Highlights
-
•
Postpartum hemorrhage incidence is increasing among nulliparous women than in multiparous women.
-
•
Erythrocyte suspension transfusion before birth, soft-birth canal avulsion, general anesthesia administration before delivery, and maternal anemia are major factors influencing PPH in nulliparous and multiparous women’s groups.
-
•
Thrombophlebitis is a major influencing factor associated with PPH among nulliparous women.
Keywords: Incidence, Influencing factors, Postpartum hemorrhage, Nulliparous, Multiparous
Abstract
Objectives
Postpartum hemorrhage (PPH) is a common cause of maternal death worldwide, but data on PPH incidence and influencing factors for nulliparous and multiparous women is scarce. So, the study aimed to assess the differences in PPH incidence and influencing factors between nulliparous and multiparous women.
Methods
A multicenter retrospective cohort study was conducted among women who gave birth at ≥ 28 weeks of gestation in Hunan Province, China, from January 2017 to December 2018. Logistic regression assessed PPH-influencing factors, and the receiver operating characteristic curve (ROC curve) assessed the predictive performance of identified factors.
Results
A total of 144,845 postpartum women were included in the study. The incidence of PPH (blood loss ≥ 500 ml) was 2.1 % and 1.7 % for nulliparous and multiparous women, respectively. Among the nulliparous and multiparous women, similar influencing factors of PPH included erythrocyte suspension transfusion before childbirth, anemia, soft-birth canal avulsion, Cesarean-section, placenta abruption, and general anesthesia administration before birth. Thrombophlebitis was associated [aOR 18.46(1.67–20.31)] with PPH among only the nulliparous women, while instrument-assisted birth [aOR 1.95(1.16–3.28)] and gestational hypertension [aOR 1.57(1.13–2.19)] were associated with PPH among only the multiparous women. The areas under the ROC-curve for the overall-cohort, nulliparous, and multiparous groups were [0.829(0.821–0.838)], [0.828(0.815–0.840)] and [0.833(0.822–0.844)], respectively.
Conclusion
PPH incidence is higher among nulliparous women than among multiparous women, but influencing factors vary relatively by parity. The study findings provide new insights into the use of different approaches to PPH prevention for nulliparous and multiparous women in clinical practice.
1. Introduction
Postpartum hemorrhage (PPH) is defined as blood loss of 500 ml or more within 24 h following birth, and severe PPH is defined as blood loss of 1000 ml or more within 24 h following childbirth (Who, 2009). PPH is a common cause of maternal mortality that accounts for about one-quarter (25 %) of all maternal deaths globally (Say et al., 2014, Geller et al., 2006, Knight M, Callaghan WM, Berg C, et al. Trends in postpartum hemorrhage in high resource countries: a review and recommendations from the International Postpartum Hemorrhage Collaborative Group. BMC Pregnancy Childbirth., 2009). According to the World Health Organization's (WHO) and existing research, 14 million women develop PPH annually, 127,000 die, and half of these deaths occur in Africa and Asia (Sosa et al., 2009, Who, 2012, Khan et al., 2006, Hogan et al., 2010). Despite recent advances in obstetric emergency care and preventive measures in China, PPH remains the leading cause of maternal death (32 %) (Feng et al., 2010).
Globally, there is growing public concern over the rising incidence of PPH (blood loss ≥ 500 ml), with estimates ranging from 1.47 to 18 % among women of mixed parity in published studies (Magann et al., 2005, Devine, 2009, Magann et al., 2005). Even though nulliparous and multiparous women make up a large part of the birthing population, there is limited information about the incidence of PPH in these women’s groups. Only a few nulliparous studies (Bais et al., 2004, Govindappagari et al., 2020) assessed PPH incidence, with reported estimates ranging from 4.2 % to 19.0 %, but no study has assessed PPH incidence in multiparous women.
Although there are studies (Li et al., 2021, Liu et al., 2021;21(1):332., Xu et al., 2018, Wei et al., 2020) reporting PPH incidence among the general birthing population in China, with estimates ranging from 0.81 % to 15.4 %, no study has assessed the differences in PPH incidence for nulliparous and multiparous women. Although PPH accounts for the vast majority of maternal deaths worldwide, most PPH-associated maternal deaths are preventable if influencing factors are identified early and prompt measures are implemented (Sosa et al., 2009, Who, 2012, Khan et al., 2006). Therefore, with nulliparous and multiparous women making up a large part of the birthing population, it is essential to assess factors influencing PPH among these women’s groups to help prevent PPH and its associated complications. Accordingly, several population-based studies from Latin America, Australia, the Netherlands, and the United States have assessed factors influencing PPH among women of mixed parity (Sosa et al., 2009, Magann et al., 2005, Ford et al., 2007, Chang et al., 2011, Kramer et al., 2013). However, only a few studies (Bais et al., 2004, Govindappagari et al., 2020, Dionne MD, Deneux-Tharaux C, Dupont C, et al. Duration of Expulsive Efforts and Risk of Postpartum Hemorrhage in Nulliparous Women: A Population-Based Study. PLoS One., 2015) have assessed factors influencing PPH among nulliparous women; no study has assessed factors influencing PPH among multiparous women. The few nulliparous studies reported relatively different influencing factors of PPH (including abnormal third-stage labor, expulsive efforts, mild thrombocytopenia, epidural analgesia, and prophylactic postpartum oxytocin administration), assessed few risk variables, and used a smaller sample size compared to our current study. In China, there are studies (Li et al., 2021, Liu et al., 2021;21(1):332., Xu et al., 2018, Wei et al., 2020) reporting a few similar PPH influencing factors (including macrosomia, placenta previa and abruption) and some different influencing factors (including obesity (Li et al., 2021), conception through in vitro fertilization, maternal age < 18 years (Liu et al., 2021;21(1):332.), repeated cesarean section (Xu et al., 2018), and lateral perineotomy (Wei et al., 2020) of PPH among the general birthing population. However, there is no information on whether or not these influencing factors are the same or different for nulliparous and multiparous women.
As a result, this study aimed to assess the differences in PPH incidence and influencing factors for nulliparous and multiparous women for the first time to provide a scientific basis for using different approaches to preventing PPH in these women's groups.
2. Methods
2.1. Data Sources, Inclusion, and exclusion criteria
The multicenter hospital-based retrospective cohort study collected data from medical records at 18 randomly selected health facilities (5 general hospitals and 13 maternal-child healthcare hospitals) within Hunan Province, South China. This study was performed following the Principles of the Declaration of Helsinki. Our study followed the institution’s guidelines for protection of human subjects concerning their safety and privacy. Ethical approval was granted by the Ethics and Research Committee of the Xiangya School of Public Health, Central South University (No. XYGW − 2023–52).
The sum of 144,845 pregnant women admitted to hospitals in Hunan Province, South China, from January 1, 2017 to December 31, 2018, who gave birth at ≥ 28 weeks of gestation were included into the study, while those without data on the exact gestational age at birth were excluded. The study data was collected using China's validated Annex 1 Critical Maternal Surveillance Questionnaire (Appendix A). Complete medical information for each birth, including maternal sociodemographic and obstetric factors (including maternal age, marital status, education, gravidity, type of pregnancy, place of birth, antenatal care visits, mode of birth, gestational age, and birth weight) were assessed. From the time of admission until the time of discharge, we assessed comorbidities or complications that occurred. These included anemia, diabetes, hepatopathy, gestational hypertension, blood clot formation disorder, low platelet count(<50,000 µl), systemic infection, HELLP syndrome, puerperal/postpartum infection, pre-eclampsia/eclampsia, placenta previa and abruption, soft birth canal avulsion, and thrombophlebitis. We also evaluated medical measures, including hysterectomy, administration of general anesthesia before childbirth, administration of magnesium sulfate before birth, erythrocyte suspension before delivery, and platelet transfusion. We categorized comorbidities or complications during pregnancy and childbirth, as well as the medical measure variables, as “yes” or “no.”.
2.2. Definitions
This study's primary outcome was PPH, defined as greater than or equal to 500 ml of blood loss after childbirth. Using WHO guidelines, PPH was classified as moderate (for blood loss of 500–999 ml following birth) or severe (for blood loss of 1000 ml or more following birth) (Who, 2009). Following the WHO recommendations, anemia was diagnosed in pregnancy when the concentration of hemoglobin was less than 110 g/L (<11 g/dL) (Shi et al., 2022;5(2):e2147046.). The diagnosis of diabetes followed the International Association of Diabetes and Pregnancy Study Group (IADPSG) 2010 criteria (Association and of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, 2010), while the diagnosis of hypertensive disease of pregnancy (gestational hypertension and preeclampsia/eclampsia) followed the 2009 recommendation of the International Society for the Study of Hypertension in Pregnancy (ISSHP) (Brown et al., 2001;20(1):IX-XIV.). The diagnosis of hepatopathy was made following the recommendations in the American College of Gastroenterology's (ACG) Clinical Guidelines for Liver Disease in Pregnancy (Tran et al., 2016). Placenta previa was diagnosed using ultrasonography and magnetic resonance imaging (MRI), showing that the placental margin reached the internal cervical orifice after 28 weeks of gestation. Furthermore, placental abruption was diagnosed based on positive signs of vaginal bleeding and uterine tachysystole, which indicated partial or complete placenta detachment from the uterus after 20 weeks of gestation but before delivery. Soft birth canal avulsion refers to obstetric trauma that occurs during labor and delivery, affecting the birth canal and soft tissue. Amniotic fluid embolism was defined as a severe but rare obstetric emergency in which amniotic fluid enters the bloodstream of a pregnant mother) (McDonnell et al., 2015). In our study, thrombophlebitis (a condition characterized by blood clot formation in a vein that causes inflammation and pain) included deep vein thrombosis (DVT) of the legs and pelvis. Diagnosing thrombophlebitis relies on a combination of clinical evaluation and diagnostic tests. If an initial Doppler ultrasound (a noninvasive method that uses sound waves to visualize blood flow) was negative, additional investigation, including serial compression ultrasound or magnetic resonance venography (which employs magnetic resonance imaging technology to provide detailed vein images, detect clots or obstructions, and assess vein-related conditions), was performed for the diagnosis of thrombophlebitis in pregnancy. The diagnostic procedures followed the American Society of Hematology 2018 guidelines for managing venous thromboembolism in pregnancy (Bates et al., 2018).
2.3. Statistical analysis
A descriptive statistic was used to calculate the frequency, means, percentages, and incidence of PPH in the study. A Multivariate logistic regression was performed to identify PPH-influencing factors for nulliparous and multiparous women. Specifically, forward stepwise logistic regression analysis was used to calculate the adjusted odds ratio(aOR) and 95 % confidence interval(CI). The study adjusted for maternal sociodemographic and obstetric factors (maternal age, marital status, educational status, and gravidity). The entry and removal points were set at 0.10 and 0.15 during the analysis, respectively. A two-sided probability p-value of ≤ 0.05 was considered statistically significant for this study. Also, the receiver operating characteristic curve (ROC curve) was used to examine the predictive performance of the identified influencing factors of PPH. Subgroup analysis using single and combined influencing factors of PPH was performed in the overall study cohort and within the nulliparous and multiparous women's groups. All the statistical analyses were performed using IBM SPSS Statistics Version 26.0 (IBM Corp., Chicago, IL, USA).
3. Results
3.1. Descriptive Statistics
A total of 144,845 postpartum women were included in this study; 60,686(41.9 %) were nulliparous women, and 84,159(58.1 %) were multiparous women. The study included more women (45.0 %) aged 30–34. The majority of the women (98.5 %) were married or cohabiting, most (64.0 %) had secondary school education, and a majority (63.5 %) of the women were primigravida. Furthermore, 64.9 % of the women had 4–9 ANC visitation records, and most (51.7 %) used county-level hospitals as a place of birth. Most (55.7 %) of the women had a normal vaginal delivery (see Table 1).
Table 1.
Sociodemographic and Obstetric Characteristics for Nulliparous and Multiparous Women in Hunan, China (2017–2018).
| Variables | Total (%) | Nulliparous | Multiparous | P-value |
|---|---|---|---|---|
| 144,845(100.0 %) | 60,686(41.9 %) | 84,159(58.1 %) | ||
| Maternal age | 0.040 | |||
| < 25 years | 6912(4.8) | 5481(9.0) | 1431(1.7) | |
| 25 – 29 years | 30905(21.3) | 19486(32.1) | 11419(13.6) | |
| 30 – 34 years | 65177(45.0) | 29086(47.9) | 36091(42.9) | |
| ≥ 35 years | 41851(28.9) | 6633(10.9) | 35218(41.8) | |
| Marital status | 0.035 | |||
| Single/Divorced/Widowed | 2134(1.5) | 1427(2.4) | 707(0.8) | |
| Married/Cohabiting | 142711(98.5) | 59259(97.6) | 83452(99.2) | |
| Educational status | 0.003 | |||
| Primary/Illiteracy | 1086(0.7) | 343(0.6) | 743(0.9) | |
| Secondary | 92647(64.0) | 34983(57.6) | 57664(68.5) | |
| Tertiary | 51112(35.3) | 25360(41.8) | 25752(30.6) | |
| Gravidity | < 0.001 | |||
| Nulligravida (O/none) | 40593(28.0) | 40462(66.7) | 131(0.2) | |
| Primigravida (1 pregnancy) | 91931(63.5) | 19537(32.2) | 72394(86.0) | |
| Multigravida (≥2 pregnancies) | 12321(8.5) | 687(1.1) | 11634(13.8) | |
| Number of ANC visits | < 0.001 | |||
| 0–3 ANC visits | 7697(5.3) | 2165(3.6) | 5532(6.6) | |
| 4–9 ANC visits | 94017(64.9) | 37902(62.5) | 56115(66.7) | |
| ≥ 10 ANC visits | 43131(29.8) | 20619(34.0) | 22512(26.7) | |
| Place of birth | 0.002 | |||
| Provincial/Municipal hospital | 69994(48.3) | 34416(56.7) | 35578(42.3) | |
| County-level hospital | 74851(51.7) | 26270(43.3) | 48581(57.7) | |
| Mode of Childbirth | < 0.001 | |||
| Normal vaginal birth/SVD | 80686(55.7) | 37747(62.2) | 42939(51.0) | |
| Cesarean section | 60226(41.6) | 21608(35.6) | 38618(45.9) | |
| Instrumental/Assisted childbirth | 3933(2.7) | 1331(2.2) | 2602(3.1) |
ANC: Antenatal Care, SVD: Spontaneous Vaginal Delivery.
Significant at P < 0.05.
3.2. Incidence of Postpartum Hemorrhage (PPH)
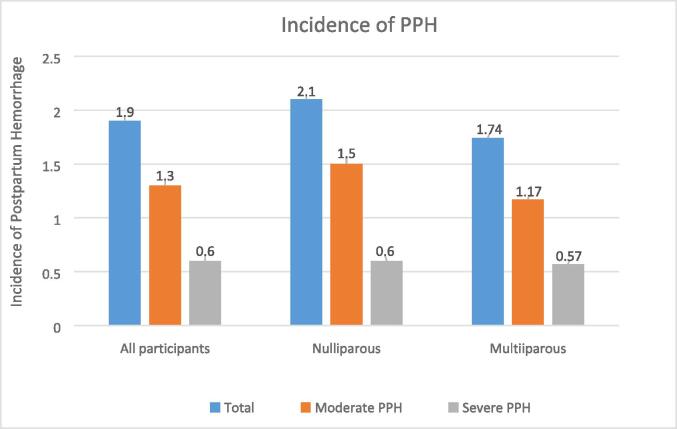
As in Fig. 1., 2,724 women had PPH (postpartum blood loss ≥ 500 ml), giving an overall PPH incidence of 1.9 %. From the subgroup analysis, moderate PPH incidence was 1.3 %, and severe PPH incidence was 0.6 %. Furthermore, 1,259 nulliparous women had PPH, while 1,465 multiparous women had PPH. Nulliparous women had a slightly higher PPH incidence (2.1 %) than multiparous women (1.7 %).
Fig. 1.
Incidence of Postpartum Hemorrhage in Nulliparous and Multiparous Women in Hunan, China (2017–2018).
3.3. Univariate analysis on potential influencing factors of PPH
In Table 2, a univariate analysis was performed to assess each independent variable relationship to PPH in the nulliparous and multiparous women's groups. Maternal age, gravidity, ANC visits, the model of birth, types of pregnancy, birth weight, anemia, hepatopathy, blood clot formation disorder, platelet count < 50,000 µl, puerperal infection, pre-eclampsia/eclampsia, placenta previa, placenta abruption, soft birth canal avulsion, thrombophlebitis, hysterectomy, general anesthesia used before birth, magnesium sulfate before birth, erythrocyte suspension transfusion before birth, and platelet transfusion were all significantly related to PPH in both nulliparous and multiparous women's groups. However, education level, marital status, place of childbirth, gestational age, and systemic infection had significant relationship to PPH among only the nulliparous women. In contrast, gestational hypertension, HELLP syndrome, and amniotic fluid embolism were significantly related to PPH among only the multiparous women.
Table 2.
Univariate Analysis Comparing Factors Significantly Associated with Postpartum Hemorrhage between Nulliparous and Multiparous Women in Hunan, China (2017–2018).
| Variables | Total |
Nulliparous (n = 60,686) |
Multiparous (n = 84,159) |
||||
|---|---|---|---|---|---|---|---|
| n (%) | PPH | No PPH | P-value | PPH | No PPH | P-value | |
| Maternal age | < 0.001 | 0.016 | |||||
| < 25 years | 6912(4.8) | 111(8.8) | 5370(9.0) | 35(2.4) | 1396(1.7) | ||
| 25 – 29 years | 30905(21.3) | 359(28.5) | 19127(32.2) | 191(13.0) | 11228(13.6) | ||
| 30 – 34 years | 65177(45.0) | 604(48.0) | 28482(47.9) | 585(39.9) | 35506(42.9) | ||
| ≥ 35 years | 41851(28.9) | 185(14.7) | 6448(10.9) | 654(44.6) | 34564(41.8) | ||
| Marital status | 0.006 | 0.214 | |||||
| Single/Divorced/Widowed | 2134(1.5) | 15(1.2) | 1412(2.4) | 8(0.5) | 699(0.8) | ||
| Married/Cohabiting | 142711(98.5) | 1244(98.8) | 58015(97.6) | 1457(99.5) | 81995(99.2) | ||
| Educational status | 0.004 | 0.700 | |||||
| Primary/Illiteracy | 1086(0.7) | 5(0.4) | 338(0.6) | 13(0.9) | 730(0.9) | ||
| Secondary | 92647(64.0) | 671(53.3) | 34312(57.7) | 989(67.5) | 56675(68.5) | ||
| Tertiary | 51112(35.3) | 583(46.3) | 24777(41.7) | 463(31.6) | 25289(30.6) | ||
| Gravidity | 0.001 | < 0.001 | |||||
| Nulligravida (O/none) | 40593(28.0) | 782(62.1) | 39680(66.8) | 3(0.2) | 128(0.2) | ||
| Primigravida (1 pregnancy) | 91931(63.5) | 456(36.2) | 19081(32.1) | 1177(80.3) | 71217(86.1) | ||
| Multigravida (≥2 pregnancies) | 12321(8.5) | 21(1.7) | 666(1.1) | 285(19.5) | 11349(13.7) | ||
| Number of ANC visits | < 0.001 | < 0.001 | |||||
| 0–3 ANC visits | 7697(5.3) | 17(1.4) | 2148(3.6) | 61(4.2) | 5471(6.6) | ||
| 4–9 ANC visits | 94017(64.9) | 801(63.6) | 37101(62.4) | 1037(70.8) | 55078(66.6) | ||
| ≥ 10 ANC visits | 43131(29.8) | 441(35.0) | 20178(34.0) | 367(25.1) | 22145(26.8) | ||
| Place of birth | <0.001 | 0.986 | |||||
| Provincial/Municipal hospital | 69994(48.3) | 778(61.8) | 33638(56.6) | 619(42.3) | 34959(42.3) | ||
| County-level hospital | 74851(51.7) | 481(38.2) | 25789(43.4) | 846(57.7) | 47735(57.7) | ||
| Mode of Childbirth | <0.001 | < 0.001 | |||||
| Normal Vaginal delivery/SVD | 80686(55.7) | 950(75.5) | 36797(61.9) | 946(64.6) | 41993(50.8) | ||
| Cesarean section | 60226(41.6) | 292(23.2) | 21316(35.9) | 489(33.4) | 38129(46.1) | ||
| Instrumental/Assisted delivery | 3933(2.7) | 17(1.4) | 1314(2.2) | 30(2.0) | 2572(3.1) | ||
| Gestational age (Weeks) | 0.041 | 0.333 | |||||
| Term (37–41 weeks) | 131155(90.5) | 1169(92.9) | 54168(91.2) | 1303(88.9) | 74515(90.1 | ||
| Preterm (<37 weeks) | 13513(9.3) | 87(6.9) | 5186(8.7) | 160(10.9) | 8080(9.8) | ||
| Post-term (≥42 weeks) | 177(0.1) | 3(0.2) | 73(0.1) | 2(0.1) | 99(0.1) | ||
| Type of pregnancy | <0.001 | < 0.001 | |||||
| Singleton fetus Pregnancy | 141752(97.9) | 1188(94.4) | 57606(96.9) | 1410(96.2) | 81548(98.6) | ||
| Multiple fetus Preg. (≥2) | 3093(2.1) | 71(5.6) | 1821(3.1) | 55(3.8) | 1146(1.4) | ||
| Birth weight | <0.001 | < 0.001 | |||||
| Normal birth weight (2500-3999 g) | 126041(87.0) | 1071(85.1) | 52222(87.9) | 1172(80.0) | 71576(86.6) | ||
| Low birth weight (<2500 g) | 11133(7.7) | 68(5.4) | 4662(7.8) | 116(7.9) | 6287(7.6) | ||
| Large birth weight (≥4000 g) | 7671(5.3) | 120(9.5) | 2543(4.3) | 177(12.1) | 4831(5.8) | ||
| Anemia | 40717(28.1) | 954(75.8) | 15274(25.7) | < 0.001 | 1097(74.9) | 23392(28.3) | < 0.001 |
| Diabetes | 15709(10.8) | 151(12.0) | 6144(10.3) | 0.057 | 174(11.9) | 9240(11.2) | 0.397 |
| Hepatopathy | 3110(2.1) | 38(3.0) | 1092(1.8) | 0.002 | 49(3.3) | 1931(2.3) | 0.011 |
| Gestational hypertension | 2987(2.1) | 33(2.6) | 1381(2.3) | 0.489 | 47(3.2) | 1526(1.8) | < 0.001 |
| Blood clot formation disorder | 51(0.0) | 5(0.4) | 11(0.0) | < 0.001 | 16(1.1) | 19(0.0) | < 0.001 |
| Low Platelet count < 50,000 µl | 42(0.0) | 3(0.2) | 15(0.0) | < 0.001 | 6(0.4) | 18(0.0) | < 0.001 |
| Systemic infection/Sepsis | 2123(1.5) | 29(2.3) | 898(1.5) | 0.023 | 16(1.1) | 1180(1.4) | 0.283 |
| HELLP Syndrome | 45(0.0) | 1(0.1) | 14(0.0) | 0.212 | 2(0.1) | 28(0.0) | 0.039 |
| Puerperal infection | 108(0.1 | 5(0.4) | 57(0.1) | 0.001 | 3(0.2) | 43(0.1) | 0.013 |
| Preeclampsia/Eclampsia | 3326(2.3) | 50(4.0) | 1540(2.6) | 0.002 | 57(3.9) | 1679(2.0) | < 0.001 |
| Placenta previa | 1652(1.1) | 18(1.4) | 431(0.7) | 0.004 | 86(5.9) | 1117(1.4) | <0.001 |
| Placenta abruption | 589(0.4) | 13(1.0) | 223(0.4) | < 0.001 | 38(2.6) | 315(0.4) | < 0.001 |
| Soft birth canal avulsion | 2171(1.5) | 95(7.5) | 1071(1.8) | < 0.001 | 71(4.8) | 934(1.1) | < 0.001 |
| Amniotic fluid embolism | 5(0.0) | 0(0.0) | 1(0.0) | 0.884 | 1(0.1) | 3(0.0) | < 0.001 |
| Thrombophlebitis | 12(0.0) | 2(0.2) | 4(0.0) | < 0.001 | 1(0.1) | 5(0.0) | 0.005 |
| Hysterectomy | 2265(1.6) | 40(3.2) | 826(1.4) | < 0.001 | 44(3.0) | 1355(1.6) | < 0.001 |
| General anesthesia used before childbirth | 68032(47.0) | 471(37.4) | 25944(43.7) | < 0.001 | 615(42.0) | 41002(49.6) | < 0.001 |
| Magnesium sulfate used before childbirth | 2029(1.4) | 30(2.4) | 921(1.5) | 0.019 | 42(2.9) | 1036(1.3) | < 0.001 |
| Erythrocyte suspension transfusion before childbirth | 1027(0.7) | 184(14.6) | 144(0.2) | < 0.001 | 298(20.3) | 401(0.5) | < 0.001 |
| Platelet transfusion | 69(0.0) | 8(0.6) | 20(0.0) | < 0.001 | 10(0.7) | 31(0.0) | < 0.001 |
ANC: Antenatal Care, Preg.: Pregnancy, SVD: Spontaneous Vaginal Delivery.
Significance at P < 0.0.
3.4. Multivariate analysis on influencing factors of PPH
Furthermore, forward stepwise logistic regression analysis was used to calculate the adjusted odds ratios (aORs) and 95 % confidence intervals for factors influencing PPH. Erythrocyte suspension transfusion before birth was the predominant influencing factor for PPH in the overall study cohort and within the subgroups of nulliparous and multiparous women. Other similar influencing factors of PPH in both nulliparous and multiparous women’s groups were low ANC (0–3) visits, anemia, soft-birth canal avulsion, Cesarean-section, placenta abruption, general anesthesia administration before birth, low birth weight (<2500 g), and hysterectomy. However, low ANC (0–3) visits [aOR 2.90(1.65–5.08), p ≤ 0.001], anemia [aOR 8.41(7.34–9.64), p ≤ 0.001], soft birth canal avulsion [aOR 4.01(3.11–5.16), p ≤ 0.001], and erythrocyte suspension transfusion before childbirth [aOR 48.67(36.43–65.04), p ≤ 0.001] had increased odds for PPH among the nulliparous women compared to the multiparous women, while Cesarean section [aOR 5.81(4.63–7.27), p ≤ 0.001], placenta abruption [aOR 3.62(2.31–5.66), p ≤ 0.001], and general anesthesia administration before birth [aOR 1.63(1.33–2.01), p ≤ 0.001] had increased odds for PPH among the multiparous than in the nulliparous women. Additionally, thrombophlebitis [aOR 18.46 (1.67–20.31), p < 0.05] was associated with PPH among only the nulliparous women, while instrument-assisted birth [aOR 1.95(1.16–3.28), p < 0.05] and gestational hypertension [aOR 1.57(1.13–2.19), p < 0.005] were associated with PPH among only the multiparous women (Table 3).
Table 3.
Multivariate Logistic Regression Analysis Comparing Influencing Factors of Postpartum Hemorrhage between Nulliparous and Multiparous Women in Hunan, China (2017–2018).
| Variables |
Nulliparous (n = 60,686) |
Multiparous (n = 84,159) |
||
|---|---|---|---|---|
| aOR | 95 % CI | aOR | 95 % CI | |
| Number of ANC visits | ||||
| 0–3 ANC visits | 2.90 | (1.65–5.08)*** | 1.85 | (1.35–2.53)*** |
| 4–9 ANC visits | 0.86 | (0.76–0.99) | 0.84 | (0.74–0.96) |
| ≥10 ANC visits (Ref.) | 1(ref.) | 1(ref.) | 1(ref.) | 1(ref.) |
| Mode of Childbirth | ||||
| Normal vaginal birth/SVD(Ref.) | 1(ref) | 1(ref.) | 1(ref.) | 1(ref.) |
| Cesarean section birth | 5.02 | (4.03–6.26)*** | 5.81 | (4.63–7.27)*** |
| Instrument-assisted birth | 1.49 | (0.82–2.73) | 1.95 | (1.16–3.28)* |
| Gestational age (weeks) | ||||
| Term (37–41 weeks) (Ref.) | 1(ref.) | 1(ref.) | – | – |
| Preterm (<37 weeks) | 1.42 | (0.99–2.02) | – | – |
| Post-term (≥42 weeks) | 0.28 | (0.08–0 0.95)* | – | – |
| Birth weight | ||||
| Normal birth weight (2500-3999 g) (Ref.) | 1(ref.) | 1(ref.) | 1(ref.) | 1(ref.) |
| Low birth weight (<2500 g) | 2.08 | (1.39–3.11)*** | 2.09 | (1.6–2.72)*** |
| Large birth weight (≥4000 g) | 0.34 | (0.28–0.43)*** | 0.44 | (0.367––0.52)*** |
| Anemia | ||||
| Yes | 8.41 | (7.34–9.64)*** | 6.78 | (5.98–7.69)*** |
| No (Ref.) | 1(ref.) | 1(ref.) | 1(ref.) | 1(ref.) |
| Gestational hypertension | ||||
| Yes | – | – | 1.57 | (1.13–––2.19)** |
| No (Ref.) | – | – | 1(ref.) | 1(ref.) |
| Placenta abruption | ||||
| Yes | 2.93 | (1.46–––5.84)** | 3.62 | (2.31–––5.66)*** |
| No (Ref.) | 1(ref.) | 1(ref.) | 1(ref.) | 1(ref.) |
| Soft birth canal avulsion | ||||
| Yes | 4.01 | (3.11–––5.16)*** | 3.66 | (2.77–––4.82)*** |
| No (Ref.) | 1(ref.) | 1(ref.) | 1(ref.) | 1(ref.) |
| Thrombophlebitis | ||||
| Yes | 18.46 | (1.67–––20.31)* | – | – |
| No (Ref.) | 1(ref.) | 1(ref.) | – | – |
| Hysterectomy | ||||
| Yes | 2.50 | (1.67–––3.75)*** | 2.59 | (1.73–––3.89)*** |
| No (ref.) | 1(ref.) | 1(ref.) | 1(ref.) | 1(ref.) |
| General anesthesia used before birth | ||||
| Yes | 1.42 | (1.19–––1.69)*** | 1.63 | (1.33–––2.01)*** |
| No (Ref.) | 1(ref.) | 1(ref.) | 1(ref.) | 1(ref.) |
| Erythrocyte suspension transfusion before birth | ||||
| Yes | 48.67 | (36.43–––65.04)*** | 46.58 | (37.72–––57.54)*** |
| No (Ref.) | 1(ref.) | 1(ref.) | 1(ref.) | 1(ref.) |
ANC: Antenatal Care, SVD: Spontaneous Vaginal Delivery, Ref: Reference category. From Anemia to erythrocyte suspension transfusion before birth, each variable is categorized as “Yes and No,” and “No” is the reference. Adjusted for maternal sociodemographic and obstetric characteristics (maternal age, marital status, educational status, and gravidity). *** =P ≤ 0.001, **=P ≤ 0.005, *=P < 0.05.
3.5. Predictive performance
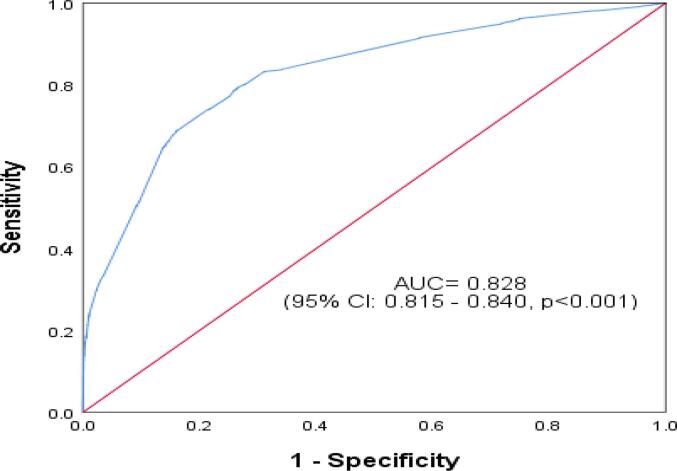
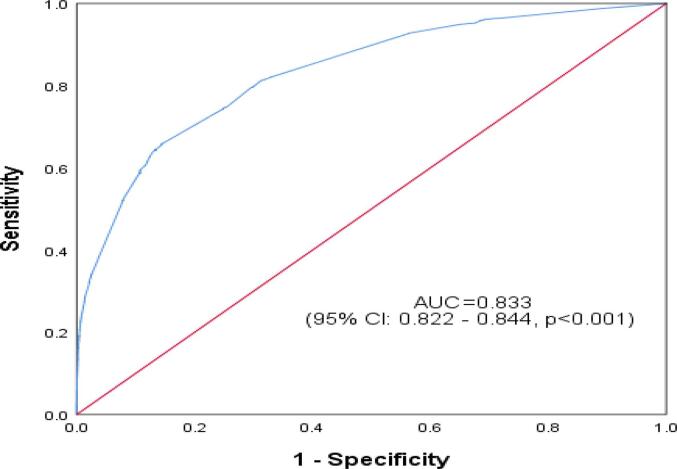
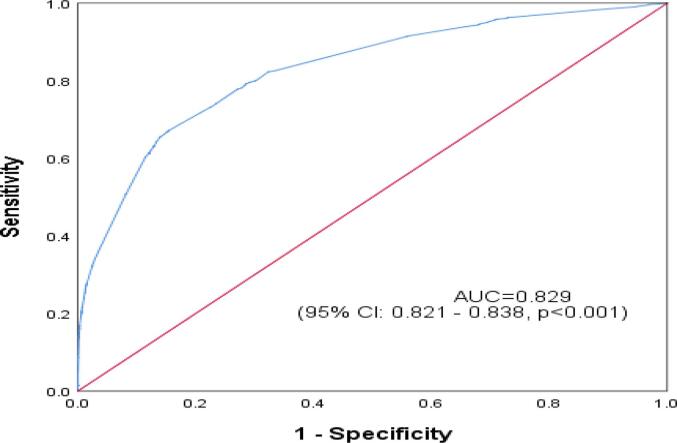
The ROC curve reflected the model's predictive performance using a single factor and combined influencing factors for PPH in the overall study cohort and within the nulliparous and multiparous subgroups (Appendix Table A1, Table A2, Table A3, Table A4). In the predictive model for the overall study cohort and within the subgroups, anemia and erythrocyte suspension transfusion before birth were two variables that had the highest AUC from the single factor analysis. The performance of the single-factor analysis was relatively unsatisfactory, with an AUC ranging from 0.50 to 0.75. However, from the combined factors analysis, the AUC for the overall study cohort 0.829(0.821–0.838), p < 0.001], the nulliparous group [0.828(0.815–0.844), p < 0.001], and the multiparous group [0.833(0.822–0.844), p < 0.001] were all satisfactory (Fig. 2a., Fig. 2b., Fig. 2c.A–C).
Fig. 2a.
Receiver Operating Characteristic Curve for Predictive Performance of Combined Influencing Factors of Postpartum Hemorrhage among Nulliparous Women in Hunan, China (2017–2018).
Fig. 2b.
Receiver Operating Characteristic Curve for Predictive Performance of Combined Influencing Factors of Postpartum Hemorrhage among Multiparous Women in Hunan, China (2017–2018).
Fig. 2c.
Receiver Operating Characteristic Curve for Predictive Performance of Combined Influencing Factors of Postpartum Hemorrhage among both Nulliparous and Multiparous women in Hunan, China (2017–2018).
4. Discussion
4.1. PPH incidence
The study assessed the differences in PPH incidence and influencing factors among nulliparous and multiparous women. Multiparous women had a lower PPH incidence of 1.7 % compared to 2.1 % PPH incidence among the nulliparous women. Although there are a few studies reporting PPH incidence among nulliparous women, no study has specifically assessed the incidence of PPH among multiparous women. So, compared to the few nulliparous studies (Bais et al., 2004, Govindappagari et al., 2020) on PPH, the incidence of PPH among nulliparous women in our study is much lower, which is probably because China recently launched a comprehensive health reform system that focuses on expanding primary care capacity, extending and improving obstetric emergency care, and delivering vital public health services to everyone (Meng et al., 2019). Furthermore, compared to multiparous women, the higher incidence of PPH among nulliparous women in our study can partly be explained by the fact that nulliparous women lack previous pregnancy and birthing experiences, have lower awareness about the importance of attending regular prenatal assessments, and have little knowledge about proper nutritional diet and exercise, which can influence the occurrence of PPH. So, healthcare professionals are encouraged to frequently conduct pregnancy-related health education for all women of childbearing age, with particular attention to nulliparous women, for the prevention of pregnancy complications such as PPH.
4.2. Influencing factor of PPH
Our study identified some similar and a few different factors influencing PPH in the nulliparous and multiparous women’s groups. Low ANC (0–3) visits had an increased risk for PPH in the nulliparous women than multiparous women. The lower risk for PPH associated with ANC visits among the multiparous women can be explained by previous pregnancy and birthing experiences. Because multiparous women have prior pregnancy experience, they are more likely to follow antenatal healthcare education than nulliparous women (WHO recommended interventions for improving maternal and newborn health. Geneva: World Health Organization Department of Making Pregnancy Safer;, 2009, WHO recommendations on antenatal care for a positive pregnancy experience. Geneva, Switzerland: World Health Organization;, 2016), which decrease their risk of PPH. Inconsistent with a previous study's (Imai, 2020) finding, our study found that anemia and soft birth canal avulsion have an increased odds for PPH in nulliparous women than in multiparous women. It can partly be explained that because nulliparous women have never been pregnant or have never given birth before, they are more likely to have poor nutritional, iron, and folate supplementation habits, which leads to anemia. Anemia in pregnancy can lead to reduced blood volume and impaired coagulation function, which increases the risk of bleeding after birth. Considering the influence of anemia on PPH, it's crucial to provide health education and support for preventing and controlling anemia in all women's groups. Also, the increased risk of PPH due to soft birth canal avulsion among nulliparous women is because the levator ani muscle (which determines the size and shape of the birth canal) often gets extensively stretched during vaginal delivery, causing an increased risk of unnoticed soft birth canal trauma, mostly in nulliparous women due to the first birthing experience, which increases the risk of PPH (Dietz, 2013). During labor and delivery, clinicians should be more vigilant in detecting soft birth canal trauma for prompt intervention.
Furthermore, erythrocyte suspension transfusion before birth was identified as the predominant influencing factor of PPH in both nulliparous and multiparous women’s groups, for the first time. Erythrocyte suspension transfusion before birth was performed due to severe maternal anemia (hemoglobin concentration less than 7 g/dL in pregnancy) and profuse intrapartum bleeding as a result of maternal complications, including placenta previa and abruption. A total of 267 (16.2 %) placenta previa (malposition) and 57 (9.7 %) placenta abruptions were included in the group of erythrocyte suspension transfusions before birth (see appendix Table A.5). Hemoglobin is essential for carrying oxygen and nutrients in the blood, and its deficiency can lead to serious health problems for both the mother and the fetus. Accordingly, severe maternal anemia and placenta abnormalities can increase the risks of fetus's growth restriction, stillbirth, preterm birth, low birth weight, PPH, higher blood transfusion rate, and maternal-neonatal mortality (Shi et al., 2022;5(2):e2147046., Downes et al., 2017, Jing et al., 2018). Our finding highlights the critical importance of addressing severe maternal anemia and placental abnormalities early during pregnancy to prevent serious health risks to both the mother and the fetus. Consistent with a previous study (Xu et al., 2021), our study found that cesarean section and placenta abruption are associated with an increased risk of PPH among multiparous women than in nulliparous women. It is partly because multiparous women are often of advanced age (≥35 years). With advanced age and multiparity, the uterus becomes weaker, contracts poorly after birth, and takes longer to heal, increasing PPH risk (Sauer, 2015). Also, because of their older age, multiparous women are at increased risk for placenta abruption, which is most likely associated with decreased uterine blood flow, uteroplacental hypoperfusion, and placental infarctions leading to PPH (Martinelli et al., 2018, Ananth et al., 1996). Interestingly, our study found that general anesthesia administration before birth has higher odds of PPH among multiparous women than nulliparous women. Our finding is supported by existing research (Magann et al., 2005, Chang et al., 2011, Butwick et al., 2014) reports that general anesthesia administration before delivery can increase the risk of PPH. It is partly because general anesthetics can slow postpartum myometrium contraction, increasing PPH risk (Yoo et al., 2006). The effect is more pronounced in multiparous women, who are often older, than in nulliparous women. It is probably because, with advancement in maternal age, the uterus of women becomes looser over time, decreasing its postpartum contractility potential, which increases the risk of PPH (Sauer, 2015, Martinelli et al., 2018, Ananth et al., 1996). Thrombophlebitis was associated with an increased risk of PPH among the nulliparous women but had no association with PPH among the multiparous women. This is the first study reporting thrombophlebitis as an influencing factor for PPH in nulliparous women. For pregnant women with thrombophlebitis, including deep vein thrombosis (DVT), heparin (such as unfractionated heparin (UFH) and low molecular weight heparin (LMWH)) is used for both treatment and prevention of clot progression to reduce the risk of pulmonary embolism (Bates et al., 2016). However, anticoagulants used in pregnancy can increase the risk of PPH due to their blood-thinning effects, inhibiting thrombus and clot formation (Sirico et al., 2019). Also, though infrequent, thrombophlebitis can increase the risk of PPH due to a few interconnected physiological mechanisms. First, disruption of the normal blood flow through veins due to thrombophlebitis can damage the walls of the veins, especially in cases like septic pelvic thrombophlebitis, increasing the risk of PPH (Shi et al., 2021). Secondly, during pregnancy and postpartum, women's blood is in a hypercoagulable state. This hypercoagulability is protective against excessive bleeding but can increase the risk of thrombophlebitis and complications like diffuse intravascular coagulation, which can increase the risk of PPH (Li et al., 2023). Therefore, it is crucial to closely monitor pregnant women with thrombophlebitis, including DVT, to early identify and treat any complications that may arise as a result of thrombophlebitis or anticoagulant use. Instrument-assisted birth and gestational hypertension were associated with increased odds of PPH among the multiparous women but had no association with PPH among the nulliparous women. Our finding can partly be explained by the fact that multiparous women are often older, and with older maternal age comes an increased risk of pregnancy complications such as high blood pressure and abnormal fetus size, leading to instrument-assisted birth that increases the risk of PPH (Behrens et al., 2017). Inconsistent with a previous study (Sosa et al., 2009), our study revealed that low birth weight (<2500 g) and hysterectomy are influencing factors that have nearly similar odds of PPH in nulliparous and multiparous women. These findings require further research to understand the mechanisms by which low birth weight and hysterectomy can increase the risk of PPH.
4.3. Risk assessment tools
Risk assessment tools are available to help identify 60–85 % of women who will experience obstetric hemorrhage (Dilla et al., 2013). The ROC curve was used to evaluate the model's accuracy in this study. Single-factor and combined-factor analyses were used to measure how well the model fit. During the single-factor analysis, each identified PPH influencing factor was paired against PPH in the prediction model in the overall study cohort and within the nulliparous and multiparous subgroups. From the single-factor analysis, the variables had a relatively unsatisfactory AUC between 0.5 and 0.7 (Appendix Table A2, Table A3, Table A4). When combined-factors analysis was used for the whole study group and the two subgroups of nulliparous and multiparous women, the AUC was greater than 0.8 (Appendix Table A.1). The findings show that single influencing factor is a poor predictor of PPH compared to combined influencing factors. Therefore, more attention should be directed to all women with single or multiple influencing factors of PPH for prompt intervention because PPH may occur in women without even a single influencing factor.
4.4. Strengths and limitation
This study presents several strengths. First, it is the first to compare the incidence and factors influencing PPH between nulliparous and multiparous women, offering novel insights for PPH prevention. Second, its unique random sampling method minimized bias, while the large sample size enhanced the findings' generalizability. Finally, the sequential modeling of many possible factors that affect PPH by stratification in both univariate and multivariate analyses increase the chance of finding new influencing factors of PPH. Also, our study has a few limitations. First, the limitations of a retrospective cohort design apply to this study. Lastly, due to data limitations, the study could not assess certain potential PPH-influencing factors, including assisted reproductive technologies (ART) dietary and behavioral factors that could influence the study conclusion.
5. Conclusion
The incidence of PPH was higher in nulliparous women than multiparous women, but influencing factors varied relatively by parity. The study findings provide new insights for the use of different approaches to PPH prevention for nulliparous and multiparous women in clinical practice to ensure better maternal-child safety.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 81172680).
CRediT authorship contribution statement
Prince L. Bestman: Writing – review & editing, Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Musa Nget: . Edwina M. Kolleh: Writing – review & editing, Methodology, Investigation, Data curation. Eva Moeng: . Tesfit Brhane: Writing – review & editing, Visualization, Methodology, Investigation. Jun qun Fang: Writing – review & editing, Visualization, Supervision, Conceptualization. Jiayou Luo: Writing – review & editing, Visualization, Validation, Supervision, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We gratefully acknowledge the participating health facilities in Hunan Province for their invaluable support and cooperation, and we extend special thanks to Yao Zhenzhen for meticulously proofreading our manuscript.
Contributor Information
Prince L. Bestman, Email: plbestman@gmail.com.
Jun qun Fang, Email: jufang3497@163.com.
Jiayou Luo, Email: jiayouluo@126.com.
Appendix
.
Table A1.
Adjusted OR and AUC with 95 % CI in the Predictive Model for Combined Influencing Factors of PPH among Pregnant Women in Hunan, China, during 2017–2018 (n = 144,845).
| Group | AUC | 95 % Confidence Interval |
P-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Overall study cohort | 0.829 | 0.821 | 0.838 | <0.001 |
| Nulliparous group | 0.828 | 0.815 | 0.840 | <0.001 |
| Multiparous group | 0.833 | 0.822 | 0.844 | <0.001 |
AUC: Area under the curve, OR: Odd ratio, PPH: Postpartum hemorrhage.
Table A2.
Adjusted OR and AUC with 95 % CI in the Predictive Model for Influencing Factors of PPH among Pregnant Nulliparous and Multiparous Women in Hunan, China, during 2017–2018 (n = 144,845).
| Variable |
Multivariate logistic regression analysis |
Single factor analysis in predictive model |
||||
|---|---|---|---|---|---|---|
| AOR | 95 % CIs | P-value | AUC | 95 % CIs | P-value | |
| Number of ANC | 0.502 | 0.481–––0.503 | 0.150 | |||
| 0–3 ANC visits | 2.15 | 1.63–––2.83 | ≤0.001 | |||
| 4–9 ANC visits | 0.85 | 0.77–––0.93 | ≤0.001 | |||
| ≥ 10 ANC visits (Ref.) | Ref. | |||||
| Mode of Childbirth | 0.571 | 0.561–––0.581 | ≤0.001 | |||
| Normal vaginal birth/SVD(Ref.) | Ref. | |||||
| Cesarean section | 7.82 | 6.86–––8.91 | ≤0.001 | |||
| Instrumental/Assisted | 1.87 | 1.26–––2.77 | 0.002 | |||
| Gestational age (in weeks) | 0.501 | 0.490–––0.512 | 0.886 | |||
| Term (37–41 weeks) (Ref.) | Ref. | |||||
| Preterm (<37 weeks) | 1.41 | 1.12–––1.76 | 0.003 | |||
| Post-term (≥42 weeks) | 0.47 | 0.18–––1.20 | 0.110 | |||
| Birth weight | 0.500 | 0.462–––0.505 | ≤0.001 | |||
| Normal birth weight (2500-3999 g) (Ref.) | Ref. | |||||
| Low birth weight (<2500 g) | 1.48 | 1.14–––1.92 | 0.003 | |||
| Large birth weight (≥4000 g) | 0.41 | 0.36––0.47 | ≤0.001 | |||
| Anemia | 0.741 | 0.731–––0.750 | ≤0.001 | |||
| Yes | 7.02 | 6.39–––7.70 | ≤0.001 | |||
| No (Ref.) | Ref. | |||||
| Gestational hypertension | 0.504 | 0.493–––0.515 | 0.425 | |||
| Yes | 1.49 | 1.16––1.91 | 0.002 | |||
| No (Ref.) | Ref. | |||||
| Placenta abruption | 0.507 | 0.496–––0.519 | 0.181 | |||
| Yes | 3.02 | 2.04–––4.47 | ≤0.001 | |||
| No (Ref.) | Ref. | |||||
| Soft birth canal avulsion | 0.523 | 0.512–––0.535 | ≤0.001 | |||
| Yes | 4.15 | 3.45–––4.99 | ≤0.001 | |||
| No (Ref.) | Ref. | |||||
| Thrombophlebitis | 0.501 | 0.490–––0.511 | 0.926 | |||
| Yes | 11.96 | 1.81–––78.95 | 0.010 | |||
| No (Ref.) | Ref. | |||||
| Hysterectomy | 0.508 | 0.497–––5.19 | 0.165 | |||
| Yes | 2.87 | 2.16–––3.81 | ≤0.001 | |||
| No (Ref.) | Ref. | |||||
| General anesthesia used before birth | 0.511 | 0.500––0.522 | 0.050 | |||
| Yes | 2.87 | 2.56–––3.22 | ≤0.001 | |||
| No (Ref.) | Ref. | |||||
| Erythrocyte suspension transfusion before birth | 0.604 | 0.592–––0.617 | ≤0.001 | |||
| Yes | 54.06 | 46.25–––63.19 | ≤0.001 | |||
| No (Ref.) | ||||||
ANC: Antenatal Care, OR: Odd ratio, AUC: Area under the curve, CI: Confidence Interval, SVD: Spontaneous Vaginal Delivery, Ref.: Reference category, PPH: Postpartum Hemorrhage.
Table A3.
Adjusted OR and AUC with 95 % CI in the Predictive Model for Influencing Factors of PPH among Pregnant Nulliparous Women in Hunan, China, during 2017–2018 (n = 60686).
| Variable | Multivariate logistic regression analysis | Single factor analysis in predictive model | ||||
|---|---|---|---|---|---|---|
| AOR | 95 % CIs | P-value | AUC | 95 % CIs | P-value | |
| Number of ANC | 0.501 | 0.472–––0.504 | 0.136 | |||
| 0–3 ANC visits | 2.90 | 1.65–––5.08 | ≤0.001 | |||
| 4–9 ANC visits | 0.86 | 0.76–––0.99 | 0.065 | |||
| ≥ 10 ANC visits (Ref.) | Ref. | |||||
| Mode of Childbirth | 0.567 | 0.552–––0.583 | ≤0.001 | |||
| Normal vaginal birth/SVD(Ref.) | Ref. | |||||
| Cesarean section | 5.02 | 4.03–––6.26 | ≤0.001 | |||
| Instrumental/Assisted | 1.49 | 0.82–––2.73 | 0.149 | |||
| Gestational age (in weeks) | 0.508 | 0.492–––0.524 | 0.328 | |||
| Term (37–41 weeks) (Ref.) | Ref. | |||||
| Preterm (<37 weeks) | 1.42 | 0.99–––2.02 | 0.104 | |||
| Post-term (≥42 weeks) | 0.28 | 0.08–––0 0.95 | 0.046 | |||
| Birth weight | 0.503 | 0.466–––0.507 | 0.039 | |||
| Normal birth weight (2500-3999 g) (Ref.) | Ref. | |||||
| Low birth weight (<2500 g) | 2.08 | 1.39–––3.11 | ≤0.001 | |||
| Large birth weight (≥4000 g) | 0.34 | 0.28–––0.43 | ≤0.001 | |||
| Anemia | 0.751 | 0.737–––0.765 | ≤0.001 | |||
| Yes | 8.41 | 7.34–––9.64 | ≤0.001 | |||
| No (Ref.) | Ref. | |||||
| Placenta abruption | 0.503 | 0.487–––0.519 | 0.689 | |||
| Yes | 2.93 | 1.46–––5.84 | ≤0.002 | |||
| No (Ref.) | Ref. | |||||
| Soft birth canal avulsion | 0.529 | 0.512–––0.546 | ≤0.001 | |||
| Yes | 4.01 | 3.11–––5.16 | ≤0.001 | |||
| No (Ref.) | Ref. | |||||
| Thrombophlebitis | 0.501 | 0.485–––0.517 | 0.926 | |||
| Yes | 18.46 | (1.67–––20.31) | 0.011 | |||
| No (Ref.) | Ref. | |||||
| Hysterectomy | 0.509 | 0.493–––0.525 | 0.277 | |||
| Yes | 2.50 | 1.67–––3.75 | ≤0.001 | |||
| No (Ref.) | Ref. | |||||
| General anesthesia used before birth | 0.500 | 0.453–––0.505 | ≤0.001 | |||
| Yes | 1.42 | 1.19–––1.69 | ≤0.001 | |||
| No (Ref.) | Ref. | |||||
| Erythrocyte suspension transfusion before birth | 0.604 | 0.576–––0.612 | ≤0.001 | |||
| Yes | 48.67 | 36.43–––65.04 | ≤0.001 | |||
| No (Ref.) | Ref. | |||||
ANC: Antenatal Care, OR: Odd ratio, AUC: Area under the curve, CI: Confidence Interval, SVD: Spontaneous Vaginal Delivery, Ref: Reference category, PPH: Postpartum hemorrhage.
Table A4.
Adjusted OR and AUC with 95 % CI in the Predictive Model for Influencing Factors of PPH among Pregnant Multiparous Women in Hunan, China, during 2017–2018 (n = 84,159).
| Variable |
Multivariate logistic regression analysis |
Single factor analysis in predictive model |
||||
|---|---|---|---|---|---|---|
| AOR | 95 % CIs | P-value | AUC | 95 % CIs | P-value | |
| Number of ANC | 0.509 | 0.485–––0.513 | 0.905 | |||
| 0–3 ANC visits | 1.85 | 1.35–––2.53 | ≤0.001 | |||
| 4–9 ANC visits | 0.84 | 0.74–––0.96 | 0.068 | |||
| ≥ 10 ANC visits (Ref.) | Ref. | |||||
| Mode of Childbirth | 0.569 | 0.555–––0.584 | ≤0.001 | |||
| Normal vaginal birth/SVD(Ref.) | Ref. | |||||
| Cesarean section | 5.81 | 4.63–––7.27 | ≤0.001 | |||
| Instrumental/Assisted | 1.95 | 1.16–––3.28 | 0.041 | |||
| Birth weight | 0.501 | 0.449–––0.508 | ≤0.001 | |||
| Normal birth weight (2500-3999 g) (Ref.) | Ref. | |||||
| Low birth weight (<2500 g) | 2.09 | 1.60–––2.72 | ≤0.001 | |||
| Large birth weight (≥4000 g) | 0.44 | 0.367––0.52 | ≤0.001 | |||
| Anemia | 0.733 | 0.720–––0.746 | ≤0.001 | |||
| Yes | 6.78 | 5.98–––7.69 | ≤0.001 | |||
| No (Ref.) | Ref. | |||||
| Gestational hypertension | 0.507 | 0.492–––0.522 | 0.370 | |||
| Yes | 1.57 | 1.13–––2.19 | 0.003 | |||
| No (Ref.) | Ref. | |||||
| Placenta abruption | 0.511 | 0.496–––0.526 | 0.146 | |||
| Yes | 3.62 | 2.31–––5.66 | ≤0.001 | |||
| No (Ref.) | Ref. | |||||
| Soft birth canal avulsion | 0.519 | 0.503–––0.534 | 0.015 | |||
| Yes | 3.66 | 2.77–––4.82 | ≤0.001 | |||
| No (Ref.) | Ref. | |||||
| Hysterectomy | 0.507 | 0.492–––0.522 | 0.370 | |||
| Yes | 2.59 | 1.73–––3.89 | ≤0.001 | |||
| No (Ref.) | Ref. | |||||
| General anesthesia used before birth | 0.502 | 0.447–––0.507 | ≤0.001 | |||
| Yes | 1.63 | 1.33–––2.01 | ≤0.001 | |||
| No (Ref.) | Ref. | |||||
| Erythrocyte suspension transfusion before birth | 0.613 | 0.596–––0.630 | ≤0.001 | |||
| Yes | 46.58 | 37.72–––57.54 | ≤0.001 | |||
| No (Ref.) | Ref. | |||||
ANC: Antenatal Care, AOR: Adjusted odd ratio, AUC: Area under the curve, CI: Confidence Interval, SVD: Spontaneous Vaginal Delivery, Ref: Reference category, PPH: Postpartum Hemorrhage.
‘
Table A5.
Incidence of Placental previa and abruption in the group of erythrocyte suspension transfusion before birth among Nulliparous and Multiparous women in Hunan, China, during 2017–2018 (n = 144845).
| Variable |
Total |
Nulliparous (n = 60686) |
Multiparous (n = 84,159) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Erythrocyte suspension transfusion before birth |
P-value |
Erythrocyte suspension transfusion before birth |
P-value |
||||||
| n(%) | Yes | No | n(%) | Yes | No | ||||
|
Placenta previa |
<0.001 | <0.001 | |||||||
| Yes | 1652(1.1) | 449(0.7) | 55(12.2) | 394(87.8) | 1203(1.4) | 212(17.6) | 991(82.4) | ||
| No | 143193(98.9) | 60237(99.3) | 273(0.5) | 59964(99.5) | 82956(98.6) | 487(0.6) | 82469(99.4) | ||
| Placenta abruption | <0.001 | <0.001 | |||||||
| Yes | 589(0.4) | 236(0.4) | 10(4.2) | 226(95.8) | 353(0.4) | 47(13.3) | 306(86.7) | ||
| No | 144256(99.6) | 60450(99.6) | 318(0.5) | 60132(99.5) | 83806(99.6) | 652(0.8) | 83154(99.2) | ||
Data availability
The data that has been used is confidential.
References
- Ananth C.V., Wilcox A.J., Savitz D.A., Bowes W.A., Jr, Luther E.R. Effect of maternal age and parity on the risk of uteroplacental bleeding disorders in pregnancy. Obstet Gynecol. 1996;88(4 Pt 1):511–516. doi: 10.1016/0029-7844(96)00236-0. [DOI] [PubMed] [Google Scholar]
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676-682. doi:10.2337/dc09-1848. [DOI] [PMC free article] [PubMed]
- Bais J.M., Eskes M., Pel M., Bonsel G.J., Bleker O.P. Postpartum haemorrhage in nulliparous women: incidence and risk factors in low and high risk women. A Dutch population-based cohort study on standard (> or = 500 ml) and severe (> or = 1000 ml) postpartum haemorrhage. Eur J Obstet Gynecol Reprod Biol. 2004;115(2):166–172. doi: 10.1016/j.ejogrb.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Bates S.M., Middeldorp S., Rodger M., James A.H., Greer I. Guidance for the treatment and prevention of obstetric-associated venous thromboembolism. J Thromb Thrombolysis. 2016;41(1):92–128. doi: 10.1007/s11239-015-1309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates S.M., Rajasekhar A., Middeldorp S., et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy. Blood Adv. 2018;2(22):3317–3359. doi: 10.1182/bloodadvances.2018024802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens I., Basit S., Melbye M., et al. Risk of post-pregnancy hypertension in women with a history of hypertensive disorders of pregnancy: nationwide cohort study. BMJ. 2017;358 doi: 10.1136/bmj.j3078. Published 2017 Jul 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M.A., Lindheimer M.D., de Swiet M., Van Assche A., Moutquin J.M. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP) Hypertens Pregnancy. 2001;20(1):IX-XIV. doi: 10.1081/PRG-100104165. [DOI] [PubMed] [Google Scholar]
- Butwick A.J., Carvalho B., El-Sayed Y.Y. Risk factors for obstetric morbidity in patients with uterine atony undergoing caesarean delivery. Br J Anaesth. 2014;113(4):661–668. doi: 10.1093/bja/aeu150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.C., Wang I.T., Chen Y.H., Lin H.C. Anesthetic management as a risk factor for postpartum hemorrhage after cesarean deliveries. Am J Obstet Gynecol. 2011;205(5):462.e1–462.e4627. doi: 10.1016/j.ajog.2011.06.068. [DOI] [PubMed] [Google Scholar]
- Devine P.C. Obstetric hemorrhage. Semin Perinatol. 2009;33(2):76–81. doi: 10.1053/j.semperi.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Dietz H.P. Pelvic floor trauma in childbirth. Aust N Z J Obstet Gynaecol. 2013;53(3):220–230. doi: 10.1111/ajo.12059. [DOI] [PubMed] [Google Scholar]
- Dilla A.J., Waters J.H., Yazer M.H. Clinical validation of risk stratification criteria for peripartum hemorrhage. Obstet Gynecol. 2013;122(1):120–126. doi: 10.1097/AOG.0b013e3182941c78. [DOI] [PubMed] [Google Scholar]
- Dionne MD, Deneux-Tharaux C, Dupont C, et al. Duration of Expulsive Efforts and Risk of Postpartum Hemorrhage in Nulliparous Women: A Population-Based Study. PLoS One. 2015;10(11):e0142171. Published 2015 Nov 10. doi:10.1371/journal.pone.0142171. [DOI] [PMC free article] [PubMed]
- Downes K.L., Shenassa E.D., Grantz K.L. Neonatal Outcomes Associated With Placental Abruption. Am J Epidemiol. 2017;186(12):1319–1328. doi: 10.1093/aje/kwx202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X.L., Zhu J., Zhang L., et al. Socio-economic disparities in maternal mortality in China between 1996 and 2006. BJOG. 2010;117(12):1527–1536. doi: 10.1111/j.1471-0528.2010.02707.x. [DOI] [PubMed] [Google Scholar]
- Ford J.B., Roberts C.L., Simpson J.M., Vaughan J., Cameron C.A. Increased postpartum hemorrhage rates in Australia. Int J Gynaecol Obstet. 2007;98(3):237–243. doi: 10.1016/j.ijgo.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Geller S.E., Adams M.G., Kelly P.J., Kodkany B.S., Derman R.J. Postpartum hemorrhage in resource-poor settings. Int J Gynaecol Obstet. 2006;92(3):202–211. doi: 10.1016/j.ijgo.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Govindappagari S., Moyle K., Burwick R.M. Mild Thrombocytopenia and Postpartum Hemorrhage in Nulliparous Women With Term, Singleton. Vertex Deliveries. Obstet Gynecol. 2020;135(6):1338–1344. doi: 10.1097/AOG.0000000000003861. [DOI] [PubMed] [Google Scholar]
- Hogan M.C., Foreman K.J., Naghavi M., et al. Maternal mortality for 181 countries, 1980–2008: a systematic analysis of progress towards Millennium Development Goal 5. Lancet. 2010;375(9726):1609–1623. doi: 10.1016/S0140-6736(10)60518-1. [DOI] [PubMed] [Google Scholar]
- Imai K. Parity-based assessment of anemia and iron deficiency in pregnant women. Taiwan J Obstet Gynecol. 2020;59(6):838–841. doi: 10.1016/j.tjog.2020.09.010. [DOI] [PubMed] [Google Scholar]
- Jing L, Wei G, Mengfan S, Yanyan H. Effect of site of placentation on pregnancy outcomes in patients with placenta previa. PLoS One. 2018;13(7):e0200252. Published 2018 Jul 17. doi:10.1371/journal.pone.0200252. [DOI] [PMC free article] [PubMed]
- Khan K.S., Wojdyla D., Say L., Gülmezoglu A.M., Van Look P.F. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367(9516):1066–1074. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- Knight M, Callaghan WM, Berg C, et al. Trends in postpartum hemorrhage in high resource countries: a review and recommendations from the International Postpartum Hemorrhage Collaborative Group. BMC Pregnancy Childbirth. 2009;9:55. Published 2009 Nov 27. doi:10.1186/1471-2393-9-55. [DOI] [PMC free article] [PubMed]
- Kramer M.S., Berg C., Abenhaim H., et al. Incidence, risk factors, and temporal trends in severe postpartum hemorrhage. Am J Obstet Gynecol. 2013;209(5):449.e1–449.e4497. doi: 10.1016/j.ajog.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Li S, Gao J, Liu J, et al. Incidence and Risk Factors of Postpartum Hemorrhage in China: A Multicenter Retrospective Study. Front Med (Lausanne). 2021;8:673500. Published 2021 Aug 23. doi:10.3389/fmed.2021.673500. [DOI] [PMC free article] [PubMed]
- Li N, Liu Y, Yun A, Song S. Correlation of Platelet Function with Postpartum Hemorrhage and Venous Thromboembolism in Patients with Gestational Hypertension Complicated with Diabetes [retracted in: Comput Math Methods Med. 2023 Sep 27;2023:9783435]. Comput Math Methods Med. 2022;2022:2423333. Published 2022 Jul 18. doi:10.1155/2022/2423333. [DOI] [PMC free article] [PubMed] [Retracted]
- Liu C.N., Yu F.B., Xu Y.Z., et al. Prevalence and risk factors of severe postpartum hemorrhage: a retrospective cohort study. BMC Pregnancy Childbirth. 2021;21(1):332. doi: 10.1186/s12884-021-03818-1. Published 2021 Apr 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magann E.F., Evans S., Hutchinson M., Collins R., Lanneau G., Morrison J.C. Postpartum hemorrhage after cesarean delivery: an analysis of risk factors. South Med J. 2005;98(7):681–685. doi: 10.1097/01.SMJ.0000163309.53317.B8. [DOI] [PubMed] [Google Scholar]
- Magann E.F., Evans S., Hutchinson M., Collins R., Howard B.C., Morrison J.C. Postpartum hemorrhage after vaginal birth: an analysis of risk factors. South Med J. 2005;98(4):419–422. doi: 10.1097/01.SMJ.0000152760.34443.86. [DOI] [PubMed] [Google Scholar]
- Martinelli KG, Garcia ÉM, Santos Neto ETD, Gama SGND. Advanced maternal age and its association with placenta praevia and placental abruption: a meta-analysis. Cad Saude Publica. 2018;34(2):e00206116. Published 2018 Feb 19. doi:10.1590/0102-311X00206116. [DOI] [PubMed]
- McDonnell N, Knight M, Peek MJ, et al. Amniotic fluid embolism: an Australian-New Zealand population-based study. BMC Pregnancy Childbirth. 2015;15:352. Published 2015 Dec 24. doi:10.1186/s12884-015-0792-9. [DOI] [PMC free article] [PubMed]
- Meng Q, Mills A, Wang L, Han Q. What can we learn from China's health system reform?. BMJ. 2019;365:l2349. Published 2019 Jun 19. doi:10.1136/bmj.l2349. [DOI] [PMC free article] [PubMed]
- Sauer M.V. Reproduction at an advanced maternal age and maternal health. Fertil Steril. 2015;103(5):1136–1143. doi: 10.1016/j.fertnstert.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2(6):e323-e333. doi:10.1016/S2214-109X(14)70227-X. [DOI] [PubMed]
- Shi Q, Gandi DS, Hua Y, et al. Postpartum septic pelvic thrombophlebitis and ovarian vein thrombosis after caesarean section: a rare case report. BMC Pregnancy Childbirth. 2021;21(1):561. Published 2021 Aug 17. doi:10.1186/s12884-021-04037-4. [DOI] [PMC free article] [PubMed]
- Shi H, Chen L, Wang Y, et al. Severity of Anemia During Pregnancy and Adverse Maternal and Fetal Outcomes. JAMA Netw Open. 2022;5(2):e2147046. Published 2022 Feb 1. doi:10.1001/jamanetworkopen.2021.47046. [DOI] [PMC free article] [PubMed]
- Sirico A., Saccone G., Maruotti G.M., et al. Low molecular weight heparin use during pregnancy and risk of postpartum hemorrhage: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2019;32(11):1893–1900. doi: 10.1080/14767058.2017.1419179. [DOI] [PubMed] [Google Scholar]
- Sosa C.G., Althabe F., Belizán J.M., Buekens P. Risk factors for postpartum hemorrhage in vaginal deliveries in a Latin-American population. Obstet Gynecol. 2009;113(6):1313–1319. doi: 10.1097/AOG.0b013e3181a66b05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TT, Ahn J, Reau NS. ACG Clinical Guideline: Liver Disease and Pregnancy [published correction appears in Am J Gastroenterol. 2016 Nov;111(11):1668]. Am J Gastroenterol. 2016;111(2):176-196. doi:10.1038/ajg.2015.430. [DOI] [PubMed]
- Wei Q., Xu Y., Zhang L. Towards a universal definition of postpartum hemorrhage: retrospective analysis of Chinese women after vaginal delivery or cesarean section: A case-control study. Medicine (baltimore). 2020;99(33):e21714. doi: 10.1097/MD.0000000000021714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Who . World Health Organization; Geneva: 2009. Guidelines for the Management of Postpartum Haemorrhage and Retained Placenta. [PubMed] [Google Scholar]
- Who . World Health Organization; Geneva: 2012. Recommendations for the Prevention and Treatment of Postpartum Haemorrhage. [PubMed] [Google Scholar]
- WHO recommendations on antenatal care for a positive pregnancy experience. Geneva, Switzerland: World Health Organization; 2016. [PubMed]
- WHO recommended interventions for improving maternal and newborn health. Geneva: World Health Organization Department of Making Pregnancy Safer; 2009.
- Xu C, Zhong W, Fu Q, et al. Differential effects of different delivery methods on progression to severe postpartum hemorrhage between Chinese nulliparous and multiparous women: a retrospective cohort study [published correction appears in BMC Pregnancy Childbirth. 2021 Jan 19;21(1):64]. BMC Pregnancy Childbirth. 2020;20(1):660. Published 2020 Oct 31. doi:10.1186/s12884-020-03351-7. [DOI] [PMC free article] [PubMed]
- Xu C., Fu Q., Tao H.B., et al. Effect of Cesarean Section on the Severity of Postpartum Hemorrhage in Chinese Women: The Shanxi Study. Curr Med Sci. 2018;38(4):618–625. doi: 10.1007/s11596-018-1922-1. [DOI] [PubMed] [Google Scholar]
- Yoo K.Y., Lee J.C., Yoon M.H., et al. The effects of volatile anesthetics on spontaneous contractility of isolated human pregnant uterine muscle: a comparison among sevoflurane, desflurane, isoflurane, and halothane. Anesth Analg. 2006;103(2) doi: 10.1213/01.ane.0000236785.17606.58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.