Abstract
How do microtubules, which maintain and direct polarity of many eukaryotic cells, regulate polarity of blood neutrophils? In sharp contrast to most cells, disrupting a neutrophil's microtubule network with nocodazole causes it to polarize and migrate [Niggli, V. (2003) J. Cell Sci. 116, 813–822]. Nocodazole induces the same responses in differentiated HL-60 cells, a model neutrophil cell line, and reduces their chemotactic prowess by causing them to pursue abnormally circuitous paths in migrating toward a stationary point source of an attractant, f-Met-Leu-Phe (fMLP). The chemotactic defect stems from dramatic nocodazole-induced imbalance between the divergent, opposed fMLP-induced “backness” and “frontness” signals responsible for neutrophil polarity. Nocodazole (i) stimulates backness by increasing Rho- and actomyosin-dependent contractility, as reported by Niggli, and also (ii) impairs fMLP-dependent frontness: pseudopods are flatter, contain less F-actin, and show decreased membrane translocation of PH-Akt-GFP, a fluorescent marker for 3′-phosphoinositide lipids. Inhibiting backness with a pharmacologic inhibitor of a Rho-dependent kinase substantially reverses nocodazole's effects on chemotaxis, straightness of migration paths, morphology, and PH-Akt-GFP translocation. Thus, microtubules normally balance backness vs. frontness signals, preventing backness from reducing the strength of pseudopods and from impairing directional migration.
Keywords: chemotaxis, PIP3, pseudopod, Rho GTPases
The critical roles of cell polarity and directional migration in host defense, wound healing, and development depend on complex interplay between the actin and microtubule cytoskeletons (1–7). Formation and breakdown of actin filaments create polarized asymmetry of the cell's front and back to generate forces required for movement and to regulate adhesion to extracellular matrix and other cells. Locations of the microtubule-organizing center (MTOC) and microtubules radiating from it are thought to determine or maintain orientation of cell polarity, in part by directing vesicular traffic and membrane localization of cytoskeletal regulators that control strength and spatial distribution of actin assemblies (4–6). In turn, actin-associated proteins at the cell cortex can stabilize or enhance depolymerization at the plus ends of microtubules, perhaps thereby designating destinations for delivery of regulatory molecules, such as guanine nucleotide exchange factors (GEFs) for Rho GTPases (1, 2).
Compared with most vertebrate cells, blood neutrophils polarize, move, and turn more rapidly over time scales of 30–90 sec rather than minutes or hours. As compared with the actin assemblies at the neutrophil's front and back that are critical for polarity, migration, and directionality (8–14), roles of the neutrophil's microtubule network in these processes are less well understood. Microtubules of a polarized neutrophil radiate toward its leading and trailing edges from an MTOC located just behind, just in front of, or between lobes of the nucleus (15–17). Microtubule-depolymerizing drugs like colchicine and nocodazole induce neutrophils to polarize and crawl in random directions, in the absence of chemoattractant (18, 19); in contrast, the drugs weaken or abolish polarity and migration induced by these drugs in most vertebrate cells. The unusual response to microtubule disruption is accompanied by elevated activities of a small GTPase, Rho, and Rho-dependent phosphorylation of myosin light chains (18, 19), which probably account for the induction of polarity and migration, because both are substantially inhibited by exposure to Y-27632, a pharmacologic inhibitor of a Rho-dependent kinase, p160-ROCK (19). Finally, neutrophils whose microtubules were disrupted by colchicine oriented correctly in a shallow gradient of attractant (9) and reoriented correctly toward an attractant from a micropipette placed one cell length away from the cell (8); in a more stringent test of direction-finding ability, microtubule disruption caused neutrophils to migrate toward spores of Candida albicans in abnormally contorted migration paths with wide turns (20).
To explore the contributions of microtubules to orientation and directional migration, we have studied behavior of differentiated HL-60 (dHL-60) cells, a model neutrophil-like cell line, in gradients of f-Met-Leu-Phe (fMLP), a tripeptide attractant. We find that microtubules normally enhance accuracy and stability of orientation by balancing two spatially distinct and functionally opposed actin assemblies controlled by signaling pathways that diverge downstream of the fMLP receptor: “frontness,” or protrusive F-actin at the front of the cell, regulated by a trimeric G protein, Gi, the small GTPase Rac, and 3′-phosphoinositides such as PIP3 and protrusion of F-actin (11–13); “backness,” or contractile actin-myosin complexes at the back and sides, regulated by different trimeric G proteins, G12 and G13, Rho, p160-ROCK, phosphorylated myosin light chains, and myosin II (14). Effects of nocodazole on frontness and directionality are reversed by a p160-ROCK inhibitor, indicating that nocodazole-induced enhancement of backness impairs directionality by inhibiting frontness.
Materials and Methods
Most materials and methods used in this work have been described in detail in previous work from this laboratory (12, 14); these include culture and differentiation of HL-60 cells, expression of exogenous cDNAs by stable and transient transfection, micropipette assays, fixation and staining for actin, and the Rho-Rhotekin pull-down assay for Rho-GTP. Transwell chemotaxis was assessed as described in ref. 21. Cellular actin and particulate PH-Akt-GFP were measured as described by refs. 22 and 23, respectively. All methods are described in further detail in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Antibodies and Reagents. Sources of reagents and antibodies are described in Supporting Materials and Methods.
Cell Culture, Transfection, and Drug Treatment. Information on transient transfection can be found in Supporting Materials and Methods.
Chemotaxis Assays and Microscopic Analysis. Treated cells and their immunofluorescence analysis are described in Supporting Materials and Methods.
Rho A-GTP Pull-Down Assay. A description of the pull-down assay and the suspension of dHL-60 cells is described in Supporting Materials and Methods.
Actin Polymerization Assay. Procedures concerning the reaction of DHL 60 cells with actin polymerization assay are described in Supporting Materials and Methods.
Assay of Membrane PH-Akt-GFP. Procedures for the interaction of cells stably expressing PH-Akt-GFP can be found in Supporting Materials and Methods.
Results
Intact Microtubules Are Necessary for Efficient Chemotaxis. In the absence of added chemoattractant, nocodazole induced polarity in ≈80% of neutrophils from human peripheral blood (19). Nocodazole induced similar polarity in dHL-60 cells, although in a smaller proportion of cells (21%; Table 1, which is published as supporting information on the PNAS web site). Colchicine, a second microtubule-disrupting agent, induced similar morphologic polarity and migration of dHL-60 cells (data not shown).
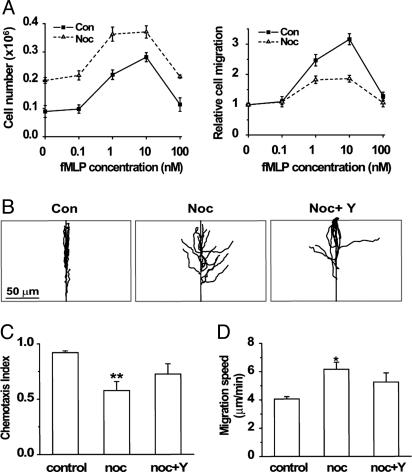
In keeping with nocodazole's ability to induce polarity in the absence of chemoattractant, it doubled the number of dHL-60 cells that migrated, in the absence of chemoattractant, through a filter between the upper and lower chambers of a Transwell device (Fig. 1A Left). In the absence of nocodazole, graded concentrations of fMLP in the lower chamber induced normal dHL-60 cells to migrate from the upper chamber, as reported in ref. 12. In cells exposed to nocodazole, fMLP induced migration to the lower chamber as well (Fig. 1 A Left), albeit less efficiently: at a maximally effective concentration (10 nM), fMLP induced a 0.7-fold stimulation of migration in nocodazole-treated cells vs. 2.1-fold in controls (Fig. 1 A Right).
Fig. 1.
Disrupting microtubules impairs chemotaxis. (A) Transwell migration of dHL-60 cells. Cells treated in suspension with or without nocodazole were allowed to migrate from the upper chamber into the lower chamber of a 24-transwell apparatus (see Supporting Materials and Methods). Lower chambers contained buffer with the indicated concentrations of fMLP. (Left) The number of cells that migrated into the lower chambers. (Right) Relative responses to the indicated concentrations of fMLP: number of control or nocodazole-treated cells were normalized relative to their migration in the absence of fMLP (1.0). Each shows the mean value ± 2 SEM of four separate experiments. (B) Trajectories of cells migrating toward fMLP in a micropipette. Trajectories were tracked with softworx software and realigned relative to the correct direction (directly upward on the y axis). The micropipette was positioned at the center of the top horizontal line (see Movies 1 and 2). (Scale bar: 50 μM.) (C) Chemotactic indices of the cells in B. Bars represent measurements of control, nocodazole-treated (noc), and nocodazole-plus Y-27632-treated cells (noc + Y) cells. Each bar shows the mean chemotactic index ± 2 SEM of n = 15 cells, assessed in three separate experiments; **, mean that differs from that of control cells by P < 0.01. Chemotactic index values were calculated as depicted in Fig. 4. (D) Migration speeds of cells shown in B and C. Bars represent the length of the migration path divided by the travel time. (Note: for cells that stopped migrating upon reaching the micropipette, migration speeds were calculated for times <10 min.) Bars show the mean speed ± 2 SEM of n = 15 cells tested in each group in three different experiments. *, results that differ from controls, at P < 0.05.
In keeping with these results, nocodazole stimulated migratory behavior but impaired directional migration toward a source of attractant. Control dHL-60 cells polarized rapidly and migrated almost straight toward a point source of fMLP supplied by micropipette, as reported in ref. 14, whereas migration paths of nocodazole-treated cells exhibited broad turns and less accurate orientation toward the fMLP source (Movies 1 and 2, which are published as supporting information on the PNAS web site). Fig. 1B depicts these differences in directional behavior by plotting migration paths in comparison to the direct trajectory toward the micropipette. The chemotactic index (the ratio of migration in the correct direction to the actual length of the migration path; see Fig. 4, which is published as supporting information on the PNAS web site) of control cells was much greater than that of nocodazole-treated cells (0.91 vs. 0.58, respectively, P < 0.01; Fig. 1C). Despite their impaired directionality, nocodazole-treated cells migrated ≈50% faster than controls (6.2 vs. 4.1 μm per min, P < 0.05; Fig. 1D).
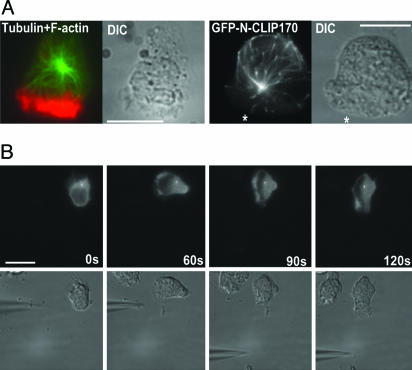
Locations and Behavior of Microtubules and the MTOC. After polarization in response to a 3-min treatment with a uniform concentration (100 nM) of fMLP, 88% of control dHL-60 cells (36 of 41 examined; Table 1) showed prominent F-actin in pseudopods. Arrays of microtubules radiated from a single origin toward back, sides, and front of the cells (Fig. 2A Left); in cells migrating toward a source of chemoattractant, this apparent origin was usually located just posterior to the nucleus. Microtubules directed toward the back and sides were much more numerous than those directed toward the front, as described in ref. 17. In 46 of 51 cells examined, front-directed microtubules terminated at (or before reaching) the cytoplasmic edge of the pseudopod (i.e., F-actin fluorescence); in 5 cells, penetration into the pseudopod could not be assessed, because the apparent origin of the microtubule array was located within or in front of the nucleus, very close to the pseudopod.
Fig. 2.
Microtubules and the MTOC in migrating dHL60 cells. (A) Location and dynamic behavior of microtubules. Left and Left Center show a cell that was stimulated with a uniform concentration (100 nM) of fMLP for 3 min and then fixed and stained for tubulin (green) and F-actin (red) as described in Supporting Materials and Methods. Fluorescence and Nomarski images are shown. Right Center and Right show a cell that transiently expressed GFP-N-Clip170 2 min after challenge with a point source of fMLP (10 μM). *, direction of the micropipette. GFP fluorescence and the corresponding Nomarski image are shown. GFP-N-Clip170 fluorescence in this migrating cell is shown in Movie 4. (B) Location of the MTOC in a polarizing, moving cell. A cell transiently expressing GFP-arrestin-3 was challenged with a micropipette containing 10 μM fMLP, placed in the position shown at time = 0. GFP-arrestin-3 fluorescence and the corresponding Nomarski image are shown. The fMLP source was moved immediately after the 60-sec image to the position shown in Left Center; the pseudopod quickly followed the micropipette, whereas the MTOC remained stationary. (Scale bars: 10 μm.)
Transient expression of a GFP-tagged version of the N-terminal domain of Clip170 (24), which binds to growing ends of microtubules (25), revealed the dynamic behavior of microtubules in living cells: comet-like streaks of GFP-N-Clip170 originated at a common origin (presumably the MTOC) and moved at ≈3 μm/min toward the cell's periphery (Fig. 2 A Right; Movies 3 and 4, which are published as supporting information on the PNAS web site), comets directed toward the back of the cell reached all of the way to the cell periphery and often traveled an additional distance (≈1–3 μm) in a direction parallel to the membrane, and comets directed toward the front of the cell stopped before entering the pseudopod (see Movie 4).
Although the MTOC plays a role in orienting polarity of many cells (4), such a steering role has not been stringently tested in neutrophils (previous studies located the neutrophil MTOC only in fixed cells; see refs. 15 and 16). We fortuitously discovered that a regulator of G protein-coupled receptors, GFP-tagged arrestin-3, serves as a useful MTOC marker in dHL-60 cells, where it colocalizes with the center of the microtubule array and with an MTOC protein (Fig. 5, which is published as supporting information on the PNAS web site).
Experiments with transiently expressed GFP-arrestin-3 (Fig. 2B; Fig. 6, which is published as supporting information on the PNAS web site) showed that the MTOC of dHL-60 cells follows the pseudopod and does not direct its orientation. In dHL-60 cells migrating toward a point source of fMLP, the MTOC marker was usually located behind the nucleus (10 of 12 cells; in two cells it overlapped the nucleus) and moved only after the pseudopod. The cell in Fig. 2B polarized and began to migrate toward the initial position of the micropipette, with its MTOC marker behind the nucleus; relocation of the micropipette at 60 sec induced a 90° change in orientation of the cell's pseudopod within the ensuing 60 sec, whereas the marker remained in precisely its original position. Five other cells subjected to a moving fMLP source similarly showed reorientation of the pseudopod that preceded movement of the MTOC marker by at least 30 sec (one is shown in Fig. 6); Dictyostelium discoideum amoebae migrating toward a moving point source of attractant exhibited similar temporal lags in MTOC reorientation (26).
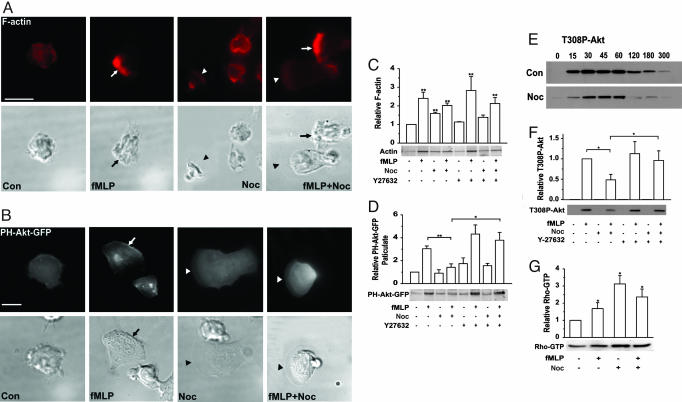
Microtubule Disruption Alters Morphology, F-Actin, and Behavior of dHL-60 Cells. Nocodazole substantially altered the distribution (Fig. 3A) and accumulation (Fig. 3C) of F-actin. In the absence of fMLP, nocodazole alone increased the total amount of cellular F-actin (Fig. 3C) and strikingly altered morphology: a prominent circumferential ring of F-actin was seen in almost all unpolarized nocodazole-treated cells but rarely in unpolarized controls (Fig. 3A and Table 1). These rings probably contain most of the increased accumulation of cellular F-actin (Fig. 3C), because pseudopods of most nocodazole-treated cells that polarized without fMLP appeared flat and thin, with little F-actin fluorescence. This phenotype contrasts with the prominent F-actin in the apparently thicker pseudopods of control cells that polarized in response to fMLP alone (arrows in Fig. 3A indicate the front edge of pseudopods). Pseudopods of nocodazole-treated cells exposed to fMLP showed either of two distinct morphologies (Fig. 3A and Table 1): (i) prominent, thick pseudopods with F-actin fluorescence like that of fMLP-treated control cells, or (ii) flat, thin pseudopods with little F-actin, similar to those of cells that polarized in response to nocodazole alone. In time-lapse movies, individual nocodazole-treated cells alternated between these two morphologies after polarizing in response to fMLP (data not shown).
Fig. 3.
Microtubule disruption alters backness and frontness signals. (A) Cellular distribution of F-actin. Cells preincubated with or without nocodazole (Noc) were exposed (where indicated) to 100 nM fMLP for 3 min (fMLP or fMLP + Noc) and then fixed and stained for F-actin (red). Fluorescence and the corresponding Nomarski images are shown. Arrows point to normal leading edges (irregular ruffles), and arrowheads point to flat, actin-poor lamellae seen at the front-most edges of nocodazole-treated cells. (Scale bar: 10 μm.) Treatment protocols are described in Supporting Materials and Methods. (B) Cellular distribution of PH-Akt-GFP. Cells stably expressing PH-Akt-GFP were pretreated with or without nocodazole and exposed to fMLP as described in A. Arrows point to the leading edge. Fluorescence and Nomarski images are shown. (Scale bar: 10 μm.) (C) Quantitation of F-actin. Cells pretreated with no drug, nocodazole, Y-27632, or nocodazole plus Y-27632 were exposed to 100 nM fMLP for 3 min, and F-actin in Triton X-100 insoluble fractions was quantitated as described in Supporting Materials and Methods. Results are normalized to the density of F-actin in unstimulated control cells (1.0). Bars show the mean ± 2 SEM of n = 4 experiments; **, statistical difference from control at P < 0.01. Coomassie-stained 42-kDa bands of actin from a representative experiment are also shown. (D) Association of PH-Akt-GFP with the particulate fraction. Suspensions of cells stably expressing PH-Akt-GFP were treated for 1 min with or without fMLP after exposure to inhibitors as described in C. PH-Akt-GFP in particulate fractions was assayed as described in the Supporting Materials and Methods. Results are normalized to the density of PH-Akt-GFP band in the unstimulated lane (1.0). The immunoblot shows the result of a representative experiment, and the bars show mean blot densities ± 2 SEM of n = 4 separate experiments. the double and single asterisks indicate pairs of conditions for which the respective results differ significantly at P < 0.01 or P < 0.05, respectively. (E) Time course of fMLP-stimulated Akt phosphorylation. Immunoblots show relative amounts of Akt phosphorylated at Thr-308. Upper and Lower show phospho-Akt densities in immunoblots of extracts from cells treated, respectively, without or with nocodazole and then stimulated with fMLP (100 nM) for the indicated times (seconds). Results are representative of two separate experiments. (F) Akt phosphorylation. Cell suspensions were pretreated, as indicated, without or with nocodazole, Y-27632, or nocodazole plus Y-27632 and then exposed to 100 nM fMLP for 1 min, where indicated. Results are normalized to the density of staining in the lane representing fMLP treatment of control cells (1.0). The immunoblot shows the result of a representative experiment; graphs show the mean blot densities ± 2 SEM of n = 4 separate experiments. *, pairs of conditions that differed significantly at P < 0.05. (G) Nocodazole increases Rho activity. Cell suspensions were treated with or without nocodazole and exposed to uniform fMLP for 1 min as indicated. Rho-GTP was determined by a GST-RBD pull-down assay as described in ref. 14. Results are normalized to the density of the Rho band in the unstimulated lane (1.0). The immunoblot shows the result of a representative experiment, and graphs show mean blot densities ± 2 SEM of n = 4 separate experiments. *, statistical differences from the control at P < 0.05.
The flat, actin-poor pseudopods of polarized nocodazole-treated cells suggested that microtubule disruption may weaken frontness signals. In accord with this idea, fluorescence of PH-Akt-GFP, a PIP3 probe, appeared weak or absent in pseudopods of cells that polarized in response to nocodazole alone, relative to control cells polarized in response to fMLP (Fig. 3B). In nocodazole-treated cells polarized in response to fMLP, PH-Akt-GFP accumulation was also weak in thin, flat pseudopods (Fig. 3B), but in thicker-appearing pseudopods (data not shown), accumulation was similar to that of fMLP-treated control cells.
Because fluorescence of PH-Akt-GFP in photomicrographs may not precisely reflect differences in PIP3 concentration at the plasma membrane, we assessed PIP3 accumulation more quantitatively: cells were treated with nocodazole and/or fMLP, followed by measurement of stably expressed PH-Akt-GFP in particulate extracts (Fig. 3D) or of PIP3-dependent phosphorylation of endogenous Akt (Figs. 3 E and F). By both assays, nocodazole alone failed to activate the PIP3 pathway despite its ability to trigger morphologic polarity in a substantial proportion of cells; nocodazole treatment also reduced the PIP3 response to fMLP, in keeping with our inference from reduced intensities of F-actin or PH-Akt-GFP seen by microscopy.
Microtubule Disruption: Rho-Dependent Effects on Frontness Signals and Chemotaxis. Niggli (19) reported elevated accumulation of Rho and p160-ROCK in membrane extracts of cells treated with colchicine and that pharmacological inhibition of p160-ROCK prevented microtubule-disrupting agents from inducing neutrophil polarity and migration. Effects of microtubule disruption on key components of the backness response triggered by fMLP, in combination with the ability of backness signals to inhibit frontness in dHL-60 cells (14), suggested that nocodazole might impair frontness and chemotaxis of dHL-60 cells by enhancing Rho-dependent backness signals. In keeping with this idea, Rho activity in dHL-60 cells, like that of human neutrophils (19), increases in response to nocodazole (Fig. 3G): the drug alone increased cellular content of the GTP-bound active form of Rho 2.5-fold relative to control cells. Although fMLP alone elevated Rho-GTP in control dHL-60 cells as reported in ref. 14, the attractant slightly reduced the elevated Rho-GTP content of nocodazole-treated cells.
Effects of Y-27632, an inhibitor of p160-ROCK, indicated that nocodazole-induced elevation of Rho-dependent backness accounts for the decreased frontness responses to fMLP: Y-27632 prevented nocodazole from inhibiting fMLP-dependent PIP3 responses, including accumulation of PH-Akt-GFP in particulate fractions of fMLP-treated cells (Fig. 3D) and fMLP-stimulated phosphorylation of endogenous Akt (Fig. 3F).
Reversal of nocodazole-induced inhibition of frontness signals by Y27632 suggested that an excess of backness, relative to frontness, could account for the chemotaxis defect of microtubule-disrupted cells. Indeed, in micropipette experiments Y-27632 strikingly improved the apparent straightness of migration trajectories of nocodazole-treated cells (Figs. 1B) and partially reversed nocodazole-induced reduction of the chemotactic index (Fig. 1C). The inhibitor also partially reversed the nocodazole-induced increase in migration speed (Fig. 1D).
Discussion
Contributions from both the actin and microtubule cytoskeletons are absolutely necessary for yeast cells, fibroblasts, neuroglia, and neurons to polarize and point in the right direction (1–6). In at least one case, microtubules are not only necessary, but can also suffice for a cell polarity and correct orientation: cytochalasin D, an inhibitor of actin polymerization, fails to prevent cultured astrocytes from correctly orienting their MTOCs and microtubule-supported projections toward a wound in the cell monolayer (4). The neutrophil's microtubules clearly play a very different role, because microtubule disruption induces neutrophil polarity rather than preventing it, and impairs, but does not abolish, interpretation of chemoattractant gradients (Fig. 1; ref. 20). These findings raise three interesting questions, discussed below.
How Do Microtubules Suppress Neutrophil Polarity? Microtubules probably suppress neutrophil polarity in several ways, of which the most critical is inhibition of Rho activity: microtubule disruption causes Rho activation (Fig. 3G; ref. 19), inducing morphologic polarity and migration that are, in turn, prevented by inhibiting a Rho-dependent protein kinase (19). Microtubules may normally suppress Rho activity by sequestering (and thereby inactivating) Rho GEFs, several of which associate with intact microtubules in various cell types (27–30). Indirect evidence strongly supports the idea that microtubules do suppress activity of one such GEF, Rho GEF-H1 (31). Rho GEF-H1 mutants that cannot associate with microtubules activate cellular Rho in HeLa cells much more effectively than does the intact GEF, and a dominant-negative Rho GEF-H1 mutant prevents the Rho-dependent morphologic changes induced by microtubule disruption.
In contrast to sequestering and inhibiting Rho GEFs, however, intact microtubules are also thought to deliver such GEFs to appropriate membrane locations, thereby exerting positive regulation of Rho activity. A recent observation in Drosophila S2 cells led to a proposal that delivery of Rho GEF may be regulated by a trimeric G protein (7). In these cells DRhoGEF2 binds to EB1, a protein that specifically associates with the plus (growing) ends of microtubules; expressing a constitutively active mutant of concertina, the α subunit of a trimeric G protein in the fly, causes DRhoGEF2 to dissociate from microtubule plus ends and induces Rho-dependent morphologic changes, mediated by myosin II, identical to those induced by overexpressed exogenous DRhoGEF2. The proposal was not rigorously tested, partly because a ligand and receptor capable of activating endogenous concertina in these cells have not been identified. Moreover, although RNA interference-mediated knockdown of EB1 caused DRhoGEF2 to disappear from microtubule plus ends, the prediction that concertina cannot activate Rho-dependent morphology in the absence of EB1 was not tested (7).
This attractive possibility nonetheless merits further exploration in dHL60 cells, where fMLP triggers Rho-dependent backness through mammalian G12α and G13α (14), two homologs of concertina. To be explicit, we speculate that fMLP induces backness by activating G12α and G13α, which in turn induces dissociation and activation of Rho GEFs brought to the plasma membrane on growing plus ends of microtubules, where it is bound to EB1 (or an EB1-like protein). This idea would account for our observation (Fig. 2 A and Movie 4) that most microtubule plus ends grow toward the plasma membrane at the back and sides of polarized dHL60 cells and for a report (19) that says abolishing microtubule dynamics with taxol reduces backness functions (membrane association of p160-ROCK and tail retraction) in human neutrophils.
Contrasting Roles of Microtubules in Neutrophils and Other Cells. In most cultured cells, Rho GEF isoforms bind to microtubules (27–30), and microtubule disruption increases Rho activity (32) and stimulates its downstream effects on the cytoskeleton (33). Why, then, does microtubule disruption impair polarity of most cells (34, 35) but induce it in neutrophils instead (18, 19)? The difference probably reflects the neutrophil's unusual capacity for rapid, self-organizing polarity, based on robust responses of opposed actin assemblies, protrusive F-actin and contractile actin–myosin complexes, mediating frontness and backness, respectively (14), to spatially uniform stimuli. Such stimuli trigger distinct signals to activate frontness and backness, which segregate into spatially separate cell regions that oppose and balance one another in the new polarized steady state.
As noted above, nocodazole induces polarity primarily by activating backness (Rho- and myosin II-based contraction; refs. 18 and 19); frontness is detectable but weak, producing flat, thin pseudopods that contain less F-actin or PIP3 than those of normal cells polarized in response to fMLP (Fig. 3). Nocodazole may trigger polarity of a neutrophil just as transiently applied external pressure on one side induces polarity of a cytoplast prepared from fish keratocytes (36). In the latter case, a radially symmetrical cell fragment rapidly polarizes by accumulating contractile actin–myosin complexes, parallel to the plasma membrane, on the side where the pressure is applied and protrusive F-actin, perpendicular to the membrane, on the other; the polarized cytoplast then crawls away, its polarity sustained long after removal of the initiating stimulus. The different actin assemblies at the front and back of cytoplasts and, by extension, neutrophils, probably sustain polarity because they are mechanically incompatible locally but reciprocally reinforce one another at a distance.
In such a scenario, we propose that nocodazole-induced Rho activation in neutrophils, like localized external pressure on a cytoplast (36), causes actin–myosin assemblies to array in parallel to the plasma membrane, thereby stimulating them to contract; initial slight, stochastic asymmetry in Rho-dependent contraction at one side of the cell, pushes the opposite side outwards, relieving membrane tension and enhancing formation of radially directed actin polymers in a protruding front (37). If protrusion at the front in turn enhances contraction at the back and vice versa, the cell maintains its polarity and migrates forward. Frontness of a cell polarized primarily by activating Rho would be weaker than that of a cell polarized in response to fMLP, which promotes not only Rho-dependent backness but in addition activates a PIP3- and actin-dependent positive feedback loop at the front.
The cytoskeletal machinery and cellular signaling pathways of professionally crawling cells like neutrophils and fish keratoplasts are poised to organize polarity in response to small perturbations. Neutrophils polarize in response not only to fMLP and microtubule disruption, but also to exposure to a uniform extracellular concentration of exogenous PIP3 (13, 38) or to a pharmacologic inhibitor of the μ isoform of calpain, a cytoplasmic protease (39). Mechanisms of these polarity responses are poorly understood but merit further study. We speculate that exogenous PIP3 may induce polarity in a fashion reciprocal to what we propose for nocodazole: that is, exogenous PIP3 stochastically stimulates protrusive frontness a bit more strongly on one side of the cell, and the mechanochemical coupling machinery responds by promoting contraction on the other side.
How Do Microtubules Enhance Directional Migration? The neutrophil's microtubule cytoskeleton plays ancillary roles not only in its ability to polarize in response to fMLP but also in its interpretation of an fMLP gradient. In a normal neutrophil with intact microtubules, the MTOC follows a turning pseudopod instead of pointing it in the correct direction (Fig. 2B). Moreover, nocodazole-treated neutrophils clearly sense the gradient of fMLP supplied by a stationary micropipette and can turn toward a moving fMLP source, although they follow meandering paths in doing so (Fig. 1 B and C; ref. 20).
How, then, do the neutrophil's microtubules perform their ancillary, but not essential, role in direction finding? Although the mechanistic details remain poorly understood, our results strongly suggest that nocodazole impairs chemotactic efficiency of dHL-60 cells by reducing the PIP3 and F-actin content of their pseudopods. We suspect that the weakening of pseudopods reflects their local inhibition by Rho-dependent backness, induced by microtubule disruption. In this scenario, by disrupting microtubules, nocodazole releases Rho GEFs indiscriminately throughout the cell, allowing them to promote backness everywhere, including the leading edge; in contrast plus ends of growing microtubules deliver Rho GEFs predominantly to the back and sides of normal polarized cells, where most of the plus ends are directed (Fig. 3). Accordingly, inhibiting nocodazole-induced backness with the p160-ROCK inhibitor increases PIP3 at the front of the cell (Fig. 3 C and D) and allows the cells crawl in straighter paths toward an fMLP source (Fig. 1 B and C). Because the backness-inhibiting agent does not completely restore directionality (Fig. 1C), it is possible that microtubule disruption also diminishes microtubule-dependent transport to the pseudopod of vesicles carrying key frontness components, including phosphatidylinositol 3-kinases, GEFs for Rac and Cdc42, actin-polymerizing machinery, and membrane lipids.
Supplementary Material
Acknowledgments
We thank Ron Vale (University of California, San Francisco) for the GFP-N-Clip170 plasmid, Marc G. Caron (Duke University) for the GFP-arrestin-3 plasmid, and Wendell Lim and Dyche Mullins for useful comments on the manuscript. This work was supported in part by National Institutes of Health Grant GM 27800 (to H.R.B.). A.V.K. was a postdoctoral researcher of the Fond National de la Recherche Scientifique of Belgium and is now a postdoctoral fellow of American Heart Association. F.W. was supported by National Institutes of Health Training Grant HL07713, and J.X. was supported by a fellowship from the Leukemia and Lymphoma Society.
Author contributions: H.R.B. designed research; J.X., F.W., A.V.K., and M.R. performed research; M.R. contributed new reagents/analytic tools; J.X., F.W., A.V.K., and H.R.B. analyzed data; and J.X. and H.R.B. wrote the paper.
Abbreviations: dHL-60, differentiated HL-60; fMLP, f-Met-Leu-Phe; GEF, guanine nucleotide exchange factor; MTOC, microtubule-organizing center.
References
- 1.Rodriguez, O. C., Schaefer, A. W., Mandato, C. A., Forscher, P., Bement, W. M. & Waterman-Storer, C. M. (2003) Nat. Cell Biol. 5, 599–609. [DOI] [PubMed] [Google Scholar]
- 2.Wittmann, T. & Waterman-Storer, C. M. (2001) J. Cell Sci. 114, 3795–3803. [DOI] [PubMed] [Google Scholar]
- 3.Daub, H., Gevaert, K., Vandekerckhove, J., Sobel, A. & Hall, A. (2001) J. Biol. Chem. 276, 1677–1680. [DOI] [PubMed] [Google Scholar]
- 4.Etienne-Manneville, S. & Hall, A. (2001) Cell 106, 489–498. [DOI] [PubMed] [Google Scholar]
- 5.Etienne-Manneville, S. & Hall, A. (2003) Nature 421, 753–756. [DOI] [PubMed] [Google Scholar]
- 6.Kodama, A., Lechler, T. & Fuchs, E. (2004) J. Cell Biol. 167, 203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogers, S. L., Rogers, G. C., Sharp, D. J. & Vale, R. D. (2002) J. Cell Biol. 158, 873–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zigmond, S. H., Levitsky, H. I. & Kreel, B. J. (1981) J. Cell Biol. 89, 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zigmond, S. H. (1977) J. Cell Biol. 75, 606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiner, O. D., Servant, G., Parent, C. A., Devreotes, P. N. & Bourne, H. R. (2000) in Cell Polarity: Frontiers in Molecular Biology, ed. Drubin, D. G. (Oxford Univ. Press, Oxford) pp. 201–239.
- 11.Srinivasan, S., Wang, F., Glavas, S., Ott, A., Hofmann, F., Aktories, K., Kalman, D. & Bourne, H. R. (2003) J. Cell Biol. 160, 375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang, F., Herzmark, P., Weiner, O. D., Srinivasan, S., Servant, G. & Bourne, H. R. (2002) Nat. Cell Biol. 4, 513–518. [DOI] [PubMed] [Google Scholar]
- 13.Weiner, O. D., Neilsen, P. O., Prestwich, G. D., Kirschner, M. W., Cantley, L. C. & Bourne, H. R. (2002) Nat. Cell Biol. 4, 509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu, J., Wang, F., Van Keymeulen, A., Herzmark, P., Straight, A., Kelly, K., Takuwa, Y., Sugimoto, N., Mitchison, T. & Bourne, H. R. (2003) Cell 114, 201–214. [DOI] [PubMed] [Google Scholar]
- 15.Anderson, D. C., Wible, L. J., Hughes, B. J., Smith, C. W. & Brinkley, B. R. (1982) Cell 31, 719–729. [DOI] [PubMed] [Google Scholar]
- 16.Schliwa, M., Pryzwansky, K. B. & Euteneuer, U. (1982) Cell 31, 705–717. [DOI] [PubMed] [Google Scholar]
- 17.Eddy, R. J., Pierini, L. M. & Maxfield, F. R. (2002) Mol. Biol. Cell 13, 4470–4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keller, H. U. & Niggli, V. (1993) Cell Motil. Cytoskeleton 25, 10–18. [DOI] [PubMed] [Google Scholar]
- 19.Niggli, V. (2003) J. Cell Sci. 116, 813–822. [DOI] [PubMed] [Google Scholar]
- 20.Allan, R. B. & Wilkinson, P. C. (1978) Exp. Cell Res. 111, 191–203. [DOI] [PubMed] [Google Scholar]
- 21.Arai, H., Tsou, C.-L. & Charo, I. F. (1997) Proc. Natl. Acad. Sci. USA 94, 14495–14499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lacalle, R. A., Gomez-Mouton, C., Barber, D. F., Jimenez-Baranda, S., Mira, E., Martinez, A. C., Carrera, A. C. & Manes, S. (2004) J. Cell Sci. 117, 6207–6215. [DOI] [PubMed] [Google Scholar]
- 23.Steimle, P. A., Yumura, S., Cote, G. P., Medley, Q. G., Polyakov, M. V., Leppert, B. & Egelhoff, T. T. (2001) Curr. Biol. 11, 708–713. [DOI] [PubMed] [Google Scholar]
- 24.Diamantopoulos, G. S., Perez, F., Goodson, H. V., Batelier, G., Melki, R., Kreis, T. E. & Rickard, J. E. (1999) J. Cell Biol. 144, 99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez, F., Diamantopoulos, G. S., Stalder, R. & Kreis, T. E. (1999) Cell 96, 517–527. [DOI] [PubMed] [Google Scholar]
- 26.Ueda, M., Graf, R., MacWilliams, H. K., Schliwa, M. & Euteneuer, U. (1997) Proc. Natl. Acad. Sci. USA 94, 9674–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Horck, F. P., Ahmadian, M. R., Haeusler, L. C., Moolenaar, W. H. & Kranenburg, O. (2001) J. Biol. Chem. 276, 4948–4956. [DOI] [PubMed] [Google Scholar]
- 28.Ren, Y., Li, R., Zheng, Y. & Busch, H. (1998) J. Biol. Chem. 273, 34954–34960. [DOI] [PubMed] [Google Scholar]
- 29.Tatsumoto, T., Xie, X., Blumenthal, R., Okamoto, I. & Miki, T. (1999) J. Cell Biol. 147, 921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glaven, J. A., Whitehead, I., Bagrodia, S., Kay, R. & Cerione, R. A. (1999) J. Biol. Chem. 274, 2279–2285. [DOI] [PubMed] [Google Scholar]
- 31.Krendel, M., Zenke, F. T. & Bokoch, G. M. (2002) Nat. Cell Biol. 4, 294–301. [DOI] [PubMed] [Google Scholar]
- 32.Ren, X. D., Kiosses, W. B. & Schwartz, M. A. (1999) EMBO J. 18, 578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Enomoto, T. (1996) Cell Struct. Funct. 21, 317–326. [DOI] [PubMed] [Google Scholar]
- 34.Glasgow, J. E. & Daniele, R. P. (1994) Cell Motil. Cytoskeleton 27, 88–96. [DOI] [PubMed] [Google Scholar]
- 35.Goldman, R. D. (1971) J. Cell Biol. 51, 752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verkhovsky, A. B., Svitkina, T. M. & Borisy, G. G. (1999) Curr. Biol. 9, 11–20. [DOI] [PubMed] [Google Scholar]
- 37.Kolega, J. (1986) J. Cell Biol. 102, 1400–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niggli, V. (2000) FEBS Lett. 473, 217–221. [DOI] [PubMed] [Google Scholar]
- 39.Lokuta, M. A., Nuzzi, P. A. & Huttenlocher, A. (2003) Proc. Natl. Acad. Sci. USA 100, 4006–4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.