Abstract
Adherence to ciliated respiratory epithelial cells is considered a critical early step in Bordetella pathogenesis. For Bordetella pertussis, the etiologic agent of whooping cough, several factors have been shown to mediate adherence to cells and cell lines in vitro. These putative adhesins include filamentous hemagglutinin (FHA), fimbriae, pertactin, and pertussis toxin. Determining the precise roles of each of these factors in vivo, however, has been difficult, due in part to the lack of natural-host animal models for use with B. pertussis. Using the closely related species Bordetella bronchiseptica, and by constructing both deletion mutation and ectopic expression mutants, we have shown that FHA is both necessary and sufficient for mediating adherence to a rat lung epithelial (L2) cell line. Using a rat model of respiratory infection, we have shown that FHA is absolutely required, but not sufficient, for tracheal colonization in healthy, unanesthetized animals. FHA was not required for initial tracheal colonization in anesthetized animals, however, suggesting that its role in establishment may be dedicated to overcoming the clearance action of the mucociliary escalator.
Investigations aimed at understanding the precise roles of individual bacterial virulence factors in host recognition, colonization of specific sites on or within host tissues, and persistence in the face of constitutive and adaptive immune defenses are often hampered by the fact that different factors may serve similar, overlapping, or redundant functions during the course of infection. Real as well as artifactual redundancies may be especially pronounced in in vitro studies or studies using surrogate hosts in which natural interactions are approximated by unnatural experimental conditions. For Bordetella pertussis, the etiologic agent of whooping cough, several potential virulence factors have been identified. These include fimbriae of at least three serotypes (9, 34, 44), an outer membrane protein called pertactin (40), a 220-kDa molecule that is both secreted and cell-associated called filamentous hemagglutinin (FHA) (37), a bifunctional adenylate cyclase/hemolysin (AC/HLY) (22), pertussis toxin (PTX) (3), the tracheal colonization factor TcfA (11), and a serum resistance factor (Brk) (10). Studies using a variety of mammalian cells and cell lines have indicated that at least four of these (FHA, fimbriae, pertactin, and PTX) can mediate attachment of B. pertussis to eukaryotic cells in vitro. Consequently, roles as adhesins have been proposed (2, 5, 14, 19–21, 23, 33, 35, 36, 43, 44, 49–51). In vivo evidence for these roles, however, has been difficult to obtain due in part to the lack of natural animal models for B. pertussis. Distinguishing the functions of FHA and fimbriae has been further complicated by the fact that the genes and operons encoding these factors are intermingled on the Bordetella chromosome (28, 56).
The goal of this work is to investigate the role of FHA in infection of a natural host and to compare these results with those obtained from in vitro studies and from experiments using B. pertussis in mouse models. We have focused our studies of this putative adhesin and other virulence determinants on Bordetella bronchiseptica, a Bordetella species very closely related to B. pertussis that naturally infects a variety of mammals, including rabbits, rats, and guinea pigs (15). B. bronchiseptica expresses nearly the same set of virulence factors as B. pertussis, including FHA, fimbriae, pertactin, and AC/HLY (15). As in B. pertussis, the B. bronchiseptica genes required for FHA synthesis and processing (fhaB and fhaC) flank the fimbrial biogenesis (fimBCD) operon and are positively regulated by the products of the bvgAS locus. BvgA and BvgS comprise a two-component signal transduction system that mediates a biphasic transition between infectious (Bvg+) and noninfectious (Bvg−) phases in response to specific environmental conditions (for reviews, see references 6, 47, and 52). Like all known protein virulence factors identified in B. pertussis and B. bronchiseptica, FHA is expressed only in the Bvg+ phase (46).
We addressed problems associated with genetic complexity and potential redundancy of other factors by constructing two types of FHA mutants (Fig. 1A). To determine the requirement of FHA for specific phenotypes, we constructed a mutant with an in-frame deletion in the FHA structural gene (fhaB) and compared this strain with the wild type in vitro and in vivo. To determine the sufficiency of FHA for specific phenotypes, and to eliminate complications due to the presence of other factors with potentially compensatory functions, an ectopic expression approach was taken. We have previously used this approach to investigate the role of Bvg-mediated repression during Bordetella pathogenesis (1). Here, we constructed a strain in which FHA is expressed ectopically in the Bvg− phase, in the absence of the constellation of adhesins and toxins with which it is normally expressed. The phenotypic properties of these two types of mutants provide compelling evidence that FHA does indeed function as an important adhesion in vivo. The data suggest FHA-mediated attachment to tracheal respiratory epithelia allows Bordetella to overcome constitutive defense mechanisms operative in the trachea, including the clearance action of the mucociliary escalator.
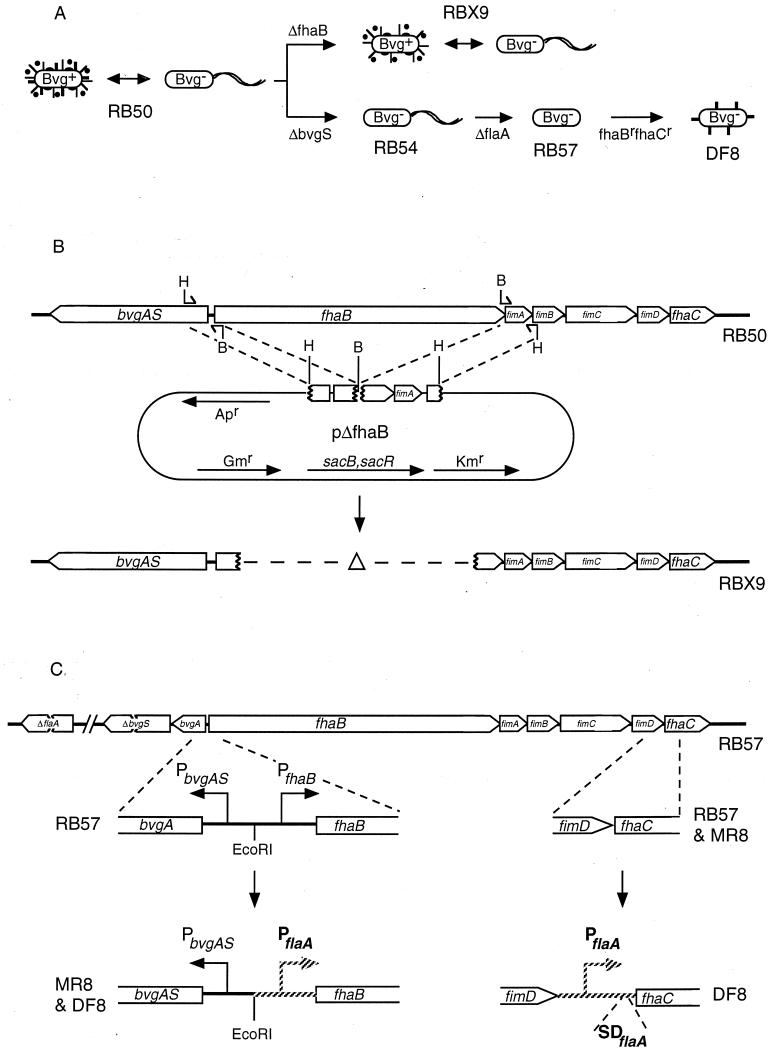
FIG. 1.
(A) Schematic representation of the two experimental approaches. RB50 alternates between the Bvg+ phase, characterized by the expression of FHA (thick lines), fimbriae (thin lines), pertactin (solid ovals), and AC/HLY (solid circles), and the Bvg− phase, characterized by the expression of flagella (wavy lines). Deletion of the FHA structural gene (ΔfhaB) results in a strain, RBX9, which is identical to RB50 except that it lacks FHA. Deletion of BvgS (ΔbvgS) locks RB50 in the Bvg− phase (RB54), and deletion of the flagellin structural gene (ΔflaA) results in lack of flagella (RB57). Addition of Bvg− phase-specific promoters to fhaB and fhaC (fhaBrfhaCr) results in ectopic expression of FHA in a Bvg− phase-locked strain (DF8). (B) Construction of the ΔFHA strain, RBX9. bvgAS, fhaB, fimA, fimB, fimC, fimD, and fhaC are contiguous on the Bordetella chromosome. Oligonucleotides containing either a HindIII (H) or a BamHI (B) restriction endonuclease cleavage site at one end were used to amplify DNA fragments by PCR, which were then joined and cloned into our allelic exchange vector to create plasmid pΔfhaB. Antibiotic resistance genes and the sacB and sacR genes encoded on the plasmid are indicated. Two consecutive homologous recombination events occurring on each side of the deletion junction result in deletion of all but the first four and last five codons of fhaB from the B. bronchiseptica chromosome. (C) Construction of the FHAr strain, DF8. RB57 contains deletion mutations in bvgS, locking it in the Bvg− phase, and flaA, rendering it unable to synthesize flagella. Replacement of the native fhaB promoter (PfhaB) with the Bvg− phase-specific flagellin promoter (PflaA; hatched lines) allows expression of fhaB in RB57. Insertion of the flagellin promoter plus the flagellin ribosome binding site (SDflaA) allows expression of fhaC. Both fhaB and fhaC are required for expression, processing, and localization of FHA.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
B. bronchiseptica RB50, RB53, RB54, and RB57 have been described elsewhere (1, 7). RBX9, MR8, and DF8 are derivatives of RB50 and RB57. Their construction is described below. B. bronchiseptica strains were grown on Bordet-Gengou agar (BG; Becton Dickinson Microbiology Systems) supplemented with 7.5% sheep blood or in Stainer-Scholte broth (45). Escherichia coli DH5α was used for all cloning steps. E. coli was grown in L broth or on L agar. When appropriate, the culture media were supplemented with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; 20 μg ml−1), ampicillin (100 μg ml−1), gentamicin (40 μg ml−1), or streptomycin (40 μg ml−1).
DNA methods.
Standard methods were used for preparation of plasmid DNA, restriction enzyme digestions, agarose gel electrophoresis, DNA ligations, and other DNA manipulations (41). Restriction enzymes, calf intestinal alkaline phosphatase, T4 DNA polymerase, and T4 DNA ligase were from Promega Corp. (Madison, Wis.), Boehringer Mannheim (Indianapolis, Ind.), New England Biolabs (Beverly, Mass.), or Bethesda Research Laboratories (Gaithersburg, Md.) and were used according to the manufacturer’s directions.
Construction of strains.
All mutations were delivered to the chromosome by allelic exchange using the sacBR-based allelic exchange system described elsewhere (1, 30). The ΔfhaB strain, RBX9, was generated by using plasmid pΔfhaB, which was constructed as follows. A 1.3-kb DNA fragment extending from the middle of bvgA to the third codon of fhaB was amplified from the chromosome of B. bronchiseptica RB50 by PCR. The oligonucleotides used for PCR (5′GCGAAGCTTATAGGTGACGTCGAACGG3′ and 5′GCGGATCCGTGTTCATATTCCGACCAG3′) were designed such that HindIII and BamHI sites would be generated at the 5′ and 3′ ends, respectively. Subsequent sequence analysis of B. bronchiseptica fhaB revealed a T in place of A six nucleotides from the end of the forward (former) primer. An additional amino acid is encoded within the BamHI restriction endonuclease recognition site. In a similar fashion, a 1.2-kb DNA fragment extending from the fifth to the last codons of fhaB to the middle of fimB was amplified with BamHI and HindIII sites at its 5′ and 3′ ends, respectively. The oligonucleotides used in this reaction were GCGGATCCAACAAATAGGTAGACGCTG and GCGAAGCTTCCCGTCACAAGCGTATGT. The PCR products were cut with BamHI and HindIII then joined at their BamHI sites and cloned into the HindIII site of our allelic exchange vector, creating pΔfhaB (Fig. 1B). Allelic exchange using this plasmid resulted in the deletion of all but four codons at the 5′ end and five codons at the 3′ end of fhaB.
To construct a strain expressing FHA under Bvg− phase conditions, we began with strain RB57, a nonmotile, Bvg− phase-locked derivative of RB50 containing deletion mutations in bvgS and flaA (1). To promote fhaB transcription in this strain, a 220-bp fragment encompassing the native fhaB promoter was replaced with a 114-bp fragment encompassing the flaA promoter by using allelic exchange. The flaA promoter fragment contains nucleotides −200 to −86 relative to the flaA translational start site. The transcriptional start site is at position −102, and this fragment joins fhaB 30 bp 5′ to the translational start site for fhaB. The resulting strain was called MR8 (Fig. 1C). To promote transcription and translation of fhaC, the same flaA promoter fragment plus 12 bp at the 3′ end containing the flaA ribosome binding site was inserted between the penultimate and last codons of fimD such that the 3′ fusion junction occurs exactly at the start codon of fhaC (Fig. 1C). Introduction of this promoter into strain MR8 resulted in the construction of strain DF8.
In vitro adherence assay.
Rat lung epithelial (L2) cells (ATCC CCL-149) were grown in F12K medium containing 10% fetal calf serum on coverslips in standard 12-well tissue culture plates. The L2 cells were used when they reached 50 to 80% confluency. The culture medium was removed and replaced with Stainer-Scholte broth containing 108 CFU of the bacterial strain to be tested (multiplicity of infection of 500); the plates were spun at 200 × g for 5 min and then incubated for 30 min at 37°C. The cells were then washed four times with Hanks’ balanced salts solution, fixed with methanol, stained with Giemsa stain, and visualized by light microscopy.
Respiratory infection of Wistar rats.
Wistar rats were obtained at 3 to 4 weeks of age from B & K Laboratories (Fremont, Calif.). Two animals from each lot were euthanized by halothane inhalation upon arrival and determined to be free of B. bronchiseptica infection upon necropsy. For standard, small-volume inoculations, rats were lightly anesthetized with aerosolized halothane, and a 5-μl droplet containing the inoculum was deposited on the external nares. Inocula were grown at 37°C in Stainer-Scholte broth and normalized by optical density at 600 nm. The number of CFU delivered was determined by plating dilutions of the inocula on BG-blood agar. Rats were monitored daily for signs of respiratory distress. At designated time points postinoculation, rats were sacrificed by halothane inhalation. The three right lung lobes, 1 cm of mid-trachea, the larynx, and the nasal septum were removed and homogenized in phosphate-buffered saline, and aliquots were plated onto BG-blood agar and incubated at 37°C for 2 days.
To determine the 50% infective doses (ID50s) for RB50 and RBX9, groups of 10 rats were inoculated with 20 or 200 CFU of either RB50 or RBX9 and colonization of the nasal cavity was determined 10 days later. All animals were found to be colonized, indicating the ID50s for RB50 and RBX9 were ≤20 CFU.
Large-volume intranasal inoculation of Wistar rats.
Three- to four-week-old female Wistar rats were inoculated with 106 CFU of B. bronchiseptica delivered in a 50-μl volume while the rats were either lightly anesthetized with halothane or sedated by intramuscular injection of ketamine (80 mg/kg of body weight) and xylazine (10 mg/kg). Animals were sacrificed 24 h or 5 days later, and a 1-cm section of trachea from midway between the larynx and the lungs was removed and homogenized. Aliquots were plated on BG-blood agar, and colonies were counted after 2 days of incubation at 37°C.
Intratracheal inoculation of Wistar rats.
Three- to four-week-old female Wistar rats were anesthetized by intramuscular injection of ketamine (80 mg/kg) and xylazine (10 mg/kg). The trachea was exposed by making a small incision in the skin with a sterile scalpel, and then 20 μl of phosphate-buffered saline containing 105 bacteria was delivered directly into the lumen of the trachea via a 25-gauge needle. The incisions were closed by using surgical staples, and the animals were monitored while recovering from anesthesia. Rats were sacrificed 24 h, 5 days, or 30 days postinoculation, and the number of CFU per centimeter of trachea was determined as described above.
SDS-PAGE and Western immunoblotting.
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and Western immunoblotting were performed as described previously (7). Whole-cell lysates and concentrated supernatants were prepared from an equivalent number of cells for each strain as determined by measuring the optical density at 600 nm.
ELISA.
Enzyme-linked immunosorbent assays (ELISAs) were performed as described previously (7). The anti-FHA antibody used was a polyclonal rabbit antibody obtained from Lederle-Praxis (West Henrietta, N.Y.). Fab1541 is a polyclonal rabbit anti-Fim3 antibody generated against B. pertussis Fim3 (gift from F. Mooi). The secondary antibody used was a horseradish peroxidase-conjugated goat anti-rabbit antibody (Amersham).
RESULTS
Construction and characterization of ΔfhaB and FHAr mutants.
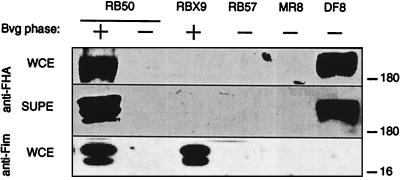
To investigate the role of FHA in Bordetella pathogensis, we constructed two types of B. bronchiseptica mutants. RBX9 contains a large in-frame deletion in fhaB, the FHA structural gene, such that only four and five codons remain at the 5′ and 3′ ends, respectively (Fig. 1 and Materials and Methods). FHA production in RBX9 was assessed by Western blot analysis using a polyclonal anti-FHA antibody (courtesy of Lederle-Praxis). This antibody recognizes a family of high-molecular-weight polypeptides present in whole-cell lysates or supernatants of wild-type B. bronchiseptica (RB50) grown under Bvg+ phase conditions and absent in lysates or supernatants of RB50 grown under Bvg− phase conditions (Fig. 2). These polypeptides were not detected in lysates or supernatants of RBX9 grown in the Bvg+ phase (Fig. 2).
FIG. 2.
Western immunoblot analysis of whole-cell lysates (WCE) and culture supernatants (SUPE) of wild-type B. bronchiseptica strain RB50 grown under Bvg+ and Bvg− phase conditions and RBX9, the ΔFHA strain, grown under Bvg+ phase conditions. RB57, the ΔbvgS ΔflaA strain, and its derivatives MR8, which contains a flagellin promoter upstream of fhaB, and DF8, which also contains a flagellin promoter upstream of fhaC, are locked in the Bvg− phase. Polypeptides contained in lysates and supernatants were separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and probed with either polyclonal anti-FHA antibody (anti-FHA) or monoclonal anti-Fim3 antibody (anti-Fim). Sizes are indicated in kilodaltons on the right.
Since genes required for fimbrial biogenesis are located between fhaB and fhaC (Fig. 1), we also determined the effect of the fhaB deletion on expression of fimbriae. Fab1541 is an anti-Fim3 antibody generated against B. pertussis fimbriae. This antibody recognizes two polypeptide species of approximately 22 and 24 kDa that are present in whole-cell lysates of RB50 grown under Bvg+ phase conditions but not in lysates of cells grown under Bvg− phase conditions (Fig. 2). These polypeptides were also present in lysates of RBX9 grown under Bvg+ phase conditions (Fig. 2), demonstrating that the fhaB deletion did not abrogate fimbrial expression.
Comparison of RBX9 with wild-type B. bronchiseptica allows us to investigate the requirement of FHA for specific phenotypes. To address the issue of sufficiency, we constructed a second type of mutant, one in which FHA is synthesized in the absence of all other Bvg+ phase factors (Fig. 1A and C). The ΔbvgS mutation in strain RB54 renders it locked in the Bvg− phase, unable to express Bvg+ phase genes and operons (7). RB54 does, however, constitutively express Bvg− phase phenotypes such as motility. We have previously shown that ectopic expression of motility in the Bvg+ phase is detrimental to the development of infection (1). This defect was specifically due to the expression of flagella since deletion of the flagellin structural gene restored virulence (1). We therefore deleted the flagellin structural gene from the chromosome of RB54 to generate a strain (RB57) that expresses neither flagellin nor Bvg+ phase factors. To induce expression of fhaB in this strain, the native fhaB promoter was replaced with the Bvg− phase specific flaA promoter by allelic exchange (Fig. 1 and Materials and Methods). Since FhaC is required for processing and export of FHA (24, 38), a second flaA promoter was introduced upstream of fhaC, near the end of the fimD gene (Fig. 1 and Materials and Methods). FHA was detected in whole-cell lysates and supernatants of strain DF8, which contains both flaA promoters, but not MR8, which contains the flaA promoter in place of the fhaB promoter but not the second flaA promoter upstream of fhaC (Fig. 2). Expression of fhaB and fhaC is therefore necessary and sufficient for synthesis, processing, and export of FHA by Bvg− phase B. bronchiseptica.
FHA mediates adherence to L2 cells.
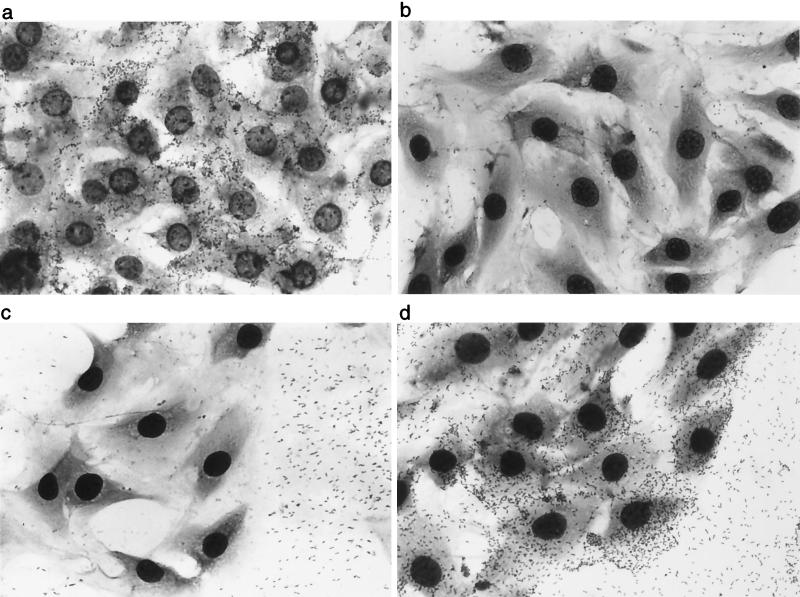
FHA has been reported to mediate adherence of B. pertussis to many cell types in vitro (2, 21, 23, 32, 33, 35, 36, 43, 50, 51). To investigate the contribution of FHA to B. bronchiseptica adherence in vitro, we compared our wild-type and mutant strains for the ability to adhere to a rat lung epithelial (L2) cell line in a standard adherence assay (58). RB50 could be detected in association with L2 cells only when grown under Bvg+ phase conditions (Fig. 3a). Interestingly, while RB50 grown under Bvg− phase conditions, and RB54 grown under any condition, did not adhere to L2 cells, both strains did adhere to the glass coverslip that was exposed between the cells (Fig. 3C). This phenomenon may reflect surface hydrophobicity differences between Bvg+ and Bvg− phase bacteria (12). RBX9 grown under Bvg+ phase conditions did not adhere to L2 cells (Fig. 3b). FHA is therefore required for binding to these cells. The FHAr strain adhered both to the L2 cells and to the exposed glass coverslip (Fig. 3d). The numbers of DF8 and RB50 bacteria adherent to L2 cells were nearly identical (data not shown), indicating that FHA functionality in DF8 was similar to FHA functionality in RB50, at least with regard to mediating adherence to L2 cells. These results demonstrate that FHA is not only required but also sufficient for mediating attachment to L2 cells. These results also show that the property of the Bvg− phase that allows adherence to the glass coverslip is not altered by the expression of FHA, nor does the ability, or lack of ability, to adhere to glass correlate with adherence to cells.
FIG. 3.
Adherence of B. bronchiseptica strains to rat lung epithelial (L2) cells used in a standard adherence assay with RB50 (a), RBX9 (b), RB57 (c), or DF8 (d).
FHA is required but not sufficient for tracheal colonization in a rat model of respiratory infection.
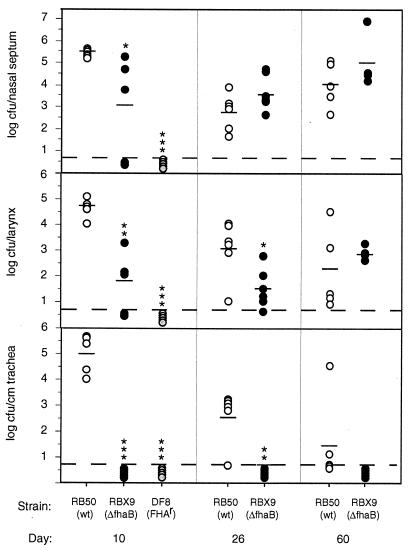
To investigate the role of FHA in establishment of respiratory tract infection, we inoculated groups of 3-week-old female Wistar rats with 500 CFU of either RB50 or RBX9 delivered in a 5-μl droplet to the external nares. Colonization of various sites in the respiratory tract was assessed on days 10, 26, and 60 postinoculation. Consistent with our previous reports (1, 8, 30), RB50 efficiently established respiratory infection in all rats; 104 to 106 CFU were recovered per nasal septum, larynx, and centimeter of trachea in all RB50-inoculated animals at day 10 postinoculation (Fig. 4). In contrast, RBX9 showed a decreased ability to colonize; averages of 103 and 102 CFU were recovered from the nasal septa and larynxes, respectively, at day 10, and bacteria were not recovered from the tracheas of any RBX9-inoculated animals (Fig. 4). At days 26 and 60 postinoculation, while the difference in ability to colonize the nasal cavity between RB50 and RBX9 had disappeared, bacteria were still not recovered from the tracheas of any RBX9-inoculated rats. We conclude that FHA is absolutely required for colonization of the trachea when inocula are administered in a small volume to the external nares. Although we did not recover RBX9 from any site in two of five animals at day 10 in this experiment, in a separate experiment we determined the ID50 for RBX9 to be less than 20 CFU, the same as obtained for RB50.
FIG. 4.
Colonization of the rat respiratory tract by RB50, RBX9, and DF8. Groups of five or six 3-week-old female Wistar rats were inoculated with approximately 500 CFU of RB50 or RBX9 or with 106 CFU of DF8. Animals were sacrificed on day 10, 26, or 60 postinoculation, and the number of CFU colonizing various sites in the respiratory tract was determined. Each symbol represents log CFU per nasal septum, larynx or centimeter of trachea recovered from a single animal, and short horizontal lines represent the mean for each group. Open circles, RB50; solid circles, RBX9; gray circles, DF8. Statistical significance is designated with asterisks (one asterisk = P < 0.05; two asterisks = P < 0.001; three asterisks = P < 0.0001. Dashed lines represent the lower limit of detection.
We also inoculated rats with the FHAr strain at a high dose (106 CFU) and determined colonization levels at day 10 post-inoculation. Not surprisingly, this strain was not recovered from any site in any animal. This result demonstrates that although FHA is required for colonization of the trachea, it is not sufficient for establishment of respiratory infection by Bvg− phase bacteria even when given at a dose more than 105 times higher than the ID50 for wild-type B. bronchiseptica. Consistent with this conclusion, we have recently shown that fimbriae are also important for tracheal colonization, although their presence appears to be less critical than that of FHA for establishment of tracheal colonization (31).
Antibody response.
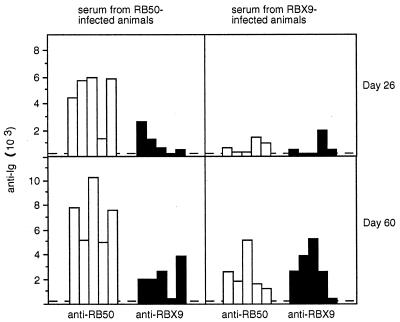
The serum antibody response to infection by RB50 and RBX9 was measured by Western blotting and ELISA. In the Western blot analyses, whole-cell lysates and supernatants of wild-type B. bronchiseptica strain RB50 were separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and probed with serum from infected animals. The antibody profiles of RB50- and RBX9-infected animals were qualitatively similar except for a lack of anti-FHA antibody in RBX9-infected animals (data not shown). Consistent with this result, serum antibody titers were similar in RB50- and RBX9-infected animals when RBX9 whole cells were used as the antigen in an ELISA but were much higher in RB50-infected animals when RB50 whole cells were used as the antigen (Fig. 5). This result suggests that FHA serves as a potent antigen but is not necessary for the induction of a humoral immune response to other Bordetella antigens.
FIG. 5.
Serum anti-Bordetella antibody titers. Open bars represent antibody titers in sera as determined by ELISA with RB50 used as the antigen; solid bars represent titers determined with RBX9 used as the antigen. Graphs show titers in sera from RB50- and RBX9-infected animals, collected at day 26 or 60 postinoculation, as indicated.
FHA is required for initial, efficient establishment of tracheal colonization in vivo.
Time course studies of rat respiratory tract colonization by B. bronchiseptica have shown that when inoculated intranasally with a small volume (5 μl), rats become colonized in the nasal cavity by day 5 whereas tracheal colonization is not consistently detected until day 10 (1). We interpret these observations to indicate that at least in this experimental model of infection, bacteria in the nasal cavity serve as a reservoir from which the trachea is seeded. This further suggests that establishment of tracheal colonization requires that bacteria be able to (i) move from the nasal cavity to the trachea, (ii) avoid clearance by the mucociliary escalator, by forming strong, specific attachments to ciliated epithelial cells and/or by paralyzing the cilia, and (iii) resist or avoid constitutive host immune defenses that normally function to eliminate bacteria from this site. Lack of tracheal colonization by RBX9, therefore, implicates FHA as important for one or more of these steps.
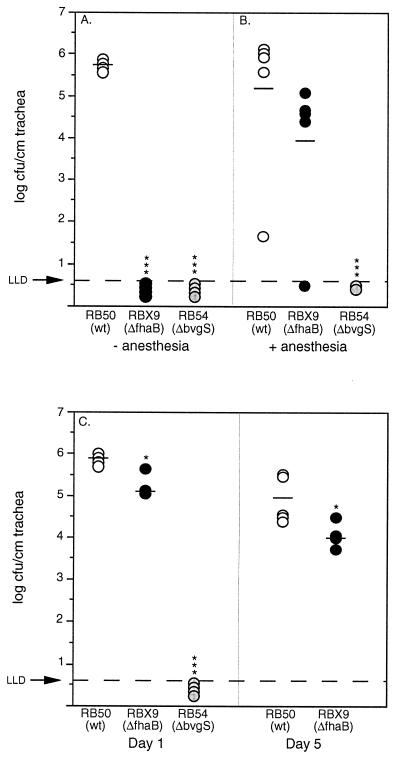
To address these issues, we performed two types of experiments. In the first, bacteria were delivered to the trachea via intranasal inoculation by administering 106 CFU of either RB50, RBX9, or RB54 in a large volume (50 μl) to the external nares. Previous studies indicate that about 10% of the inoculum is present in the trachea 1 h postinoculation with this protocol. At 24 h postinoculation, all rats inoculated with RB50 contained about 106 CFU/cm of trachea (Fig. 6A). In marked contrast, bacteria were not recovered from the trachea of any animal inoculated with RBX9 or RB54. This result indicates that without FHA, B. bronchiseptica is unable to establish colonization in the trachea even when the requirement for first establishing colonization in the nasal cavity is removed. FHA therefore appears to play a crucial role in overcoming constitutive host defense mechanisms operative in the trachea.
FIG. 6.
Colonization of the trachea following intranasal inoculation of anesthetized or unanesthetized rats or intratracheal inoculation of anesthetized rats. Groups of five 3-week-old female Wistar rats were inoculated by delivering 106 CFU of RB50, RBX9, or RB54 in a 50-μl volume to the external nares of unanesthetized (A) or anesthetized (B) rats or by injecting 106 CFU of RB50, RBX9, or RB54 in a 20-μl volume into the trachea of anesthetized rats (C). Animals were sacrificed at 24 h or 5 days postinoculation, as indicated. Each symbol represents the number of CFU per centimeter of trachea from a single animal, and horizontal bars show the mean for each group. Open circles, RB50; solid circles, RBX9; gray circles, RB54. The horizontal dashed lines represent the lower limit of detection. Statistical significance is designated with asterisks (one asterisk = P < 0.05; three asterisks = P < 0.0001.
To deliver bacteria more directly, and more precisely, into the trachea, and to avoid passage of the inoculum through the nasal cavity, we performed experiments in which the tracheas of anesthetized animals were exposed surgically and bacteria were delivered in a 20-μl volume into the lumen of the trachea via a 25-gauge needle. At 24 h postinoculation, RB50 and RBX9 were present in the trachea at about 106 and 105 CFU/cm, respectively (Fig. 6C), while RB54 was not recovered from the trachea of any animal. Both RB50 and RBX9 were still present in the trachea at 5 days postinoculation, although at levels about 1 log lower than at 24 h (Fig. 6C). In contrast to the data presented above, this result indicates that FHA is not absolutely required for tracheal colonization.
A possible explanation for these apparently contradictory results is that FHA is required for tracheal colonization only when bacteria first pass through the nasal cavity. Alternatively, the requirement for FHA in tracheal colonization may somehow be eliminated by anesthesia or by direct injection of bacteria into the trachea. General anesthesia can result in decreased airway protection by reducing respiratory rate, suppressing gag and cough reflexes and inhibiting mucociliary clearance (27). To test the hypothesis that constitutive host defense mechanisms normally operative in the trachea are compromised in anesthetized rats to the extent that FHA is not required for establishment of colonization at this site, we repeated the intranasal inoculation experiments using anesthetized rats. In this experiment, both RB50 and RBX9, but not RB54, were present in high numbers in the trachea 24 h postinoculation (Fig. 6B). FHA therefore appears to be required for establishment of tracheal colonization in uncompromised animals but is not required for bacteria to persist in the trachea for up to 5 days once tracheal colonization has been established. These data support the hypothesis that FHA functions as an important adhesin which mediates efficient initial adherence to tracheal epithelial cells, allowing B. bronchiseptica to overcome the clearance action of the mucociliary escalator. Interestingly, RBX9 was not recovered from the tracheas of intratracheally inoculated rats 30 days postinoculation (data not shown) suggesting FHA also plays a role in long-term tracheal persistence.
FHA does not complement the tracheal colonization defect in trans.
FHA contains several types of binding domains, and secreted FHA has been demonstrated to bind both bacterial cell surfaces as well as host cell surfaces in vitro, suggesting that it may function as a bridge between the bacterium and the host (48). It has even been suggested that by acting in trans, FHA may mediate adherence of other bacteria to host tissues and thus may contribute to secondary infections (48). To investigate this possibility in vivo, we coinfected six rats with approximately 300 CFU of RBX9 and RB58 (17), a ΔcyaA derivative of RB50. RB58 was chosen for this experiment because it is easily distinguished from RBX9 on BG-blood agar due to its lack of hemolysis. RB58 does not differ from the wild type in its ability to synthesize and secrete FHA (data not shown). Although the importance of AC/HLY in resisting the action of neutrophils and macrophages is apparent in mouse models (17, 25), RB58 does not differ from the wild type in its ability to colonize the rat trachea at day 10 following low-volume intranasal inoculation (17). In all six animals coinoculated with RBX9 and RB58, only RB58 was recovered from the trachea (approximately 103 to 105 CFU per cm). Although we did not quantitate nasal cavity colonization in this experiment, swabbing the nasal turbinates exposed by dissection indicated that RBX9 and RB58 were present in approximately equal numbers in the nasal cavity. These results indicate that FHA is not capable of conferring tracheal colonization ability to an FHA− strain in trans in vivo.
DISCUSSION
FHA is an unusually large, highly immunogenic, hairpin-shaped molecule which has been included as a primary component in acellular pertussis vaccines (42). It is synthesized as a 367-kDa precursor, FhaB, which is modified at its N terminus (24) and cleaved at its C terminus (38) to form the mature 220-kDa FHA protein. Although efficiently secreted by a process requiring the outer membrane protein FhaC, a significant amount of FHA remains associated with the cell surface (38). In vitro studies using a variety of mammalian cell types suggest that FHA possess at least four distinct attachment activities, and four separate FHA binding domains have been identified. The Arg-Gly-Asp (RGD) triplet (36), situated in the middle of FHA and localized to one end of the proposed hairpin structure (29), stimulates adherence to monocytes/macrophages and possibly other leukocytes via leukocyte response integrin/integrin-associated protein and complement receptor type 3 (CR3) (23, 36, 43). The CR3 recognition domain in FHA has yet to be identified. FHA also possesses a carbohydrate recognition domain (CRD) which mediates attachment to ciliated respiratory epithelial cells as well as to macrophages (35, 43). Finally, FHA displays a lectin-like activity for heparin and other sulfated carbohydrates which can mediate adherence to nonciliated epithelial cell lines in vitro (33). The heparin binding site is distinct from the CRD and RGD sites and is required for FHA-mediated hemagglutination (33).
Evidence for FHA-dependent phenotypes in vivo has been more difficult to obtain. Using a rabbit model, Saukkonen et al. found fewer FHA mutants than wild-type bacteria in the lungs 24 h after intratracheal inoculation (43). Based on in vitro-determined binding characteristics of the various mutants used in their study, they inferred that wild-type B. pertussis cells were adhering to both ciliated epithelial cells and macrophages, and competition experiments with lactose and anti-CR3 antibody suggested both CRD- and RGD-dependent binding was involved (43). Using mouse models, others have found FHA mutants to be indistinguishable from the wild type in the ability to persist in the lungs but defective for tracheal colonization (13, 26, 34). Still others, also using mouse models, have observed no difference between FHA mutants and the wild type (16, 25, 39, 54, 55). The difficulty in achieving a complete and detailed understanding of the role of FHA during B. pertussis infection probably reflects not only the absence of a natural animal host (other than humans) but the complexity of this molecule and its associated biological activities.
We have chosen to study the function of FHA and other Bordetella virulence factors by using B. bronchiseptica and natural-host animal models. The ability to study infection in the context of a natural bacterium-host interaction may be particularly important for investigating putative adhesins, as such molecules are expected to bind in a highly specific manner to host receptors. The extremely close phylogenetic relatedness of B. pertussis and B. bronchiseptica (53), and of the virulence factors they express, suggests that fundamentally similar pathogenic strategies are used and that common virulence factors perform analogous functions. However, the long-term, asymptomatic infections that B. bronchiseptica typically establishes in rabbits and rats differ significantly from the acute, symptomatic infections that B. pertussis causes in infants and young children. Thus, our results, together with those obtained with B. pertussis, contribute to a comparative analysis of the similarities and differences in the infectious cycles of Bordetella species and represent an opportunity to use experiments of nature as a guide to understanding fundamental features of bacterium-host interactions.
By constructing a B. bronchiseptica strain with a nonpolar, in-frame deletion in fhaB and comparing it with the wild type, we have demonstrated that FHA is absolutely required for colonization of the trachea in our rat model of respiratory infection. These results are in agreement with some of the studies done with B. pertussis (13, 26, 34). Together with previous observations (1, 57), our results support the hypothesis that the colonized nasal cavity represents a reservoir from which bacteria that eventually colonize the trachea are seeded. The ability to resist the clearing action of the mucociliary escalator requires efficient adherence to ciliated tracheal epithelial cells. Our results suggest that FHA provides this ability; without FHA, B. bronchiseptica is unable to establish tracheal colonization. The fact that FHA was not required for establishment of tracheal colonization in anesthetized animals, in which mucociliary clearance and lower airway protection are presumably compromised, is consistent with this hypothesis. Other Bvg+ phase factors, however, are apparently required for tracheal colonization since neither the Bvg− phase-locked mutant nor the FHAr mutant was able to establish tracheal colonization, even in anesthetized animals.
While our results strongly suggest that FHA functions as an important adhesin, we do not propose that adherence is the only in vivo role for FHA. When the requirement for FHA in establishment of tracheal colonization was removed by using anesthetized rats, a role for FHA in persistence was revealed. Tracheal colonization may be a dynamic process in which microcolonies are continuously being established, cleared, and reestablished. Persistence at this site, therefore, may require that the bacteria continuously be able to resist both constitutive and adaptive host defense mechanisms. FHA has been shown to mediate adherence to macrophages and possibly other leukocytes in vitro (23, 35, 36, 43), a function which could have immunomodulatory effects, either by acting directly on host cell signal transduction systems or by aiding in the delivery of secreted toxins, such as AC/HLY (4, 23). It has also been suggested that secreted FHA could bind to receptors on phagocytic cells, thus blocking their ability to recognize and engulf FHA-bearing Bordetella (23, 36). Our observation that FHA may contribute to persistence is consistent with these data, and we are currently investigating the cellular immune response to Bordetella infection to address this issue. We are also constructing strains expressing FHA molecules with specific mutations in the various binding motifs. Comparison of these mutants in intranasally versus intratracheally inoculated rats should allow us to separate adherence and immunomodulatory functions in vivo.
The distinction between nasal cavity colonization and tracheal colonization is an important one. The ability to colonize the nasal cavity, but not the trachea, is a phenotype that RBX9 shares with several other B. bronchiseptica mutants, including one multiply deficient in pertactin, AC/HLY, fimbriae, and FHA (1, 8, 31). While these observations demonstrate stricter requirements for colonizing the trachea compared to the nasal cavity, the nasal cavity is not an entirely permissive environment since Bvg− phase bacteria are unable to establish infection at this site. Moreover, mutants that are able to colonize the nasal cavity, but not the trachea, still induce a Bordetella-specific serum antibody response. In fact, except for a lack of anti-FHA antibodies, the serum antibody profile generated in RBX9-infected animals was indistinguishable from that of RB50-infected animals. Thus, induction of an humoral immune response may not necessarily require colonization of immune-privileged sites. However, additional evidence indicates that interactions that occur with bacteria capable of colonizing the trachea differ qualitatively from those that occur with bacteria that are limited to the nasal cavity. In contrast to wild-type bacteria, mutants that are unable to establish tracheal colonization fail to induce protective immunity against superinfection by wild-type B. bronchiseptica (31). Also, mutants that do not colonize the trachea are unable to establish lethal infections in SCID/beige mice while wild-type B. bronchiseptica kill these immunodeficient hosts within about 50 days postinoculation (17, 18). The ability to colonize the normally sterile trachea, therefore, appears to reflect an interaction that is potentially pathogenic and against which protective immunity will be generated. Understanding the molecular basis for these different bacterium-host interactions will contribute to our understanding of respiratory bacterial pathogenesis as well as to the development of more efficacious vaccines.
ACKNOWLEDGMENTS
M.H.Y. and S.M. contributed equally to this work.
We thank members of our laboratories for helpful discussions and comments on the manuscript and F. R. Mooi and Lederle Praxis for antibodies.
We are supported by grants from the NIH (AI38417 to J.F.M. and AI39587 to D.A.R.) and postdoctoral fellowships (a research training grant from the American Lung Association of California to J.B. and a postdoctoral fellowship from the Damon Runyon-Walter Winchell Foundation to M.H.Y.).
REFERENCES
- 1.Akerley B J, Cotter P A, Miller J F. Ectopic expression of the flagellar regulon alters development of the Bordetella-host interaction. Cell. 1995;80:611–620. doi: 10.1016/0092-8674(95)90515-4. [DOI] [PubMed] [Google Scholar]
- 2.Arico B, Nuti S, Scarlato V, Rappuoli R. Adhesion of Bordetella pertussis to eukaryotic cells requires a time-dependent export and maturation of filamentous hemagglutinin. Proc Natl Acad Sci USA. 1993;90:9204–9208. doi: 10.1073/pnas.90.19.9204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arico B, Rappuoli R. Bordetella parapertussis and Bordetella bronchiseptica contain transcriptionally silent pertussis toxin genes. J Bacteriol. 1987;169:2847–2853. doi: 10.1128/jb.169.6.2847-2853.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boschwitz J S, Batanghari J W, Kedem H, Relman D A. Bordetella pertussis infection of human monocytes inhibits antigen-dependent CD4 T cell proliferation. J Infect Dis. 1997;176:678–686. doi: 10.1086/514090. [DOI] [PubMed] [Google Scholar]
- 5.Brennan M J, Hannah J H, Leininger E. Adhesion of Bordetella pertussis to sulfatides and to the GalNAc beta 4Gal sequence found in glycosphingolipids. J Biol Chem. 1991;266:18827–18831. [PubMed] [Google Scholar]
- 6.Cotter P A, Miller J F. BvgAS dependent phenotypic modulation of Bordetella species. In: Rappuoli R, Scarlato V, Arico B, editors. Signal transduction and bacterial virulence. R. G. Austin, Tex: Landes; 1995. pp. 21–42. [Google Scholar]
- 7.Cotter P A, Miller J F. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect Immun. 1994;62:3381–3390. doi: 10.1128/iai.62.8.3381-3390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotter P A, Miller J F. A mutation in the Bordetella bronchiseptica bvgS gene results in reduced virulence and increased resistance to starvation, and identifies a new class of Bvg-regulated antigens. Mol Microbiol. 1997;24:671–685. doi: 10.1046/j.1365-2958.1997.3821741.x. [DOI] [PubMed] [Google Scholar]
- 9.Cuzzoni A, Pedroni P, Riboli B, Grandi G, de Ferra F. Nucleotide sequence of the fim3 gene from Bordetella pertussis and homology to fim2 and fimX gene products. Nucleic Acids Res. 1990;18:1640. doi: 10.1093/nar/18.6.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez R C, Weiss A A. Cloning and sequencing of a Bordetella pertussis serum resistance locus. Infect Immun. 1994;62:4727–4738. doi: 10.1128/iai.62.11.4727-4738.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finn T M, Stevens L A. Tracheal colonization factor: a Bordetella pertussis secreted virulence determinant. Mol Microbiol. 1995;16:625–634. doi: 10.1111/j.1365-2958.1995.tb02425.x. [DOI] [PubMed] [Google Scholar]
- 12.Fish F, Navon Y, Goldman S. Hydrophobic adherence and phase variation in Bordetella pertussis. Med Microbiol Immunol. 1987;176:37–46. doi: 10.1007/BF00189407. [DOI] [PubMed] [Google Scholar]
- 13.Geuijen C A, Willems R J, Bongaerts M, Top J, Gielen H, Mooi F R. Role of the Bordetella pertussis minor fimbrial subunit, FimD, in colonization of the mouse respiratory tract. Infect Immun. 1997;65:4222–4228. doi: 10.1128/iai.65.10.4222-4228.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geuijen C A, Willems R J, Mooi F R. The major fimbrial subunit of Bordetella pertussis binds to sulfated sugars. Infect Immun. 1996;64:2657–2665. doi: 10.1128/iai.64.7.2657-2665.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodnow R A. Biology of Bordetella bronchiseptica. Microbiol Rev. 1980;44:722–738. doi: 10.1128/mr.44.4.722-738.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodwin M S, Weiss A A. Adenylate cyclase toxin is critical for colonization and pertussis toxin is critical for lethal infection by Bordetella pertussis in infant mice. Infect Immun. 1990;58:3445–3447. doi: 10.1128/iai.58.10.3445-3447.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harvill, E. T., P. A. Cotter, and J. F. Miller. Probing the function of a bacterial virulence factor by manipulating host immunity. Submitted for publication.
- 18.Harvill, E. T., P. A. Cotter, and J. F. Miller. Unpublished data.
- 19.Hazenbos W L, Geuijen C A, van den Berg B M, Mooi F R, van Furth R. Bordetella pertussis fimbriae bind to human monocytes via the minor fimbrial subunit FimD. J Infect Dis. 1995;171:924–929. doi: 10.1093/infdis/171.4.924. [DOI] [PubMed] [Google Scholar]
- 20.Hazenbos W L, van den Berg B M, Geuijen C W, Mooi F R, van Furth R. Binding of FimD on Bordetella pertussis to very late antigen-5 on monocytes activates complement receptor type 3 via protein tyrosine kinases. J Immunol. 1995;155:3972–3978. [PubMed] [Google Scholar]
- 21.Hazenbos W L, van den Berg B M, van’t Wout J W, Mooi F R, van Furth R. Virulence factors determine attachment and ingestion of nonopsonized and opsonized Bordetella pertussis by human monocytes. Infect Immun. 1994;62:4818–4824. doi: 10.1128/iai.62.11.4818-4824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hewlett E L, Weiss A A, Crane J K, Pearson R D, Anderson H J, Myers G A, Evans W S, Hantske L L, Kay H D, Cronin M J. Bordetella extracytoplasmic adenylate cyclase: actions as a bacterial toxin. Dev Biol Stand. 1985;61:21–26. [PubMed] [Google Scholar]
- 23.Ishibashi Y, Claus S, Relman D A. Bordetella pertussis filamentous hemagglutinin interacts with a leukocyte signal transduction complex and stimulates bacterial adherence to monocyte CR3 (CD11b/CD18) J Exp Med. 1994;180:1225–1233. doi: 10.1084/jem.180.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacob-Dubuisson F, Buisine C, Mielcarek N, Clement E, Menozzi F D, Locht C. Amino-terminal maturation of the Bordetella pertussis filamentous haemagglutinin. Mol Microbiol. 1996;19:65–78. doi: 10.1046/j.1365-2958.1996.349883.x. [DOI] [PubMed] [Google Scholar]
- 25.Khelef N, Zychlinsky A, Guiso N. Bordetella pertussis induces apoptosis in macrophages: role of adenylate cyclase-hemolysin. Infect Immun. 1993;61:4064–4071. doi: 10.1128/iai.61.10.4064-4071.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura A, Mountzouros K T, Relman D A, Falkow S, Cowell J L. Bordetella pertussis filamentous hemagglutinin: evaluation as a protective antigen and colonization factor in a mouse respiratory infection model. Infect Immun. 1990;58:7–16. doi: 10.1128/iai.58.1.7-16.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konrad F, Marx T, Schrag M, Kilian J. Combination anesthesia and bronchial transport velocity. Effects of anesthesia with isoflurane, fentanyl, vecuronium, and oxygen-nitrous oxide breathing on bronchial mucus transport. Anaesthesist. 1997;46:403–407. doi: 10.1007/s001010050417. [DOI] [PubMed] [Google Scholar]
- 28.Locht C, Bertin P, Menozzi F D, Renauld G. The filamentous haemagglutinin, a multifaceted adhesion produced by virulent Bordetella spp. Mol Microbiol. 1993;9:653–660. doi: 10.1111/j.1365-2958.1993.tb01725.x. [DOI] [PubMed] [Google Scholar]
- 29.Makhov A M, Hannah J H, Brennan M J, Trus B L, Kocsis E, Conway J F, Wingfield P T, Simon M N, Steven A C. Filamentous hemagglutinin of Bordetella pertussis: a bacterial adhesin formed as a 50-nm monomeric rigid rod based on a 19-residue repeat motif rich in beta strands and turns. J Mol Biol. 1994;241:110–124. doi: 10.1006/jmbi.1994.1478. [DOI] [PubMed] [Google Scholar]
- 30.Martinez de Tejada G, Miller J F, Cotter P A. Comparative analysis of the virulence control systems of Bordetella pertussis and Bordetella bronchiseptica. Mol Microbiol. 1996;22:895–908. doi: 10.1046/j.1365-2958.1996.01538.x. [DOI] [PubMed] [Google Scholar]
- 31.Mattoo, S., P. A. Cotter, and J. F. Miller. Unpublished data.
- 32.Menozzi F D, Boucher P E, Riveau G, Gantiez C, Locht C. Surface-associated filamentous hemagglutinin induces autoagglutination of Bordetella pertussis. Infect Immun. 1994;62:4261–4269. doi: 10.1128/iai.62.10.4261-4269.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menozzi F D, Mutombo R, Renauld G, Gantiez C, Hannah J H, Leininger E, Brennan M J, Locht C. Heparin-inhabitable lectin activity of the filamentous hemagglutinin adhesin of Bordetella pertussis. Infect Immun. 1994;62:769–778. doi: 10.1128/iai.62.3.769-778.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mooi F R, Jansen W H, Brunings H, Gielen H, van der Heide H G J, Walvoort H C, Guinee P A M. Construction and analysis of Bordetella pertussis mutants defective in the production of fimbriae. Microb Pathog. 1992;12:127–135. doi: 10.1016/0882-4010(92)90115-5. [DOI] [PubMed] [Google Scholar]
- 35.Prasad S M, Yin Y, Rodzinski E, Tuomanen E I, Masure H R. Identification of a carbohydrate recognition domain in filamentous hemagglutinin from Bordetella pertussis. Infect Immun. 1993;61:2780–2785. doi: 10.1128/iai.61.7.2780-2785.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Relman D, Tuomanen E, Falkow S, Golenbock D T, Saukkonen K, Wright S D. Recognition of a bacterial adhesion by an integrin: macrophage CR3 (alpha M beta 2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell. 1990;61:1375–1382. doi: 10.1016/0092-8674(90)90701-f. [DOI] [PubMed] [Google Scholar]
- 37.Relman D A, Domenighini M, Tuomanen E T, Rappuoli R, Falkow S. Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proc Natl Acad Sci USA. 1989;86:2634–2641. doi: 10.1073/pnas.86.8.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Renauld-Mongenie G, Cornette J, Mielcarek N, Menozzi F D, Locht C. Distinct roles of the N-terminal and C-terminal precursor domains in the biogenesis of the Bordetella pertussis filamentous hemagglutinin. J Bacteriol. 1996;178:1053–1060. doi: 10.1128/jb.178.4.1053-1060.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts M, Cropley I, Chatfield S, Dougan G. Protection of mice against respiratory Bordetella pertussis infection by intranasal immunization with P.69 and FHA. Vaccine. 1993;11:866–872. doi: 10.1016/0264-410x(93)90363-3. [DOI] [PubMed] [Google Scholar]
- 40.Roberts M, Fairweather N F, Leininger E, Pickard D, Hewlett E L, Robinson A, Hayward C, Dougan G, Charles I G. Construction and characterization of Bordetella pertussis mutants lacking the vir-regulated P.69 outer membrane protein. Mol Microbiol. 1991;5:1393–1404. doi: 10.1111/j.1365-2958.1991.tb00786.x. [DOI] [PubMed] [Google Scholar]
- 41.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 42.Sato H, Fukumi H, Kimura M. Development of a pertussis component vaccine in Japan. Lancet. 1984;i:122–126. doi: 10.1016/s0140-6736(84)90061-8. [DOI] [PubMed] [Google Scholar]
- 43.Saukkonen K, Cabellos C, Burroughs M, Prasad S, Tuomanen E. Integrin-mediated localization of Bordetella pertussis within macrophages: role in pulmonary colonization. J Exp Med. 1991;173:1143–1149. doi: 10.1084/jem.173.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Savelkoul P H, de Kerf D P, Willems R J, Mooi F R, van der Zeijst B A, Gaastra W. Characterization of the fim2 and fim3 fimbrial subunit genes of Bordetella bronchiseptica: roles of Fim2 and Fim3 fimbriae and flagella in adhesion. Infect Immun. 1996;64:5098–5105. doi: 10.1128/iai.64.12.5098-5105.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stainer D W, Scholte M J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970;63:211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- 46.Stibitz S, Weiss A A, Falkow S. Genetic analysis of a region of the Bordetella pertussis chromosome encoding filamentous hemagglutinin and the pleiotropic regulatory locus vir. J Bacteriol. 1988;170:2904–2913. doi: 10.1128/jb.170.7.2904-2913.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stock J B, Surette M G, Levit M G, Park P. Two-component signal transduction systems: structure-function relationships and mechanisms of catalysis. In: Hoch J, Silhavy T, editors. Two-component signal transduction. Washington, D.C: ASM Press; 1995. pp. 25–51. [Google Scholar]
- 48.Tuomanen E. Piracy of adhesins: attachment of superinfecting pathogens to respiratory cilia by secreted adhesins of Bordetella pertussis. Infect Immun. 1986;54:905–908. doi: 10.1128/iai.54.3.905-908.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tuomanen E, Towbin H, Rosenfelder G, Braun D, Larson G, Hansson G C, Hill R. Receptor analogs and monoclonal antibodies that inhibit adherence of Bordetella pertussis to human ciliated respiratory epithelial cells. J Exp Med. 1988;168:267–277. doi: 10.1084/jem.168.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tuomanen E, Weiss A. Characterization of two adhesins of Bordetella pertussis for human ciliated respiratory-epithelial cells. J Infect Dis. 1985;152:118–125. doi: 10.1093/infdis/152.1.118. [DOI] [PubMed] [Google Scholar]
- 51.Tuomanen E, Weiss A, Rich R, Zak F, Zak O. Filamentous hemagglutinin and pertussis toxin promote adherence of Bordetella pertussis to cilia. Dev Biol Stand. 1985;61:197–204. [PubMed] [Google Scholar]
- 52.Uhl M A, Miller J F. The Bordetella BvgAS signal transduction system. In: Silhavy T, Hoch J, editors. Signal transducing genetic switches. Washington, D.C: ASM Press; 1995. pp. 333–349. [Google Scholar]
- 53.van der Zee A, Mooi F, Van Embden J, Musser J. Molecular evolution and host adaptation of Bordetella spp.: phylogenetic analysis using multilocus enzyme electrophoresis and typing with three insertion sequences. J Bacteriol. 1997;179:6609–6617. doi: 10.1128/jb.179.21.6609-6617.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weiss A A, Goodwin M S. Lethal infection by Bordetella pertussis mutants in the infant mouse model. Infect Immun. 1989;57:3757–3764. doi: 10.1128/iai.57.12.3757-3764.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weiss A A, Hewlett E L, Myers G A, Falkow S. Pertussis toxin and extracytoplasmic adenylate cyclase as virulence factors of Bordetella pertussis. J Infect Dis. 1984;150:219–222. doi: 10.1093/infdis/150.2.219. [DOI] [PubMed] [Google Scholar]
- 56.Willems R J, van der Heide H G, Mooi F R. Characterization of a Bordetella pertussis fimbrial gene cluster which is located directly downstream of the filamentous haemagglutinin gene. Mol Microbiol. 1992;6:2661–2671. doi: 10.1111/j.1365-2958.1992.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 57.Yoda H, Nakayama K, Nakagawa M. Experimental infection of Bordetella bronchiseptica to rabbits. Jikken Dobutsu. 1982;31:113–118. [PubMed] [Google Scholar]
- 58.Yuk M H, Harvill E T, Miller J F. The bvgAS virulence control system regulates type III secretion in Bordetella bronchiseptica. Mol Microbiol. 1998;28:945–959. doi: 10.1046/j.1365-2958.1998.00850.x. [DOI] [PubMed] [Google Scholar]