Abstract
Diabetes retinopathy prevention necessitates early detection, monitoring, and treatment. Non-invasive optical coherence tomography (OCT) shows structural changes in the retinal layer. OCT image evaluation necessitates retinal layer segmentation. The ability of our automated retinal layer segmentation to distinguish between normal, non-proliferative (NPDR), and proliferative diabetic retinopathy (PDR) was investigated in this study using quantifiable biomarkers such as retina layer smoothness index (SI) and area (S) in horizontal and vertical OCT images for each zone (fovea, superior, inferior, nasal, and temporal). This research includes 84 eyes from 57 individuals. The study shows a significant difference in the Area (S) of inner nuclear layer (INL) and outer nuclear layer (ONL) in the horizontal foveal zone across the three groups (p < 0.001). In the horizontal scan, there is a significant difference in the smoothness index (SI) of the inner plexiform layer (IPL) and the upper border of the outer plexiform layer (OPL) among three groups (p < 0.05). There is also a significant difference in the area (S) of the OPL in the foveal zone among the three groups (p = 0.003). The area (S) of the INL in the foveal region of horizontal slabs performed best for distinguishing diabetic patients (NPDR and PDR) from normal individuals, with an accuracy of 87.6%. The smoothness index (SI) of IPL in the nasal zone of horizontal foveal slabs was the most accurate at 97.2% in distinguishing PDR from NPDR. The smoothness index of the top border of the OPL in the nasal zone of horizontal slabs was 84.1% accurate in distinguishing NPDR from PDR. Smoothness index of IPL in the temporal zone of horizontal slabs was 89.8% accurate in identifying NPDR from PDR patients. In conclusion, optical coherence tomography can assess the smoothness index and irregularity of the inner and outer plexiform layers, particularly in the nasal and temporal regions of horizontal foveal slabs, to distinguish non-proliferative from proliferative diabetic retinopathy. The evolution of diabetic retinopathy throughout severity levels and its effects on retinal layer irregularity need more study.
Subject terms: Diagnostic markers, Diagnostic markers, Eye diseases
Introduction
Diabetic retinopathy is the primary cause of vision loss among adults of working age. The increasing diabetic population will contribute to a significant public health concern regarding visually impaired individuals1. Diabetes is associated with changes in the retinal microvasculature and structure, which can gradually result in visual impairment. The prevention of vision loss caused by diabetic retinopathy necessitates prompt diagnosis, frequent monitoring, and timely therapeutic intervention2,3. One major obstacle is the identification of diabetic individuals who are at risk of developing retinopathy and advancing to vision-threatening conditions such as macular edema or proliferative diabetic retinopathy3–5.
Diabetic retinopathy can be categorized into two primary classes: nonproliferative and proliferative. The term “proliferative” pertains to the presence or absence of neovascularization, which denotes abnormal growth of blood vessels in the retina6. Non-proliferative diabetic retinopathy NPDR refers to the initial stage of disease in which neovascularization is absent. As the disease advances, it can develop into proliferative diabetic retinopathy PDR, characterized by neovascularization and a higher risk of significant visual complications7,8.
The progression of diabetic retinopathy can be averted through effective metabolic control, timely detection, and early treatment of DR. Fluorescein angiography (FA) is the preferred imaging technique for identifying retinal neovascularization and differentiating between NPDR and PDR9,10. Fluorescein angiography (FA) is an invasive imaging technique that necessitates the administration of intravenous dye and is associated with systemic and allergic complications11. Optical coherence tomography (OCT) is a non-invasive imaging technique used to visualize structural changes in the retina and choroid. It is commonly employed in the examination of different retinal diseases including diabetic retinopathy, age-related macular degeneration (AMD), retinal vein occlusion, and vitreomacular interface disorders9. OCT offers a variety of biomarkers for diagnosing, treating, and monitoring retinal changes in patients. Another advantage of this imaging modality is its ability to provide high-resolution images of the retinal layers structure, distinguishing it from FA9,12.
The healthy retina exhibits an organized and layered structure on spectral-domain OCT (SD-OCT), which is characterized by diverse reflectance patterns. With the advancement of SD-OCT knowledge, various imaging biomarkers have been proposed to assess visual prognosis. These include central retinal thickness, which provides insight into the entire retina, as well as the attenuation of the ellipsoid zone or external limiting membrane, which indicates photoreceptors dysfunction13,14. Additionally, the disorganization of the retinal inner layers (DRIL) is defined as the inability to distinguish between the ganglion cell layer–inner plexiform layer complex, inner nuclear layer, and outer plexiform layer. The inner retinal layers encompass axons, bipolar cells, and amacrine cell nuclei, all crucial for transmitting visual signals from photoreceptors to the ganglion cell layer. The DRIL indicates impairment in these components, resulting in abnormal visual signal processing15. Similarly, the presence and localization of hyperreflective foci (HF) within the retina may have a similar prognostic value16. The utilization of new OCT biomarkers could enhance risk stratification, aid in characterizing disease morphology, optimize the use of specific therapies such as anti-vascular endothelial growth factor (VEGF) therapy, improve prognosis counseling, and provide more precise inclusion criteria for therapeutic trials17,18.
Segmentation of retinal layers is necessary for evaluating OCT images of the retinal structure. There are various methods to accomplish this task, including manual, semi-automated, or fully automated approaches19–23. In a recent study, an automated technique was developed to serve as a dependable, expeditious, and user-friendly method for segmenting the inner plexiform layer (IPL) and outer plexiform layer (OPL), as well as estimating the smoothness index (SI) inside these specific layers17. Automated segmentation methods are advantageous due to their ability to operate continuously and independently of user experience, thereby mitigating the time-consuming and low repeatability nature of manual segmentation procedures17,19,23.
The IPL, or inner synaptic layer, comprises synaptic connections formed by the axons of bipolar cells and the dendrites of ganglion cells. The OPL, or outer synaptic layer, consists of synapses connecting photoreceptor cells with cells from the inner nuclear layer. The external limiting membrane (ELM) forms the junctional complex between Müller glia and photoreceptor cells24. We postulated that synaptic or junctional levels might be the initial sites of manifestation for abnormalities in the irregularity of retinal layers. The purpose of this study was to determine how well our previously developed automated retinal layer segmentation performed in differentiating between normal, NPDR, and PDR using quantified biomarkers like irregularity of hyperreflective retinal layers through machine learning techniques.
Methods
The study was conducted at Farabi Eye Hospital, Tehran University of Medical Sciences, Iran, and followed the Declaration of Helsinki’s principles. The institutional review board of Tehran University of Medical Sciences approved the study (IR.TUMS.FARABIH.REC.1400.028). The patients all provided written informed consent.
Both normal subjects without diabetes mellitus and patients with diabetic retinopathy were recruited. All patients with DR underwent fluorescein angiography (Heidelberg Engineering, Heidelberg, Germany) and SD-OCT (RTVue XR 100 Avanti device manufactured by Optovue, Inc. in Fremont, CA, USA) at baseline. Patients diagnosed with diabetic retinopathy were categorized into two groups: NPDR and PDR. This categorization was determined by the presence or absence of neovascularization in the retina or optic disc, as observed during a dilated fundus examination, as well as the presence of dye leakage on fluorescein angiography (FA). Therefore, the retinopathy stage was consensually determined by two experienced retina specialists (H. R. E. and E. K. P.) after evaluating fundus examination findings and fluorescein angiography images.
Patients with diabetic macular edema (DME) were excluded due to its potential to induce irregularities in the retinal layer structure, which could introduce bias into the findings of this study. Other exclusion criteria encompassed the existence of exudate and fibrovascular proliferation in the macular region, as well as other macular diseases such as age-related macular degeneration, macular dystrophies, and vitreomacular interface disorders. Additionally, uveitis, uncontrolled glaucoma, severe media opacity, visual acuity below 20/200, and refractive error exceeding + 3 or less than − 3 were also considered as exclusion criteria. Participants with a documented medical background involving prior PRP (panretinal photocoagulation), macular photocoagulation, intravitreal anti-VEGF (vascular endothelial growth factor) injections, or intraocular surgery, with the exception of cataract extraction, were not included in the study. The study excluded pictures of low quality, defined as having a signal strength index (SSI) below 40 as shown by the quality evaluation report generated by the RTVue program. The images were obtained using both horizontal and vertical raster patterns over the foveal region measuring 8 mm by 12 mm. All images had dimensions of 256 × 728 pixels.
The retinal layers were segmented automatically using the method described in our recently published study17. The automated procedure entailed mitigating artifacts and enhancing image quality through the utilization of nonlocal algorithms, notably Gaussian filters with a kernel size of 11 × 11. Subsequently, Gabor filters with two distinct kernel sizes of 6 × 6 were employed to specifically focus on the retinal area. The details were previously described in our published paper, where we supplied the Gabor filter formula and associated parameters in a companion table17. In all horizontal OCT scans, an unwanted region known as the optic disc is evident. We automatically trimmed images in all horizontal scans to eliminate the optic disc in SI segmentation and quantification. The boundaries of the retinal layers were then recognized using Support Vector Regression (SVR), a well-known machine learning approach. SVR is a supervised learning technique that employs points on layers and a prediction function. In the event of mislabeling, the model predicts and substitutes the wrong values, which is a big benefit of utilizing SVR.
The data was translated into a higher-dimensional space known as a kernel because our model used a nonlinear function. In SVR implementation, many kernels such as polynomials, sigmoid, Radial Basis Functions (RBF), and wavelet functions are used. The kernel used has a significant impact on the efficacy of SVR. Notably, the kernel-based method can use feature mapping with an infinite number of dimensions. The wavelet kernel is supposed to generate more appropriate outcomes heuristically. As a result, the algorithm extracted the retina’s hyperreflective layers (IPL, OPL, and EZ).
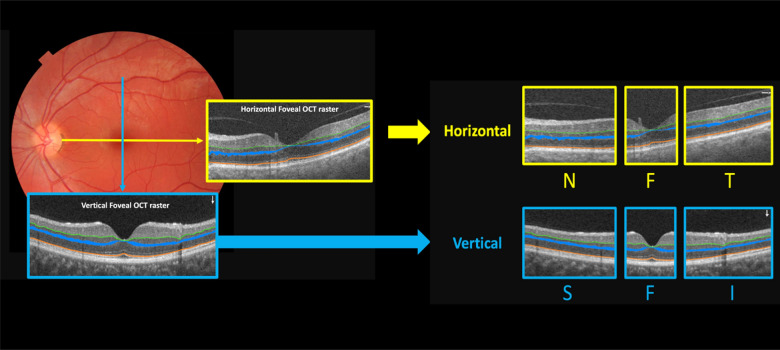
The IPL and EZ were depicted as a thin line, while the OPL was depicted as a thick band with an upper and border. The horizontal cross-section OCT images were segmented and extracted into three zones: nasal, foveal (comprises 20% of the central pixels equally located on both the left and right sides of the fovea) and temporal. The vertical cross-sectional OCT images were divided into superior, fovea (comprises 20% of the central pixels equally located on both the upper and lower sides of the fovea), and inferior zones in vertical diameter. Figure 1 displays the divisions and patterns of the cross-sections.
Figure 1.
The provided illustration depicts the cross-sectional sections of the fovea and their respective subdivisions. The blue arrow indicates a vertical slab, while the yellow arrow indicates a horizontal slab. Both the horizontal and vertical slabs are subdivided into three zones. The horizontal cuts are labeled as N for nasal, F for fovea, and T for temporal. Similarly, the vertical cuts are labeled as S for superior, F for fovea, and I for inferior.
In the next stage, the biomarkers, specifically the smoothness index (SI), and area (S) of retina layers were calculated individually for each zone (fovea, superior, inferior, nasal, and temporal).
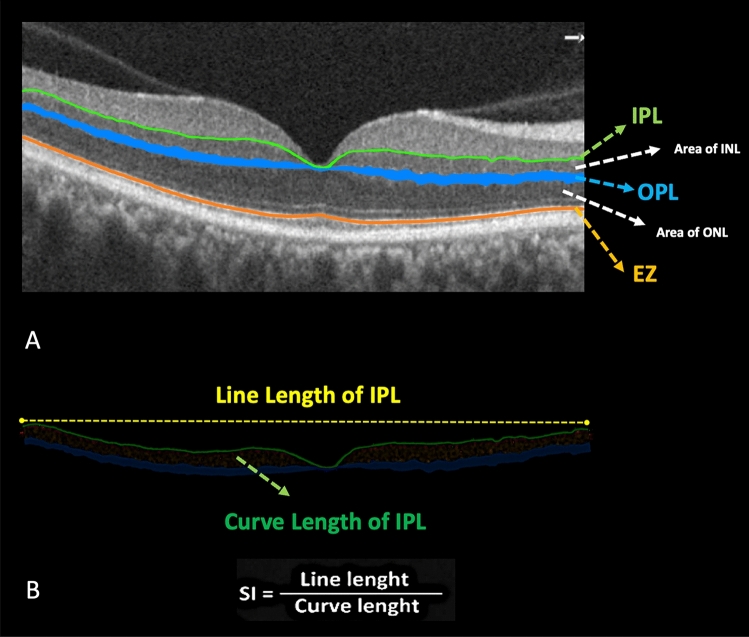
The SI of each layer was determined by dividing the line length (LL) of that retinal layer, which represents the direct distance between the start and end points of the layer in a specific zone, by the curve length (CL), which represents the actual length of the segmented layer in that zone. The SI was calculated for IPL, OPL, and EZ extracted bands. The OPL measurements involved calculations of the SI for the upper borders. The formula for calculating the SI is depicted in Fig. 2.
Figure 2.
(A) illustrates the zones and areas that have been assessed in the present investigation. The white dashed arrows depict areas (S) of the inner nuclear layer (INL) and outer nuclear layer (ONL). The inner plexiform layer (IPL) is depicted as a linear representation by the green arrow. The Blue band represents the outer plexiform layer (OPL). The upper and lower boundaries of the outer plexiform layer (OPL) have been assessed individually, and the measurement of the distance between these boundaries has been designated as the OPL area. Moreover, the orange line represents the ellipsoid zone (EZ). (B) In this section, we have presented the calculation of the smoothness index (SI). The line length, shown by the yellow dashed line, represents the Euclidean distance between the starting and ending points of the IPL. On the other hand, the curve length, depicted by the green curve, represents the actual length of the IPL.
The area of the inner nuclear layer (INL) can be determined by calculating the area between the inner plexiform layer (IPL) and the upper boundary of the outer plexiform layer (OPL). The area of the outer plexiform layer (OPL) was determined by calculating the area between the upper and lower borders of the OPL. The area of the outer nuclear layer (ONL) was determined by calculating the region between the lower boundary of the outer plexiform layer (OPL) and the ellipsoid zone (EZ). Figure 2 illustrates the IPL, OPL, and EZ extracted layers as well as the INL, OPL and ONL area.
Diabetic retinopathy was classified as either normal, NPDR, or PDR based on the severity of the disease. Each person’s SI and area (S) of the segmented layers were automatically measured in the OCTs’ aforementioned zones, and the results were compared across groups.
Ethical approval
This study has been approved by the local institutional review board of Tehran University of Medical Sciences (IR.TUMS.FARABIH.REC.1400.028).The study was performed in accordance with the Helsinki Declaration of 1964, and its later amendments.
Informed consent
All patients provided informed consent to participate in the study.
Statistical methods
To present the data, we used mean, standard deviation (SD), median and range, frequency, and percentage. To compare variables between groups considering the correlation of measurements in two eyes, we used a generalized estimating equation (GEE). All the analysis was adjusted based on age and sex. To assess the diagnostic ability of the variables we used the ROC curve and the associated 95% CI. The Spearman correlation coefficient was applied to evaluate the association of the quantitative variables. Statistical analysis was performed with SPSS (IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp). A p-value less than 0.05 is considered statistically significant.
Results
In the present investigation, a total of 84 eyes from 57 patients were included. Table 1 presents the demographic distribution of various groups of patients participating in the present investigation. The participants’ average age was 52 ± 11.2 years, with 36 eyes (42.8%) belonging to males.
Table 1.
Presents the demographic data among various groups of patients participating in the present investigation.
| Normal (n = 33) | NPDR (n = 36) | PDR (n = 15) | Total (n = 84) | p-value | |
|---|---|---|---|---|---|
| Men, n (%) | 17 (51.5) | 14 (38.8) | 5 (33.3) | 36 (42.8) | 0.12a |
| Right eye, n (%) | 15 (45.4) | 19 (52.7) | 9 (60) | 43 (51.1) | 0.76a |
| Age (years), mean ± SD | 45 ± 11.3 | 56 ± 13.1 | 57 ± 9.6 | 52 ± 11.2 | 0.42b |
| log MAR BCVA, mean ± SD | 0.12 ± 0.12 | 0.36 ± 0.42 | 0.45 ± 0.47 | 0.31 ± 0.33 | 0.09b |
BCVA best-corrected visual acuity, NPDR non-proliferative diabetic retinopathy, PDR proliferative diabetic retinopathy.
aChi-square tests.
bIndependent t-test.
The first group included 33 normal optical coherence tomography (OCT) scans obtained from a sample of 33 individuals, with 51.5% of the subjects identifying as male. The second group included 36 optical coherence tomography (OCT) images obtained from a group of 36 individuals diagnosed with NPDR, of whom 38.8% were male. The final cohort included a total of 15 optical coherence tomography (OCT) images obtained from 15 individuals, of whom 33.3% were male, all diagnosed with PDR.
Tables 2 and 3 present the average and standard deviation of measurements for each variable across three distinct groups (normal, NPD, and PDR). Additionally, these tables include pairwise comparisons among the three groups.
Table 2.
In horizontal optical coherence tomography slabs, this table presents the average and standard deviation of observations for each variable across three separate groups (normal, NPDR, and PDR).
| Group | p-value | Multiple comparisions | ||||||
|---|---|---|---|---|---|---|---|---|
| Normal | NPDR | PDR | P1 | P2 | P3 | |||
| Horizontal slabs—foveal zone | S_INL.H.fovea | 3357.44 ± 866.07 | 5179.56 ± 1169.94 | 5316.13 ± 1598.35 | < 0.001 | < 0.001 | < 0.001 | 0.91578 |
| S_ONL.H.fovea | 24,879.87 ± 3237.89 | 29,462.08 ± 5319.02 | 29,361.67 ± 2845.06 | < 0.001 | < 0.001 | < 0.001 | 0.99998 | |
| S_OPL.H.fovea | 2307.19 ± 724.41 | 2604.81 ± 906.79 | 2599.07 ± 1132.69 | 0.36402 | 0.4238 | 0.8186439 | 0.99886 | |
| SI_IPL.H.fovea | 0.91 ± 0.02 | 0.91 ± 0.02 | 0.91 ± 0.02 | 0.63898 | 0.96141 | 0.9112877 | 0.71803 | |
| SI_OPL_up.H.fovea | 0.93 ± 0.02 | 0.94 ± 0.02 | 0.93 ± 0.01 | 0.45537 | 0.71204 | 0.999999 | 0.62501 | |
| SI_EZ.H.fovea | 0.95 ± 0.01 | 0.95 ± 0.01 | 0.94 ± 0.03 | 0.2394 | 0.54337 | 0.7918036 | 0.3984 | |
| Horizontal slabs—temporal zone | S_INL.H.Temporal | 20,271.5 ± 3098.11 | 21,607.47 ± 3732.75 | 21,666.21 ± 3334.99 | 0.61386 | 0.69012 | 0.9465641 | 0.98409 |
| S_ONL.H.Temporal | 54,562.81 ± 6418.32 | 53,070.61 ± 8910.94 | 57,912.64 ± 8575.12 | 0.31518 | 0.796 | 0.3751907 | 0.96437 | |
| S_OPL.H.Temporal | 12,490.53 ± 1566 | 11,237.22 ± 1747.79 | 11,354.5 ± 3125.23 | 0.06509 | 0.05956 | 0.6756801 | 0.95759 | |
| SI_IPL.H.Temporal | 0.93 ± 0.01 | 0.93 ± 0.01 | 0.9 ± 0.02 | < 0.001 | 0.76157 | < 0.001 | < 0.001 | |
| SI_OPL_up.H.Temporal | 0.93 ± 0.01 | 0.93 ± 0.01 | 0.91 ± 0.02 | 0.00196 | 0.48475 | 0.0014818 | 0.01183 | |
| SI_EZ.H.Temporal | 0.94 ± 0.02 | 0.94 ± 0.01 | 0.93 ± 0.02 | 0.37693 | 0.71234 | 0.9406616 | 0.51626 | |
| Horizontal slabs—nasal zone | S_INL.H.Nasal | 22,617.88 ± 3607.72 | 23,088.25 ± 3417.13 | 23,575.07 ± 3849.62 | 0.21948 | 0.7038 | 0.2577146 | 0.77395 |
| S_ONL.H.Nasal | 58,425.69 ± 7715.16 | 60,214.31 ± 12,619.52 | 60,470.47 ± 7431.86 | 0.32646 | 0.65075 | 0.8607225 | 0.40356 | |
| S_OPL.H.Nasal | 13,122.72 ± 2189.31 | 12,177.72 ± 2775.48 | 12,287.4 ± 2189.84 | 0.05676 | 0.13685 | 0.1066176 | 0.96035 | |
| SI_IPL.H.Nasal | 0.93 ± 0.01 | 0.93 ± 0.01 | 0.89 ± 0.03 | < 0.001 | 0.72501 | < 0.001 | < 0.001 | |
| SI_OPL_up.H.Nasal | 0.93 ± 0.01 | 0.93 ± 0.01 | 0.9 ± 0.03 | 0.00392 | 0.99918 | 0.0037228 | 0.00281 | |
| SI_EZ.H.Nasal | 0.94 ± 0.01 | 0.94 ± 0.01 | 0.94 ± 0.02 | 0.81829 | 0.98439 | 0.8987655 | 0.94468 | |
Also provides pairwise comparisons between the three groups. We utilized a generalized estimating equation (GEE) to compare variables between groups while accounting for the correlation of data in two eyes.
S surface area, SI smoothness index, INL inner nuclear layer, ONL outer nuclear layer, IPL inner plexiform layer, OPL outer plexiform layer, EZ ellipsoid zone, up upper boundary of IPL.
Statistically significant outcomes are shown in bold font. P1: normal vs NPDR, P2: normal vs PDR, P3: NPDR vs PDR.
Table 3.
In vertical optical coherence tomography slabs, this table presents the average and standard deviation of observations for each variable across three separate groups (normal, NPDR, and PDR).
| Group | p-value | Multiple comparisions | ||||||
|---|---|---|---|---|---|---|---|---|
| Normal | NPDR | PDR | P1 | P2 | P3 | |||
| Vertical slabs—foveal zone | S_INL.V.fovea | 6733 ± 1753 | 5856 ± 1402 | 7022 ± 2247 | 0.038 | 0.138 | 0.989 | 0.111 |
| S_onl.V.fovea | 29,166 ± 3910 | 27,450 ± 4772 | 26,275 ± 3956 | 0.026 | 0.269 | 0.032 | 0.75 | |
| S_OPL.V.fovea | 4453 ± 1009 | 4193 ± 1228 | 5711 ± 1575 | 0.003 | 0.888 | 0.015 | 0.002 | |
| SI_IPL.V.fovea | 0.918 ± 0.026 | 0.912 ± 0.024 | 0.914 ± 0.031 | 0.566 | 0.636 | 0.934 | 0.982 | |
| SI_OPL_up.V.fovea | 0.942 ± 0.016 | 0.936 ± 0.015 | 0.924 ± 0.034 | 0.066 | 0.253 | 0.125 | 0.476 | |
| SI_EZ.V.fovea | 0.946 ± 0.011 | 0.949 ± 0.013 | 0.937 ± 0.034 | 0.233 | 0.742 | 0.503 | 0.309 | |
| Vertical slabs—inferior zone | S_INL.V.INF | 22,476 ± 3243 | 23,461 ± 3611 | 24,563 ± 4926 | 0.261 | 0.589 | 0.364 | 0.825 |
| S_onl.V.INF | 50,752 ± 6300 | 47,902 ± 10,501 | 53,080 ± 11,555 | 0.167 | 0.37 | 0.71 | 0.229 | |
| S_OPL.V.INF | 13,038 ± 2148 | 14,512 ± 2950 | 16,054 ± 2926 | 0.001 | 0.074 | 0.001 | 0.23 | |
| SI_IPL.V.INF | 0.927 ± 0.015 | 0.928 ± 0.013 | 0.932 ± 0.013 | 0.584 | 0.998 | 0.698 | 0.764 | |
| SI_OPL_up.V.INF | 0.932 ± 0.013 | 0.93 ± 0.015 | 0.926 ± 0.017 | 0.418 | 0.813 | 0.525 | 0.919 | |
| SI_EZ.V.INF | 0.942 ± 0.018 | 0.938 ± 0.015 | 0.942 ± 0.014 | 0.722 | 0.843 | 0.998 | 0.911 | |
| Vertical slabs—superior zone | S_INL.V.SUP | 22,792 ± 3087 | 22,941 ± 4454 | 23,060 ± 3145 | 0.973 | 1 | 0.994 | 0.998 |
| S_onl.V.SUP | 52,050 ± 6720 | 51,708 ± 8842 | 54,366 ± 10,127 | 0.661 | 0.998 | 0.777 | 0.755 | |
| S_OPL.V.SUP | 14,599 ± 2191 | 15,487 ± 3507 | 16,400 ± 3161 | 0.12 | 0.486 | 0.166 | 0.778 | |
| SI_IPL.V.SUP | 0.936 ± 0.015 | 0.931 ± 0.015 | 0.929 ± 0.02 | 0.284 | 0.488 | 0.401 | 0.955 | |
| SI_OPL_up.V.SUP | 0.934 ± 0.015 | 0.928 ± 0.029 | 0.929 ± 0.02 | 0.378 | 0.552 | 0.626 | 0.993 | |
| SI_EZ.V.SUP | 0.946 ± 0.013 | 0.946 ± 0.014 | 0.942 ± 0.021 | 0.8 | 1 | 0.885 | 0.898 | |
Also provides pairwise comparisons between the three groups. We utilized a generalized estimating equation (GEE) to compare variables between groups while accounting for the correlation of data in two eyes.
S surface area, SI smoothness index, INL inner nuclear layer, ONL outer nuclear layer, IPL inner plexiform layer, OPL outer plexiform layer, EZ ellipsoid zone, up upper boundary of IPL.
Statistically significant outcomes are shown in bold font. P1: normal vs NPDR, P2: normal vs PDR, P3: NPDR vs PDR.
The analysis of the tables reveals a statistically significant difference in the area (S) of the INL and ONL in the horizontal foveal zone among the three groups (p < 0.001). However, upon conducting pairwise comparisons, this difference is observed only between the normal group and the NPDR group (p < 0.001), as well as between the normal group and the PDR group (p < 0.001). No significant difference is observed between the NPDR and PDR groups.
Regarding variables in the temporal zone, there is a statistically significant difference in the smoothness index (SI) of the IPL and the upper border of the OPL among three groups in the horizontal scan (p < 0.001 and p = 0.0019 for IPL and OPL, respectively). However, upon pairwise comparison, this difference is only observed between the normal and PDR group (p < 0.05), as well as between the NPDR and the PDR group (p < 0.05), but not between the normal and NPDR groups.
Regarding the variables in the horizontal nasal zone, A significant statistical difference can be observed in the smoothness index (SI) of the IPL and the upper border of the OPL among the three groups (p < 0.05). However, upon conducting pairwise comparisons, this difference is only observed between the normal and PDR group (p < 0.05), as well as between the NPDR group and the PDR group (p < 0.05), but not between the normal and NPDR groups.
Regarding the variables pertaining to the vertical slabs, the area (S) of the OPL in the foveal zone exhibited a statistically significant difference among the three groups (p = 0.003). However, upon conducting pairwise comparisons, this difference was only observed between the normal and PDR group (p = 0.015), as well as between the NPDR and the PDR group (p = 0.002), but not between the normal and NPDR groups.
The remaining variables, as indicated in Tables 2 and 3, did not exhibit statistically significant changes or demonstrated significant changes only between two specific groups in the pairwise comparison.
In order to evaluate the diagnostic efficacy of the variables, we employed the receiver operating characteristic (ROC) curve and calculated the corresponding 95% confidence interval (CI).
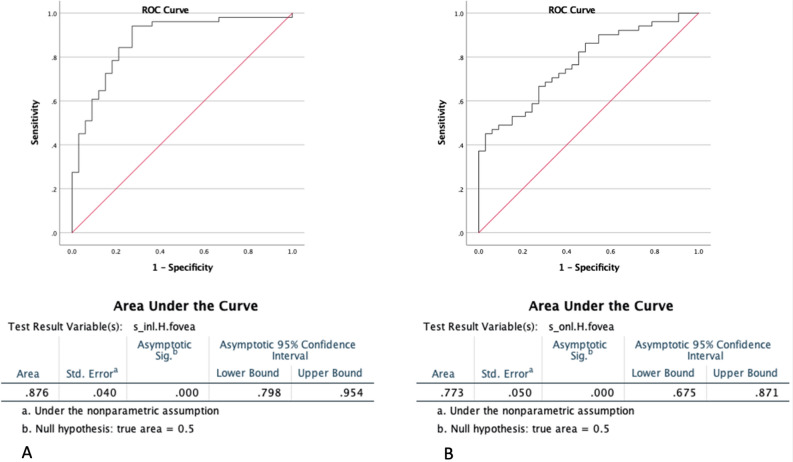
For discriminating diabetic patients (NPDR and PDR) from normal patients, the area (S) of the inner nuclear layer (INL) in the foveal region of horizontal slabs demonstrated the highest performance, achieving an accuracy of 87.6% (CI 0.798–0.954) (Fig. 3A). The area of ONL in the foveal region of horizontal slabs demonstrated an accuracy of 77.3% (confidence interval: 0.675–0.871) in distinguishing between those with normal health conditions and those diagnosed with Diabetes (Fig. 3B).
Figure 3.
The receiver operating characteristic curves (ROC curves). (A) To differentiate between diabetic patients (NPDR and PDR) and normal patients, the study found that the area (S) of the inner nuclear layer (INL) in the foveal zone of horizontal slabs exhibited the most effective performance. This measure achieved an accuracy rate of 87.6% (confidence interval 0.798–0.954). (B) The accuracy of discriminating between individuals with normal health conditions and those diagnosed with Diabetes, based on the area of the outer nuclear layer (ONL) in the foveal zone of horizontal slabs, was found to be 77.3% (confidence interval 0.675–0.871).
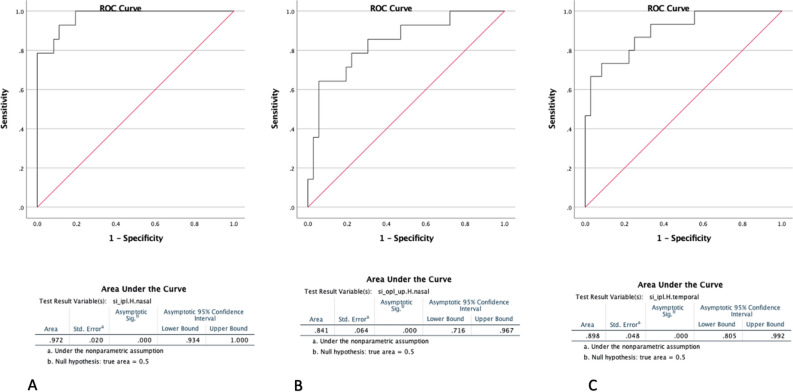
In the context of discriminating between patients with PDR and NPDR, the smoothness index (SI) of IPL in the nasal zone of horizontal foveal slabs demonstrated the highest level of performance, achieving an accuracy of 97.2% (confidence interval 0.934–1.00) (Figure-4A). The accuracy of discriminating between patients with NPDR and patients with PDR based on the smoothness index of the upper border of the OPL in the nasal zone of horizontal slabs was found to be 84.1% (CI 0.716–0.967) (Fig. 4B). The study found that the smoothness index of IPL in the temporal zone of horizontal slabs had an accuracy of 89.8% (CI 0.805–0.992) in distinguishing between patients with NPDR and those with PDR (Fig. 4C).
Figure 4.
The receiver operating characteristic curves (ROC curves). (A) In the context of discriminating between patients with PDR and NPDR, the smoothness index (SI) of IPL in the nasal zone of horizontal foveal slabs achieved the highest level of performance, with an accuracy of 97.2% (confidence interval 0.934–1.00). (B) Demonstrates that the accuracy of distinguishing between patients with NPDR and patients with PDR based on the smoothness index of the upper border of the OPL in the nasal zone of horizontal slabs was 84.1% (CI 0.716–0.967). (C) Demonstrates that the smoothness index of IPL in the temporal zone of horizontal slabs distinguished between patients with NPDR and those with PDR with an accuracy of 89.8% (CI 0.805–0.992).
The vertical receiver operating characteristic (ROC) curves, used to distinguish between normal and diabetic individuals, as well as between patients with PDR and NPDR, did not exhibit an area under the curve (AUC) over 80% for any of the variables.
Figure 5 depicts the segmentation of IPL, OPL, and ELM in three groups of patients (normal, NPDR, and PDR) who participated in the current study.
Figure 5.
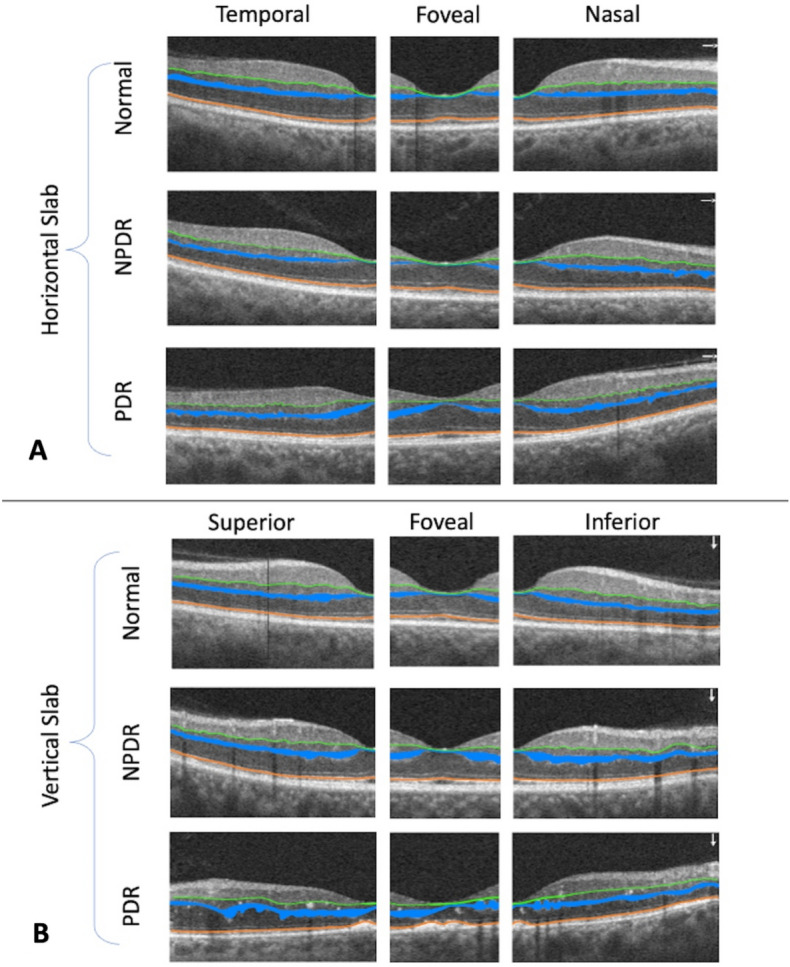
Illustrates the segmentation of IPL (shown by a green line), OPL (represented by a blue region), and ELM (shown by an orange line) in three groups of patients (normal, NPDR, and PDR) in both horizontal (A) and vertical (B) OCT slabs.
Discussion
Diabetic retinopathy is a microvascular condition that arises from diabetes mellitus, exhibiting distinctive alterations in both the structural and functional aspects of the retinal layers25–27. Optical Coherence Tomography (OCT) has emerged as a highly important modality for non-invasive imaging of retinal layers, offering exceptional resolution and enabling meticulous examination of structural modifications. Emerging optical coherence tomography (OCT) technologies, namely spectral domain OCT (SD-OCT) and swept-source OCT (SS-OCT), offer improved visibility of retinal layers, hence facilitating a more precise assessment of irregularity and area of retinal layers28,29. Nevertheless, it is imperative to tackle obstacles such as segmentation errors and discrepancies across optical coherence tomography (OCT) instruments in order to provide uniform measurements across diverse clinical environments17,19,21.
In the present investigation, employing a pre-existing automated system for the segmentation of hyperreflective retinal layers, we assessed the irregularity and surface area of the inner retinal layers (such as the inner plexiform layer and inner nuclear layer) as well as the outer retinal layers (such as the outer plexiform layer and outer nuclear layer).
The study investigated differences in smoothness index (SI) between three groups in the IPL and upper border of the OPL, both in temporal and nasal regions. Statistical significance was found, with significant differences between normal and PDR groups, as well as between NPDR and PDR groups, but not between normal and NPDR groups. Notably, the SI of IPL in the nasal area of horizontal foveal slabs achieved 97.2% accuracy in distinguishing PDR from NPDR, while the SI of the upper border of the OPL in nasal horizontal slabs was 84.1% accurate in differentiating NPDR from PDR. Moreover, the SI of IPL in the temporal area of horizontal slabs accurately distinguished NPDR from PDR with an 89.8% accuracy. The presence of irregularity can be associated with the formation of microaneurysms, impaired blood flow in capillaries, and blood-retinal barrier disruption which cause edema, all of which are indicative of the disease’s severity30. The utilization of irregularity measurements as biomarkers in the assessment of diabetic retinopathy can exhibit potential for disease monitoring and therapeutic evaluation31. The prompt identification of anomalies and alterations in layer areas might potentially enhance the prompt execution of intervention strategies. Moreover, the use of these markers holds promise in facilitating the prediction of illness progression and the tailoring of personalized treatment approaches. The assessment of the regularity (smoothness) of retinal layers has thus far been conducted through semi-automated or manual methods in the context of ERM. However, the algorithm used in this study presents an opportunity to explore this potential biomarker of retinal layers in different vascular and structural diseases of the retina32.
It is noteworthy that our findings indicate that the area (S) of the inner nuclear layer (INL) and outer nuclear layer (ONL) in the foveal region of horizontal slabs exhibit the most superior performance in distinguishing diabetic patients (with [NPDR] and [PDR]) from normal patients. Specifically, the area (S) of the INL achieved an accuracy of 87.6%, while the area (S) of the ONL achieved an accuracy of 77.3%.
The measurement of the surface area of certain layers inside the retina, such as the ganglion cell layer (GCL) and the inner plexiform layer (IPL), can yield significant information on cellular density and structural changes. Prior studies have presented findings that suggest a notable correlation between the reduced surface area in these specific layers and the presence of neuronal degeneration and thinning33–35. The investigation of retinal thickness in individuals diagnosed with diabetic retinopathy (DR) in comparison to those without the condition demonstrates considerable heterogeneity in the findings. This variability may be attributed to the dynamic nature of the disease progression and the lack of a consistent pattern or trend. This observation is supported by other studies36–39. The reduction in neural tissue leads to a drop in thickness, while there is a possibility of an increase in thickness owing to vascular permeability and inflammation. This potential rise may offset the impact of neurodegeneration on macular thickness, as proposed by Sugimoto et al.40. However, it should be noted that an increase in total retinal thickness alone does not provide enough evidence to dismiss the possibility of a related neurodegenerative process. The exclusive reliance on macular thickness as a measure for assessing initial alterations in the retinal structure of individuals with type 2 diabetes mellitus (DM2) is inadequate, since it lacks the necessary sensitivity to identify changes in the microstructure of retinal layers36,37,41,42. In light of this rationale, our study aimed to assess the thickness of both horizontal and vertical retinal layers inside foveal slabs in individuals with varying degrees of diabetic retinopathy, while excluding those with any indications of diabetic macular edema, thereby distinguishing our research from prior investigations. Based on the findings of the present study, it appears that the horizontal (not vertical) area of the inner nuclear layer (INL) and outer nuclear layer (ONL) inside the central foveal zone exhibit more precision in distinguishing between those with normal retinal health and those diagnosed with diabetes. In general, the measurement of retinal layers area at various depths holds promise as biomarkers that could be used to track the development and advancement of diabetic retinopathy (DR)43.
Our concept for differentiating NPDR and PDR by evaluating anomalies in hyperreflective retinal layers is based on the application of OCT biomarkers such as disorganization of retinal layers (DRIL). On optical coherence tomography (OCT), DRIL is distinguished by the difficulty to clearly determine the boundaries of the inner nuclear layer (INL), outer plexiform layer (OPL), and ganglion cell layer-inner plexiform layer (GCL-IPL) complex. We expected that changes in the smoothness of the retinal layers would occur in patients with severe stages of diabetic retinopathy before the formation of DRIL, as opposed to patients with milder stages of diabetic retinopathy or normal patients without diabetic retinopathy. Because recent research found that DRIL is strongly related with areas of ischemic damage, which corresponds to places where there is no flow in the superficial, middle, and deep capillary plexus, this advancement could be due to variables such as increasing degrees of ischemia15,16,30,44–48. To verify this theory, we conducted a cross-sectional study on diabetic retinopathy patients who did not have macular edema. However, until we did a larger, more thoroughly controlled study, we were unable to apply our findings to all diabetic patients.
This study is subject to certain limitations, which is a common characteristic of studies with a small sample size. The initial investigation was carried out on a limited cohort of patients. Consequently, the primary obstacle of differentiating between individuals with severe NPDR and PDR will be tackled in subsequent research of greater magnitude, including a bigger sample size. It is important to note that people with retinal edema or vitreous hemorrhage were excluded from the study due to the limited visual clarity and presence of artifacts in assessing retinal layers. However, it should be acknowledged that these patients do exhibit the underlying pathophysiology of diabetic retinopathy. Consequently, a portion of diabetes patients in routine clinical settings may not get any advantages from the methodology employed in the present study. Due to the extensive distribution of vascular changes resulting from diabetes, it has been shown that over 50% of lesions associated with Diabetic Retinopathy (DR) are situated beyond the boundaries of the seven-standard Early Treatment Diabetic Retinopathy Study (ETDRS) fields. Prior research has indicated that the existence of peripheral retinal lesions may indicate a higher level of severity in diabetic retinopathy (DR) in around 9 to 15% of eyes49,50. In the present investigation, the researchers were unable to get ultrawide-field fluorescein angiography (UWF-FA) and instead employed conventional fluorescein angiography in all subjects. Additional research is necessary to directly compare the findings of the present investigation, which relied on more precise angiography tests utilizing ultra-widefield fluorescein angiography (UWF-FA)51. Furthermore, it should be noted that the HbA1C levels of the participants included in the study were not adjusted, a factor that has the potential to impact the outcomes of the present investigation.
Our previously published investigation established the segmentation method employed in this experiment, which contains a full discussion of its strengths and limits17. The present research focuses on applying the algorithm to determine if a patient is NPDR, PDR, or normal. We compiled relevant literature in a Supplementary Table (Supplementary File 1) and compared datasets and the extent of automation among the offered methods. It is worth mentioning that most previous study used datasets from healthy eyes, but our proposed method was used on a diverse population of patients with PDR, NPDR, and normal eyes. Furthermore, as previously investigated and published in our prior investigation 17, the SVR-based segmentation approach is totally automated, providing speed. However, our method, like prior image processing-based segmentation algorithms, may not perform well when layer irregularities and oscillations are significant and layers are indistinguishable, as in DRIL. Another limitation of our study is that the proposed algorithm used in the current study does not segment all retinal layers.
Given that OCT tilt can potentially introduce or eliminate pixels at the start or end of the curve and the straight line in certain patients, we propose that this impact may not be as significant since it affects both lines. However, in order to confirm this hypothesis, it is necessary to evaluate the SI measurement in patients with tilted and flattened OCT sections.
In summary, the evaluation of the smoothness index and irregularity of the inner and outer plexiform layers using optical coherence tomography, particularly in the nasal and temporal regions of horizontal foveal slabs, has the potential to be utilized as a biomarker for distinguishing between non-proliferative and proliferative diabetic retinopathy. Further research is required to examine the inherent progression of diabetic retinopathy across various levels of severity and its impact on the irregularity of distinct retinal layers.
Supplementary Information
Author contributions
All authors contributed to the study conception and design. All authors read and approved the final manuscript.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-54535-6.
References
- 1.Aiello LP, et al. Diabetic retinopathy. Diabetes Care. 1998;21:143–156. doi: 10.2337/diacare.21.1.143. [DOI] [PubMed] [Google Scholar]
- 2.Stratton IM, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Early Treatment Diabetic Retinopathy Study Research Group Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:766–785. [PubMed] [Google Scholar]
- 5.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch. Ophthalmol. 1984;102:520–526. doi: 10.1001/archopht.1984.01040030398010. [DOI] [PubMed] [Google Scholar]
- 6.Jung EE, et al. Association of the pattern of retinal capillary non-perfusion and vascular leakage with retinal neovascularization in proliferative diabetic retinopathy. J. Curr. Ophthalmol. 2021;33:56–61. doi: 10.4103/JOCO.JOCO_234_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vorob’eva IV. Modern approach to early diagnosis and pathogenetic treatment of diabetic retinopathy. Vestn. Oftalmol. 2016;132:60–67. doi: 10.17116/oftalma2016132560-67. [DOI] [PubMed] [Google Scholar]
- 8.Update on Diagnosis and Treatment of Diabetic Retinopathy: A Consensus Guideline of the Working Group of Ocular Health (Spanish Society of Diabetes and Spanish Vitreous and Retina Society). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5488240/. [DOI] [PMC free article] [PubMed]
- 9.Nanegrungsunk O, Patikulsila D, Sadda SR. Ophthalmic imaging in diabetic retinopathy: A review. Clin. Exp. Ophthalmol. 2022;50:1082–1096. doi: 10.1111/ceo.14170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mustafi D, Saraf SS, Shang Q, de Koo LCO. New developments in angiography for the diagnosis and management of diabetic retinopathy. Diabetes Res. Clin. Pract. 2020;167:108361. doi: 10.1016/j.diabres.2020.108361. [DOI] [PubMed] [Google Scholar]
- 11.Falavarjani KG, Sarraf D. Optical coherence tomography angiography of the retina and choroid; current applications and future directions. J. Curr. Ophthalmol. 2017;29:1–4. doi: 10.1016/j.joco.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwan CC, Fawzi AA. Imaging and biomarkers in diabetic macular edema and diabetic retinopathy. Curr. Diab. Rep. 2019;19:95. doi: 10.1007/s11892-019-1226-2. [DOI] [PubMed] [Google Scholar]
- 13.Forooghian F, et al. Relationship between photoreceptor outer segment length and visual acuity in diabetic macular edema. Retina. 2010;30:63–70. doi: 10.1097/IAE.0b013e3181bd2c5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelosini L, et al. Optical coherence tomography may be used to predict visual acuity in patients with macular edema. Investig. Ophthalmol. Vis. Sci. 2011;52:2741–2748. doi: 10.1167/iovs.09-4493. [DOI] [PubMed] [Google Scholar]
- 15.Das R, Spence G, Hogg RE, Stevenson M, Chakravarthy U. Disorganization of inner retina and outer retinal morphology in diabetic macular edema. JAMA Ophthalmol. 2018;136:202–208. doi: 10.1001/jamaophthalmol.2017.6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suciu C-I, Suciu V-I, Nicoara S-D. Optical coherence tomography (angiography) biomarkers in the assessment and monitoring of diabetic macular edema. J. Diabetes Res. 2020;2020:6655021. doi: 10.1155/2020/6655021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saeidian J, et al. Automated assessment of the smoothness of retinal layers in optical coherence tomography images using a machine learning algorithm. BMC Med. Imaging. 2023;23:1. doi: 10.1186/s12880-023-00976-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhende M, Shetty S, Parthasarathy MK, Ramya S. Optical coherence tomography: A guide to interpretation of common macular diseases. Indian J. Ophthalmol. 2018;66:20–35. doi: 10.4103/ijo.IJO_902_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montazerin M, et al. Livelayer: A semi-automatic software program for segmentation of layers and diabetic macular edema in optical coherence tomography images. Sci. Rep. 2021;11:13794. doi: 10.1038/s41598-021-92713-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Automatic Detection of Retinal Regions Using Fully Convolutional Networks for Diagnosis of Abnormal Maculae in Optical Coherence Tomography Images. https://pubmed.ncbi.nlm.nih.gov/31111697/. [DOI] [PMC free article] [PubMed]
- 21.Chakravarty A, Sivaswamy J. A supervised joint multi-layer segmentation framework for retinal optical coherence tomography images using conditional random field. Comput. Methods Progr. Biomed. 2018;165:235–250. doi: 10.1016/j.cmpb.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Kugelman J, Alonso-Caneiro D, Read SA, Vincent SJ, Collins MJ. Automatic segmentation of OCT retinal boundaries using recurrent neural networks and graph search. Biomed. Opt. Express. 2018;9:5759–5777. doi: 10.1364/BOE.9.005759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukherjee S, et al. Retinal layer segmentation in optical coherence tomography (OCT) using a 3D deep-convolutional regression network for patients with age-related macular degeneration. Biomed. Opt. Express. 2022;13:3195–3210. doi: 10.1364/BOE.450193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu SM. Synaptic organization of the vertebrate retina: General principles and species-specific variations: The Friedenwald lecture. Investig. Ophthalmol. Vis. Sci. 2010;51:1263–1274. doi: 10.1167/iovs.09-4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khalili Pour E, et al. Automated machine learning-based classification of proliferative and non-proliferative diabetic retinopathy using optical coherence tomography angiography vascular density maps. Graefes Arch. Clin. Exp. Ophthalmol. 2023;261:391–399. doi: 10.1007/s00417-022-05818-z. [DOI] [PubMed] [Google Scholar]
- 26.Lechner J, O’Leary OE, Stitt AW. The pathology associated with diabetic retinopathy. Vis. Res. 2017;139:7–14. doi: 10.1016/j.visres.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Sinclair SH, Schwartz SS. Diabetic retinopathy—An underdiagnosed and undertreated inflammatory, neuro-vascular complication of diabetes. Front. Endocrinol. 2019;10:843. doi: 10.3389/fendo.2019.00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Velthoven MEJ, Faber DJ, Verbraak FD, van Leeuwen TG, de Smet MD. Recent developments in optical coherence tomography for imaging the retina. Prog. Retin. Eye Res. 2007;26:57–77. doi: 10.1016/j.preteyeres.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Aumann S, Donner S, Fischer J, Müller F. Optical coherence tomography (OCT): Principle and technical realization. In: Bille JF, editor. High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics. Springer; 2019. [PubMed] [Google Scholar]
- 30.Ishibashi T, et al. Association between disorganization of retinal inner layers and visual acuity after proliferative diabetic retinopathy surgery. Sci. Rep. 2019;9:12230. doi: 10.1038/s41598-019-48679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alasil T, et al. Relationship between optical coherence tomography retinal parameters and visual acuity in diabetic macular edema. Ophthalmology. 2010;117:2379–2386. doi: 10.1016/j.ophtha.2010.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho KH, Park SJ, Cho JH, Woo SJ, Park KH. Inner-retinal irregularity index predicts postoperative visual prognosis in idiopathic epiretinal membrane. Am. J. Ophthalmol. 2016;168:139–149. doi: 10.1016/j.ajo.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 33.Ezhilvendhan K, Shenoy A, Rajeshkannan R, Balachandrachari S, Sathiyamoorthy A. Evaluation of macular thickness, retinal nerve fiber layer and ganglion cell layer thickness in patients among type 2 diabetes mellitus using optical coherence tomography. J. Pharm. Bioallied Sci. 2021;13:S1055–S1061. doi: 10.4103/jpbs.jpbs_165_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodrigues EB, et al. Diabetes induces changes in neuroretina before retinal vessels: A spectral-domain optical coherence tomography study. Int. J. Retina Vitreous. 2015;1:4. doi: 10.1186/s40942-015-0001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.PincelliNetto M, et al. Macular inner retinal layer thinning in diabetic patients without retinopathy measured by spectral domain optical coherence tomography. Med. Hypothesis Discov. Innov. Ophthalmol. 2018;7:133–139. [PMC free article] [PubMed] [Google Scholar]
- 36.Brown JC, et al. Detection of diabetic foveal edema: Contact lens biomicroscopy compared with optical coherence tomography. Arch. Ophthalmol. 2004;122:330–335. doi: 10.1001/archopht.122.3.330. [DOI] [PubMed] [Google Scholar]
- 37.Clerck EEBD, et al. New ophthalmologic imaging techniques for detection and monitoring of neurodegenerative changes in diabetes: A systematic review. Lancet Diabetes Endocrinol. 2015;3:653–663. doi: 10.1016/S2213-8587(15)00136-9. [DOI] [PubMed] [Google Scholar]
- 38.Trento M, et al. Vision related quality of life in patients with type 2 diabetes in the EUROCONDOR trial. Endocrine. 2017;57:83–88. doi: 10.1007/s12020-016-1097-0. [DOI] [PubMed] [Google Scholar]
- 39.Vujosevic S, Midena E. Retinal layers changes in human preclinical and early clinical diabetic retinopathy support early retinal neuronal and Müller cells alterations. J. Diabetes Res. 2013;2013:e905058. doi: 10.1155/2013/905058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugimoto M, et al. Detection of early diabetic change with optical coherence tomography in type 2 diabetes mellitus patients without retinopathy. Ophthalmologica. 2005;219:379–385. doi: 10.1159/000088382. [DOI] [PubMed] [Google Scholar]
- 41.Oshitari T, Hanawa K, Adachi-Usami E. Changes of macular and RNFL thicknesses measured by stratus OCT in patients with early stage diabetes. Eye. 2009;23:884–889. doi: 10.1038/eye.2008.119. [DOI] [PubMed] [Google Scholar]
- 42.Browning DJ, Fraser CM, Clark S. The relationship of macular thickness to clinically graded diabetic retinopathy severity in eyes without clinically detected diabetic macular edema. Ophthalmology. 2008;115:533–539. doi: 10.1016/j.ophtha.2007.06.042. [DOI] [PubMed] [Google Scholar]
- 43.Wanek J, et al. Alterations in retinal layer thickness and reflectance at different stages of diabetic retinopathy by en face optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2016;57:341–347. doi: 10.1167/iovs.15-18715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spaide RF. Volume-rendered optical coherence tomography of retinal vein occlusion pilot study. Am. J. Ophthalmol. 2016;165:133–144. doi: 10.1016/j.ajo.2016.02.037. [DOI] [PubMed] [Google Scholar]
- 45.Balaratnasingam C, et al. Visual acuity is correlated with the area of the foveal avascular zone in diabetic retinopathy and retinal vein occlusion. Ophthalmology. 2016;123:2352–2367. doi: 10.1016/j.ophtha.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 46.Dodo Y, Murakami T, Uji A, Yoshitake S, Yoshimura N. Disorganized retinal lamellar structures in nonperfused areas of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2015;56:2012–2020. doi: 10.1167/iovs.14-15924. [DOI] [PubMed] [Google Scholar]
- 47.Nicholson L, et al. Diagnostic accuracy of disorganization of the retinal inner layers in detecting macular capillary non-perfusion in diabetic retinopathy. Clin. Exp. Ophthalmol. 2015;43:735–741. doi: 10.1111/ceo.12557. [DOI] [PubMed] [Google Scholar]
- 48.Onishi AC, Ashraf M, Soetikno BT, Fawzi AA. Multilevel ischemia in disorganization of the retinal inner layers on projection-resolved optical coherence tomography angiography. Retina. 2019;39:1588–1594. doi: 10.1097/IAE.0000000000002179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Price LD, Au S, Chong NV. Optomap ultrawide field imaging identifies additional retinal abnormalities in patients with diabetic retinopathy. OPTH. 2015;9:527–531. doi: 10.2147/OPTH.S79448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silva PS, et al. Peripheral lesions identified by mydriatic ultrawide field imaging: Distribution and potential impact on diabetic retinopathy severity. Ophthalmology. 2013;120:2587–2595. doi: 10.1016/j.ophtha.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 51.Ghasemi Falavarjani K, Wang K, Khadamy J, Sadda SR. Ultra-wide-field imaging in diabetic retinopathy; an overview. J. Curr. Ophthalmol. 2016;28:57–60. doi: 10.1016/j.joco.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.