Highlights
-
•
Novel phase II Trial: adrenomedullin in patients with CADASIL.
-
•
Vasoactive peptide therapy for hereditary small vessel disease.
-
•
AdrenoMedullin for CADASIL: a multicenter evaluation of efficacy and safety.
-
•
Non-invasive cerebral blood flow assessment with MRI-ASL.
-
•
Promising insights into CADASIL management potential.
Keywords: Adrenomedullin, Arterial spin labeling, Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, Cerebral blood flow, Clinical trial
Abstract
Background
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), the most common form of hereditary cerebral small vessel disease (SVD), currently lacks disease-modifying treatments. Adrenomedullin (AM), a vasoactive peptide with angiogenic, vasodilatory, anti-inflammatory, and anti-oxidative properties, shows potential effects on the neuro-glial-vascular unit.
Objective
The AdrenoMedullin for CADASIL (AMCAD) study aims to assess the efficacy and safety of AM in patients with CADASIL.
Sample size
Overall, 60 patients will be recruited.
Methods
The AMCAD is a multicenter, investigator-initiated, single-arm phase II trial. Patients with a confirmed CADASIL diagnosis, based on NOTCH3 genetic testing, will receive an 8-h AM treatment (15 ng/kg/min) for 14 days following a baseline assessment (from day 1 to day 14). Follow-up evaluations will be performed on days 15, 28, 90, and 180.
Study outcomes
The primary endpoint is the cerebral blood flow change rate in the frontal cortex, evaluated using arterial spin labeling magnetic resonance imaging, from baseline to day 28. Summary statistics, 95% confidence intervals, and a one-sample t-test will be used for analysis.
Conclusion
The AMCAD study aims to represent the therapeutic potential of AM in patients with CADASIL, addressing an unmet medical need in this challenging condition.
Clinical Trial Registration
jRCT 2,051,210,117 (https://jrct.niph.go.jp/en-latest-detail/jRCT2051210117).
1. Introduction
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is the most common hereditary cerebral small vessel disease (SVD) and is caused by mutations in NOTCH3. CADASIL leads to the development of lacunar infarcts and vascular cognitive impairment, but disease-modifying treatments are currently lacking [1]. White matter (WM) hyperintensities on magnetic resonance imaging (MRI) are the earliest and most common MRI abnormalities observed in patients with CADASIL. Importantly, the WM lesions are not pathologically uniform conditions but rather diverse abnormalities in the brain [2]. Reduced cerebral blood flow (CBF) is a contributing factor to the development of WM lesions [3]. Furthermore, disruptions in the differentiation of oligodendrocyte precursor cells (OPCs) into mature oligodendrocytes also play a role in the formation of WM lesions. This suggests that preventing impaired oligovascular unit function is a promising therapeutic approach for CADASIL [4].
Adrenomedullin (AM) is a bioactive peptide initially discovered in human pheochromocytoma [5]. Within the cerebral vasculature, endothelial cells are the primary source of AM secretion, and AM can have multiple sites of action on the neuro-glial-vascular unit [6] by binding to a heterodimer receptor composed of the calcitonin receptor-like receptor (CLR) and receptor activity-modifying protein (RAMP) type 2 or 3 [7]. Based on these modes of action, AM has exhibited angiogenic, vasodilatory, anti-inflammatory, and anti-oxidative properties and has shown promise in mitigating WM damage in a mouse model of cerebral hypoperfusion [8,9]. Consistent with the angiogenic properties of AM, deletions of AM or RAMP2 lead to severe brain edema with hemorrhage and fetal lethality due to vascular fragility [10,11]. AM promotes the differentiation of OPCs into mature oligodendrocytes, even under hypoxic conditions, and protects against WM pathology [7,12].
Therefore, we hypothesized that AM can restore the disrupted oligovascular unit in CADASIL through its essential effects on OPCs and endothelial cells, leading to oligovascular protection. To establish the clinical proof-of-concept of AM against CADASIL, an investigator-initiated clinical trial, the AdrenoMedullin for CADASIL (AMCAD) study, was designed. Thus, AM is expected to be an innovative therapeutic drug for CADASIL.
2. Material and methods
2.1. Trial design
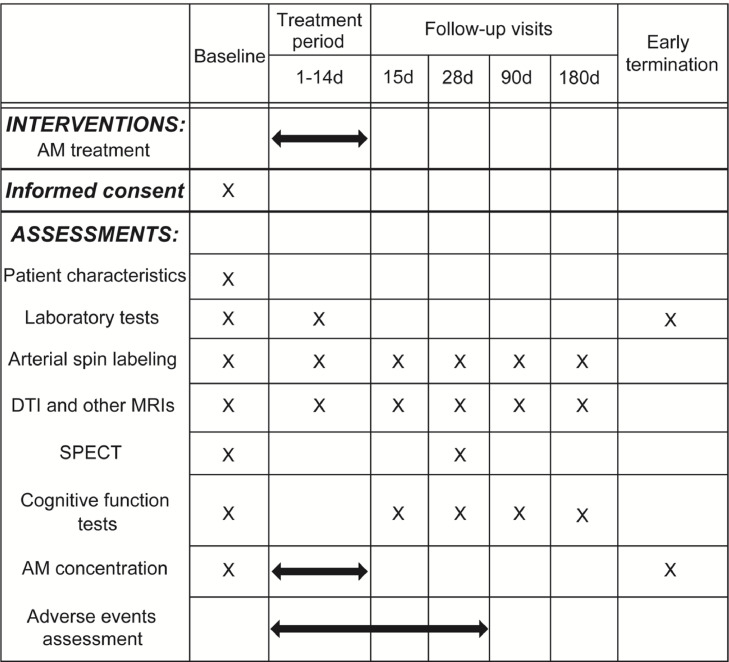
The AMCAD study is an investigator-initiated, multicenter, phase II single-arm trial. Approval for the study protocol and related documents was granted by the institutional review board of the National Cerebral and Cardiovascular Center and Mie University. This study will be conducted in accordance with the Helsinki Declaration as revised in 2013, the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use Guidelines for Good Clinical Practice, and Japan Pharmaceutical Affairs Law. Written informed consent will be secured from all participants prior to the initiation of study procedures by the investigators. The AMCAD study is scheduled to run from November 2021 to March 2024. The schedule of interventions and assessments in the AMCAD study is detailed in Fig. 1.
Fig. 1.
The schedule of interventions and assessments in the AMCAD study. Abbreviations: AM, Adrenomedullin; DTI, Diffusion tensor imaging; SPECT, Single photon emission computed tomography.
2.2. Eligibility
The study will enroll 60 patients with CADASIL who have pathogenic NOTCH3 mutations. Detailed eligibility criteria are listed in Table 1.
Table 1.
Eligibility criteria.
| Inclusion criteria | |
| 1) | Written informed consent for participation in clinical trial |
| 2) | Age between 20 and 90 years at the time of obtaining consent |
| 3) | Diagnosis of CADASIL after confirming NOTCH3 gene mutation by genetic testing |
| 4) | Mini-Mental State Examination (MMSE) score of 10–27 points or Trail Making Test-B score of average (age adjustment) +1.5 standard deviation or higher |
| Exclusion criteria | |
| 1) | Patients who cannot perform cognitive function tests (owing to deafness, blindness, severe cognitive impairment with MMSE score <10 points, etc.) |
| 2) | Patients who receive treatment with prohibited drugs or prohibited therapy within 12 weeks from the time of registration |
| 3) | Patients who started to take concomitant restriction drugs including calcium channel blockers or changed the dosage of concomitant restriction drugs within the past 4 weeks from the time of registration |
| 4) | MMSE score with an improvement of ≥ 4 points conducted twice at least 4 weeks apart during the time of screening (in patients receiving concomitant restriction drugs at the time of registration) |
| 5) | Active infections requiring antibiotic treatment |
| 6) | Physical disability equivalent to modified Rankin Scale score of 5 |
| 7) | Severe impaired consciousness (Japan Coma Scale score of ≥ 100) |
| 8) | Severe renal dysfunction (estimated glomerular filtration rate <30 mL/min/1.73m2) |
| 9) | Severe liver damage (aspartate aminotransferase or alanine aminotransferase ≥100 IU/L) |
| 10) | Diagnosis of cerebral infarction, intracranial hemorrhage, transient ischemic attack, or cerebral aneurysm with high probability of rupture within 12 weeks from the time of registration |
| 11) | Occlusion or severe stenosis of the intracranial main artery or carotid artery |
| 12) | Significant electrocardiogram abnormalities (atrioventricular block of 2–3°, QRS interval ≥120 ms, QTcB ≥450 ms) at the time of registration, or a history of acute coronary syndrome or acute heart failure within the 12 weeks from the time of registration |
| 13) | Systolic blood pressure <100 mmHg |
| 14) | Heart rate <45 beats/min or ≥120 beats/min |
| 15) | Drug abuse or alcoholism |
| 16) | Patients who cannot undergo head magnetic resonance imaging |
| 17) | Active solid malignant tumor |
| 18) | Patients who do not give consent to contraception from the date of obtaining consent until the end of the safety evaluation period |
| 19) | Pregnant, lactating, and possibly pregnant individuals |
| 20) | Patient who participated in another clinical trial within the past 24 weeks from the time of registration |
| 21) | Other patients judged ineligible for this study by the investigators |
2.3. Endpoints
The primary endpoint is the CBF change rate in the frontal cortex evaluated by arterial spin labeling (ASL) magnetic resonance imaging (MRI), from baseline to day 28. A central committee for imaging evaluation, comprising trained neurologists and neuroradiologists, will independently conduct the CBF assessments. Additionally, we will evaluate WM integrity, WM hyperintensities, and brain atrophy as secondary or exploratory endpoints following the recommendations outlined in the Framework for Clinical Trials in Cerebral SVD released by the International Society of Vascular Behavioral and Cognitive Disorders [13]. The secondary and exploratory endpoints are detailed in Table 2.
Table 2.
Primary, secondary, and exploratory endpoints.
| Primary endpoint | |
| 1) | Cerebral blood flow change rate in the frontal cortex, evaluated by arterial spin labeling magnetic resonance imaging (MRI), from baseline to day 28 |
| Secondary endpoints | |
| 1) | Serious adverse events for which a causal relationship cannot be denied |
| 2) | Cerebral blood flow change rate in the frontal cortex, evaluated by arterial spin labeling MRI, from baseline to 8 h after starting adrenomedullin administration and from baseline to days 15, 90 and 180 |
| 3) | Cerebral blood flow change rate, evaluated by arterial spin labeling MRI, from baseline to 8 h after starting adrenomedullin administration and from baseline to days 15, 28, 90 and 180 (whole brain mean and each area) |
| 4) | Mean diffusivity change rate in the white matter, evaluated by diffusion tensor imaging from baseline to 8 h after starting adrenomedullin administration and from baseline to days 15, 28, 90 and 180 (whole brain mean and each area) |
| 5) | Fractional anisotropy change rate in the white matter, evaluated by diffusion tensor imaging from baseline to 8 h after starting adrenomedullin administration and from baseline to days 15, 28, 90 and 180 (whole brain mean and each area) |
| 6) | Trail Making Test-A and B score change from baseline to days 15, 28, 90 and 180 |
| 7) | Montreal cognitive assessment score change from baseline to days 15, 28, 90 and 180 |
| 8) | Digit span and digit symbol subtests of Wechsler Adult Intelligence Scale-Fourth edition score change from baseline to days 15, 28, 90 and 180 |
| 9) | Occurrence of cerebral infarction at 8 h after starting adrenomedullin administration and on days 15, 28, 90 and 180 |
| 10) | Cerebral blood flow change rate in the frontal lobe, evaluated by single photon emission computed tomography, from baseline to day 28 |
| Other endpoints | |
| 1) | Clinical examinations |
| 2) | Vital signs |
| 3) | Cognitive function tests: Disability Assessment for Dementia, Clinical Dementia Rating, Center for Epidemiologic Studies Depression Scale and Apathy scale |
| Exploratory endpoints | |
| 1) | Adrenomedullin blood concentration on days 1, 7, and 14 |
| 2) | Adrenomedullin dose intensity at 8 h after starting adrenomedullin administration and on day 15 |
| 3) | Confirmation of robustness of primary endpoint using different image analysis program |
| 4) | Volume change rate of the white matter lesion, evaluated by fluid-attenuated inversion recovery image, from baseline to 8 h after starting adrenomedullin administration and from baseline to days 15, 28, 90 and 180 |
| 5) | Volume change rate of the whole brain, evaluated by 3D T1WI, from baseline to 8 h after starting adrenomedullin administration and from baseline to days 15, 28, 90 and 180 |
2.4. Intervention
All enrolled patients will receive 15 ng/kg/min AM for 8 h/day for 14 days (from day 1 to day 14). AM will be produced in accordance with the guidelines for good manufacturing practices. Bulk AM powder, chemically synthesized at the Peptide Institute in Osaka, Japan, will be dissolved in a solution containing d-mannitol and formulated as a freeze-dried material at Fuji Yakuhin in Toyama, Japan. Each vial of AM containing 500 μg AM and 50 mg d-mannitol will be stored at temperatures ranging from 2°C to 8°C. AM will be administered intravenously. In cases where systolic blood pressure falls below 100 mmHg or severe headache or palpitations occur, dose reduction or discontinuation of AM will be implemented.
2.5. Imaging protocol
All MRI examinations at each facility will be conducted using a single 3-T MRI scanner with a 32-channel head coil (Ingenia Elition X, Philips, Netherlands). All slices will be parallel to the anterior commissure - posterior commissure (AC-PC) line. CBF will be evaluated using pseudo-continuous ASL (pCASL) following the consensus recommendations [14,15]. The assessments will take place before and 8 h after the initiation of AM treatment, and on days 15, 28, 90, and 180. The sequence parameters that will be employed for pCASL are as follows: axial scan plane, echo time (TE) / repetition time (TR) = 12 / 4219 ms, field-of-view (FOV): 240 × 240 mm, voxel size: 3.75 × 3.75 × 6 mm3, 26 slices; labeling duration, 1800 ms; post-labeling delay, 2000 ms; flip angle, 90°; and dynamic scans, 8 times. The sequence parameters that will be employed for 3D T1 weighted images (3D T1WI) are as follows: sagittal scan plane, TE / TR = 2.3 / 8.0 ms, FOV: 250 × 250 mm, voxel size: 1 × 1 × 1 mm3, 320 slices, inversion time: 1050 ms, and flip angle: 8° The 3D fluid-attenuated inversion recovery (FLAIR) sequence parameters that will be used are as follows: axial scan plane, TE/TR = 285 / 4800 ms, FOV: 230 × 198 mm2, voxel size: 1 × 1 × 1.2 mm3, 250 slices, and inversion time: 1650 ms. Diffusion tensor imaging (DTI) data will be acquired with spin-echo-planar imaging with the following parameters: axial scan plane, TE/TR = 99/10,794 ms, FOV: 256 × 256 mm, voxel size: 2 × 2 × 2 mm3, 80 slices, b-value: 1000 s/mm2, including b0 images and 32 gradient directions, and flip angle: 90°
2.6. Measurement of regional cerebral blood flow
Image processing for the structural and ASL images will be performed using ExploreASL, a dedicated toolbox that provides an automated analysis pipeline for single- or multicenter ASL studies [16]. This toolbox [17] is based on MATLAB (MathWorks, MA, USA), statistical parametric mapping (SPM) 12 (Wellcome Trust Centre for Neuroimaging, University College London, UK), and diffeomorphic anatomical registration analysis using exponentiated lie algebra (DARTEL) [18]. Structural processing, requiring T1WI and FLAIR images, will employ lesion segmentation tool-based WM hyperintensity lesion-filling [19] of the 3D T1WI. The 3D T1WI will be segmented into gray matter (GM), WM, and cerebrospinal fluid (CSF) and registered to the standard Montreal Neurological Institute (MNI) space. The ASL images will undergo processing involving motion correction, motion outlier detection, and rigid-body registration to the GM segmentation derived from the 3D T1WI. The average GM CBF will be calculated for the GM regions of interest (ROIs). The ROIs will be defined as voxels with a partial GM content of over 70%. The average CBF for the whole brain cortex, entire deep WM, and each specific cortex (frontal lobe, temporal lobe, occipital lobe, parietal lobe, caudate, putamen, and thalamus) will be defined using Hammers atlas [20]. CBF change rate (%) will be calculated using the following formula:
2.7. Diffusion tensor imaging (DTI) data processing
FSL v6.0.3 (Functional MRI of the BRAIN Software Library [FMRIB]) [21] will be used for DTI data preprocessing. Head motion artifacts and eddy current distortions will be corrected using eddy correct. Following the correction, fractional anisotropy (FA) and mean diffusivity (MD) will be computed using dtifit. Subsequently, the FA and MD images will be spatially normalized to the MNI space using FMRIB's linear image registration tool and FMRIB's non-linear image registration tool. Regional diffusion values will be extracted from the Johns Hopkins University DTI-based WM atlas [22], and the whole-brain FA and MD values will be calculated by averaging the values across regions. DTI analysis will be conducted independently by the central committee for imaging evaluations.
2.8. Single photon emission computed tomography (SPECT)
SPECT will be performed using a technetium-99 m ethyl cysteinate dimer (99mTc-ECD) (PDRadiopharma Inc., Tokyo, Japan). The dual-head SPECT systems Symbia Evo (Siemens Healthcare, Tokyo, Japan), Discovery NM/CT670, or NM 830 (GE Healthcare, Tokyo, Japan) equipped with a low-energy high-resolution collimator will be used. The mean CBF will be quantified using the Patlak plot method [23,24]. The brain perfusion index (BPI) will be calculated using the time-activity curves of the aortic arch and brain. Quantitative CBF images will be obtained from the reconstructed images using Patlak plot graphical analysis and Lassen's correction algorithm [23,25,26]. SPECT analysis will be performed independently by the central committee for imaging evaluations.
2.9. Cognitive testing
Neuropsychological tests will be administered before the initiation of AM treatment and on days 15, 28, 90, and 180. The tests will include Trail Making Test-A/B, Montreal Cognitive Assessment, Digit span and digit symbol subtests of Wechsler Adult Intelligence Scale-Fourth Edition, Disability Assessment for Dementia, Clinical Dementia Rating, Center for Epidemiologic Studies Depression Scale, and Apathy scale. Additionally, a Mini-Mental State Examination is planned for during the eligibility assessments.
2.10. Safety profile
The safety of AM will be assessed by monitoring adverse events (AEs) that may occur between days 1 and 28. AEs will be categorized by the primary investigator based on expectedness, seriousness, severity, and causality. Serious AEs encompass events resulting in death, life-threatening situations, inpatient hospitalization, extended hospital stays, or substantial persistent disabilities. The severity of AEs will be determined based on their interference with daily activities. Causality was designated as "not ruled out" or "ruled out." An independent data monitoring committee can discuss serious AEs and potential protocol revisions.
2.11. Data collection
A web-based electronic data capture system will gather clinical data from patient medical records. Trained personnel assigned to data management will perform quality control at every step to ensure data reliability. The principal investigator is responsible for the integrity and accuracy of the data analysis.
2.12. Statistical analyses
Efficacy analysis will be performed for full analysis and per-protocol sets. The full analysis set will include all participants who receive at least one protocol treatment and have at least one observed efficacy endpoint after study entry. The per-protocol set encompasses participants within the full analysis set without major protocol deviations. A safety analysis will be performed for the safety analysis set, which will comprise participants receiving the protocol treatment at least once.
The primary endpoint is the CBF change rate in the frontal cortex, evaluated using ASL MRI, from baseline to day 28. Summary statistics and 95% confidence intervals will be calculated and a one-sample t-test will be employed for primary endpoint analysis. Furthermore, summary statistics and 95% confidence intervals will be computed for secondary and exploratory endpoints.
Statistical software SAS, release version 9.4 or higher (SAS Institute Inc., Cary, NC, USA), will be used for all statistical analyses. The significance level will be set at Two-sided p < 0.05.
2.13. Sample size estimates
Basic experiments have indicated that AM gradually restores reduced CBF by approximately 10%–15% [9]. Using this as a reference, it was deemed reasonable to assume that treating patients with CADASIL using AM for 14 days would yield an approximately 10% CBF improvement after 28 days. In a mouse model, the standard deviation of the CBF increment from the baseline at 14 days following common carotid artery occlusion was 6.37% [9]. Although comprehensive data on CBF changes in human patients with CADASIL were not available, a one-sample t-test at a two-sided significance level of 5% with an expected difference of 10% and a standard deviation of 6.37% at 2 weeks post-treatment indicated that a sample size of approximately 40 participants would yield almost 100% statistical power. Considering the feasibility and dropout rates, the target enrollment has been set at 60 participants.
2.14. Rationale for dose selection
In several mouse models of chronic cerebral hypoperfusion and cerebral infarction [9,27], AM was intraperitoneally injected at a rate of 50 ng/h, corresponding to approximately 15 ng/kg/min in humans, which is the dose of AM in the current AMCAD study. Several studies [9,27,28,29] have demonstrated that achieving a four-fold increase in AM blood concentration from the baseline through treatment is necessary to obtain beneficial effects against chronic cerebral ischemia. A phase I study reported the pharmacodynamic properties and safety of AM treatment at doses of 3, 9, and 15 ng/kg/min over a 12-h period in healthy adults. The study showed that a four-fold increase in AM blood concentration was achieved with the 15 ng/kg/min dosage, but not the 9 ng/kg/min dose [30]. In another study, 15 ng/kg/min AM treatment for 27 h resulted in an over four-fold increase in AM concentration in patients with chronic ischemic stroke. A phase II trial demonstrated the safety of 15 ng/kg/min AM treatment for 8 h over 14 days in patients with ulcerative colitis [31]. Based on these findings, we decided to employ a treatment protocol of 15 ng/kg/min AM for 8 h daily for 14 days.
3. Discussion
In this multicenter, phase II study, we aim to investigate whether AM increases CBF in patients with CADASIL. We will enroll patients with a Mini-Mental State Examination score of 10–27 points or a Trail Making Test part-B time equal to or higher than 1.5 with standard deviations above the average for their ages (Table 1). These inclusion criteria are based on a previous clinical trial involving donepezil treatment for CADASIL patients [32] and indicate that AM will be administered to patients with mild or moderate CADASIL.
The primary endpoint of this study will be the CBF change rate in the frontal cortex, evaluated using ASL. CBF reduction in the cortex was reported in transgenic NOTCH3R169C mice [33], and pronounced in the frontal area on SPECT images in patients with CADASIL [34]. CADASIL is a vascular disease of the brain but not exclusively of the white matter. Consistent with this, a previous ASL study, 6% of CBF decline per 2 years in the cortex was demonstrated in patients with CADASIL [35]. ASL is advantageous as it uses endogenous tracers [14] and does not require the injection of radioisotopes or gadolinium compounds. Multiple studies have shown that ASL is a precise and reliable method for quantitatively measuring CBF. Recent developments have further enhanced the accuracy of ASL with improved pulse sequences, resulting in enhanced signal-to-noise ratios and spatial resolution [36]. Several studies [37], including a clinical trial [38], showed that ASL could provide unparalleled advantages with high reliability (intraclass correlation coefficient > 0.95) and low within-participant variability (coefficient of variation ≤ 7.5%). For evaluating CBF, [15O]-water positron emission tomography (PET) has occasionally been used. However, the scan–rescan reproducibility of ASL in the same subjects is consistently better than that of [15O]-water PET [36], which is crucial for accurately interpreting longitudinal studies.
Image processing for the structural and ASL images will be performed using ExploreASL. The utilization of T1WI and FLAIR images for structural processing allows the application of separate models to GM and WM, leading to further enhanced reproducibility of CBF measurement by ASL [16]. These advancements have led to the widespread adoption of ASL in both research and clinical settings. In the PASTIS trial, in which tadalafil was administered to patients with sporadic SVD, ASL was employed as the primary endpoint [39,40]. Another study reported that cerebral perfusion status in patients with CADASIL, as measured by ASL, exhibited a stronger association with global cognitive function than WM integrity, as measured by DTI [41]. On the other hand, ASL is a technique that magnetically labels arterial blood at the level of the bilateral carotid arteries, implying that accurately assessing CBF in the posterior circulation region is challenging. Based on these findings, we concluded that measuring cortical CBF changes in the frontal lobe by using ASL is the most suitable method for evaluating effects of AM in patients with CADASIL.
This study will also assess the safety of AM in patients with CADASIL by monitoring AEs that may occur between days 1 and 28, because the half-life of AM is only less than 30 min [30]. There is a growing body of evidence supporting the safety of AM, based on phase I studies involving healthy volunteers [30] and phase II trials involving patients with ulcerative colitis and Crohn's disease [31,42]. However, the safety of AM in cerebrovascular diseases remains uncertain. AM, known for its ability to induce vasodilation and lower blood pressure [7], may theoretically worsen cerebral hypoperfusion and infarcts. The angiogenic properties of AM may raise concerns about an increased risk of cerebral hemorrhage, which is a common manifestation of CADASIL, particularly in East Asian populations [43]. Although our preclinical experiments with mice overexpressing AM in the systemic circulation have shown restoration of CBF without any damaging effects on the brain, the safety of AM in patients should be confirmed through clinical trials.
4. Conclusions
The AMCAD study will uncover the efficacy and safety of AM in patients with CADASIL.
Funding
This study was funded by Japan Agency for Medical Research and Development (AMED), grant number JP21ek0109516. This study was also funded by the SICORP A*STAR-AMED, grant number JP17jm0210053.
CRediT authorship contribution statement
Kazuo Washida: Writing – original draft, Methodology, Investigation. Satoshi Saito: Writing – review & editing, Project administration, Methodology, Investigation. Tomotaka Tanaka: Project administration, Methodology. Yuriko Nakaoku: Project administration, Methodology. Hiroyuki Ishiyama: Project administration, Methodology. Soichiro Abe: Project administration, Methodology. Takehito Kuroda: Project administration, Methodology. Shinsaku Nakazawa: Project administration, Methodology. Chikage Kakuta: Project administration, Methodology. Katsuhiro Omae: Methodology, Investigation, Formal analysis. Kenta Tanaka: Investigation, Formal analysis. Manabu Minami: Methodology, Investigation, Formal analysis. Yoshiaki Morita: Project administration, Methodology. Tetsuya Fukuda: Project administration, Methodology. Akihiro Shindo: Project administration, Investigation. Takakuni Maki: Methodology, Investigation. Kazuo Kitamura: Project administration, Methodology, Investigation. Hidekazu Tomimoto: Supervision, Project administration, Investigation. Toshihiko Aso: Project administration, Methodology, Investigation. Masafumi Ihara: Writing – review & editing, Supervision, Project administration, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
The authors declare that Kitamura K. own stock in Himuka AM Pharma Corporation, a company aiming to develop adrenomedullin and its derivatives as a novel drug.
Acknowledgments
We would like to thank Ms. Imazato, Mr. Kakuta, Ms. Hirase, Ms. Ohashi, and Ms. Wada for their technical assistances.
References
- 1.Chabriat H., Joutel A., Dichgans M., Tournier-Lasserve E., Bousser MG. Cadasil. Lancet Neurol. 2009;8(7):643–653. doi: 10.1016/s1474-4422(09)70127-9. [DOI] [PubMed] [Google Scholar]
- 2.Rajani R.M., Ratelade J., Domenga-Denier V., et al. Blood brain barrier leakage is not a consistent feature of white matter lesions in CADASIL. Acta Neuropathol. Commun. 2019;7(1):187. doi: 10.1186/s40478-019-0844-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang R., Zhang J., Shang J., Wang F., Yan X. Effects of different regional cerebral blood flow on white matter hyperintensity in CADASIL patients. J. Biomed. Res. 2022;36(5):368–374. doi: 10.7555/jbr.36.20220006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaucker A., Mercurio S., Sternheim N., Talbot W.S., Marlow F.L. notch3 is essential for oligodendrocyte development and vascular integrity in zebrafish. Dis. Model Mech. 2013;6(5):1246–1259. doi: 10.1242/dmm.012005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitamura K., Kangawa K., Kawamoto M., et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem. Biophys. Res. Commun. 1993;192(2):553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- 6.Hase Y., Horsburgh K., Ihara M., Kalaria R.N. White matter degeneration in vascular and other ageing-related dementias. J. Neurochem. 2018;144(5):617–633. doi: 10.1111/jnc.14271. [DOI] [PubMed] [Google Scholar]
- 7.Ihara M., Washida K., Yoshimoto T., Saito S. Adrenomedullin: a vasoactive agent for sporadic and hereditary vascular cognitive impairment. Cereb. Circ. Cogn. Behav. 2021;2 doi: 10.1016/j.cccb.2021.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shibata M., Ohtani R., Ihara M., Tomimoto H. White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke. 2004;35(11):2598–2603. doi: 10.1161/01.Str.0000143725.19053.60. [DOI] [PubMed] [Google Scholar]
- 9.Maki T., Ihara M., Fujita Y., et al. Angiogenic and vasoprotective effects of adrenomedullin on prevention of cognitive decline after chronic cerebral hypoperfusion in mice. Stroke. 2011;42(4):1122–1128. doi: 10.1161/strokeaha.110.603399. [DOI] [PubMed] [Google Scholar]
- 10.Ichikawa-Shindo Y., Sakurai T., Kamiyoshi A., et al. The GPCR modulator protein RAMP2 is essential for angiogenesis and vascular integrity. J. Clin. Invest. 2008;118(1):29–39. doi: 10.1172/jci33022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shindo T., Kurihara Y., Nishimatsu H., et al. Vascular abnormalities and elevated blood pressure in mice lacking adrenomedullin gene. Circulation. 2001;104(16):1964–1971. doi: 10.1161/hc4101.097111. [DOI] [PubMed] [Google Scholar]
- 12.Maki T., Takahashi Y., Miyamoto N., et al. Adrenomedullin promotes differentiation of oligodendrocyte precursor cells into myelin-basic-protein expressing oligodendrocytes under pathological conditions in vitro. Stem Cell Res. 2015;15(1):68–74. doi: 10.1016/j.scr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markus H.S., van Der Flier W.M., Smith E.E., et al. Framework for clinical trials in cerebral small vessel disease (FINESSE): a review. JAMA Neurol. 2022;79(11):1187–1198. doi: 10.1001/jamaneurol.2022.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alsop D.C., Detre J.A., Golay X., et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn. Reson. Med. 2015;73(1):102–116. doi: 10.1002/mrm.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steketee R.M., Mutsaerts H.J., Bron E.E., et al. Quantitative functional arterial spin labeling (fASL) MRI–sensitivity and reproducibility of regional CBF changes using pseudo-continuous ASL product sequences. PLoS ONE. 2015;10(7) doi: 10.1371/journal.pone.0132929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mutsaerts H., Petr J., Groot P., et al. ExploreASL: an image processing pipeline for multi-center ASL perfusion MRI studies. Neuroimage. 2020;219 doi: 10.1016/j.neuroimage.2020.117031. [DOI] [PubMed] [Google Scholar]
- 17.Explore ASL. Secondary explore ASL. https://github.com/ExploreASL/ExploreASL. (accessed 10 October 2023).
- 18.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt P., Gaser C., Arsic M., et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. Neuroimage. 2012;59(4):3774–3783. doi: 10.1016/j.neuroimage.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 20.Hammers A., Allom R., Koepp M.J., et al. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum. Brain Mapp. 2003;19(4):224–247. doi: 10.1002/hbm.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkinson M., Beckmann C.F., Behrens T.E., Woolrich M.W., Smith SM. Fsl. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Mori S., Oishi K., Jiang H., et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40(2):570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuda H., Yagishita A., Tsuji S., Hisada K. A quantitative approach to technetium-99m ethyl cysteinate dimer: a comparison with technetium-99m hexamethylpropylene amine oxime. Eur. J. Nucl. Med. 1995;22(7):633–637. doi: 10.1007/bf01254564. [DOI] [PubMed] [Google Scholar]
- 24.Patlak C.S., Blasberg R.G., Fenstermacher J.D. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J. Cereb. Blood Flow Metab. 1983;3(1):1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- 25.Friberg L., Andersen A.R., Lassen N.A., Holm S., Dam M. Retention of 99mTc-bicisate in the human brain after intracarotid injection. J. Cereb. Blood Flow Metab. 1994;14:S19–S27. Suppl 1. [PubMed] [Google Scholar]
- 26.Lassen N.A., Andersen A.R., Friberg L., Paulson O.B. The retention of [99mTc]-d,l-HM-PAO in the human brain after intracarotid bolus injection: a kinetic analysis. J. Cereb. Blood Flow Metab. 1988;8(6):S13–S22. doi: 10.1038/jcbfm.1988.28. [DOI] [PubMed] [Google Scholar]
- 27.Miyashita K., Itoh H., Arai H., et al. The neuroprotective and vasculo-neuro-regenerative roles of adrenomedullin in ischemic brain and its therapeutic potential. Endocrinology. 2006;147(4):1642–1653. doi: 10.1210/en.2005-1038. [DOI] [PubMed] [Google Scholar]
- 28.Iimuro S., Shindo T., Moriyama N., et al. Angiogenic effects of adrenomedullin in ischemia and tumor growth. Circ. Res. 2004;95(4):415–423. doi: 10.1161/01.RES.0000138018.61065.d1. [DOI] [PubMed] [Google Scholar]
- 29.Nishikimi T., Mori Y., Kobayashi N., et al. Renoprotective effect of chronic adrenomedullin infusion in Dahl salt-sensitive rats. Hypertension. 2002;39(6):1077–1082. doi: 10.1161/01.hyp.0000018910.74377.93. [DOI] [PubMed] [Google Scholar]
- 30.Kita T., Kaji Y., Kitamura K. Safety, tolerability, and pharmacokinetics of adrenomedullin in healthy males: a randomized, double-blind, Phase 1 clinical trial. Drug Des Devel. Ther. 2020;14:1–11. doi: 10.2147/DDDT.S225220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kita T., Ashizuka S., Ohmiya N., et al. Adrenomedullin for steroid-resistant ulcerative colitis: a randomized, double-blind, placebo-controlled phase-2a clinical trial. J. Gastroenterol. 2021;56(2):147–157. doi: 10.1007/s00535-020-01741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dichgans M., Markus H.S., Salloway S., et al. Donepezil in patients with subcortical vascular cognitive impairment: a randomised double-blind trial in CADASIL. Lancet Neurol. 2008;7(4):310–318. doi: 10.1016/S1474-4422(08)70046-2. [DOI] [PubMed] [Google Scholar]
- 33.Joutel A., Monet-Leprêtre M., Gosele C., et al. Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. J. Clin. Invest. 2010;120(2):433–445. doi: 10.1172/jci39733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mellies J.K., Bäumer T., Müller J.A., et al. SPECT study of a German CADASIL family: a phenotype with migraine and progressive dementia only. Neurology. 1998;50(6):1715–1721. doi: 10.1212/wnl.50.6.1715. [DOI] [PubMed] [Google Scholar]
- 35.Moreton F.C., Cullen B., Dickie D.A., et al. Brain imaging factors associated with progression of subcortical hyperintensities in CADASIL over 2-year follow-up. Eur. J. Neurol. 2021;28(1):220–228. doi: 10.1111/ene.14534. [DOI] [PubMed] [Google Scholar]
- 36.Fan A.P., Jahanian H., Holdsworth S.J., Zaharchuk G. Comparison of cerebral blood flow measurement with [15O]-water positron emission tomography and arterial spin labeling magnetic resonance imaging: a systematic review. J. Cereb. Blood Flow Metab. 2016;36(5):842–861. doi: 10.1177/0271678x16636393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu B., Lou X., Wu X., Ma L. Intra- and interscanner reliability and reproducibility of 3D whole-brain pseudo-continuous arterial spin-labeling MR perfusion at 3T. J. Magn. Reson. Imaging. 2014;39(2):402–409. doi: 10.1002/jmri.24175. [DOI] [PubMed] [Google Scholar]
- 38.Binnie L.R., Pauls M.M.H., Benjamin P., et al. Test-retest reliability of arterial spin labelling for cerebral blood flow in older adults with small vessel disease. Transl. Stroke Res. 2022;13(4):583–594. doi: 10.1007/s12975-021-00983-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pauls M.M.H., Clarke N., Trippier S., et al. Perfusion by arterial spin labelling following single dose tadalafil in small vessel disease (PASTIS): study protocol for a randomised controlled trial. Trials. 2017;18(1):229. doi: 10.1186/s13063-017-1973-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pauls M.M.H., Binnie L.R., Benjamin P., et al. The PASTIS trial: testing tadalafil for possible use in vascular cognitive impairment. Alzheim. Dement. 2022;18(12):2393–2402. doi: 10.1002/alz.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yin X., Zhou Y., Yan S., Lou M. Effects of cerebral blood flow and white matter integrity on cognition in CADASIL patients. Front Psych. 2018;9:741. doi: 10.3389/fpsyt.2018.00741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kita T., Ashizuka S., Takeda T., et al. Adrenomedullin for biologic-resistant Crohn's disease: a randomized, double-blind, placebo-controlled phase 2a clinical trial. J. Gastroenterol. Hepatol. 2022;37(11):2051–2059. doi: 10.1111/jgh.15945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim Y., Bae J.S., Lee J.Y., et al. Genotype and phenotype differences in CADASIL from an asian perspective. Int. J. Mol. Sci. 2022;23(19) doi: 10.3390/ijms231911506. [DOI] [PMC free article] [PubMed] [Google Scholar]