Abstract
Silver nanoparticles are the extensively utilized among all nanoparticles due to their antibacterial and wound healing properties making them highly suitable for medical and pharmaceutical applications. The field of nanoparticle toxicity is an emerging field and the present study aims to assess the biochemical, hematological and genotoxicity in Oreochromis mossambicus exposed to different concentrations of silver nanoparticles for 7 and 14 days. Silver nanoparticles were synthesized by reduction of silver nitrate using trisodium citrate and was characterized using X-ray diffraction, SEM, HRTEM and DLS. Hematological parameters like RBC, WBC, Hb, HCT and MCV and for biochemical analysis, antioxidant enzymes SOD, CAT and GPX and serum enzymes AST, ALT, ACP, ALP and LDH were analyzed. Genotoxicity was studied using comet assay. Results obtained showed decrease in erythrocytes, HCT, Hb and MCV while an increase was noted in WBC on day 7 and 14. The antioxidant enzymes SOD, CAT and GPx showed a decrease and the lipid peroxidation product MDA was elevated. The serum enzymes AST, ALT, ACP ALP and LDH showed an increased activity when compared to control. DNA damage was evident by an increase in % TDNA. The results indicate hematological, biochemical and genotoxicity of silver nanoparticles that might be mediated through ROS generation in O. mossambicus.
Keywords: silver nanoparticle, Oreochromis mossambicus, toxicity, genotoxicity, hematological, biochemical
Introduction
The rapid emergence and development of nanotechnology driven by growing markets for products that incorporate these materials, has intensified concerns regarding their potential impact on environment. Evaluation of nanomaterial toxicity is an essential prerequisite for the development of safer nanomaterials and the rapid expansion of nanotechnology industry. The unique chemistry of engineered nanoparticles makes it extremely difficult for the prediction of their mechanisms of toxicity and bioavailability is exceedingly challenging. Consequently, the creation of advanced robust and easily accessible risk assessment tools for emerging pollutants such as nanoparticle is imperative for determining the health risks of commercial nanomaterials, as well as providing industry with information of development of safer nanomaterials and products. To bridge the paucity of literature on the fate, bioavailability, and biological effects of nanomaterials within the environment it is imperative to construct appropriate experiments for testing and screening these nanomaterials. Nanoparticles reach the aquatic environment during production use and disposal.1 The uptake within non-target organisms like fish has been reported.2 Size and increased surface area are the two characteristic features of nanoparticles toxicity.3 Silver nanoparticles are extensively utilized due of their antibacterial activity and wound healing properties, rendering silver nanoparticles the most frequently utilized nanoparticle in medical and pharmaceutical applications.4 These nanoparticles are being used in food storage containers, detergents, liquid fabric softeners, wound dressings, cosmetics, fabrics and clothing, sporting goods etc.5 The extensive use of silver nanoparticle leads to large scale production that increases its discharge into aquatic and terrestrial environment.6
Silver nanoparticles pose potential risk to aquatic organisms, especially fish therefore are keen area of interest.7 Toxicity studies of silver nanoparticles show huge discrepancy owing to lack of characterization of the nanoparticles.8 The toxicity of silver nanoparticle is contingent upon transformation in biological and environmental media, surface oxidation, release of silver ions and its interaction with biological macromolecules.9 Nanoscale materials often have different surface to volume ratio and exponential increasing reactivity when compared to their bulk counterpart. The characterization of nanoparticles plays a major role in evaluating the toxicity of silver particles. In tilapia, silver nanoparticles exposure resulted in damage to fish gill tissue, leading to cellular impairment, elevated silver accumulation, oxidative stress, and modifications in enzymatic and non-enzymatic factors.10 Iswarya et al.11 discovered that silver nanoparticles synthesized with β-1,3-glucan binding protein enhanced wound healing, efficacy against bacterial infections, and immunity in fish. Nevertheless, exposure to silver nanoparticles in fish decreased enzyme activity and antioxidant levels in the liver and gills, leading to the accumulation of detrimental free radicals and disruption in the oxidative and antioxidant systems.12 The use of AgNPs caused genetic expression, antioxidant activity, and blood characteristic alterations in fish.13 While silver nanoparticles had minimal impact on tilapia performance, they reduced water pollution and provided protection against bacterial infection. It is important to note that silver nanoparticles can also cause inflammation and tissue damage.14 Exposure to silver nanoparticles resulted in a decrease in phagocytic activity, red blood cell count, and hematocrit, as well as changes in metallothionein expression in different organs.15 Sarkar et al.16 observed thinning of fish intestinal walls, swollen mucosal layer, and increased catalase expression when treated with silver nanoparticles.
The present study characterizes the shape and size of nanoparticles through the utilization of diffraction scanning electron microscope and dynamic light scattering. Exposure to toxic elements elicit stressed condition in an organism that requires energy for the metabolic activities to eliminate the detrimental compound. Enzymes are the first indicators of stress in an organism hence in the present study the serum marker enzymes aspartate transaminase (AST), alanine transaminase (ALT), acid phosphatase (ACP), Alkaline phosphatase (ALP) and lactate dehydrogenase (LDH) along with antioxidant enzymes superoxide dismutase (SOD), catalase (CAT) and glutathione peroxide (GPx) and lipid peroxidation content MDA are analyzed. The present study is an attempt to study the hematological, biochemical and genotoxicity of silver nanoparticles in O. mossambicus.
Material and methods
Silver nanoparticle synthesis
0.1 M silver nanoparticles were synthesized through the co-precipitation of silver nitrate (AgNO3) and trisodium citrate (Na3C6H5O7) using a chemical reduction technique. To begin, 500 mL beaker was filled with 20 mL of 1% trisodium citrate. The burette was then filled with 1% AgNO3. Gradually, drop by drop, the AgNO3 was added to the trisodium citrate while continuously mixing the solution vigorously. Once a volume of 200 mL was reached, the burette was refilled, and the mixture was heated on an induction stove until it reached a temperature of 90 °C. The heating process was stopped once the color of the solution changed from yellow to brown. Finally, 1 g of this resulting powder attained was separated and placed in a desiccator for further analysis.
Characterization of silver nanoparticles
Analyzing the effectiveness of nanoparticles involves considering their physicochemical properties. In order to understand the properties and sizes of silver nanoparticles in an aqueous suspension, techniques such as X-Ray diffraction (XRD), scanning electron microscope (SEM),High Resolution Transmission electron microscopy (HRTEM), and dynamic light scattering (DLS) were used for characterization.
Experimental setup
The ethical committee granted approval for conducting the experiments using O. mossambicus in this study. The experiment utilized 300 O. mossambicus fish, each measuring approximately 15 ± 2 cm in length and weighing around 90 ± 3 g. Prior to commencement of the experiment fish were acclimated to laboratory conditions for a period of two weeks. The laboratory sustained a continuous water system with aeration, a 12–12 light to dark photo period, and a temperature of 26 ± 2 °C. The fish were provided with a commercial feed that contained 8% lipids, 35% protein, 50% carbohydrate, 2.5% fiber, and 1.2% vitamins and minerals. The feeding regimen involved administering the fish 10% of their body weight twice a day.
Experimental design
After a period of 2 weeks allocated for adjustments to their environment, the fish were divided into five distinct cohorts. Each cohort comprised three sets of 20 fish placed in a 50 L glass tank. The first cohort acted as the control group, with no nanoparticles added, and was maintained in triplicate. The remaining four cohorts were considered treatment groups, each exposed to different concentrations of silver nanoparticles: 0.5 mg/L, 5 mg/L, 10 mg/L, and 20 mg/L.
Sample collection
Blood samples were procured from fish on day 7 and 14 by cardiac puncture. The first set of samples was placed in EDTA tubes for hematological analysis, while the second set was transferred to sterile centrifuge tubes without anticoagulant. The blood in the second set was left to clot and then centrifuged at 5,000 rpm to segregate the serum for biochemical analysis.
Hematological analysis
The enumeration of red blood cell was performed by using a haemocytometer and hayem’s fluid for fish blood, while the white blood cell quantification was done utilizing WBC diluting fluid. The hemoglobin content was determined using the cyanmethemoglobin calorimetric method. The hemotocrit (Hct) value was calculated using the wintrobe’s hemotocrit method.
Biochemical analysis
Superoxide dismutase (SOD) activity was analyzed using a modified procedure based on Das et al.17 One unit of enzyme activity was defined as the amount of SOD that inhibited 50% of nitrite formation under the assay conditions. Catalase (CAT) activity was determined following the method of Sinha.18 The quantification of CAT was expressed as the number of moles of H2O2 decomposed per minute per milligram of protein. Glutathione peroxidase (GPx) activity was measured according to the procedure described by Rotruck et al.19 The enzyme activity was expressed as the number of moles of Glutathione (GSH) oxidized per minute per milligram of protein. Activity of Aspartate aminotransferase (AST) was assessed using the method outlined by Reitman and Frankel.20 Alanine transaminase (ALT)and Acid phosphatase (ACP) activity were studied. Alkaline phosphatase (ALP) activity was determined using the approach described by Varley.21 Lactate dehydrogenase (LDH) activity was measured following the method established by King.22
Comet assay
The alkaline comet assay was performed for analysis of DNA damage according to the method of Paulraj and Behari.23 Assessed cell viability using the trypan blue exclusion assay and performed the entire experiment in a dark environment to circumvent DNA damage from sunlight exposure. Blood samples were obtained from control and treated fish on day 7 and 14, and triplicate slides were prepared for each sample in each experiment. A mixture of 10 μL blood sample and 65 μL of 0.5% low melting point agarose (LMPA) dissolved in phosphate buffered saline (PBS) was layered onto a frosted microscope slide, coated in 1% normal melting point agarose. After solidifying at 4 °C for 15 min, an additional layer of 1% low melting point agarose was spread over. The cells were then lysed in a lysing buffer at 4 °C for 2–12 h. Electrophoretic separation was performed in an electrophoresis buffer solution for 30 min at 20 V and 300 mA, followed by neutralization in three washing steps with 0.4 M tris–HCl (pH 7.5). The slides were fixed in 96% ethanol, rehydrated in ultrapure water, and stained with ethidium bromide solution. The scoring of DNA damage was conducted using a fluorescence microscope and Komet 5.0 image analysis software. A total of 50 cells were scored per gel, with duplicate slides prepared for each treatment and independently coded and scored. The %tail DNA was chosen as the most relevant and widely used parameter out of the 34 possible measurements.
Statistical analysis
We utilized the statistical software Origin 8 for Windows to conduct the statistical analysis. The data are expressed as the mean ± standard deviation. Before proceeding with the analysis, we checked whether the data satisfied the assumptions of homogeneity and normality. In case these assumptions were met, we performed a One-way analysis of variance (ANOVA). Conversely, if the assumptions were not satisfied, we carried out the non-parametric Kruskal-Wallis test. The significance level was set at p < 0.05.
Results
Characterization of silver nanoparticles
The X-ray diffraction (XRD) pattern illustrated in Fig. 1 indicates that the silver nanoparticle possesses a crystalline structure. The crystallite size has been estimated from the XRD pattern using the Scherrer’s equation, [where D is the grain size, λ the wavelength of X-ray (1.54056 Å), B the full width at half maxima of the diffraction peak (in radian)]. The presence of sharp structural peaks in XRD patterns suggested the nano-crystalline nature of the particles. For characterization of size, size distribution and shape were assessed by Scanning Electron microscopy (SEM), TECH NAI-12 with an accelerating voltage of 120 kV. Microfilms were prepared by placing a drop of the respective nanoparticle suspension onto a copper SEM grid and drying at room temperature overnight. To obtain a representative view, a minimum of 5 images were collected for each sample. The images in Fig. 2 display scanning electron microscope (SEM) photographs of the produced silver nanoparticles. The range was calculated by measuring over 10 nanoparticles in random fields of view. The range of silver nanoparticles was 126–160 nm. Images were captured using High resolution Transmission electron microscopy (HRTEM, JOEL Japan JEM 2100) at 200KV model with LaB6 electron gun (Fig. 3). Dynamic light scattering (DLS) of silver nanoparticle (100 mg/L) was conducted using a Malvern zetasizer model 3,000 (Malvern Instruments Limited, Worcestershire, UK) and the results are plotted as a graph Fig. 4). The size distribution ranges from 200 nm to 380 nm where 50% of the particles were between 180,200 nm. The average silver hydrodynamic diameter was 192.3 nm.
Fig. 1.
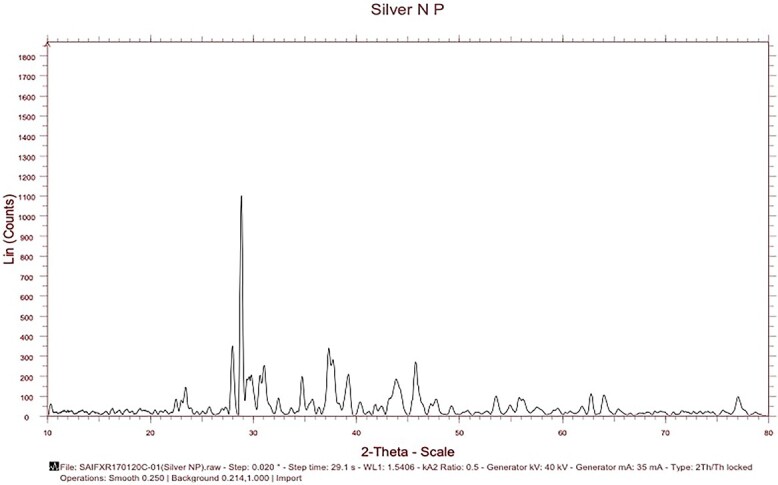
Xray diffraction studies of silver nanoparticle.
Fig. 2.

Scanning electron microscopy of silver nanoparticles.
Fig. 3.

High resolution transmission electron microscopy of silver nanoparticles.
Fig. 4.

Particle size distribution of silver nanoparticles (100 mg/L) dispersed in water using dynamic light scattering. Values are means of three replicates.
Hematological
The RBC, haemoglobin, MCV and Hct values showed a significant (P < 0.05) decrease the level on exposure to different concentrations of silver nanoparticles. The decrease was dose and time-dependent (P < 0.05) when compared to control. The lower concentration of 0.5 mg/L did not show any significant changes. The WBC count was significantly (P < 0.05) elevated on 7th day and 14th today in all the three concentrations (5 mg/L, 10 mg/L and 20 mg/L) but the decrease was not dose-dependent and was not statistically significant (Table 1).
Table 1.
Haematological parameters of Oreochromis mossambicus exposed to different concentration of silver nanoparticle and control.
| Exposure | Concentration | RBC (106mm3) | WBC (103mm3) | Hb (g %) | HCT (%) | MCV (μ3) |
|---|---|---|---|---|---|---|
| Control | 1.2 + 0.12d | 8.6 + 1.4a | 4.2d | 25.6b | 72 + 2.4c | |
| 0.5 mg/L | 1.23 + 0.11d | 8.4 + 1.6a | 4.2d | 24.9b | 70 + 4.0c | |
| Day 7 | 5 mg/L | 0.98 + 0.09c | 10.7 + 2.1b | 3.6c | 22.2a | 68.2 + 3.6c |
| 10 mg/L | 0.91 + 0.07b | 10.8 + 2.3b | 3.2b | 21.9a | 66 + 4.2b | |
| 20 mg/L | 0.81 + 0.07a | 11.6 + 2.1c | 2.6a | 19.8a | 62.2 + 3.2a | |
| Control | 1.21 + 0.09d | 8.5 + 1.1a | 4.2d | 25.3b | 72.2 + 2.6c | |
| 0.5 mg/L | 1.25 + 0.08d | 8.6 + 1.3a | 4.4d | 24.7b | 72.0 + 4.2c | |
| Day 14 | 5 mg/L | 0.96 + 0.04c | 10.4 + 2.0b | 3.6c | 21.9a | 66.2 + 3.6b |
| 10 mg/L | 0.88 + 0.04b | 11.1 + 1.8c | 3.0b | 20.3a | 64 + 2.8a | |
| 20 mg/L | 0.73 + 0.08a | 11.2 + 1.6c | 2.6a | 20.9a | 64.2 + 3.2a |
Values are presented as mean + SD for three replicates. Lower case letter represents statistically (P < 0.05) different groups (one way ANOVA).
Biochemical analysis
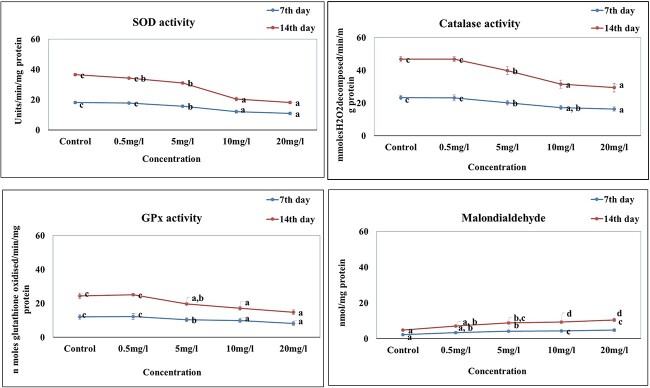
The effect of silver nanoparticles on liquid peroxidation is shown in Fig. 5a. A significant (P < 0.05) elevated lipids peroxidation level was noted on 7th and 14th day of experiment when compared to control. The lower concentration of 0.5 mg/L did not show any significant change. Results indicated that silver nanoparticle induced dose and time-dependent significant increase (P < 0.05). The effect of different concentration of silver nanoparticles on SOD activity showed a significant (P < 0.05) decrease except 0.5 mg/L when compared to control (Fig. 5b). The catalase activity also showed a significant decrease (P < 0.05) in activity of silver nanoparticle concentrations on 7th and 14th day of exposure except 0.5 mg/L (Fig. 5c). Glutathione peroxidase (GPx) showed a time and dose dependent a significant decrease (P < 0.05) in all the concentrations of silver nanoparticle exposed groups. The lower dose of 0.5 mg/L e did not show significant activity (Fig. 5d).
Fig. 5.
Oxidative stress markers of Oreochromis mossambicus exposed to different concentration of silver nanoparticle and control. A) Superoxide dismutase activity (SOD) B) catalase activity (CAT) C) glutathione peroxidase (GPx) and D) malondialdehyde (MDA). Values are presented as mean + SD. Lower case letter represents statistically (P < 0.05) different groups (one way ANOVA).
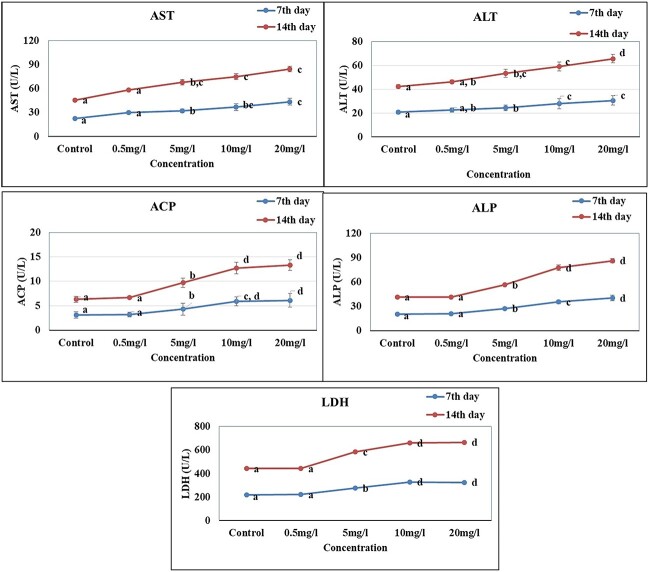
Changes in serum enzymes of aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), acid phosphatase (ACP) and lactate dehydrogenase (LDH) in O. mossambicus exposed to silver nanoparticles were studied on day 7 and 14 of exposure. The AST and ALT activity where significantly (P < 0.05) elevated in all concentrations of 0.5 mg/L, 5 mg/L, 10 mg/L and 20 mg/L when compared to control (Fig. 6a and b). Exposure to Silver nanoparticles showed a significant increase (P < 0.05) both in ALP (Fig. 6c) and ACP (Fig. 6d) in the serum level of O. mossambicus whereas normal levels were recorded in control group. The activity of ACP and ALP recorded for lower concentration of 0.5 mg/L did not show any significant activity. Lactate dehydrogenase (LDH) enzyme activity on exposure to silver nanoparticles showed a significant (P < 0.05) elevated activity in in 5 mg/L, 10 mg/L and 20 mg/L but was not dose-dependent (Fig. 6e).
Fig. 6.
Serum enzymes of Oreochromis mossambicus exposed to different concentration of silver nanoparticle. A) Aspartate transaminase activity (AST) B) alanine transaminase activity (ALT) C) acid phosphatase (ACP) D) alkaline phosphatase (ALP) E) lactate dehydrogenase (LDH). Values are presented as mean + SD. Lower case letter represents statistically (P < 0.05) different groups (one way ANOVA).
DNA damage to blood cells exposed to silver nanoparticle was quantified as %TDNA utilizing the comet assay. %TDNA increased with higher concentration of silver nanoparticle, which were significant when compared to control group (P > 0.05). %TDNA in the low concentration group (05 and 5 mg/L) did not increase significantly but was slightly higher compared to the control group (Fig. 7).
Fig. 7.

DNA strand breaks in blood cells (% tail DNA) of Oreochromis mossambicus exposed to different concentration of silver nanoparticle and control. Values are presented as mean + SD. Lower case letter represents statistically (P < 0.05) different groups (one way ANOVA).
Discussion
Increasing production of nanoparticles, applications in diverse fields, novel technologies and methods of synthesis of some of the contributing factors in the ever-increasing contamination of these nanoparticles in aquatic and terrestrial environment. The small size and high surface area raise concern regarding the toxicity of nanoparticle. Silver nanoparticles were found to impair cellular structures, induce oxidative stress and alter gene expression.24,25 In zebrafish embryos, silver nanoparticle interfere with ionocytes hindering their functioning.26 Furthermore, silver nanoparticle have been shown to have negative impacts on hair cells27 and blood cells in fish, causing changes in hematologic parameters and histological damage.28,29 The Nrf2-Keap pathway was identified as being involved in the response to silver nanoparticlesss.30
WBC count in silver nanoparticle exposed fish showed a significant increase which may be due to the production of antibodies as a primary immune response to cope with foreign particle entry into fish31 or decrease in nonspecific immunity of the fish32 or it might be a normal reaction of fish to substances that alter their normal physiological processes as stated by Kanwal et al.33 Decrease in hemoglobin content may have stemmed from an increase in oxidation of hemoglobin to make hemoglobin or release of O2− radical due to silver nanoparticle toxicity. The decreased hemoglobin might have resulted in decreased oxygen supply to tissues Kanwal et al.33 The haematocrit values did not show any significant changes. The hematological changes on exposure to toxic compounds indicate the toxic stress of fish.34 Similar results like increased WBC and platelets, and decreased RBCs, Hb, hematocrit and mean cellular hemoglobin concentration (MCHC) decreased were reported by Khan et al.35 when he worked on zinc nanoparticle toxicity on O. mossambicus. Conversely, Remya et al.36 reported that exposure of Labeo rohita to Iron oxide nanoparticles significantly increased hemoglobin (Hb) content, red blood cell (RBC) count and hematocrit (Ht) values. MCV and HCT also showed a decrease that was dose dependent. A similar decrease in MCV was found in Tilapia mossambica exposed to copper nanoparticles.37
Catalase (CAT) is an endogenous antioxidant enzyme present in all biological tissues facilitating conversion of hydrogen peroxide to oxygen and water for prevention of cell damage.38,39 A decrease in catalase activity may be attributed to binding of toxicants to –SH groups of enzymes, or increased H2O2 and/or superoxide radical.40 The reduction in SOD enzyme activity on exposure to silver nanoparticle might be due to utilization of this enzyme as an antioxidant for the conversion of the free radical formed in (O2−) to H2O2.41
The prime role of glutathione peroxidase (GPx) is protection of cell membrane from damage against lipid peroxidation by termination of free radical propagation.42 The decrease of GPx on exposure to silver nanoparticles may be the result of failure of antioxidant defence mechanism of the body to counteract the free radical propagation. The SOD, CAT and GPx activity are considered as the first line defence mechanism of the body to counteract against the reactive oxygen species resulting from oxidative stress caused by silver nanoparticles.43
The toxicity of silver nanoparticle has been confirmed by a significant increase in serum enzymes aspartate transaminase (ALT), alanine transaminase (ALT), acid phosphatase (ACP), alkaline phosphatase (ALP) and lactate dehydrogenase (LDH). Alanine transaminase and aspartate transaminases are recognized as enzyme markers indicating hepatic damage and liver dysfunction.44 An elevated level of alanine transaminase activity might be due to mitochondrial membrane damage of hepatocytes.45,46 The increased level of transaminases indicates depletion of energy and mobilization of free amino acids for glucose production.47 This elevated transaminase connotes depletion of total carbohydrates during stress and the high energy demand that leads to depletion of protein and release of free amino acids for glucose production. The increase level of transaminase coupled with reactive oxygen species generation and elevated lipid peroxidation indicates the liver cell membrane permeability.
The phosphatases acid phosphatase (ACP) and alkaline phosphatase (ALP) showed increased activity in silver nanoparticle exposed O. mossambicus. Increase in Alkaline phosphatase might be due to hepatic tissue damage and dysfunction. Alkaline phosphatase (ALP) plays a key role in phosphate hydrolysis and membrane transport in biological systems and elevation in alkaline phosphatase (ALP) may be the result of change in cell membrane permeability. Changes within the cellular and subcellular level of lysosome results in an increase in acid phosphatase activity. An elevation of acid phosphatase (ACP) indicates change in lysosomal membrane permeability.48 Lysosomes are primarily concerned with integration and elimination of toxicants and an impairment of detoxification ability of hepatocytes increases acid phosphatase (ACP) activity.49 Lactate dehydrogenase enzyme activity exhibited an increase in all the silver nanoparticle treatment groups when compared to control groups. Dehydrogenase enzymes confront conversion of lactate to pyruvate. Under anaerobic conditions the lactate dehydrogenase converts pyruvate to lactate and this significant increase in lactate dehydrogenase may be an indication of anaerobic activity.50,51
DNA damage is the result of exposure to nanoparticles either directly interacting with DNA or the nanoparticles causing oxidative stress that may further induce DNA damage as evidenced by Comet assay. Nanoparticles have shown to induce chromosomal abberations, mutations and DNA strand breaks.52 In comet assay the tail indicates the extent of damage in single stranded DNA. It was found that the largest tail DNA were noted in nanoCeO2 with highest concentration. Similar studies were reported for other nanoparticle like TiO2 and CuO2 that caused DNA damage.53,54
The potential of silver nanoparticles might enter cells to diffusion or endocytosis leading to mitochondrial dysfunction and generation of reactive oxygen species that cause cytoplasmic imbalance and inhibition of cell proliferation. The dose dependent activity was not evidenced in the study though deviations were based on concentration. Thus, the present study provides substantiated evidence for hematological, biochemical and genotoxicity of silver nanoparticles in O. mossambicus.
Conclusion and future perspectives
It is concluded from this work that O. mossambicus is particularly susceptible to the deleterious effects of silver nanoparticles. These effects are observed on different organs and at organelle level and effecting multiple metabolic and physiological pathways. Confirmed to be hemotoxic, genotoxic, and hepatotoxic in nature. Though studies confirm its antibacterial nature bioaccumulation remains a significant concern. Benefic effects couldn’t surpass the toxicity so it’s always advisable for restricted use of silver nanoparticles. Further research is warranted to elucidate the molecular mechanisms underlying the toxicity of silver nanoparticles at various organelle levels.
Contributor Information
Gisha Sivan, Division of Medical Research, SRM Medical College Hospital and Research Centre, SRM Institute of Science and Technology, Chennai, Tamil Nadu 603203, India.
Rajesh Pamanji, Department of Microbiology, Pondicherry University, Puducherry 605014, India.
Srikanth Koigoora, Vignan's Foundation for Science, Technology and Research (Deemed to be University), Vadlamudi, Andhra Pradesh 560075, India.
Nimila Joseph, St Alberts College, Ernakulam, Kerala 682016, India.
Joseph Selvin, Department of Microbiology, Pondicherry University, Puducherry 605014, India.
Author contributions
Gisha Sivan: Conceptualization, Methodology, Data curation, Writing - Original Draft,Visualization, Investigation, ValidationRajesh Pamanji: Data curation, Writing- Reviewing and Editing, Visualization, SoftwareSrikanth Koigoora.: Supervision, Project administration, ValidationNimila Joseph: Data Curation, ValidationJoseph shelvin: Supervision, Validation.
Conflict of interest statement
All the authors declare that they have no conflict of interest.
References
- 1. Pamanji R, Kumareshan TN, Priya SL, Sivan G, Selvin J. Exploring the impact of antibiotics, microplastics, nanoparticles, and pesticides on zebrafish gut microbiomes: insights into composition, interactions, and health implications. Chemosphere. 2024:349:140867. [DOI] [PubMed] [Google Scholar]
- 2. Aziz S, Abdullah S, Anwar H, Latif F, Mustfa W. Effect of engineered nickel oxide nanoparticles on antioxidant enzymes in freshwater fish, Labeo rohita. Pak Vet J. 2021:41(03):424–428. [Google Scholar]
- 3. Huang YW, Cambre M, Lee HJ. The toxicity of nanoparticles depends on multiple molecular and physicochemical mechanisms. Int J Mol Sci. 2017:18(12):2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tian X, Jiang X, Welch C, Croley TR, Wong T-Y, Chen C, Fan S, Chong Y, Li R, Ge C, et al. Bactericidal effects of silver nanoparticles on lactobacilli and the underlying mechanism. ACS Appl Mater Interfaces. 2018:10(10):8443–8450. [DOI] [PubMed] [Google Scholar]
- 5. Wei L, Lu J, Xu H, Patel A, Chen Z-S, Chen G. Silver nanoparticles: synthesis, properties, and therapeutic applications. Drug Discov Today. 2015:20(5):595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Taju G, Abdul Majeed S, Nambi KSN, Sahul Hameed AS. In vitro assay for the toxicity of silver nanoparticles using heart and gill cell lines of Catla catla and gill cell line of Labeo rohita. Comp Biochem Physiol C: Toxicol Pharmacol. 2014:161:41–52. [DOI] [PubMed] [Google Scholar]
- 7. Khoshnamvand M, Hao Z, Fadare OO, Hanachi P, Chen Y, Liu J. Toxicity of biosynthesized silver nanoparticles to aquatic organisms of different trophic levels. Chemosphere. 2020:258:127346.Epub 2020 Jun 10. PMID: 32544815. [DOI] [PubMed] [Google Scholar]
- 8. Gliga AR, Skoglund S, Odnevall Wallinder I, Fadeel B, Karlsson HL. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: the role of cellular uptake, agglomeration and ag release. Part Fibre Toxicol. 2014:11(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McShan D, Ray PC, Yu H. Molecular toxicity mechanism of nanosilver. J Food Drug Anal. 2014:22(1):116–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sibiya A, Gopi N, Jeyavani J, Mahboob S, Al-Ghanim KA, Sultana S, Mustafa A, Govindarajan M, Vaseeharan B. Comparative toxicity of silver nanoparticles and silver nitrate in freshwater fish Oreochromis mossambicus: a multi-biomarker approach. Comp Biochem Physiol C: Toxicol Pharmacol. 2022:259:109391. [DOI] [PubMed] [Google Scholar]
- 11. Iswarya A, Anjugam M, Gopi N, Shanthi S, Govindarajan M, Alharbi NS, Kadaikunnan S, Alharbi MS, Sivakamavalli J, Vaseeharan B. β-1,3-Glucan binding protein-based silver nanoparticles enhance the wound healing potential and disease resistance in Oreochromis mossambicus against Aeromonas hydrophilla. Microb Pathog. 2022:162:105360. [DOI] [PubMed] [Google Scholar]
- 12. Govindasamy R, Rahuman AA. Histopathological studies and oxidative stress of synthesized silver nanoparticles in Mozambique tilapia (Oreochromis mossambicus). J Environ Sci. 2012:24(6):1091–1098. [DOI] [PubMed] [Google Scholar]
- 13. Mansour WAA, Abdelsalam NR, Tanekhy M, Khaled AA, Mansour AT. Toxicity, inflammatory and antioxidant genes expression, and physiological changes of green synthesis silver nanoparticles on Nile tilapia (Oreochromis niloticus) fingerlings. Comp Biochem Physiol C: Toxicol Pharmacol. 2021:247:109068. [DOI] [PubMed] [Google Scholar]
- 14. Aly SM, Eissa AE, Abdel-Razek N, El-Ramlawy AO. The antibacterial activity and immunomodulatory effect of naturally synthesized chitosan and silver nanoparticles against pseudomonas fluorescence infection in Nile tilapia (Oreochromis niloticus): an in vivo study. Fish Shellfish Immunol. 2023:135:108628. [DOI] [PubMed] [Google Scholar]
- 15. Thummabancha K, Onparn N, Srisapoome P. Analysis of hematologic alterations, immune responses and metallothionein gene expression in Nile tilapia (Oreochromis niloticus) exposed to silver nanoparticles. J Immunotoxicol. 2016:13(6):909–917. [DOI] [PubMed] [Google Scholar]
- 16. Sarkar B, Jaisai M, Mahanty A, Panda P, Sadique M, Nayak BB, Gallardo G, Thakur D, Bhattacharjee S, Dutta J. Optimization of the sublethal dose of silver nanoparticle through evaluating its effect on intestinal physiology of Nile tilapia (Oreochromis niloticus L.). J Environ Sci Health A. 2015:50(8):814–823. [DOI] [PubMed] [Google Scholar]
- 17. Das K, Samanta L, Chainy G. A modified spectrophotometric assay of superoxide dismutase using nitrite formation by superoxide radicals. Indian J Biochem Biophys. 2000:37:201–204. http://nopr.niscpr.res.in/handle/123456789/15379. [Google Scholar]
- 18. Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972:47(2):389–394. [DOI] [PubMed] [Google Scholar]
- 19. Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973:179(4073):588–590. [DOI] [PubMed] [Google Scholar]
- 20. Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic Oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957:28(1):56–63. [DOI] [PubMed] [Google Scholar]
- 21. Varley H. Practical clinical biochemistry. 4th ed. New Delhi: Arnold Heinman. (India) Ltd.; 1975. p. 465. [Google Scholar]
- 22. King J. Practical clinical enzymology. NewYork: D. Van Nostrand; 1965. p. 372. [Google Scholar]
- 23. Paulraj R, Behari J. Single strand DNA breaks in rat brain cells exposed to microwave radiation. Mutat Res Fundam Mol Mech Mutagen. 2006:596(1–2):76–80. [DOI] [PubMed] [Google Scholar]
- 24. Bermejo-Nogales A, Fernández M, Fernández-Cruz ML, Navas JM. Effects of a silver nanomaterial on cellular organelles and time course of oxidative stress in a fish cell line (PLHC-1). Comp Biochem Physiol C: Toxicol Pharmacol. 2016:190:54–65. [DOI] [PubMed] [Google Scholar]
- 25. Bruneau A, Turcotte P, Pilote M, Gagné F, Gagnon C. Fate of silver nanoparticles in wastewater and immunotoxic effects on rainbow trout. Aquat Toxicol. 2016:174:70–81. [DOI] [PubMed] [Google Scholar]
- 26. Horng J-L, Lee C-Y, Liu S-T, Hung G-Y, Lin L-Y. Differential effects of silver nanoparticles on two types of mitochondrion-rich ionocytes in zebrafish embryos. Comp Biochem Physiol C: Toxicol Pharmacol. 2022:252:109244. [DOI] [PubMed] [Google Scholar]
- 27. Yen H-J, Horng J-L, Yu C-H, Fang C-Y, Yeh Y-H, Lin L-Y. Toxic effects of silver and copper nanoparticles on lateral-line hair cells of zebrafish embryos. Aquat Toxicol. 2019:215:105273. [DOI] [PubMed] [Google Scholar]
- 28. Joo HS, Kalbassi MR, Johari SA. Hematological and histopathological effects of silver nanoparticles in rainbow trout (Oncorhynchus mykiss)—how about increase of salinity? Environ Sci Pollut Res. 2018:25(16):15449–15461. [DOI] [PubMed] [Google Scholar]
- 29. Shabrangharehdasht M, Mirvaghefi A, Farahmand H. Effects of nanosilver on hematologic, histologic and molecular parameters of rainbow trout (Oncorhynchus mykiss). Aquat Toxicol. 2020:225:105549. [DOI] [PubMed] [Google Scholar]
- 30. Osborne OJ, Mukaigasa K, Nakajima H, Stolpe B, Romer I, Philips U, Lynch I, Mourabit S, Hirose S, Lead JR, et al. Sensory systems and ionocytes are targets for silver nanoparticle effects in fish. Nanotoxicology. 2016:10(9):1276–1286. [DOI] [PubMed] [Google Scholar]
- 31. Bantu N, Chaithnaya Karri K, Krishnan Vk G, Kumari N, Vakita R. Histological alteration in different tissues of Indian major carp, Labeo rohita (Hamilton) exposed to Profenofos 50% EC and Carbosulfan 25% EC formulations. J Biol Today’s World. 2017:6(3). 10.15412/J.JBTW.01060301. [DOI] [Google Scholar]
- 32. Shaluei F, Hedayati A, Jahanbakhshi A, Kolangi H, Fotovat M. Effect of subacute exposure to silver nanoparticle on some hematological and plasma biochemical indices in silver carp (Hypophthalmichthys molitrix). Hum Exp Toxicol. 2013:32(12):1270–1277. [DOI] [PubMed] [Google Scholar]
- 33. Kanwal Z, Raza MA, Manzoor F, Riaz S, Jabeen G, Fatima S, Naseem S. A comparative assessment of Nanotoxicity induced by metal (silver, nickel) and metal oxide (cobalt, chromium) nanoparticles in Labeo rohita. Nano. 2019:9(2):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kavitha C, Ramesh M, Kumaran SS, Lakshmi SA. Toxicity of Moringa oleifera seed extract on some hematological and biochemical profiles in a freshwater fish, Cyprinus carpio. Exp Toxicol Pathol. 2012:64(7–8):681–687. [DOI] [PubMed] [Google Scholar]
- 35. Khan GB, Akhtar N, Khan MF, Ullah Z, Tabassum S, Tedesse Z. Toxicological impact of zinc nano particles on tilapia fish (Oreochromis mossambicus). Saudi J Biol Sci. 2022:29(2):1221–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Remya AS, Ramesh M, Saravanan M, Poopal RK, Bharathi S, Nataraj D. Iron oxide nanoparticles to an Indian major carp, Labeo rohita: impacts on hematology, iono regulation and gill Na+/K+ ATPase activity. J King Saud Univ Sci. 2015:27(2):151–160. [Google Scholar]
- 37. Siddiqui SA, Noorjahan CM. Toxicity of copper nanoparticle on haematology and biochemistry of fish, Tilapia mossambica. Int Res J Pharm. 2018:9(10):121–124. [Google Scholar]
- 38. Parlak V. Evaluation of apoptosis, oxidative stress responses, AChE activity and body malformations in zebrafish (Danio rerio) embryos exposed to deltamethrin. Chemosphere. 2018:207:397–403. [DOI] [PubMed] [Google Scholar]
- 39. Vasylkiv OY, Kubrak OI, Storey KB, Lushchak VI. Catalase activity as a potential vital biomarker of fish intoxication by the herbicide aminotriazole. Pestic Biochem Physiol. 2011:101(1):1–5. [Google Scholar]
- 40. Ruas CBG, Carvalho CDS, De Araújo HSS, Espíndola ELG, Fernandes MN. Oxidative stress biomarkers of exposure in the blood of cichlid species from a metal-contaminated river. Ecotoxicol Environ Saf. 2008:71(1):86–93. [DOI] [PubMed] [Google Scholar]
- 41. Abhijith BD, Ramesh M, Poopal RK. Responses of metabolic and antioxidant enzymatic activities in gill, liver and plasma of Catla catla during methyl parathion exposure. J Basic Appl Zool. 2016:77:31–40. [Google Scholar]
- 42. Van Der Oost R, Beyer J, Vermeulen NPE. Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol. 2003:13(2):57–149. [DOI] [PubMed] [Google Scholar]
- 43. Hoseinifar SH, Yousefi S, Van Doan H, Ashouri G, Gioacchini G, Maradonna F, Carnevali O. Oxidative stress and antioxidant defense in fish: the implications of probiotic, prebiotic, and synbiotics. Rev Fish Sci Aquacult. 2021:29(2):198–217. [Google Scholar]
- 44. Venkateswara Rao J. Sublethal effects of an organophosphorus insecticide (RPR-II) on biochemical parameters of tilapia, Oreochromis mossambicus. Comp Biochem Physiol C: Toxicol Pharmacol. 2006:143(4):492–498. [DOI] [PubMed] [Google Scholar]
- 45. Contreras-Zentella ML, Hernández-Muñoz R. Is liver enzyme release really associated with cell necrosis induced by oxidant stress? Oxidative Med Cell Longev. 2016:2016:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McGill MR. The past and present of serum aminotransferases and the future of liver injury biomarkers. EXCLI J. 2016:15:817–828. ISSN 1611-2156. 10.17179/EXCLI2016-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chatterjee N, Pal AK, Das T, Mohammed MS, Sarma K, Venkateshwarlu G, Mukherjee SC. Secondary stress responses in Indian major carps Labeo rohita (Hamilton), Catla catla (Hamilton) and Cirrhinus mrigala (Hamilton) fry to increasing packing densities. Aquac Res. 2006:37(5):472–476. [Google Scholar]
- 48. Sivan G, Radhakrishnan CK. Renal lysosomal functions on exposure to Scatophagus argus venom in experimental mice. Toxicol Mech Methods. 2007:17(9):519–526. [DOI] [PubMed] [Google Scholar]
- 49. Donohue TM, Osna NA, Kharbanda KK, Thomes PG. Lysosome and proteasome dysfunction in alcohol-induced liver injury. Liver Res. 2019:3(3–4):191–205. [Google Scholar]
- 50. Anadón, A., Castellano, V., & Martínez-Larrañaga, M. R. (2014). Biomarkers in drug safety evaluation. In: Gupta RC (Ed.) Biomarkers in toxicology San Diego, USA. pp. 923–945. 10.1016/B978-0-12-404630-6.00055-5. [DOI] [Google Scholar]
- 51. Laganá G, Barreca D, Calderaro A, Bellocco E. Lactate dehydrogenase inhibition: biochemical relevance and therapeutical potential. Curr Med Chem. 2019:26(18):3242–3252. [DOI] [PubMed] [Google Scholar]
- 52. Kaur K, Kaur R. Occupational pesticide exposure, impaired DNA repair, and diseases. Indian J Occup Environ Med. 2018:22(2):74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hou J, Liu H, Wang L, Duan L, Li S, Wang X. Molecular toxicity of metal oxide nanoparticles in Danio rerio. Environ Sci Technol. 2018:52(14):7996–8004. [DOI] [PubMed] [Google Scholar]
- 54. Mottola F, Iovine C, Santonastaso M, Carfora V, Pacifico S, Rocco L. Evaluation of zebrafish DNA integrity after individual and combined exposure to TiO2 nanoparticles and lincomycin. Toxics. 2022:10(3):132.PMID: 35324757; PMCID: PMC8954801. [DOI] [PMC free article] [PubMed] [Google Scholar]