Abstract
Meningoencephalitis is a serious and often fatal complication of Listeria monocytogenes infection. The aim of the present study was to analyze the role of internalin A (InlA) and B, which are involved in the invasion of L. monocytogenes into cultivated host tissue cells, and that of phosphatidylcholine-specific phospholipase C (PlcB), which mainly promotes the direct cell-to-cell spread of L. monocytogenes, in murine cerebral listeriosis by use of an InlA/B (ΔinlAB2)- and a PlcB (ΔplcB2)-deficient isogenic deletion mutant strain and the wild-type (WT) L. monocytogenes EGD. Listeria strains were directly applied to the brain, a technique which has been employed previously to study the pathogenesis of cerebral listeriosis (D. Schlüter, S. B. Oprisiu, S. Chahoud, D. Weiner, O. D. Wiestler, H. Hof, and M. Deckert-Schlüter, Eur. J. Immunol. 25:2384–2391, 1995). We demonstrated that PlcB, but not InlA or InlB, is an important virulence factor in cerebral listeriosis. Nonimmunized mice infected intracerebrally with the ΔplcB2 strain survived significantly longer and had a reduced intracerebral bacterial load compared to mice infected with the ΔinlAB2 strain or WT bacteria. In addition, immunization with the WT prior to intracerebral infection significantly increased the survival rate of mice challenged intracerebrally with the ΔplcB2 strain compared to that of mice infected with the WT or ΔinlAB2 strain. Histopathology revealed that the major difference between the various experimental groups was a significantly delayed intracerebral spread of the ΔplcB2 mutant strain, indicating that cell-to-cell spread is an important pathogenic feature of cerebral listeriosis. Interestingly, irrespective of the Listeria mutant used, the apoptosis of hippocampal and cerebellar neurons and an internal hydrocephalus developed in surviving mice, indicating that these complications are not dependent on the virulence factors InlA/B and PlcB. In conclusion, this study points to PlcB as a virulence factor important for the intracerebral pathogenesis of murine L. monocytogenes meningoencephalitis.
Listeria monocytogenes is a gram-positive ubiquitous living bacterium that can be isolated from soil, decaying plants, and food and is able to infect a wide variety of organisms, including humans and rodents. L. monocytogenes is the causative agent of human listeriosis, which is normally acquired by the consumption of contaminated food, particularly unpasteurized dairy products (11). Persons at risk include the elderly, the immunocompromised, and pregnant women. An important pathogenic feature of this facultative intracellular pathogen is its capacity to infect a broad range of host cells, and in vitro studies have revealed that L. monocytogenes induces its own uptake into nonprofessional phagocytic cells, including enterocytes, fibroblasts, dendritic cells, hepatocytes, and endothelial cells (reviewed in references 4 and 7). After internalization, L. monocytogenes escapes from the phagosome into the cytosol of infected cells, recruits actin filaments, and is capable of actin-based motility and cell-to-cell spread.
Recently, a chromosomal genetic locus, the internalin operon, has been identified, whose gene products are involved in entry into various cell types (6, 13, 27). The gene products of this operon, InlA and InlB (InlA/B), are cell wall-associated proteins of 88 and 65 kDa, respectively (27). InlA associates with the bacterial cell wall by a conventional cell wall anchoring motif (25), while the cell wall association of InlB requires a larger segment, comprising the last 232 amino acid residues of the C terminus (3). A striking structural feature of both proteins is the presence of consecutive leucine-rich repeats, present 15 times in InlA and 7 times in InlB (5, 27). Homology searches have revealed that InlA and InlB are members of a superfamily of leucine-rich repeat-containing proteins found only in pathogenic bacteria (24, 29, 39). It has been shown previously that InlA and InlB are differentially required for entry into various tissue and culture cell lines (reviewed in references 4 and 7). InlA mediates the uptake into the epithelium-like cell line Caco-2 via interaction with host cell E-cadherin (30), whereas the eukaryotic receptor for the bacterial ligand InlB is still unknown. It has been shown that pathogenic Listeria also harbors an internalin-like protein gene (irpA), encoding a secreted protein of 30 kDa (5, 10). Recently, four more internalin-like proteins have been found in L. monocytogenes; however, the role of these proteins in infection is at present unclear (8).
The capacity of L. monocytogenes to spread efficiently from cell to cell appears to be dependent on the product of the plcB gene, a secreted phospholipase with broad-substrate specificity, particularly for phosphatidylcholine. Thus, the highly purified 29-kDa mature form of this enzyme (termed PlcB or PC-PLC) cleaves a variety of phospholipids, including sphingomyelin, in detergent micelles, liposomes, and biological membranes (15, 16, 31). The plcB gene is part of the lecithinase operon, consisting of the metalloprotease mpl, actin recruiting factor actA, and plcB (48), and mutations of the plcB gene have resulted in a partial defect in cell-to-cell spread and reduced virulence (35, 41, 42). Transmission electron microscopic studies revealed that plcB-deficient mutant strains accumulate in double-membrane vesicles in newly infected cells which form as a consequence of cell-to-cell spread (48). Thus, infection of host cells by L. monocytogenes can occur by two distinct mechanisms: direct bacterial invasion of the cells in an internalin-mediated fashion and cell-to-cell spread (9). The intracellular lifestyle along with direct cell-to-cell spread is responsible for T cell-mediated immunity to cure the infection, whereas antibodies, albeit induced, are not important for elimination of the bacteria (20, 28).
Whereas most experimental studies with animals have focused on the role of bacterial virulence factors in systemic listeriosis, which involves predominantly the liver and the spleen, the pathogenesis of cerebral listeriosis, a common and life-threatening complication of human listeriosis, has remained largely unexplored (43). Previous studies in an established murine model of cerebral listeriosis (12, 26) have revealed that in the brain most of the bacteria reside intracellularly in plexus epithelial cells, ependymal cells, and neurons as well as macrophages (37). The intracellular location of L. monocytogenes indicates that bacterial virulence factors promoting its entry into host cells or facilitating its spread from cell to cell may significantly contribute to the pathogenesis and severity of cerebral listeriosis. The aim of this study was to examine the roles of InlA and InlB, necessary for invasion, and that of PlcB, necessary for the efficient spread of Listeria cells, in experimental murine cerebral listeriosis. In the present study we have used Listeria mutants lacking InlA and InlB (ΔinlAB2) or PlcB (ΔplcB2). Our data clearly demonstrate that PlcB, but not InlA/B, is an important virulence factor contributing to murine cerebral listeriosis.
MATERIALS AND METHODS
Bacterial strains and media.
The weakly hemolytic L. monocytogenes EGD serotype 1/2a was originally obtained from G. B. Mackaness and served as the parental strain for construction of the ΔplcB2 (18) and ΔinlAB2 (32) mutant strains. Listeria strains were grown in brain heart infusion broth (Difco, Freiburg, Germany) at 37°C, with vigorous shaking.
Mice.
Female C57BL/6 mice (6 to 8 weeks old) were obtained from Harlan-Winkelmann (Borchen, Germany). Animals were kept under special pathogen-free conditions before infection with L. monocytogenes.
Experimental procedure.
Mice were infected intracerebrally (i.c.) with 4 × 102 organisms of L. monocytogenes EGD (wild type [WT]), ΔinlAB2 (InlA/B deficient), and ΔplcB2 (lecithinase deficient) strains. In addition, experimental groups were actively immunized by intraperitoneal injection of 5 × 103 L. monocytogenes EGD cells 14 days prior to i.c. infection. The survival rates of the mice in each experimental group were monitored.
The i.c. bacterial load was determined at days 1, 3, and 5 postinfection (p.i.) by plating 10-fold serial dilutions of brain tissue on tryptose agar (Difco). CFU were counted after incubation at 37°C for 24 h. In addition, spleens and livers from five immunized mice were used to assess the bacterial load 14 days after intraperitoneal immunization prior to i.c. challenge infection. After incubation of spleen and liver tissue for 24 h, no CFU could be recovered from these animals.
For histology, mice were sacrificed by CO2 asphyxiation and immediately thereafter perfused intracardially with 4% paraformaldehyde in phosphate-buffered saline (pH 7.4) at days 1, 3, 5, and 10 p.i. Brains were dissected and incubated in 4% paraformaldehyde in phosphate-buffered saline at 4°C for 24 h and subsequently embedded in paraffin.
Histology.
For histology, sections (4 μm each) were cut from paraffin-embedded brain tissue. Specimens were stained with hemalum and eosin, cresyl violet, and Giemsa solution (Merck, Darmstadt, Germany). In addition, L. monocytogenes was demonstrated immunohistochemically by incubating deparaffinized sections with a polyclonal rabbit anti-L. monocytogenes antiserum (Difco) followed by peroxidase-labelled goat anti-rabbit immunoglobulin G F(ab′)2 fragments (Jackson-Dianova, Hamburg, Germany). Peroxidase reaction products were visualized by 3,3′-diamonobenzidine tetrahydrochloride (Sigma, Deisenhofen, Germany), and H2O2 was used as the cosubstrate.
Histopathological alterations were evaluated in specimens stained with hemalum and eosin, cresyl violet, and Giemsa solution as well as in L. monocytogenes immunohistochemically stained sections obtained from various regions of the brain, including the basal ganglia, the hippocampus, the hypothalamus, the cerebellum, the brain stem, the ventricular system at the levels of the lateral, third, and fourth ventricles, the subarchnoid space at the level of the forebrain, the cerebellar cisterns, and the brain stem. To compare the intensity of cerebral listeriosis between the various groups, a semiquantitative grading system was introduced as follows: no pathological alterations, −; single bacteria and small inflammatory infiltrates, +; clusters of bacteria and large infiltrates with no tissue destruction, ++; and huge amounts of bacteria and large numbers of infiltrates with parenchymal destruction, +++. For the in situ detection of DNA fragmentation, the TUNEL method (14) was employed with paraffin sections. The In Situ Cell Death Detection kit (Boehringer, Mannheim, Germany) was used according to the manufacturer’s instructions. Negative control experiments were carried out without terminal transferase in the TUNEL reaction mixture.
Statistics.
The Wilcoxon test was used to compare statistical differences between the survival times of the various experimental groups as well as differences in the i.c. bacterial load at various time points after infection. P values of <0.05 were considered significant. Each experiment was performed at least twice.
RESULTS
Survival rates.
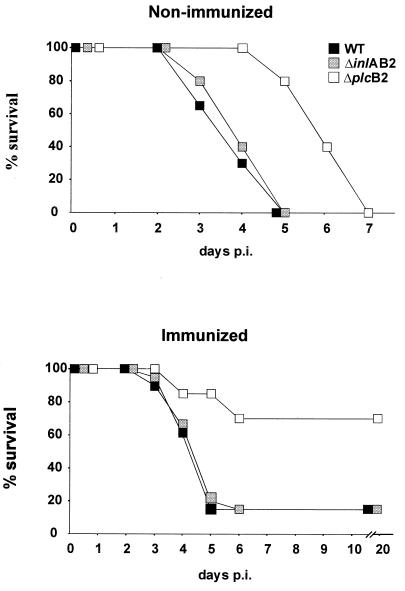
Following i.c. infection with the L. monocytogenes WT and ΔinlAB2 mutant strains, all nonimmunized mice succumbed to the disease by day 5 p.i. (Fig. 1). In addition, although mice infected with the L. monocytogenes ΔplcB2 mutant strain inevitably showed a fatal outcome caused by central nervous system (CNS) listeriosis (Fig. 1), death was significantly delayed in these animals (P < 0.05 for ΔplcB2 versus WT and ΔinlAB2).
FIG. 1.
Survival rates of nonimmunized (upper panel) and immunized (lower panel) mice infected i.c. with L. monocytogenes WT, ΔinlAB2, and ΔplcB2. Nonimmunized mice infected i.c. with ΔplcB2 survived significantly longer than mice infected with WT or ΔinlAB2 (P < 0.05). Immunized mice infected i.c. with ΔplcB2 had a significantly increased survival rate compared to that of mice infected with WT or ΔinlAB2 L. monocytogenes (P < 0.05). Data represent survival rates of 10 mice per experimental group. The results of one of two experiments which gave comparable results are shown.
Mice actively immunized with WT L. monocytogenes were significantly protected from i.c. infection with the ΔplcB2 strain compared to nonimmunized animals (P < 0.05 [Fig. 1]). In contrast, immunization did not significantly protect mice i.c. infected with either WT or ΔinlAB2, and mortality rates did not differ significantly from those of nonimmunized mice infected with WT and the ΔinlAB2 mutant strain. Thus, survival rates of these mice were significantly reduced compared with those of immunized mice infected with ΔplcB2 (Fig. 1 [P < 0.05]). Notably, after day 6 p.i., no more animals succumbed to the disease, irrespective of experimental group.
Kinetics of bacterial load.
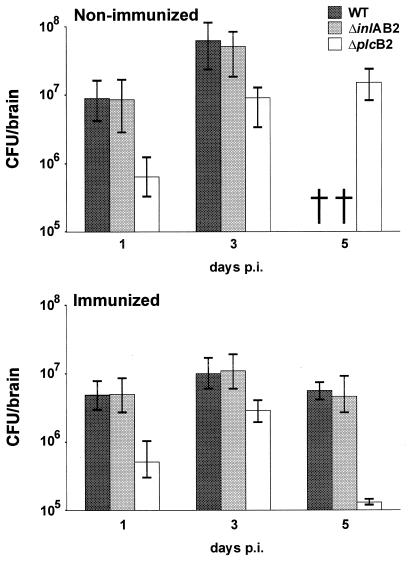
Determination of the i.c. bacterial load at days 1, 3, and 5 p.i. revealed that nonimmunized and immunized mice infected with WT and ΔinlAB2 had comparable amounts of bacteria in their brains. In both the nonimmunized and immunized mice in these groups, the i.c. bacterial load increased from day 1 to day 3 p.i. Interestingly, immunized WT- and ΔinlAB2-infected mice reduced the i.c. bacterial load only moderately from days 3 to 5 p.i. (Fig. 2).
FIG. 2.
Parasitic load of nonimmunized (upper panel) and immunized (lower panel) mice i.c. infected with L. monocytogenes WT, ΔinlAB2, and ΔplcB2. At each time point after infection, the i.c. bacterial load of mice infected with ΔplcB2 was significantly lower than that of mice infected with WT or ΔinlAB2 (P < 0.05). †, mice were already deceased. Five mice per group were analyzed, and the median ± the highest and lowest values of each group are shown.
A comparison of WT- and ΔinlAB2-infected mice with ΔplcB2-infected animals revealed that the bacterial load in the brains of ΔplcB2-infected mice was significantly reduced (Fig. 2). This observation was made for both immunized and nonimmunized groups throughout the course of disease. Although the number of bacteria increased in the brains of ΔplcB2-infected nonimmunized mice from days 1 to 3 p.i., the bacterial load was significantly lower compared to those in nonimmunized WT- and ΔinlAB2-infected mice (P < 0.05 for ΔplcB2 versus WT and ΔinlAB2). In addition, immunized ΔplcB2-infected mice had a significantly reduced i.c. bacterial load at days 1, 3, and 5 p.i. compared to immunized WT- and ΔinlAB2-infected mice (P < 0.05 for ΔplcB2 versus WT and ΔinlAB2). Interestingly, analysis of nonimmunized and immunized ΔplcB2-infected mice revealed that immunization, which significantly protected these mice from the fatal course of disease, did not result in a significant reduction of the i.c. bacterial load before day 5 p.i.
Histopathology.
After i.c. application of L. monocytogenes WT, ΔinlAB2, and ΔplcB2 strains, all mice developed cerebral listeriosis. Whereas the same neuroanatomical structures were affected in all groups, there were major differences in the kinetics and severity of inflammation (Table 1).
TABLE 1.
Semiquantitative histopathological grading of cerebral listeriosis in mice infected with L. monocytogenes WT, ΔinlAB2, or ΔplcB2
| Day p.i. | Listeria strain | Results for indicated group of micea
|
|||||
|---|---|---|---|---|---|---|---|
| Nonimmunized
|
Immunized
|
||||||
| Meningitis | Encephalitis | Ventriculitis | Meningitis | Encephalitis | Ventriculitis | ||
| 1 | WT | + | + | ++ | + | − | ++ |
| ΔinlAB2 | + | + | ++ | ++ | + | ++ | |
| ΔplcB2 | + | + | + | + | + | + | |
| 3 | WT | +++ | ++ | +++ | +++ | +++ | +++ |
| ΔinlAB2 | +++ | ++ | +++ | ++ | ++ | +++ | |
| ΔplcB2 | + | + | ++ | + | + | + | |
| 5 | WT | DEC | DEC | DEC | ++ | ++ | ++ |
| ΔinlAB2 | DEC | DEC | DEC | + | ++ | +++ | |
| ΔplcB2 | + | ++ | ++ | + | + | + | |
Three mice per group were analyzed, and results from one of two experiments with similar results are shown. −, no pathological alterations; +, presence of single bacteria and small inflammatory infiltrates; ++, clusters of bacteria and large infiltrates in the absence of tissue destruction; +++, large numbers of bacteria and infiltrates with tissue destruction; DEC, deceased.
In general, following the i.c. application of L. monocytogenes onto the rostral basal ganglia, nonimmunized WT- and ΔinlAB2-infected mice developed similar pathologies. At day 1 p.i., bacteria and inflammatory cells were present in the ventricular system associated with the choroid plexus and the ependyma. The ΔplcB2 mutant strain was observed in slightly reduced numbers in the same structures. However, there was no evidence of an invasion of the ependyma by the ΔplcB2 strain at this stage of the disease.
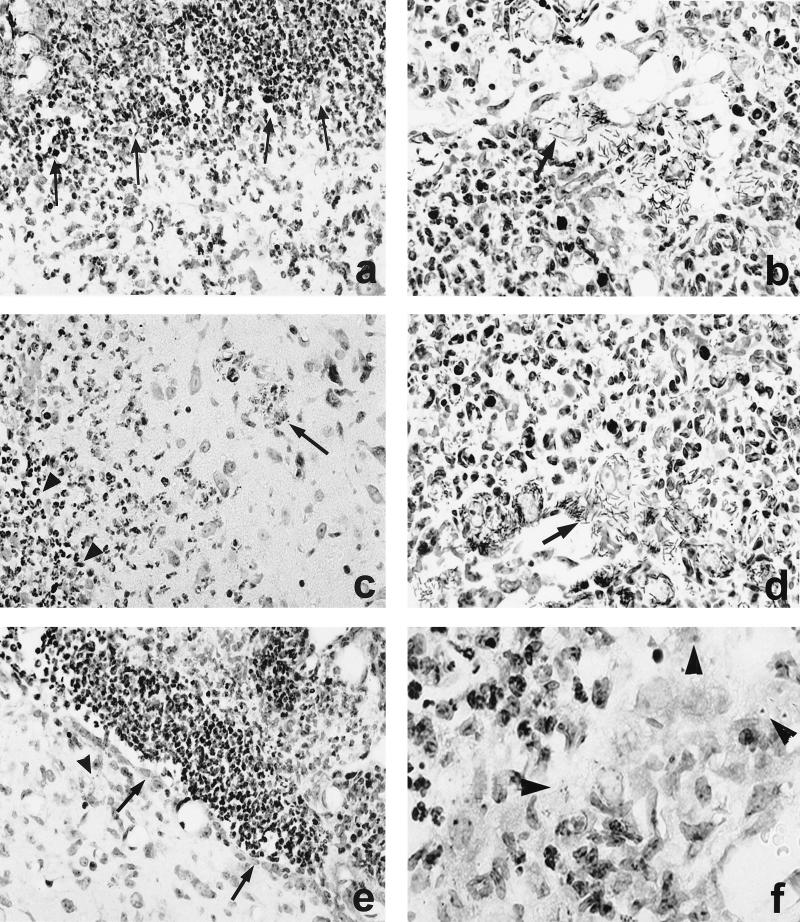
At day 3 p.i., cerebral listeriosis had significantly progressed in all experimental groups. L. monocytogenes WT and ΔinlAB2 strains had caused severe meningitis and ventriculitis. The lateral, third, and fourth ventricles were completely obstructed by large numbers of polymorphonuclear leukocytes and macrophages (Fig. 3a and c). Interestingly, numerous elongated, rod-shaped WT bacteria and ΔinlAB2 mutants adhered to plexus epithelial cells and formed large clusters (Fig. 3b and d). They had destroyed the choroid plexus and the ependyma (Fig. 3a to d), allowing the bacteria access to the periventricular tissue, where inflammatory infiltrates were associated with the bacteria and small foci of necrosis (Fig. 3a and c). Invasion of WT and the ΔinlAB2 strain was most prominent in the brain stem, where bacteria were also detected in the cytoplasm of neurons. These Listeria-infected neurons were surrounded by neutrophils and macrophages (Fig. 3c).
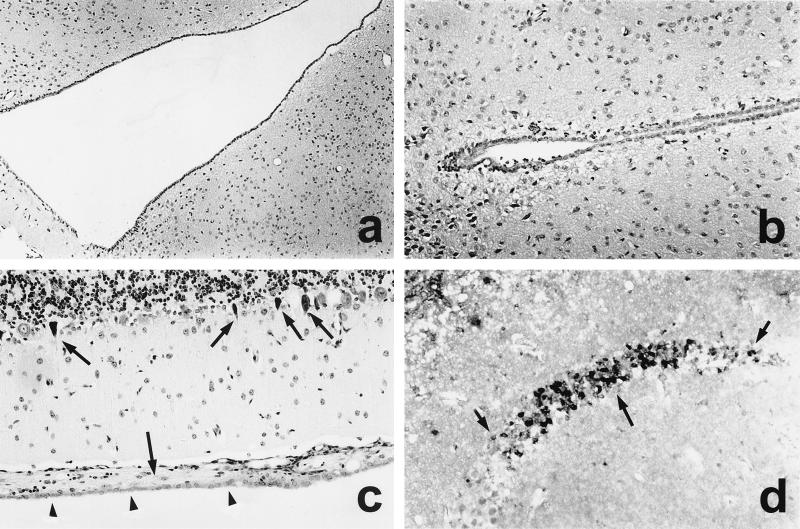
FIG. 3.
CNS pathology of nonimmunized mice i.c. infected with L. monocytogenes WT (a and b), ΔinlAB2 (c and d), and ΔplcB2 (e and f) at day 3 p.i. (a) Severe empyema of the ventricle (area above the arrows). The ependymal wall is largely destroyed and barely discernible, and the periventricular brain stem is infiltrated by numerous neutrophils and macrophages. (b) L. monocytogenes WT cluster in the largely destroyed plexus. The arrow points to a group of remarkably elongated WT bacteria. (c) ΔinlAB2 has also largely destroyed the ependyma of the ventricular wall (area left of the arrowheads) and has invaded the adjacent brain parenchyma. Brain stem neurons are surrounded by inflammatory leukocytes (arrow). (d) ΔinlAB2 cluster in the partially necrotic choroid plexus. Note that the elongated shape of the ΔinlAB2 strain (arrow) is identical to that of the WT in panel b. (e) ΔplcB2 has also infected the fourth ventricle, and the bacteria are accompanied by intraventricular neutrophils and macrophages. In contrast to WT (a) and ΔinlAB2 (c), the ependymal lining is largely intact (arrows). Very few bacteria are detectable in the periventricular tissue (arrowhead). (f) Some ΔplcB2 are detectable either as single or small groups of coccoid bacteria (arrowheads) in the choroid plexus. The choroid plexus is infiltrated by neutrophils and macrophages, but in contrast to the images shown in panels b and d, its structure is largely preserved. Specimens in panels a to f were stained with cresyl violet. Magnification is as follows: for panels a and e, ×260; b and d, ×1,250; c, ×520; f, ×1,470.
In contrast to WT and ΔinlAB2, the ΔplcB2 mutant strain caused a course of disease that was significantly less severe (Fig. 3e). The number of ΔplcB2 bacteria was much lower than those of WT and ΔinlAB2, and consequently there were fewer inflammatory infiltrates. Interestingly, ΔplcB2 bacteria did not take an elongated shape or form large clusters in the ventricular system but were isolated or present in small groups (Fig. 3f). The choroid plexus was much less severely destroyed, and the intact ependymal layer precluded significant bacterial invasion of the adjacent brain tissue (Fig. 3e). Up to day 5 p.i., when mice infected with WT and ΔinlAB2 had already succumbed to necrotizing brain stem encephalitis, the disease was still progressing in animals infected with the ΔplcB2 strain. At this stage of infection, histopathological alterations of ΔplcB2-infected mice were comparable to those in mice infected with WT and ΔinlAB2 bacteria at day 3 p.i.
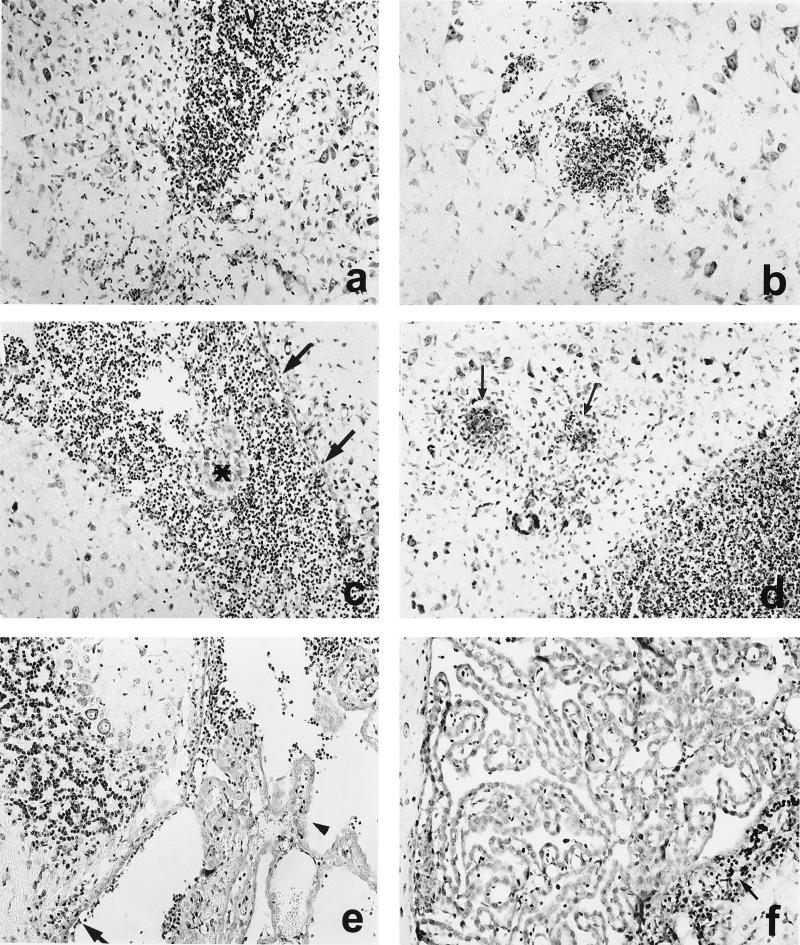
With either bacterial strain, the anatomic structures that were affected in actively immunized mice were the same as those affected in nonimmunized mice. However, the severity of cerebral listeriosis, particularly in mice infected with mutant strain ΔplcB2, was reduced (Table 1 and Fig. 4). The involvement of the periventricular basal ganglia, the fourth ventricle, and the adjacent brain stem tissue, especially, was less severe (Fig. 4a to d). Nevertheless, most of the immunized mice infected with the WT and ΔinlAB2 strains still developed progressive encephalitis, although it was somewhat delayed, and subsequently the vast majority of these animals succumbed to the disease.
FIG. 4.
CNS pathology of immunized mice i.c. infected with L. monocytogenes WT (a and b), ΔinlAB2 (c and d), and ΔplcB2 (e and f) at days 3 (a, c, and e) and 5 (b, d, and f) p.i. (a) Inflammation of the lateral ventricle (V) in a mouse infected with L. monocytogenes WT. Additional infiltrates are present in the adjacent brain parenchyma. (b) Small, circumscribed infiltrates are present in the brain stem in the vicinity of neurons. (c) In ΔinlAB2-infected mice, inflammation is also largely confined to the lateral ventricle, and small parts of the choroid plexus are preserved (asterisk). A significant part of the ependyma is still intact (arrows). (d) From days 3 to 5 p.i., disease progressed in ΔinlAB2-infected mice. Purulent ventriculitis and focal brain stem encephalitis (arrows) developed. (e) Only discrete infiltrates were detectable in the largely normal fourth ventricle in a ΔplcB2-infected mouse at day 3 p.i. The choroid plexus was largely preserved (arrowhead), and ventricular empyema was absent. The ependyma was only focally destroyed, and small, single infiltrates were present in the periventricular tissue (arrow). (f) At day 5 p.i., the inflammation was largely resolved, and only small residual infiltrates were present (arrow) in the wall of the fourth ventricle. Specimens in panels a to f were stained with cresyl violet. Magnification, ×260.
Immunized ΔplcB2-infected mice also developed meningitis and ventriculitis; however, these diseases were much less severe than in nonimmunized ΔplcB2-infected or immunized WT- or ΔinlAB2-infected animals (Fig. 4e and Table 1). The most-striking finding was the lack of disease progression from days 3 to 5 p.i. in most animals (Fig. 4f and Table 1). In addition, only a small number of immunized ΔplcB2-infected mice developed brain stem encephalitis, and inflammation was restricted mostly to the periventricular brain tissue. In particular, necrosis did not occur in the brains of these animals. Interestingly, the ΔplcB2 mutant strain was observed less often in neurons than were bacteria from the other experimental groups.
At day 10 p.i., surviving mice had developed postinflammatory internal hydrocephalus of the lateral, third, and fourth ventricles (Fig. 5a) due to partial destruction of the ependymal lining and formation of delicate sheets of glial-ependymal tissue in the fourth ventricle, irrespective of the injected Listeria mutant (Fig. 5c).
FIG. 5.
CNS complications in L. monocytogenes meningoencephalitis at day 10 p.i. (a and b) A severe obstructive hydrocephalus developed at day 10 p.i. Note the massive enlargement of the third ventricle (a) compared to the normal size of the third ventricle of an immunized mouse i.c. infected with ΔplcB2 at day 1 p.i. (b). (c) Postinflammatory scarring of the ventricular wall of the fourth ventricle. Subependymal formation of a membranous gliotic tissue (large arrow). In this area, the ependyma is largely intact (arrowheads). In addition, some Purkinje cells in the adjacent cerebellum have small, pyknotic nuclei with condensed chromatin (small arrows), which is characteristic of apoptotic cells. (d) Apoptotic, TUNEL-positive neurons with small, pyknotic nuclei in the CA1 segment of the hippocampus (large arrow). Note the relatively sharp demarcation from the adjacent hippocampal segments (small arrows). Specimens in panels a to c were stained with cresyl violet, and panel d shows TUNEL staining. Magnification for panels a to d, ×260. Images shown in panels a, c, and d are from immunized mice i.c. infected with ΔplcB2 at day 10 p.i. Similar observations were made about the brains of surviving mice infected with L. monocytogenes WT and ΔinlAB2 at day 10 p.i.
Apoptosis.
To determine whether apoptosis of CNS cells contributes to the pathogenesis of cerebral listeriosis and whether apoptosis is influenced by virulence factors of L. monocytogenes, brain sections from the various experimental groups were analyzed by the TUNEL technique.
At day 1 p.i., no apoptotic cells were detectable in the brains of any of the Listeria-infected mice. Starting at day 3 p.i., apoptosis of hippocampal neurons was observed in all experimental groups. The vast majority of neurons of the CA1 segment of the hippocampus exhibited small, pyknotic nuclei and an eosinophilic cytoplasm, and these neurons were strongly labelled in the TUNEL reaction. Apoptotic neurons were confined to the CA1 segment and were sharply demarcated from the adjacent intact hippocampus (Fig. 5d). In addition, surviving mice exhibited a small but significant number of apoptotic Purkinje cells in the cerebellum at day 10 p.i. (Fig. 5c). In contrast to hippocampal and cerebellar neurons, other structures of the CNS exhibited only a marginal number of apoptotic cells. Thus, the destruction of the plexus epithelium, the ependymal layer as well as parts of the brain parenchyma, was due to L. monocytogenes-induced necrosis rather than to apoptosis. Furthermore, at days 3 and 5 p.i., some apoptotic inflammatory cells in the ventricular lumen were detected by TUNEL labelling.
DISCUSSION
In this study we have assessed the properties contributing to the virulence of the inlA, inlB, and plcB genes of L. monocytogenes in the CNS following the crossing of the blood-brain barrier (BBB). We employed L. monocytogenes isogenic deletion mutant strains lacking the main virulence factors necessary for invasion and cell-to-cell spread, inlA/B and plcB, respectively.
We demonstrated that PlcB, but not InlA or InlB, is an important determinant of the virulence of L. monocytogenes in cerebral listeriosis. The delayed intracerebral spread and reduced multiplication of the L. monocytogenes ΔplcB2 mutant strain indicated that cell-to-cell spread, which is dependent mainly on lecithinase activity (48), is important for the rapid dissemination of the bacterium in the brain. Consequently, nonimmunized mice infected i.c. with ΔplcB2 survived significantly longer than mice infected with the WT and ΔinlAB2 strains. However, it should be stressed that although its spread was delayed, ΔplcB2 finally gained access to the same anatomic structures of the CNS, i.e. the plexus epithelium, the ependyma, the leptomeninges, and the brain parenchyma, as the WT and ΔinlAB2 strains did. Thus, PlcB seems to be required for efficacious and fulminant infection in cerebral listeriosis.
In immunized mice the delayed spread of ΔplcB2 enabled the immune system to prevent a significant spread of the bacterium to the brain stem, resulting in an outcome that was significantly more favorable, with an increased survival rate, compared to that of mice infected with WT and ΔinlAB2. The improved prognosis of immunized L. monocytogenes ΔplcB2-infected mice can be explained by two complementary factors. For infection of host cells to be ongoing, the ΔplcB2 mutant strain must continuously leave the host cells and enter the extracellular space. This enables macrophages, stimulated by immunization-induced L. monocytogenes-specific T cells, to phagocytize and degrade extracellular ΔplcB2 mutant listeriae. In fact, previous studies have demonstrated that systemic immunization results in the increased activation of i.c. macrophages in cerebral listeriosis (37). In addition, the lack of cell-to-cell spread results in the reduced growth of L. monocytogenes ΔplcB2 in the brain, independent of a Listeria-specific T-cell response (Fig. 2, nonimmunized mice). Therefore, immunization-induced Listeria-specific T cells have to combat a significantly reduced number of i.c. bacteria in mice infected with the ΔplcB2 strain compared to the number in mice infected with the WT or the ΔinlAB2 strain, significantly facilitating control and elimination of the pathogen. These findings extend those of previous in vitro studies, which have indicated that the lecithinase expressed by mature PlcB is essential for cell-to-cell spread in various tissue culture cell lines (35, 42, 48).
In contrast, the cell wall-associated virulence factors InlA/B were unimportant for the efficient spread and multiplication of L. monocytogenes in the brain. Thus, while InlA is necessary in mediating the invasion of intestinal Caco-2 cells, and InlB is required for the invasion of hepatocytes and human umbilical cord endothelial cells (6, 13, 30, 32), these virulence factors are dispensable for the efficient spread and multiplication of L. monocytogenes in the brain. In addition, the experiments of Gregory et al. (17) have revealed that InlA and InlB are not required for the entry of L. monocytogenes into hepatic cells in vivo.
In the present study, L. monocytogenes was directly applied to the brain, a technique which is widely used to study the pathogenesis of cerebral listeriosis (12, 26, 36, 37) as well as that of other bacterial (reviewed in reference 46) and viral (26, 45) cerebral infections. This approach guaranteed that i.c. listeriosis started at the same time point in all groups of mice, with exactly the same amounts of the various Listeria mutants, a prerequisite for evaluating the roles of the different virulence factors for the i.c. pathogenesis of listeriosis in the CNS. However, it must be remembered that cerebral listeriosis is, in general, a food-borne disease acquired by the consumption of contaminated food. After the intestinal barrier has been passed, bacteremia enables L. monocytogenes to cross the BBB and to infect the CNS, generating characteristic cerebral symptoms (1). The specific listerial determinants that are required for the crossing of the intestinal barrier and the infection of the CNS are still unknown. Two general mechanisms for traversing the BBB can be envisaged as follows: first, transcytosis through the endothelial cells lining the barrier, and second, paracellular infection, either directly or via infected phagocytic cells between vascular endothelial cells. We have recently shown that L. monocytogenes can invade endothelial cells directly and that this invasion is mediated by the inlB gene product (32). Other studies showed that the interaction of Neisseria meningitidis with components of the BBB correlates with the increased expression of PilC (34) and that the outer membrane protein A of Escherichia coli contributes to the invasion of brain microvascular endothelial cells (33). Both bacterial proteins may be important factors for the crossing of the BBB, allowing pathogens access to the normally sterile CNS. Another mechanism by which bacteria might gain access to the CNS is the modulation of the BBB permeability during systemic bacterial infections such as those caused by nitric oxide, tumor necrosis factor alpha (21, 23, 38, 40), and bacterial products (44, 47). We speculate that paracellular forms of listerial transfer may also occur via changes in tight junction physiology, probably mediated by the action of listeriolysin.
In the present study, a common feature of cerebral listeriosis was the capacity to induce the apoptosis of neurons in the hippocampus. Interestingly, the apoptosis of hippocampal neurons was confined to the CA1 segment, which is particularly vulnerable to a variety of lesions. Purkinje cells in the cerebellum, which are also highly vulnerable to stress, underwent apoptosis as well. Since the same apoptotic changes were observed in the mice infected with the ΔinlAB2 and ΔplcB2 mutant strains, these virulence factors of L. monocytogenes are not critical for the induction of apoptosis in neurons. The fact that the apoptosis of hippocampal neurons was also observed in other experimental models of bacterial meningitis, including pneumococcal and streptococcal meningitis (2, 49), indicates that a common pathogenic mechanism underlies the induction of neuronal apoptosis in bacterium-induced meningoencephalitis. Candidate molecules, which have been shown to induce neuronal apoptosis in experimental group B streptococcal meningitis as well as in neuronal cell cultures stimulated with Streptococcus pneumoniae or group B streptococcal cell walls, are tumor necrosis factor alpha and nitric oxide (2, 22), which are also produced in cerebral listeriosis (26, 36). The assumption that immune reactions may be critically involved in the induction of neuronal apoptosis is further evidenced by our observation that apoptotic cells were often observed in the vicinity of inflammatory infiltrates. In addition, it has been demonstrated recently that a main virulence factor from L. monocytogenes, listeriolysin, is able to induce the apoptosis of mouse dendritic cells (19). Since the ΔinlAB2 and ΔplcB2 mutant strains we used in this study produce listeriolysin (data not shown), we can assume that this toxin may also contribute to the apoptotic process of neurons in the hippocampus and cerebellum.
Furthermore, a common feature of all surviving mice, irrespective of the type of L. monocytogenes that was applied, was the development of a hydrocephalus. This hydrocephalus was caused by the destruction of the ependymal lining and the formation of sheets of glial-ependymal scar tissue in the ventricles, interfering with cerebrospinal fluid outflow. Since the development of a hydrocephalus was independent of the listerial virulence factors InlA/B and PlcB, the infection and subsequent destruction of specific anatomic structures such as the ependyma seems to be the main pathogenic factor. In this respect, murine CNS listeriosis closely resembles human bacterial meningitis, in which postinflammatory hydrocephalus is a common complication.
In conclusion, the present study identifies PlcB as a virulence factor in CNS listeriosis, which is important in determining the course and final outcome of the disease. Since the ΔplcB2 mutant strain was not completely attenuated, additional, as-yet-undefined virulence factors may play a significant role in the i.c. pathogenesis of this life-threatening disease.
ACKNOWLEDGMENTS
The expert technical assistance of Nadja Kaefer and Andrea Rang is gratefully acknowledged.
This work was supported in part by a grant from the Deutsche Forschungsgemeinschaft through SFB 535 (TP/A5) to E.D.
REFERENCES
- 1.Berche P. Bacteremia is required for invasion of the murine central nervous system by Listeria monocytogenes. Microb Pathog. 1995;18:323–336. doi: 10.1006/mpat.1995.0029. [DOI] [PubMed] [Google Scholar]
- 2.Bogdan I, Leib S L, Bergeron M, Chow L, Täubner M G. Tumor necrosis factor-alpha contributes to apoptosis in hippocampal neurons during experimental group B streptococcal meningitis. J Infect Dis. 1997;176:693–697. doi: 10.1086/514092. [DOI] [PubMed] [Google Scholar]
- 3.Braun L, Dramsi S, Dehoux P, Bierne H, Lindahl G, Cossart P. InlB: an invasion protein of Listeria monocytogenes with a novel type of surface association. Mol Microbiol. 1997;25:285–294. doi: 10.1046/j.1365-2958.1997.4621825.x. [DOI] [PubMed] [Google Scholar]
- 4.Chakraborty T, Wehland J. The host cell infected with Listeria monocytogenes. In: Kaufmann S H E, editor. Host response to intracellular pathogens. R. G. Austin, Tex: Landes Company; 1997. pp. 271–290. [Google Scholar]
- 5.Domann E, Zechel S, Lingnau A, Hain T, Darji A, Nichterlein T, Wehland J, Chakraborty T. Identification and characterization of a novel PrfA-regulated gene in Listeria monocytogenes whose product, IrpA, is highly homologous to internalin proteins, which contain leucine-rich repeats. Infect Immun. 1997;65:101–109. doi: 10.1128/iai.65.1.101-109.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dramsi S, Biswas I, Maguin E, Braun L, Mastroeni P, Cossart P. Entry of Listeria monocytogenes into hepatocytes requires expression of inlB, a surface protein of the internalin multigene family. Mol Microbiol. 1995;16:251–261. doi: 10.1111/j.1365-2958.1995.tb02297.x. [DOI] [PubMed] [Google Scholar]
- 7.Dramsi S, Lebrun M, Cossart P. Molecular and genetic determinants interfere in invasion of mammalian cells by Listeria monocytogenes. Curr Top Microbiol Immunol. 1996;209:61–77. doi: 10.1007/978-3-642-85216-9_4. [DOI] [PubMed] [Google Scholar]
- 8.Dramsi S, Dehoux P, Lebrun M, Goossens P L, Cossart P. Identification of four new members of the internalin multigene family of Listeria monocytogenes EGD. Infect Immun. 1997;65:1615–1625. doi: 10.1128/iai.65.5.1615-1625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drevets D A, Sawyer R T, Potter T A, Campbell P. Listeria monocytogenes infects human endothelial cells by two distinct mechanisms. Infect Immun. 1995;63:4268–4276. doi: 10.1128/iai.63.11.4268-4276.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelbrecht F, Chun S-K, Ochs C, Hess J, Lottspeich F, Goebel W, Sokolovic Z. A new PrfA-regulated gene of Listeria monocytogenes encoding a small, secreted protein which belongs to the family of internalins. Mol Microbiol. 1996;21:823–837. doi: 10.1046/j.1365-2958.1996.541414.x. [DOI] [PubMed] [Google Scholar]
- 11.Farber J M, Peterkin P I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991;55:476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frei K, Nadal D, Pfister H W, Fontana A. Listeria meningitis: identification of a cerebrospinal fluid inhibitor of macrophage listericidal function as interleukin-10. J Exp Med. 1993;178:1255–1261. doi: 10.1084/jem.178.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaillard J-L, Berche P, Frehel C, Gouin E, Cossart P. Entry of Listeria monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell. 1991;65:1127–1141. doi: 10.1016/0092-8674(91)90009-n. [DOI] [PubMed] [Google Scholar]
- 14.Gavrieli Y, Sherman Y, Ben-Sasson S A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geoffroy C, Raveneau J, Beretti J-L, Lecroisey A, Vazquez-Boland J-A, Alouf J E, Berche P. Purification and characterization of an extracellular 29-kilodalton phospholipase C from Listeria monocytogenes. Infect Immun. 1991;59:2382–2388. doi: 10.1128/iai.59.7.2382-2388.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldfine H, Johnston N C, Knob C. Nonspecific phospholipase C of Listeria monocytogenes activity on phospholipids in Triton X-100-mixed micelles and in biological membranes. J Bacteriol. 1993;175:4298–4306. doi: 10.1128/jb.175.14.4298-4306.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregory S H, Sagnimeni A J, Wing E J. Expression of the inlAB operon by Listeria monocytogenes is not required for entry into hepatic cells in vivo. Infect Immun. 1996;64:3983–3986. doi: 10.1128/iai.64.10.3983-3986.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guzman C A, Domann E, Rohde M, Bruder D, Darji A, Weiss S, Wehland J, Chakraborty T, Timmis K N. Apoptosis of mouse dendritic cells is triggered by listeriolysin, the major virulence determinant of Listeria monocytogenes. Mol Microbiol. 1996;20:119–126. doi: 10.1111/j.1365-2958.1996.tb02494.x. [DOI] [PubMed] [Google Scholar]
- 19.Guzman C A, Rohde M, Chakraborty T, Domann E, Hudel M, Wehland J, Timmis K. Interaction of Listeria monocytogenes with mouse dendritic cells. Infect Immun. 1995;63:3665–3673. doi: 10.1128/iai.63.9.3665-3673.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufmann S H E. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 21.Kim K S, Wass C A, Cross A S. Blood-brain barrier permeability during the development of experimental bacterial meningitis in the rat. Exp Neurol. 1997;145:253–257. doi: 10.1006/exnr.1997.6458. [DOI] [PubMed] [Google Scholar]
- 22.Kim K S, Wass C A, Cross A S, Opal S M. Modulation of blood-brain barrier permeability by tumor necrosis factor and antibody to tumor necrosis factor in the rat. Lymphokine Cytokine Res. 1992;11:293–298. [PubMed] [Google Scholar]
- 23.Kim Y S, Täuber M G. Neurotoxicity of glia activated by gram-positive bacterial products depends on nitric oxide production. Infect Immun. 1996;64:3148–3153. doi: 10.1128/iai.64.8.3148-3153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobe B, Deisenhofer J. The leucine-rich repeat: a versatile binding motif. Trends Biochem Sci. 1994;19:415–421. doi: 10.1016/0968-0004(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 25.Lebrun M, Mengaud J, Ohayon H, Nato F, Cossart P. Internalin must be on the bacterial surface to mediate entry of Listeria monocytogenes into epithelial cells. Mol Microbiol. 1996;21:579–592. doi: 10.1111/j.1365-2958.1996.tb02566.x. [DOI] [PubMed] [Google Scholar]
- 26.Leist T P, Frei K, Kam-Hansen S, Zinkernagel R M, Fontana A. Tumor necrosis factor α in cerebrospinal fluid during bacterial, but not viral, meningitis. Evaluation in murine model infections and in patients. J Exp Med. 1988;167:1743–1748. doi: 10.1084/jem.167.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lingnau A, Domann E, Hudel M, Bock M, Nichterlein T, Wehland J, Chakraborty T. Expression of the Listeria monocytogenes EGD inlA and inlB genes, whose products mediate bacterial entry into tissue culture cell lines, by PrfA-dependent and -independent mechanisms. Infect Immun. 1995;63:3896–3903. doi: 10.1128/iai.63.10.3896-3903.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mackaness G B. Cellular resistance to infection. J Exp Med. 1962;116:381–406. [PubMed] [Google Scholar]
- 29.Makhov A M, Hannah J H, Brennan M J, Trus B L, Kocsis E, Conway J F, Wingfield P T, Simon M N, Steven A C. Filamentous hemagglutinin of Bordetella pertussis. A bacterial adhesin formed as a 50-nm monomeric rigid rod based on a 19-residue repeat motif rich in beta strands and turns. J Mol Biol. 1994;241:110–124. doi: 10.1006/jmbi.1994.1478. [DOI] [PubMed] [Google Scholar]
- 30.Mengaud J, Ohayon H, Gounon P, Mege R M, Cossart P. E-cadherin is the receptor for internalin, a surface protein required for entry of Listeria monocytogenes into epithelial cells. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 31.Niebuhr K, Chakraborty T, Köllner P, Wehland J. Production of monoclonal antibodies to the phosphatidylcholine-specific phospholipase C of Listeria monocytogenes, a virulence factor for this pathogen. Med Microbiol Lett. 1993;2:9–16. [Google Scholar]
- 32.Parida S K, Domann E, Rohde M, Müller S, Darji A, Hain T, Wehland J, Chakraborty T. Internalin B is essential for adhesion and mediates the invasion of Listeria monocytogenes into human endothelial cells. Mol Microbiol. 1998;28:81–93. doi: 10.1046/j.1365-2958.1998.00776.x. [DOI] [PubMed] [Google Scholar]
- 33.Prasadarao N V, Wass C A, Weiser J N, Stins M F, Huang S H, Kim K S. Outer membrane protein A of Escherichia coli contributes to invasion of brain microvascular endothelial cells. Infect Immun. 1996;64:146–153. doi: 10.1128/iai.64.1.146-153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pron B, Taha M K, Rambaud C, Fournet J C, Pattey N, Monnet J P, Musilek M, Beretti J L, Nassif X. Interaction of Neisseria meningitidis with the components of the blood-brain barrier correlates with an increased expression of pilC. J Infect Dis. 1997;176:1285–1292. doi: 10.1086/514124. [DOI] [PubMed] [Google Scholar]
- 35.Raveneau J, Geoffroy C, Beretti J-L, Gaillard J-L, Alouf J E, Berche P. Reduced virulence of a Listeria monocytogenes phospholipase-deficient mutant obtained by transposon insertion into the zinc metalloprotease gene. Infect Immun. 1992;60:916–921. doi: 10.1128/iai.60.3.916-921.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlüter D, Chahoud S, Lassmann H, Hof H, Deckert-Schlüter M. Intracerebral targets and immunomodulation of murine Listeria monocytogenes meningoencephalitis. J Neuropathol Exp Neurol. 1996;55:14–24. doi: 10.1097/00005072-199601000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Schlüter D, Oprisiu S B, Chahoud S, Weiner D, Wiestler O D, Hof H, Deckert-Schlüter M. Systemic immunization induces protective CD4+ and CD8+ T cell-mediated immune responses in murine Listeria monocytogenes meningoencephalitis. Eur J Immunol. 1995;25:2384–2391. doi: 10.1002/eji.1830250839. [DOI] [PubMed] [Google Scholar]
- 38.Sharief M K, Ciardi M, Thompson E J. Blood-brain barrier damage in patients with bacterial meningitis: association with tumor necrosis factor-alpha but not interleukin-1 beta. J Infect Dis. 1992;166:350–358. doi: 10.1093/infdis/166.2.350. [DOI] [PubMed] [Google Scholar]
- 39.Shevchenko D V, Akins D R, Robinson E, Li M, Popova T G, Cox D L, Radolf J D. Molecular characterization and cellular localization of TpLRR, a processed leucine-rich repeat protein of Treponema pallidum, the syphilis spirochete. J Bacteriol. 1997;179:3188–3195. doi: 10.1128/jb.179.10.3188-3195.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shukla A, Dikshit M, Srimal R C. Nitric oxide modulates blood-brain barrier permeability during infections with an inactivated bacterium. Neuroreport. 1995;6:1629–1632. doi: 10.1097/00001756-199508000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Smith G A, Marquis H, Jones S, Johnston N C, Portnoy D A, Goldfine H. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect Immun. 1995;63:4231–4237. doi: 10.1128/iai.63.11.4231-4237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Songer J G. Bacterial phospholipases and their role in virulence. Trends Microbiol. 1997;5:156–161. doi: 10.1016/S0966-842X(97)01005-6. [DOI] [PubMed] [Google Scholar]
- 43.Southwick F S, Purich D L. Intracellular pathogenesis of listeriosis. N Engl J Med. 1996;334:770–776. doi: 10.1056/NEJM199603213341206. [DOI] [PubMed] [Google Scholar]
- 44.Spellerber B, Prassad S, Cabellos C, Burroughs M, Cahill P, Tuomanen E. Penetration of the blood-brain barrier: enhancement of drug delivery and imaging by bacterial glycopeptides. J Exp Med. 1995;182:1037–1043. doi: 10.1084/jem.182.4.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stohlman S A, Bergmann C C, Lin M T, Cua D J, Hinton D R. CTL effector function within the central nervous system requires CD4+ T cells. J Immunol. 1998;160:2896–2904. [PubMed] [Google Scholar]
- 46.Tunkel A R, Scheld W M. Pathogenesis and pathophysiology of bacterial meningitis. Clin Microbiol Rev. 1993;6:118–136. doi: 10.1128/cmr.6.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tuomanen E. Entry of pathogens into the central nervous system. FEMS Microbiol Rev. 1996;18:289–299. doi: 10.1111/j.1574-6976.1996.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 48.Vazquez-Boland J-A, Kocks C, Dramsi S, Ohayon H, Geoffroy C, Mengaud J, Cossart P. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect Immun. 1992;60:219–230. doi: 10.1128/iai.60.1.219-230.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zysk G, Brück W, Gerber J, Bruck Y, Prange H W, Nau R. Anti-inflammatory treatment influences neuronal apoptotic cell death in the detate gyrus in experimental pneumococcal meningitis. J Neuropathol Exp Neurol. 1996;55:722–728. doi: 10.1097/00005072-199606000-00006. [DOI] [PubMed] [Google Scholar]