Highlights
-
•
Ketone bodies are critical markers of metabolic status.
-
•
Sensitive, reliable mass spectrometry clinical assay was developed to quantify ketones.
-
•
Health-critical ketones measured: α, β, γ -hydroxybutyrate, β-hydroxyisobutyrate, acetoacetate.
-
•
Thorough stability study and validation demonstrates high accuracy and precision.
-
•
Highly robust via optimized extraction efficacy and stability.
Keywords: Ketone bodies, Beta-hydroxybutyrate, Acetoacetate, LC-MS/MS, Clinical diagnostics, Mitochondrial disease testing
Abstract
Objectives
Ketone bodies (KBs) serve as important energy sources that spare glucose, providing the primary energy for cardiac muscle, skeletal muscle during aerobic exercise, and the brain during periods of catabolism. The levels and relationships between the KBs are critical indicators of metabolic health and disease. However, challenges in separating isomeric KBs and concerns about sample stability have previously limited their clinical measurement.
Methods
A novel 6.5-minute liquid chromatography-mass spectrometry-based assay was developed, enabling the precise measurement of alpha-, beta- and gamma-hydroxybutyrate, beta-hydroxyisobutyrate, and acetoacetate. This method was fully validated for human serum and plasma samples by investigating extraction efficiency, matrix effects, accuracy, recovery, intra- and inter-precision, linearity, lower limit of quantitation (LLOQ), carryover, specificity, stability, and more. From 107 normal samples, reference ranges were established for all analytes and the beta-hydroxybutyrate/acetoacetate ratio.
Results
All five analytes were adequately separated chromatographically. An extraction efficiency between 80 and 120 % was observed for all KBs. Accuracy was evaluated through spike and recovery using 10 random patient samples, with an average recovery of 85–115 % for all KBs and a coefficient of variation of ≤ 3 %. Coefficients of variation for intra- and inter-day imprecision were < 5 %, and the total imprecision was < 10 %. No significant interferences were observed. Specimens remained stable for up to 6 h on ice or 2 h at room temperature.
Conclusions
The developed method is highly sensitive and robust. It has been validated for use with human serum and plasma, overcoming stability concerns and providing a reliable and efficient quantitative estimation of ketone bodies.
Introduction
Ketone bodies (KBs) are crucial energy sources that spare glucose. They primarily serve as the main energy source for cardiac muscle, skeletal muscle during aerobic exercise, and the brain during periods of catabolism [1], [2], [3]. Ketones are synthesized from fatty acids and ketogenic amino acids in the liver and are oxidized in cardiac muscle, skeletal muscle, and brain during catabolism to conserve glucose. The measurement of serum or plasma beta-hydroxybutyrate (BHB) and acetoacetate (AcAc) concentrations provides insights into fatty acid β-oxidation and ketogenic amino acid catabolism [4], [5], [6]. Atypical ketones, such as alpha-hydroxybutyrate (AHB) and beta-hydroxyisobutyrate (BHIB), possess diagnostic significance in certain pathophysiological conditions. For example, elevated levels of AHB have been observed in individuals with increased intracellular reduced nicotinamide adenine dinucleotide (NADH) and decreased oxidized nicotinamide adenine dinucleotide (NAD+). These elevated levels indicate conditions such as mitochondrial disease or hypoxia [7], [8], as well as early-stage insulin resistance [9], [10]. Likewise, BHIB, a biomarker of valine catabolism in skeletal muscle [11], is elevated in patients with 3-hydroxyisobutyral-CoA Hydrolase (HIBCH) deficiency, a specific type of mitochondrial disease. Gamma-hydroxybutyrate (GHB) is a marker for succinate semialdehyde dehydrogenase deficiency (SSADH) [12], although it can also originate from exogenous intake [13], [14].
BHB and AcAc have been analyzed in biological matrices in a variety of ways, including global metabolomics studies using nuclear magnetic resonance spectroscopy [15], [16], [17], liquid chromatography-mass spectrometry [18], [19], [20], and—most commonly—by gas chromatography paired with mass spectrometry [3], [21], [22]. However, accurately measuring the ratio between BHB and AcAc in a clinical setting has been challenging due to the absence of an appropriate internal standard, stability, and sensitivity limitations of AcAc [2], [23], [24], [25]. In order to overcome these limitations, a rapid liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based assay has been developed. This new method does not require derivatization and has a low sample volume requirement. The assay facilitates precise measurement of BHB, AcAc, and their ratio, as well as other KB isomers, including AHB, GHB, and BHIB (Fig. 1). The method developed is highly sensitive, robust, and has been validated with just 10 µL of human serum or plasma. It effectively overcomes stability concerns and offers a reliable and efficient quantitative estimation of KBs.
Fig. 1.
Molecular structures of the five targets that comprise the ketone body panel assay including their molecular weight showing the inclusion of four isomers.
Overall, understanding the dynamics of KBs, including AcAc, BHB, AHB, GHB, and BHIB, is crucial for comprehending energy metabolism and its implications in various physiological and pathological conditions [26]. The development of this LC-MS/MS assay for directly measuring the BHB/AcAc ratio represents a significant advancement, providing a reliable and efficient method for quantifying KBs [27]. This assay aids not only in indirectly assessing mitochondrial function and redox state, but also in quantitatively measuring AHB, GHB, and BHIB, potentially expanding the diagnostic capabilities for mitochondrial-related disorders. The availability of this novel clinical assay offers promise for improving the diagnosis, monitoring, and understanding of mitochondrial dysfunction and related pathophysiological conditions. Furthermore, the availability of this novel assay opens new possibilities for research and clinical applications in the field of energy metabolism.
Materials and Methods
Chemicals and reagents
LCMS grade water and methanol were purchased from Honeywell, while LCMS grade acetonitrile, sodium hydroxide solution (10 N), and hydrochloric acid solution (6 N) were obtained from Fisher Scientific. Acetic acid (ACS grade), lithium acetoacetate (90 %), DL-Beta-Hydroxybutyric acid sodium salt (98 %), and DL-2- Hydroxybutyric acid sodium salt (97 %), DL-sodium-B-Hydroxyisobutyrate (96 %) were sourced from Sigma Aldrich. Santa Cruz Biotech provided the 4-hydroxybutyric acid methyl ester, and Cambridge Isotope supplied the sodium DL-3-hydroxybutyrate [3,4,4,4-D4, 98 %] at 1 mg/mL in water (95 %), ethyl acetoacetate [1,2,3,4–13C4, 99 %] (98 %), and sodium borodeuteride [D4, 99 %] (95 %). Dialyzed serum FBS (one shot) was obtained from Gibco.
Stock solutions, working solutions, calibrators, and quality controls
Individual stock solutions of AcAc, BHB, AHB, GHB, and BHIB were prepared at 50 mM in water. All five KB stock solutions were mixed equally to create a 10 mM KB-Mix, which was used to prepare dilutions for working solutions, calibrators, and quality controls (QCs). Stock solutions of the internal standards (ISs) (i.e., AcAc-IS and BHB-IS) were prepared at 5 mM in water and a mixture of these two ISs was created at 1 mM by combining the stock solutions. Due to unavailability of commercially produced GHB and AcAc-IS during the method development and validation, both compounds were synthesized in-house by hydrolyzing 4-hydroxybutyric acid methyl ester and ethyl acetoacetate [13C4], respectively [28]. Further details are provided in the Supporting Information.
Nine calibrators were prepared in water using the KB-Mix, with concentrations equivalent to serum samples: 0.0025, 0.005, 0.01, 0.025, 0.05, 0.1, 0.25, 0.5, and 1.5 mM. Due to the lack of KB-free matrices, three levels of QC materials were prepared in pooled dialyzed fetal bovine serum, serving as a surrogate matrix. The same materials were used for the precision study. Blank dialyzed serum was tested to ensure that KB levels were below the lowest calibrator and QC. All stock solutions, mixtures, dilutions, and QC materials were stored at −80 °C in small aliquots to minimize free-thaw cycles.
Specimens
All plasma and serum specimens included in this study were received at the Children’s Hospital of Philadelphia for Metabolic Lab testing, BHB testing, or routine well-visits. Upon receipt, specimens were spun down, aliquots were created, and stored at −80 °C prior to analysis. One aliquot was used for routine clinical testing, other aliquots were utilized for LC-MS/MS KB comparison testing and validation studies. Additional whole blood samples were drawn via venipuncture from consenting healthy donors to perform stability testing. The Children’s Hospital of Philadelphia Institutional Review Board (IRB)/Ethics Committee provided a determination of exemption for this study (IRB 23–021290).
Sample preparation
A straightforward protein precipitation was performed, adapted from Puchalska, et al. [25] 10 µL of serum or plasma was transferred into a microcentrifuge tube, 10 µL of 25 µM IS mix was added, followed by 30 µL of water. All samples were thoroughly homogenized prior to adding 200 µL of ice-cold extraction solution (50 % methanol in water, v/v). A pellet was created by centrifugation at 15,000 x g, chilled at 4 °C for 10 min; 200 µL of the supernatant was transferred into a new tube and dried down under nitrogen flow at room temperature. Finally, the samples were reconstituted with 160 µL 0.0125 % acetic acid in water (v/v) and injected onto the column. Throughout the sample preparation, samples, QCs, and reagents remained on ice unless otherwise noted.
LC-MS/MS method
Analyses were performed on a Waters Acquity Classic UPLC system equipped with a binary pump, coupled to a Waters Xevo TQS triple quadrupole MS utilizing electrospray ionization (ESI). Separation of the analytes was carried out on a Waters Cortecs UPLC T3 (100x2.1 mm, 1.6 µm) column at 17.5 °C. 0.0125 % acetic acid in water was selected as mobile phase A and 0.0125 % acetic acid in water:methanol (60:40) as mobile phase B (v/v). The solvent gradient started at 0 % B, increasing to 10 % B and then 20 % B at 0.5 and 1.9 min, respectively. At 2.0 min mobile phase B was increased to 30 % and at 3.0 min to 90 % and held constant for one minute. Re-equilibration of the column was performed by reducing B back to 0 %; total run time was 6.5 min. The flow rate was 0.34 mL/min; however, from 2 to 4.5 min this was reduced to 0.22 mL/min. 3 µL of sample material was injected onto the column; following each injection a needle wash was performed using 80 % methanol in water (strong wash) followed by 5 % methanol in water (weak wash) (v/v).
Ionization was carried out in negative mode using 0.8 kV; other optimized MS parameters were: nebulizer pressure at 7 bar, source gas flow of 165 L/H at 130 °C, and desolvation gas flow of 700 L/H at 425 °C. Experiments were performed using multiple reaction monitoring (MRM), and optimized MS parameters and ion transitions, including applied collision energies, are listed in Table 1.
Table 1.
MS parameters and MS/MS transitions of the ketone bodies and their respective IS.
| Analyte | RT | Description | Q1 (m/z) | Q3 (m/z) | Cone (V) | Collision energy (V) | Dwell time (ms) |
|---|---|---|---|---|---|---|---|
| AcAc | 1.39 | Quantifier | 101.02 | 57.03 | 25 | 9 | 80 |
| Qualifier | 101.02 | 57.08 | 25 | 6 | 80 | ||
| AcAc-C4 | 1.39 | Quantifier | 105.04 | 60.04 | 25 | 9 | 80 |
| Qualifier | 105.04 | 60.09 | 25 | 6 | 80 | ||
| BHB | 1.86 | Quantifier | 103.04 | 59.01 | 25 | 9 | 20 |
| Qualifier | 103.04 | 59.06 | 25 | 6 | 20 | ||
| BHB-D4 | 1.82 | Quantifier | 107.06 | 59.01 | 25 | 9 | 20 |
| Qualifier | 107.06 | 59.06 | 25 | 6 | 20 | ||
| AHB | 1.77 | Quantifier | 103.04 | 57.03 | 25 | 9 | 20 |
| Qualifier | 103.04 | 57.08 | 25 | 6 | 20 | ||
| GHB | 1.67 | Quantifier | 103.04 | 57.03 | 25 | 9 | 20 |
| Qualifier | 103.04 | 57.08 | 25 | 6 | 20 | ||
| BHIB | 2.01 | Quantifier | 103.04 | 73.03 | 25 | 9 | 20 |
| Qualifier | 103.04 | 73.08 | 25 | 6 | 20 |
Method validation
The LC-MS/MS method was fully validated and tested by examining extraction efficiency, matrix effects, accuracy, recovery, intra- and inter-precision, linearity, lower limit of quantitation (LLOQ), diluent selection, dilution, carryover, specificity, stability, specimen tube selection, and assay robustness, in accordance with the Clinical and Laboratory Standards Institute (CLSI) C62-A guidelines [29].
Extraction efficiency or recovery was determined by comparing spiked dialyzed serum pre- and post-extraction at three levels in triplicate. Matrix effects were investigated by comparing spiked dialyzed serum post-extraction versus a neat standard at the expected concentration post-extraction. A similar experiment was conducted with pooled serum. Two pooled serum samples were analyzed - spiked and non-spiked. The difference between the two samples was compared against the neat standard; both experiments were performed in triplicate.
Accuracy and recovery were evaluated by analyzing 10 random serum patient samples pre- and post-spike with a 0.05 mM KB mixture. For BHB comparison, forty-three random serum patient samples were measured using an FDA-approved enzymatic assay (Stanbio LiquiColor, performed on Ortho Diagnostics Vitros 4600), and the results were compared with those from the new LC-MS/MS KB assay.
Intra- and inter-assay precision studies were performed over 20 days, with each day involving a duplicate extraction, and each replicate was injected twice. Three levels (low, medium, and high) of spiked dialyzed serum were evaluated.
Linearity and LLOQ were determined by analyzing 11 mixtures of the lowest and highest spiked dialyzed serum (0.00125–1.5 mM) in triplicate. Subsequently, at the anticipated LLOQ level and slightly above (i.e., 0.00125 and 0.0025 mM), five replicates of each concentration were prepared in dialyzed serum and analyzed for five days to measure the imprecision and bias.
Diluent selection, dilution maximum, and carryover were established by spiking pooled patient serum at 0.1 mM and performing five dilutions between 2 and 100x in triplicate using water and dialyzed serum. Additionally, a 10 mM KB mix was spiked into pooled serum. First, a blank was measured, followed by the spiked sample, and then three blanks. The presence of analytes in the blanks was assessed. The same strategy was carried out for the system check sample at the LLOQ level. The system check was analyzed prior to and post the 10 mM spiked sample, and the percent deviation of the reported peak area was measured.
The specificity of the assay was established by measuring dialyzed serum to ensure the absence of the targets, in addition to conducting a comprehensive interference study. This study included 16 common therapeutic drugs as well as unconjugated bilirubin (icterus), conjugated bilirubin, hemoglobin (hemolysis), triglycerides (lipemic), and protein levels using the Assurance Interference Kits from Sun Diagnostics. Pooled patient serum was spiked with all KBs at two levels, 0.1 mM (low) and 1.0 mM (high), and exposed to each potential interferent. Bias compared with the control was then measured.
Specimen stability was determined through the full testing process, including pre-spin down, post-spin down, and freeze–thaw cycles, as well as extract stability. For the pre-spin down study, four serum separator tube (SST) samples were drawn from six donors and were each kept under different conditions (2 or 6 h on ice, and 2 or 6 h at room temperature). Following an immediate refrigerated spin down, the serum was stored at −80 °C for analysis. In the post-spin down study, two sample pools of newly drawn and spun down serum (SST) and plasma (NaHeparin) were spiked with GHB, since it is absent in most healthy subjects. Aliquots were stored in duplicate for each time point at various temperatures. Serum and plasma aliquots at time zero (control) were prepared for immediate analysis after aliquoting. Other samples were moved from their storage temperature to a −80 °C freezer at designated time points for analysis upon completion of the study. In addition to time zero, the study evaluated time points of 6 h, 18 h, and 24 h at room temperature, and 1, 2, 3, 4, 7, 17, and 90 days at 4 °C, −20 °C, and −80 °C. Freeze-thaw stability was assessed using the same pooled serum and plasma used for the post-spin down stability study, with aliquots subjected to up to 5 cycles of thawing and refreezing. Extract stability was evaluated by keeping a batch of 10 random patient samples in the autosampler at 4 °C after the initial analysis and re-running them over the next four days. This experiment was replicated with another batch where three aliquots of each extract, post-initial analysis, were stored at −20 °C and reanalyzed after 1, 2, and 5 days.
The performance of the matrix and specimen collection tube was examined by measuring pooled serum (SST) and plasma (NaHeparin). Each sample pool was spiked with GHB at different concentrations due to its absence in these pooled samples. Mixtures at different ratios in serum and plasma (i.e., 3:1, 1:1, and 1:3) were prepared in triplicate. The percent deviation from the expected concentration was calculated to assess differences in performance. In addition, blood was drawn from two donors using four different collection tubes (SST or Gold Top, Red Top, NaHeparin or Green Top, and EDTA or Purple Top) to compare the percent deviation of reported concentrations.
Throughout the validation process, the robustness of the assay was evaluated by testing three different column lots, two different LC-MS/MS platforms of the same model, and several brands of pipettes and tips.
Data analysis
Throughout the method development phase, MassLynx and TargetLynx V4.2 software from Waters were utilized to acquire data, optimize parameters, and batch process. All validation data collected using the final LC-MS/MS method were analyzed and curated with Ascent V4 from Indigo BioAutomation. Validated customized rules were applied to reduce processing times and human errors. Statistical data analysis was performed with R 4.2.3 [30]. Deming regression was performed with the assumption of equal variance using the “mcr” package [31]. Assessment of inter- and intra-day precision was performed using the “VCA” package [32].
Results and discussion
Method development
A 6.5-minute LC-MS/MS method was developed and validated for the quantification of AcAc, BHB, AHB, GHB, and BHIB in serum and plasma using protein precipitation. A previously developed method was adapted to improve separation and peak shape and reduce the analysis time [25]. All five analyte peaks were adequately separated chromatographically. For example, AHB and GHB are isomers and share the same MS/MS transition; however, their LC peaks were baseline resolved at 1.77 and 1.67 min, respectively. Lowering the column temperature was an important step in the chromatographic development to separate all isomers. As shown in Fig. 2, at 25 °C, minimal separation between GHB and BHB was observed; however, at a lower temperature, increased separation was observed. In the final method, the column temperature was set at 17.5 °C using a column chiller (+/−0.5 °C) to achieve appropriate separation while maintaining a sufficiently low viscosity of the mobile phase to avoid pressure issues, which were observed at lower temperatures. Additionally, the flow rate was reduced to 0.22 mL/min between 2 and 4.5 min to mitigate the pressure increase due to increased viscosity. Chromatography performance was measured over 472 injections across 20 batches, including calibrators, QCs, and unknowns, reporting a percent coefficient of variation (%CV) of <1 % for all analytes. Details are listed in the Supporting Information.
Fig. 2.
TICs show that reducing the column temperature increased the separation power of the KB isomers; however, the viscosity and system pressure also increased. At 17.5 °C optimum separation and flow rate were established. KB mix at 0.025 mM.
KBs are small molecules that easily fragment, and due to their small size, only one or two suitable fragments were recorded with the application of minimal collision energy. As shown in Table 1, unique fragments were selected for the four KB isomers whenever possible. Selecting adequate qualifier ions with sufficient abundance and a similar background was difficult, so different collision energies were applied to produce a qualifier ion. For example, hydroxybutyrate ions may produce fragments with m/z 57 and m/z 85; however, due to the inadequate intensity of m/z 85 and the presence of background noise, only m/z 57 was used. To avoid instrument errors, a slight mass offset was applied, which is negligible on a unit resolution MS [33]. In this scenario ion ratios can still be monitored to ensure fragmentation is consistent. Unfortunately, this approach may provide less analytical specificity than a true qualifier ion if the ratio of quantifier transition to pseudo-qualifier transition does not change significantly in the presence of an interferant.
Extraction efficiency and matrix effects were constantly monitored during the method development phase. An extraction efficiency between 95 and 105 % was observed for all KBs, except AcAc. The latter is the least stable, but still reported an extraction efficiency between 80 and 95 %, which was within our aim of 80–120 %. The optimized protein precipitation workflow, using an extra reconstitution step, demonstrated acceptable matrix effect for all analytes, averaging between 1.5 and 13.9 % in dialyzed serum and pooled serum. See Supporting Information for a full overview.
Accuracy and precision
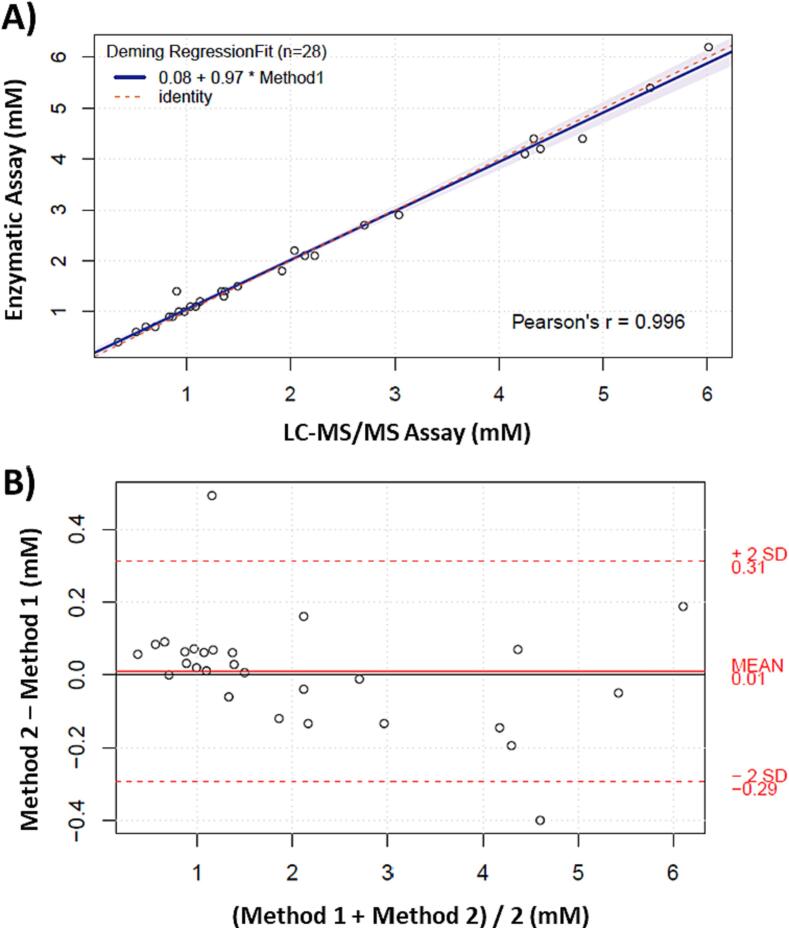
Accuracy was evaluated through spike and recovery using 10 random patient samples. Table 2 presents the data, demonstrating an average recovery between 85 and 115 % for all compounds being studied, with a tight %CV of ≤ 3 %. AHB, GHB, and BHIB exhibited slightly lower recoveries compared to the other KBs, which may be due to the absence of a suitable IS. Additionally, accuracy was assessed by analyzing three levels of QCs across 20 batches. The mean values of each level for all KBs demonstrated accuracy between 85 and 115 %. Further, the comparison between BHB measurements using an existing FDA-approved enzymatic assay (Stanbio LiquiColor, performed on Ortho Vitros 4600) in the Core Laboratory of the Children’s Hospital of Philadelphia and the new LC-MS/MS method showed a strong correlation. Out of the 43 samples compared, 13 had values below 0.3 mM (LLOQ) in the enzymatic assay, while all these samples were still within the analytical measurement range (AMR) in the LC-MS/MS method, with reported values below 0.3 mM. Moreover, samples with assigned values in both methods were compared using the Deming regression fit, resulting in a Pearson's R of 0.996 and a slope of 0.97 (Fig. 3A). The variance was equally distributed across the measured concentrations, and a mean bias of 0.01 was observed (Fig. 3B).
Table 2.
Spike and recovery results from random serum samples (n = 10) spiked with 0.05 mM of each analyte.
| Analyte | Recoveryrange (%) | Average recovery (%) | %CV |
|---|---|---|---|
| AcAc | 96.2–104.5 | 99.9 | 2.2 |
| BHB | 98.7–107.9 | 102.7 | 3.0 |
| AHB | 93.0–98.0 | 95.3 | 2.1 |
| GHB | 81.5–88.1 | 85.7 | 2.1 |
| BHIB | 84.6–91.4 | 87.5 | 2.0 |
Fig. 3.
Method comparison of BHB between the newly developed LC-MS/MS ketone body panel (method 1) and CHOP’s enzymatic assay (method 2). A) Deming regression plot with 95% interval in purple with Jackknife method, and B) Difference plot showing estimated bias. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The precision study was carried out at low, medium, and high QC levels. Intra-precision was determined by assessing the injection precision and the replicate extraction precision within-day. Inter-precision was determined by analyzing the same QC materials over a span of 20 days to calculate between-day precision. The reported %CV values for intra- and inter-imprecision were < 5 %, and the total imprecision was < 10 %, which was within our target acceptance criteria (Table 3).
Table 3.
Precision study results of 20 days, 2 replicates sample preparations, and 2 injections in dialyzed serum at three levels.
| Level | Spiked Concentration (mM) | Total (%CV) | Inter-assay | Intra-assay |
|
|---|---|---|---|---|---|
| Day (%CV) | Replicate (%CV) | Injection (%CV) | |||
| AcAc Low | 0.005 | 5.1 | 2.5 | 0.0 | 4.4 |
| AcAc Med | 0.05 | 3.8 | 2.8 | 1.8 | 1.7 |
| AcAc High | 0.5 | 2.8 | 1.5 | 2.0 | 1.3 |
| BHB Low | 0.005 | 4.2 | 2.3 | 1.8 | 3.1 |
| BHB Med | 0.05 | 3.3 | 1.3 | 2.3 | 2.0 |
| BHB High | 0.5 | 4.2 | 2.3 | 1.8 | 3.1 |
| AHB Low | 0.005 | 6.3 | 3.9 | 3.5 | 3.4 |
| AHB Med | 0.05 | 5.3 | 2.6 | 4.5 | 1.1 |
| AHB High | 0.5 | 3.7 | 1.2 | 3.3 | 1.3 |
| GHB Low | 0.005 | 6.3 | 4.2 | 1.6 | 4.4 |
| GHB Med | 0.05 | 4.8 | 2.5 | 3.7 | 1.8 |
| GHB High | 0.5 | 4.1 | 3.1 | 1.8 | 1.8 |
| BHIB Low | 0.005 | 4.2 | 3.5 | 1.2 | 2.0 |
| BHIB Med | 0.05 | 4.0 | 1.5 | 3.6 | 1.0 |
| BHIB High | 0.5 | 3.0 | 1.9 | 2.0 | 1.1 |
Linearity and LLOQ
Eleven-point curves were created using a mix of 0.0125 mM and 1.5 mM samples in triplicate, representing equal intervals. Nominal values were plotted against the measured concentrations for each analyte. AcAc and BHB demonstrated linearity across the tested range, while AHB, GHB, and BHIB exhibited linearity up to 1.35 mM, likely due to the absence of an IS. The correlation coefficients were > 0.9993, and the calculated residuals reported a bias of < 15 % for all analytes.
The LLOQ was established by measuring five replicate extractions over five days at two levels. At 0.00125 mM, all analytes reported a CV of < 15 %, but a calculated bias of > 15 %. At 0.0025 mM, AcAc, AHB, and GHB reported a CV and bias of < 15 %, while BHB and BHIB performed well at 0.0050 mM. Given the assigned nominal values to the calibrators, appropriate analytical measurement ranges (AMRs) were set and listed in Table 4. Integrated chromatograms at LLOQ level are displayed in Fig. 4.
Table 4.
Established analytical measurement range (AMR), clinically reportable range (CRR), and clinical reference range.
| Analyte | AMR (mM) | CRR (mM) | Reference Range (mM) |
|---|---|---|---|
| AcAc | 0.0025 – 1.5 | 0.0025 – 75.0 | 0.0072–0.2409 |
| BHB | 0.0050 – 1.5 | 0.0050 – 75.0 | 0.0078–0.4101 |
| BHB/AcAc | – | – | 0.4757–2.5654 |
| AHB | 0.0025 – 0.5 | 0.0025 – 25.0 | 0.0106–0.1031 |
| GHB | 0.0025 – 0.5 | 0.0025 – 25.0 | <0.0025 |
| BHIB | 0.0050 – 0.5 | 0.0050 – 25.0 | <0.0050–0.0439 |
Fig. 4.
Integrated chromatograms at LLOQ levels for each analyte.
Diluent, dilution maximum, and carryover
Dilutions were performed in triplicate using both dialyzed serum and water at 2, 5, 10, 50, and 100x. All targets demonstrated an imprecision and bias of < 15 % when using either diluent up to 50x dilution. Based on the established maximum dilution of 50x, a clinically reportable range (CRR) was determined and listed in Table 4, using the upper limit of the AMR and the maximum dilution.
Throughout the validation process, no carryover was observed in the blank sample following the highest calibrator. Moreover, blank samples analyzed after a spiked patient sample with 10 mM KB mix, values were reported < LLOQ. Furthermore, when comparing peak areas of a system check sample at the LLOQ level measured before and after analysis of the 10 mM spiked sample, the deviation was <20 %.
Specificity
Twenty-one interferences were tested using pooled serum that was spiked at both low and high concentrations of KBs. No significant interferences were observed, and all five analytes reported a bias of <10 % for the individually surveyed interferences. The only exceptions were high levels of triglycerides and protein, which resulted in a bias of <15 %.
Stability
Due to the known limited stability of acetoacetate, it was important to conduct a comprehensive stability study to ensure the accuracy of the assay. The stability of the KBs was assessed from the time of sample collection until centrifugation at the laboratory, with the aim of evaluating any potential biases in the measurements. When comparing the stability of specimens kept on ice for 6 h or at room temperature (RT) for 2 h to those kept on ice for 2 h (which represents the fastest typical time from blood draw to receipt in our clinical setting), the average bias for all KBs remained below 15 %. However, when samples were kept at RT for a longer period after collection, the bias increased from 14.5 % (2 h at RT) to 25.9 % (6 h at RT) for AcAc. Additionally, the bias for the BHB/AcAc ratio increased from 16.6 % (2 h at RT) to 37.1 % (6 h at RT). Nevertheless, the remaining analytes demonstrated biases of less than 10 % across all studied time points. Notably, GHB was excluded from this experiment due to its absence in most healthy subjects and the challenges associated with accurately spiking freshly drawn blood without knowledge of the exact volume. Detailed results are provided in the Supporting Information.
Additionally, the stability of all KBs, including GHB, was evaluated after spinning down the serum and plasma samples at various temperatures: RT, 4 °C, −20 °C, and −80 °C. The results showed that all analytes, except AcAc, remained stable for up to 24 h in both serum and plasma when stored at RT. However, although AcAc displayed a bias of less than 10 % for up to 6 h, but this bias significantly increased to over 35 % after this time point in both plasma and serum. Conversely, at 4 °C, −20 °C, and −80 °C, all analytes, except AcAc, demonstrated stability for at least 90 days in both serum and plasma, with biases below 17 %. AcAc maintained stability for 2 days at 4 °C, 7 days at −20 °C, and at least 90 days at −80 °C. The stability profile of AcAc is depicted in Fig. 5.
Fig. 5.
Stability plot of AcAc in serum and plasma at (A) room temperature, (B) 4 °C, −20 °C, and −80 °C, error bars are 1xSD, n = 2.
Additionally, the impact of freeze–thaw cycles on analyte stability was examined. The results indicated that all analytes remained stable for at least 5 cycles in both serum and plasma, with biases below 20 %. However, AHB exhibited an upward trend immediately after the first cycle in both matrices, and after two cycles, a steady bias between 15 and 20 % was observed, particularly in serum. A comprehensive overview of the stability results can be found in Table 5. Finally, the stability of a fully extracted batch was assessed at 4 °C for four days and at −20 °C for five days. BHB demonstrated stability under both storage conditions, whereas AcAc remained stable for one day at 4 °C, but achieved full stability at −20 °C. AHB and BHIB displayed stability for one day at 4 °C, but were not stable at −20 °C. The post-extraction stability of GHB was not determined as none of the tested batches contained GHB in patient samples. Further details regarding the stability experiments, including stability plots, can be found in the Supporting Information.
Table 5.
Overview KB stability, bias < 20 % was set as cutoff.
| Analyte | From draw tospin-down |
From spin-down to storage |
Freeze-thaw cycles | ||||
|---|---|---|---|---|---|---|---|
| RT | 4 °C | RT | 4 °C | −20 °C | −80 °C | ||
| AcAc | 6H | 2H | 6H | 2 days | 7 days | At least 90 days |
At least 5 |
| BHB | At least 6H |
At least 6H |
At least 24H | At least 90 days |
At least 90 days |
At least 90 days |
At least 5 |
| AHB | At least 6H |
At least 6H |
At least 24H | At least 90 days |
At least 90 days |
At least 90 days |
At least 5 |
| GHB | At least 6H |
At least 6H |
At least 24H | At least 90 days |
At least 90 days |
At least 90 days |
At least 5 |
| BHIB | At least 6H |
At least 6H |
At least 24H | At least 90 days |
At least 90 days |
At least 90 days |
At least 5 |
Matrix and specimen collection tubes
To evaluate the commutability of different matrices, pooled serum and plasma samples were spiked and mixed in various ratios. The objective was to investigate any potential bias between the two matrices. The results showed that all analytes exhibited a bias of less than 10 %. In general, absolute bias was slightly less in serum (Table 6, Table 7).
Table 6.
Matrix comparison of spiked serum SST and plasma NaHeparin collected samples; calculated %bias towards the expected KB concentrations after mixing the sample pools.
| Serum: Plasma Ratio | 3: 1 | 1: 1 | 1: 3 |
|---|---|---|---|
| AcAc | 3.9 | 4.7 | 5.1 |
| BHB | 4.6 | 7.9 | 7.3 |
| AHB | 1.9 | 3.1 | 5.7 |
| GHB | 1.6 | 5.8 | 8.3 |
| BHIB | 4.7 | 4.1 | 8.5 |
Table 7.
Comparison of freshly drawn blood in four different collection tubes, %bias is calculated towards the serum SST tube as control. D1 and D2 are donor 1 and 2.
| Analyte | Plasma (NaHeparin) |
Plasma (EDTA) |
Serum (Red Top) |
|||
|---|---|---|---|---|---|---|
| D1 | D2 | D1 | D2 | D1 | D2 | |
| AcAc | −13.7 | 6.5 | −4.8 | 13.6 | 1.0 | −5.8 |
| BHB | −5.5 | 7.7 | 15.8 | 17.9 | 3.0 | −2.5 |
| AHB | −2.5 | 3.7 | −5.2 | 4.0 | −2.6 | 4.0 |
| GHB | ND | ND | ND | ND | ND | ND |
| BHIB | 1.9 | 0.0 | 18.5 | 13.7 | −1.5 | 1.0 |
In addition to matrix mixing, blood samples were freshly drawn into different specimen collection tubes, namely SST, Red Top, NaHeparin, and EDTA tubes, from two donors. Since most of the validation was performed using serum from SSTs, it was considered as the control. For AcAc and AHB, the bias was below 15 % across all four tested tubes. BHB and BHIB showed good agreement between the NaHeparin and Red Top tubes. However, a bias greater than 15 % was observed for the EDTA tube (Table 7). GHB was not detected in any of the tubes from either donor. Based on these findings, the EDTA tubes were deemed unsuitable and were excluded from the list of acceptable collection tubes.
Reference range
New reference ranges for all analytes and the BHB/AcAc ratio have been established for patient diagnosis. Initially, 156 specimens were analyzed; after a chart review, ultimately 107 samples were included in the reference range. Samples were excluded due to factors such as recent vomiting, extreme fasting, eating disorders, or the presence of interfering metabolic diseases. The newly determined reference ranges, representing the central 95 % of included samples, are presented in Table 4. Additional details regarding the distribution of each analyte among the reference samples can be found in the Supporting Information.
Conclusions
A rapid and robust LC-MS/MS-based clinical assay was developed for the quantification of KBs, including AcAc and BHB, as well as three additional isomers of hydroxybutyrate (i.e., AHB, GHB, and BHIB). The method exhibited minimal imprecision, acceptable bias in accuracy testing, and high specificity with respect to common interferences. Notably, the instability of AcAc was overcome with strict guidelines for sample handling. Despite limited stability, the validation study identified three acceptable sample collection tubes (i.e., SST, Red Top, and NaHeparin). This flexibility in collection tubes allows for minimizing collection volumes when blood is collected for multiple simultaneous tests. Finally, a reference range was established for all five KBs, in addition to the BHB/AcAc ratio. The reference range of the BHB/AcAc ratio and AHB are expected to be valuable in diagnosing and monitoring mitochondrial dysfunction, aiding in the accurate assessment and diagnosis of these associated conditions. This assay also provides highly sensitive measurement of gamma-hydroxybutyrate which will be useful in diagnosing and monitoring patients with succinate semialdehyde dehydrogenase deficiency.
Full disclosures
Dr. Kemperman has nothing to disclose.
Dr. Ganetzky receives consulting fees from Nurture Genomics and Minovia Therapeutics. She receives grant funding from the Champ Foundation, Saol therapeutics, NIGMS and NICHD.
Dr. Master has the following disclosures: Employment or Leadership: ADLM, Consultant or Advisory Role: MEDACorp, Stock Ownership: None declared., Honoraria: Korean Society for Laboratory Medicine and Weill Cornell Medicine, Research Funding: National Institutes of Health., Expert Testimony: Expert witness fees from the US Attorney’s Office, Northern California District., Patents: US patent 11,473,131 B2 (Methods for Detecting Genomic DNA Methylation)., Other Remuneration: Support for attending meetings and/or travel from MSACL, AACC, KSLM.
Ethics statement
The Children’s Hospital of Philadelphia Institutional Review Board (IRB)/Ethics Committee provided a determination of exemption for this study (IRB 23–021290).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements and Funding
The authors would like to thank the support of the Children’s Hospital of Philadelphia’s Mitochondrial Frontier Program.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jmsacl.2024.01.004.
Contributor Information
Rebecca D. Ganetzky, Email: ganetzkyr@chop.edu.
Stephen R. Master, Email: masters@chop.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Koeslag J.H., Noakes T.D., Sloan A.W. Post-exercise ketosis. J. Physiol. 1980;301(1):79–90. doi: 10.1113/jphysiol.1980.sp013190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoon H.-R. Simultaneous determination of plasma lactate, pyruvate, and ketone bodies following tert-butyldimethylsilyl derivatization using GC-MS-SIM. Biomed. Sci. Lett. 2015;21(4):241–247. doi: 10.15616/BSL.2015.21.4.241. [DOI] [Google Scholar]
- 3.Mikkelsen K.H., Seifert T., Secher N.H., Grøndal T., Van Hall G. Systemic, cerebral and skeletal muscle ketone body and energy metabolism during acute hyper-D-β-hydroxybutyratemia in post-absorptive healthy males. J. Clin. Endocrinol. Metab. 2015;100(2):636–643. doi: 10.1210/jc.2014-2608. [DOI] [PubMed] [Google Scholar]
- 4.Miyazaki T., Honda A., Ikegami T., Iwamoto J., Monma T., Hirayama T., Saito Y., Yamashita K., Matsuzaki Y. Simultaneous quantification of salivary 3-hydroxybutyrate, 3-hydroxyisobutyrate, 3-hydroxy-3-methylbutyrate, and 2-hydroxybutyrate as possible markers of amino acid and fatty acid catabolic pathways by LC–ESI–MS/MS. SpringerPlus. 2015;4(1):494. doi: 10.1186/s40064-015-1304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grillet P.-E., Badiou S., Lambert K., Sutra T., Plawecki M., Raynaud De Mauverger E., Brun J.-F., Mercier J., Gouzi F., Cristol J.-P. Biomarkers of redox balance adjusted to exercise intensity as a useful tool to identify patients at risk of muscle disease through exercise test. Nutrients. 2022;14(9):1886. doi: 10.3390/nu14091886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corkey B.E., Deeney J.T. The Redox Communication Network as a Regulator of Metabolism. Front. Physiol. 2020;11 doi: 10.3389/fphys.2020.567796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma R., Reinstadler B., Engelstad K., Skinner O.S., Stackowitz E., Haller R.G., Clish C.B., Pierce K., Walker M.A., Fryer R., Oglesbee D., Mao X., Shungu D.C., Khatri A., Hirano M., De Vivo D.C., Mootha V.K. Circulating markers of NADH-reductive stress correlate with mitochondrial disease severity. J. Clin. Invest. 2021;131(2):e136055. doi: 10.1172/JCI136055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pirinen E., Auranen M., Khan N.A., Brilhante V., Urho N., Pessia A., Hakkarainen A., Kuula J., Heinonen U., Schmidt M.S., Haimilahti K., Piirilä P., Lundbom N., Taskinen M.-R., Brenner C., Velagapudi V., Pietiläinen K.H., Suomalainen A. Niacin cures systemic NAD+ deficiency and improves muscle performance in adult-onset mitochondrial myopathy. Cell Metab. 2020;31(6):1078–1090.e5. doi: 10.1016/j.cmet.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Tripathy D., Cobb J.E., Gall W., Adam K.-P., George T., Schwenke D.C., Banerji M., Bray G.A., Buchanan T.A., Clement S.C., Henry R.R., Kitabchi A.E., Mudaliar S., Ratner R.E., Stentz F.B., Reaven P.D., Musi N., Ferrannini E., DeFronzo R.A. A novel insulin resistance index to monitor changes in insulin sensitivity and glucose tolerance: The ACT NOW study. J. Clin. Endocrinol. Metab. 2015;100(5):1855–1862. doi: 10.1210/jc.2014-3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Syed Ikmal S.I.Q., Zaman Huri H., Vethakkan S.R., Wan Ahmad W.A. Potential biomarkers of insulin resistance and atherosclerosis in type 2 diabetes mellitus patients with coronary artery disease. Int. J. Endocrinol. 2013;2013:1–11. doi: 10.1155/2013/698567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avogaro A., Bier D.M. Contribution of 3-hydroxyisobutyrate to the measurement of 3-hydroxybutyrate in human plasma: comparison of enzymatic and gas-liquid chromatography-mass spectrometry assays in normal and in diabetic subjects. J. Lipid Res. 1989;30(11):1811–1817. doi: 10.1016/S0022-2275(20)38227-4. [DOI] [PubMed] [Google Scholar]
- 12.Divry P., Baltassat P., Rolland M.O., Cotte J., Hermier M., Duran M., Wadman S.K. A new patient with 4-hydroxybutyric aciduria, a possible defect of 4-aminobutyrate metabolism. Clin. Chim. Acta. 1983;129(3):303–309. doi: 10.1016/0009-8981(83)90033-5. [DOI] [PubMed] [Google Scholar]
- 13.Elliott S.P. Gamma Hydroxybutyric Acid (GHB) concentrations in humans and factors affecting endogenous production. Forensic Sci. Int. 2003;133(1–2):9–16. doi: 10.1016/S0379-0738(03)00043-4. [DOI] [PubMed] [Google Scholar]
- 14.Dahl S.R., Olsen K.M., Strand D.H. Determination of Gamma-Hydroxybutyrate (GHB), Beta-Hydroxybutyrate (BHB), Pregabalin, 1,4-Butane-Diol (1,4BD) and Gamma-Butyrolactone (GBL) in Whole Blood and Urine Samples by UPLC–MSMS. J. Chromatogr. B. 2012;885–886:37–42. doi: 10.1016/j.jchromb.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Wang L., Huang S., Zhu T., Ge X., Pei C., Hong G., Han L. Metabolomic Study on Iohexol-Induced Nephrotoxicity in Rats Based on NMR and LC–MS Analyses. Chem. Res. Toxicol. 2022;35(2):244–253. doi: 10.1021/acs.chemrestox.1c00299. [DOI] [PubMed] [Google Scholar]
- 16.Wallace M., Hashim Y.-Z.-H.-Y., Wingfield M., Culliton M., McAuliffe F., Gibney M.J., Brennan L. Effects of Menstrual Cycle Phase on Metabolomic Profiles in Premenopausal Women. Hum. Reprod. 2010;25(4):949–956. doi: 10.1093/humrep/deq011. [DOI] [PubMed] [Google Scholar]
- 17.Lanza I.R., Zhang S., Ward L.E., Karakelides H., Raftery D., Nair K.S. Quantitative Metabolomics by 1H-NMR and LC-MS/MS Confirms Altered Metabolic Pathways in Diabetes. PLoS ONE. 2010;5(5):e10538. doi: 10.1371/journal.pone.0010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He F., Zhai J., Zhang L., Liu D., Ma Y., Rong K., Xu Y., Ma J. Variations in Gut Microbiota and Fecal Metabolic Phenotype Associated with Fenbendazole and Ivermectin Tablets by 16S RRNA Gene Sequencing and LC/MS-Based Metabolomics in Amur Tiger. Biochem. Biophys. Res. Commun. 2018;499(3):447–453. doi: 10.1016/j.bbrc.2018.03.158. [DOI] [PubMed] [Google Scholar]
- 19.Van Hall, G.; Afshar, M. Lc-Ms/Ms Method for Quantitative Profiling of Ketone Bodies, Α-Keto Acids, Lactate, Pyruvate and Their Stable Isotopically Labeled Tracers in Human Plasma: An Analytical Panel for Clinical Metabolic Kinetics and Interactions; preprint; SSRN, 2023. https://doi.org/10.2139/ssrn.4435908. [DOI] [PubMed]
- 20.Lewis G.D., Farrell L., Wood M.J., Martinovic M., Arany Z., Rowe G.C., Souza A., Cheng S., McCabe E.L., Yang E., Shi X., Deo R., Roth F.P., Asnani A., Rhee E.P., Systrom D.M., Semigran M.J., Vasan R.S., Carr S.A., Wang T.J., Sabatine M.S., Clish C.B., Gerszten R.E. Metabolic signatures of exercise in human plasma. Sci. Transl. Med. 2010;2:33. doi: 10.1126/scitranslmed.3001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sánchez-Illana Á., Núñez-Ramiro A., Cernada M., Parra-Llorca A., Valverde E., Blanco D., Moral-Pumarega M.T., Cabañas F., Boix H., Pavon A., Chaffanel M., Benavente-Fernández I., Tofe I., Loureiro B., Fernández-Lorenzo J.R., Fernández-Colomer B., García-Robles A., Kuligowski J., Vento M., HYPOTOP Study Group, Cordeiro M., Arriaga M., Ureta-Velasco N., Caballero M.A., Fernández C., Castilla Y., Ferreira J.F., Lubián-López S.P., Jaraba P., López de Heredia J. Evolution of energy related metabolites in plasma from newborns with hypoxic-ischemic encephalopathy during hypothermia treatment. Sci. Rep. 2017;7(1):17039. doi: 10.1038/s41598-017-17202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paik M.-J., Cho E.-Y., Kim H., Kim K.-R., Choi S., Ahn Y.-H., Lee G. Simultaneous clinical monitoring of lactic acid, pyruvic acid and ketone bodies in plasma as methoxime/tert-butyldimethylsilyl derivatives by gas chromatography-mass spectrometry in selected ion monitoring mode. Biomed. Chromatogr. 2008;22(5):450–453. doi: 10.1002/bmc.966. [DOI] [PubMed] [Google Scholar]
- 23.Pacenti M., Dugheri S., Traldi P., Degli Esposti F., Perchiazzi N., Franchi E., Calamante M., Kikic I., Alessi P., Bonacchi A., Salvadori E., Arcangeli G., Cupelli V. New automated and high-throughput quantitative analysis of urinary ketones by multifiber exchange-solid phase microextraction coupled to fast gas chromatography/negative chemical-electron ionization/mass spectrometry. J. Autom. Methods Manag. Chem. 2010;2010:1–13. doi: 10.1155/2010/972926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carragher F.M., Bonham J.R., Smith J.M. Pitfalls in the measurement of some intermediary metabolites. Ann. Clin. Biochem. Int. J. Lab. Med. 2003;40(4):313–320. doi: 10.1258/000456303766476968. [DOI] [PubMed] [Google Scholar]
- 25.Puchalska P., Nelson A.B., Stagg D.B., Crawford P.A. Determination of ketone bodies in biological samples via rapid UPLC-MS/MS. Talanta. 2021;225 doi: 10.1016/j.talanta.2020.122048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sørensen L.K., Rittig N.F., Holmquist E.F., Jørgensen K.A., Jørgensen J.O.L., Møller N., Johannsen M. Simultaneous Determination of β-Hydroxybutyrate and β-Hydroxy-β-Methylbutyrate in human whole blood using hydrophilic interaction liquid chromatography electrospray tandem mass spectrometry. Clin. Biochem. 2013;46(18):1877–1883. doi: 10.1016/j.clinbiochem.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Gill E.L., Wang J., Viaene A.N., Master S.R., Ganetzky R.D. Methodologies in mitochondrial testing: diagnosing a primary mitochondrial respiratory chain disorder. Clin. Chem. 2023:hvad037. doi: 10.1093/clinchem/hvad037. [DOI] [PubMed] [Google Scholar]
- 28.Beylot M., Beaufrere B., Normand S., Riou J.P., Cohen R., Momex R. Determination of human ketone body kinetics using stable-isotope labelled tracers. Diabetologia. 1986;29(2):90–96. doi: 10.1007/BF00456116. [DOI] [PubMed] [Google Scholar]
- 29.Clarke W., Molinaro R.J. Clinical and Laboratory Standards Institute; Wayne, Penn: 2014. Liquid Chromatography-Mass Spectrometry Methods: Approved Guideline. [Google Scholar]
- 30.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, 2023. https://www.r-project.org/.
- 31.Potapov, S.; Model, F.; Schuetzenmeister, A.; Manuilova, E.; Dufey, F.; Raymaekers, J. Mcr: Method Comparison Regression, 2023. https://CRAN.R-project.org/package=mcr.
- 32.Schuetzenmeister, A.; Dufey, F. VCA: Variance Component Analysis, 2022. https://CRAN.R-project.org/package=VCA.
- 33.Kushnir M.M., Rockwood A.L., Nelson G.J., Yue B., Urry F.M. Assessing analytical specificity in quantitative analysis using tandem mass spectrometry. Clin. Biochem. 2005;38(4):319–327. doi: 10.1016/j.clinbiochem.2004.12.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.