To the Editor:
Accurately estimating extracellular volume status and establishing a euvolemic target weight are critical to caring for people treated with dialysis. However, physical examination findings of lung crackles and peripheral edema are often unreliable markers of volume status.1 Lung ultrasound is a low-risk, relatively inexpensive tool that may improve volume status assessment as well as ambulatory blood pressure, lung congestion, and cardiac indices in the outpatient dialysis setting.2, 3, 4, 5
To date, there are limited data about the agreement of lung ultrasound and volume status measures, such as physical examination and bioimpedance, in the hospital setting. Hospitalized hemodialysis patients are complex and often experience fluid shifts, heightened vasodilatory and vasoconstrictive states, blunted capillary refill, and inflammation-mediated capillary leak. These factors may affect lung ultrasound accuracy as well as its agreement with other volume status measures in the hospital. We conducted a prospective study among hospitalized patients with kidney failure treated with hemodialysis to examine the diagnostic accuracy of lung ultrasound-measured extracellular volume status, compared to the designated reference standard of bioimpedance spectroscopy and, separately, to physical examination.6,7 We hypothesized that lung ultrasound would demonstrate good agreement with bioimpedance spectroscopy. In addition, we sought to identify clinically relevant scoring thresholds for pulmonary edema to inform more time-efficient lung ultrasound.
Patients receiving maintenance hemodialysis hospitalized at University of North Carolina Hospitals (Chapel Hill, NC) from November 2019 to September 2021 were eligible. Major exclusion criteria included limb amputation(s), pacemaker, and severe lung pathology. After providing informed consent and within 30-120 minutes of the end of a hemodialysis treatment, participants underwent 3 volume assessments: standardized physical examination, bioimpedance spectroscopy (SFB7, Impedimed Limited), and lung ultrasound (Vscan, GE Healthcare). Participants were classified as hypervolemic or normo/hypovolemic based on examination findings. In each patient, the 3 examinations were performed by unique clinicians blinded to the results of the other examinations and hemodialysis treatment data. The diagnostic agreement between lung ultrasound and bioimpedance, and, separately, physical examination was assessed by calculating percent agreement and kappa statistics. Using bioimpedance-classified volume status as the reference standard, we computed the sensitivity and specificity of 28-point lung ultrasound-classified volume status. In separate analyses, we used Akaike information criterion to evaluate goodness of fit for abbreviated 4- and 8-point lung ultrasound examinations relative to the 28-point examination. See Item S1 for detailed methods.
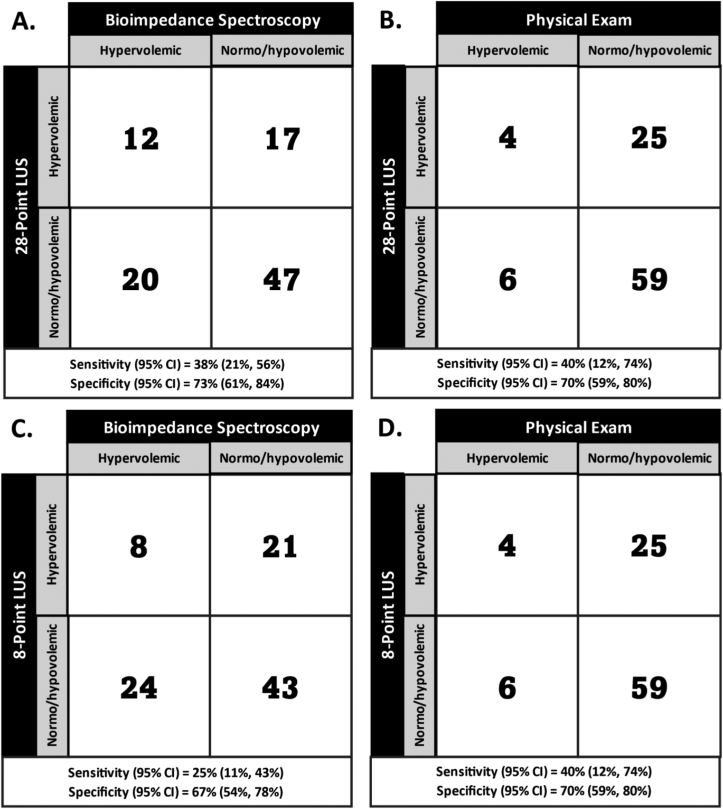
Of the 167 people offered participation, 100 (60%) individuals were enrolled, and 96 (96%) enrolled individuals completed all 3 examinations. Participants had a mean age of 56 ± 14 years and time on dialysis of 5.7 ± 5.1 years; 44 (46%) were female. The most common reasons for hospitalization were infection in 21 participants (22%) and cardiovascular causes in 16 participants (17%) (Table S1). Among the 96 participants with 3 examinations, lung ultrasound-classified volume status (hypervolemic vs normo/hypovolemic) agreed with bioimpedance-classified volume status in 59 (62%) participants (Table 1). Lung ultrasound-classified volume status agreed with physical examination-classified volume status in 63 (66%) participants. In analyses considering components of the physical examination, lung ultrasound-classified volume status agreed with the presence (vs absence) of pulmonary crackles and, separately, an S3 heart sound in 67 (70%) and 66 (69%) of participants, respectively. Among the 36 participants with no missing values for lung ultrasound (ie, no field obscured by cardiomegaly, subcutaneous fat, or catheter dressing), lung ultrasound-classified volume status agreed with bioimpedance-classified volume status in 21 participants (58%) and, separately, physical examination-classified volume status in 27 (75%) participants. Using bioimpedance-classified volume status as the reference standard, 28-point lung ultrasound-classified volume status had a sensitivity (95% confidence interval [CI]) of 38% (21%-56%) and a specificity (95% CI) of 73% (61%-84%) (Fig 1).
Table 1.
Agreement Between Lung Ultrasound and Bioimpedance Spectroscopy and Physical Examinationa
| Cohort | 28-point LUS |
8-point LUS |
||||
|---|---|---|---|---|---|---|
| Agreement Count (95% CI) | Agreement Percentage a (95% CI) | Kappa Statistic (95% CI) | Agreement Count (95% CI) | Agreement Percentage a (95% CI) | Kappa Statistic (95% CI) | |
| All patients with 3 examinations (N=96) | ||||||
| Bioimpedance spectroscopy | 59 (45 to 76) | 62% (51 to 71) | 0.11 (-0.092 to 0.32) | 51 (38 to 67) | 53% (43 to 63) | -0.08 (-0.27 to 0.11) |
| Physical examinationb | 63 (48 to 81) | 66% (55 to 75) | 0.022 (-0.15 to 0.2) | 63 (48 to 81) | 66% (55 to 75) | 0.022 (-0.15 to 0.2) |
| Physical examination components | ||||||
| Lung crackles | 67 (52 to 85) | 70% (59 to 79) | 0.095 (-0.057 to 0.25) | 67 (52 to 85) | 71% (61 to 79) | 0.095 (-0.06 to 0.25) |
| Jugular venous distension | 57 (43 to 74) | 59% (49 to 69) | 0.05 (-0.14 to 0.24) | 58 (44 to 75) | 60% (50 to 70) | 0.059 (-0.13 to 0.25) |
| S3 heart sound | 66 (51 to 84) | 69% (58 to 78) | 0.026 (-0.072 to 0.12) | 66 (51 to 84) | 69% (58 to 78) | 0.025 (-0.072 to 0.12) |
| Excluding patients with lung ultrasounds with any missing values (n=36) | ||||||
| Bioimpedance spectroscopy | 21 (13 to 32) | 58% (41 to 74) | 0.1 (-0.21 to 0.41) | 14 (8 to 23) | 39% (24 to 56) | -0.31 (-0.59 to -0.029) |
| Physical examinationb | 27 (18 to 39) | 75% (57 to 87) | 0.21 (-0.11 to 0.52) | 24 (15 to 36) | 67% (49 to 81) | 0.014 (-0.24 to 0.27) |
| Physical examination components | ||||||
| Lung crackles | 26 (17 to 38) | 72% (55 to 85) | 0.082 (-0.18, 0.34) | 25 (16 to 37) | 69% (52 to 83) | 0.066 (-0.17 to 0.31) |
| Jugular venous distension | 26 (17 to 38) | 72% (55 to 85) | 0.26 (-0.041, 0.56) | 24 (15 to 36) | 67% (49 to 81) | 0.076 (-0.19 to 0.34) |
| S3 heart sound | 26 (17 to 38) | 72% (55 to 85) | 0 (0,0) | 25 (16 to 37) | 69% (52 to 83) | 0 (0 to 0) |
| Excluding patients with lung ultrasounds with missing values in “non-usual” fields (n=59)c | ||||||
| Bioimpedance spectroscopy | 35 (24 to 49) | 59% (46, 72) | 0.092 (-0.17, 0.35) | 28 (19, 40) | 47% (34, 61) | -0.29 (-0.53, -0.049) |
| Physical examinationb | 38 (27 to 52) | 64% (51, 76) | 0.056 (-0.16, 0.27) | 37 (26, 51) | 63% (49, 75) | -0.012 (-0.23, 0.2) |
| Physical examination components | ||||||
| Lung crackles | 39 (28 to 53) | 66% (53 to 76) | 0.092 (-0.082 to 0.27) | 38 (27 to 52) | 64% (51 to 76) | 0.098 (-0.093 to 0.29) |
| Jugular venous distension | 35 (24 to 49) | 59% (46 to 72) | 0.067 (-0.14 to 0.27) | 35 (24 to 49) | 59% (46 to 72) | 0 (-0.19 to 0.19) |
| S3 heart sound | 37 (26 to 51) | 63% (49 to 75) | -0.034 (-0.099 to 0.031) | 36 (25 to 50) | 61% (47 to73) | -0.039 (-0.11 to 0.036) |
Abbreviations: CI, confidence interval; LUS, lung ultrasound.
Agreement was assessed by counting the number of participants for whom the volume status determined by lung ultrasound and the assessment in each row agreed. This was then divided by the total number of participants to determine agreement percentage.
Overall clinician assessment of volume status based on standardized physical examination findings.
Some ultrasound imaging fields (eg, intercostal spaces 2 and 3 on right midclavicular zone, intercostal spaces 3 and 4 on left parasternal zone, and intercostal spaces 2, 3 and 4 on left midclavicular zone) are more commonly obscured by an enlarged heart or dialysis catheter dressing. Missing values in “non-usual” fields denotes instances in which there were missing values in fields other than those most commonly missing.
Figure 1.
2×2 contingency tables comparing 28- (A and B) and 8-point (C and D) lung ultrasound classification of pulmonary edema status to both bioimpedance spectroscopy (A and C) and standardized physical examination (B and D).
Abbreviation: LUS, lung ultrasound.
Comparing 4-point and, separately, 8-point lung ultrasound thresholds to standard 28-point lung ultrasound, 8-point examinations demonstrated the best goodness of fit, outperforming all 4-point examinations (Fig 1, Fig S1).
We found that posthemodialysis lung ultrasound classification of volume status did not agree strongly with bioimpedance spectroscopy or physical examination in hospitalized hemodialysis patients. To date, studies of lung ultrasound in people receiving hemodialysis have been conducted primarily in the outpatient setting and have shown varying degrees of agreement between lung ultrasound and clinical assessment, echocardiographic features, natriuretic peptide levels, inferior vena cava measurements, and bioimpedance spectroscopy. In general, lung ultrasound and bioimpedance spectroscopy display good agreement, particularly postdialysis.8 However, the hospital setting presents unique challenges, and physiologic changes of acute illness, such as increased capillary leak and intercompartmental fluid shifts, among other factors, complicate volume assessment. Such acute changes may explain the relatively weak agreement between lung ultrasound and bioimpedance observed in our study. Unlike bioimpedance spectroscopy, lung ultrasound does not measure total body water, making it potentially less accurate in the setting of frequent fluid shifts. Hospitalized hemodialysis patients are often not at steady state due to factors, such as active infections, recent surgeries, decompensated heart failure, intravenous fluid administration, and administration of vasoactive medications. Accordingly, in the immediate posthemodialysis period, lung ultrasound alone may not be the ideal approach to volume assessment in hospitalized hemodialysis patients. However, lung ultrasound for acute indications, such as dyspnea and/or hypoxia, may have utility and requires further study.
In conclusion, we found that posthemodialysis lung ultrasound classification of volume status did not agree strongly with classification of volume status by bioimpedance spectroscopy or physical examination in hospitalized hemodialysis patients. We also showed that an 8-point lung ultrasound examination is a potential alternative to the more time-consuming 28-point lung ultrasound examination, a finding important to enhancing the uptake of lung ultrasound in busy hospital settings. Further investigation into the accuracy of lung ultrasound for volume assessment in the hospital hemodialysis setting is needed.
Article Information
Authors’ Contributions
Research idea and study design: EC, JF; data acquisition: MT, CG; data analysis/interpretation: EC, JF, MA; statistical analysis: MA, RG, QL; supervision or mentorship: JF. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This work was supported by an unrestricted, investigator-initiated research grant (UG2019-001) from Renal Research Institute (RRI), a subsidiary of Fresenius Kidney Care, North America. RRI played no role in study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Financial Disclosure
In the last 2 years, JEF has received speaking honoraria from the American Society of Nephrology and multiple universities. She serves on a medical advisory board for Fresenius Medical Care, North America and a scientific advisory board and a Data and Safety Monitoring Committee for NIDDK. In the last 2 years, MMA has received honoraria from the American Society of Nephrology and the International Society of Nephrology. When this study was conducted, MMA was employed by the University of North Carolina at Chapel Hill. As of August 1, 2023, she is employed by Aetion, Inc. and holds stock options in the company.
Acknowledgments
The authors acknowledge Drs. Gargi Priamvada Sharma, Surya Manivannan, Jia Yi, and Sheikh Bilal Khalid as well as research coordinators Anne Froment, Jordan Ormond Foster, Emmie Cole, and Julia Narendra for their assistance with study activities.
Peer Review
Received May 26, 2023. Evaluated by _ external peer reviewers, with direct editorial input from an Associate Editor and the Editor-in-Chief. Accepted in revised form September 10, 2023.
Footnotes
Figure S1: Thresholds for B-line score-based pulmonary edema classification from 4-point (Panel A) and 8-point (Panel B) lung ultrasounds compared to 28-point classification.
Item S1. Detailed methods.
Table S1. Participant Characteristics.
Supplementary Material
Figure S1; Item S1; Table S1.
References
- 1.Torino C., Gargani L., Sicari R., et al. The agreement between auscultation and lung ultrasound in hemodialysis patients: the LUST study. Clin J Am Soc Nephrol. 2016;11(11):2005–2011. doi: 10.2215/CJN.03890416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vitturi N., Dugo M., Soattin M., et al. Lung ultrasound during hemodialysis: the role in the assessment of volume status. Int Urol Nephrol. 2014;46(1):169–174. doi: 10.1007/s11255-013-0500-5. [DOI] [PubMed] [Google Scholar]
- 3.Loutradis C., Sarafidis P.A., Ekart R., et al. Ambulatory blood pressure changes with lung ultrasound-guided dry-weight reduction in hypertensive hemodialysis patients: 12-month results of a randomized controlled trial. J Hypertens. 2021;39(7):1444–1452. doi: 10.1097/HJH.0000000000002818. [DOI] [PubMed] [Google Scholar]
- 4.Zoccali C., Torino C., Mallamaci F., et al. A randomized multicenter trial on a lung ultrasound-guided treatment strategy in patients on chronic hemodialysis with high cardiovascular risk. Kidney Int. 2021;100(6):1325–1333. doi: 10.1016/j.kint.2021.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Loutradis C., Papadopoulos C.E., Sachpekidis V., et al. Lung ultrasound-guided dry-weight reduction and echocardiographic changes in clinically euvolemic hypertensive hemodialysis patients: 12-month results of a randomized controlled trial. Hellenic J Cardiol. 2022;64:1–6. doi: 10.1016/j.hjc.2021.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Basile C., Vernaglione L., Di Iorio B., et al. Development and validation of bioimpedance analysis prediction equations for dry weight in hemodialysis patients. Clin J Am Soc Nephrol. 2007;2(4):675–680. doi: 10.2215/CJN.00240107. [DOI] [PubMed] [Google Scholar]
- 7.Chertow G.M., Lazarus J.M., Lew N.L., Ma L., Lowrie E.G. Development of a population-specific regression equation to estimate total body water in hemodialysis patients. Kidney Int. 1997;51(5):1578–1582. doi: 10.1038/ki.1997.216. [DOI] [PubMed] [Google Scholar]
- 8.Covic A., Siriopol D., Voroneanu L. Use of lung ultrasound for the assessment of volume status in CKD. Am J Kidney Dis. 2018;71(3):412–422. doi: 10.1053/j.ajkd.2017.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1; Item S1; Table S1.