Summary
Pluripotent stem cell-based therapy for retinal degenerative diseases is a promising approach to restoring visual function. A clinical study using retinal organoid (RO) sheets was recently conducted in patients with retinitis pigmentosa. However, the graft preparation currently requires advanced skills to identify and excise suitable segments from the transplantable area of the limited number of suitable ROs. This remains a challenge for consistent clinical implementations. Herein, we enabled the enrichment of wild-type (non-reporter) retinal progenitor cells (RPCs) from dissociated ROs using a label-free ghost cytometry (LF-GC)-based sorting system, where a machine-based classifier was trained in advance with another RPC reporter line. The sorted cells reproducibly formed retinal spheroids large enough for transplantation and developed mature photoreceptors in the retinal degeneration rats. This method of enriching early RPCs with no specific surface antigens and without any reporters or chemical labeling is promising for robust preparation of graft tissues during cell-based therapy.
Keywords: ghost cytometry, label-free cell sorting, retinal progenitor cell, retinal organoid, regenerative therapy, transplantation, retinal degeneration
Graphical abstract

Highlights
-
•
We enriched RPCs using LF-GC
-
•
LF-GC-based-sorted RPCs consistently developed retinal tissues for transplantation
-
•
LF-GC-based sorting achieved nearly 10 times efficiency in retinal graft preparation
-
•
LF-GC-enriched hiPSC-RPC grafts structurally matured in retinal degeneration animals
Mandai and colleagues show that their label-free ghost cytometry-based sorting system optimized by retinal progenitor cell (RPC)-reporter hESC line as a training sample successfully enriched early RPCs dissociated from non-labeled hiPSC-derived retinal organoids. They also show that their system provides the stable and robust production of retinal grafts, which structurally matured in retinal degeneration nude rat eyes.
Introduction
Cell-based therapy is considered a promising strategy for restoring visual function in retinal degenerative diseases, such as retinitis pigmentosa (Pearson et al., 2012; Singh et al., 2013; Gasparini et al., 2019; Ribeiro et al., 2021; Mandai et al., 2017). We obtained proof-of-concept data using human embryonic stem cell (hESC)/human induced pluripotent stem cell (hiPSC)-derived retinal organoids (ROs) in end-stage retinal degeneration animal models, including those exhibiting graft photoreceptor maturation, host-graft synapse formation, and light signal transmission to host retinal ganglion cells (Mandai et al., 2017; Iraha et al., 2018; Tu et al., 2019). In a recent clinical study, hiPSC-derived RO sheets were used in patients with retinitis pigmentosa (jRCTa050200027). Currently, however, graft sheet preparation requires a highly skilled technician to identify and excise an adequate area for transplantation, which is normally less than 1.0 mm2, far less than the desired target area. Moreover, the efficiency of the production of ROs that contain substantial areas for graft excision varies from hiPSC line to line, and ROs with small retinal parts cannot be utilized, making it difficult to generalize the procedure for robust graft production.
One strategy to resolve these problems is to collect and enrich RPCs from all ROs, regardless of the RPC content, to ultimately produce stable retinal tissues of the desired form and size. We previously confirmed that mouse iPSC-derived early RPCs on differentiation day (DD) 11–13 can reconstruct retinal lamination after transplantation (Assawachananont et al., 2014). Thus, DD11–13 seem to be an appropriate time for collecting RPCs to reconstruct retinal structures. DD11–13 of mouse ROs are almost equivalent to DD25–30 in hiPSC-derived RO (Llonch et al., 2018), which were selected for RPC enrichment. On DD25–30, RAX is an essential transcription factor for eye-field fate decisions and establishment of the retina, as well as an RPC marker (Osakada et al., 2008; Muranishi et al., 2012). RPC enrichment at such an early stage is challenging when using conventional cell sorting technologies, such as fluorescence-activated cell sorting (FACS) or magnetic-activated cell sorting, owing to the large heterogeneity in gene expression among RPCs (Trimarchi et al., 2008; Cepko, 2014). Consequently, no specific surface antigens as a whole have been reported (Lakowski et al., 2015; Kaewkhaw et al., 2016; Gagliardi et al., 2018). Additionally, given the labor and potential chemical toxicity of molecular staining on the final product, it is better avoided (Roederer, 2001; Progatzky et al., 2013). Although some studies have demonstrated label-free sorting of mature photoreceptors based on cell stiffness and viscosity (Stone et al., 2020) or bright-field images (Herbig et al., 2022), achieving the practical application of these technologies in terms of quality control and clinical-scale throughput remains challenging. Therefore, we used ghost cytometry (GC) to perform label-free automated RPC enrichment.
GC is a recently developed high-content flow cytometric approach that enables fast and accurate analysis of cells based on their high-resolution structural information down to the subcellular levels (Ota et al., 2018, 2020). In its label-free mode, GC acquires the structural information of cells as multiparametric temporal signals, called ghost motion imaging (GMI) waveforms, by flowing cells through a structured light illumination (Ugawa et al., 2021). Label-free GMI (LF-GMI) waveforms include forward- and backward-scattering and bright-field GMI (fsGMI, bsGMI, and bfGMI, respectively) waveforms. fsGMI and bsGMI waveforms provide high-resolution information compared with forward and backward scattering (FSC and BSC, respectively) signals of conventional flow cytometry, while bfGMI waveforms are analogous to bright-field microscopy images. By directly analyzing these LF-GMI waveforms using machine learning without image reconstruction, LF-GC-based cell sorting enables the accurate and high-throughput enrichment of target cells without using any biological stains, making it applicable in regenerative medicine.
In this study, we applied an LF-GC-based sorting system to enrich hESC/hiPSC-derived RPCs for robust graft preparation in retinal regenerative therapies. Based on our initial observation that Venus strong-positive cells of DD25 Rax::Venus hESC-derived ROs can form circumferential retinal spheroids (C-spheroids) (Figure 1), we first used the Rax::Venus cell line to generate a classifier and sorted Rax::Venus cells to evaluate the system. Second, we performed LF-GC-based sorting on the hESC-Crx::Venus cell line, which has the same genetic background as the Rax::Venus cell line but does not express Venus fluorescence at the time of cell sorting, to test whether the sorted cells showed enhanced RPC marker gene expression and could form consistent retinal spheroids. Third, we applied the same system to hiPSC-derived RO cells to determine whether we could reproducibly enrich RPCs that formed consistent retinal spheroids. Finally, we transplanted hiPSC-derived LF-GC-based sorted retinal spheroids into a nude rat retinal degeneration model and evaluated graft maturation and synaptic integration via immunohistochemistry 4–5 months after transplantation.
Figure 1.
Characteristic spheroid morphologies after FACS-sorted progenitor cell re-aggregation
(A and B) Phenotypes and FCM profiling of D6-BMP4 (A) and no-BMP4 organoids (B). D6-BMP4 organoids were those subjected to BMP treatment on DD6, whereas no-BMP4 organoids were not subjected to BMP treatment. (Top right) FSC-A and Venus profiles. (Bottom right) FSC-A and Venus profiles in contour plots. We set the Venus++ line by referring to the contour plot of Venus intensity profiles of D6-BMP4 (A).
(C–E) FSC and SSC profiles of Venus++ FACS-sorted cells, Venus+/++ FACS-sorted cells, and unsorted cells. (Top) FSC-A and Venus profiles. (Bottom) FSC-A and SSC-A profiles. None of the FACS-sorted cells (red dots) were in the specific FSC/SSC plot regions.
(F) Characteristic spheroid morphologies after re-aggregation of FACS-sorted Venus ++, FACS-sorted Venus +/++, and original unsorted cells.
(G) Phenotypes of Rax::Venus-based spheroid assays on DD60. We performed six independent FACS experiments based on Venus intensities (#01-#06). Each color box and bar represents the samples indicated by the same color.
(H–M) Immunostaining images of each spheroid on DD60–70 with antibodies against Venus (green) (H), VSX2 (red) (I and M), PAX6 (white) (J), BRN3 (red) (K), CRX (white) (L), EMX2 (white) (M), and DAPI (blue) (H–L).
(N and O) Immunostaining images of each spheroid on DD60–70 at high magnification. Venus ++ and Venus+/++ spheroids expressed PAX6 (white) (N) and BRN3 (red) (O) on the basal side of the cell layer and VSX2 (red) (N) in the apical layer. They expressed CRX (white) (O), mainly on the apical side. The unsorted spheroids did not contain VSX2/BRN3-positive cells.
Scale bars, 500 μm (A, B, and F), 200 μm (I–N), and 100 μm (N and O).
Results
Rax::Venus-positive RPCs present characteristic spheroid morphologies after re-aggregation
We first tested whether Rax::Venus expression was an appropriate marker for RPC enrichment using cells dissociated from ROs on DD25 in the Rax::Venus ESC line. Based on the initial observation of RPCs reproducing RO-like structures via re-aggregation, we investigated whether RPCs with different Rax::Venus intensities exhibited distinct spheroid phenotypes. Human ESCs were differentiated using a modified method involving a serum-free floating culture of embryoid body-like aggregates with quick aggregation (SFEBq) and bone morphogenetic protein 4 (BMP4) treatment as previously described (Nakano et al., 2012; Kuwahara et al., 2015, 2019). Rax::Venus cells consistently developed into ROs of polarized Venus-positive and Venus-negative components on DD25 (Figure 1A). Cells that did not differentiate into Rax::Venus-positive organoids without BMP4 treatment were used as negative controls for FACS (no-BMP4; Figure 1B). Venus++ cells were defined on the flow cytometry (FCM) density plot, as shown in Figure 1A. The dissociated cells from Rax::Venus ROs on DD25 were sorted into either Venus++ or Venus+/++ populations by setting different Venus intensity thresholds, which were not in specific FSC/BSC plot regions (Figures 1C and 1D). Venus++ (Figure 1C), Venus+/++ (Figure 1D), and unsorted cells (Figure 1E) formed different types of spheroids by their morphology and Venus expression patterns, which were evaluated using bright-field and fluorescence imaging (Figures 1F and 1G). On DD60–70, Venus++ cells consistently developed characteristic spheroids with uniform Rax::Venus expression over the entire circumference in six independent trials (C-spheroid, hereafter; Figures 1F, left, and 1G), whereas Venus+/++ cells mainly developed into polarized spheroids consisting of Rax::Venus-positive and -negative parts (P-spheroid, hereafter; Figures 1F, middle, and 1G). Unsorted cells mainly developed into Rax::Venus-negative non-retinal tissues on DD60-70 (N-spheroid, hereafter; Figures 1F, right, and 1G). During immunohistological examination, Rax::Venus-positive C-spheroids and the RAX-positive portion of the P-spheroids revealed neural epithelium-like structures consisting of VSX2-positive cells apically and PAX6-positive cells basally, with CRX-positive photoreceptor precursor cells predominantly apically and BRN3-positive retinal ganglion cells basally. These findings suggest the formation of structured retinal tissue (Figures 1H–1O). In contrast, Venus-negative tissues were also negative for VSX2, BRN3, and CRX, but contained cells positive for PAX6 and EMX2, which are markers of general neural progenitor cells (Figures 1H–1O). C-spheroids and the neural epithelium-like part of P-spheroids seemed ideal and adequate, respectively, for retinal transplantation. These results indicate that we can utilize the aggregate phenotype patterns as a spheroid assay to determine whether we have obtained the desired RPC population from ROs and that Rax::Venus++ cells are good candidates for selecting RPCs.
Classifier for LF-GC-based sorting generated using Rax::Venus RO cells
Next, we generated a classifier for LF-GC-based sorting using DD25 Rax::Venus RO cells as training samples. In the GC setup, five LF optical signals—fsGMI, bsGMI, and bfGMI waveforms, along with the FSC and BSC from each cell in the training sample—were acquired together with Venus-fluorescent signals (Figure 2A). After defining the live-cell gate in the FSC-H/BSC-H plot (Figure 2B), the Venus++ cell gate was defined in the FSC-H/Venus-H plot (Figure 2C). The cells outside the Venus++ cell gate in the FSC-H/Venus-H plot were defined as others. Using two of the three GMI waveforms (Figures 2D–2F), together with the FSC-H and BSC-H intensities of 700–800 cells randomly selected from each gate (Venus++ cells or others), a classifier was trained based on the support vector machine (SVM) algorithm (Figures 2G–2J). The mean percentage of Venus++ cells to total cells in the live-cell gate was 68.8 ± 7.65% (range, 57.3%–77.9%; n = 10). To evaluate the accuracy of the trained classifier, we calculated the area under the receiver operating characteristic curve (AUROC). The mean AUROC was 0.73 ± 0.02 (range, 0.71–0.76; n = 10); in 6 of the 10 trials, the combination of fsGMI and bsGMI waveforms as well as FSC-H and BSC-H intensities provided the highest AUROC (Figure 2K). During sorting, the cells in the live-cell gate were classified as positive or negative based on a trained classifier. The decision boundary for SVM scores in each experiment was determined so that the recovery rate (number of cells predicted to be positive/number of cells in the live-cell gate) estimated from the precision and recall curve was approximately 10%–20% (Figure 2I; Table S1); cells with an SVM score of 0.7–1.5 (decision boundary) or higher were classified as positive cells, while other were classified as negative cells (Table S1). During sorting, only positive cells were collected as Sorted samples; all other cells were collected as Waste samples (Figure 2L); the input cell suspension was preserved on ice as Control samples.
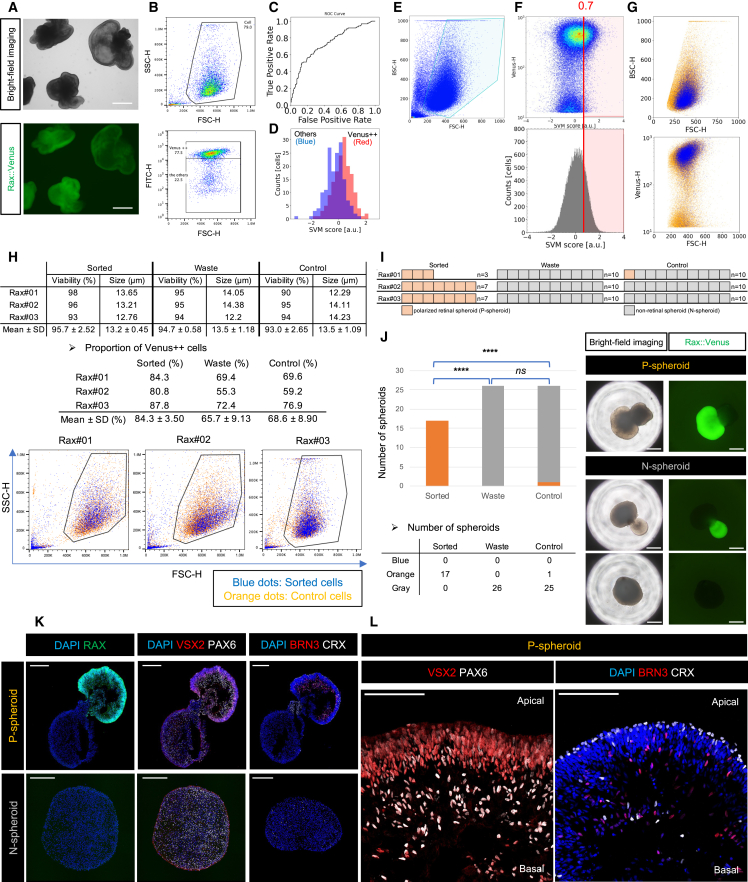
Figure 2.
LF-GC-based sorting system
(A) Scheme used for obtaining the LF-GMI waveforms. The LF-GMI waveforms and Venus fluorescence signals (ground truth labels) of Rax::Venus cells were acquired using a GC setup.
(B) FSC and BSC profiles of the Rax::Venus cell line (Rax#02). The blue gate indicates the live-cell gate.
(C) FSC and Venus profiles of the cells in the live-cell gate. The green gate represents the Venus++ cell gate. The cells outside the Venus++ cell gate in the live-cell gate were defined as others.
(D–F) Representative GMI waveforms of Venus++ and others. Twenty cells are displayed in each GMI waveform: fsGMI (D), bsGMI(E), and bfGMI (F).
(G) Receiver operating characteristic curve for the classification of Venus++ cells and other cells using fsGMI, bsGMI waveforms, FSC-H, and BSC-H. The AUROC was 0.76.
(H) SVM score histogram in classifier evaluation. (Red) Venus++ cells. (Blue) Others.
(I) Precision (purity) and recall (recovery) rates of Venus++ cells for SVM scores. (Red) Precision rate (%). (Blue) recall rate (%).
(J) Scheme of training a classifier using Venus++ cells as the training sample. Venus ++ and others were identified using flow cytometry gating. Using the identified LF-GMI waveforms, the classifier was trained based on the SVM algorithm.
(K) AUROC scores from all experiments using two of the three GMI waveforms and the percentage of Venus++ cells in the Rax::Venus cell line. (Purple circle) AUROC of bsGMI, fsGMI, FSC, and BSC. (Blue triangle) AUROC of bfGMI, fsGMI, FSC-H, and BSC-H. (Yellow cross) AUROC of bsGMI, bfGMI, FSC, and BSC. (Gray bar plot) Percentage of Venus++ cells to total cells in the live-cell gate.
(L) Scheme of LF-GC-based sorting for RPC enrichment. In sorting, each cell was classified as positive or negative in real time based on the trained classifier using FSC-H, BSC-H, and LF-GMI waveforms. Subsequently, the positive cells were sorted and collected as Sorted samples.
LF-GC-based sorting system enriched RPCs from Rax::Venus ROs
We first performed three LF-GC-based sorting experiments (Rax#01–#03) using a spheroid assay with DD25 Rax::Venus ROs from independent differentiation batches (Figures 3A and 3B). The classifiers for LF-GC-based sorting were trained using Venus++ cells in each experiment (Figures 3C and 3D). During sorting, dead cells were excluded by gating, and cells in the live-cell gate (Figure 3E) were analyzed based on the trained classifier. In Rax#01, the SVM decision boundary was set to 0.7 (Figure 3F); cells with an SVM score of 0.7 or higher were classified as positive cells, which were collected as Sorted samples (Figure 3G). The cell viability and average cell size calculated using Countess II did not differ among the Sorted, Waste, and Control groups (p = 0.366 and 0.886, respectively; Figure 3H, top). Flow cytometry reanalysis after LF-GC-based sorting showed that Venus++ cells were enriched in the Sorted samples compared with those in the Waste and Control samples (Figure 3H, middle). According to the spheroid assay, P-spheroids reproducibly developed from the Sorted group, whereas the other groups mainly became Venus-negative N-spheroids (Figures 3I and 3J). In the Sorted group, the proportions of spheroids with retinal regions large enough for transplantation (referred to as the graft index hereafter) was significantly higher at 100% (17/17) than in the other groups (both p < 0.0001) (Figure 3J). Histological examination showed that Venus-positive tissues of P-spheroids (orange boxes and bars in Figures 3I and 3J) exhibited retinal epithelial structures consisting of VSX2-positive apical cells and PAX6-positive basal cells and contained cells expressing retina-specific markers, such as CRX and BRN3 (Figures 3K, top and 3L). In contrast, Venus-negative N-spheroids (gray boxes and bars in Figures 3I and 3J) did not express RAX, VSX2, or BRN3 (Figure 3K, bottom).
Figure 3.
Characteristic spheroid morphologies after re-aggregation of cells sorted by LF-GC-based sorting in Rax::Venus cell line
(A) Representative phenotypes of D6-BMP4 and no-BMP4 organoids (Rax#01). (Top) Bright-field image; (bottom) Venus-fluorescent image.
(B) FCM profiles of D6-BMP4 organoids. (Top) FSC-H and SSC-H profiles; (bottom) FSC-H and Venus profiles.
(C) A receiver operating characteristic curve for the classification of Venus++ cells and others. The AUROC for this classification was 0.73.
(D) SVM score histogram for the classification of Venus++ cells and others. Red and blue colors in the histogram correspond to Venus++ cells and others.
(E) Scatterplot of FSC and BSC. Cells in live-cell gates (green) were sorted based on the generated classifier.
(F) SVM score of the classifier and Venus intensity (top) or the number of cells (bottom). Cells with SVM scores of ≥0.7 (red) were sorted.
(G) Scatterplot of FSC and BSC (top) and FSC and Venus intensity (bottom) for the cells in the live-cell gate. Blue indicated the cells predicted to be positive according to the LF-GC-based sorting system, and orange indicated others.
(H) Characteristics of Sorted, Waste, and Control cells after LF-GC-based sorting experiments for Rax::Venus cell line (Rax#01-#03). Cell viability and average cell size (top), and the proportion of Venus++ cells (middle). The scatterplot (bottom: FSC-H and SSC-H profiles) showed that Sorted cells (blue dots) of all experiments were not in the specific FSC/SSC plot regions.
(I and J) Phenotypes of spheroid assays of Rax#01-#03 on DD60-70. One box corresponds with one spheroid (I) and each color box (I) and bar (J) represent the samples indicated by the same color. ∗∗∗∗p < 0.0001; ns, not significant. Fisher’s exact test with Bonferroni correction.
(K) Immunostaining of each spheroid phenotype on DD60–70 with antibodies against Venus (green, left), VSX2 (red, middle), PAX6 (white, middle), BRN3 (red, right), CRX (white, right), and DAPI (blue).
(L) Immunostaining of Venus-positive tissues in P-spheroids on DD60–70 at high magnification for VSX2 (red, left), PAX6 (white, left), BRN3 (red, right), and CRX (white, right).
Scale bars, 500 μm (A and J), 200 μm (K), and 100 μm (L).
LF-GC-based sorting system enriched RPCs from Crx::Venus ROs without Venus expression
Crx::Venus ROs did not show Venus fluorescence approximately DD25 (Figures 4A and 4B). Therefore, we investigated how LF-GC-based sorting works on these hESC line-derived RPCs without Venus expression using three independent differentiation batches (Crx#01–03). We selected a decision boundary for the SVM scores in each sorting experiment such that the recovery rate was approximately 10%–20%, as explained above (Figures 4C–4E; Table S1). Again, the cell viability and average cell size did not show any statistically significant differences among the Sorted, Waste, or Control groups (p = 0.916 and 0.572, respectively) (Figure 4F, top). In the Sorted group, the expression of specific marker genes for RPCs, such as RAX, VSX2, and SIX3, increased, while those of non-retinal genes, such as EMX2, FOXG1, and HOXB2, decreased compared with those in the Control group. This trend was reversed in the Waste group (Figure 4F, bottom). According to the spheroid assay, the Sorted spheroids mainly comprised Crx::Venus-positive retinal tissues with a graft index of 92% (23/25), which was significantly higher than that of the other groups (both p < 0.0001) (Figures 4G and 4H). Histological examination demonstrated that Venus-positive structures in C- and P-spheroids (blue and orange boxes/bars, respectively, in Figures 4G and 4H) comprised VSX2-positive apical cells and PAX6-positive basal cells, and contained cells that expressed retina-specific markers such as CRX and BRN3 (Figures 4I and 4J). In contrast, N-spheroids rarely expressed VSX2, CRX, or BRN3, but included some PAX6-positive and EMX2-positive cells (Figure 4K). In some Crx::Venus lots with poor retinal differentiation, all spheroids in the Sorted, Waste, and Control groups became non-retinal tissues.
Figure 4.
Characteristic spheroid morphologies after re-aggregation of the cells sorted by LF-GC-based sorting in Crx::Venus cell line
(A) Representative phenotypes of Crx::Venus-derived organoids (Crx#03). (Top) Bright-field image. (Bottom) Venus-fluorescent image.
(B) FSC and Venus profiles to evaluate fluorescence intensity (Crx#03).
(C) Scatterplot of FSC and BSC (Crx#03). SVM score histograms for the classification of target cells and others were calculated for the cells within the live-cell gate (green).
(D) SVM score of the classifier and the number of cells (Crx#03). Cells with SVM scores of ≥1.0 (red) were sorted.
(E) Scatterplot of FSC and BSC for the cells in the live-cell gate (C). Blue indicated the cells predicted to be positive in the LF-GC-based sorting system, and orange indicated the negative cells.
(F) Characteristics of Sorted, Waste, and Control cells after LF-GC-based sorting experiments for Crx::Venus cell line (Crx#01-#03). Cell viability and average cell size (top), and gene expressions of each sample (bottom). Changes in gene expressions are shown as a fold change in gene expressions of Sorted cells (blue) and Waste cells (orange) compared with those of Control cells (black).
(G and H) Summary of phenotypes in spheroid assays of Crx::Venus cell line on DD60-70. One box corresponds with one spheroid (G) and each color box (G) and bar (H) represent the samples with the same color. C-spheroids and P-spheroids were significantly more abundant in the Sorted group. ∗∗∗∗p < 0.0001; ns, not significant. Fisher’s exact test with Bonferroni correction.
(I and J) Immunostaining images of C-spheroids and P-spheroids with low (left) and high (right) magnification on DD60–70. PAX6 (white) and BRN3 (magenta, bottom) were expressed on the basal side, while VSX2 (magenta, top) and CRX (green) were expressed mainly on the apical side.
(K) Immunostaining images of N-spheroids on DD60–70. Some cells are positive for PAX6 (white, top left) and EMX2 (white, top right).
Scale bars, 500 μm (A and H), 200 μm (left, I, left, J, and K), and 100 μm (right, I and J).
LF-GC-based sorting system enriched target RPCs from unlabeled hiPSC-derived ROs
LF-GC-based sorting was then applied to four independent differentiation batches of hiPSC-WJs531 RO cells on DD25 (Figure 5A) for spheroid assay experiments (WJs#01–04). The WJs531-hiPSC ROs have no reporter Venus expression (Figure 5B). In each experiment, we selected a decision boundary applied to the SVM scores of the classifier such that the recovery rate was approximately 10%–20% (Figures 5C–5E; Table S1). Cell viability and average cell size did not differ significantly among the Sorted, Waste, or Control groups (p = 0.929 and 0.847, respectively) (Figure 5F, top). The overall gene expression patterns after LF-GC-based sorting among the three groups were similar to those in the Crx::Venus cell line (Figure 5F, bottom). According to the spheroid assay, WJs531 Sorted cells demonstrated good reproducibility in the consistent production of C- and P-spheroids with retinal regions large enough for transplantation, with a graft index of 91.4% (32/35), which was significantly higher compared with the other groups (both p < 0.0001) (Figures 5G and 5H). These C-spheroids and P-spheroids consistently and uniformly exhibited a characteristic retinal epithelial-like structure, expressing VSX2 and CRX in the outer layer and PAX6 and BRN3 in the inner layer (Figures 5I and 5J). N-spheroids, which were predominantly observed in the Waste and Control groups, rarely expressed VSX2 and BRN3 but expressed EMX2 and PAX6, as seen in other cell lines (Figure 5K). The results for WJs#02 (Figure 5G) suggested that LF-GC-based sorting could generate C-spheroids if we set the decision boundary for SVM scores to a higher value on adequate cell lines that efficiently differentiate RPCs. Thus, with WJs#03–04, we set the decision boundary for SVM scores to 1.5, to adjust the expected purity of the target cells after sorting. In WJs#04, while all Waste and Control spheroids differentiated into N-spheroids, some Sorted spheroids differentiated into C-spheroids (Figure 5G). The cell recovery rates after LF-GC-based sorting were 9.38 and 9.8% for WJs#03 and WJs#04, respectively (Table S1). In contrast, we obtained 9 of 192 ROs (4.7%) suitable for transplantation using the conventional SFEBq methods with the WJs531 cell line in two independent experiments (Figure S1), suggesting robust graft production after LF-GC-based sorting.
Figure 5.
Characteristic spheroid morphologies after re-aggregation of cells sorted by LF-GC-based sorting in hiPSC-WJs531 cell line
(A) Phenotypes of WJs531-derived organoids (WJs#04). (Left) Bright-field image. (Right) Fluorescent image.
(B) FSC and Venus profiles of WJs#04.
(C) Scatterplot of FSC and BSC (WJs#04). SVM score histograms for the classification of target cells and others were calculated for the cells within the live-cell gate (green).
(D) SVM score of the classifier and the number of cells. Cells with SVM scores of ≥1.5 (red line) were sorted (WJs#04).
(E) Scatterplot of FSC and BSC for the cells in the green gate in (C). Blue indicated the cells predicted to be positive in the LF-GC-based sorting system, whereas orange indicated the other cells.
(F) Characteristics of Sorted, Waste, and Control cells after LF-GC-based sorting experiments for WJs531 cell line (WJs#01-#04). Cell viability and average cell size (top), and gene expressions of each sample (bottom). Changes in gene expressions are shown as a fold change in gene expressions of Sorted cells (blue) and Waste cells (orange) compared with those of Control cells (black).
(G and H) Phenotypes of spheroid assays of WJs#01–04 on DD60–70. One box corresponds with one spheroid (G) and each color box (G) and bar (H) represent the samples with the same color. C-spheroids and P-spheroids were significantly more abundant in the Sorted group. ∗∗∗∗p < 0.0001; ns, not significant. Fisher’s exact test with Bonferroni correction.
(I and J) Immunostaining images of C-spheroids and P-spheroids with low (left) and high (right) magnification on DD60–70. PAX6 (white, top) and BRN3 (magenta, bottom) are expressed on the basal side of the cell layer, whereas VSX2 (red, top), CRX (white, bottom), and RCVRN (green, bottom) are expressed on the apical side.
(K) Immunostaining images of N-spheroids on DD60–70. N-spheroids comprised cells that were negative for VSX2 (magenta, top), BRN3 (magenta, bottom), and RCVRN (green, bottom).
Scale bars, 500 μm (A and H), 200 μm (left, I, left, J, and K), and 100 μm (right, I and J).
Human iPSC-derived LF-GC-based-sorted retinal spheroids showed graft maturation and synaptic integration in an animal model of retinal degeneration
Finally, we transplanted hiPSC-WJs531 spheroids on DD60-70, as done in previous studies (Iraha et al., 2018; Tu et al., 2019), into the subretinal space of 6-month-old SD-Foxn1 Tg(S334ter)3LavRrrc nude rats, which are immunodeficient end-stage retinal degeneration model rats (Seiler et al., 2014). Retinal epithelial regions excised from C- and P-spheroids and non-retinal tissues from the Waste and Control spheroids were transplanted (Figure 6A). Postoperative optical coherence tomography imaging demonstrated the presence of retinal grafts three months after transplantation (Figures 6B and 6C), while irregularly shaped low-density areas were observed in the non-retinal grafts (Figure 6D). Immunohistochemical images at 160–170 days after transplantation (graft DD230–240) revealed the engraftment of human tissue that was positive for the human nuclei marker HuNu (Figures 6E and 6F). Transplanted C- and P-spheroids developed mature photoreceptors and retinal bipolar cells (Figures 6E and 6F), whereas non-retinal spheroids of the Waste and Control groups developed few photoreceptors (Figure 6G). Most of the graft photoreceptor layers formed rosettes with the outer segments directed inward, as indicated by the expression of RHO (RetP1), OPN1LW/OPN1MW (L/M-cone opsins), and OPN1SW (S-cone opsin) (Figure 6H). Some SCGN-positive host cone bipolar cells extended dendrites toward graft photoreceptor rosettes, potentially forming synaptic connections (Figure 6I). Presynaptic Ribeye proteins expressed adjacent to host bipolar cell dendrite tips further indicated these potential synaptic connections (Figures 6I and 6J).
Figure 6.
Maturation and synaptic integration of hiPSC-WJs531-derived graft tissues after transplantation into the subretinal space of the retinal degeneration model nude rats
(A) Schematic illustration summarizing the transplantation in the present study. All types of spheroids were cut and transplanted subretinally on DD60–70.
(B–D) In vivo optical coherence tomography imaging of nude rat eyes 3 months after transplantation of the C-, P-, and N-spheroids (B, C, and D, respectively). The grafts were observed subretinally, as indicated by blue dotted lines (B–D). Graft viability was achieved after transplantation, although some irregularly shaped low-density areas (orange arrows) were present in the graft (D).
(E and F) Representative immunostaining images after transplantation of C-spheroids (E) and P-spheroids (F). RCVRN-positive (green, top) photoreceptors, many of which formed rosette structures, and SCGN-positive (white, top) cone bipolar cells were observed at the transplantation site.
(G) Representative immunostaining images after transplantation of N-spheroids. Some graft-derived cells differentiate into nonretinal tissues and form irregularly shaped structures in the degenerating host retina.
(H) Maturation of the graft photoreceptors with outer segment formation, as indicated by the expression of RHO (green), OPN1LW/OPN1MW (white), and OPN1SW (magenta).
(I and J) Ribbon synapse formation at the graft photoreceptor axon terminals. Potential contacts were found between the dendritic tips of extending SCGN-positive (white) host cone bipolar cells and RCVRN-positive (green) graft photoreceptors. Magnified views of the orange boxes are shown in (J), showing ribeye protein (CTBP2, red) expression by graft photoreceptors at the host-graft interface.
Scale bars, 500 μm (A), 200 μm (B–G), 100 μm (H and I), and 10 μm (J).
Discussion
In this study, we first confirmed that C-spheroids with uniform Venus expression, considered ideal for transplantation, were obtained by sorting and aggregating Rax::Venus++ RPCs. To sort RPCs and prepare grafts for transplantation therapy without biological labeling, we optimized the conditions of the LF-GC-based sorting system for target RPCs using GMI waveforms with FSC/BSC as the training dataset. By employing a spheroid assay as a subsequent phenotypic marker to evaluate whether the target cells were correctly selected, we confirmed that almost all LF-GC-based sorted spheroids had sufficiently large retinal areas for transplantation on DD60-70 under bright-field imaging and histological examinations, whereas Waste and Control spheroids became mainly non-retinal tissue. Moreover, retina-associated genes RAX, VSX2, and SIX3 were enriched, while non-retinal genes EMX2, FOXG1, and HOXB2 were decreased in LF-GC-based sorted samples from both the Crx::Venus ESC and WJs531 iPSC lines. Namely, we showed that the LF-GC classifier practically enriched the target RPCs from ROs derived from two different cell lines without Venus fluorescence. Although these sorted RPCs were used to create spheroids in this study, they may be used more effectively for transplantation in the future by applying them to different graft forms, such as retinal sheets.
In the hiPSC-WJs531 cell line, we could create C- and P-spheroids suitable for transplantation from almost all the LF-GC-based sorted samples (graft index of >90%) by selecting optimized decision boundaries applied to the SVM scores of the classifiers such that the recovery rate was approximately 10%–20%. According to our calculation, since a single RO on DD25 consists of an average of 1.25 × 105 cells (Table S2), we can produce one retinal spheroid (1.20 × 104 cells) (see experimental procedures) with 10% recovery, and each spheroid can produce one or two transplantation pieces with P- or C-spheroids, respectively. Considering that less than 5% of the total ROs were suitable for transplantation with this cell line when we used the conventional SFEBq method (Figure S1), the current LF-GC approach allows us to increase the graft production by ×10 efficiency and considerably reduces the labor involving the human judgment during graft preparation. However, it is noteworthy that the proportion of each spheroid phenotype after sorting varies from one cell line to another because the proportion of target RPCs in the original samples differs among cell lines. Hence, our optimized LF-GC-based sorting system is expected to exert robust efficiency in graft preparation by choosing adequate cell lines that can consistently produce sufficient RPCs.
Histological examination revealed that grafted retinas from hiPSC-derived retinal spheroids showed potency similar to that of hESC/hiPSC-derived RO patches in graft maturation and potential synaptic connections between host bipolar cells and graft photoreceptors (Iraha et al., 2018; Tu et al., 2019). Interestingly, transplantation of non-retinal tissues derived from N-spheroids resulted in the development of photoreceptors and retinal interneurons, in addition to undesired structures involving one that looked like the ciliary body, which is a peripheral marginal structure of the retina, in the degenerating host retina after transplantation. While suggesting that post-transplantation environments may strongly induce transplanted cells toward retinal differentiation (Liu et al., 2023), our results indicate the importance of removing inappropriate cells from transplantation because they may cause undesired proliferation or impede the proper function of graft photoreceptor cells. Spheroids prepared using our optimized LF-GC-based sorting system may be clinically applicable to patients with retinal degeneration in anticipation of effective outcomes.
In this study, the accuracy of the trained classifiers resulted in moderate AUROC scores (0.73 ± 0.02) (Swets, 1988; Fischer et al., 2003), leading to the formation of P-spheroids from the Sorted cells in many trials. The moderate score can be attributed to two factors: first, differentiation from ESCs/iPSCs toward RPCs is a continuous process with gradually changing morphological phenotypes; second, the ground truth label based on Venus intensity is imperfect for annotating RPCs in training the machine learning-based classifier. Another limitation was the number of cells available for generating the classifier. In GC-based cell sorting, a field-programmable gate array (FPGA) circuit equipped with a pre-implemented machine learning model returns real-time classification results for each cell to the microfluidic device. However, the current FPGA’s limited data processing capacity during real-time analysis restricts the amount of cell data available for model training. Upgrading to a more powerful FPGA in the future could expand the maximum number of data points accessible for real-time analysis and the number of cell data available for training, which would lead to improved classification accuracy (Figure S2).
Unexpectedly, the expression of some retina-associated genes such as OTX2 and CRX decreased in the Sorted samples, regardless of the cell line. This is probably because OTX2 and CRX are expressed not only in photoreceptor precursors (Sridhar et al., 2020; Swaroop et al., 2010) but also in RPE cells (Esumi et al., 2009). In the present study, we used ROs developed by SFEBq with a modified induction-reversal method, which turns the cell fate of the neural retina (NR) into RPE from DD18 to DD22 followed by reverse induction into NR again. We performed LF-GC-based sorting of ROs on DD25; therefore, induced RPE precursors may have remained present at the time of sorting. Moreover, photoreceptor precursor cells expressing CRX rarely existed on DD25 (Figures 4A and 4B) (Llonch et al., 2018). Thus, it is reasonable that the expression of OTX2 and CRX decreased in the Sorted cells, suggesting that such undesirable RPE precursors were successfully removed via LF-GC-based sorting.
In conclusion, our optimized LF-GC-based sorting system consistently enriched RPC using different hESC/iPSC lines with no fluorescent labeling or specific surface antigens at the early differentiation stage, which is difficult to achieve using conventional sorting methods. With some cell lines, LF-GC-based enrichment achieved the production of over 90% transplantable spheroids that are easily processed for graft excision. This novel approach enables the stable and robust production of retinal grafts by using consistent criteria and sparing the ambiguous human judgment and time-consuming processes traditionally performed by humans. By expanding the dataset for training, enhancing machine learning algorithms, and improving device specifications, we believe that we can improve the classification model’s accuracy, robustness, and yield, making this approach practical for cell manufacturing in regenerative cell therapy in the future.
Experimental procedures
Resource availability
Corresponding author
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead corresponding author, Michiko Mandai (e_lab.mandai@kcho.jp). For LF-GC-associated information and inquiries, please contact the corresponding author, Yoko Kawamura (yoko@thinkcyte.com).
Materials availability
This study did not generate new unique reagents.
Data and code availability
The raw GMI waveform data are available on Zenodo (https://zenodo.org/record/7955662).
Tissue dissociation for cell sorting
ROs on DD25 were washed with phosphate-buffered saline (PBS, Fujifilm Wako Pure Chemical) at room temperature (RT) and dissociated using dispersed solutions for neuronal cells (Fujifilm Wako Pure Chemical Corporation) at 37°C for 60–70 min with gentle pipetting. After centrifugation and supernatant removal, the cell pellets were resuspended in Stain Buffer (PBS containing 2.0% fetal bovine serum with 2 mM EDTA [Nacalai]) and filtered through a 40-μm cell strainer (pluriSelect) to remove cell clumps. Cells were counted using Countess II (Thermo Fisher Scientific), and the cell viability and average cell size were calculated. Dissociated cells were preserved in Stain Buffer at 4°C in the dark until cell sorting.
Cell sorting: FACS and LF-GC-based sorting
FACS
FACS was performed on the Rax::Venus cell line using a FACSAria II (BD Biosciences). For FCM analysis (Figures 1A–1E), at least 10,000 events were analyzed using the FACSDiva or FlowJo software (BD Biosciences). Dead cells, cell debris, and cell multiplets were excluded from the analysis and sorting by gating with forward scatter and side scatter as indicators. Cells were sorted into Venus++ and Venus+/++ fractions using the FACSAria with FACSDiva software.
Cell preparation for LF-GC-based sorting
Dissociated cells were fixed using 4% paraformaldehyde (Thermo Fisher Scientific) for 20 min at RT to determine the sorting delay time in LF-GC-based sorting. After being washed with PBS, the cells were incubated with LIVE/DEAD Fixable Green Dead Cell Stain Kit (FG, Thermo Fisher Scientific) over 60 min at RT. Stained cells were centrifuged and washed with 2% bovine serum albumin (BSA) in PBS. After counting the cells using Countess II, the cell concentration was adjusted to 5.0 × 105 cells/mL with 2% BSA/PBS.
LF-GC-based sorting
Figures 2A, 2J, and 2L show the workflow for LF-GC-based sorting in this study. Rax::Venus cells (5.0 × 105 cells/mL) were first flowed as a training sample to the GC setup to collect the training data. In the GC setup, when the cells pass through a structured light illumination pattern with a wavelength of 405 nm in a microfluidic channel, three LF-GMI (fsGMI, bsGMI, and bfGMI) waveforms were acquired. A spot at the 637 nm wavelength was utilized to acquire conventional LF modalities such as FSC and BSC, which is SSC’s analogy and can be used interchangeably with SSC. For model training and sorting, we selected four LF modalities, including two LF-GMI waveforms, FSC-H and BSC-H, to maximize model performance. Emission signals at a wavelength of 525 nm were also recorded to detect Venus fluorescence intensities for annotating cell data based on conventional flow cytometry gating schemes in supervised machine learning analysis. Live cells were identified in the FSC/BSC scatterplot to remove debris and dead cells (Figure 2B), and Venus++ cells were identified in the FSC/Venus scatterplot (Figure 2C). The thresholds of Venus intensity were adjusted to obtain as many RPCs as possible (Figure S3). We adopted the Venus threshold, which is the same as the Venus++ gate in FACS sorting (Figures 1A, 2C, and S3A), owing to the highest purity and recovery of the RPC (Figure S3). The cells outside the Venus++ gate in the FSC/Venus plot were defined as others. For each class (Venus++ cells or others), data from 700 to 800 randomly selected cells were divided into training and evaluation datasets, with which a machine-learning model was trained based on the SVM algorithm and subsequently evaluated using the AUROC. After passing through a 40-μm cell strainer (Funakoshi), the samples (Rax::Venus, Crx::Venus, or WJs531 cell lines) were flowed and sorted based on the trained model without using fluorescence labels. We selected different SVM decision boundaries for each sorting experiment to obtain high-purity positive cells, as shown in Table S1 (SVM scores ≥ decision boundary: positive prediction; SVM scores < decision boundary: negative prediction). The cells were collected in vials containing 50% CELLOTION (Takara Bio Inc.)/50% NR differentiation medium for 2 h and subsequently concentrated via centrifugation and cultured in a 96-well plate. We defined the sorted cells as Sorted, others as Waste, and the original cells preserved at 4°C in the dark as Control and checked each group’s cell viability and average cell size using Countess II. We processed 2.0–4.0 × 106 cells within a 50-min time frame using the sorter system, with approximately 10%–20% of them being sorted into the collection tube. Hence, the system’s throughput in sorting reached a maximum of approximately 1,300 cells per second.
Retinal spheroid formation and differentiation
After cell sorting, cells were centrifuged and the supernatants were removed; cell pellets were resuspended with NR differentiation medium with 10 μM Y-27632 (Wako Pure Chemical Industries), 3 μM CHIR99021 (Bio Vision), 300 nM SAG (Enzo Biochem), and 100 ng/mL human recombinant FGF8 (Fujifilm Wako Pure Chemical) and counted with Countess II (WO/2023/090427). The cells were plated in a 96-well plate at a density of 12,000 cells/well and cultured at 37°C in a humidified atmosphere of 5% CO2. The medium was replaced every 3 days.
Statistical analysis
All values are presented as mean ± standard deviation (range). Fisher’s exact test was used to compare the distribution of categorical variables between groups. For the continuous variables, the normality and homoscedasticity of the data distribution were assessed using the Shapiro–Wilk test and Bartlett’s test. Depending on the data distribution, one-way ANOVA or the Kruskal-Wallis test was used to compare variables between groups. In the post hoc test, the Bonferroni correction was used for multiple comparisons. Two-sided p values of <0.05 were considered statistically significant in all statistical analyses.
The procedures for hESC/iPSC maintenance, retinal differentiation of hESCs and hiPSCs, RNA extraction and reverse transcription real-time quantitative PCR, transplantation into nude rats, and immunohistological procedures are described in supplemental experimental procedures.
Acknowledgments
We thank K. Kawai for her help in cell culture, S. Yamasaki for his advice on cell culture and experiments, J. Sho and T. Senba for supporting the animal experiments, R. Miyazaki for cell preparation, and H. Takemoto for technical support. We thank Editage (www.editage.jp) for English language editing. The Center for iPS Cell Research and Application (CiRA) provided the human iPSC-WJs531 line. This research was supported by the Japan Agency for Medical Research and Development (AMED), grant JP13bm0204002.
Author contributions
Conceptualization: S.O., M.Mandai., and M.T.; methodology: Y.I., H.N., T.M., Y.K., K.T., and M.Mandai.; software: H.N., Y.K., and S.O.; validation: Y.I., H.N., T.M., and Y.K.; formal Analysis: Y.I., H.N., T.M., Y.K., and M.Mandai.; investigation: Y.I., H.N., T.M., Y.K., M.Matsumura., Y.M., and K.T.; resources: Y.I., H.N., T.M., Y.K., M.Matsumura., Y.M., K.T., and K.N.; data curation: Y.I., H.N., T.M., and Y.K.; writing—original draft: Y.I., H.N., and Y.K.; writing—review and editing: Y.I., H.N., T.M., Y.K., S.O., and M.Mandai.; visualization: Y.I. and H.N.; supervision: S.O., K.N., M.Mandai., and M.T.; project administration: S.O., K.N., M.Mandai., and M.T.; funding acquisition: M.T. All authors reviewed and approved the final manuscript.
Declaration of interests
T.M. and M. Matsumura are employees of Vision Care Inc. M.T. is the founder and president of Vision Care Inc. S.O. is a founder and shareholder of ThinkCyte K.K. H.N., Y.K., K.T., and Y.M. are employees of ThinkCyte K.K. and have shares of stock options of ThinkCyte K.K. S.O. and Y.K. have filed patent applications related to this study. All other authors declare they have no competing interests.
Published: January 4, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2023.12.001.
Contributor Information
Tomohiro Masuda, Email: tomohiro_masuda@kcho.jp.
Yoko Kawamura, Email: yoko@thinkcyte.com.
Michiko Mandai, Email: e_lab.mandai@kcho.jp.
Supplemental information
References
- Assawachananont J., Mandai M., Okamoto S., Yamada C., Eiraku M., Yonemura S., Sasai Y., Takahashi M. Transplantation of embryonic and induced pluripotent stem cell-derived 3D retinal sheets into retinal degenerative mice. Stem Cell Rep. 2014;2:662–674. doi: 10.1016/j.stemcr.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko C. Intrinsically different retinal progenitor cells produce specific types of progeny. Nat. Rev. Neurosci. 2014;15:615–627. doi: 10.1038/nrn3767. [DOI] [PubMed] [Google Scholar]
- Esumi N., Kachi S., Hackler L., Jr., Masuda T., Yang Z., Campochiaro P.A., Zack D.J. BEST1 expression in the retinal pigment epithelium is modulated by OTX family members. Hum. Mol. Genet. 2009;18:128–141. doi: 10.1093/hmg/ddn323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J.E., Bachmann L.M., Jaeschke R. A readers’ guide to the interpretation of diagnostic test properties: clinical example of sepsis. Intensive Care Med. 2003;29:1043–1051. doi: 10.1007/s00134-003-1761-8. [DOI] [PubMed] [Google Scholar]
- Gagliardi G., Ben M’Barek K., Chaffiol A., Slembrouck-Brec A., Conart J.B., Nanteau C., Rabesandratana O., Sahel J.A., Duebel J., Orieux G., et al. Characterization and transplantation of CD73-positive photoreceptors isolated from human iPSC-derived retinal organoids. Stem Cell Rep. 2018;11:665–680. doi: 10.1016/j.stemcr.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini S.J., Llonch S., Borsch O., Ader M. Transplantation of photoreceptors into the degenerative retina: current state and future perspectives. Prog. Retin. Eye Res. 2019;69:1–37. doi: 10.1016/j.preteyeres.2018.11.001. [DOI] [PubMed] [Google Scholar]
- Herbig M., Tessmer K., Nötzel M., Nawaz A.A., Santos-Ferreira T., Borsch O., Gasparini S.J., Guck J., Ader M. Label-free imaging flow cytometry for analysis and sorting of enzymatically dissociated tissues. Sci. Rep. 2022;12:963. doi: 10.1038/s41598-022-05007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraha S., Tu H.Y., Yamasaki S., Kagawa T., Goto M., Takahashi R., Watanabe T., Sugita S., Yonemura S., Sunagawa G.A., et al. Establishment of immunodeficient retinal degeneration model mice and functional maturation of human ESC-derived retinal sheets after transplantation. Stem Cell Rep. 2018;10:1059–1074. doi: 10.1016/j.stemcr.2018.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaewkhaw R., Swaroop M., Homma K., Nakamura J., Brooks M., Kaya K.D., Chaitankar V., Michael S., Tawa G., Zou J., et al. Treatment paradigms for retinal and macular diseases using 3-D retina cultures derived from human reporter pluripotent stem cell lines. Invest. Ophthalmol. Vis. Sci. 2016;57:ORSFl1–ORSFl11. doi: 10.1167/iovs.15-17639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara A., Ozone C., Nakano T., Saito K., Eiraku M., Sasai Y. Generation of a ciliary margin-like stem cell niche from self-organizing human retinal tissue. Nat. Commun. 2015;6:6286. doi: 10.1038/ncomms7286. [DOI] [PubMed] [Google Scholar]
- Kuwahara A., Yamasaki S., Mandai M., Watari K., Matsushita K., Fujiwara M., Hori Y., Hiramine Y., Nukaya D., Iwata M., et al. Preconditioning the initial state of feeder-free human pluripotent stem cells promotes self-formation of three-dimensional retinal tissue. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-55130-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowski J., Gonzalez-Cordero A., West E.L., Han Y.T., Welby E., Naeem A., Blackford S.J.I., Bainbridge J.W.B., Pearson R.A., Ali R.R., Sowden J.C. Transplantation of photoreceptor precursors isolated via a cell surface biomarker panel from embryonic stem cell-derived self-forming retina. Stem Cell. 2015;33:2469–2482. doi: 10.1002/stem.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.V., Santiago C.P., Sogunro A., Konar G.J., Hu M.W., McNally M.M., Lu Y.C., Flores-Bellver M., Aparicio-Domingo S., Li K.V., et al. Single-cell transcriptome analysis of xenotransplanted human retinal organoids defines two migratory cell populations of nonretinal origin. Stem Cell Rep. 2023;18:1138–1154. doi: 10.1016/j.stemcr.2023.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llonch S., Carido M., Ader M. Organoid technology for retinal repair. Dev. Biol. 2018;433:132–143. doi: 10.1016/j.ydbio.2017.09.028. [DOI] [PubMed] [Google Scholar]
- Mandai M., Fujii M., Hashiguchi T., Sunagawa G.A., Ito S.I., Sun J., Kaneko J., Sho J., Yamada C., Takahashi M. iPSC-derived retina transplants improve vision in rd1 end-stage retinal-degeneration mice. Stem Cell Rep. 2017;8:69–83. doi: 10.1016/j.stemcr.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranishi Y., Terada K., Furukawa T. An essential role for Rax in retina and neuroendocrine system development. Dev. Growth Differ. 2012;54:341–348. doi: 10.1111/j.1440-169X.2012.01337.x. [DOI] [PubMed] [Google Scholar]
- Nakano T., Ando S., Takata N., Kawada M., Muguruma K., Sekiguchi K., Saito K., Yonemura S., Eiraku M., Sasai Y. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10:771–785. doi: 10.1016/j.stem.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Osakada F., Ikeda H., Mandai M., Wataya T., Watanabe K., Yoshimura N., Akaike A., Sasai Y., Takahashi M. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat. Biotechnol. 2008;26:215–224. doi: 10.1038/nbt1384. [DOI] [PubMed] [Google Scholar]
- Ota S., Horisaki R., Kawamura Y., Ugawa M., Sato I., Hashimoto K., Kamesawa R., Setoyama K., Yamaguchi S., Fujiu K., et al. Ghost cytometry. Science. 2018;360:1246–1251. doi: 10.1126/science.aan0096. [DOI] [PubMed] [Google Scholar]
- Ota S., Sato I., Horisaki R. Implementing machine learning methods for imaging flow cytometry. Microscopy (Oxf) 2020;69:61–68. doi: 10.1093/jmicro/dfaa005. [DOI] [PubMed] [Google Scholar]
- Pearson R.A., Barber A.C., Rizzi M., Hippert C., Xue T., West E.L., Duran Y., Smith A.J., Chuang J.Z., Azam S.A., et al. Restoration of vision after transplantation of photoreceptors. Nature. 2012;485:99–103. doi: 10.1038/nature10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Progatzky F., Dallman M.J., Lo Celso C. From seeing to believing: labelling strategies for in vivo cell-tracking experiments. Interface Focus. 2013;3 doi: 10.1098/rsfs.2013.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro J., Procyk C.A., West E.L., O’Hara-Wright M., Martins M.F., Khorasani M.M., Hare A., Basche M., Fernando M., Goh D., et al. Restoration of visual function in advanced disease after transplantation of purified human pluripotent stem cell-derived cone photoreceptors. Cell Rep. 2021;35 doi: 10.1016/j.celrep.2021.109022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roederer M. Spectral compensation for flow cytometry: visualization artifacts, limitations, and caveats. Cytometry. 2001;45:194–205. doi: 10.1002/1097-0320(20011101)45:3<194::aid-cyto1163>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Seiler M.J., Aramant R.B., Jones M.K., Ferguson D.L., Bryda E.C., Keirstead H.S. A new immunodeficient pigmented retinal degenerate rat strain to study transplantation of human cells without immunosuppression. Graefes Arch. Clin. Exp. Ophthalmol. 2014;252:1079–1092. doi: 10.1007/s00417-014-2638-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M.S., Charbel Issa P., Butler R., Martin C., Lipinski D.M., Sekaran S., Barnard A.R., Maclaren R.E. Reversal of end-stage retinal degeneration and restoration of visual function by photoreceptor transplantation. Proc. Natl. Acad. Sci. USA. 2013;110:1101–1106. doi: 10.1073/pnas.1119416110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar A., Hoshino A., Finkbeiner C.R., Chitsazan A., Dai L., Haugan A.K., Eschenbacher K.M., Jackson D.L., Trapnell C., Bermingham-McDonogh O., et al. Single-cell transcriptomic comparison of human fetal retina, hPSC-derived retinal organoids, and long-term retinal cultures. Cell Rep. 2020;30:1644–1659.e4. doi: 10.1016/j.celrep.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone N.E., Voigt A.P., Cooke J.A., Giacalone J.C., Hanasoge S., Mullins R.F., Tucker B.A., Sulchek T. Label-free microfluidic enrichment of photoreceptor cells. Exp. Eye Res. 2020;199 doi: 10.1016/j.exer.2020.108166. [DOI] [PubMed] [Google Scholar]
- Swaroop A., Kim D., Forrest D. Transcriptional regulation of photoreceptor development and homeostasis in the mammalian retina. Nat. Rev. Neurosci. 2010;11:563–576. doi: 10.1038/nrn2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swets J.A. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- Trimarchi J.M., Stadler M.B., Cepko C.L. Individual retinal progenitor cells display extensive heterogeneity of gene expression. PLoS One. 2008;3:e1588. doi: 10.1371/journal.pone.0001588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu H.Y., Watanabe T., Shirai H., Yamasaki S., Kinoshita M., Matsushita K., Hashiguchi T., Onoe H., Matsuyama T., Kuwahara A., et al. Medium- to long-term survival and functional examination of human iPSC-derived retinas in rat and primate models of retinal degeneration. EBioMedicine. 2019;39:562–574. doi: 10.1016/j.ebiom.2018.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa M., Kawamura Y., Toda K., Teranishi K., Morita H., Adachi H., Tamoto R., Nomaru H., Nakagawa K., Sugimoto K., et al. In silico-labeled ghost cytometry. Elife. 2021;10 doi: 10.7554/eLife.67660. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw GMI waveform data are available on Zenodo (https://zenodo.org/record/7955662).