Abstract
It was believed that food poisoning in Osaka in 2000 was due to small amounts of staphylococcal enterotoxin A (SEA) in reconstituted milk. Results of this study clearly indicate that SEH was also present in the raw material of reconstituted milk, indicating that the food poisoning was caused by multiple staphylococcal enterotoxins.
Exotoxins produced by Staphylococcus aureus are enterotoxins and elicit an emetic response. Fourteen staphylococcal enterotoxins, designated staphylococcal enterotoxin A (SEA), SEB, SEC, SED, SEE, SEG, SEH, SEI, SEJ, SEK, SEL, SEM, SEN, and SEO, have been identified (2, 4, 7, 9, 10, 11, 13). However, only the first five (SEA through SEE) can be detected using a commercial kit. Moreover, as commercial kits are generally employed to detect the presence of staphylococcal enterotoxins in food poisoning, SEG, SEH, or SEI was rarely detected and implicated as the source of food poisoning.
A mass outbreak of food poisoning caused by the consumption of reconstituted milk occurred in Osaka, Japan, in June 2000, and more than 10,000 cases were reported (6). A small amount of SEA and sea gene were detected in the reconstituted milk and the skim milk powder, which was the raw material for the reconstituted milk (6). In an outbreak of food poisoning in United States, caused by SEA present in chocolate milk, 200 ng or less SEA was presumed to be the cause (3). Although it was considered that the outbreak of food poisoning in Osaka could have been caused by SEA, the quantity of SEA detected, i.e., approximately 80 ng, was insufficient to cause food poisoning on such a large scale. Hence, we investigated the possibility of staphylococcal enterotoxins other than SEA as the cause of the outbreak.
We got 11 batches of skim milk powder, which were the raw material of the food causing the outbreak. Ten of these batches were manufactured on 1 April 2000 and designated SM1, SM100, SM200, SM300, SM400, SM500, SM600, SM700, SM800, and SM830 in the order of manufacture, and the 11th batch, manufactured on 10 April 2000, was designated SM500 (10/April).
Since the manufacturing process of skim milk required heat treatment for 3 s at 130°C, viable S. aureus strains were not isolated from the skim milk powder samples. However, Gram staining of these samples revealed the presence of gram-positive cocci in large numbers. The skim milk solution (10%, wt/vol) reconstituted in sterile water was centrifuged at 12,000 rpm for 3 min, and the DNA was extracted from the precipitate using a DNeasy tissue kit (QIAGEN GmbH, Hilden, Germany). PCR was performed to detect the sea, seb, sec, sed, see, seg, seh, and sei genes (5, 8). The seb, sec, sed, and see genes were not detected in any of the samples, the sea and seh genes were detected in 10 samples, and the seg and sei genes were detected in 7 samples (Fig. 1 and Table 1).
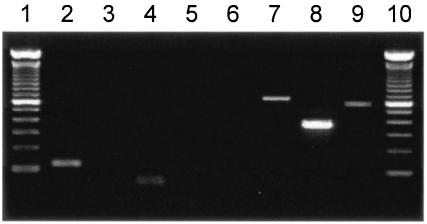
FIG. 1.
Detection of sea, seb, sec, sed, see, seg, seh, and sei genes in SM600 by PCR. Lane 1, 100-bp size marker; lane 2, sea; lane 3, seb; lane 4, sec; lane 5, sed; lane 6, see; lane 7, seg; lane 8, seh; lane 9, sei; lane 10, 100-bp size marker. The sea, seg, seh, and sei genes were detected in SM600.
TABLE 1.
Detection of se-genes encoding SEs in skim milk powder by PCR
| Sample | Presencea of:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| sea | seb | sec | sed | see | seg | seh | sei | |
| SM1 | − | − | − | − | − | − | − | − |
| SM100 | + | − | − | − | − | + | + | + |
| SM200 | + | − | − | − | − | + | + | + |
| SM300 | + | − | − | − | − | − | + | − |
| SM400 | + | − | − | − | − | − | + | − |
| SM500 | + | − | − | − | − | − | + | − |
| SM600 | + | − | − | − | − | + | + | + |
| SM700 | + | − | − | − | − | + | + | + |
| SM800 | + | − | − | − | − | + | + | + |
| SM830 | + | − | − | − | − | + | + | + |
| SM500 (10/April) | + | − | − | − | − | + | + | + |
−, not detected; +, detected.
Since the skim milk samples that contained the sea gene also showed the presence of the seh gene and the seh gene showed stronger expression of enterotoxin compared to that shown by the seg or sei gene (8), there was a high possibility that the skim milk contained more SEH than SEG or SEI. We tried to detect SEH by Western blotting in SM600 and SM700, which had a comparatively larger quantity of SEA (6). One hundred microliters of skim milk solution (10%, wt/vol), prepared in phosphate-buffered saline (137 mM NaCl, 8.1 mM Na2HPO4, 2.68 mM KCl, 1.47 mM KH2PO4 [pH 7.4]), was centrifuged at 15,000 rpm for 30 min. Ten microliters of 2× sample buffer (0.5 M Tris-HCl [pH 6.8], 20% glycerol, 4% sodium dodecyl sulfate, 12% [vol/vol] 2-mercaptoethanol, 0.02% bromophenol blue) was added to 10 μl of the supernatant. The mixture was then heated at 95°C for 3 min and separated in a 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis minigel. We used anti-SEH antibody (SEHS-1; Toxin Technology, Sarasota, Florida) for SEH detection. SEH was detected in both SM600 and SM700 (Fig. 2).
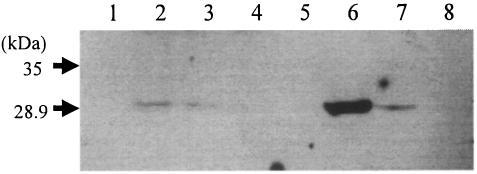
FIG. 2.
Western blot analysis with polyclonal antibody for SEH in skim milk powder. Lane 1, SM1; lane 2, SM600; lane 3, SM700; lane 4, SM500 (10/April); lane 5, skim milk powder (negative control); lane 6, skim milk powder containing SEH at 100 ng/g; lane 7, skim milk powder containing SEH at 10 ng/g; lane 8, skim milk powder containing SEA at 100 ng/g.
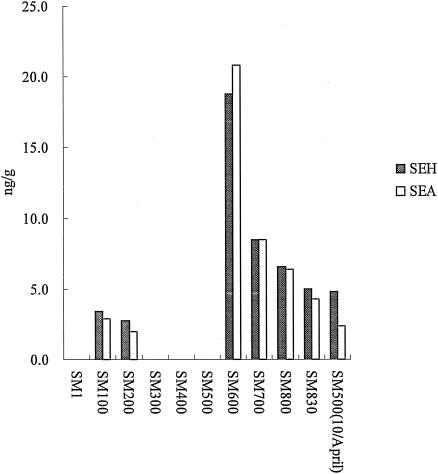
Subsequently, the quantitative detection of SEH was measured in all the samples using enzyme-linked immunosorbent assay (ELISA). The ELISA modified by Su and Wong (12) was performed. For this, anti-SEH antibody (SEHS-1; Toxin Technology)-coated polystyrene microtiter plates (Maxisorp; Nunc, Roskilde, Denmark) were sequentially incubated with skim milk samples and standards (3.125, 6.25, 12.5, 25, and 50 ng/ml of SEH prepared in phosphate-buffered saline), peroxidase-conjugated anti-SEH antibody (LSEHC-1; Toxin Technology), and TMB solution (Promega, Madison, Wisconsin). A 10% solution of each sample was measured three times by ELISA, and the concentration of SEH was determined based on the average value. SEH was detected in the range of 2.8 to 18.8 ng/g in seven samples (Table 2). The quantity of SEH in each sample was almost similar to that of SEA (Fig. 3).
TABLE 2.
Amount of SEH in each sample of skim milk powder determined by ELISA
| Sample | Amt of SEH (ng/g) |
|---|---|
| SM1 | NDa |
| SM100 | 3.4 |
| SM200 | 2.8 |
| SM300 | ND |
| SM400 | ND |
| SM500 | ND |
| SM600 | 18.8 |
| SM700 | 8.5 |
| SM800 | 6.6 |
| SM830 | 5.0 |
| SM500 (10/April) | 4.8 |
ND, not detected.
FIG. 3.
Amounts of SEA and SEH in all 11 samples. The amount of SEH corresponds to that of SEA in all the samples.
Many of the S. aureus strains with the seh gene had either the sea or the seb genes (8). Since these strains produce not only SEH but also SEA or SEB in many cases, the influence of only SEH on food poisoning cannot be accurately evaluated. The 50% effective dose of SEA is 1 μg in humans and 5 μg in monkeys, and it has been reported that approximately 100 to 200 ng of SEA is sufficient for the onset of food poisoning (1, 3). On the other hand, since the 50% effective dose of SEH in humans or monkeys has not been accurately determined, the toxicity of SEH to humans cannot be compared to the toxicity of SEA. However, it can be presumed that the outbreak of food poisoning in Osaka was caused by small amounts of SEA and SEH, since SEA and SEH were present in almost equal quantities in all the skim milk powder samples and 30 μg of SEH elicited emetic responses in monkeys between 1.5 to 3 h after its administration (11).
It was believed that this food poisoning was caused by small amounts of SEA in reconstituted milk (6). Therefore, after the outbreak of food poisoning in Osaka a method to concentrate SEA present in the skim milk powder or reconstituted milk was developed so that even small amounts of SEA could be detected. However, since only small amounts of SEA were detected in the samples, it is presumed that other staphylococcal enterotoxins might have contributed to the outbreak. Hence, in case of food poisoning due to S. aureus enterotoxin, it is essential to employ methods other than that used by commercial kits since SEG, SEH, and SEI cannot be detected by these kits.
REFERENCES
- 1.Bergdoll, M. S. 1979. Staphylococcal infections, p. 443-494. In H. Riemann and F. L. Bryan (ed.), Food-borne infections and intoxications, 2nd ed. Academic Press, Inc., New York, N.Y.
- 2.Bergdoll, M. S. 1983. Enterotoxins, p. 559-598. In C. S. F. Easton and C. Adlam (ed.), Staphylococci and staphylococcal infections. Academic Press, London, United Kingdom.
- 3.Evenson, M. L., M. W. Hinds, R. S. Bernstein, and M. S. Bergdoll. 1988. Estimation of human dose of staphylococcal enterotoxin A from a large outbreak of staphylococcal food. Int. J. Food Microbiol. 31:311-316. [DOI] [PubMed] [Google Scholar]
- 4.Jarraud, S., M. A. Peyrat, A. Lim, A. Tristan, M. Bes, C. Mougel, J. Etienne, F. Vandenesch, M. Bonneville, and G. Lina. 2001. egc, a highly prevalent operon of enterotoxin gene, forms a putative nursery of superantigens in Staphylococcus aureus. J. Immunol. 166:669-677. [DOI] [PubMed] [Google Scholar]
- 5.Johnson, W. M., S. D. Tyler, E. P. Ewan, F. E. Ashton, D. R. Pollard, and K. R. Rozee. 1991. Detection of genes for enterotoxins, exfoliative toxins, and toxic shock syndrome toxin 1 in Staphylococcus aureus by the polymerase chain reaction. J. Clin. Microbiol. 29:426-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ministry of Health and Welfare, Osaka City. 2001. A massive outbreak of Staphylococcus aureus enterotoxin A associated with milk product. Food Sanitation Res. 51:17-36. [Google Scholar]
- 7.Munson, S. H., M. T. Tremaine, M. J. Beteley, and R. A. Welch. 1998. Identification and characterization of staphylococcal enterotoxin type G and I from Staphylococcus aureus. Immunity 66:3337-3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omoe, K., M. Ishikawa, Y. Shimoda, D. L. Hu, S. Ueda, and K. Shinagawa. 2002. Detection of seg, seh, and sei genes in Staphylococcus aureus isolates and determination of the enterotoxin productivities of S. aureus isolates harboring seg, seh, or sei genes. J. Clin. Microbiol. 40:857-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orwin, P. M., D. Y. M. Leung, H. L. Donahue, R. P. Novick, and P. M. Schlievert. 2001. Biochemical and biological properties of staphylococcal enterotoxin K. Infect. Immun. 69:360-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren, K., J. D. Bannan, V. Pancholi, A. L. Cheung, J. C. Robbins, V. A. Fischetti, and J. B. Zabriskie. 1994. Characterization and biological properties of a new staphylococcal enterotoxin. J. Exp. Med. 180:1675-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su, Y.-C., and A. C. L. Wong. 1995. Identification and purification of a new staphylococcal enterotoxin, H. Appl. Environ. Microbiol. 61:1438-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su, Y.-C., and A. C. L. Wong. 1995. Detection of staphylococcal enterotoxin H by an enzyme-linked immunosorbent assay. J. Food Prot. 59:327-330. [DOI] [PubMed] [Google Scholar]
- 13.Zhang, S., J. J. Iandolo, and G. C. Stewart. 1998. The enterotoxin D plasmid of Staphylococcus aureus encodes a second enterotoxin determinant (sej). FEMS Microbiol. Lett. 168:227-233. [DOI] [PubMed] [Google Scholar]