Abstract
Serologic parameters of cat scratch disease (CSD) were evaluated by Western blot analysis. Sera from patients with serologically confirmed CSD antigen were screened for immunoglobulin (Ig) isotype-specific as well as IgG subclass-specific reactivity against Bartonella henselae whole-cell antigen. Bartonella-negative control sera were used to determine baseline antibody activity. Heterogeneous B. henselae-specific IgG reactivity with numerous protein bands, ranging from >150 to <17 kDa, was observed. Though individual banding patterns were variable, one approximately 83-kDa B. henselae protein (Bh83) was immunoreactive with all CSD sera tested, suggesting it is a conserved antigen during infection. Bh83 was not recognized by reference human antisera against Rickettsia rickettsii, Chlamydia group positive, Treponema pallidum, Orientia tsutsugamushi, Fransciscella tularensis, Ehrlichia chaffeensis, Mycoplasma pneumoniae, and Escherichia coli, although other cross-reactive proteins were evident. Significantly, CSD sera failed to recognize the 83-kDa protein when tested against Bartonella quintana antigen, though sera from B. quintana-infected patients did react to Bh83. This cross-reactivity suggests epitope conservation during infection with B. henselae or B. quintana. Western blot analysis further revealed similar banding patterns when B. henselae was reacted against the Ig isotypes IgG and IgG1 and both secretory and alpha chains of IgA. Neither IgM nor IgE reacted significantly to Bartonella antigen by our Western blot analysis. Dissection of the antibody response at the IgG subclass level indicated that prominent antigen recognition was limited to IgG1. These observations provide insight into induced immunity during CSD and provide evidence for conserved epitope expression during infection with B. henselae or B. quintana.
The spectrum of human disease and pathologic syndromes observed to be associated with Bartonella henselae, an alpha-2 proteobacterium, has been progressively expanding since its identification in 1992 (18, 27). Granulomatous and vasculoproliferative diseases stemming from this emerging pathogen have since been described in both immunosuppressed and immunocompetent patients. Implicated in the etiology of cutaneous bacillary angiomatosis, bacillary hepatic peliosis and its parenchymal variant, endocarditis, and fever with persistent bacteremia (2, 9, 10, 12, 23, 24, 28), B. henselae is most notably recognized for its role as the primary etiologic agent of cat scratch disease (CSD) (5, 14). Afflicting an estimated 24,000 persons in the United States annually (8), CSD is characterized by a broad range of clinical symptoms manifested in varying degrees of severity depending largely on the immune status of the host. Infected patients present with subacute regional lymphadenopathy after inoculation, low-grade fever, anorexia, and malaise. Such manifestations are typically self-limiting and resolve untreated within several weeks in the immunocompetent host. It has become clear, though, that individuals with a depressed cellular immune response succumb to more-severe, atypical manifestations of CSD, including systemic complications of multiorgan involvement, particularly of the spleen and liver, and involvement of the central nervous system (2, 21, 28). Although B. henselae is a cause of human disease with a wide spectrum of severity, little is known regarding pathogenicity and immunity induced during infection.
B. henselae is a fastidious, gram-negative bacillus that may require an incubation period as long as 5 weeks to culture axenically. Consequently, serologic methods, such as indirect fluorescent-antibody assay (IFA) and enzyme immunoassay (EIA), have been the most-practical and least-invasive means of clinical diagnosis (3, 19). Widely accepted as a diagnostic assay, IFA is routinely used to confirm B. henselae infection (4). However, when the whole bacterial cell antigen is used, IFA is unable to differentiate species-specific serologic reactivity from cross-reactivity with other antigens of phylogenetic proximity, namely, Bartonella quintana (4, 11). Modifications to improve the efficacy of serologic detection methods are pending a more-comprehensive understanding of the factors influencing both the pathogenesis of infection due to B. henselae and the evoked human immune response.
The purpose of this study was to dissect the humoral immune response to B. henselae antigen in patients with clinically and laboratory-diagnosed CSD (positive by IFA) by Western blot analysis. In evaluation of the B. henselae proteins recognized following infection, an 83-kDa immunodominant protein was identified that was recognized by all seropositive patient samples tested. Furthermore, we have provided an in-depth characterization of the immunoglobulin (Ig) isotype and IgG subclass response in CSD patients. The findings, which elucidate serologic responses to B. henselae infection, provide insight into the immunity induced by this pathogen.
(This work was presented in part at the 13th Sesqui-Annual Meeting of the American Society for Rickettsiology [abstract 14], September 1997, Champion, Pa.)
MATERIALS AND METHODS
Human sera.
Human sera were selected nationwide from among suspected CSD patient samples submitted to the Centers for Disease Control and Prevention (CDC, Atlanta, Ga.) for confirmative diagnosis. Sera were stored at 4°C and heat inactivated at 56°C prior to serologic testing. Evidence of infection with B. henselae and/or B. quintana was determined by IFA as previously described (4, 19). Test samples (n = 54) were selected for this study on the basis of seropositivity (IFA IgG titers of ≥64) to both B. henselae and B. quintana, with titers ranging from 64 to 8,193 (Table 1). Sera with negative IFA titers (IgG titers of ≤32) to Bartonella spp. (n = 15) were used as controls. The sera used in the figures of Western blots are representative of the reactivity seen in all sera tested. Human antisera against the following bacterial strains obtained from the reference serum bank of the CDC or the CDC rickettsial zoonoses laboratory stocks were tested for cross-reactivity to B. henselae as follows: Rickettsia rickettsii (spotted fever group), Chlamydia group positive sera (≥1:32; CDC no. CS0022), Treponema pallidum (CDC no. BS1505 and BS30612), Orientia tsutsugamushi (scrub typhus agent), Fransciscella tularensis (≥1:320; CDC no. BS0864), Ehrlichia chaffeensis, Mycoplasma pneumoniae (CDC no. MS2204), and Escherichia coli. PCR-confirmed B. quintana-infected human sera (n = 4) were also included.
TABLE 1.
Human serum specimens received at the CDC for diagnosis of CSD by IFA
| Serum no. | IFA titera for:
|
|
|---|---|---|
| B. henselae | B. quintana | |
| S1 | 31 | 31 |
| S2 | 31 | 31 |
| S3 | 128 | 128 |
| S4 | 64 | 512 |
| S5 | 512 | 8,192 |
| S6 | 31 | 31 |
| S7 | 8,192 | 8,192 |
| S8 | 8,193 | 8,193 |
| S9 | 8,193 | 8,193 |
| S10 | 31 | 31 |
| S11 | 31 | 31 |
| S12 | 2,048 | 8,192 |
| S13 | 2,048 | 8,192 |
| S14 | 512 | 512 |
| S15 | 8,192 | 8,193 |
| S16 | 8,193 | 8,193 |
| S17 | 31 | 31 |
| S18 | 2,048 | 2,048 |
Titers are reported as the reciprocal of serum dilutions; 31 is the convention for <32. An intense signal at 8,192 (the highest dilution tested) is considered to be >8,192. Thus, 8,193 is the convention for >8,192.
Antigen preparation.
Strains of B. henselae (Houston-1) or B. quintana (OK-90-268) were cultivated on heart infusion agar supplemented with 5% defibrinated rabbit blood (BBL, Cockeysville, Md.). Plates inoculated with Bartonella were incubated for 3 to 5 days at 32°C in the presence of 5% CO2. Bacterial cells were harvested and suspended in brain heart infusion media by gently scraping plates with a sterile loop. The cells were then collected via centrifugation and suspended in phosphate-buffered saline solution (PBS). CFU of harvested Bartonella cultures were titrated on blood agar plates before being inactivated by gamma irradiation (5 × 105 rad) and stored at −70°C until use.
For cell culture-derived antigen, B. henselae or B. quintana cells were cocultivated with antibiotic-free Vero cell monolayers maintained in MEM complete medium (minimal essential medium supplemented with l-glutamine, HEPES buffer, and 10% fetal calf serum). Vero cell monolayers were inoculated with 106 CFU of Bartonella. Cell cultures were incubated at 32°C with 5% CO2 for 2 to 4 days postinoculation. At harvest, the medium was discarded, and cocultivated cells were collected by gentle rocking with glass beads to detach Vero cells. Cultures were inactivated by gamma irradiation and stored at −70°C prior to use.
Western blotting.
Whole-cell lysate suspensions of Bartonella were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis as described below. Protein concentration of B. henselae stock lysates was determined by a bicinchoninic acid protein reagent assay (Pierce, Rockford, Ill.), with bovine serum albumin as a standard. Cell proteins (∼7.5 mg/ml) from a 125-μl aliquot of whole-cell agar-grown Bartonella antigen were then centrifuged for 10 min at 13,000 rpm. The cell pellet was solubilized and lysed in 1X Tris-glycine SDS sample buffer (Novex, San Diego, Calif.) and 10% beta-mercaptoethanol for 10 min at 100°C. The resulting suspension was separated by SDS-PAGE in a 4 to 20% gradient polyacrylamide Tris-glycine single-well minigel (Novex) for 2 h at 120 V. A prestained broad-range molecular weight protein marker (Bio-Rad) was used as a standard. Following electrophoresis, proteins were electrophoretically transferred to a 0.45-μm-pore-size nitrocellulose membrane (Bio-Rad) for 2 h at 90 V in transfer buffer (Novex). Membranes were then blocked overnight at 4°C in PBS containing 0.1% Tween 20 (PBST) and 5% skim milk. Membranes were subsequently washed four times, for 10 min each, in PBST and incubated with a 1:100 dilution of the test sera in PBST-5% skim milk solution for 1 h at room temperature by using a Mini Protean II Multiscreen System (Bio-Rad). After four additional washes in PBST, bound antibody was reacted with a 1:5,000 dilution of horseradish peroxidase-labeled anti-human Ig (Kirkegaard & Perry, Gaithersburg, Md.) of one of the following: IgG (heavy and light chains), IgG1, IgG2, IgG3, IgG4, IgM, IgE, IgA (secretory), and IgA (alpha) diluted in PBST-10% skim milk and incubated for 1 h at room temperature. Membranes were then washed as before, and antigens were detected with a TMB (3,3′, 5,5′ tetramethyl benzidine) membrane substrate developer (Kirkegaard & Perry).
RESULTS
Western blot for IgG.
Sixty-nine human serum specimens were used, 54 of which were derived from patients with laboratory-diagnosed Bartonella infection, as indicated by IFA seropositivity to B. henselae or B. quintana antigens; the remaining 15 samples were from control patients for whom negative IFA results were obtained. Sera included in the figures are listed in Table 1, with corresponding IFA titers to both B. henselae and B. quintana.
Western blotting Vero cell culture-derived, as well as blood agar-derived, B. henselae antigen yielded multiple bands predominantly in a range of 17 kDa to greater than 150 kDa (Fig. 1), with one band occurring at ∼6 kDa. A particular concentration of bands was noted in the 50- to 150-kDa region of the membrane. CSD sera visually react to approximately 10 to 15 B. henselae-specific agar-prepared antigenic proteins. Banding patterns exhibit variability depending on individual serum reactivity. In addition to total IgG (heavy and light chains), B. henselae antigen was reacted against sera from CSD patients for detection of the following IgG-specific fragments: IgG (F[ab]), IgG (F[ab′]2), and IgG (Fc). Each of these tests for IgG components yielded immunoblot patterns indistinguishable from those of total IgG (heavy and light chains) (data not shown).
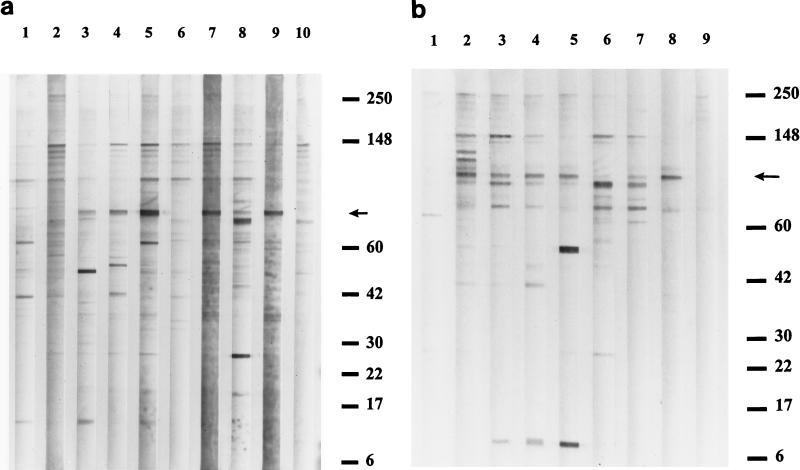
FIG. 1.
(a) Western blot analysis of serum IgG (heavy and light chains) activity reacting with SDS-PAGE-separated proteins of B. henselae whole-cell antigen cocultivated with Vero cells. Lane numbers are indicated at the top; serum reference numbers are provided below, and corresponding IFA results are listed in Table 1. Lanes 1, 2, 6, and 10, IgG activity in control sera from patients with negative Bartonella IFA titers (sera no. S1, S2, S6, S10 respectively). Lanes 3, 4, 5, 7, 8, 9, IgG recognition of antigenic proteins by serum from patients with IFA titers positive for CSD (sera no. S3, S4, S5, S7, S8, S9 respectively). (b) IgG Western blot reacting with B. henselae whole-cell antigen prepared on rabbit blood agar plates. Lanes 1 and 9, antibody activity in seronegative control specimens (sera no. S11 and S17 respectively). Lanes 2 through 8, antigen recognition revealed by CSD-positive patient serum IgG (sera no. S12, S13, S14, S15, S8, S16, S9 respectively). Positions of molecular size standards (in kilodaltons) are indicated at the right. An arrow in each panel denotes the location of Bh83, recognized exclusively by all CSD IFA-positive sera tested.
Analysis of differences between serum reactivity to blood agar-derived and Vero cell-derived B. henselae antigen revealed that Vero cell-derived antigen yielded more numerous bands than agar-grown antigen. However, this result may have been caused by reactivity against Vero protein alone (see Discussion). With regard to B. henselae-specific reactivity, differences between antigen preparations were unremarkable by SDS-PAGE and Western blot analysis.
Despite heterogeneous reactivity to B. henselae antigen, an approximately 83-kDa protein was immunoreactive with all human sera that were positive by IFA analysis (Fig. 1). Bh83 was not recognized in Western blots by any of the IFA seronegative sera tested (Fig. 1a, lanes 1, 2, 6, and 10 and Fig. 1b, lanes 1 and 9).
Serologic reactivity with other pathogens.
Significant cross-reactivity was observed from all sera tested (Fig. 2). Recognition of 10 to 15 B. henselae bands was observed with all antisera tested (data for E. chaffeensis, M. pneumonia, and E. coli not shown). Two Rickettsia group antisera demonstrated the least amount of cross-reactivity with B. henselae antigen; in particular, the spotted fever group Rickettsia (R. rickettsii) yielded only weak activity with two bands in the 200-kDa region. T. pallidum and Chlamydia each reacted strongly to B. henselae antigens in the range of approximately 45 and 75 kDa, respectively. Despite the extensive recognition of numerous antigenic proteins, however, none of the cross-reactive bacterial antisera recognized the 83-kDa band of B. henselae.
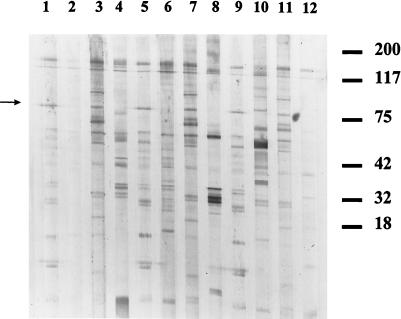
FIG. 2.
Western blot analysis of IgG (heavy and light chains) of various sera reacting with other bacterial pathogens for reactivity to B. henselae agar-derived antigen. Lanes 1, 3, 5, 7, 9, and 11, IFA-positive CSD sera; lane 2, R. rickettsii; lane 4, F. tularensis; lane 6, F. tularensis negative control; lane 8, T. pallidum; lane 10, Chlamydia; lane 12, Rickettsia prowazekii. Positions of molecular size standards (in kilodaltons) are indicated at the right. An arrow denotes the location of Bh83.
Antibody type reactivity.
Western blot analysis of human serum reactivity against whole-cell B. henselae antigen indicates a strong IgG reaction of a heterogeneic nature against total antigen, with a specific reaction to Bh83 (Fig. 1). To determine which human subclasses of IgG were responsible for these interactions, Western blot assays specific for IgG1, IgG2, IgG3, and IgG4 were performed. Classes and subclasses of Igs reactive with B. henselae are summarized in Table 2. Despite multiple-banding patterns in assays detecting total IgG against B. henselae antigen (Fig. 1), little B. henselae reactivity was evidenced by either IgG2, IgG3, or IgG4. A prominent reaction against B. henselae antigen among the IgG subclasses tested was limited to IgG1. Immunoblot banding patterns with anti-IgG1 conjugate were extremely similar to those produced when anti-total IgG (heavy and light chains) was used, suggesting that IgG1 is the primary IgG subclass induced during CSD infection.
TABLE 2.
Western blot analysis of antibody types against B. henselae antigen
| Antibody type | Reactivity against:
|
|
|---|---|---|
| B. henselaea | Bh83 | |
| Total IgG (heavy and light chains) | + | + |
| IgG1 | + | + |
| IgG2 | − | − |
| IgG3 | − | − |
| IgG4 | − | − |
| IgAb | + | + |
| IgM | − | − |
| IgE | − | − |
Multiple bands appear as with total IgG (heavy and light chains) analysis, as seen in Fig. 1.
Alpha chain- as well as secretory chain-specific activity.
In addition to dissection of the antibody response at the IgG subclass level, Western blot analysis was employed to detect differences among the Ig isotypes represented in the sera after a mounted immune response upon infection with B. henselae (Table 2). The presence of B. henselae-specific IgA (secretory chain), IgA (alpha chain), IgE, and IgM antibodies was determined in CSD sera. Both the secretory and alpha chains of IgA demonstrated multiband recognition of B. henselae whole-cell antigen in 15 IFA-seropositive samples tested. IgA reactivity varied with sera tested, being heterogeneous with similar protein band reactivity compared with total IgG. Despite the lesser degree of antigen recognition, however, the 83-kDa B. henselae protein (Bh83) was recognized by IgA antibodies in all CSD-positive sera tested. IgM recognition of B. henselae antigen was essentially absent, with only two very faint bands in 2 of the 25 CSD IFA-positive serum samples tested. Additionally, IgE antibodies against B. henselae antigen were absent in 25 CSD IFA-positive serum samples tested.
Cross-reactivity between B. henselae and B. quintana.
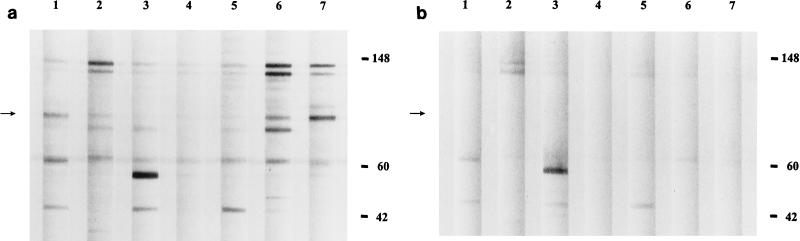
Whole-cell B. henselae and B. quintana have been repeatedly shown to cross-react by IFA, presumably due to close phylogenetic relatedness. We endeavored to assess the level of cross-reactivity between these two Bartonella species by Western blot analysis. The experimental design allowed for the crossing of the following four variables: B. henselae antigen, B. quintana antigen, B. henselae-positive sera, and B. quintana-positive sera (PCR confirmed). Reflecting the outcomes of repeated IFAs, each reaction yielded cross-reactivity to some degree. Sera from PCR-confirmed B. quintana infections reacted with B. henselae and to a lesser extent with B. quintana antigen preparations by Western blotting (Fig. 3). Included among the B. henselae immunoreactive antigens recognized by B. quintana antisera was Bh83 (Fig. 3a, lanes 1 to 4). However, when B. quintana antigen preparations were screened with either B. henselae- or B. quintana-reactive human sera, reaction to the 83-kDa protein was absent (Fig. 3b). B. quintana antigen, therefore, appears to lack expression of an 83-kDa antigen. Despite the absence of reactivity of B. henselae-positive sera to an 83-kDa protein in B. quintana antigen, other B. quintana antigens between 6 and 200 kDa in size do react, supporting the cross-reactivity seen by IFA.
FIG. 3.
Western blot analysis of IgG (heavy and light chains) of IFA-positive CSD sera (lanes 1 to 7) reacted against B. henselae (a) and B. quintana (b) antigens. Lanes 1 to 4 contain sera from patients with PCR-confirmed B. quintana infection. Positions of molecular size standards (in kilodaltons) are indicated at the right. An arrow in each panel denotes the location of Bh83.
DISCUSSION
The analysis of human humoral immune responses to B. henselae infection has been expanded by performing Western blot analysis on sera from patients with CSD. In addition to identifying reactive B. henselae antigens, the involvement of Ig classes and subclasses in the human humoral response to Bartonella infection was assessed. Since current IFAs for the diagnosis of CSD are based on IgG levels in the sera, total IgG reactivity against both Vero cell-grown and agar-grown B. henselae antigen by immunoblot analysis was initially tested. These data indicate that human humoral responses to B. henselae infection regarding protein reactivity vary from patient to patient. Similar to other reports, proteins in the range of 50 to 200 kDa were observed (8, 14). This range of B. henselae-reactive proteins compares with previously recognized proteins of approximately 17, 48, 69, 97, and 116 kDa and with multiple protein bands at approximately 200 kDa (1, 7, 13, 16, 26). However, CSD serum reactivity to these proteins did not occur in all samples tested in this study. Additionally, with some proteins, such as the multiple bands at approximately 200 kDa and the 116-kDa proteins described by Litwin et al. (13), reactivity was observed with our IFA-negative control sera as well (Fig. 1). In contrast, one protein, described here as Bh83, has been reactive with all IFA-positive human sera tested to date, while it is not recognized with IFA-negative control sera (Fig. 1). In addition, the antigen preparation may affect the number of reactive bands observed. In fact, this study reveals that Vero-derived antigen yields more numerous bands than agar-derived antigen (Fig. 1a versus b). This may be the result of nonspecific reactivity of human sera to Vero cell protein alone, since Bartonella IFA-negative sera show reactivity to bands in the Vero-derived antigen preparations that are absent in agar-derived antigen preparations (Fig. 1a, lane 10 versus Fig. 1b, lane 9). This, however, does not alter B. henselae-specific band reactivity in these preparations.
Serologically, cross-reactivity between Bartonella species and other bacterial pathogens has been reported (6, 11, 15). Analyses of whole-cell B. henselae antigen reactivity against antisera to R. rickettsii (spotted fever group), Chlamydia group, T. pallidum, O. tsutsugamushi, F. tularensis, E. chaffeensis, M. pneumoniae, and E. coli demonstrated extensive cross-reactivity, with complex banding patterns, including recognition of multiple B. henselae proteins (Fig. 2). Interestingly, despite the seemingly high cross-reactivity to B. henselae proteins, none of these CDC reference sera recognized Bh83.
In addition to intergenus cross-reactivity, cross-reactivity between B. henselae and B. quintana has been extensively described (4, 11). The results of our studies reflect the cross-reactivity observed in previous IFAs and EIAs. This cross-reaction includes B. quintana sera (PCR positive for B. quintana) reacting with B. henselae antigen in a heterogeneous manner, including activity against Bh83 (Fig. 3). However, positive CSD sera (positive to B. henselae antigen, including activity against Bh83) does not detect an 83-kDa protein in B. quintana antigen preparations. In fact, even when screened with B. quintana-positive sera, B. quintana antigen preparations fail to exhibit an 83-kDa protein. This pattern of reactivity may indicate a common component of in vivo gene expression of Bh83, since both B. henselae- and B. quintana-positive sera contain antibodies reactive with this protein, whereas B. quintana antigen fails to express it (Fig. 3). This suggests at least two possible scenarios. The first is that despite a lack of Bh83 expression in B. quintana antigen, B. quintana does express Bh83 in vivo, resulting in a Bh83-specific humoral response. The second is that B. quintana contains an allelic gene which expresses a protein possessing antigenic attributes similar to those of Bh83 but a different molecular weight. Further analysis of gene expression may help to elucidate the antigen expression from B. henselae and B. quintana in vivo and in vitro.
In comparing CSD serum reaction to B. henselae antigen by IFA and Western blot analysis, a correlation may be made regarding reactivity. However, neither relative strength nor number of reactive bands is directly correlative to either of the Bartonella IFA titer values obtained for each patient. For example, a comparison of the immunoreactivities of sera S14 (IFA Bartonella titers both 512) and S16 (IFA titers both >8,192) in Fig. 1b reveals that higher-titer sera do not necessarily correspond to stronger or more-numerous bands relative to lower-titer sera. These data suggest that IFA remains favorable for Western blot analysis in the determination of IgG titer activity. It may be that the proteins responsible for IFA-positive activity are limited to a subset of outer membrane proteins that react upon Western blot analysis of all sera but to a varying degree regarding total IgG activity.
In this study we further characterized the humoral immunological response to whole-cell B. henselae antigen by analyzing the distribution of IgG subclass antibodies in the sera of CSD patients. As the four antibody subclasses comprising human IgG are antigenically distinct, with differing physiochemical properties conferring different biological functions, including complement-fixing activity and interaction with other proteins (17), assessment of IgG subclass production in association with B. henselae infection may be indicative of disease activity and pathogenicity. In fact, the selective distribution of IgG subclasses expressed during humoral immune responses can provide details of the biological nature of the specific antigens against which the response is targeted. It is known, for example, that bacterial protein antigens preferentially induce IgG1 antibodies in human T cell-dependent responses, with concordant variable levels of amplification of IgG3 and IgG4 (17). In contrast, elicitation of antibodies in T cell-independent responses to polysaccharide antigens, such as lipopolysaccharide, is largely restricted to the IgG2 subclass of IgG (17). Western blot analysis here indicates that in addition to activity with total IgG, reaction to B. henselae total antigen, and specifically to Bh83, appears to be primarily isolated to the IgG1 isotype of IgG (Table 2). IgG2, IgG3, and IgG4 did not react significantly to B. henselae antigen (Table 2). The fact that the IgG antibodies present in the sera of our CSD patients were predominantly of the IgG1 subclass with no evidence of IgG2 production suggests that the humoral response to B. henselae is directed against protein domains of the organism rather than against carbohydrate antigens. In addition to IgG1, the presence of reactive IgA in patient sera may be an indication of a mucosal component of B. henselae immunity, particularly since secretory IgA was detected (Table 2). In contrast to results for IgG and IgA, we failed to detect B. henselae-specific serum IgM, although it has been described by others in cases of CSD (13, 22, 25, 29). However, the lack of IgM antibodies coupled with the presence of IgG may suggest a convalescent status of our infected serum samples.
IgG1 and IgG3 are the predominant IgG subclasses involved in functions such as complement activation (primarily IgG3), mediation of antibody-dependent cytotoxicity, and attachment to cell membranes through Fc receptors (opsonization, primarily IgG1), which ultimately enhance phagocytosis (17). A lack of IgG3 and high levels of IgG1 activity against B. henselae in CSD infection suggest increased opsonization activity during CSD rather than complement fixation. In fact, Bartonella-specific sera failed to enhance antibody-mediated complement fixation in vitro compared with nonimmune sera (20), supporting a lack of B. henselae-specific IgG3. As IgG1 represents the most-abundant subclass in healthy human serum, comprising 65% of total IgG antibodies (17), it is possible that these intrinsic antibodies are sufficient to activate complement and commence the cascade leading to bactericidal activity in vitro. If so, preexisting IgG1 antibody levels in the sera may play a role as a determinant of clinical disease severity, duration, and symptomatology in CSD. This may fit particularly well considering the atypical severe disease associated with immunocompromised, B. henselae-infected patient status. The anti-B. henselae IgG1 subclass may be involved in immunity against CSD by participation in the aforementioned functions. However, this does not preclude the potential role played by other arms of the human immune response, such as production of specific secretory IgA at the mucosal level or cellular-mediated immune mechanisms in protection against CSD.
Historically, a role for cellular-mediated immunity in CSD has been apparent. Results of the Hanger-Rose skin test, entailing the intradermal injection of inactivated B. henselae antigen for use in traditional CSD diagnostics, have indicated the presence of cellular-mediated delayed-type hypersensitivity in 95 to 98% of infected individuals (25). Furthermore, the development of severe disease involving internal organs, such as the spleen and liver, and the central nervous system among predominantly immunocompromised patients makes it unlikely that CSD progresses without cellular immune induction. The relative importance of humoral and cellular-mediated immunity in protection against CSD remains unclear. Perhaps a combination of IgG1-mediated opsonization and T-cell effector activation is required for clearance of B. henselae and the elimination of disease. Continued progress in immune characterization of CSD is critical in our efforts to decipher the complex nature of B. henselae infections.
REFERENCES
- 1.Anderson B E, Lu E, Jones D C, Regnery R L. Characterization of a 17-kilodalton antigen of Bartonella henselae reactive with sera from patients with cat scratch disease. J Clin Microbiol. 1995;33:2358–2365. doi: 10.1128/jcm.33.9.2358-2365.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson B E, Neuman M A. Bartonella spp. as emerging human pathogens. Clin Microbiol Rev. 1997;10:203–219. doi: 10.1128/cmr.10.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barka N E, Hadfield T, Patnaik M, Schwartzman W A, Peter J B. EIA for detection of Rochalimaea henselae-reactive IgG, IgM, and IgA antibodies in patients with suspected cat-scratch disease. J Infect Dis. 1993;167:1503–1504. doi: 10.1093/infdis/167.6.1503. [DOI] [PubMed] [Google Scholar]
- 4.Dalton M J, Robinson L E, Cooper J, Regnery R L, Olson J G, Childs J E. Use of Bartonella antigens for serologic diagnosis of cat-scratch disease at a national referral center. Arch Intern Med. 1995;155:1670–1676. [PubMed] [Google Scholar]
- 5.Dolan M J, Wong M T, Regnery R L. Syndrome of Rochalimaea henselae adenitis suggesting cat scratch disease. Ann Intern Med. 1993;118:331–336. doi: 10.7326/0003-4819-118-5-199303010-00002. [DOI] [PubMed] [Google Scholar]
- 6.Drancourt M, Mainardi J L, Brouqui P, Vandenesch F, Carta A, Lehnert F, Etienne J, Goldstein F, Acar J, Raoult R. Bartonella (Rochalimaea) quintana endocarditis in three homeless men. N Engl J Med. 1995;332:419–423. doi: 10.1056/NEJM199502163320702. [DOI] [PubMed] [Google Scholar]
- 7.Engbæk K, Uttenthal L O, Koch C. Immunopurified extracellular Bartonella henselae antigen for detecting specific antibodies by enzyme immunoassay. APMIS. 1997;105:941–950. [PubMed] [Google Scholar]
- 8.Jackson L A, Perkins B A, Wenger J D. Cat scratch disease in the United States: an analysis of three national databases. Am J Public Health. 1993;83:1707–1711. doi: 10.2105/ajph.83.12.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koehler J E. Bartonella infections. Adv Ped Infect Dis. 1996;11:1–27. [PubMed] [Google Scholar]
- 10.Koehler J E, Quinn F D, Gerger T G, LeBoit P E, Tappero J W. Isolation of Rochalimaea species from cutaneous and osseous lesions of bacillary angiomatosis. N Engl J Med. 1992;327:1625–1631. doi: 10.1056/NEJM199212033272303. [DOI] [PubMed] [Google Scholar]
- 11.La Scola B, Raoult D. Serological cross-reactions between Bartonella quintana, Bartonella henselae, and Coxiella burnetii. J Clin Microbiol. 1996;34:2270–2274. doi: 10.1128/jcm.34.9.2270-2274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liston T E, Koehler J E. Granulomatous hepatitis and necrotizing splenitis due to Bartonella henselae in a patient with cancer: case report and review of hepatosplenic manifestations of Bartonella infection. Clin Infect Dis. 1996;22:951–957. doi: 10.1093/clinids/22.6.951. [DOI] [PubMed] [Google Scholar]
- 13.Litwin C M, Martins T B, Hill H R. Immunologic response to Bartonella henselae as determined by enzyme immunoassay and Western blot analysis. Am J Clin Pathol. 1997;108:202–209. doi: 10.1093/ajcp/108.2.202. [DOI] [PubMed] [Google Scholar]
- 14.Loutit J S. Bartonella infections. Curr Clin Top Infect Dis. 1997;17:269–290. [PubMed] [Google Scholar]
- 15.Maurin M, Eb F, Etienne J, Raoult D. Serological cross-reactions between Bartonella and Chlamydia species: implications for diagnosis. J Clin Microbiol. 1997;35:2283–2287. doi: 10.1128/jcm.35.9.2283-2287.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maurin M, Roux V, Stein A, Ferrier F, Viraben R, Raoult D. Isolation and characterization by immunofluorescence, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Western blot, restriction fragment length polymorphism-PCR, 16S rRNA gene sequencing, and pulsed-field gel electrophoresis of Rochalimaea quintana from a patient with bacillary angiomatosis. J Clin Microbiol. 1994;32:1166–1171. doi: 10.1128/jcm.32.5.1166-1171.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papadea C, Check I J. Human immunoglobulin G and immunoglobulin G subclasses: biochemical, genetic, and clinical aspects. Crit Rev Clin Lab Sci. 1989;27:27–58. doi: 10.3109/10408368909106589. [DOI] [PubMed] [Google Scholar]
- 18.Regnery R L, Anderson B E, Clarridge J E, Rodriguez-Barradas M C, Jones D C, Carr J H. Characterization of a novel Rochalimaea species, R. henselae sp. nov., isolated from blood of a febrile, human immunodeficiency virus-positive patient. J Clin Microbiol. 1992;30:265–274. doi: 10.1128/jcm.30.2.265-274.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Regnery R L, Olson J G, Perkins B A, Bibb W. Serological response to Rochalimaea henselae antigen in suspected cat-scratch disease. Lancet. 1992;339:1443–1445. doi: 10.1016/0140-6736(92)92032-b. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Barradas M C, Bandres J C, Hamill R J, Trial J, Clarridge J E, Baughn R E, Rossen R D. In vitro evaluation of the role of humoral immunity against Bartonella henselae. Infect Immun. 1995;63:2367–2370. doi: 10.1128/iai.63.6.2367-2370.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartzman W A, Patnaik P, Barka N E, Peter J B. Rochalimaea antibodies in HIV-associated neurologic disease. Neurology. 1994;44:1312–1316. doi: 10.1212/wnl.44.7.1312. [DOI] [PubMed] [Google Scholar]
- 22.Schwartzman W A, Patnaik M, Angulo F J, Visscher B R, Miller E N, Peter J B. Bartonella (Rochalimaea) antibodies, dementia, and cat ownership among men infected with human immunodeficiency virus. Clin Infect Dis. 1995;21:954–959. doi: 10.1093/clinids/21.4.954. [DOI] [PubMed] [Google Scholar]
- 23.Slater L N, Welch D F, Min K W. Rochalimaea henselae causes bacillary angiomatosis and peliosis hepatitis. Arch Intern Med. 1992;152:602–606. [PubMed] [Google Scholar]
- 24.Stewart B A. Human infection with Bartonella species. Clin Microbiol Infect. 1997;3:677–689. doi: 10.1111/j.1469-0691.1997.tb00478.x. [DOI] [PubMed] [Google Scholar]
- 25.Szelc-Kelly C M, Goral S, Perez-Perez G I, Perkins B A, Regnery R L, Edwards K M. Serologic responses to Bartonella and Afipia antigens in patients with cat scratch disease. Pediatrics. 1995;96:1137–1142. [PubMed] [Google Scholar]
- 26.Welch D F, Hensel D M, Pickett D A, San Joaquin V H, Robinson A, Slater L N. Bacteremia due to Rochalmiaea henselae in a child: practical identification of isolates in the clinical laboratory. J Clin Microbiol. 1993;31:2381–2386. doi: 10.1128/jcm.31.9.2381-2386.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welch D F, Pickett D A, Slater L N, Steigerwalt A G, Brenner D J. Rochalimaea henselae sp. nov., a cause of septicemia, bacillary angiomatosis, and parenchymal bacillary peliosis. J Clin Microbiol. 1992;30:275–280. doi: 10.1128/jcm.30.2.275-280.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong M T, Dolan M J, Lattuada C P, Regnery R L, Garcia M L, Mokulis E C, LaBarre R C, Ascher D P, Delmar J A, Kelly J W, Leigh D R, McRae A C, Reed J B, Smith R E, Melcher G P. Neuroetinitis, aseptic meningitis, and lymphadenitis associated with Bartonella (Rochalimaea) henselae infection in immunocompetent patients and patients infected with human immunodeficiency virus type 1. Clin Infect Dis. 1995;21:352–360. doi: 10.1093/clinids/21.2.352. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida H, Kusaba N, Omachi K, Miyazaki N, Yamawaki M, Tsuji Y, Nakahara K, Sumino M, Noudomi M, Shimokawa Y, Tanikawa K. Serological study of Bartonella henselae in cat scratch disease in Japan. Microbiol Immunol. 1996;40:671–673. doi: 10.1111/j.1348-0421.1996.tb01126.x. [DOI] [PubMed] [Google Scholar]