Abstract
Fall armyworm (FAW), Spodoptera frugiperda (J.E. Smith) has significantly affected maize crop yields, production efficiency, and farmers’ incomes in the Indian Eastern Gangetic Plains region since it was first observed in India in 2018. A lack of awareness by maize growers of the appropriate selection, method, and timing of insecticide application not only creates a barrier to sustainable FAW control but also contributes to increased environmental pollution, reduced human health and increased production costs. We demonstrated that FAW inflicted the most damage in early whorl growth stage of maize, regardless of whether chemical insecticides were applied. FAW egg masses and larvae collected from maize fields in which no insecticides had been sprayed showed high parasitism rates by parasitoid wasps; in contrast fields that had been sprayed had much lower rates of parasitism on FAW. Ten hymenopteran parasitoids were observed in maize fields across the study region, suggesting a diversity of natural methods to suppress FAW in maize at different growth stages. These included two FAW egg parasitoids and eight FAW larval parasitoids. Microplitis manilae Ashmead was the most abundant FAW larval parasitoid species, and Telenomus cf. remus was the dominant FAW egg parasitoid species. Endemic FAW parasitoids such as those observed in this study have great potential as part of a sustainable, cost-effective agroecological management strategy, which can be integrated with other methods to achieve effective control of FAW.
Subject terms: Plant sciences, Zoology
Introduction
Fall armyworm (FAW), Spodoptera frugiperda (J. E. Smith), is a noctuid polyphagous generalist insect native to tropical and subtropical areas of the Americas. FAW was first reported in India in 2018 when it was observed in a maize crop in the state of Karnataka1,2. Since then, FAW has been observed widely across India3,4 and other Asian countries5.
FAW inflicts significant losses on many agricultural crops6. Maize is one of its most preferred hosts, and globally average yield losses of between 17 and 36% resulting from FAW infestation have been observed7. FAW infestation results in economic losses of USD 9.4 billion annually across Africa8 and increased food insecurity, particularly in low- and middle-income countries where maize is a staple food crop9.
The Eastern Gangetic Plain (EGP) spans the Indian states of Bihar, West Bengal, eastern Uttar Pradesh, and Assam as well as parts of the Nepal Terai, and northwestern Bangladesh. The EGP is home to approximately 450 million people, most of whom are resource-poor small and marginal farmers who depend on agriculture for food and livelihood security10. The region ranks high in poverty, food insecurity and climatic vulnerability11–14. Traditionally, crop production in the EGP is rice-based, with approximately 6.5 m ha each year under either rice-rice or rice–wheat cropping systems, whereby monsoon-season rice is followed by irrigated wheat or rice (boro rice) in the dry season. Only some other crops, such as mustard and pulses, are cultivated after monsoon rice, and jute is also cultivated after boro rice. Over the last two decades, the new cropping system has emerged as rice-maize (covers > 1.0 m ha) in EGP due to its high yield potential in widespread agro-ecologies and climatic conditions of the region. The dry-season maize (winter maize) and spring maize in West Bengal and Bihar increased significantly. Maize is more productive, profitable, and more climatically adaptive and more water, energy, and labor efficient than boro rice12,14. Within India, EGP contributes almost 20% of the total maize-grown areas15. Thus, FAW is a critical threat to maize farmers within the EGP, and to national food security.
Smallholder farmers generally apply chemical insecticides to control FAW, although many have limited knowledge about appropriate insecticides or how or when to apply them. FAW is resistant to carbamate, organophosphate, and pyrethroid insecticides16,17. The use of inappropriate chemicals, methods and/or times of application does not effectively control FAW. However, it also contributes to environmental pollution, negatively affects human health, and increases production costs.
An alternative strategy, integrated pest management (IPM), is a more promising method for smallholder farmers to manage FAW than chemical insecticides18,19. IPM encourages the timely and efficient use of both nonchemical and chemical agents to suppress pests within an agroecological environment without completely eliminating them from an agroecosystem, and often by providing conditions in which the pests’ natural enemies thrive20. IPM may include specific timing and techniques for crop establishment, nutrient management, variety selection, chemical and nonchemical pesticides, and pest behavior manipulation21–24.
There are large numbers of potential FAW parasitoids in the world, and this paves the way to regulate the pest sustainably and economically in farmers’ fields25,26. Previous research in the American continents and the Caribbean resulted in the identification of more than 150 parasitoid species that had the potential to suppress FAW in the maize fields and diverse crops habitats, and thus to be a key part of IPM of FAW to minimize crop yield loss27,28.
Effective pest suppression through IPM techniques, such as using native parasitoids, is an emerging and potentially vast area of investigation in India and across southern Asia. There has been limited research on FAW parasitoids in southern India2,29,30; however no systematic investigation has been conducted in the large maize-growing areas of the Indian EGP into the potential to suppress FAW through IPM, and there is currently very little information for farmers and others on the use of FAW parasitoids to suppress and manage the pest in the maize-producing regions of the EGP.
The aims of this research were: (1) to assess the incidence and severity of FAW in key maize-growing regions within the Indian EGP; (2) to describe the suppression of FAW by endemic pest-parasitoids; and thus (3) to improve the understanding of existing endemic FAW natural enemy species in the Indian EGP, particularly those of the parasitoid complex. To address these aims farmers’ fields were surveyed to quantify the presence of FAW in the EGP and the effect on maize plants. Parasitoids culled from FAW samples were analyzed molecularly and morphologically. This research was conducted in Bihar and West Bengal, India, and has applications elsewhere across the EGP and southern Asia more broadly, in maize-growing areas that are vulnerable to FAW and where parasitoids may form a key part of IPM strategies.
Results
FAW distribution, incidence, and severity of damage in maize
FAW was observed in all ten districts surveyed and in maize in three different phenological growth stages. Rice was the preceding crop in nine districts, while farmers of one district, Katihar, grew maize after jute. A significant (p < 0.0001) incidence and severity of FAW damage was observed in maize at the early whorl, late whorl, and reproductive stages (Table 1). There were no differences in FAW incidence or severity between the districts, indicating the widespread nature of infestation throughout the EGP. Moreover, interactions between districts and crop growth stages were also not significant in terms of both the incidence and severity of FAW damage to maize crops.
Table 1.
One-way analyses of variance in FAW incidence and severity of damage to maize crops in ten districts of the Indian Eastern Gangetic Plains.
| Y | Source | DF | SS | MSS | F Value | p Value |
|---|---|---|---|---|---|---|
|
Damage incidence (Plant damage %) |
District | 9 | 1644.77 | 182.75 | 1.80 | 0.078 |
| Stage | 2 | 15,017.88 | 7508.94 | 73.34 | < 0.0001 | |
| District*stage | 18 | 969.46 | 53.86 | 0.53 | 0.937 | |
| Damage severity | District | 9 | 12.57 | 1.40 | 1.05 | 0.408 |
| Stage | 2 | 128.95 | 64.47 | 55.07 | < 0.0001 | |
| District*Stage | 18 | 10.55 | 0.59 | 0.44 | 0.975 |
The highest average FAW damage incidence (38.19%) and average FAW damage severity score (3.98) were observed in maize plants in the early whorl stage, and the greatest plant damage (50–65%) was observed at this time, approximately four to five weeks after sowing (Fig. 1a,b and Supplementary Tables S1). Maize plants at the late whorl stage had moderate damage incidence from FAW (28.97%), with a damage severity score of 2.92. Plants in the reproductive stage had the lowest damage incidence (7.63%) and damage severity score (1.17).
Figure 1.
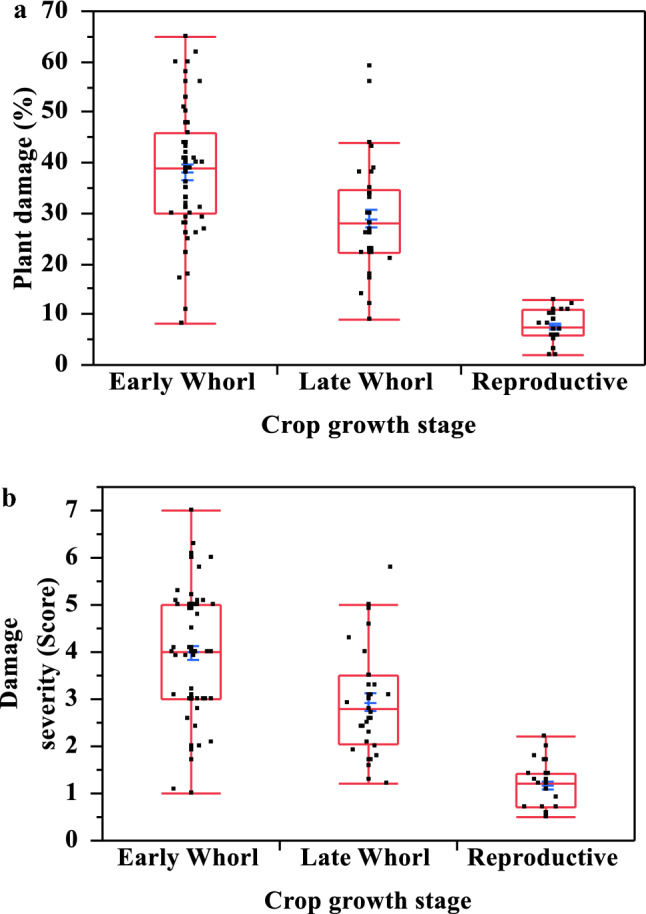
Maize damage (a) and its severity score (b) by FAW at different crop phenological growth stages (n = 124). Dispersion of observed values of damage incidence (plant damage %) and its severity score in different crop growth stages represented in box plot diagrams. Significant variation (p < 0.0001) observed in damage incidence and severity score among crop growth phenological stages viz. early-whorl, late-whorl and reproductive stages. Tukey HSD at 5% interval also confirms significant variation between early-whorl and late-whorl stages (p = 0.0002, p < 0.0001), early-whorl and reproductive stages (p < 0.0001), and late-whorl and reproductive stages (p < 0.0001). Early growth stages suffered the highest damage incidence 38.19 ± 1.30% (8–65%), along with a severity score 3.98 ± 0.14 (1.00–7.00).
The incidence and severity of FAW infestation were spatially variable across the sample locations within the EGP (Supplementary Table S2). The incidence of average FAW damage varied between 22.71 and 41.29%, with the highest and lowest incidences observed in the Dakshin Dinajpur and Murshidabad districts of West Bengal, where damage severity scores of 3.53 and 3.03, respectively, were recorded. The highest damage severity score (4.02) was recorded in Katihar, Bihar, and the lowest severity score (2.85) was recorded in Coochbehar, West Bengal. Between 18 and 71% of farmers at any sample location applied insecticides. There was no significant difference in the FAW damage incidence or damage severity score in maize in the early whorl stage between crops with or without insecticide application: fields where no insecticide was applied had 38.88% damage incidence and a damage severity score of 4.07, while those where insecticide had been applied had a damage incidence of 37.50% and a damage severity score of 3.89 (Supplementary Tables S3, S4).
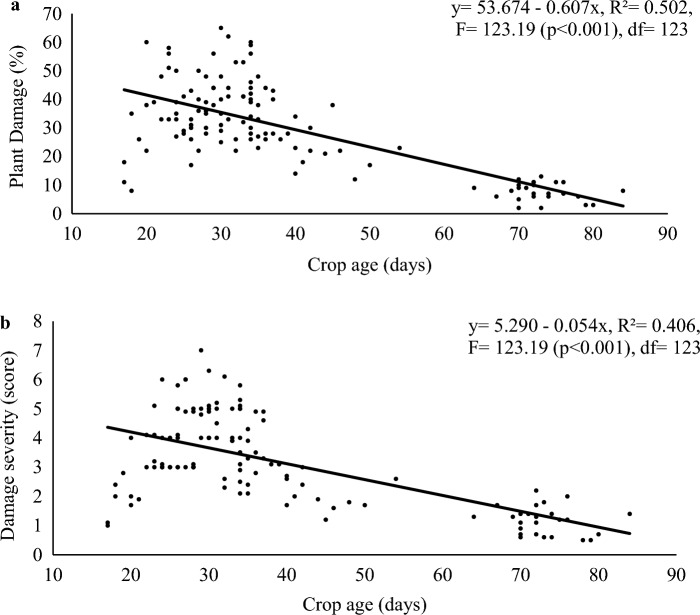
There was a negative correlation between the age of the maize crop and its damage incidence (r = − 0.71, n = 124 and p < 0.0001). A similar trend was observed between maize crop age and damage severity (r = − 0.64, n = 124 and p < 0.0001). Damage incidence and damage severity were positively correlated (r = 0.79, n = 124 and p < 0.0001). Regression analyses further confirmed that both damage incidence and severity by FAW declined with the age of the maize plants (Fig. 2a,b).
Figure 2.
Maize plant damage (a) and its severity score (b) from FAW at different crop ages (n = 124). Regression analyses indicated a decline in damage incidence (plant damage %) and severity score with increased crop age.
Molecular and morphological identification of parasitoids
Overall, 2743 FAW larvae were collected from maize plants across the 93 survey locations in ten districts, and 146 egg masses were collected from 73 locations in nine of these districts. Of the collected larvae, 215 died due to infection by entomopathogens or other unknown factors. The remaining 2528 larvae and the collected egg masses were reared in the laboratory to identify which parasitoid species engaged in parasitism in the Indian EGP. This identification was performed through molecular analysis of the parasitoid species, and comparison with reference accession species in the National Centre for Biotechnology Information, and further morphological identification was performed (Table 2).
Table 2.
Hymenopteran parasitoids that emerged at different growth stages of FAW collected from maize crops in the Indian EGP and identified through molecular analyses and morphological studies.
| Sl. no | Parasitoid name | Host stage affected | GenBank accession No | Similarity with other accessions (%) | Reference accessions |
|---|---|---|---|---|---|
| 1 |
Trichogramma chilonis (Trichogrammatidae)§ |
Egg | OQ849581 | 100 | |
| 2 |
Telenomus cf. remus (Scelionidae) |
Egg | OP288795 | 99.77 | |
| 3 |
Chelonus formosanus (Braconidae) |
Egg-Larvae | OP278928 | 100 |
MT906644 NC060869 |
| 4 |
Campoletis chlorideae (Ichneumonidae) |
Larvae | OP269829 | 98.11 | |
| 5 |
Charops bicolor (Ichneunmonidae) |
Larvae | OP393900 | 100 | MW506958 OP898533 JF866230 |
| 6 |
Cotesia ruficrus (Braconidae) |
Larvae | OP288793 | 99.33 | KY837624 KY836674 |
| 7 |
Microplitis manilae (Braconidae) |
Larvae | OP288794 | 99.70 | HM406523 |
| 8 |
Microplitis prodeniae (Braconidae) |
Larvae | OR053831 | 100 | MW739950 |
| 9 |
Euplectrus spp. (Eulophidae) |
Larvae | OR053833 | 92.04 | MT949367 |
| 10 |
Temelucha spp. (Ichneumonidae) |
Larvae | OQ848503 | 99.03 | MN525186 |
§within parenthesis represents the family of the species.
The ten parasitoid species identified are hymenopteran: four are from the family Braconidae, three are from the family Ichneumonidae, and one species each was observed from the Trichogrammatidae, Scelionidae, and Eulophidae families. Two parasitoid species were observed infesting FAW egg masses, while eight were observed at the larval stage.
Seven hymenopteran parasitoid species were identified up to the species level, and their morphological keys are presented in Supplementary Table S5. Two parasitoids were found in FAW egg masses: Telenomus cf. remus Nixon, and Trichogramma chilonis Ishii (Supplementary Figs. S1, S2). The parasitoid species identified through molecular analysis as Telenomus remus shows 99.77% similarity with the Indian reference accessions (ON923739 and MN879316) and other Asian (KY835081, ON737907 and MT906647) and African accessions (OP93200, MT949366 and MT465126). However, species identification could not be morphologically confirmed, so it is described here as Te. cf. remus.
Two species of parasitoids belonging to the genus Microplitis were observed in FAW larvae: Microplitis prodeniae Rao and Kurian (Supplementary Fig. S3) and Microplitis manilae Ashmead (Supplementary Fig. S4). Other parasitoid species observed in FAW larvae were two braconids, Chelonus formosanus Sonan (Supplementary Fig. S5) and Cotesia ruficrus (Haliday) (Supplementary Fig. S6); three ichneumonids, Campoletis chlorideae Uchida (Supplementary Fig. S7), Charops bicolor (Szepligeti) (Supplementary Fig. S8) and Temelucha spp. (Supplementary Fig. S9); and one eulophid; Euplectrus spp. (Supplementary Fig. S10a,b). The hatched eggs of Euplectrus spp. were observed to develop into yellowish-green parasitoid larvae, which attached to the dorsum of the host FAW caterpillar (Supplementary Fig. S10c). The Euplectrus spp. larvae had crawled to the underside of the dead FAW host larva and spun a loose cocoon before pupation (Supplementary Fig. S10d).
Rate and relative abundance of egg and larval parasitoids
Altogether, 2743 FAW larvae were collected from the field. Out of these collected larvae, 215 larvae died because of other factors such as infection by the entomopathogens, parasitic nematodes, physical injuries and other unknown factors. Of the remaining 2528 FAW larvae reared in the laboratory, 234 died by parasitism from one of the eight identified species of larval parasitoids, and of the 146 egg masses collected, two egg parasitoid species parasitized 23 egg masses. Thus, the overall parasitism rates were 9.26% and 15.75% for the FAW larval and egg stages, respectively. Other factors contributed to 8.57% of FAW mortality, as noted at the start of this paragraph. Therefore, the dominance of the hymenopteran group of parasitoids over other factors inflicting mortality at the field level population of the pest was clearly visible.
Of the two egg parasitoids, a greater overall mean parasitism rate (8.90%) of egg masses was observed in Te. cf. remus and a lower rate (1.37%) in Tr. chilonis, while the two parasitoids together had a parasitism rate of 5.48% (Table 3). Egg masses covered with fewer tufts of hair were parasitized by Tr. chilonis alone or together with Te. cf. remus. FAW egg masses collected from four locations, Dakshin Dinajpur, Uttar Dinajpur and Coochbehar in West Bengal and Purnea in Bihar, were not infested by either of these two parasitoid species. The Murshidabad district (West Bengal) had the highest rate of FAW egg mass parasitism (27.27%) by Te. cf. remus, while both parasitoids together imposed high rates of parasitism on egg masses in the Birbhum (West Bengal, 15.38%) and Kishanganj (Bihar, 25.00%) districts.
Table 3.
Egg parasitoids and their parasitism rate of FAW egg masses observed across the maize-producing region of the Indian Eastern Gangetic Plains.
| Locations | No. of egg masses collected | No. of egg masses parasitized | Observed egg mass parasitoid(s) | Parasitism (%) |
|---|---|---|---|---|
| Birbhum, West Bengal | 26 | 3 | Te. cf. remus | 11.54 |
| 1 | Tr. chilonis | 3.85 | ||
| 4 | Te. cf. remus + Tr. chilonis | 15.38 | ||
| Murshidabad, West Bengal | 11 | 3 | Te. cf. remus | 27.27 |
| 1 | Te. cf. remus + Tr. chilonis | 9.09 | ||
| Malda, West Bengal | 28 | 5 | Te. cf. remus | 17.86 |
| Katihar, Bihar | 15 | 1 | Tr. chilonis | 6.67 |
| Kishanganj, Bihar | 12 | 2 | Te. cf. remus | 16.67 |
| 3 | Te. cf. remus + Tr. chilonis | 25.00 | ||
| Others (4 districts) | 64 | 0 | - | 0.00 |
| Overall mean | 146 | 13 | Te. cf. remus | 8.90 |
| 2 | Tr. chilonis | 1.37 | ||
| 8 | Te. cf. remus + Tr. chilonis | 5.48 |
Te. cf. remus = Telenomus cf. remus, Tr. chilonis = Trichogramma chilonis.
Across the maize-producing districts in which the study was conducted, there was significant geographic variability (p < 0.0001) in the presence of FAW larval hymenopteran parasitoids (Fig. 3a and Supplementary Table S6), and the effect of applying chemical insecticides on the parasitoids (p = 0.0017) was observed (Fig. 3b and Supplementary Table S7). The highest average parasitism rate was observed in Birbhum, West Bengal (30.90%), which was significantly higher than that in all other districts except Kishanganj, Bihar (18.70%). Moderate levels of parasitism were observed in Murshidabad (10.97%) and Katihar (8.60%), while all other districts recorded low rates of parasitism, with the lowest (2.56%) observed in Purnea, Bihar.
Figure 3.
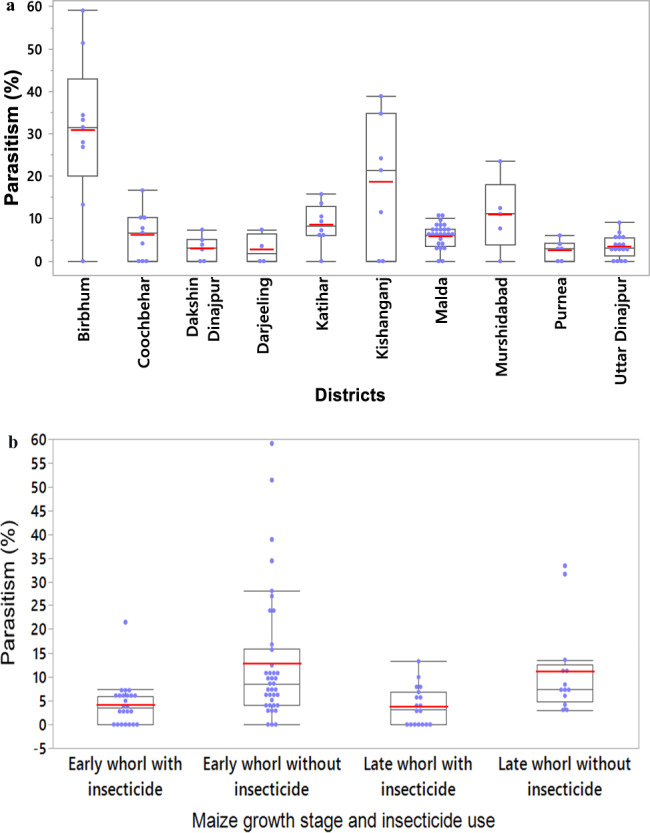
Parasitism rate of FAW (a) across the Indian Eastern Gangetic Plains (n = 93, p < 0.0001) and (b) affected by applying chemical insecticides (n = 93, p = 0.0017). Birbhum district showed the highest parasitism rate 30.90 ± 2.55% (0.0–59.09%). Crop growth stages without applying chemical insecticides also recorded significantly higher parasitism rates, with the highest rate of 12.90 ± 1.63% (0.0–59.09%) observed in the early whorl stage.
The application of chemical insecticides was noticeably low in Birbhum, where chemical insecticides had not been applied in approximately 82% of surveyed locations. In contrast, farmers in Dakshin Dinajpur, Uttar Dinajpur and Malda districts relied more on chemicals to control FAW, with between 60 and 70% of the surveyed locations in these districts having applied chemical insecticides.
FAW larvae sampled from early- and late-whorl stage maize without any insecticide applied had the highest parasitism rates (12.90 and 11.28%, respectively). Where insecticides had been applied, parasitism rates were considerably lower: 4.20 and 3.89% in early- and late-whorl stage maize, respectively.
The parasitism rate of individual species varied widely between locations (Table 4). The Birbhum district recorded the highest rate of parasitism by M. manilae (13.89%), and additional parasitism from an additional three species (Ch. formosanus, 7.54%; C. chlorideae, 4.37%; and Euplectrus spp., 3.57%). Rates of parasitism by Cha. bicolor (7.65%) and M. prodeniae (1.76%) were highest in the Kishanganj district, while the parasitoid Co. ruficrus was recorded solely in the Malda district, and Temelucha spp. only in Murshidabad district. More than one species of parasitoid was observed infesting FAW larvae in all districts except Darjeeling, where M. manilae was the only parasitoid found infesting the larval stage of the FAW host.
Table 4.
Parasitism rates of parasitoid species on FAW larvae collected from different maize-growing regions within the Indian Eastern Gangetic Plains.
| Location | Number of larvae reared | Parasitoid observed | Parasitoid family | Parasitism rate (%) |
|---|---|---|---|---|
| Birbhum, West Bengal | 252 | Ch. formosanus | Braconidae | 7.54 |
| C. chlorideae | Ichneumonidae | 4.37 | ||
| Euplectrus spp. | Eulophidae | 3.57 | ||
| Cha. bicolor | Ichneumonidae | 4.37 | ||
| M. manilae | Braconidae | 13.89 | ||
| Murshidabad, West Bengal | 97 | C. chlorideae | Ichneumonidae | 3.09 |
| Cha. bicolor | Ichneumonidae | 5.15 | ||
| Temelucha spp. | Ichneumonidae | 2.06 | ||
| Malda, West Bengal | 663 | Ch. formosanus | Braconidae | 0.60 |
| C. chlorideae | Ichneumonidae | 0.30 | ||
| Co. ruficrus | Braconidae | 0.60 | ||
| Euplectrus spp. | Eulophidae | 0.60 | ||
| Cha. bicolor | Ichneumonidae | 1.06 | ||
| M. prodeniae | Braconidae | 1.21 | ||
| M. manilae | Braconidae | 1.66 | ||
| Dakshin Dinajpur, West Bengal | 166 | Ch. formosanus | Braconidae | 0.60 |
| M. manilae | Braconidae | 2.41 | ||
| Uttar Dinajpur, West Bengal | 509 | C. chlorideae | Ichneumonidae | 0.20 |
| Euplectrus spp. | Eulophidae | 0.79 | ||
| Cha. bicolor | Ichneumonidae | 0.98 | ||
| M. prodeniae | Braconidae | 0.59 | ||
| M. manilae | Braconidae | 1.18 | ||
| Darjeeling, West Bengal | 98 | M. manilae | Braconidae | 3.06 |
| Coochbehar, West Bengal | 150 | C. chlorideae | Ichneumonidae | 2.00 |
| M. manilae | Braconidae | 4.00 | ||
| Katihar, Bihar | 231 | Ch. formosanus | Braconidae | 0.87 |
| Euplectrus spp. | Eulophidae | 3.03 | ||
| M. prodeniae | Braconidae | 0.87 | ||
| M. manilae | Braconidae | 3.46 | ||
| Purnea, Bihar | 192 | Cha. bicolor | Ichneumonidae | 1.04 |
| M. manilae | Braconidae | 1.56 | ||
| Kishanganj, Bihar | 170 | Euplectrus spp. | Eulophidae | 3.53 |
| Cha. bicolor | Ichneumonidae | 7.65 | ||
| M. prodeniae | Braconidae | 1.76 | ||
| M. manilae | Braconidae | 10.0 |
Ch. formosonus = Chelonus formosanus, C. chlorideae = Campoletis chlorideae, Co. ruficrus = Cotesia ruficrus, Cha. bicolor = Charops bicolor, M. manilae = Microplitis manilae, M. prodeniae = Microplitis prodeniae.
Microplitis manilae was the most abundant parasitoid species observed infesting FAW in maize crops across the Indian Eastern Gangetic Plains: it was observed in nine of the ten surveyed districts and had a relative abundance of 39.74% (Fig. 4). Another widely observed species was Cha. bicolor (observed in six districts and with a relative abundance of 18.38%); Euplectrus spp. (five locations, relative abundance 12.82%); Ch. formosanus (four locations, relative abundance 11.11%) and C. chlorideae (five locations, relative abundance 8.55%). Microplits prodeniae, with a relative abundance of 6.84%, was observed only in a small region in the neighboring districts of Malda and Uttar Dinajpur in West Bengal and Katihar and Kishanganj in Bihar.
Figure 4.
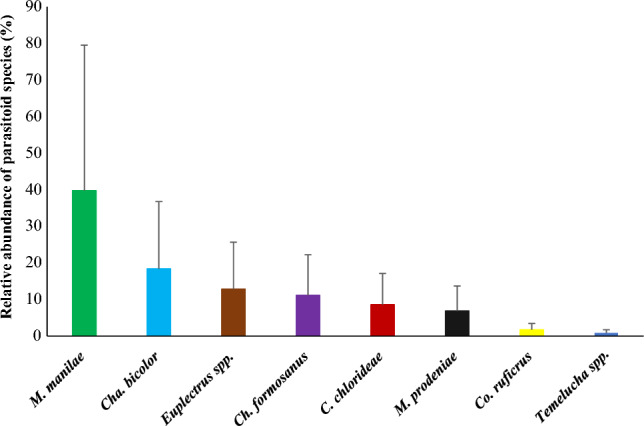
Relative abundance of parasitoids of FAW larvae observed in maize crop in the Indian Eastern Gangetic Plains. Out of 234 parasitized larvae, 93, 43, 30, 26, 20, 16, 4 and 2 larvae died for infestation of M. manilae, Cha. bicolor, Euplectrus spp., Ch. formosanus, C. chlorideae, M. prodeniae, Co. ruficrus and Temelucha spp. respectively. Microplitis manilae was recorded as the most dominant larval parasitoid of FAW in the region.
Discussion
In this research we observed greater FAW damage to maize in early growth stages (the early-whorl, V3–V6), with both damage incidence and severity declining in maize plants in the late-whorl and reproductive stages. Similar results have been observed elsewhere in southern Asia29,31–33. However, little damage to reproductive tissues may have a greater impact on yield than the foliar damage in the early-whorl growth stages. The crop can compensate for the damage at early stages34.
Moderate to high FAW damage was observed in all locations. Two locations, where relatively high use of insecticides was recorded, had greater FAW damage in maize than was observed in locations where no insecticides were applied. The apparent ineffectiveness of the insecticides on FAW suppression may be due to their improper selection, timing, and/or method of application, or to their poor efficiency against FAW. Other research has also demonstrated that the efficacy of chemicals in controlling FAW in maize primarily depends on appropriate insecticide selection and application. Baudron et al.23 also reported higher FAW infestation when insecticides were applied than in control plots.
Across ten study locations and from maize crops at different phenological growth stages we observed ten hymenopteran parasitoids: two that attacked FAW egg masses and eight that parasitized FAW larvae. While the mean parasitism rates were highest (12.90%) in FAW collected from early-whorl stage maize grown without insecticide application, the overall mean parasitism rates varied between 2.56 and 30.90% across the collection sites. Elsewhere, parasitism rates in FAW have been observed, between 3.6 and 9.2% in Uganda, and between 13.8 and 39.4% across the Americas35–38. The relatively high rate of parasitism observed in this study at Birbhum (30.90%) may have been a result of reduced use of broad-spectrum insecticides relative to other study locations.
We observed that both the parasitoids Te. cf. remus and Tr. chilonis parasitize FAW egg masses: Te. cf. remus attacks those egg masses that are covered in dense tufts of hair, while Tr. Chilonis, which are less hairy: the complimentary effect of these species has also been observed elsewhere39,40. Telenomus remus has been observed as a main FAW egg parasitoid in America and across Africa in both field and laboratory conditions25,41. Other researchers2,42 have also observed Te. remus parasitizing FAW at field level in India. Another FAW egg parasitoid, Tr. chilonis., has been observed elsewhere in maize crops with relatively high parasitism rates in India with a parasitism rate of 12.84–56.25%29,43, and in China with parasitism rates of 10.7–31.4%44: this parasitoid may also be useful in maize growing regions in eastern India.
Two konobiont endoparasitoids from the genus Microplitis were observed in the FAW larvae samples from the survey locations. Microplitis manilae, an important parasitoid of the armyworm group45, was the most abundant (39.74%) FAW larval parasitoid observed in the Indian EGP, with the highest parasitism rates, of up to 13.89% at Birbhum. A second parasitoid, M. prodeniae, was also observed in FAW larvae at four different study locations in the Indian Eastern Gangetic Plains, with parasitism rates between 0.59 and 1.76%. Microplitis prodeniae has elsewhere been recognized as a monophagous solitary parasitoid of Spodoptera litura46,47. The present report revealed that it also effectively parasitized Spodoptera exigua48. Our observation of M. prodeniae parasitizing FAW larvae in the Indian Eastern Gangetic Plains is similar to the findings of researchers from Rajasthan in western India (GenBank accession no. OP898529) and in China (GenBank accession numbers MW739950 and MW250775). As FAW becomes widespread across maize crops in Asia and outcompetes the native Asian armyworm S. litura in maize habitats49, there is potential for M. prodeniae, which has been effective as a biological control of S. litura, to also parasitize and thus suppress FAW throughout the region. Further research is needed to confirm this potential.
Other major FAW parasitoids observed in the study region were Cha. bicolor, C. chlorideae, Ch. formosanus, Temelucha spp., and Euplectrus spp., with relative abundances between 8.55 and 18.38%. Campoletis chlorideae, a solitary larval endoparasitoids that is a common parasitoid of FAW in the Indian subcontinent has elsewhere been demonstrated as an effective biological control of FAW2,29. Chelonus formosanus are egg-larval solitary koinobiont endoparasitoids that have been observed as one of the dominant FAW parasitoids in northern and northwestern India, with a maximum suppression rate of 16.40%43,50,51. Cotesia ruficrus (Haliday) has been observed to demonstrate considerable parasitism on FAW larvae elsewhere in India52, and different parasitoid species of the genus Cotesia have also been recorded to attack FAW larvae in other countries36,53. Charops bicolor, which is an important parasitoid of other obnoxious crop pests, such as yellow stem borer54 and S. litura55, is found to suppress FAW in the eastern states of India. Other parasitoid species of the genus Charops were observed as potential parasitoids of FAW by many researchers35,41. Temelucha spp. are endolarval hymenopteran parasitoids that have previously been shown to be effective against FAW larvae in India50,56 and other countries27. Similarly, Euplectrus spp., a konobiont ectoparasitoid, has been shown to effectively parasitize FAW larvae in India56. Other species of the genus Euplectrus, such as E. laphygmae and E. platyhypenae, have also been reported as FAW parasitoids from Africa57 and Argentina37, respectively.
FAW has particularly large negative impacts on maize growers’ production and profitability across Africa and Asia8,58. The extent of damage by FAW is greater outside the native areas due to the absence of naturally occurring biological control agents or native biological control agents of recently invaded areas that have not yet adopted the pest as their host59. The search for, and conservation of, parasitoids in agroecologies where FAW has relatively recently arrived is of prime importance to identify cost-effective, ecologically sustainable strategies with which FAW can be controlled in farmers’ fields. Parasitoids may be used effectively as a biological control of an invasive pest through either conservation or augmentative release30. Farmers' management practices in maize production are a key component of the cultivation of beneficial parasitoids and their rates of parasitism of FAW. It is likely that insect parasitism of FAW will increase in maize-producing regions of South Asia, as the spread of FAW itself continues. The use of chemical synthetic insecticides has been observed to negatively affect parasitism rates on FAW, and indiscriminate use of chemical insecticides imposes a negative impact on these natural, biological pest control methods60. It is imperative that effective, endemic FAW parasitoid species are identified and conserved38,61 by using selective insecticides and other agroecological approaches in crop management practices21,62.
We identified ten parasitoid species distributed across our sample sites in West Bengal and Bihar: two egg masses and eight larval parasitoids. Of the FAW larval parasitoids, Microplitis manilae was the most abundant, while Te. cf. remus was the most prevalent of the two FAW egg parasitoids. In field conditions where no chemical insecticides were applied, samples taken from maize in the early-whorl growth stage had higher rates of FAW larval parasitism than samples taken from maize in later growth stages. Similarly, FAW egg masses collected from locations where chemical insecticides were not applied had higher rates of parasitism. In contrast, maize plants observed in the early whorl stage to which synthetic chemical insecticides had been applied had comparable FAW damage incidence to that observed in maize plants where no insecticides had been applied.
Telenomus remus inflicted natural control of the FAW egg masses in Africa and Asia25,29,40,41 and has been found very effective for augmentative biological control in Latin America63. It may also be used for augmentative biological control in the newly invaded geographical regions including the study area. Trichogramma chilonis is already in use in India for augmentative release of other lepidopteran pests in paddy and sugarcane fields64,65 and may also be tested against FAW in farmers’ fields. Further attempts to identify and select suitable natural enemies of FAW for enhanced biological control in maize should consider two main characteristics: high efficacy to the host; and high host specificity to avoid nontarget effects34. Microplitis manilae, which generally infests Spodoptera spp. may also be of use and should be further investigated. At the same time, Microplitis prodeinae, which was considered as solitary parasitoid of S. litura, started adopting FAW as its host. All ten parasitoid species observed in this study naturally control the FAW population in the maize field of eastern India and this parasitoid complex may be the game changer in the near future. The conservation of these parasitoids in the maize habitats to achieve effective and sustainable suppression of FAW at early growth stages of the crop requires a combined application of all possible agro-ecological management strategies, including the use of botanicals, biopesticides, intercrops, trap crops, and so on, without using chemical pesticides in the first 30 to 45 days of the crop growth stages.
Method
All experimental research on cultivated plants and insects described here complied with relevant institutional, national and international guidelines and legislation.
Fall armyworm survey and assessment of damage
Farmers’ fields were surveyed between October 2021 and March 2022 to quantify the incidence and effects of FAW on farmers’ maize crops in two Indian states, West Bengal and Bihar. The survey was conducted in over 142 different locations in ten districts of these two states, in a north–south gradient at approximately 23.5–26.5°N and centered around 88°E (Fig. 5). The survey covered approximately 40% of the maize cultivated area in Bihar and West Bengal.
Figure 5.
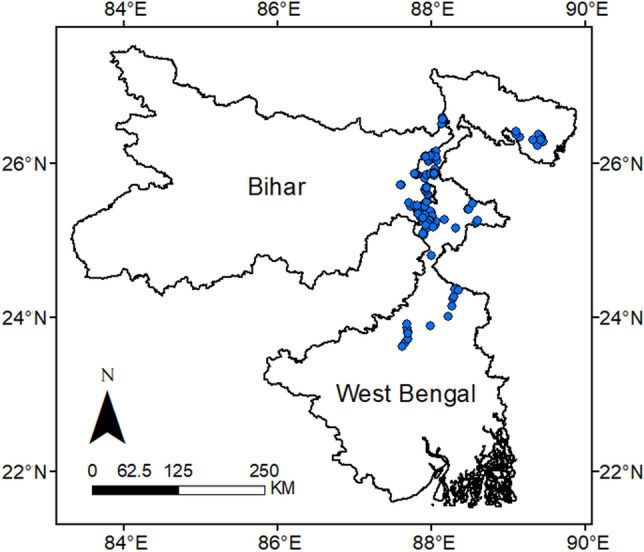
GPS coordinates of survey locations in the eastern gangetic plains of India.
FAW infestation was determined through the presence of fresh frass and feeding injuries on maize whorls and leaves. One hundred twenty-four scouting locations had FAW incidence. The methodology of McGrath et al.66 was followed, examining maize plants for ‘W Scouting patterns’ in the early- (VE to V6: emergence to six leaves stage) and late- (V7 to VT: seven leaves to initiation of tasseling stage) whorl phenological growth stages, and for ‘ladder patterns’ in the reproductive (tasseling and silking) stages. At each sampling location, five spots of 4.0 m row length with 3 rows were randomly selected from within a maize field. Twenty plants were randomly selected from each spot and used to quantify the maize infestation damage by expressing the number of plants infested by FAW out of the hundred plants examined as a percentage.
Ten maize plants (two each from the five spots) were randomly selected from each sampling location, and the severity of FAW damage on the newly grown plant parts (i.e., whorls and furls) was quantified using the simple day-independent scale described by Davis and Williams67 and suggested by Toepfer et al.68, which uses Eq. (1):
| 1 |
where I = severity of FAW damage, Z = highest possible damage score (here 9), N = number of plants observed, n = number of plants that have a ‘v’ value, v = value (score) of the crop damage, where 0 is no visible signs of damage and 9 is almost total destruction of whorl and furled leaves.
FAW egg masses and larvae were collected from early- and late-whorl stages of maize plants at all locations where they were observed and grouped into those that had or had not been sampled from maize treated with chemical insecticides based on information gathered from farmers. FAW gathered from maize where insecticides had been applied at least 25 days prior were deemed to be in the “noninsecticide group”; those gathered from maize where insecticides had been applied within 25 days were the “insecticide group”. Mostly the first to fourth larval instars were collected through semidestructive sampling by pulling apart the damaged leaf whorls. Egg masses were collected from 7- to 21- day-old plants. Farmer’s cropping system management information was also collected from each survey sample plot.
Collection of FAW and rearing for emergence of parasitoids
The field collected FAW egg masses and larvae were morphologically identified69 and reared under laboratory conditions following standard protocols52. The larvae were placed individually in 100 ml transparent plastic rearing containers with small pin holes at the top cover to allow ventilation while containing the larvae. Maize leaves were rinsed with double distilled water, cut into small pieces, and transferred into rearing containers. Filter paper (Whatman grade 1, size 110 mm) was placed at the bottom of the containers to absorb excess moisture produced by the maize leaves and larvae, and frass. The larvae were reared on maize leaves in the laboratory under room conditions (27 ± 3 °C, 75 ± 5% RH, and 12:12 h photoperiod). The larvae were examined every 24 h for emerged parasitoids which were collected, placed in 70% ethanol, and then subjected to molecular identification. Parasitoids were photographed using a stereo zoom microscope (make: Lieca S9i).
Molecular identification of parasitoids
High-quality DNA was obtained from parasitoids and then submitted to the National Centre for Biotechnology Information to determine GenBank accession numbers and match them to reference accessions through BLAST analysis. DNA was extracted from the parasitoid insects using Qiagen D Neasy® kits, following the manufacturer’s protocol. The DNA extracts were subjected to polymerase chain reaction (PCR) amplification of a 700 bp region near the 5′ terminus end of the COX1 gene following the standard protocol70. The primers used were as follows: forward primer (LCO 1490: 5′- GGTCAACAAATCATAAAGATATTGG-3′), and reverse primer (HCO 2198: 5′- TAAACTTCAGGGT GACCAAAAAATCA-3′). PCRs were carried out in 96-well plates with a 50 µL reaction volume containing: 5 µL GeNeiTM Taq buffer, 1 µL GeNeiTM 10 mM dNTP mix, 2.5 µL (20 pmol/µL) forward primer, 2.5 µL (20 pmol/µL) reverse primer, 1 µL GeNeiTM Taq DNA polymerase (1 U/µL), 2 µL DNA (50 ng/µL), and 36 µL sterile water. Thermocycling consisted of an initial denaturation at 95 °C for 3 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 50 °C for 30 and extension at 72 °C for 45 s followed by another 3 min at 72 °C. PCR analysis was performed using a C1000™ Thermal Cycler. The amplified products were analyzed by 1.5% agarose gel electrophoresis. The amplified products were purified to remove contaminants and then sequenced using a BDT v3.1 Cycle sequencing kit on an ABI 3730xl Genetic Analyzer. The consensus sequence of the COI gene was generated from forward and reverse sequence data using aligner software. The COI gene sequence was used to carry out BLAST with the ‘nr’ database of NCBI GenBank database. Using a maximum identity score, the first ten sequences were selected and aligned using a multiple alignment software program. A cluster W Distance matrix was generated using an RDP database, and the phylogenetic tree was constructed using MEGA 6. The amplified products were sequenced by Barcode Biosciences Private Limited, Bengaluru, India. This was further compared with the BOLD (Barcode of Life Data System) to confirm their similarity with the other barcoded parasitoid specimens across the world.
Morphological study of parasitoids
Parasitoids were identified, and sample specimens were preserved in the laboratories of Visva Bharati University and the National Insect Museum of the Indian Council of Agricultural Research-National Bureau of Agricultural Insect Resources, India. The morphological analysis of two parasitoid species under the genus Microplitis was conducted using the taxonomic keys outlined by Gupta71, and species identification was determined using previously published descriptions72,73. The standard morphological descriptions were also followed for Co. ruficrus74; Cha. bicolor75; C. chlorideae76; Che. formosanus77; Te. cf. remus78; and Tr. chilonis79,80. The morphology of the genera Temelucha and Euplectrus were matched with the general description proposed by Townes81 and Hansson et al.82, respectively.
Parasitoid effectiveness
The number of FAW larvae collected was calculated by subtracting from the total number of larvae those that had died from other causes besides parasitoids, such as infection by entomopathogens, predators, insecticides, physical injury, or unknown causes. Next, the parasitism rate (PR) and relative abundance (RA) were calculated using the methodology of Pair et al.61 and Agboyi et al.83, respectively. The percentage of FAW killed by parasitoids was calculated for each location by dividing the number of parasitoids that emerged by the total number of FAW larvae collected and multiplying by 100. The PR of each parasitoid species was calculated using Eq. (2):
| 2 |
where Lp is the number of FAW larvae parasitized and TL is the total number of FAW larvae collected.
The RA of each species was determined using Eq. (3):
| 3 |
where Ni is the number of individuals of a given species and Nt is the total number of all parasitoids that were recorded.
Data analysis
Data were graphed and examined for correlations and regressions using R version 4.3.0. Analysis of variance (ANOVA) was conducted using the statistical analysis system (SAS) software (Version 9.4) on plant damage incidence and damage severity, using field plot location, crop growth stage and their interaction as factors. Similarly, ANOVA was also performed on the parasitism rate across the locations and the impact of chemical insecticides on the parasitism rate at different crop growth stages. The normality assumption of analysis of variance (ANOVA) was tested using the Shapiro Wilk test84. Pooled treatment adjusted means under different parameters were compared using Tukey’s honest significant difference (HSD) test (p ≤ 0.05).
Supplementary Information
Acknowledgements
The authors gratefully acknowledge Barcode Biosciences Private Limited, Bengaluru, India for sequencing DNA samples. The authors are also grateful to Dr. Arun Kumar Barik, Dean, Palli Siskha Bhavana, Visva Bharati University, Shantiniketan, Bolpur, West Bengal, India and Dr. Ashok Choudhury, Director of Research, Uttar Banga Krishi Viswavidyalaya, Pundibari, Coochbehar, West Bengal, India for their support and facilitation in carrying out the research work successfully. The authors are also thankful to Dr. Satya Nanda Sushil, Director of ICAR-National Bureau of Agricultural Insect Resources, Bengaluru, Karnataka, India for his constant support during this investigation.
Author contributions
S.P., N.G., P.P., N.C.: Survey works and investigation; S.B., T.D.: Conceptualization, visualization, supervision, data curation, writing original draft; A.G., H.C.: Morphological identification, sources of instruments, writing original draft; S.D.: Molecular analysis, A.Gh.: Statistical analysis, S.K.S.: writing original draft, P.M.B., M.K.G., A.M.L.: Editing of original draft.
Data availability
The dataset generated through molecular analysis during the current study is available with the following links: 1. Trichogramma chilonis (OQ849581): https://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Search&db=nucleotide&term=OQ849581.1&dopt=GenBank. 2. Telenomus cf. remus (OP288795): https://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Search&db=nucleotide&term=OP288795.1&dopt=GenBank. 3. Chelonus formosanus (OP278928): https://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Search&db=nucleotide&term=OP278928.1&dopt=GenBank. 4. Campoletis chlorideae (OP269829): https://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Search&db=nucleotide&term=OP269829.1&dopt=GenBank. 5. Charops bicolor (OP393900): https://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Search&db=nucleotide&term=OP393900.1&dopt=GenBank. 6. Cotesia ruficrus (OP288793): https://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Search&db=nucleotide&term=OP288793.1&dopt=GenBank. 7. Microplitis manilae (OP288794): https://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Search&db=nucleotide&term=OP288794.1&dopt=GenBank. 8. Microplitis prodeniae (OR053831):.https://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Search&db=nucleotide&term=OR053831.1&dopt=GenBank. 9. Euplectrus spp. (OR053833): https://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Search&db=nucleotide&term=OR053833.1&dopt=GenBank. 10. Temelucha spp. (OQ848503): https://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Search&db=nucleotide&term=OQ848503.1&dopt=GenBank. Supplementary information is also available with http://nature.com.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-54342-z.
References
- 1.Sharanabasappa S, et al. First report of the fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae), an alien invasive pest on maize in India. Pest Manag. Hortic. Ecosyst. 2018;24:23–29. [Google Scholar]
- 2.Shylesha AN, et al. Studies on new invasive pest Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) and its natural enemies. J. Biol. Control. 2018;32:1–7. doi: 10.18311/jbc/2018/21707. [DOI] [Google Scholar]
- 3.Suby SB, et al. Invasion of fall armyworm (Spodoptera frugiperda) in India: Nature, distribution, management and potential impact. Cur. Sci. 2020;119:44–51. doi: 10.18520/cs/v119/i1/44-51. [DOI] [Google Scholar]
- 4.Dhar T, et al. Occurrence of fall armyworm Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) on maize in West Bengal, India and its field life table studies. J. Entomol. Zool. Stud. 2019;7:869–875. [Google Scholar]
- 5.Rwomushana, I. Spodoptera frugiperda (fall armyworm). Invasive Species Compendium (29810). 10.1079/cabicompendium.29810 (2019).
- 6.Montezano DG, et al. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018;26:286–300. doi: 10.4001/003.026.0286. [DOI] [Google Scholar]
- 7.Overton K. Global crop impacts, yield losses and action thresholds for fall armyworm (Spodoptera frugiperda): A review. Crop Prot. 2021;145:105641. doi: 10.1016/j.cropro.2021.105641. [DOI] [Google Scholar]
- 8.Eschen R, et al. Towards estimating the economic cost of invasive alien species to African crop and livestock production. CABI Agric. Biosci. 2021;2:1–18. doi: 10.1186/s43170-021-00038-7. [DOI] [Google Scholar]
- 9.FAO. Briefing note on FAO actions on fall armyworm. www.fao.org/3/bs183/bs183e.pdf (2019).
- 10.Ericksen, P. J. et al. Mapping hotspots of climate change and food insecurity in the global tropics. 5. CCAFS report. 1–52 (2011).
- 11.Pyne S, Guha S, Das S, Ray M, Chandra H. Food insecurity in the eastern Indo-Gangetic plain: Taking a closer look. PLoS ONE. 2023;18:e0279414. doi: 10.1371/journal.pone.0279414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Islam S, et al. Conservation agriculture based sustainable intensification: Increasing yields and water productivity for smallholders of the eastern Gangetic plains. Field Crops Res. 2019;238:1–17. doi: 10.1016/j.fcr.2019.04.005. [DOI] [Google Scholar]
- 13.Gathala MK, et al. Energy-efficient, sustainable crop production practices benefit smallholder farmers and the environment across three countries in the eastern Gangetic plains, South Asia. J. Clean. Prod. 2020;246:118982. doi: 10.1016/j.jclepro.2019.118982. [DOI] [Google Scholar]
- 14.Gathala MK, et al. Enabling small holder farmers to sustainably improve their food, energy and water nexus while achieving environmental and economic benefits. Renew. Sustain. Energy Rev. 2020;120:109645. doi: 10.1016/j.rser.2019.109645. [DOI] [Google Scholar]
- 15.Indiastat. Selected state wise area, production and productivity of maize in India (2020–2021). http://www.indiastat.com/table/selected-state-wise-area-production-productivity-m/1423779 (Date of access 31052023) (2023).
- 16.Zhao YX, et al. Susceptibility of fall armyworm, Spodoptera frugiperda (JE Smith), to eight insecticides in China, with special reference to lambda-cyhalothrin. Pesticide Biochem. Physiol. 2020;168:104623. doi: 10.1016/j.pestbp.2020.104623. [DOI] [PubMed] [Google Scholar]
- 17.Gutiérrez-Moreno R, et al. Field-evolved resistance of the fall armyworm (Lepidoptera: Noctuidae) to synthetic insecticides in Puerto Rico and Mexico. J. Econ. Entomol. 2018;112:792–802. doi: 10.1093/jee/toy372. [DOI] [PubMed] [Google Scholar]
- 18.Kumar RM, et al. Sustainable management of invasive fall armyworm, Spodoptera frugiperda. Agronomy. 2022;12:2150. doi: 10.3390/agronomy12092150. [DOI] [Google Scholar]
- 19.Tambo JA, et al. Tackling fall armyworm (Spodoptera frugiperda) outbreak in Africa: An analysis of farmers’ control actions. Int. J. Pest Manag. 2019;66:298–310. doi: 10.1080/09670874.2019.1646942. [DOI] [Google Scholar]
- 20.Abbas A, et al. Biological control of fall armyworm, Spodoptera frugiperda. Agronomy. 2022;12:2704. doi: 10.3390/agronomy12112704. [DOI] [Google Scholar]
- 21.Kenis M, et al. Invasiveness, biology, ecology, and management of the fall armyworm, Spodoptera frugiperda. Entomol. Gen. 2022;43:187–241. doi: 10.1127/entomologia/2022/1659. [DOI] [Google Scholar]
- 22.Guera OGM, et al. Effectiveness of push–pull systems to fall armyworm (Spodoptera frugiperda) management in maize crops in Morelos, Mexico. Insects. 2021;12:298. doi: 10.3390/insects12040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baudron F, Zaman-Allah MA, Chaipa I, Chari N, Chinwada P. Understanding the factors influencing fall armyworm (Spodoptera frugiperda JE Smith) damage in African smallholder maize fields and quantifying its impact on yield. A case study in eastern Zimbabwe. Crop Prot. 2019;120:141–150. doi: 10.1016/j.cropro.2019.01.028. [DOI] [Google Scholar]
- 24.Midega CA, Pittchar JO, Pickett JA, Hailu GW, Khan ZRA. Climate-adapted push-pull system effectively controls fall armyworm, Spodoptera frugiperda (JE Smith), in maize in East Africa. Crop Prot. 2018;105:10–15. doi: 10.1016/j.cropro.2017.11.003R. [DOI] [Google Scholar]
- 25.Winsou JK, et al. Seasonal variations of Spodoptera frugiperda host plant diversity and parasitoid complex in southern and central Benin. Insects. 2022;13:491. doi: 10.3390/insects13060491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ordóñez-García M, et al. Occurrence of natural enemies of Spodoptera frugiperda (Lepidoptera: Noctuidae) in Chihuahua, Mexico. Fla. Entomol. 2015 doi: 10.1653/024.098.0305. [DOI] [Google Scholar]
- 27.Tchao M, et al. Literature review on the natural enemies of Spodoptera frugiperda JE Smith (Lepidoptera: Noctuidae) and the effectiveness of their use in the management of this pest in corn (Zea mays L.) IJMRA. 2022;5:1591–1601. doi: 10.5281/zenodo.6797815. [DOI] [Google Scholar]
- 28.Molina-Ochoa J, Carpenter JE, Heinrichs EA, Foster JE. Parasitoids and parasites of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas and Caribbean basin: an inventory. Fla. Entomol. 2003;86:254–289. doi: 10.1653/0015-4040(2003)086[0254:PAPOSF]2.0.CO;2. [DOI] [Google Scholar]
- 29.Navik O, et al. Damage, distribution and natural enemies of invasive fall armyworm Spodoptera frugiperda (JE smith) under rainfed maize in Karnataka. India. Crop Prot. 2021;143:105536. doi: 10.1016/j.cropro.2021.105536. [DOI] [Google Scholar]
- 30.Sharanabasappa S, et al. Natural enemies of Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae), a recent invasive pest on maize in South India. Fla. Entomol. 2019;102:619–623. doi: 10.1653/024.102.0335. [DOI] [Google Scholar]
- 31.Singh S, et al. Occurrence, distribution, damage potential, and farmers’ perception on fall armyworm, Spodoptera frugiperda (JE Smith): Evidence from the eastern Himalayan region. Sustainability. 2023;15:5681. doi: 10.3390/su15075681. [DOI] [Google Scholar]
- 32.Mutyambai DM, et al. Agronomic factors influencing fall armyworm (Spodoptera frugiperda) infestation and damage and its co-occurrence with stemborers in maize cropping systems in Kenya. Insects. 2022;13:266. doi: 10.3390/insects13030266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Supartha IW, et al. Damage characteristics and distribution patterns of invasive pest, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) on maize crop in Bali, Indonesia. Biodiv. J. Bio. Divers. 2021;22:3378–3389. doi: 10.13057/biodiv/d2206xx. [DOI] [Google Scholar]
- 34.Kenis M. Prospects of classical biological control of Spodotera frugiperda (Lepidoptera: Noctuidae) in invaded areas using parasitoids from the Americas. J. Econ. Entomol. 2023;116:331–341. doi: 10.1093/jee/toad029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otim MH, et al. Parasitoid distribution and parasitism of the fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) in different maize producing regions of Uganda. Insects. 2021;12:121. doi: 10.3390/insects12020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meagher RL, Jr, Nuessly GS, Nagoshi RN, Hay-Roe MM. Parasitoids attacking fall armyworm (Lepidoptera: Noctuidae) in sweet corn habitats. Biol. Control. 2016;95:66–72. doi: 10.1016/j.biocontrol.2016.01.006. [DOI] [Google Scholar]
- 37.Murúa MG, Molina-Ochoa J, Fidalgo P. Natural distribution of parasitoid of the larvae of the fall armyworm, Spodoptera frugiperda, Argentina. J. Insect Sci. 2009;9:1–17. doi: 10.1673/031.009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molina-Ochoa J, et al. Natural distribution of hymenopteran parasitoids of Spodoptera frugiperda (Lepidoptera: Noctuidae) larvae in Mexico. Fla. Entomol. 2004;87:461–472. doi: 10.1653/0015-4040(2004)087[0461:NDOHPO]2.0.CO;2. [DOI] [Google Scholar]
- 39.Ballal, C. R. et al. Biological control for fall armyworm management in Asia in Fall armyworm in Asia: A Guide for integrated pest management. (ed. Prasanna, B.M.) 114–137 (CIMMYT, 2021). https://hdl.handle.net/10883/21658.
- 40.Laminou SA, Ba MN, Karimoune L, Doumma A, Muniappan R. Parasitism of locally recruited egg parasitoids of the fall armyworm in Africa. Insects. 2020;11:430. doi: 10.3390/insects11070430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abang AF, et al. Natural enemies of fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) in different agro-ecologies. Insects. 2021;12:509. doi: 10.3390/insects12060509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Firake DM, Behere GT. Natural mortality of invasive fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) in maize agroecosystems of northeast India. Biol. Control. 2020;148:104303. doi: 10.1016/j.biocontrol.2020.104303. [DOI] [Google Scholar]
- 43.Keerthi MC, et al. Bio-intensive tactics for the management of invasive fall armyworm for organic maize production. Plants. 2023;12:685. doi: 10.3390/plants12030685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang L. Performance of three Trichogramma species as biocontrol agents on Spodoptera frugiperda eggs. J. Appl. Entomol. 2022;146:1019–1027. doi: 10.1111/jen.13042. [DOI] [Google Scholar]
- 45.Moghaddam MG, Butcher BA. Microplitis manilae Ashmead (Hymenoptera: Braconidae): Biology, systematics, and response to climate change through ecological niche modelling. Insects. 2023;14:338. doi: 10.3390/insects14040338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan Z, et al. Parasitism of Spodoptera litura (Lepidoptera: Noctuidae) by Microplitis prodeniae (Hymenoptera: Braconidae) Neotrop. Entomol. 2017;47:139–144. doi: 10.1007/s13744-017-0542-y. [DOI] [PubMed] [Google Scholar]
- 47.Zhou Z, Chen Z, Xu Z. Potential of trap crops for integrated management of the tropical armyworm, Spodoptera litura in tobacco. J. Insect Sci. 2010;10:117. doi: 10.1673/031.010.11701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ou-Yang YY, et al. Parasitism of two Spodoptera spp. by Microplitis prodeniae (Hymenoptera: Braconidae) J. Econ. Entomol. 2018;111:1131–1136. doi: 10.1093/jee/toy085. [DOI] [PubMed] [Google Scholar]
- 49.Song Y, et al. Interference competition and predation between invasive and native herbivores in maize. J. Pest Sci. 2021;94:1053–1063. doi: 10.1007/s10340-021-01347-6. [DOI] [Google Scholar]
- 50.Jindal J, Sharma KP, Shera PS, Cheema HK. Native parasitoids of fall armyworm Spodoptera frugiperda (JE Smith) in Maize. Ind. J. Entomol. 2022;84:865–867. doi: 10.55446/IJE.2021.72. [DOI] [Google Scholar]
- 51.Sagar D, Suroshe SS, Keerthi MC, Poorani J, Gupta A, Chandel RK. Native parasitoid complex of the invasive fall armyworm, Spodoptera frugiperda (JE Smith) from northern India. Int. J. Trop. Insect Sci. 2022;42:2773–2778. doi: 10.1007/s42690-022-00743-4. [DOI] [Google Scholar]
- 52.Gupta A, Babu SR, Kumar MS. Cotesia ruficrus (Haliday, 1834) (Hymenoptera: Braconidae) emerging as a common natural parasitoid of Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) in Indian maize fields. J. Biol. Control. 2019;33:193–196. doi: 10.18311/jbc/2019/24118. [DOI] [Google Scholar]
- 53.Koffi D, et al. Natural enemies of the fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) in Ghana. Fla. Entomol. 2020;103:85–90. doi: 10.1653/024.103.0414. [DOI] [Google Scholar]
- 54.Prasanthi G, Rath PC, Dey D. First record of Brachymeria excarinata Gahan, 1925 (Hymenoptera: Chalcididae) as a hyperparasitoid of Charops bicolor (Szepligeti, 1906) (Hymenoptera: Ichneumonidae) from India. Natl. Acad. Sci. Lett. 2023;46:87–89. doi: 10.1007/s40009-023-01204-3. [DOI] [Google Scholar]
- 55.Chiu SC, Chou LY. Hymenopterous parasitoids of Spodotera litura Fab. J. Agric. Res. China. 1976;25:227–241. [Google Scholar]
- 56.Anandhi S, Saminathan VR. New record of larval parasitoids and predatory spiders on fall armyworm Spodoptera frugiperda (JE Smith) (Noctuidae: Lepidoptera) in Tamil Nadu. J. Entomol. Zool. Stud. 2021;9:340–342. [Google Scholar]
- 57.Ogunfunmilayo A, et al. Occurrence of natural enemies of fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae) in Nigeria. PLoS ONE. 2021;16:e0254328. doi: 10.1371/journal.pone.0254328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mendesil E, et al. The invasive fall armyworm, Spodoptera frugiperda, in Africa and Asia: Responding to the food security challenge, with priorities for integrated pest management research. J. Plant Dis. Prot. 2023;130:1175–1206. doi: 10.1007/s41348-023-00777-x. [DOI] [Google Scholar]
- 59.Cruz, I. et al. Biological control and biorational pesticides for fall armyworm management, in Fall Armyworm in Africa: A Guide for Integrated Pest Management (ed. Prasanna, B.M.) 63–88 (CIMMYT, 2018). http://hdl.handle.net/10883/19204.
- 60.Agboyi LK, et al. Comparative effects of biopesticides on fall armyworm management and larval parasitism rates in northern Ghana. J. Pest Sci. 2023 doi: 10.1007/s10340-023-01590-z. [DOI] [Google Scholar]
- 61.Pair SD, Raulston JR, Sparks AN, Martin PB. Fall armyworm (Lepidoptera: Noctuidae) parasitoids: differential spring distribution and incidence on corn and sorghum in the southern United States and northeastern México. Environ. Entomol. 1986;15:342–348. doi: 10.1093/ee/15.2.342. [DOI] [Google Scholar]
- 62.Ngangambe MH, Mwatawala MW. Effects of entomopathogenic fungi (EPFs) and cropping systems on parasitoids of fall armyworm (Spodoptera frugiperda) on maize in eastern central, Tanzania. Biocontrol Sci. Technol. 2020;30:418–430. doi: 10.1080/09583157.2020.1726878. [DOI] [Google Scholar]
- 63.Colmenarez YC, Babendreier D, Wurst FRF, Vásquez-Freytez CL, de Freitas Bueno A. The use of Telenomus remus (Nixon, 1937) (Hymenoptera: Scelionidae) in the management of Spodoptera spp.: potential, challenges and major benefits. CABI Agric. Biosci. 2022;3:5. doi: 10.1186/s43170-021-00071-6. [DOI] [Google Scholar]
- 64.Sangha KS, Shera PS, Sharma S, Kaur R. On-farm impact of egg parasitoid Trichogramma spp. against lepidopteran pests in organic basmati rice. J. Biol. Control. 2018;32:116–120. doi: 10.18331/jbc/2018/16272. [DOI] [Google Scholar]
- 65.Sharma S, Shera PS, Kaur R, Sangha KS. Evaluation of augmentative biological control strategy against major borer insect pests of sugarcane—a large scale field appraisal. Egypt. J. Biol. Pest Control. 2020;30:127. doi: 10.1186/s41938-020-00330-0. [DOI] [Google Scholar]
- 66.McGrath, D. et al. Monitoring, surveillance, and scouting for fall armyworm, in Fall Armyworm in Africa: A Guide for Integrated Pest Management (ed. Prasanna, B.M.) 11–28 (CIMMYT, 2018) http://hdl.handle.net/10883/19204.
- 67.Davis FM, Ng SS, Williams WP. Visual rating scales for screening whorl-stage corn for resistance to fall armyworm. Tech. Bull. (Miss. Agric. For. Exp. Stn.) 1992;186:1–9. [Google Scholar]
- 68.Toepfer S, et al. Streamlining leaf damage rating scales for the fall armyworm on maize. J. Pest Sci. 2021;94:1075–1089. doi: 10.1007/s10340-021-01359-2. [DOI] [Google Scholar]
- 69.Huesing, J. E. et al. Integrated pest management of fall armyworm in Africa: An introduction, in Fall Armyworm in Africa: A Guide for Integrated Pest Management (ed. Prasanna, B.M.) 1–10 (CIMMYT, 2018). http://hdl.handle.net/10883/19204.
- 70.Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994;3:294–299. [PubMed] [Google Scholar]
- 71.Gupta A. Revision of the Indian Microplitis Foerster (Hymenoptera: Braconidae: Microgastrinae), with description of one new species. Zootaxa. 2013;3620:429–452. doi: 10.11646/zootaxa.3620.3.5. [DOI] [PubMed] [Google Scholar]
- 72.Rao SN, Kurian C. Descriptions of eleven new and records of fifteen known species of Ichneumonoidea (Hymenoptera Parasitica) from India. Ind. J. Entomol. 1950;12:167–190. [Google Scholar]
- 73.Ashmead WH. A list of the Hymenoptera of the Philippine Islands, with descriptions of new species. J. N. Y. Entomol. Soc. 1904;12:1–22. [Google Scholar]
- 74.Wilkinson DSA. Revision of the Indo-Australian species of the genus Apanteles (Hym. Bracon.)—Part II. Bull. Entomol. Res. 1928;19:109–146. doi: 10.1017/S0007485300020393. [DOI] [Google Scholar]
- 75.Gupta VK, Maheshwary S. Indian species of Charops Holmgren (Hymenoptera: Ichneumonidae) Orient. Insects. 1970;4:453–480. doi: 10.1080/00305316.1970.10433983. [DOI] [Google Scholar]
- 76.Wei YW, Zhou YB, Zou QC, Sheng ML. A new species of Campoletis Förster (Hymenoptera, Ichneumonidae) with a key to species known from China, Japan and South Korea. Zookeys. 2020;1004:99–108. doi: 10.3897/zookeys.1004.57913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gupta A, Lalitha Y, Varshney R, Shylesha AN, Van Achterberg C. Chelonus formosanus Sonan (Hymenoptera: Braconidae) an egg-larval parasitoid of the invasive pest Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) amenable to laboratory mass production in India. J. Entomol. Zool. Stud. 2020;8:1521–1524. [Google Scholar]
- 78.Nixon GEJ. Some Asiatic Telenominæ (Hym., Proctotrupoidea) Ann. Mag. Natl. Hist. 1937;20:444–475. doi: 10.5281/zenodo.23908. [DOI] [Google Scholar]
- 79.Nagarkatti S, Nagaraja H. The status of Trichogramma chilonis Ishii (Hym: Trichogrammatidae) Orient. Insects. 1979;13:115–117. doi: 10.1080/00305316.1979.10433549. [DOI] [Google Scholar]
- 80.Nagarkatti S, Nagaraja H. Redescriptions of some known species of Trichogramma (Hym., Trichogrammatidae), showing the importance of the male genitalia as a diagnostic character. Bull. Entomol. Res. 1971;61:13–31. doi: 10.1017/S0007485300057412. [DOI] [Google Scholar]
- 81.Townes, H. The genera of Ichneumonidae. Part 4. Memoirs of the American entomological institute. 17, 1–372 (1971).
- 82.Hansson C, Smith MA, Janzen DH, Hallwachs W. Integrative taxonomy of new World Euplectrus Westwood (Hymenoptera, Eulophidae), with focus on 55 new species from Area de Conservación Guanacaste, northwestern Costa Rica. Zookeys. 2015;485:1–236. doi: 10.3897/zookeys.485.9124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Agboyi LK, et al. Parasitoid complex of fall armyworm, Spodoptera frugiperda, in Ghana and Benin. Insects. 2020;11:68. doi: 10.3390/insects11020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples) Biometrika. 1965;52:591–611. doi: 10.2307/2333709. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset generated through molecular analysis during the current study is available with the following links: 1. Trichogramma chilonis (OQ849581): https://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Search&db=nucleotide&term=OQ849581.1&dopt=GenBank. 2. Telenomus cf. remus (OP288795): https://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Search&db=nucleotide&term=OP288795.1&dopt=GenBank. 3. Chelonus formosanus (OP278928): https://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Search&db=nucleotide&term=OP278928.1&dopt=GenBank. 4. Campoletis chlorideae (OP269829): https://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Search&db=nucleotide&term=OP269829.1&dopt=GenBank. 5. Charops bicolor (OP393900): https://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Search&db=nucleotide&term=OP393900.1&dopt=GenBank. 6. Cotesia ruficrus (OP288793): https://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Search&db=nucleotide&term=OP288793.1&dopt=GenBank. 7. Microplitis manilae (OP288794): https://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Search&db=nucleotide&term=OP288794.1&dopt=GenBank. 8. Microplitis prodeniae (OR053831):.https://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Search&db=nucleotide&term=OR053831.1&dopt=GenBank. 9. Euplectrus spp. (OR053833): https://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Search&db=nucleotide&term=OR053833.1&dopt=GenBank. 10. Temelucha spp. (OQ848503): https://www.ncbi.nlm.nih.gov/sites/entrez?cmd=Search&db=nucleotide&term=OQ848503.1&dopt=GenBank. Supplementary information is also available with http://nature.com.