Abstract
Introduction
COVID-19 caused by SARS-CoV-2, commonly presents with symptoms such as fever and shortness of breath but can also affect other organs. There is growing evidence pointing to potential eye complications. In this article, we aim to systematically review the ocular manifestations of COVID-19.
Methods
We conducted a systematic review to explore the ocular manifestations of COVID-19. We searched online databases including PubMed, Embase, Scopus, and Web of Science up to September 4, 2023. After a two-stage screening process and applying inclusion/exclusion criteria, eligible articles were advanced to the data extraction phase. The PRISMA checklist and Newcastle-Ottawa Scale (NOS) were used for quality and bias risk assessments.
Results
We selected and extracted data from 42 articles. Most of the studies were cross-sectional (n = 33), with the highest number conducted in Turkey (n = 10). The most frequent ocular manifestation was conjunctivitis, reported in 24 articles, followed by photophobia, burning, chemosis, itching, and ocular pain. Most studies reported complete recovery from these manifestations; however, one study mentioned visual loss in two patients.
Conclusion
In general, ocular manifestations of COVID-19 appear to resolve either spontaneously or with supportive treatments. For more severe cases, both medical treatment and surgery have been employed, with the outcomes suggesting that complete recoveries are attainable.
Keywords: COVID-19, Ocular manifestations, Ophthalmic complication, Eye involvement
1. Introduction
Coronavirus disease 19 (COVID-19) is caused by Severe Acute Respiratory Syndrome Coronavirus 2)SARS-CoV-2(virus, first identified in Wuhan, China, in December 2019 (Nora et al., 2020). The disease quickly spread globally and was declared a pandemic on March 11, 2020 (Khalili et al., 2020). The rapid spread of COVID-19 had devastating consequences worldwide. Mortality rates varied, with approximately 80 % of hospitalized patients and 60 % of patients in the intensive care unit (ICU) surviving (Bedford et al., 2020).
The most common symptoms in COVID-19 are fever and shortness of breath (Wiersinga et al., 2020). While the virus primarily targets the lungs and immune system, it can also damage other organs, with the severity varying among patients (Chen and Jungang, 2020). COVID-19 complicates the clinical diagnosis process due to the absence of unique pathognomonic symptoms (Jin et al., 2020). Additionally, some patients may present with gastrointestinal (Han et al., 2020), renal and even ocular symptoms (Renu et al., 2020). The eye is an organ that plays a very important role in the transmission process of COVID-19, and protecting the eyes and doing this correctly during the epidemic is a must. On the other hand, it should be taken into account that sometimes eye manifestations can be the first or even the only manifestation of COVID-19, sometimes eye manifestations are a set of symptoms that can show themselves at any stage of the disease or even after recovery (Domínguez-Varela et al., 2021, Qu et al., 2021).
Most studies have emphasized respiratory symptoms, yet there is evidence pointing to potential eye complications (Seah and Agrawal, 2020). Research indicates that keratoconjunctivitis (Hutama et al., 2022), conjunctivitis (Chen et al., 2020, Maychuk et al., 2020, Wu et al., 2020), conjunctival irritation, diplopia and cotton wool patches are among the most prevalent ophthalmic symptoms in COVID-19 patients (Khan et al., 2021). Notably, viral conjunctivitis has been observed to affect men more frequently than women (Al-Namaeh, 2021). In some instances, these ophthalmic symptoms have been among the initial clinical manifestations of COVID-19 (Baig, 2020). Most reported cases were linked to acute manifestations of COVID-19, although some chronic instances have also been documented (Mahayana et al., 2020). Understanding the frequency and nature of ocular symptoms related to COVID-19 can assist physicians in making more accurate and timely diagnoses. This study underscores the significance of recognizing the prevalence and ocular manifestations of COVID-19 to facilitate better and earlier detection of the disease. Hence, we aim to systematically examine the ocular manifestations of COVID-19 in this article.
2. Methods
In this systematic review, we provide a comprehensive overview of the ocular manifestations associated with COVID-19 infection, drawing from the current literature. The authenticity and reliability of our findings are enhanced through adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist. Bias risk is also minimized by exploiting the Newcastle-Ottawa Scale (NOS) bias assessment tool.
2.1. Data sources
We searched for specified keywords and their combinations in the online databases of PubMed, Embase, Scopus, and Web of Science. All literature published in English up until September 4, 2023, were harvested for further investigations and data extraction processes. Below are the examples of keywords and their combinations used in our database searches:
-
A.
“COVID-19” OR “SARS-CoV-2” OR “coronavirus disease 2019” OR “severe acute respiratory syndrome coronavirus 2” [Title/Abstract]
-
B.
“Ocular” OR “Ophthalmic” [Title/ Abstract]
-
C.
[A] AND [B]
2.2. Study selection
Screening and selection of the retrieved publications occurred in two steps. In the initial step, the titles and abstracts were assessed. In this step, four researchers (R.S., A.M., A.D., M.A.H.) identified relevant articles based on their titles and abstracts to be considered for the subsequent, more detailed stage. In this second stage, five other researchers (A.P., I.A.F., S.S.T.Z., P.M., A.M.V.) scrutinized the full texts of the screened articles and proceeded with extraction of necessary data.
Included publications met the following criteria: they were being original article, case series, or case reports, published in English, and underwent peer review process before publication.
We excluded the papers that met any of the following criteria: non-human such as animal or in vitro studies, experiments with insufficient published data, publications without accessible full texts, conference abstracts, duplicated papers, and editorial letters.
2.3. Data extraction
Five researchers (A.P., I.A.F., S.S.T.Z., P.M., A.M.V.) carried out extraction of essential data. This step was undertaken after the completion of the second selection phase, during which full texts were thoroughly reviewed. The extracted data is presented in Table 2. Additional checks for possible duplicate papers and extracted data were performed by other research members.
Table 2.
Description of the findings reported in eligible studies.
| Author (year) | Country | Population |
Comorbidity (%) |
Ocular Manifestations (%) | Time of onset | Treatment of ocular Manifestations | Outcome | Other results |
|---|---|---|---|---|---|---|---|---|
| Giampietro et al. (2023) | Brazil | 150 patients | Hypertension (Samaranayake et al., 2020) HIV (Domínguez-Varela et al., 2021) Obesity (Khan et al., 2021) Diabetes mellitus (Ranzenigo et al., 2021) |
Candle flame hemorrhage and cotton wool exudates (Qu et al., 2021) |
N/A | N/A | N/A | N/A |
| Firat and Kobat (2021) | Turkey | 32 patients 32 healthy |
N/A | No significant differences in terms of central choroidal thickness, central foveal thickness, nasal 500, nasal 1500, temporal 500, or temporal 500 µm distances were found between the groups. | N/A | N/A | N/A | Choroidal and retinal thicknesses were not affected in patients with recent mild COVID 19 without comorbidities. |
| Ganesh and Mohanan-Earatt (2022) | India | 13 patients | N/A | Anterior uveitis (Han et al., 2020) intermediate uveitis (Bedford et al., 2020) posterior uveitis (Nora et al., 2020) panuveitis (Khalili et al., 2020) |
Within the 6 weeks after COVID-19 diagnosis | Topical Steroids (anterior uveitis) Topical, Oral, and IV Steroids, Mycophenolate mofetil, and MTX (intermediate, posterior, and panuveitis) |
All patients responded well to treatment. Two patients had surgical treatment |
Bilateral eye involvement (Renu et al., 2020) unilateral eye involvement (Chen and Jungang, 2020) |
| Shaikh et al. (2022) | Qatar | 39 patients | Hypertension, diabetes mellitus, coronary artery disease, rheumatic arthritis, or cancer | Conjunctival hyperemia (33.3) eye pain (23.1) epiphora (20.5) burning sensation (10.3) photophobia (5.1) Conjunctivitis (5.1) |
N/A | N/A | N/A | no correlation between patients’ gender, comorbidities, and occurrence of ocular manifestations in COVID-19 patients |
| Sharma et al. (2022) | India | 658 (total) 162 (ocular) |
Diabetes mellitus followed by hypertension (18.42) | Lid swelling (8.64) Watering and irritation (25.30) Follicular conjunctivitis (7.40) blephritis, lid hyperemia, dry eye, foreign body sensation, photophobia, chemosis and blurring of the vision and peri-orbital pain |
mostly < 1 week after COVID-19 infection | N/A | 56.7 % relieved from ocular discomfort | The most significant ocular morbidity was black discoloration of lids and peri ocular skin, lid swelling, and redness and purulent discharge of conjunctivitis needed emergency ophthalmic reference |
| Shen et al. (2021) | China | Total 3198 Ocular 28 |
hypertension (28.6), Glaucoma (3.6), Nephritis (3.6), autoimmune anemia (3.6), diabetes (3.6), hepatitis B (3.6), cerebral infarction (3.6) |
Conjunctivitis (100 %) | N/A | Levofloxacin eye drop (15 = 53.6 %) Ganciclovir eye drop (2 = 7.1 %) sodium hyaluronate eye drop (1 = 3.6 %) artificial tears (1 = 3.6 %) observe (9 = 32.1 %) |
N/A | conjunctivitis was a rare and self-limited complication in adults with COVID-19 while the existence of coronavirus receptors on human ocular surface and mouse lacrimal glands indicated the risk of SARS-CoV-2 infection |
| Silveira et al. (2022) | Brazil | Total 104 Ocular 36 |
N/A | Burning (19.23), pain (11.54), foreign body sensation (7.7), hyperemia (7.7), and tearing (3.84) | concomitantly with general symptoms (77.7) one day before flu-like symptoms (11.11 %) after 3 days(11.11 %) |
eye drops for selfmedication (ketorolac trometamol and carmellose sodium)(5.71 %) | N/A | |
| Sindhuja et al. (2020) | India | Total 127 Ocular 12 |
Diabetes mellitus (Seah and Agrawal, 2020), hypertension (Renu et al., 2020), thyroid disorders (Khalili et al., 2020), pulmonary tuberculosis (Nora et al., 2020); parkinsonism (Nora et al., 2020); bronchial asthma (Nora et al., 2020), cardiovascular disorder (Nora et al., 2020) |
conjunctival congestion (66.6) ocular burning sensation (8.33), watering (8.33), hordeolum externum (8.33) |
Within 3 weeks of COVID symptoms (50 %) Before COVID symptoms (41.6 %) |
N/A | N/A | N/A |
| Stephan et al. (2021) | France | 20 | N/A | Unilateral corneal injury (Qu et al., 2021), Superficial punctate keratitis without ulcer (1 8 0), Bilateral subconjunctival hemorrhages (Chen and Jungang, 2020) | N/A | Vit A ointment (for corneal injury) | Fully resolved | Prone to eye damage because of sedation, no blinking, and poor eyelid occlusions are prone to eye damage |
| Meduri et al. (2020) | Italy | 29 patients | Hypertension, (86.2), Diabetes (62.1), Obesity (44.8), Coronary heart disease (27.5), Cerebrovascular disease, (24.1), Atrial fibrillation (24.1), Neurological disease (13.8), Cancer (Oren et al., 2021), BPCO (Oren et al., 2021), Chronic renal disease, (24.1) |
Hyperemia (24.1), Chemosis (3.4), Secretion (6.9), Lid margin hyperemia (34.5), Crusted eyelashes (24.1), Meibomian orifices abnormalities (20.7) |
N/A | N/A | Tear analysis did not reveal the presence of SARS-CoV-2. Ocular symptoms are common in patients with COVID-19 | N/A |
| Oncul et al. (2021) | Turkey | 359 | N/A | Various ocular diseases (4.5) conjunctivitis | N/A | N/A | N/A | N/A |
| Oren et al. (2021) | Turkey | Total 60 COVID-19 group 35 Control group 25 |
N/A | Macular and peripapillary retinal nerve fiber layer (RNFL) thickness measurements, each retinal layer thickness of all participants was done 14–30 days after COVID-19 symptom onset |
N/A | N/A | The mean value of central macular thickness and the mean values of the ganglion cell layer and inner nuclear layer thickness was significantly higher in the COVID-19 group |
N/A |
| Oren and Kocabas (2022) | Turkey | COVID-19 group 34 Control group 34 |
N/A | Corneal endothelial cell morphology, increase in CV value | N/A | N/A | N/A | N/A |
| Perez-Chimal et al. (2021) | USA | 15 newborns | N/A | Periorbital edema (1 0 0), Hemorrhagic conjunctivitis (73.3), Corneal edema (Ma et al., 2020), Hyaline secretion (1 0 0), Rubeosis (6.6) | N/A | N/A | N/A | N/A |
| Pirraglia et al. (2020) | Italy | 43 | N/A | No further retinal manifestation related to COVID-19 infection was found in our cohort |
ophthalmological screening was performed after a median of 21.5 days | N/A | N/A | N/A |
| Ranzenigo et al. (2021) | Italy | 53 patient | N/A | Conjunctivitis symptoms 37 Physician-assessed ocular signs 28 |
N/A | N/A | N/A | Plasma levels of Interleukin-6 were higher in patients with signs or symptoms in comparison with those without them: 43.5 pg/ml (19.7–49.4) vs. 8 pg/ ml (3.6–20.7) Red cell distribution width was also significantly higher |
| Riotto et al. (2022) | Switzerland | 172 patient | Arterial hypertension, or Dyslipidemia (Sen et al., 2021); Hypertension (10.5), Coronary disease (5.3), Diabetes (36.8) |
Cotton wool spots (CWS) and/or hemorrhages (Seah and Agrawal, 2020) | N/A | All subjects received 6 mg of dexamethasone daily since their admission | All patients were symptom-free 3 months after screening. | Diabetes history, overweight, and elevated C-reactive protein were more frequently observed among patients with retinal abnormalities, while a history of systemic hypertension was more frequently observed among patients without retinal findings |
| Rodriguez-Ares et al. (2021) | Spain | 56 patients | Hypertension (48.2), diabetes (28.6), cancer (23.2), heart disease (21.4), obesity (17.9), chronic lung disease (Khan et al., 2021) | Grittiness (16.1), ocular pain (7.1), photophobia (1.8), blurry vision (3.6), conjunctival hyperemia (3.6), itching (3.6), secretion (7.1) | Average of 7.1 days (range 1–20) before ocular testing |
N/A | No association was found between positive ocular samples and ocular symptoms | N/A. |
| Rokohl et al. (2020) | Germany | 108 patients | N/A | burning sensations (36.1), epiphora (34.3), redness (25.9) | 1.96 ± 3.17 days after the beginning of COVID-19 | N/A | Do not need treatment | N/A |
| Kumar et al. (2021) | India | 2742 | N/A | Bilateral viral conjunctivitis, orbital cellulitis secondary to pansinusitis | Ocular examinations were performed every 72 h | N/A | N/A | N/A |
| Ma et al. (2020) | China | 216 pediatric patients | N/A | Exhibited conjunctival discharge (12.5), eye rubbing (8.8), conjunctival congestion (2.3) |
N/A | Observation without treatment and minimal eye drop | Complete recovery for 41 patients and persistent eye rubbing for the other 8. | Children with systematic symptoms had a higher chance of developing ocular manifestations |
| Jiang et al. (2021) | China | 255 COVID-19 | N/A | Asthenopia (4.3), mild conjunctival congestion, and serous secretion (0.8) | ||||
| Hepokur et al. (2023) | Turkey | 16 COVID-19 17 control | N/A | Decrease in Subfoveal choroidal thickness (SFC), SFCT increase in late post-infectious period, peripapillary choroidal thickness, Decrease in choroidal stroma and blood vessels |
N/A | N/A | N/A | N/A |
| Zhou et al. (2020) | China | 243 patients | N/A | Exhibited ocular manifestations (6.6) | N/A | N/A | N/A | N/A |
| Wang et al. (2021) | China | 42 patients | N/A | Dry eye disease (61.9) | N/A | N/A | N/A | N/A |
| Dag Seker and Erbahceci Timur (2021) | Turkey | 32 COVID-19 36 control |
N/A | Thinner macular retinal nerve fiber layer (RNFL) of inner and outer nasal and outer inferior quadrants | Eye examination was performed after 60.5 ± 38.6 days after COVID-19 confirmation | N/A | N/A | Patients with ocular pain had Thinner Superonasal and inferotemporal sectors of the Peripapillary retinal nerve fiber layer |
| Layikh et al. (2021) | Iraq | 186 | N/A | Conjunctivitis (13.4) | N/A | No treatment | All symptoms disappear within a few days | No significant association between gender and conjunctivitis prevalence, significant association between conjunctivitis and severity of the disease |
| Sehgal et al. (2021) | India | 804 COVID-19 | Diabetes mellitus, hypertension, obesity (81.5) | Conjunctival hyperemia (Jin et al., 2020); follicular reaction in palpebral conjunctiva (65.6), chemosis (Bypareddy et al., 2021) |
4.52 ± 1.47 days from systemic manifestation to the onset of ocular manifestations | N/A | N/A | N/A |
| Reinhold et al. (2021) | Switzerland | 10 COVID-19 5 control |
N/A | swollen endothelial cells in congested choroidal vessels (Ma et al., 2020) |
N/A | N/A | N/A | N/A |
| Jidigam et al. (2022) | USA | 7 COVID-19 6 control group |
N/A | Hemorrhagic spots and increased vitreous, increased retinal thickness, changes in retinal microvasculature, increased inflammation, gliosis, localized density changes, and increased inflammation |
N/A | N/A | N/A | N/A |
| Wan et al. (2022) | China | 228 COVID-19 109 Control |
N/A | Meibomian gland dysfunction (MGD), ocular surface staining score, shorter Tear Break-up time in patients requiring supplementary oxygen during hospitalization | 1 new ocular manifestation, 1 week before the COVID-19 diagnosis (Maychuk et al., 2020), ocular symptoms 4 weeks following diagnosis of COVID-19 (21.5) Evaluation was done within 52.23 ± 16.12 days after their COVID-19 positive test |
N/A | N/A | N/A |
| Bayram et al. (2022) | Turkey-USA | 53 COVID-19 group 53 control group |
N/A | Outer plexiform layer thickness, choroidal thickness, low choroidal vascularity, increase in the stromal area to vascular area Significant increase in all quadrants of the peripapillary retinal nerve fiber layer (RNFL) thickness, significantly higher reflectivity of OCT echo of the choroid, and peripapillary RNFL |
Day of patient hospitalization and third month of follow-up after recovery | N/A | N/A | Enhance in the outer plexiform layer thickness, mean choroidal thickness, the stromal area to vascular area (S/V) ratio of the choroid, peripapillary retinal nerve fiber layer (RNFL) thicknesses, and The reflectivity of OCT echo of the was observed in patients compared to the control group while a decrease in choroidal vascularity was detected. |
| Abdelkader et al. (2021) | Egypt | 9 | renal failure on hemodialysis (11.1) | All patients: edema and erythema of eyelids, severe conjunctival and ciliary injection, subconjunctival hemorrhage, corneal edema and infiltration, dense inflammatory coagulum in the anterior chamber, axial proptosis, limitation of the ocular motility. Ocular B-scan ultrasonography: medium to highly reflective floaters and membranous echoes with loculated opacities in the vitreous cavity more condensed posteriorly with choroidal thickening and the retina was in place Orbital CT: mild proptosis, haziness of orbital fat, in all cases. Mucoperiosteal thickening (11.1) |
N/A | IV vancomycin, ceftazidime with oral metronidazole, Topical moxifloxacin hydrochloride 0.5 %, a topical combination of dexamethasone and tobramycin, and cycloplegic, Vitreous tap with intravitreal injection of vancomycin (1 mg/0.1 ml) and ceftazidime (2.25/0.1 ml) | 3 patients died, atrophia bulbs in 4 eyes and preserved eyeball with complete visual loss in 2 patients | N/A |
| Abrishami et al. (2020) | Iran | 142 | Cataract (7.7), diabetic retinopathy (6.3) | Tearing (23.2), red eyes (Oren et al., 2021); eye irritation (13.4), eye itching (8.5), foreign body sensation (2.8), periorbital pain (3.5), photophobia (0.7), blurred vision (0.7), conjunctival swelling (15.5), conjunctival hyperemia (28.9), Chemosis (15.5) | N/A | N/A | N/A | The percentage of patients with ≥ 1 ocular manifestations was significantly higher in those admitted to ICU compared to the non-ICU group. Among all patients, the most common finding was conjunctival hyperemia. Among ICU patients, the most common finding was chemosis. |
| Akturk Acar et al. (2022) | Turkey | 15 | N/A | Bilateral conjunctivitis (26.7), an avascular area in Zone-III (Han et al., 2020) | The first: During hospitalization following negative RT-PCR result The second: 1 month later |
Supportive treatments | N/A | N/A |
| Ahuja et al. (2020) | USA | 1 | N/A | Left eye irritation, upper eyelid swelling, erythematous, swollen and had to crust along the lashes, mild inflammation, and injection of the conjunctiva | Past 24 h | Doxycycline 100 mg to use if the symptoms worsened or did not improve | Clinically improved within six days of his initial presentation | N/A |
| Sezgin Akcay et al. (2021) | Turkey | 1083 | HTN (3 %), DM (1.3), allergic asthma (0.2 %), CHF (0.9 %), Hashimoto disease (0.3 %), CRF (0.2 %), RA (0.1), and lymphoma (0.1 %) | Sore eye or burning sensation (Jin et al., 2020), foreign body sensation ± burning sensation (3.6), red eye ± foreign body sensation, burning sensation, pain, itching (3.4) | Conjunctivitis symptoms manifested at the first and second week of disease onset in (28.5) and patients (71.4), respectively | N/A | N/A | The inpatient group had higher rates of comorbidity, ophthalmic medication, chronic ocular disease, and previous ocular surgery, but not contact lens wear compared to the outpatient group. |
| Boz et al. (2021) | Turkey | 50 | HTN (Maychuk et al., 2020), COPD (Wiersinga et al., 2020), Asthma (Wiersinga et al., 2020), DM (Khalili et al., 2020) |
Follicular conjunctivitis (Baig, 2020); Blepharoconjunctivitis (Renu et al., 2020), Blepharitis (Renu et al., 2020), Papillary conjunctivitis (7.5), Anterior uveitis (Wiersinga et al., 2020); Presence of cataract (Wiersinga et al., 2020), Presence of pterygium (Khalili et al., 2020) |
Within 2 weeks after COVID-19 infection had been confirmed | Symptomatic treatment (preservative-free artificial tears, cold compress, and lubricating ophthalmic ointment) | All resolved | N/A |
| Bypareddy et al. (2021) | India | 138 | N/A | A single streak of superficial retinal hemorrhage at the posterior pole of the fundus in the left eye of one patient (0.72) | 6 days from the infection symptoms onset | N/A | N/A | No lesions that can be attributed to COVID-19 were found in those with mild to moderate COVID-19 symptoms |
| Chen et al. (2020) | China | 535 | HTN (Chen et al., 2020), Hyperlipidaemia (1.1), DM (7.1), Cardiovascular and cerebrovascular diseases (3.3), Respiratory system disease (6.9), Hematological system disease (0.5), CKD (0.5), Chronic liver disease (4.7), Autoimmune disease (1.9) |
Without conjunctival congestion group: Conjunctival secretion (8.7), Ocular pain (3.5), Foreign body sensation (11.4), Photophobia (2.6), Blurred vision (12.8), Dry eye (20.1), Tearing (9.6), Itching (9.6), With conjunctival congestion group: Conjunctival secretion (29.6), Ocular pain (18.5), Foreign body sensation (18.5), Photophobia (11.1), Blurred vision (11.1), Dry eye (Rodriguez-Ares et al., 2021), Tearing (22.2), Itching (14.8) |
Conjunctival congestion 0–3 days in 7 patients, 4–7 days in 1 patient, 8–14 days in 6 patients, and 15–28 days in 6 patients after clinical symptoms | N/A | N/A | Conjunctival congestion lasted for an average of 5.9 ± 4.5 days. A significant association between hand–eye contact and conjunctival congestion appeared. |
| Scalinci and Trovato (2020) | Italy | 5 |
N/A | chemosis, epiphora, photophobia, Conjunctivitis | Initial presentation in all patients | Moxifloxacin eye drops four times a day for 5 more days. | Resolved | Conjunctivitis was the only sign and symptom of COVID-19 infection. |
| Gangaputra and Patel (2020) | USA | 144 COVID-19 positive 306 COVID-19 negative | N/A | Eye pain 19.4, Photophobia 13.9, flashes/floaters 11.8, blurry vision 11.1, red eyes 10.4 |
1 to 4 weeks following the results of 69 their COVID-19 testing |
N/A | 26.5 % of patients were suffering from ocular symptoms despite recovery from systemic infection |
Red eyes and epiphora were more likely to be found in COVID-19-negative patients relative to COVID-19 positive ones. |
2.4. Quality assessment and bias risk evaluation
As mentioned above, to enhance the reliability and soundness of our research, we employed the items of PRISMA checklist in this review study. Moreover, we assessed the risk of bias in the included studies using the Newcastle-Ottawa Scale (NOS). The criteria of this tool, which include selection, comparability, and exposure/outcome, have respective values of 4, 2, and 3 for individual studies, as shown in Table 1. The sum of these values gives a maximum possible score of nine for each included study.
Table 1.
Bias Risk Assessment of Included Studies Based on Newcastle-Ottawa Scale (NOS).
3. Results
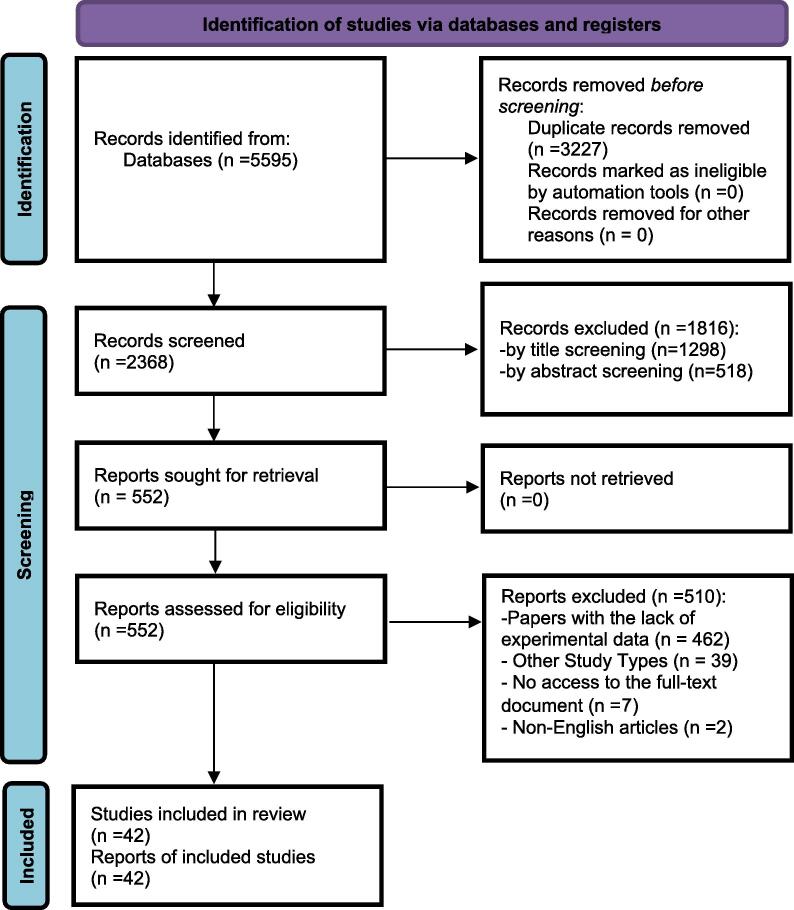
In this review, we investigated the ocular manifestations in COVID-19 patients by examining available evidence. We initially identified 5,595 articles. After eliminating 3,227 duplicates, we screened 2,368 articles based on their titles and abstracts. In the first phase of screening, 1,816 articles were excluded based on our inclusion and exclusion criteria. During the second phase of screening, 552 articles were reviewed, from which we extracted data from 42 articles. The selection process is illustrated in the PRISMA diagram (Fig. 1). Most of the studies were cross-sectional (n = 33), followed by case-control studies (n = 3), cohort studies (n = 3), case-report (n = 2), and case-series (n = 1). Most of studies have been conducted in Turkey (n = 10); followed by China (n = 7), Italy (n = 4), India (n = 6), the USA (n = 4), with two studies each from Brazil, and Switzerland. Single studies were conducted in France, Spain, Germany, Iraq, Egypt, Iran, and a multi-country study was undertaken in both Turkey and the USA.
Fig. 1.
PRISMA 2020 flow diagram of study retrieval process.
Comorbidities were reported as follow: hypertension in 14 articles, Diabetes Mellitus in 12 articles, obesity and cerebrovascular in 4 articles, cancer and asthma in 3 articles; and cardiovascular, cataract, renal disease, respiratory system disease, and dyslipidemia were each mentioned in 2 articles. Autoimmune disease, hyperlipidemia, chronic liver disease, CKD, COPD, Hashimoto disease, diabetic retinopathy, CRF, glaucoma, nephritis, thyroid disorders, and neurological disorders were each discussed in one article. Ocular manifestations were reported in the majority of the studies and were significantly more prevalent in COVID-19 patients compared to control groups. The most common ocular manifestation was conjunctivitis, mentioned in 24 articles, followed by photophobia, burning, chemosis, itching, and ocular pain in fourteen, seven, seven, six, and five articles, respectively. Less prevalent manifestations were as follows: tearing, red eyes, irritation, eye pain, blurry vision, and retinal hemorrhage. Some studies reported treatments for these manifestations, with most patients recovering fully (Giampietro et al., 2023, Silveira et al., 2022, Pirraglia et al., 2020, Wang et al., 2021, Reinhold et al., 2021, Wan et al., 2022, Abrishami et al., 2020). However, one study noted visual loss in two patients (Chen et al., 2020).
Based on a cross-sectional study of 104 COVID-19 patients of which 36 exhibited ocular symptoms, it was observed that ocular manifestations related to COVID-19 can manifest at various stages. Among those with ocular symptoms, 77.7 % developed them within three weeks of the onset of COVID-19 symptoms. Specifically, 50 % exhibited ocular symptoms concurrently with general symptoms. Additionally, 41.6 % showed ocular symptoms before the onset of COVID-19 symptoms, with 11.11 % presenting them one day before flu-like symptoms and another 11.11 % displaying them three days afterward (Silveira et al., 2022).
The interval between the ocular testing and the ophthalmological screening varied, with a median of 21.5 days (Pirraglia et al., 2020, Wan et al., 2022), an average of 7.1 days (range 1–20 days) (Rodriguez-Ares et al., 2021), and within 52.23 ± 16.12 days following a positive COVID-19 test (Wan et al., 2022). In some cases, ocular tests were performed every 72 h (Kumar et al., 2021).
Conjunctivitis symptoms appeared as particular ocular manifestations at various times, with 28.5 % of patients exhibiting them during the first week of disease onset and 71.4 % exhibiting them during the second. Some individuals developed conjunctival congestion 0–3 days after developing clinical symptoms, while others developed it 15–28 days after developing clinical symptoms (Jidigam et al., 2022). Overall, the timing and presentation of ocular symptoms in COVID-19 patients varied, indicating that ocular involvement can happen at various times over the course of the illness (Giampietro et al., 2023, Sharma et al., 2022, Oren and Kocabas, 2022, Riotto et al., 2022, Dag Seker and Erbahceci Timur, 2021).
According to the findings, conjunctivitis is a rare and self-limited consequence in adults with COVID-19. However, the presence of coronavirus receptors on the surface of the human eye and the lacrimal glands of mice raises concerns about SARS-CoV-2 infection. Individuals with ocular pain had thinner peripapillary retinal nerve fiber layers, and those with fever and ocular involvement typically had greater levels of CRP, neutrophil counts, and ESR, while having lower levels of lymphocytes. Also, the inpatient group of patients had a higher prevalence of ocular symptoms, comorbidities, chronic ocular disease, and prior ocular surgery. Also, patients with signs or symptoms had greater plasma levels of interleukin-6 and red cell distribution width, and patients with retinal abnormalities were more likely to have a history of diabetes (Baig, 2020, Firat and Kobat, 2021, Ganesh and Mohanan-Earatt, 2022, Shaikh et al., 2022, Shen et al., 2021, Sindhuja et al., 2020, Pirraglia et al., 2020, Ranzenigo et al., 2021, Dag Seker and Erbahceci Timur, 2021, Jidigam et al., 2022, Wan et al., 2022, Abdelkader et al., 2021), be overweight (Baig, 2020, Sindhuja et al., 2020, Ranzenigo et al., 2021, Dag Seker and Erbahceci Timur, 2021), or have raised C-reactive protein levels (Pirraglia et al., 2020).
4. Discussion
4.1. Overview of findings
Evidence indicates that COVID-19 exhibited a wide range of signs and symptoms. Although ocular manifestations in COVID-19 patients are typically infrequent, our systematic review aimed to evaluate these symptoms, potentially aiding in the disease's earlier diagnosis. In this systematic review, the authors encompassed 42 studies, with conjunctivitis emerging as the most frequently reported ocular symptom (n = 24 papers), followed by photophobia (n = 14), burning (n = 7), chemosis (n = 7), itching (n = 6), and ocular pain (n = 5). Some less common manifestations were as follows: tearing, red eyes, irritation, eye pain, blurry vision, and retinal hemorrhage.
4.2. Ocular manifestations
A meta-analysis involving 8,219 COVID-19 patients indicated an 11.03 % prevalence of ocular symptoms. It highlighted that foreign body sensation or dry eye was the most prevalent ocular symptom (16.0 %), followed by eye redness (13.3 %) and tearing (12.8 %). Similar to our findings, Nasiri et al. also reported that the most prevalent ocular disease was conjunctivitis (88.8 %) (Nasiri et al., 2021). Another meta-analysis among 5,717 COVID-19 patients reported that the most frequent ocular symptoms were; hyperemia of conjunctiva, conjunctival discharge, tearing and foreign body sensation. This study also emphasized that severe COVID-19 patients were 2–3 times more likely to be have ocular manifestations than mild cases (Zhong et al., 2021). Soltani et al. in their systematic review of 3,650 COVID-19 demonstrated that the prevalence of having at least one type of ocular manifestations was 23.77 %. The most frequent symptom was dry eyes (13.66 %), followed by conjunctival hyperemia (13.41 %), conjunctival congestion/conjunctivitis (9.14 %), ocular pain (10.34 %,), irritation/itching/burning sensation (9.34 %,), and foreign body sensation (5.24 %) (Soltani et al., 2022).
In our study, conjunctivitis emerged as the most frequently observed ocular symptom, reported in 24 articles. Aligned with our findings, N. Shaikh et al. (Shaikh et al., 2022), reported that 33.3 % of COVID-19 patients exhibited conjunctivitis, making it the predominant ocular manifestation, followed by eye pain at 23.1 %. Another study showed that 13.4 % of patients experienced conjunctivitis, which significantly correlated with disease severity (Shen et al., 2021). The underlying pathogenic pathways of conjunctival COVID-19 infection remain unclear. The ocular surface might serve as an entry point for the virus, either through hand-to-eye contact or exposure to aerosolized droplets. Two critical components facilitating the virus's entry into host cells are the cell surface protease enzyme (TMPRSS2) and the ACE-2 receptor. The presence of these receptors on the ocular surface is a subject of debate. However, research utilizing immunohistochemistry analysis has indicated the ACE-2 receptor's distinct presence in the conjunctiva, cornea, and limbus. Additionally, conjunctival samples have shown expression of TMPRSS2 (Zhou et al., 2020). The research showing that SARS-CoV-2 RNA may be found in the conjunctival mucosa and tears of COVID-19 patients reinforces the idea that the eye may be a potential site of infection (Mohammad Alrawashdeh et al., 2021). SARS-CoV-2 is thought to spread via the conjunctival sac tears and nasolacrimal ducts and finally proceed to the respiratory system (Qing et al., 2020). Additionally, it has been suggested that the direct viral assault from the eyes could be maintained by a strong immune response that may result in significant tissue injury. As a result, autoimmune and auto-inflammatory responses may both be at play (Gulati et al., 2020). The symptoms of conjunctivitis associated with COVID-19 are comparable to those of other viral types. Most often, the patients have chemosis, epiphora, follicular response of the tarsal conjunctiva, minor eyelid edema, watery discharge, and swollen submaxillary and preauricular lymph nodes (Bertoli et al., 2020). The COVID-19 conjunctival symptoms seem to be self-limiting. Some patients have shown symptom relief after the application of topical treatments like ganciclovir and ribavirin (Sen et al., 2021).
Regarding ocular chemosis, a notable symptom observed in various studies, G. Sehgal et al. examined 309 COVID-19 patients with ocular complications and found that 58.2 % exhibited chemosis (Sehgal et al., 2021). Another study revealed that 15.5 % out of 142 COVID-19 patients presented chemosis as an ocular symptom (Abrishami et al., 2020). Factors such as decreased venous return and elevated hydrostatic pressure resulting from positive pressure ventilation or tight endotracheal tube tape, mainly due to prolonged recumbency, are potential risk factors for conjunctival chemosis (Sen et al., 2021). Some other manifestations such as photophobia, ocular pain, itching, and burning have also been reported as ocular manifestations of COVID-19 patients (Shaikh et al., 2022, Rodriguez-Ares et al., 2021, Abrishami et al., 2020). Therefore, medical personnel in close contact with COVID-19 patients should wear face shield or eye protection glasses, given the virus's ability to adhere to the ocular surface and enter the respiratory system through the eye (Qing et al., 2020).
In addition to the direct effects of the virus on the eyes, its treatments and immunizations have similar impacts. Some medications for COVID-19, such as chloroquine and hydroxychloroquine—commonly used treatments—may pose risks to ocular health. Prolonged use of these drugs can lead to retinal toxicity, although short-term use seems safe. Interferon, another treatment option, has been linked to side effects including retinopathy, conjunctivitis, corneal ulcers, optic neuropathy, and epithelial abnormalities (Asili et al., 2021, Eissa et al., 2022). Ribavirin is also identified to have side effects such as serous retinal detachment, retinopathy, retinal vein occlusion, and ischemic optic neuropathy. Corticosteroids also have known ocular side effects in systemic treatment, which comprise glaucoma, cataracts, and central serous chorioretinopathy (Samaranayake et al., 2020).
In regards to protective measures and ocular transmission of COVID-19, Samaranayake et al. in their systematic review of 21 papers found that using layered, masks/respirators that fit on face, and protective-eyewear can curb COVID-19 transmission among healthcare workers. They also specified that a combination of intervention such as face mask and a face shield, can resist aerosol inhalation more effectively than either alone (Matos et al., 2021). Similarly, a review showed that SARS-CoV-2 can be transmitted effectively through the eyes which signifies the importance of eye-protection tools like goggles for health care workers or potential carriers of the virus (Chu et al., 2020). One study supported by the WHO for guidelines establishment on social distancing also proposed that eye protection can be effective in transmission prevention in the community setting and provides additional benefits (Davis et al., 2022). Another review also asserted that eyes are an additional entry route for SARS-CoV-2, and found that SARS-CoV-2 is most probable to be extracted from the ocular secretions of high-viral load patients that have conjunctivitis, as the ocular fluid can serve as a source of viral replication and transmission vector to corneal, conjunctival, and nasolacrimal tissue. Thus they also have signified the rule of protective eye-wear for prevention of COVID-19 transmission (Masiá et al., 2021).
4.3. Time of onset
In terms of ocular manifestation timeline, several studies have shown a correlation with the timing of the increase in immunoglobulin levels. In essence, high IgG concentrations, early seroconversion, and an elevated viral load have all been linked to severe COVID-19. It has been demonstrated that a mild infection results in delayed seroconversion and longer viral shedding. Due to the longer time being exposed to viral antigens, there is a delayed viral clearance and also increased serum levels of IgG at 6–8 weeks (Jin et al., 2020, Danthuluri and Grant, 2020). In an assessment of 27 COVID-19 patients, the onset of ocular symptoms spanned a broad timeline. For some, ocular manifestations were the initial symptom of the COVID-19 infection, while for others, these symptoms appeared anywhere from 0 to 28 days after the onset of clinical symptoms (Chen et al., 2020). Another study found that ophthalmological symptoms manifested between one and seven days following the appearance of clinical symptoms (Boz et al., 2021). The ocular symptoms may appear at any stage of the disease. Conjunctivitis, as a medical sign of COVID-19, usually appears at the beginning of the disease. If ocular pathology develops later in the clinical phase, it seems to be linked to COVID-19 being more severe. Although early detection of COVID-related ocular symptoms should limit the spread of the illness in general, evidence for direct transmission through eye mucosa is currently lacking (Danthuluri and Grant, 2020).
4.4. Outcome
Unfortunately, 26 out of 42 articles neither reported the outcome of patients nor did followed patients. However, the majority of studies that included a short follow-up reported that most of the ocular manifestations resolved spontaneously within few days or with supportive treatments. In cases of severe conditions, patients underwent medical treatments such as Vit-A/healing ointment, antibiotic eye drops, topical steroids, IV steroids, etc. and even surgery, leading to clinical improvement and complete recovery. While most symptoms disappeared and patients became symptom-free, some individuals continued to experience ocular discomfort, such as eye rubbing, for several months.
4.5. Limitation
The present study has some limitations. Firstly, the study design of included studies did not allow for reliable causal inferences. Additionally, there were few studies on some of the discussed matters, which may reduce the validity and reliability of reported outcomes. Nevertheless, this study may provide relevant insights for future research to conduct original studies and/or meta‐ analyses to precisely determine the association between COVID-19 and ocular manifestations. Furthermore, our recommendation for future studies is to continuously monitor patients in order to identify long-term effects of COVID-19 on eyes.
5. Conclusion
Although ocular manifestations of COVID-19 appear to be infrequent, they present a broad spectrum of symptoms, ranging in severity from mild eye redness or itching that may need no or only supportive treatment, to severe cases requiring medical intervention and possibly surgery. While there have been few reports of severe conditions resulting in visual loss, the majority of patients responded favorably to treatments, with full recoveries being attainable. These findings highlight the need for a comprehensive clinical assessment, encompassing not just the commonly recognized respiratory symptoms, but also potential ocular complications. Clinicians should be aware of the possibility of ocular symptoms as an early or concurrent presentation of COVID-19. Regular ophthalmic examinations, particularly for hospitalized patients, can aid in early detection and management and future policies should consider updating guidelines to include routine eye examinations for COVID-19 patients, particularly those exhibiting severe respiratory symptoms, as this could be indicative of systemic disease severity.
6. Future research
Few studies have investigated the ocular manifestations of COVID-19 among the children or the elderly, or the potential differences between sexes. In order to fill that gap in literature, the ocular symptoms among specific age or gender sub groups, more studies are needed. In addition, the exact mechanism of SARS-CoV-2 transmission via tear is unknown and uncertain thus further studies can be conducted to shed light on that part.
CRediT authorship contribution statement
SeyedAhmad SeyedAlinaghi: Writing – original draft. Esmaeil Mehraeen: Writing – original draft, Investigation, Conceptualization. Arian Afzalian: Writing – original draft. Mohsen Dashti: Writing – original draft. Afsaneh Ghasemzadeh: Writing – original draft. Ava Pashaei: Writing – original draft. Amir Masoud Afsahi: Writing – original draft. Seyed Saeed Tamehri Zadeh: Writing – original draft. Iman Amiri Fard: Writing – original draft. AmirMohammad Vafaee: Writing – original draft. Ayoob Molla: Methodology, Investigation. Ramin Shahidi: Writing – original draft. Ali Dadjou: Writing – original draft. Mohammad Amin Habibi: Writing – original draft. Pegah Mirzapour: Writing – original draft, Supervision. Omid Dadras: Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The present study was conducted in collaboration with Khalkhal University of Medical Sciences, and Tehran University of Medical Sciences.
Data availability
The data that has been used is confidential.
References
- Abdelkader M., Elshafei A.M.K., Nassar M.M., Abu Elela M.A., Abdallah R.M.A. Combined endophthalmitis and orbital cellulitis in patients with corona virus disease (COVID-19) J. Ophthalmic. Inflamm. Infect. 2021;11(1):27. doi: 10.1186/s12348-021-00258-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrishami M., Tohidinezhad F., Daneshvar R., Omidtabrizi A., Amini M., Sedaghat A., et al. Ocular Manifestations of Hospitalized Patients with COVID-19 in Northeast of Iran. Ocul. Immunol. Inflamm. 2020;28(5):739–744. doi: 10.1080/09273948.2020.1773868. [DOI] [PubMed] [Google Scholar]
- Ahuja A.S., Farford B.A., Forouhi M., Abdin R., Salinas M. The Ocular Manifestations of COVID-19 Through Conjunctivitis. Cureus. 2020;12(12):e12218. doi: 10.7759/cureus.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akturk Acar F., Esenulku M.C., Hekimoglu B. Retinal Findings of Hospitalized Neonates Recovered from COVID-19 Infection: A Prospective, Observational, Descriptive Study. J. Trop. Pediatr. 2022;68(3) doi: 10.1093/tropej/fmac036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Namaeh M. COVID-19 and conjunctivitis: a meta-analysis. Therapeutic Adv. Ophthalmol. 2021;13 doi: 10.1177/25158414211003368. 25158414211003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asili P., Mirahmad M., Tabatabaei-Malazy O., Manayi A., Haghighat E., Mahdavi M., et al. Characteristics of published/registered clinical trials on COVID-19 treatment: A systematic review. Daru. 2021;29(2):449–467. doi: 10.1007/s40199-021-00422-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig AM. Chronic COVID syndrome: Need for an appropriate medical terminology for long-COVID and COVID long-haulers. 2020. [DOI] [PubMed]
- Bayram N., Gundogan M., Ozsaygili C., Adelman R.A. Posterior ocular structural and vascular alterations in severe COVID-19 patients. Graefes Arch. Clin. Exp. Ophthalmol. 2022;260(3):993–1004. doi: 10.1007/s00417-021-05420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford J., Enria D., Giesecke J., Heymann D.L., Ihekweazu C., Kobinger G., et al. COVID-19: towards controlling of a pandemic. Lancet. 2020;395(10229):1015–1018. doi: 10.1016/S0140-6736(20)30673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoli F., Veritti D., Danese C., Samassa F., Sarao V., Rassu N., et al. Ocular Findings in COVID-19 Patients: A Review of Direct Manifestations and Indirect Effects on the Eye. J. Ophthalmol. 2020;2020:4827304. doi: 10.1155/2020/4827304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boz A.A.E., Atum M., Cakir B., Karabay O., Celik E., Alagoz G. Outcomes of the Ophthalmic Examinations in Patients Infected by SARS-CoV-2. Ocul. Immunol. Inflamm. 2021;29(4):638–641. doi: 10.1080/09273948.2020.1844904. [DOI] [PubMed] [Google Scholar]
- Bypareddy R., Rathod B.L.S., Shilpa Y.D., Hithashree H.R., Nagaraj K.B., Hemalatha B.C., et al. Fundus evaluation in COVID-19 positives with non-severe disease. Indian J. Ophthalmol. 2021;69(5):1271–1274. doi: 10.4103/ijo.IJO_3227_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Deng C., Chen X., Zhang X., Chen B., Yu H., et al. Ocular manifestations and clinical characteristics of 535 cases of COVID-19 in Wuhan, China: a cross-sectional study. Acta Ophthalmol. 2020;98(8):e951–e959. doi: 10.1111/aos.14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Jungang X. Interpretation of “the diagnosis and treatment plan for COVID-19 (the seventh trial edition)”. Her Med. 2020;39(5):613–615. [Google Scholar]
- Chen L., Liu M., Zhang Z., Qiao K., Huang T., Chen M., et al. Ocular manifestations of a hospitalised patient with confirmed 2019 novel coronavirus disease. Br. J. Ophthalmol. 2020;104(6):748–751. doi: 10.1136/bjophthalmol-2020-316304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D.K., Akl E.A., Duda S., Solo K., Yaacoub S., Schünemann H.J. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395(10242):1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dag Seker E., Erbahceci Timur I.E. COVID-19: more than a respiratory virus, an optical coherence tomography study. Int. Ophthalmol. 2021;41(11):3815–3824. doi: 10.1007/s10792-021-01952-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danthuluri V., Grant M.B. Update and Recommendations for Ocular Manifestations of COVID-19 in Adults and Children: A Narrative Review. Ophthalmol Ther. 2020;9(4):853–875. doi: 10.1007/s40123-020-00310-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis G., Li K., Thankam F.G., Wilson D.R., Agrawal D.K. Ocular transmissibility of COVID-19: possibilities and perspectives. Mol. Cell. Biochem. 2022;477(3):849–864. doi: 10.1007/s11010-021-04336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez-Varela I.A., Rodríguez-Gutiérrez L.A., Morales-Mancillas N.R., Barrera-Sánchez M., Macias-Rodriguez Y., Valdez-García J.E. COVID-19 and the eye: a review. Infect. Dis. 2021;53(6):399–403. doi: 10.1080/23744235.2021.1882697. [DOI] [PubMed] [Google Scholar]
- Eissa M., Abdelrazek N.A., Saady M. Covid-19 and its relation to the human eye: transmission, infection, and ocular manifestations. Graefes Arch. Clin. Exp. Ophthalmol. 2022;1–10 doi: 10.1007/s00417-022-05954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firat M., Kobat S. How are central foveal and choroidal thickness affected in patients with mild COVID-19 infection? Bosn. J. Basic Med. Sci. 2021;21(6):782–786. doi: 10.17305/bjbms.2021.5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh S.K., Mohanan-Earatt A. An analysis of the clinical profile of patients with uveitis following COVID-19 infection. Indian J. Ophthalmol. 2022;70(3):1000–1006. doi: 10.4103/ijo.IJO_2735_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangaputra S.S., Patel S.N. Ocular Symptoms among Nonhospitalized Patients Who Underwent COVID-19 Testing. Ophthalmology. 2020;127(10):1425–1427. doi: 10.1016/j.ophtha.2020.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giampietro B.V., Dutra S., Oliveira R.V.C., Biancardi A.L., Veloso V., Curi A.L.L. Ophthalmological Findings in Patients with SARS-CoV-2 Infection Examined at the National Institute of Infectious Diseases - INI/Fiocruz. Ocul. Immunol. Inflamm. 2023;31(1):44–47. doi: 10.1080/09273948.2021.1980812. [DOI] [PubMed] [Google Scholar]
- Gulati A., Pomeranz C., Qamar Z., Thomas S., Frisch D., George G., et al. A Comprehensive Review of Manifestations of Novel Coronaviruses in the Context of Deadly COVID-19 Global Pandemic. Am. J. Med. Sci. 2020;360(1):5–34. doi: 10.1016/j.amjms.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C., Duan C., Zhang S., Spiegel B., Shi H., Wang W., et al. Digestive symptoms in COVID-19 patients with mild disease severity: clinical presentation, stool viral RNA testing, and outcomes. Am. J. Gastroenterol. 2020 doi: 10.14309/ajg.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepokur M., Gunes M., Durmus E., Aykut V., Esen F., Oguz H. Long-term follow-up of choroidal changes following COVID-19 infection: analysis of choroidal thickness and choroidal vascularity index. Can. J. Ophthalmol. 2023;58(1):59–65. doi: 10.1016/j.jcjo.2021.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutama S.A., Alkaff F.F., Intan R.E., Maharani C.D., Indriaswati L., Zuhria I. Recurrent keratoconjunctivitis as the sole manifestation of COVID-19 infection: a case report. Eur. J. Ophthalmol. 2022;32(4):NP17–NP21. doi: 10.1177/11206721211006583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B., Li S.J., Wang W.L., Hu M., He S., Cao J., et al. Ocular manifestations and SARS-CoV-2 detection in tears and conjunctival scrape from non-severe COVID-19 patients. Int. J. Ophthalmol. 2021;14(8):1133–1137. doi: 10.18240/ijo.2021.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jidigam V.K., Singh R., Batoki J.C., Milliner C., Sawant O.B., Bonilha V.L., et al. Histopathological assessments reveal retinal vascular changes, inflammation, and gliosis in patients with lethal COVID-19. Graefes Arch. Clin. Exp. Ophthalmol. 2022;260(4):1275–1288. doi: 10.1007/s00417-021-05460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Lian J.-S., Hu J.-H., Gao J., Zheng L., Zhang Y.-M., et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69(6):1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C.C., Zhu L., Gao C., Zhang S. Correlation between viral RNA shedding and serum antibodies in individuals with coronavirus disease 2019. Clin. Microbiol. Infect. 2020;26(9):1280–1282. doi: 10.1016/j.cmi.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili M., Karamouzian M., Nasiri N., Javadi S., Mirzazadeh A., Sharifi H. Epidemiological characteristics of COVID-19: a systematic review and meta-analysis. Epidemiol. Infect. 2020;148 doi: 10.1017/S0950268820001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S.I., Versha F., Bai P., Bachani P., Nawaz M.U., Kumar L., et al. Frequency of ophthalmological findings in hospitalized COVID-19 patients. Cureus. 2021;13(5) doi: 10.7759/cureus.14942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar K.K., Sampritha U.C., Prakash A.A., Adappa K., Chandraprabha S., Neeraja T.G., et al. Ophthalmic manifestations in the COVID-19 clinical spectrum. Indian J. Ophthalmol. 2021;69(3):691–694. doi: 10.4103/ijo.IJO_3037_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layikh H.A., Hashim Z.A., Kadum A.A. Conjunctivitis and other ocular findings in patients with COVID-19 infection. Ann. Saudi Med. 2021;41(5):280–284. doi: 10.5144/0256-4947.2021.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N., Li P., Wang X., Yu Y., Tan X., Chen P., et al. Ocular Manifestations and Clinical Characteristics of Children With Laboratory-Confirmed COVID-19 in Wuhan, China. JAMA Ophthalmol. 2020;138(10):1079–1086. doi: 10.1001/jamaophthalmol.2020.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahayana I.T., Angsana N.C., Kamila A., Fatiha N.N., Sunjaya D.Z., Andajana W., et al. Literature review of conjunctivitis, conjunctival swab and chloroquine effect in the eyes: a current updates on COVID-19 and ophthalmology. J. Med Sci. (berkala Ilmu Kedokteran) 2020;52(3) [Google Scholar]
- Masiá M., Telenti G., Fernández M., García J.A., Agulló V., Padilla S., et al. SARS-CoV-2 Seroconversion and Viral Clearance in Patients Hospitalized With COVID-19: Viral Load Predicts Antibody Response. Open Forum. Infect. Dis. 2021;8(2) doi: 10.1093/ofid/ofab005. ofab005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos A.G., Sarquis I.C., Santos A.A.N., Cabral L.P. COVID-19: risk of ocular transmission in health care professionals. Rev. Bras Med. Trab. 2021;19(1):82–87. doi: 10.47626/1679-4435-2021-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maychuk D.Y., Atlas S., Loshkareva A. Ocular manifestations of coronavirus infection COVID-19 (clinical observation) Vestn. Oftalmol. 2020;136(4):118–123. doi: 10.17116/oftalma2020136041118. [DOI] [PubMed] [Google Scholar]
- Meduri A., Oliverio G.W., Mancuso G., Giuffrida A., Guarneri C., Venanzi Rullo E., et al. Ocular surface manifestation of COVID-19 and tear film analysis. Sci. Rep. 2020;10(1):20178. doi: 10.1038/s41598-020-77194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad Alrawashdeh H., Al Zubi K., Abdulmannan D.M., Al-Habahbeh O., Abu-Ismail L. Conjunctivitis as the only sign and symptom of COVID-19: A case report and review of literature. Qatar Med. J. 2021;2021(2):31. doi: 10.5339/qmj.2021.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasiri N., Sharifi H., Bazrafshan A., Noori A., Karamouzian M., Sharifi A. Ocular Manifestations of COVID-19: A Systematic Review and Meta-analysis. J. Ophthalmic Vis. Res. 2021;16(1):103–112. doi: 10.18502/jovr.v16i1.8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nora R.L.D., Putera I., Khalisha D.F., Septiana I., Ridwan A.S., Sitompul R. Are eyes the windows to COVID-19? Systematic review and meta-analysis. BMJ Open Ophthalmology. 2020;5(1):e000563. doi: 10.1136/bmjophth-2020-000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oncul H., Oncul F.Y., Alakus M.F., Caglayan M., Dag U. Ocular findings in patients with coronavirus disease 2019 (COVID-19) in an outbreak hospital. J. Med. Virol. 2021;93(2):1126–1132. doi: 10.1002/jmv.26412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren B., Aksoy Aydemir G., Aydemir E., Atesoglu H.I., Goker Y.S., Kiziltoprak H., et al. Quantitative assessment of retinal changes in COVID-19 patients. Clin. Exp. Optom. 2021;104(6):717–722. doi: 10.1080/08164622.2021.1916389. [DOI] [PubMed] [Google Scholar]
- Oren B., Kocabas D.O. Assessment of corneal endothelial cell morphology and anterior segment parameters in COVID-19. Ther. Adv. Ophthalmol. 2022;14 doi: 10.1177/25158414221096057. 25158414221096057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Chimal L.G., Cuevas G.G., Di-Luciano A., Chamartin P., Amadeo G., Martinez-Castellanos M.A. Ophthalmic manifestations associated with SARS-CoV-2 in newborn infants: a preliminary report. J. AAPOS. 2021;25(2):102–104. doi: 10.1016/j.jaapos.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirraglia M.P., Ceccarelli G., Cerini A., Visioli G., d'Ettorre G., Mastroianni C.M., et al. Retinal involvement and ocular findings in COVID-19 pneumonia patients. Sci. Rep. 2020;10(1):17419. doi: 10.1038/s41598-020-74446-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing H., Li Z., Yang Z., Shi M., Huang Z., Song J., et al. The possibility of COVID-19 transmission from eye to nose. Acta Ophthalmol. 2020;98(3):e388. doi: 10.1111/aos.14412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu J.-Y., Xie H.-T., Zhang M.-C. Evidence of SARS-CoV-2 transmission through the ocular route. Clin. Ophthalmol. 2021;687–96 doi: 10.2147/OPTH.S295283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranzenigo M., Bruzzesi E., Galli L., Castagna A., Ferrari G. Symptoms and signs of conjunctivitis as predictors of disease course in COVID-19 syndrome. J. Ophthalmic Inflamm. Infect. 2021;11(1):35. doi: 10.1186/s12348-021-00264-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold A., Tzankov A., Matter M.S., Mihic-Probst D., Scholl H.P.N., Meyer P. Ocular Pathology and Occasionally Detectable Intraocular Severe Acute Respiratory Syndrome Coronavirus-2 RNA in Five Fatal Coronavirus Disease-19 Cases. Ophthalmic Res. 2021;64(5):785–792. doi: 10.1159/000514573. [DOI] [PubMed] [Google Scholar]
- Renu K., Prasanna P.L., Gopalakrishnan A.V. Coronaviruses pathogenesis, comorbidities and multi-organ damage–A review. Life Sci. 2020;255 doi: 10.1016/j.lfs.2020.117839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riotto E., Megevand V., Megevand A., Marti C., Pugin J., Stangos A.N., et al. Retinal Manifestations in Patients with COVID-19: A Prospective Cohort Study. J. Clin. Med. 2022;11(7) doi: 10.3390/jcm11071828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Ares T., Lamas-Francis D., Trevino M., Navarro D., Cea M., Lopez-Valladares M.J., et al. SARS-CoV-2 in Conjunctiva and Tears and Ocular Symptoms of Patients with COVID-19. Vision (basel) 2021;5(4) doi: 10.3390/vision5040051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokohl A.C., Loreck N., Wawer Matos P.A., Zwingelberg S., Augustin M., Dewald F., et al. More than loss of taste and smell: burning watering eyes in coronavirus disease 2019. Clin. Microbiol. Infect. 2020;26(11) doi: 10.1016/j.cmi.2020.08.018. 1560 e5-e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaranayake L.P., Fakhruddin K.S., Ngo H.C., Chang J.W.W., Panduwawala C. The effectiveness and efficacy of respiratory protective equipment (RPE) in dentistry and other health care settings: a systematic review. Acta Odontol. Scand. 2020;78(8):626–639. doi: 10.1080/00016357.2020.1810769. [DOI] [PubMed] [Google Scholar]
- Scalinci S.Z., Trovato B.E. Conjunctivitis can be the only presenting sign and symptom of COVID-19. Idcases. 2020;20:e00774. doi: 10.1016/j.idcr.2020.e00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seah I., Agrawal R. Can the coronavirus disease 2019 (COVID-19) affect the eyes? A review of coronaviruses and ocular implications in humans and animals. Ocul. Immunol. Inflamm. 2020;28(3):391–395. doi: 10.1080/09273948.2020.1738501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal G., Bal P., Bal B., Chopra R. Pattern of ocular manifestations and the prevalence of severe acute respiratory syndrome coronavirus-2 in tears of hospitalized coronavirus disease 2019 patients. Taiwan J. Ophthalmol. 2021;11(4):380–385. doi: 10.4103/tjo.tjo_40_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen M., Honavar S.G., Sharma N., Sachdev M.S. COVID-19 and Eye: A Review of Ophthalmic Manifestations of COVID-19. Indian J. Ophthalmol. 2021;69(3):488–509. doi: 10.4103/ijo.IJO_297_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sezgin Akcay B.I., Kardes E., Kiray G., Ayaz B., Karakus Hacioglu G., Pala E. Evaluation of ocular symptoms in COVID-19 subjects in inpatient and outpatient settings. Int. Ophthalmol. 2021;41(4):1541–1548. doi: 10.1007/s10792-021-01728-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh N., Al Mahdi H., Pai A., Pathare A., Abujaber A.A., Dsliva A., et al. Ocular manifestations of COVID-19: facts and figures from a tertiary care center. Ann. Med. 2022;54(1):310–313. doi: 10.1080/07853890.2022.2029554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Yadav P., Shekhawat P., Lunia G., Taneja Y., Shekhawat K. Spectrum of Ocular Manifestations in SARS COV–2 Patients at Tertiary Care Center. Eur. J. Mol. Clinical Med. 2022:5761–5768. [Google Scholar]
- Shen J., Wu J., Yang Y., Wang P., Luo T., Guo Y., et al. The paradoxical problem with COVID-19 ocular infection: Moderate clinical manifestation and potential infection risk. Comput. Struct. Biotechnol. J. 2021;19:1063–1071. doi: 10.1016/j.csbj.2021.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira A.K.T., Lynch M.I., Medeiros C.S.L., Moraes B.T., Remigio M.C., Paiva M.M.F., et al. Ophthalmological findings in patients suspected with COVID-19 at a tertiary hospital in Pernambuco. Brazil. Arq Bras Oftalmol. 2022;85(6):614–619. doi: 10.5935/0004-2749.20220083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindhuja K., Lomi N., Asif M.I., Tandon R. Clinical profile and prevalence of conjunctivitis in mild COVID-19 patients in a tertiary care COVID-19 hospital: A retrospective cross-sectional study. Indian J. Ophthalmol. 2020;68(8):1546–1550. doi: 10.4103/ijo.IJO_1319_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltani S., Zandi M., Ahmadi S.E., Zarandi B., Hosseini Z., Akhavan Rezayat S., et al. Pooled Prevalence Estimate of Ocular Manifestations in COVID-19 Patients: A Systematic Review and Meta-Analysis. Iran J. Med. Sci. 2022;47(1):2–14. doi: 10.30476/ijms.2021.89475.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan S., Cohen F., Salviat F., Thevenin S., Devys J.M., Cochereau I., et al. Evaluation of the impact of intensive care support for COVID-19 on the ocular surface in a prospective cohort of 40 eyes. Ocul. Surf. 2021;22:13–14. doi: 10.1016/j.jtos.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan K.H., Lui G.C.Y., Poon K.C.F., Ng S.S.S., Young A.L., Hui D.S.C., et al. Ocular surface disturbance in patients after acute COVID-19. Clin. Exp. Ophthalmol. 2022;50(4):398–406. doi: 10.1111/ceo.14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Yang S., Zhang Y., Zhang X., Jiang Y., Wang X., et al. Symptoms of Dry Eye Disease in Hospitalized Patients with Coronavirus Disease 2019 (COVID-19) J. Ophthalmol. 2021;2021:2678706. doi: 10.1155/2021/2678706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. J. Am. Med. Assoc. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- Wu P., Duan F., Luo C., Liu Q., Qu X., Liang L., et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmology. 2020;138(5):575–578. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y., Wang K., Zhu Y., Lyu D., Yu Y., Li S., et al. Ocular manifestations in COVID-19 patients: A systematic review and meta-analysis. Travel Med. Infect. Dis. 2021;44 doi: 10.1016/j.tmaid.2021.102191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Duan C., Zeng Y., Tong Y., Nie Y., Yang Y., et al. Ocular Findings and Proportion with Conjunctival SARS-COV-2 in COVID-19 Patients. Ophthalmology. 2020;127(7):982–983. doi: 10.1016/j.ophtha.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Xu Z., Castiglione G.M., Soiberman U.S., Eberhart C.G., Duh E.J. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocul. Surf. 2020;18(4):537–544. doi: 10.1016/j.jtos.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.