5N1) virus in Alaskan residents. Influenza Other Resp Viruses 2014; 8:516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reses HE, Gargano JW, Liang JL, Cronquist A, et al. 2018. Risk factors for sporadic Giardia infection in the USA: A case-control study in Colorado and Minnesota. Epidemiol Infect 2018;146:1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocheleau JP, Michel P, Lindsay LR, Drebot M, et al. Emerging arboviruses in Quebec, Canada: Assessing public health risk by serology in humans, horses and pet dogs. Epidemiol Infect 2017; 145:2940–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan U, Fayer R, Xiao L. Cryptosporidium species in humans and animals: Current understanding and research needs. Parasitology 2014; 141:1667–1685. [DOI] [PubMed] [Google Scholar]
- Sampasa-Kanyinga H, Levesque B, Anassour-Laouan-Sidi E, Cote S, et al. Zoonotic infections in native communities of James Bay, Canada. Vector Borne Zoonotic Dis 2012; 12:473–481. [DOI] [PubMed] [Google Scholar]
- Sato T, Fujita H, Ohara Y, Homma M. Microagglutination test for early and specific serodiagnosis of tularemia. J Clin Microbiol 1990; 28:2372–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer YP, Casillas S, Helfrich K, Mocan D, et al. Two outbreaks of Trichinellosis linked to consumption of Walrus Meat—Alaska, 2016–2017. Morb Mort Wkly Rep 2017; 66: 692–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansfield SK, Calisher CH, Hunt AR, Winkler WG. Antibodies to arboviruses in an Alaskan population at occupational risk of infection. Can J Microbiol 1988; 34:1213–1216. [DOI] [PubMed] [Google Scholar]
- State of Alaska Section of Epidemiology. Prevalence of Diarrheal Pathogens in Two Anchorage Daycare Centers. Bulletin No. 8, May 2, 1986.
- State of Alaska Section of Epidemiology. Echinococcus in Alaska. Bulletin No. 2, February 10, 2003.
- State of Alaska Section of Epidemiology. Human and Animal Brucellosis in Alaska. Bulletin No. 31, December 28, 2011.
- State of Alaska Section of Epidemiology. Q Fever in Alaska. Bulletin No. 24, September 7, 2011.
- State of Alaska Section of Epidemiology. Q Fever in Alaska—Update. Bulletin No. 1, March 6, 2014.
- State of Alaska Section of Epidemiology. Assessment of the Potential Health Impacts of Climate Change in Alaska. Bulletin Recommendation and Reports 2018; 20:1–6. [Google Scholar]
- Taylor LH, Latham SM, Woolhouse ME. Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci 2001; 356:983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villard O, Cimon B, L’Ollivier C, Fricker-Hidalgo H, et al. Help in the choice of automated or semiautomated immunoassays for serological diagnosis of toxoplasmosis: Evaluation of nine immunoassays by the french national reference center for toxoplasmosis. J Clin Microbiol 2016; 54:3034–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters LL, Tirrell SJ, Shope RE. Seroepidemiology of California and Bunyamwera serogroup (Bunyaviridae) virus infections in native populations of Alaska. Am J Trop Med Hyg 1999; 60:806–821. [DOI] [PubMed] [Google Scholar]
- Weger S, Elkin B, Lindsay R, Bollinger T, et al. Hepatitis E virus seroprevalence in free-ranging deer in Canada. Trans-bound and Emerg Dis 2017; 64:1008–1011. [DOI] [PubMed] [Google Scholar]
- Welch TP. Risk of giardiasis from consumption of wilderness water in North America: A systematic review of epidemiologic data. Int J Infect Dis 2000; 4:100–103. [DOI] [PubMed] [Google Scholar]
- Wilson NO, Hall RL, Montgomery SP, Jones JL. Trichinellosis surveillance—United States, 2008–2012. MMWR Surveill Summ 2015; 64:1–8. [PubMed] [Google Scholar]
- Woolhouse ME, Gowtage-Sequeria S. Host range and emerging and reemerging pathogens. Emerg Infect Dis 2005; 11:1842–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. 2007. WHO Guidelines on Tularaemia. In: Tärnvik A, ed. WHO Press, Geneva Switzerland. [Google Scholar]
- Yoder JS, Harral C, Beach MJ. Cryptosporidiosis surveillance—United States, 2006–2008. MMWR Surveill Summ 2010a; 59:1–14. [PubMed] [Google Scholar]
- Yoder JS, Harral C, Beach MJ. Giardiasis Surveillance—United States, 2006–2008. MMWR Surveill Summ 2010b; 59:15–28. [PubMed] [Google Scholar]
- Zarnke RL. Serologic survey for selected microbial pathogens in Alaskan wildlife. J Wildl Dis 1983; 19:324–329. [DOI] [PubMed] [Google Scholar]
- Zarnke RL, Calisher CH, Kerschner J. Serologic evidence of arbovirus infections in humans and wild animals in Alaska. MMWR Surveill Summ 1983; 19:175–179. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wen H, Li J, Lin R, et al. Immunology and immunodiagnosis of cystic echinococcosis: An update. Clin Dev Immunol 2012; 2012:101895. [DOI] [PMC free article] [PubMed] [Google Scholar]


