Take Home Message
In this population-based study, testosterone therapy for men on active surveillance for prostate cancer did not worsen mortality.
Keywords: Testosterone, Prostate cancer, Outcomes, Active surveillance
Abstract
Background and objective
There is insufficient evidence on the oncologic risks of testosterone therapy for men with prostate cancer managed with active surveillance. We carried out a retrospective study to assess the effect of testosterone therapy on oncologic outcomes for men on active surveillance for prostate cancer.
Methods
Surveillance, Epidemiology and End Results (SEER)-Medicare linked data were used to identify men diagnosed with prostate cancer from 2008 to 2017 who were managed with active surveillance and received testosterone (n = 167) or no testosterone (n = 6658) therapy. Outcomes included conversion from active surveillance to active treatment (radical prostatectomy, cryotherapy, radiation, or androgen deprivation therapy), prostate cancer–specific mortality, and overall mortality. Statistically significant factors on univariable analysis were included in a Cox proportional-hazards regression model for multivariable analysis.
Key findings and limitations
The median age was 71 yr (interquartile range [IQR] 68–74) in the testosterone group and 72 yr (IQR 69–75) in the no-testosterone group, with corresponding median follow-up after prostate cancer diagnosis of 5.2 yr (IQR 3.4–7.8) and 4.7 yr (IQR 3.2–6.9). There were no prostate cancer–specific deaths in the testosterone group and 39 (0.6%) in the no-testosterone group. Testosterone therapy was not associated with conversion to active treatment (hazard ratio [HR] 0.66, 95% confidence interval [CI] 0.46–0.97; p = 0.033) or overall mortality (HR 1.02, 95% CI 0.68–1.53; p > 0.9).
Conclusions and clinical implications
In the first population-based, nationally representative study of testosterone therapy for men on active surveillance for prostate cancer, testosterone therapy did not increase the risk of conversion to active therapy or worsen mortality. Prospective studies are needed to confirm these findings.
Patient summary
For men on active surveillance for prostate cancer, we assessed the effect of testosterone therapy. We found that testosterone therapy did not increase the risk of proceeding to active therapy or of death from prostate cancer.
1. Introduction
In 1966, Charles Huggins won the Nobel Prize for discovering that androgen deprivation inhibits prostate cancer [1], [2]. While androgen deprivation therapy remains a mainstay of prostate cancer treatment [2], the discovery by Huggins also led to the widespread belief that testosterone directly stimulates prostate cancer in a linear fashion [1], [3], [4], [5]. This idea persisted until at least 2001, when the National Cancer Institute (NCI) rejected funding for a large testosterone therapy clinical trial because of concerns that testosterone causes prostate cancer [3]. By the mid-2000s, however, several studies demonstrated that high testosterone levels do not cause prostate cancer [3], [4].
Following these reports, Morgentaler and colleagues [3], [4], [5] proposed the saturation model as a paradigm shift for the relationship between serum testosterone and prostate cancer. The model suggests that prostate growth is sensitive to androgen concentrations at near-castrate levels, but as androgen receptors become saturated, further prostatic growth is minimal at normal and high levels of testosterone [3], [5], [6]. As the saturation model predicts, testosterone therapy and high levels of endogenous testosterone do not appear to increase the risk of prostate cancer [4], [6]. Moreover, testosterone therapy after treatment of localized prostate cancer does not appear to cause greater recurrence [4].
There is insufficient evidence on the oncologic risks of testosterone therapy for men with prostate cancer managed with active surveillance [4], [7]. Active surveillance is recommended for low- and intermediate-risk prostate cancer to avoid interventions that may worsen health-related quality of life [8]. Testosterone therapy mitigates the signs and symptoms of testosterone deficiency and improves health-related quality of life [4]. Our aim was to compare oncologic outcomes in terms of conversion to active treatment and prostate cancer–specific mortality and overall mortality of testosterone versus no testosterone therapy in the active surveillance population. To the best of our knowledge, this is the first population-based study and largest series to characterize the use of testosterone therapy for men on active surveillance for prostate cancer. We hypothesized that oncologic outcomes for men receiving testosterone therapy would be noninferior to those for men without testosterone therapy.
2. Patients and methods
2.1. Data source
The Weill Cornell Medicine institutional review board approved this study (protocol number 1510016660). We conducted a retrospective cohort study using Surveillance, Epidemiology and End Results (SEER)-Medicare linked data. SEER is supported by the NCI and captures data for 48% of the US population across 22 geographic regions [9]. SEER includes demographic, clinical, and cause-of-death information for individuals diagnosed with cancer, as well as information on cancer treatment and survival [10]. Medicare data include information on all covered health care services received by individuals from the time of Medicare eligibility until death [10].
2.2. Study cohort
The study cohort is illustrated in Figure 1. The cohort included 250 935 men aged ≥66 yr diagnosed with prostate cancer from 2008 to 2017. As previously described [11], active surveillance was defined as no active treatment for prostate cancer (radical prostatectomy, cryotherapy, radiation, or androgen deprivation therapy) within 12 mo of diagnosis and at least one of the following three surveillance strategies within 2 yr of diagnosis: one or more prostate biopsies, four or more prostate-specific antigen (PSA) tests, or four or more encounters with a physician that had prostate cancer as the primary diagnosis. For reliable comorbidity and surveillance information, we excluded men who were not enrolled in Medicare Parts A and B for at least 1 yr before and 2 yr after prostate cancer diagnosis.
Fig. 1.
Flow chart of the study cohort. PSA = prostate-specific antigen.
To assess testosterone therapy use, we excluded men who were not enrolled in Medicare Part D for at least 2 yr after prostate cancer diagnosis. Testosterone therapy use from 2008 to 2019 was determined from National Drug Code or Healthcare Common Procedure Coding System codes and included various modes of administration (injection, gel, solution, implant, patch, powder, and tablet) [12], [13]. Finally, we excluded men with missing prostate cancer information, grade group ≥3 disease, clinical stage T3 or T4 prostate cancer, or PSA >20 ng/ml because active surveillance is not recommended in these cases [8]. After applying these criteria, we identified 167 men who received testosterone therapy and 6658 men who did not receive testosterone therapy while on active surveillance.
2.3. Outcomes
The primary outcomes were conversion from active surveillance to active treatment (radical prostatectomy, cryotherapy, radiation, or androgen deprivation therapy), and prostate cancer–specific mortality and overall mortality. Secondary outcomes included demographic and prostate cancer characteristics.
2.4. Statistical analysis
Patient and cancer characteristics are summarized using descriptive statistics and compared between the two groups. The χ2 test was used for comparison of categorical variables and the Wilcoxon rank-sum test for continuous variables. Cohorts with <11 were omitted from the tables in accordance with the cell-size suppression policy of SEER-Medicare.
Overall survival was defined as time from the date of diagnosis to the date of death from any cause or the date of last follow-up. The Kaplan-Meier method was used to obtain survival probabilities and differences between groups were assessed using the log-rank test. Multivariate Cox proportional-hazards models were applied to assess the association of testosterone therapy with conversion to active treatment and overall survival, with adjustment for age and all the variables in Table 2. Hazard ratios (HRs) and their 95% confidence intervals (CIs) are reported (Table 3). All p values reported are two-sided, and p < 0.05 was considered statistically significant. All statistical analyses were performed using SAS v9.4 software (SAS Institute, Cary, NC, USA).
Table 2.
Characteristics of the study population in terms of categorical variables
| Variable and categories | Patients, n (%) |
p valuea | |
|---|---|---|---|
| No testosterone (n = 6658) |
Testosterone (n = 167) |
||
| Race/ethnicity | 0.6 | ||
| White | 5663 (85) | 149 (89)b | |
| Black | 361 (5.4) | <11 (<7)b | |
| Hispanic | 232 (3.5) | <11 (<7)b | |
| Asian | 187 (2.8) | <11 (<7)b | |
| Unknown | 215 (3.2) | <11 (<7)b | |
| Charlson comorbidity index | 0.5 | ||
| 0 | 4017 (60) | 94 (56) | |
| 1 | 1514 (22.7) | 40 (24.0) | |
| ≥2 | 1127 (16.9) | 33 (19.8) | |
| Residential population density | 0.7 | ||
| Metropolitan | 5641 (85) | 143 (86) | |
| Nonmetropolitan | 1017 (15) | 24 (14) | |
| Region | <0.001 | ||
| Northeast | 1336 (20) | 19 (11) | |
| South | 1546 (23) | 47 (28) | |
| Midwest | 875 (13) | 11 (6.6) | |
| West | 2901 (44) | 90 (54) | |
| Annual household or individual income | 0.9 | ||
| <$35 000 | 525 (7.9) | 11 (6.6) | |
| $35 000–$44 999 | 783 (12) | 21 (13) | |
| $45 000–$59 999 | 1379 (21) | 32 (19) | |
| ≥$60 000 | 3971 (60) | 103 (62) | |
| Completed at least high school in census tract of residence | 0.3 | ||
| <75% | 3020 (45) | 69 (41) | |
| 75–84.9% | 1948 (29) | 48 (29) | |
| 85–89.9% | 857 (13) | 21 (13) | |
| ≥90% | 833 (13) | 29 (17) | |
| Clinical T stage | >0.9 | ||
| cT1 | 5016 (75) | 126 (75) | |
| cT2 | 1642 (25) | 41 (25) | |
| Prostate-specific antigen | 0.018 | ||
| <10 ng/ml | 5760 (87) | 155 (93) | |
| 10–20 ng/ml | 898 (13) | 12 (7) | |
| Gleason score | 0.003 | ||
| ≤6 | 5329 (80) | 149 (89) | |
| 3 + 4 = 7 | 1329 (20) | 18 (11) | |
χ2 test.
The Surveillance, Epidemiology and End Results-Medicare data use agreement prohibits the reporting of numbers less than 11 out of concern for patient privacy.
Table 3.
Multivariable analysis of factors associated with testosterone therapy
| Variable | OR (95% CI) | p value |
|---|---|---|
| Age category | ||
| 66–69 yr | Reference | |
| 70–74 yr | 0.92 (0.74–1.14) | 0.4 |
| ≥75 yr | 0.9 (0.71–1.14) | 0.4 |
| Race/ethnicity | ||
| White | Reference | |
| Black | 0.80 (0.38–1.70) | 0.6 |
| Hispanic | 1.02 (0.49–2.14) | >0.9 |
| Asian | 1.06 (0.47–2.36) | 0.9 |
| Unknown | 0.91 (0.41–2.01) | 0.8 |
| Charlson comorbidity index | ||
| 0 | Reference | |
| 1 | 1.02 (0.80–1.30) | 0.9 |
| ≥2 | 1.21 (0.93–1.57) | 0.1 |
| RPD (nonmetropolitan vs metropolitan) | 0.98 (0.77–1.25) | 0.9 |
| Region | ||
| Northeast | Reference | |
| South | 1.50 (1.11–2.03) | 0.008 |
| Midwest | 0.65 (0.41–1.03) | 0.066 |
| West | 1.48 (1.13–1.95) | 0.005 |
| Annual household or individual income | ||
| <$35 000 | Reference | |
| $35 000–$44 999 | 1.07 (0.74–1.55) | 0.7 |
| $45 000–59 999 | 0.98 (0.71–1.35) | >0.9 |
| ≥$60 000 | 1.09 (0.79–1.51) | 0.6 |
| Completed at least HSE in CTR | ||
| <75% | Reference | |
| 75–84.9% | 0.96 (0.74–1.25) | 0.8 |
| 85–89.9% | 0.91 (0.64–1.29) | 0.6 |
| ≥90% | 1.17 (0.84–1.63) | 0.3 |
| Clinical T stage (cT2 vs cT1) | 1.0 (0.84–1.2) | >0.9 |
| PSA (10–20 vs <10 ng/ml) | 0.74 (0.56–0.99) | 0.04 |
| Gleason score (3 + 4 = 7 vs ≤6) | 0.73 (0.58–0.93) | 0.01 |
CI = confidence interval; CTR = census tract of residence; HSE = high school education; OR = odds ratio; PSA = prostate-specific antigen; RPD = residential population density.
3. Results
The median age was 71 yr (interquartile range [IQR] 68–74) in the testosterone group and 72 yr (IQR 69–75) in the no-testosterone group (Table 1), with corresponding median follow-up after prostate cancer diagnosis of 5.2 yr (IQR 3.4–7.8) and 4.7 yr (IQR 3.2–6.9). Testosterone therapy was more common in the southern and western SEER regions (p < 0.001), and for men with PSA <10 ng/ml (p = 0.018) and Gleason score ≤6 (p = 0.003; Table 2). These findings remained significant on multivariable analysis (Table 3).
Table 1.
Characteristics of the study population in terms of continuous variables
| Variable | Median (interquartile range) |
p valueb | |
|---|---|---|---|
| No testosterone (n = 6658) |
Testosterone (n = 167) |
||
| Age (yr) | 72 (69–75) | 71 (68–74) | 0.10 |
| Prostate-specific antigen at diagnosis (ng/ml) | 5.9 (4.5–8.1) | 5.2 (3.6–6.8) | <0.001 |
| Biopsies per patient (n)a | 2 (1–3) | 2 (1–3) | 0.023 |
| Prostate-specific antigen tests per patient (n)a | 2 (0–4) | 3(0–4) | 0.031 |
| Physician office visits per patient (n)a | 19 (13–27) | 25 (17–38) | <0.001 |
| Length of follow-up (yr) | 4.7 (3.2–6.9) | 5.2 (3.4–7.8) | 0.008 |
| Duration of active surveillance (yr) | 3.9 (2.6–6.1) | 4.9 (2.9–7.4) | <0.001 |
Within 2 yr of prostate cancer diagnosis.
Wilcoxon rank-sum test.
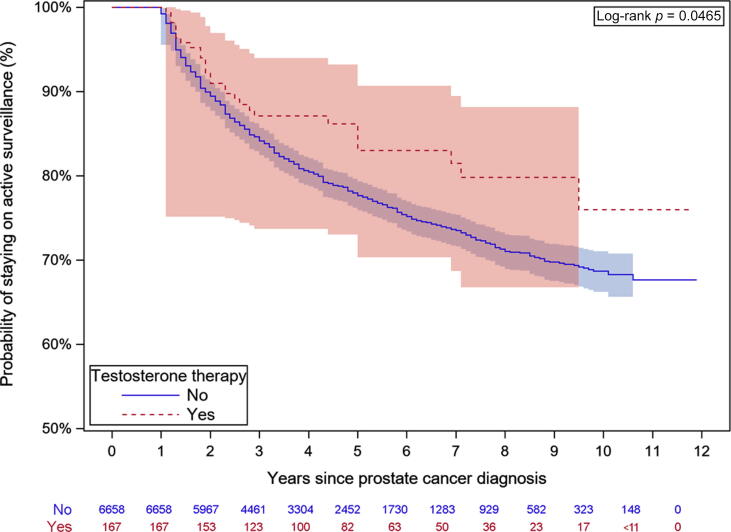
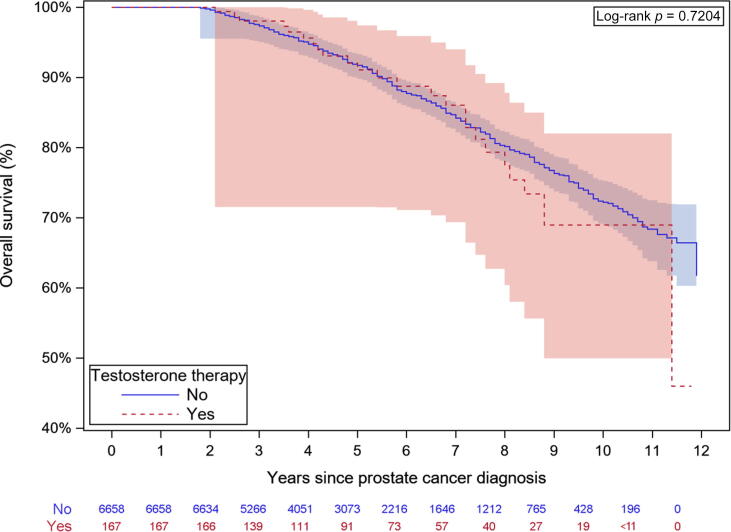
There were 28 (17%) conversions to active treatment in the testosterone group and 1455 (22%) in the no-testosterone group. There were no prostate cancer–specific deaths in the testosterone group, precluding comparison to the 39 (0.6%) prostate cancer–specific deaths in the no-testosterone group. Multivariable analysis revealed that older men and men with higher comorbidity were less likely to switch to active treatment and experienced higher overall mortality (Table 4). Higher PSA at diagnosis was associated with greater likelihood of active therapy (HR 1.21, 95% CI 1.05–1.4; p = 0.009) and overall mortality (HR 1.3, 95% CI 1.08–1.57; p = 0.005). Higher income and education were associated with lower overall mortality. The mean duration of active surveillance was 4.9 yr (IQR 2.9–7.4) in the testosterone group and 3.9 yr (IQR 2.6–6.1) in the no-testosterone group (p < 0.001). Testosterone therapy was not associated with conversion to active therapy (HR 0.66, 95% CI 0.46–0.97; p = 0.033) or overall mortality (HR 1.02, 95% CI 0.68–1.53; p > 0.9). Kaplan-Meier survival curves demonstrate that testosterone therapy was not associated with conversion to active therapy (Fig. 2) or worse mortality (Fig. 3).
Table 4.
Multivariable analysis of oncologic outcomes
| Variable | Conversion to active treatment |
Overall mortality |
||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Testosterone therapy (yes vs no) | 0.66 (0.46–0.97) | 0.033 | 1.02 (0.68–1.53) | >0.9 |
| Age category | ||||
| 66–69 yr | Reference | Reference | ||
| 70–74 yr | 0.82 (0.73–0.92) | <0.001 | 1.45 (1.18–1.79) | <0.001 |
| ≥75 yr | 0.65 (0.57–0.74) | <0.001 | 2.85 (2.35–3.46 | <0.001 |
| Race/ethnicity | ||||
| White | Reference | Reference | ||
| Black | 1.14 (0.89–1.45) | 0.3 | 0.99 (0.73–1.34) | >0.9 |
| Hispanic | 0.96 (0.73–1.29) | 0.8 | 0.67 (0.42–1.06) | 0.086 |
| Asian | 1.01 (0.74–1.38) | >0.9 | 0.75 (0.45–1.23) | 0.2 |
| Unknown | 0.91 (0.68–1.22) | 0.5 | 0.27 (0.12–0.59) | 0.001 |
| Charlson comorbidity index | ||||
| 0 | Reference | Reference | ||
| 1 | 0.99 (0.87–1.12) | 0.9 | 1.47 (1.23–1.74) | <0.001 |
| ≥2 | 0.84 (0.72–0.97) | 0.016 | 2.35 (1.98–2.79) | <0.001 |
| RPD (nonmetropolitan vs metropolitan) | 0.84 (0.71–1.00) | 0.05 | 1 (0.82–1.23) | >0.9 |
| Region | ||||
| Northeast | Reference | Reference | ||
| South | 1.35 (1.13–1.61) | 0.001 | 1.3 (1.03–1.64) | 0.024 |
| Midwest | 1.16 (0.94–1.43) | 0.2 | 1.03 (0.78–1.35) | 0.8 |
| West | 1.45 (1.24–1.71) | <0.001 | 1.09 (0.87–1.36) | 0.5 |
| Annual household or individual income | ||||
| <35 000 | Reference | Reference | ||
| $35 000–$44 999 | 1.06 (0.83–1.35) | 0.6 | 0.93 (0.71–1.22) | 0.6 |
| $45 000–$59 999 | 1.14 (0.91–1.43) | 0.24 | 0.86 (0.67–1.11) | 0.3 |
| ≥$60 000 | 1.13 (0.90–1.42) | 0.28 | 0.76 (0.58–1.00) | 0.046 |
| Completed at least HSE in CTR | ||||
| <75% | Reference | Reference | ||
| 75–84.9% | 0.99 (0.86–1.14) | >0.9 | 0.81 (0.67–0.99) | 0.036 |
| 85–89.9% | 0.93 (0.77–1.11) | 0.4 | 0.71 (0.53–0.94) | 0.016 |
| ≥90% | 0.92 (0.76–1.12) | 0.4 | 0.66 (0.49–0.89) | 0.007 |
| Clinical T stage (cT2 vs cT1) | 1.04 (0.93–1.18) | 0.48 | 1.02 (0.87–1.20) | 0.7938 |
| PSA (10–20 vs <10 ng/ml) | 1.21 (1.05–1.4) | 0.009 | 1.3 (1.08–1.57) | 0.005 |
| Gleason score (3 + 4 = 7 vs ≤6) | 0.97 (0.84–1.11) | 0.6 | 1.15 (0.92–1.43) | 0.2 |
CI = confidence interval; CTR = census tract of residence; HR = hazard ratio; HSE = high school education; PSA = prostate-specific antigen; RPD = residential population density.
Fig. 2.
Kaplan-Meier analysis of the risk of conversion to active therapy.
Fig. 3.
Kaplan-Meier analysis of overall mortality.
4. Discussion
There is a lack of research on the effects of testosterone therapy in men with untreated prostate cancer, with no prospective randomized controlled trials and only a few small retrospective studies conducted to date [14], [15], [16], [17]. We demonstrate that testosterone therapy does not appear to increase the risk of conversion to active treatment or prostate cancer–specific or overall mortality in men with untreated prostate cancer on active surveillance. These results support our hypothesis and align with the saturation model concept that prostate cells reach a limit in their capacity to respond to androgens at physiologic levels of testosterone [3], [5]. These findings have important implications for patient care, as they suggest that testosterone therapy may be safely considered in this population.
In 2019, 224 733 individuals were diagnosed with prostate cancer in the USA [18]. Approximately 20% of these diagnoses were low-risk disease, for which the recommended management is active surveillance [8], [19]. Before 2010, less than 10% of low-risk disease cases in community practice were managed with active surveillance [20]. Currently, up to 60% of cases are being managed with active surveillance and it is estimated that the optimal rate on the basis of guideline recommendations should exceed 80% [20]. On the basis of these figures, we estimate that 27 000–36 000 individuals are started on active surveillance each year. Considering that up to 25% of older men experience testosterone deficiency [4], almost 10 000 newly identified men per year could potentially improve their health-related quality of life through testosterone therapy.
Small, single-center retrospective studies have not shown a higher oncologic risk associated with testosterone therapy. Out of 13 men on active surveillance who received at least 12 mo of testosterone therapy and underwent one or more follow-up biopsies, only two experienced cancer progression [16]. Another study reported similar biopsy progression rates for up to 3 yr among men on active surveillance in groups receiving testosterone therapy (n = 28) or no testosterone therapy (n = 96) [14]. In a third study, none of eight patients on active surveillance and testosterone therapy followed for a median of 27 mo experienced clinical or pathological progression [17].
In our study, there were no prostate cancer–specific deaths in the testosterone group and 39 (0.6%) in the no-testosterone group. This finding should be interpreted within the context of currently available evidence. Some reports suggest that testosterone therapy may actually have a protective effect on prostate cancer. For example, low free testosterone levels have been linked to reclassification of higher-risk disease in men on active surveillance [21], and testosterone therapy has been associated with lower rates of aggressive (ie, high-risk, locally advanced, regional, and distant metastatic) prostate cancer [22].
It is well known that low testosterone levels are an independent risk factor for poorly differentiated prostate cancer [23]. A possible explanation may be that normal or high testosterone levels maintain prostate cell differentiation, whereas low testosterone levels lead to poorly differentiated cancer cells [22]. This mechanism may explain how high-dose testosterone therapy in men with castration-resistant prostate cancer induces prostate cell differentiation and resensitizes the cells to androgen deprivation therapy [22]. In addition, previous work suggested that at very high levels of testosterone past the saturation plateau, the proliferation of prostate cells actually decreases [6]. Bipolar testosterone therapy is based on this principle and has shown promising results as a treatment for metastatic prostate cancer [24].
While these preliminary findings may eventually lead to fundamental changes in the management of prostate cancer, more research is needed. According to the most recent American Urological Association and European Association of Urology guidelines, there is insufficient evidence to determine the risk-benefit ratio of testosterone therapy for men with a history of prostate cancer [6], [25]. TRAVERSE is an ongoing prospective, randomized, double-blind, placebo-controlled clinical trial that is assessing the efficacy response and long-term vascular events in hypogonadal men receiving testosterone replacement therapy, with the rate of high-grade prostate cancer as a secondary endpoint [26].
We also found that testosterone use was more common among men living in southern and western US regions and for men with PSA <10 ng/ml and Gleason score ≤6, which is consistent with other population-based studies [27], [28]. These findings may be because of differences in testosterone prescribing practices or patient preferences between regions and cultural groups, as well as the persistent belief that testosterone stimulates prostate cancer growth in a linear manner.
Our findings must be interpreted in the context of the study design. First, our retrospective study is hypothesis-generating. Second, the mean duration of active surveillance was longer than in many existing studies because the claims-based definition used for active surveillance implied no intervention within 2 yr of diagnosis. The testosterone group may also have had longer surveillance duration than the no-testosterone group because these individuals started with lower testosterone levels and Gleason scores. However, it is clinically significant that testosterone therapy was not associated with higher mortality. Third, active surveillance protocols used by different providers may vary, which could affect the comparability of the study groups. Fourth, the data lack clinical granularity such as testosterone serum levels, dosage of testosterone, and indications for treatment. Finally, our study population was limited to men aged ≥66 yr, so it is uncertain whether these findings are generalizable to younger men with prostate cancer.
5. Conclusions
In the first nationally representative study to examine the use of testosterone therapy for men on active surveillance for prostate cancer, testosterone therapy did not increase the risk of conversion to active therapy or worsen mortality. These findings align with the saturation model and have important implications for patient care, as they suggest that testosterone therapy may be safely considered in this population. Prospective research is needed to confirm these findings and to explore the risks and benefits of testosterone therapy for these patients.
Author contributions: Elie Kaplan-Marans had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kaplan-Marans, Zhang, Hu.
Acquisition of data: Kaplan-Marans.
Analysis and interpretation of data: Kaplan-Marans, Zhang, Hu.
Drafting of the manuscript: Kaplan-Marans, Zhang, Hu.
Critical revision of the manuscript for important intellectual content: Kaplan-Marans, Zhang, Hu.
Statistical analysis: Kaplan-Marans, Zhang, Hu.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Hu.
Other: None.
Financial disclosures: Elie Kaplan-Marans certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Acknowledgments: Jim C. Hu receives research support from the Frederick J. and Theresa Dow Wallace Fund of the New York Community Trust and salary support from grants NIH R01 CA241758, R01 CA259173, R01 CA273031; PCORI CER-2019C1-15682, and CER-2019C2-17372, and a Prostate Cancer Foundation Challenge Award.
Associate Editor: Roderick van den Bergh
References
- 1.Huggins C., Hodges C.V. Studies on prostatic cancer. Cancer Res. 1941;1:293–297. [Google Scholar]
- 2.Denmeade S.R., Isaacs J.T. A history of prostate cancer treatment. Nat Rev Cancer. 2002;2:389–396. doi: 10.1038/nrc801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgentaler A. Testosterone and prostate cancer: an historical perspective on a modern myth. Eur Urol. 2006;50:935–939. doi: 10.1016/j.eururo.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan A.L., Hu J.C., Morgentaler A., Mulhall J.P., Schulman C.C., Montorsi F. Testosterone therapy in men with prostate cancer. Eur Urol. 2016;69:894–903. doi: 10.1016/j.eururo.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgentaler A., Traish A.M. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol. 2009;55:310–320. doi: 10.1016/j.eururo.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 6.Mulhall J.P., Trost L.W., Brannigan R.E., et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol. 2018;200:423–432. doi: 10.1016/j.juro.2018.03.115. [DOI] [PubMed] [Google Scholar]
- 7.Lenfant L., Leon P., Cancel-Tassin G., et al. Testosterone replacement therapy (TRT) and prostate cancer: an updated systematic review with a focus on previous or active localized prostate cancer. Urol Oncol. 2020;38:661–670. doi: 10.1016/j.urolonc.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines®): prostate cancer. Version 1.2023. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
- 9.National Cancer Institute. SEER fact sheets. https://seer.cancer.gov/about/factsheets/.
- 10.National Cancer Institute. SEER-Medicare: brief description of the SEER-Medicare database. https://healthcaredelivery.cancer.gov/seermedicare/overview/.
- 11.Krishna S., Fan Y., Jarosek S., Adejoro O., Chamie K., Konety B. Racial disparities in active surveillance for prostate cancer. J Urol. 2017;197:342–349. doi: 10.1016/j.juro.2016.08.104. [DOI] [PubMed] [Google Scholar]
- 12.Al-Lami R.A., Graham J.E., Deer R.R., et al. Testosterone replacement therapy and rehospitalization in older men with testosterone deficiency in a postacute care setting. Am J Phys Med Rehabil. 2019;98:456–459. doi: 10.1097/phm.0000000000001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez D.S., Huang D., Tsilidis K.K., et al. The role of testosterone replacement therapy and statin use, and their combination, in prostate cancer. Cancer Causes Control. 2021;32:965–976. doi: 10.1007/s10552-021-01450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kacker R., Hult M., San Francisco I.F., et al. Can testosterone therapy be offered to men on active surveillance for prostate cancer? Preliminary results. Asian J Androl. 2016;18:16–20. doi: 10.4103/1008-682X.160270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashimoto T., Rahul K., Takeda T., et al. Prostate magnetic resonance imaging findings in patients treated for testosterone deficiency while on active surveillance for low-risk prostate cancer. Urol Oncol. 2016;34:530.e9–530.e14. doi: 10.1016/j.urolonc.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgentaler A., Lipshultz L.I., Bennett R., Sweeney M., Avila D.J., Khera M. Testosterone therapy in men with untreated prostate cancer. J Urol. 2011;185:1256–1260. doi: 10.1016/j.juro.2010.11.084. [DOI] [PubMed] [Google Scholar]
- 17.Ory J., Flannigan R., Lundeen C., Huang J.G., Pommerville P., Goldenberg S.L. Testosterone therapy in patients with treated and untreated prostate cancer: impact on oncologic outcomes. J Urol. 2016;196:1082–1089. doi: 10.1016/j.juro.2016.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Prostate cancer incidence by stage at diagnosis, United States—2001−2019. https://www.cdc.gov/cancer/uscs/about/data-briefs/no34-prostate-cancer-incidence-2001-2019.htm.
- 19.Cooperberg M., Meeks W., Fang R., Gaylis F., Catalona W., Makarov D. MP43-03 Active surveillance for low-risk prostate cancer: time trends and variation in the AUA Quality (AQUA) registry. J Urol. 2022;207(Suppl 5):e740. doi: 10.1097/JU.0000000000002609.03. [DOI] [Google Scholar]
- 20.Cooperberg M.R., Meeks W., Fang R., Gaylis F.D., Catalona W.J., Makarov D.V. Time trends and variation in the use of active surveillance for management of low-risk prostate cancer in the US. JAMA Netw Open. 2023;6:e231439. doi: 10.1001/jamanetworkopen.2023.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.San Francisco I.F., Rojas P.A., DeWolf W.C., Morgentaler A. Low free testosterone levels predict disease reclassification in men with prostate cancer undergoing active surveillance. BJU Int. 2014;114:229–235. doi: 10.1111/bju.12682. [DOI] [PubMed] [Google Scholar]
- 22.Loeb S., Folkvaljon Y., Damber J.-E., Alukal J., Lambe M., Stattin P. Testosterone Replacement Therapy and Risk of Favorable and Aggressive Prostate Cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2017;35(13):1430–1436. doi: 10.1200/JCO.2016.69.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park J., Cho S.Y., Jeong S.-H., Lee S.B., Son H., Jeong H. Low testosterone level is an independent risk factor for high-grade prostate cancer detection at biopsy. BJU Int. 2016;118(2):230–235. doi: 10.1111/bju.13206. [DOI] [PubMed] [Google Scholar]
- 24.Morgentaler A., Caliber M. Safety of testosterone therapy in men with prostate cancer. Expert Opin Drug Saf. 2019;18:1065–1076. doi: 10.1080/14740338.2019.1666103. [DOI] [PubMed] [Google Scholar]
- 25.Salonia A, Bettocchi C, Capogrosso P, et al. EAU guidelines on sexual and reproductive health. Arnhem, The Netherlands: European Association of Urology; 2023. https://uroweb.org/guidelines/sexual-and-reproductive-health/chapter/male-hypogonadism.
- 26.Bhasin S., Lincoff A.M., Basaria S., et al. Effects of long-term testosterone treatment on cardiovascular outcomes in men with hypogonadism: rationale and design of the TRAVERSE study. Am Heart J. 2022;245:41–50. doi: 10.1016/j.ahj.2021.11.016. [DOI] [PubMed] [Google Scholar]
- 27.Zhou C.K., Advani S., Chaloux M., et al. Trends and patterns of testosterone therapy among U.S. male Medicare beneficiaries, 1999 to 2014. J Urol. 2020;203:1184–1190. doi: 10.1097/JU.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen T., Li S., Eisenberg M.L. Trends in testosterone therapy use in prostate cancer survivors in the United States. J Sex Med. 2021;18:1346–1353. doi: 10.1016/j.jsxm.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]