Abstract
The goal of this work was to investigate the decomposition of azo dyes by oxidative methods, such as laccase and ultrasound treatments. Each of these methods has strong and feeble sides. The laccase treatment showed high decolorization rates but cannot degrade all investigated dyes (reactive dyes), and high anionic strength led to enzyme deactivation. Ultrasound treatment can decolorize all tested dyes after 3 h at a high energy input, and prolonged sonication leads to nontoxic ionic species, which was demonstrated by ion chromatography and toxicity assays. For the first time, it was shown that a combination of laccase and ultrasound treatments can have synergistic effects, which was shown by higher degradation rates. Bulk light absorption and ion-pairing high-performance liquid chromatography (IP-HPLC) were used for process monitoring, while with reversed-phase HPLC, a lower number of intermediates than expected by IP-HPLC was found. Liquid chromatography-mass spectrometry indicated that both acid orange dyes lead to a common end product due to laccase treatment. Acid Orange 52 is demethylated by laccase and ultrasound treatment. Further results confirmed that the main effect of ultrasound is based on ˙OH attack on the dye molecules.
Dye house effluents contain large amounts of dyes. Unbound reactive dyes undergo hydrolysis due to elevated temperature and pH values during the dyeing processes. The strong color of discharged dyes even at very small concentrations has a huge impact on the aquatic environment caused by its turbidity and high pollution strength (30). Additionally, toxic degradation products can be formed.
Azo dyes, which constitute the largest group of colorants used in industry (57), leave municipal wastewater plants highly diluted but nearly unchanged because they resist aerobic and short-term anaerobic treatment (44, 51). Only small amounts can be precipitated or adsorbed, while under anaerobic conditions azo dyes are cleaved by microorganisms, forming potentially carcinogenic aromatic amines (13). This can occur in river sediments. The fragments of azo bond cleavage can undergo autoxidation under aerobic conditions, forming colored products, as in case of Acid Orange 52, where fragments form “aniline black” by polymerization (12). Azo dyes forming “forbidden aromatic amines” are not allowed to be produced in Germany (3). Among forbidden aromatic amines are some alkylated derivatives of aniline, such as 2,4,5-trimethylaniline, o-toluidine, naphthylamine derivatives, such as 2-naphthylamine, and benzidine derivatives, like aminobiphenyls. They are toxic and, as has been proven, potential carcinogens (13). It is possible that toxic metabolites are formed after hydroxylation or oxidation by cytochrome P450 (46). This creates an urgent demand for the development of multistep treatment concepts which guarantee not only irreversible decolorization but also mineralization of azo dyes.
For our studies on dye degradation, we have chosen a laccase from the white rot fungus Trametes modesta which has previously shown potential for dye degradation (34). Laccases are well known as benzenediol:oxygen oxidoreductase (EC 1.10.3.2) and belong to the class of blue oxidases. Their typical molecular mass ranges from 60 to 85 kDa (10, 50). Laccases are capable of catalyzing a four-electron transfer reaction necessary to reduce molecular oxygen, which is used as the terminal electron acceptor, thus forming water without formation of H2O2. Laccases have a very broad substrate specificity with respect to the electron donor (54, 55).
The enzyme catalyzes the formation of free radicals by removal of a hydrogen atom from the hydroxyl group of ortho- and para-substituted mono- and polyphenolic substrates and from aromatic amines by one electron abstraction, which are capable of undergoing polymerization through radical coupling to form phenolic polymers (6, 7). Further electron withdrawal will lead to depolymerization, repolymerization, demethylation, or quinone formation (11, 19).
Artificial redox mediators, such as 2,2′-azino-bis(3-ethylthiazoline-6-sulfonate, hydroxybenzotriazole, and phenothiazines, are used to extend the substrate range of laccases, e.g., to nonphenolics subunits of lignin (8) or anthracene (25). Although mediators can enhance dye degradation by laccase (8, 9, 14, 34, 52, 56), they do not have industrial potential for this process, since their application is expensive and increases wastewater toxicity. While laccases can eventually be reused by immobilization (2), mediators are lost with the effluents. As an alternative to artificial redox mediators, we have accessed the potential of ultrasound to enhance enzymatic dye degradation.
Ultrasound has found a widespread industrial application, such as for surface treatment, soldering, and formation of emulsions (38, 48). Its application in organic chemistry has also become increasingly important, especially for the synthesis of organometallic compounds (29). When aqueous solutions are exposed to ultrasound, transient cavitations are formed due to compression and rarefaction of the bulk water. The collapse of cavities produces locally high pressure and temperature peaks (500 bar, 5,000 K). Under these extreme conditions, hydroxyl radicals and hydrogen atoms are formed by scission of the H-O bond (48). Eighty percent of these species recombine before exiting the cavitation bubble, which represents the main drawback of this method (15, 16). Hydrophilic and volatile compounds react inside the bulk water with the ejected hydroxyl radicals and at the layer between cavitation and bulk water with supercritical water. Hydrophobic and highly volatile compounds are pyrolyzed inside the cavitation bubble (24). Ultrasonication of azo dyes is said to lead to nitro and nitroso aromatics (27). Their acute and chronic toxicity and carcinogenic properties render them a high pollution potential (39, 43). Therefore, it is of outstanding interest to find a combination with another treatment to enhance dye degradation, avoiding the formation of toxic degradation products. For the first time we report on a combined treatment of azo dyes with ultrasound and laccases.
MATERIALS AND METHODS
Chemicals.
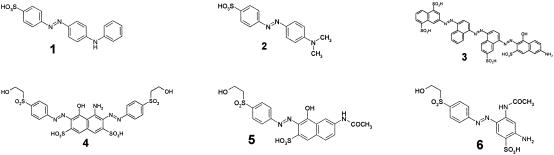
Six water-soluble azo dyes have been investigated (Fig. 1): Acid Orange 5 (Aldrich), Acid Orange 52 (Merck), Direct Blue 71 (Aldrich), Reactive Black 5 (DyStar), Reactive Orange 16 (DyStar), and Reactive Orange 107 (DyStar). All other used chemicals (pro analysis [p.a.] quality) were from Sigma; high-performance liquid chromatography (HPLC) solvents used in gradient-grade quality were from Merck. All three reactive dyes were hydrolyzed at 70°C and pH 12 for 24 h, followed by neutralization. All experiments were carried out at a 100 μM initial dye concentration.
FIG. 1.
Structures of used azo dyes which were used as sodium salt. 1, Acid Orange 5; 2, Acid Orange 52; 3, Direct Blue 71; 4, Reactive Black 5H; 5, Reactive Orange 16H; 6, Reactive Orange 107H. The letter H indicates the hydrolyzed form made from the sulfonyl ester derivative.
Laccase production and assay.
Laccase from Trametes modesta was produced and purified as previously reported (33). Enzyme activity was determined by monitoring the formation of the dimeric oxidation product from 2,6-dimethoxyphenol as described previously (4).
Dye degradation studies.
Ultrasound treatment was performed with an ultrasound device K 80 (Meinhardt Ultraschalltechnik, Leipzig, Germany) at 850 kHz, 30°C (303K) in continuous operation mode in a stirred batch reactor. Power input was set at P = 90 W (4.1 W cm−2) and P = 120 W (5.5 W cm−2) according to the manufacturer's data, which corresponded a power uptake of 84 and 124 W and a calorimetric uptake of 24 and 50 W, respectively. The enzymatic treatment was performed at 40°C, pH 4.5, acetate buffer 50 mM, and 5 × 10−9-kat/ml laccase activity. Reactions were stopped by addition of 10 mM NaF, final concentration (54). The simultaneous treatment was carried out at 40°C and pH 4.5. The samples were kept frozen and were centrifuged at 10,000 × g for 10 min to remove suspended particles prior to following analysis.
Analysis.
UV-visible region (VIS) spectrometry was done with a Perkin-Elmer Lambda 10 spectrometer (Boston, MA). Spectra were recorded between 190 and 800 nm at a scan rate of 240 nm min−1. Ion-pairing (IP)-HPLC analysis was carried out with a LaChrom System, Merck HITACHI, DAD L-7450A (180 to 850 nm), RP-select B (5 μM; 125 by 4 mm) LiChroshere 60 column (Merck KgaA, Germany). Eluent A, 1 mM tetrabutylammonium hydrogen sulfate in water plus 10% (vol/vol) CH3CN; eluent B, CH3CN. Gradient profile: 0 to 5 min 100% A, 5- to 30-min linear gradient plus 2% min−1 B, 30- to 34-min linear gradient plus 10% min−1 B, 34 to 36 min 90% B, 36-min to 37-min return to 0% B, 37 to 45 min 0% B. Eluent flow rate was set at 1 ml min−1 at 40°C oven temperature. For chloride inhibition studies, laccase preincubated 5 min at 0 to 100 mM NaCl was added to a 100 μM solution of Direct Blue 71.
For liquid chromatography (LC)-mass spectrometry (MS), lyophilized solutions were dissolved in a quarter of their initial volumes. LC (reverse-phase [RP]-HPLC), LiChrosphere WP300, 5 μm, 250 by 5; A, 10 mM ammonium acetate plus 10% (vol/vol) CH3CN; B, acetonitrile (CH3CN), gradient as described above; detector, PDA 800; flux rate, 0.8 ml min−1, oven temperature was set at 40°C, Software Chromeleon: MS, negative ESI; ionization parameters, 350°C, 50 psi, sheath gas, 10 liters min−1, 3,000 V; trap, accumulation 50,000 μs, 30 to 1,500 m/z; fragmentation, 30%; width, 10 m/z, 40,000 μs; LC/MSD trap software, version 4.1 Agilent.
Toxicity of degradation products was measured by the respiratory inhibition of Pseudomonas putida according to a modified Deutsche Industrie Norm (DIN) standard 38412 L27 protocol. The harvested cells were incubated 90 min at room temperature at moderate aeration. Each assay mix was preincubated 15 min at 100 liters/h air. To monitor the blank activity decay, every fourth analysis was carried out by monitoring blank activity.
RESULTS
Treatment of azo dyes with laccase.
UV-VIS spectroscopy showed that the azo dyes were decolorized by the enzymatic treatment at different rates. Acid Orange 52 and Direct Blue 71 showed the highest decolorization rates (more than 50% decolorization after 2 h) at the absorption maximum in the visual region. Reactive Orange 16 and Reactive Orange 107 showed no decolorization. Formation of bands at 320 and 350 nm was found in the cases of Acid Orange 5 and Acid Orange 52, respectively. Generally, there was no significant decrease of absorption maxima in the UV region (240 to 310 nm).
IP-HPLC analysis indicated that Acid Orange 5, Acid Orange 52, and Direct Blue 71 were decomposed to an extent of 65 to 90% after 1 h of treatment and to an extent of 90 to 100% after 2 h of treatment. All reactive dyes seemed to be resistant against laccase treatment. IP-HPLC analysis further showed that laccase treatment of Acid Orange 5 (420 nm/27′) led to four intermediates (387/19′, 337/23′, 319/25′, and 440/30′), with one showing a high retention time compared to the parent peak. Acid Orange 52 (444 nm/24′) also formed four intermediates (386/20′, 420/22′, 435/25′, and 417/27′). After 2 h, both acid orange dyes were nearly completely degraded, while after the same time Direct Blue 71 (570 nm/26′) showed a residual dye concentration of 10 μM and the formation of several intermediates. The faster-eluting intermediates absorbed in the UV region (227/17′, 235/18′), slower-eluting intermediates absorbed in the visible region (444/27′, 555/28′, 560/29′). Both acid orange dyes and Direct Blue 71 showed high degradation rates (Table 1). In contrast to the other reactive dyes, Reactive Black 5 H (567 nm/21′) formed one intermediate by laccase treatment (515/20′). There was no degradation detectable in the case of both Reactive Orange dyes (484 nm/18′ and 444/18′).
TABLE 1.
LE UV-VIS and IP-HPLC dataa for laccase treatment
| Dye | Degradation rate measured by:
|
|
|---|---|---|
| UV-VIS | IP-HPLC | |
| Acid Orange 5 | −0.24 ± 0.05 | −1.16 ± 0.13 |
| Acid Orange 52 | −0.36 ± 0.05 | −2.06 ± 0.37 |
| Direct Blue 71 | −1.7 ± 0.1 | −4.85 ± 0.30 |
| Reactive Black 5H | −(6 ± 3) × 10−3 | −(5 ± 0) × 10−2 |
| Reactive Orange 16H | Not degraded | Not degraded |
| Reactive Orange 107H | Not degraded | Not degraded |
k value (h−1); n = 3 (average ± SE).
Concordant IP-HPLC and UV-VIS data illustrate good or excellent degradation of Acid Orange 5 and 52 and Direct Blue 71 by laccases and poor or no degradation of the reactive dyes. The comparison between HPLC and UV-VIS data showed that formation of one peak in the UV-VIS spectra corresponds to several peaks observed by HPLC. Additionally, UV-VIS showed a very large blank absorption below 230 nm, making it impossible to detect intermediates in this region.
At the beginning, Direct Blue 71 showed a pseudo-first-order degradation rate, which changed to higher order after 15 min at 5 × 10−9 kat/ml laccase activity. A “double addition” conducted at 1 × 10−9 kat/ml enzymatic activity was used to proof the possible occurrence of inhibitor effects by intermediates due to the fact that laccase degradation of this dye led to a residual dye concentration. After 80% decolorization, the same amount of dye was added and subsequent decolorization showed the same kinetic rate as before. The formation of intermediates also showed reproducible rates.
A chloride concentration range from 11 to 47 mM corresponding to a “real concentration” in textile wastewater (41) led to a significant reduction of the decolorization rate to 29 to 15% of its initial values (data not shown), indicating a significant inhibition of the enzyme.
Ultrasound treatment.
After 1.3 h of ultrasound treatment, Acid Orange 5 was almost degraded, as indicated by UV-VIS spectroscopy. At 220 nm and 330 nm, there was an absorption increase detectable. No formation of intermediates was detected for Acid Orange 52. Higher initial concentrations caused a zero-order degradation. Direct Blue 71 underwent complete decolorization after 23 h. Eighty percent of Reactive Black 5 was decolorized after 3.5 h. Complete decolorization was achieved after 9 h. Reactive Orange 16 showed nearly complete decolorization after 23 h. Reactive Orange 107 exhibited a moderate decolorization rate. The absorption maxima in the visual region decreased much faster than in the UV region.
IP-HPLC analysis indicated that the ultrasound treatment was able to achieve complete decolorization of all investigated dyes at different degradation rates. After 1 h, 25 to 80% decolorization and after 2 h, 45 to 97% decolorization were achieved. IP-HPLC analysis further showed for both acid orange dyes the formation of at least five colored degradation products (AcO5, 420/22′, 430/23′, 420/24′, 441/26′; AcO52, 354/18′, 319/21′, 420/22′, 342/23′, 468/25′) which were degraded after 7 h of treatment. Direct Blue 71 and Reactive Black 5 H showed the slowest degradation (Table 2) but also a pronounced intermediate formation (373/18′, 228/19′, 561/27′, and 350/20′, 573/22′, and 555/25′, respectively). Reactive Orange 16 H formed one intermediate (279/16′). There were no intermediates detected in the case of Reactive Orange 107 H. IP-HPLC did not confirm the assumption that higher initial color concentrations cause a zero-order degradation.
TABLE 2.
| Dye | Dye concn and degradation rate withc:
|
|
|---|---|---|
| UV-VIS | IP-HPLC | |
| Acid Orange 5 | 54 μM; −1.6 ± 0.2d | −1.21 ± 0.43 |
| Acid Orange 52 | 100 μM; −0.67 ± 0.07d | −1.66 ± 0.03 |
| 300 μM; −75 ± 2e | ||
| Direct Blue 71 | 100 μM; −24 ± 6e | −0.30 ± 0.11 |
| Reactive Black 5H | 550 μM; −46 ± 4e | −0.36 ± 0.05 |
| Reactive Orange 16H | −0.44 ± 0.08 | |
| Reactive Orange 107H | −0.69 ± 0.43 | |
c0 = 100 μM.
P = 90 W.
k value; *, n = 3 (average ± SE).
First order (h−1).
Zero order (μM h−1).
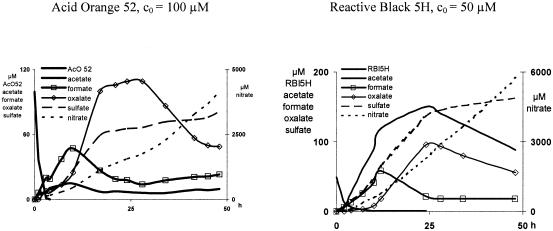
The ion-chromatographic investigations showed good degradation of all azo dyes to ionic species. Monocarboxylic acids (acetic and formic) have been found in traces. Moreover, acetate and oxalate underwent further degradation. During ultrasound treatment of azo dyes, the acetate formation showed linear increases and reached a maximum after 5 to 30 h (Fig. 2). While Acid Orange 5 and 52 and Direct Blue 71 reached around 2 to 4 percent of the theoretical possible concentration, all reactive dyes showed significantly higher concentrations (9 to 43 percent).
FIG. 2.
Reaction products resulting from ultrasound treatment (P = 90 W) of azo dyes as quantified by ion chromatography.
An acetate blank (starting concentration [c0] = 200 μm) showed a linear decrease from the beginning (data not shown). Formate, nitrate, and sulfate were found as end products and oxalate and nitrite as intermediates. During sonication, ionic species (formate and oxalate) detected by ion chromatography accounted for 13 to 29 percent of the degraded carbon.
Carbonate nearly coeluted with nitrate and was therefore not quantifiable. Formate formation was marked by a linear increase of up to 40 to 100 μM. It is noteworthy that the water blank showed 12 μM formate after 18 h.
The concentration evolution of nitrate was nearly linear from the beginning, and there was no correlation to the dye degradation. The initial oxalate formation correlates with the dye concentration, because at least 90 percent of the dye had to be degraded until the formation of oxalate started (2 to 10 h). After 20 to 34 h, a pronounced peak occurred, which was followed by a concentration decrease. These top concentrations correspond to 7 to 30 percent of the theoretical concentrations. Generally, the delayed oxalate decrease was linked to a stop of the formate decrease.
Pure oxalate solution (c0 = 100 μM) showed rather small concentration decreases during the first 10 h, indicating a rather bad degradability. Afterwards, a linear decrease was observed.
The sulfate formation showed two distinct linear increases and did not correlate with the dye degradation. After 48 h, 76 to 120% of the theoretical concentration was reached.
Combination of laccase and ultrasound treatment.
Since both laccase and ultrasound treatment led to efficient degradation only of selected dyes, the effect of a combination of these methods was investigated. In case of simultaneous treatment, the substrates were treated with laccase and ultrasound irradiation simultaneously.
During this treatment, laccase was deactivated to a similar extent (P = 90 W; t50 = 19 h) as without ultrasound action (t50 = 21 h). A higher energy input, however, caused a higher deactivation rate (P = 120 W; t50 = 5.0 ± 0.5 h).
IP-HPLC data showed (Table 3) that in all cases the simultaneous treatment gave at least the same degradation rates as the individual treatments. In the combined treatment, Direct Blue 71 and Reactive Orange 107 showed the most pronounced improvements. The reaction rates were higher than the sum of the degradation rates of laccase or ultrasound treatment alone.
TABLE 3.
| Dye | Degradation rate with:
|
||
|---|---|---|---|
| Laccase | Ultrasound | Combination | |
| Acid Orange 5 | −1.16 ± 0.13 | −1.21 ± 0.43 | −1.73 ± 0.21 |
| Acid Orange 52 | −2.06 ± 0.37 | −1.66 ± 0.03 | −2.39 ± 0.18 |
| Direct Blue 71 | −4.85 ± 0.30 | −0.30 ± 0.11 | −7.18 ± 1.05 |
| Reactive Black 5H | −0.05 ± 0.00 | −0.36 ± 0.05 | −0.30 ± 0.04 |
| Reactive Orange 16H | Not degraded | −0.44 ± 0.08 | −0.45 ± 0.17 |
| Reactive Orange 107H | Not degraded | −0.69 ± 0.43 | −1.29 ± 0.26 |
P = 90 W.
IP-HPLC data: k value (h−1), n = 3 (average ± SE).
A sequential combination treatment beginning with ultrasound followed by laccase treatment was advantageous in the case of Reactive Black 5. This dye is scarcely decolorized by laccase (decoloration rate k = −0.05 h−1) and moderately decolorized by ultrasound (−0.28). A preceding 1-h (−0.46) and 3-h (−0.44) ultrasound treatment showed the most pronounced effects during the subsequent laccase treatment.
LC-MS. (i) LC-MS of pure azo dyes.
LC-MS analysis showed for all pure azo dyes a corresponding m/z ratio [M-H]−. Acid Orange 52 and Reactive Orange 16H showed small amounts of a [2 M-2H+Na]− and a [2 M-H]− species, respectively. Direct Blue 71 and Reactive Black 5H showed [M-2H]2− and a [2 M-3H]3− species, which is common for polysulfonated dyes (23, 45).
(ii) Structure of degradation products.
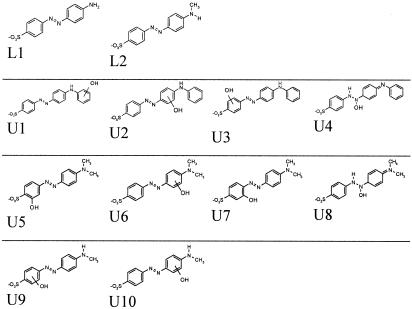
Laccase treatment of Acid Orange 5 showed at least six RP-HPLC-separated intermediates. Common features in tandem mass spectrometry (MS/MS), such as the loss of 80 and 64 units and m/z 156, which can be found in case of m/z 276 (Fig. 3, L1), 368, and 366, indicated a sulfonatobenzene structure. 318 showed the loss of 64 units. Laccase treatment of Acid Orange 52 resulted in at least seven intermediates. Interestingly, m/z 290 (L2) was found in traces from the beginning on in the dye solution. Common features in MS/MS, such as the loss of 80 and 64 units and an occurring m/z 156, were found in the case of 276, 290, 409, and 395, indicating a sulfonatobenzene structure. 289 showed the loss of 64 units. Direct Blue 71 showed at least two LC separated intermediates. The initial assumption of a common laccase product of Acid Orange 5 and Acid Orange 52 was confirmed by the MS analysis of 4-(4-aminophenylazo)-benzenesulfonic acid. It showed identical retention times, identical m/z rations (m/z 276), and identical MS/MS fragmentation compared to the occurring product peak in the solution of the treated dyes.
FIG. 3.
Structure of LC-MS-detectable intermediates originated by laccase and ultrasound treatment of Acid Orange 5 and Acid Orange 52.
Ultrasound treatment of Acid Orange 5 showed five RP-HPLC-separable intermediates, with four having an m/z 368 (Fig. 3, U1, U2, U3, and U4)m, indicating a hydroxylation [M-H+O]− and one having m/z 320. Different hydroxylation sites were confirmed by different MS/MS data. MS/MS of three m/z 368 and the m/z 320 intermediates showed a loss of 80 and 64 units and an m/z 156. The fourth one shows an m/z 186 subunit. Acid Orange 52 showed 12 intermediates. m/z 320 indicated a hydroxylation [M-H+O]− at different hydroxylation sites (U5, U6, U7, and U8), which was confirmed by different MS/MS data thereof. Furthermore, m/z 290 and 276 were ascribed to a sequential demethylation, and m/z 306 was ascribed to a demethylation and subsequent hydroxylation (U9 and U10).
Toxicity of dye degradation products.
Aquatic toxicity was measured based on the respiratory inhibition of Pseudomonas putida. The laccase treatment showed in the case of both acid orange dyes a significant increase of the apparent toxicity, which was more pronounced in case of Acid Orange 52. Direct Blue 71 was detoxified after 4 h after passing through a slight maximum. Reactive Black 5H, which was hardly decolorized by laccase, showed a small toxicity increase after 6 h. Reactive Orange 16H and Reactive Orange 107H have not been investigated, because these dyes were not decolorized by laccase treatment.
Ultrasound treatment revealed that generally the highest toxicity was measured at the highest concentration of intermediates. As the ultrasound treatment proceeded, slightly different scenarios occurred. A prolonged treatment—6 to 12 h—led to a detoxification, which was reached fastest by Reactive Black 5H. Both acid orange dyes and Reactive Orange 107H showed after 7 and 4 h, respectively, the complete degradation of all HPLC-detectable substances. At this moment the aquatic toxicity was around 10 to 15%, which declined to 5% after 12 h. A solution of Direct Blue 71 showed after 12 h no toxicity but contained two UV active intermediates passing through a concentration maximum. Reactive Black 5H and Reactive Orange 16H showed a good correlation between the apparent toxicity and the HPLC-detectable substances.
DISCUSSION
In this study we have shown that acid and direct azo dyes are degraded by laccases while reactive azo dyes seemed to be resistant. In contrast to several studies on dye degradation in the literature, we have used HPLC analysis in addition to UV-VIS spectroscopy for monitoring of the reaction. We have clearly shown that UV-VIS spectroscopy alone can lead to misinterpretation of the actual degradation process.
Acid Orange 52 was degraded twice as fast as Acid Orange 5 by the laccase from T. modesta. This can be explained by the N,N-dimethyl group of the dye, which increases the electronic density of the nitrogen atom. A laccase from Trametes trogii preferably oxidized phenols with o-, p-oriented groups in ortho and/or para positions. This effect is more pronounced in cases of -OH, -OCH3, and -NH2 groups because of lone electron pairs and therefrom its electron-donating character. This suggests that only an electron-rich phenolic ring can be oxidized by laccase (18). Laccase treatment of Acid Orange 5 and 52 should stop after the first attack. The enzymatic degradation with fungal laccase leads to formation of quinones and hydroperoxides, which are not degradable (11, 22, 40, 42). However, Acid Orange 5, Acid Orange 52, and Direct Blue 71 showed the highest degradation rates and very pronounced product formation detected by UV/VIS and IP-HPLC. The lack of these intermediates in the case of Reactive Black 5H suggests on the one hand that its fragments have been degraded very fast, because they still posses -OH, -NHR groups which can be attacked once again by laccase. On the other hand, these fragments may possess absorption maxima below the HPLC detection limit of 180 nm.
Double addition of Direct Blue 71 showed identical pseudo-first-order degradation rates and reproducible formation of intermediates. This observation excludes the possibility of laccase inhibition by intermediates, but still these intermediates may possess -OH, -NH2 groups, which may compete with the parent dye molecule for the enzyme’s catalytical centers.
Our findings suggest that the highest laccase degradation rates correlate with the accessibility of the amine groups. Reactive Black 5 was hardly decolorized, which was in contrast to results previously found (1, 34). This dye undergoes tautomerization to a ketohydrazine derivative (37), which is also possible for Reactive Orange 16, hence leading to worse accessibility of the amino group by sterical hindrance. Both acid orange dyes and their intermediates—except one—are degraded completely. These observations may be explained by the broad substrate specificity of laccase or the possible action of internal mediators, which are intermediates formed after the first laccase attack.
Due to the laccase treatment, both acid orange dyes exhibited significant toxicity increases during the first 6 h, which were more pronounced in case of Acid Orange 52. LC-MS proved a common product of these dyes to be most probably 4-(4-aminophenylazo)-benzenesulfonic acid, which was not further degraded. The two main intermediates of Direct Blue 71 are possibly products. After 2 h, the apparent toxicity reached its highest value and declined afterwards very fast to 0%. Although Reactive Black 5H was degraded very slowly, the aquatic toxicity slightly increased. Possibly aromatic amines are formed. Various textile dyes have been decolorized by a Trametes hirsuta laccase to an extent of 80% and showed no general rule in detoxification tendencies (2). In contrast, a laccase from Rhizoctonia praticola showed detoxification of cresols and chlorophenol compounds by cross-linking them with naturally occurring phenols (7).
The degradation rate of Direct Blue 71 was reduced to 29 to 15% of its initial values by chloride concentrations commonly found in textile wastewater. The enzyme laccase generally is very sensitive to halide ions and small anions (18, 53), which can bind on the T2/T3 trinuclear copper cluster site inhibiting the internal electron transfer.
Ultrasound degradation of Acid Orange 52 and Direct Blue 71 followed by UV-VIS showed a zero-order decolorization rate at a high initial dye concentration, which was not confirmed by IP-HPLC. As the reaction proceeds, the pseudo-first-order degradation changes to higher orders independently of the initial dye concentration. The enrichment of low-molecular-weight species, like oxalic acid, seems to be responsible for that observation, since they are more difficult to degrade and may act as quencher (47). Independent investigations reported in the literature have shown that total organic carbon decreases until a residual concentration is left around 20% for Reactive Black 5, which was ascribed to oxalate (49). In the case of Acid Orange 52, there has been found 50% residual total organic carbon concentration (27), which confirms what has been stated above.
LC-MS proved monohydroxylation and bihydroxylation of the azo dyes ([M-H]− → [M-H+O]−, [M-H+2O]−) before their decolorization. Vinodgopal et al. (49) described the attack of nonvolatile Reactive Black 5 in the “bulk” water by ˙OH radicals which destroy the chromophoric system through azo bond cleavage. Our experiments showed that Acid Orange 52 was demethylated. Joseph et al. (27) assumed that the ˙OH attack on the same dye leads to hydroxyl amines (which were not detected by MS analysis), followed by subsequent oxidation, leading to nitroso and nitro aromatic compounds. They observed dicarboxylic, succinic, and acetic acid as final products. Galindo et al. (17) found that Acid Orange 52 was demethylated by UV-H2O2 treatment—a treatment which produced OH radicals—and carboxylic acids and aliphatic compounds have been formed as final products. Different mechanisms, including the attack of the azo link bearing carbon, leading to phenyl derivative radicals, have also been postulated (37). Phenol degradation led to catechol, hydroquinone, and para-benzoquinone as primary products. In addition to CO2, carboxylic acids, such as oxalic, maleic, formic, propionic, and acetic acid, were identified as final products (5). Hydroxylation occurs at different sites of the target molecule, which was confirmed by LC-MS/MS results (m/z 156, 186). Ipso attacks on the N,N-dimethyl group of Acid Orange 52 (m/z 277) take place. Peller et al. (35) found out that 2,4-dichlorophenoxyacetic acid is first exposed to an ipso attack of OH radicals on the ether substituent, followed by dechlorination.
Generally, it is difficult to predict the further degradation pathways of these intermediates, because their fate depends on their physical and chemical properties. Further reactions may occur inside the cavitation, in the hypercritical water layer, or in the “bulk” water. As a matter of fact, the degree of mineralization to gaseous compounds strongly depends on the on the gas mixture (5, 36). Acid Orange 52 was degraded at 1.7 h−1 (c0 = 100 μM), which is in good agreement with results found by other investigators (2.4 to 3.0 h−1 at 500 kHz, 50 W, 288 K; c0 = 10 μM) (27).
The highest acetate concentrations were reached by reactive dyes (RBl5H, RO16H, and RO107H) which possess C2 groups (-SO2-C2H4-OH and -NH-CO-CH3, respectively), which indicated that these C2 groups are essential for acetate formation. Acetate intermediate was most probably degraded to formate. Gutierrez et al. (21) have found that under argon saturation, acetate is degraded to succinic, glyoxylic, and glycolic acids. Further, the formation of CO2, CO, and CH4 depends on the initial acetate concentration. During the first 10 h of azo dye degradation, possibly formate was formed through the primary ˙OH attack on the C1 and C2 groups. Later-occurring formate may be formed by acetate and oxalate degradation. The final nitrate concentration is much higher than the theoretical, because the N2 oxidation originated from air-saturated solutions (48). Therefore, no quantification of nitrate originating from dyes was possible.
The late oxalate formation indicated its origin in primary intermediates and not in the azo dyes themselves. The concentration profile indicated that oxalate was an intermediate which was possibly degraded to formate. Pure oxalate was hardly degraded during the first 10 h. Possibly, oxalate was attacked by the accumulating hydrogen peroxide.
Sulfate was formed through the attack on -SO3H and -SO2- groups. If one of these groups is more easily accessible by ˙OH attack, the flexion point in the concentration profile should lie on a stoichiometric point. The experimental data do not support this assumption. Sulfate, a product of ultrasound treatment, hardly scavenges hydroxyl radicals (31). The formation of significant concentrations of carbon-containing substances (formate and oxalate) in the case of pure water may be explained by the dissolved amount of CO2. It is well known that carbon dioxide is in equilibrium with carbonate and hydrocarbonate (32). The latter is transformed by ˙OH attack to a carbonate radical (17), which possibly recombines and disproportionates to an oxalate ion.
Our work shows that the aquatic toxicity is not clearly linked to the concentration of detected intermediates. There is a unanimous opinion among a lot of researchers that ultrasound and advanced oxidation process (AOP) treatment of azo dyes leads to toxic intermediates (17, 47). In general, the increase in the UV region is linked with the increase of aromatic substances which are per se defined as toxic. As a matter of fact, this assumption is not supported by any experimental data. Therefore, it must not be concluded that the degradation of the UV-active substances is linked to detoxification. A bioluminescence assay with Vibrio fischeri was used for the determination of toxicity and indicated that the increasing toxicity after complete degradation of sodium pentachlorophenolate by ultrasound occurred due to the formation of hydrogen peroxide (20). These experimental data differ from our data, which clearly demonstrated that P. putida used for determination of aquatic toxicity was a lot more insensitive towards hydrogen peroxide, most likely due to catalases constitutively expressed by this organism (28).
The simultaneous treatment did not decolorize all dyes to the same beneficial extent. Direct Blue 71 and Reactive Orange 107 showed the most pronounced increases, which indicated synergistic effects of the simultaneous treatment. Ultrasonication possibly created intermediates acting as internal mediators for laccase. Mediators are assumed to oxidize nonsubstrate molecules or enhance degradation rates by undergoing a redox cycle between laccase enzyme and the target molecule (25, 26, 34). The small k value increases, and the lower concentration of intermediates in the cases of both acid orange dyes may be due to the competition for hydroxyl radicals by these intermediates. The observed intermediate features were not the same as with the single methods. Some intermediates do not occur, while new ones can be observed. This was more pronounced in the case of Direct Blue 71. At the higher energy input level (P = 120 W) only, the ultrasound treatment caused significant laccase deactivation, which enables the simultaneous combination at the low energy input.
Conclusion.
Laccase enzyme treatment and ultrasonication are two oxidizing processes which are capable of degrading azo dyes. It was clearly shown that laccase treatment does not degrade the azo dyes to the same extent. But it was able to degrade the hereby-formed intermediates of the well-degradable dyes. The two acid orange dyes showed a common product formed by laccase treatment. It was proven by RP-HPLC-MS that it was a primary amine, most probably a 4-(4-aminophenylazo)-benzenesulfonic acid. Ultrasound degraded all formed intermediates, which showed the more unspecific nature of the degradation mechanism.
RP-HPLC-MS demonstrated that the degradation by ultrasound was caused by OH radical attack and led to an ipso substitution and/or addition (hydroxylation) at different sites of the target molecules.
Our findings show the possibility of saving time and energy by applying a simultaneous combination of laccase and ultrasound treatments for decolorization of azo dyes. Ongoing investigations focus on the structures of the intermediates formed by laccase and ultrasound to elucidate the degradation mechanisms.
Acknowledgments
We thank M. Senhold (UAS Cologne) for performing ion chromatography analysis and A. Erlacher (TU Graz), A. Plum (UAS Cologne), and M. Schäfer (University of Cologne) for support during mass spectrometry measurements and valuable discussions. We further thank the DAAD and ÖAD for scholarship support of M. Tauber.
The European Commission is acknowledged for financial support to project NMP2-CT-2003-505892 through the European Community Sixth Framework Programme.
REFERENCES
- 1.Abadulla, E., K.-H. Robra, G. M. Gübitz, L. M. Silva, and A. Cavaco-Paulo. 2000. Enzymatic decolorization of textile dyeing effluents. Text. Res. J. 70:409-414. [Google Scholar]
- 2.Abadulla, E., T. Tzanov, S. Costa, K.-H. Robra, A. Cavaco-Paulo, and G. M. Gübitz. 2000. Decolorization and detoxification of textile dyes with a laccase from Trametes hirsuta. Appl. Environ. Microbiol. 66:3357-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bedarfsgegenständeverordnung. Fassung 23. December 1997. Bundesgesetzblatt I 1998.
- 4.Benfield, G., S. M. Bocks, K. Bromley, and B. R. Brown. 1964. Studies of fungal and plant laccases. Phytochemistry 3:79-88. [Google Scholar]
- 5.Berlan, J., F. Trabelsi, H. Delams, A. M. Wilhelm, and J. F. Petrignani. 1994. Oxidative degradation of phenol in aqueous media using ultrasound. Ultrason. Sonochem. 1:97-102. [Google Scholar]
- 6.Bollag, J. M. 1992. Enzymes catalyzing oxidative coupling reactions of pollutants. Metal Ions Biol. Syst. 28:205-217. [Google Scholar]
- 7.Bollag, J. M., K. L. Shuttleworth, and D. H. Anderson. 1988. Laccase-mediated detoxification of phenolic compounds. Appl. Environ. Microbiol. 54:3086-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bourbonnais, R., M. G. Paice, B. Freiermuth, E. Bodie, and S. Bornemann. 1997. Reactivities of various mediators and laccases with Kraft pulp and lignin model compounds. Appl. Environ. Microbiol. 63:4627-4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourbonnais, R., M. G. Paice, R. Reid, P. Lanthier, and M. Yaguchi. 1995. Lignin oxidation by laccase isozymes from Trametes versicolor and role of the mediator ABTS in Kraft lignin depolymerization. Appl. Environ. Microbiol. 61:1876-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calvo, A. M., J. L. Copa-Patino, O. Alonso, and A. E. González. 1998. Studies of the production and characterization of laccase activity in the basidiomycete Coriolopsis gallica, an efficient decolorizer. Arch. Mirobiol. 171:31-36. [DOI] [PubMed] [Google Scholar]
- 11.Chivukula, M., and V. Renganathan. 1995. Phenolic azo dye oxidation by laccase from Pyricularia oryzae. Appl. Environ. Microbiol. 34:4374-4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christen, H. R., and F. Vögtle. 1989. Grundlagen der organischen Chemie, p. 566. Salle und Sauerländer Verlag, Frankfurt am Main, Germany.
- 13.Chung, K.-T., and C. E. Cerniglia. 1992. Mutagenicity of azo dyes: structure-activity relationships. Mutat. Res. 277:201-220. [DOI] [PubMed] [Google Scholar]
- 14.Fabbrini, M., C. Galli, and P. Gentili. 2002. Comparing the catalytic efficiency of some mediators of laccase. J. Mol. Catal. B Enzym. 16:231-240. [Google Scholar]
- 15.Fischer, C.-H., E. J. Hart, and A. Henglein. 1986. H/D exchange in the D2-H2O system under the influence of ultrasound. J. Phys. Chem. 90:222-224. [Google Scholar]
- 16.Fischer, C.-H., E. J. Hart, and A. Henglein. 1986. Ultrasonic irradiation of water in the presence of 18,18O2: isotope exchange and isotopic distribution of H2O2. J. Phys. Chem. 90:1954-1956. [Google Scholar]
- 17.Galindo, C., P. Jaques, and A. Kalt. 2000. Photodegradation of the aminobenzene acid orange 52 by three AOPs: UV/H2O2, UV/TiO2 and VIS/TiO2. Comparative mechanistic and kinetic investigations. J. Photochem. Photobiol. A Chem. 130:35-47. [Google Scholar]
- 18.Garzillo, A. M. V., M. C. Colao, C. Caruso, C. Caporale, D. Celletti, and V. Buonocore. 1998. Laccase from white-rot fungus Trametes trogii. Appl. Microbiol. Biotechnol. 49:545-551. [DOI] [PubMed] [Google Scholar]
- 19.Goncalves, M. L., and W. Steiner. 1996. Purification and characterization of laccase from a newly isolated wood decaying fungus. ACS Symp. Ser. 655:258-266. [Google Scholar]
- 20.Gonze, E., Y. Gonthier, and A. Bernis. 2002. Characterization of ultrasonic activity in high frequency reactors for waste water treatment, p. 41-59. In U. Neis (ed.), Reports on sanitary engineering. Ultrasound in environmental engineering. Technische Universität Hamburg-Harburg, Hamburg, Germany.
- 21.Gutierrez, M., A. Henglein, and C.-H. Fischer. 1986. Hot spot kinetics of the sonolysis of aqueous acetate solutions. Int. J. Radiat. Biol. 50:313-321. [DOI] [PubMed] [Google Scholar]
- 22.Heinfling, A., M. Bergbauer, and U. Szewzyk. 1997. Biodegradation of azo and phthalocyanine dyes by Trametes versicolor and Bjerkandera adusta. Appl. Microbiol. Biotechnol. 48:261-266. [Google Scholar]
- 23.Holčapek, M., P. Jandera, and P. Zderadicka. 2001. High performance liquid chromatography-mass spectrometric analysis of sulfonated dyes and intermediates. J. Chromatogr. A 926:175-186. [DOI] [PubMed] [Google Scholar]
- 24.Hua, I., R. H. Höchemer, and M. R. Hoffmann. 1995. Sonolytic hydrolysis of p-nitrophenyl acetate: the role of supercritical water. J. Phys. Chem. 99:2335-2342. [Google Scholar]
- 25.Johannes, C., A. Majcherczyk, and A. Hüttermann. 1996. Degradation of anthracene by laccase of Trametes versicolor in the presence of different mediator compounds. Appl. Microbiol. Biotechnol. 46:313-317. [DOI] [PubMed] [Google Scholar]
- 26.Johannes, C., and A. Majcherczyk. 2000. Natural mediators in the oxidation of polycyclic aromatic hydrocarbons by laccase mediator systems. Appl. Environ. Microbiol. 66:524-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joseph, J. M., H. Destaillats, H.-M. Hung, and M. R. Hoffmann. 2000. The sonochemical degradation of azobenzene and related azo dyes: rate enhancements via Fenton′s reactions. J. Phys. Chem. A 104:301-307. [Google Scholar]
- 28.Katsuwon, J., and A. J. Anderson. 1992. Characterization of catalase activities in a root-colonizing isolate of Pseudomonas putida. Can. J. Microbiol. 38:1026-1032. [Google Scholar]
- 29.Ley, S. V., and C. M. R. Low. 1989. Ultrasound in synthesis. Springer-Verlag, Berlin, Germany.
- 30.Lin, S. H., and C. M. Lin. 1993. Treatment of textile waste effluents by ozonisation and chemical coagulation. Water Res. 27:1743-1748. [Google Scholar]
- 31.Matthews, R. W., H. A. Mahiman, and T. J. Sworski. 1972. Elementary processes in radiolysis of aqueous sulfuric-acid solutions—determination of both GOH and GSO4. J. Phys. Chem. 76:1265-1272. [Google Scholar]
- 32.Mortimer, C. E. 1987. Chemie, Das Basiswissen der Chemie, p. 447-465. Georg Thieme Verlag Stuttgart, New York, N.Y.
- 33.Nyanhongo, G. S., J. Gomes, G. M. Gübitz, R. Zvauya, J. Read, and W. Steiner. 2002. Optimization of some variables for the production of laccase from a newly isolated strain of Trametes modesta. Biores. Technol. 84:259-263. [DOI] [PubMed] [Google Scholar]
- 34.Nyanhongo, G. S., J. Gomes, G. M. Gübitz, R. Zvauya, J. Read, and W. Steiner. 2002. Decolorization of textile dyes by laccase from a newly isolated strain of Trametes modesta. Water Res. 36:1449-1456. [DOI] [PubMed] [Google Scholar]
- 35.Peller, J., O. Wiest, and P. V. Kamat. 2001. Sonolysis of 2,4-dichlorophenoxyacetic acid in aqueous solutions. Evidence for *OH radical mediated degradation. J. Phys. Chem. 105:3176-3181. [Google Scholar]
- 36.Petrier, C., Y. Jiang, A. Francony, and M. F. Lamy. 1999. Aromatics and chloroaromatics sonochemical degradation: yields and by-products, p. 23-37. In A. Thiem and U. Neis (ed.), Reports on sanitary engineering. Ultrasound in environmental engineering. Technische Universität Hamburg-Harburg, Hamburg, Germany.
- 37.Pham, T. L. H., W. Rotard, A. Preiss, and M. Elend. 2001. Möglichkeiten und Grenzen der LC-MS Analyse von Farbstoffmetaboliten, SFB 193, oral presentation/handout. Biologische Abwasserreinigung, Kolloquium an der TU Berlin, Berlin, Germany.
- 38.Rasanu, N., E. Chirila, V. Marza, and S. Dobrinas. 2000. Descompunerea unor coloranti disaoici in camp ultrasonor. Rev. Chim. 51:349-353. [Google Scholar]
- 39.Rickert, D. E. 1987. Metabolism of nitroaromatic compounds. Drug Metab. Rev. 18:23-53. [DOI] [PubMed] [Google Scholar]
- 40.Rodríguez, E., M. A. Pickard, and R. Vazquez-Duhalt. 1999. Industrial dye decolorization by laccases from ligninolytic fungi. Curr. Microbiol. 38:27-32. [DOI] [PubMed] [Google Scholar]
- 41.Scharf, S. 1995. Wasserbelastung durch Textilveredlungsbetriebe, Technologische Aspekte und Messungen bei fünf Direkteinleitern. Wien. Monographien des Umweltbundesamtes M 68:107. [Google Scholar]
- 42.Schliephake, K., D. E. Mainwaring, G. T. Lonergan, I. K. Jones, and W. L. Baker. 2000. Transformation and degradation of the disazo dye Chicago Sky Blue by a purified laccase from Pycnoporus cinnabarinus. Enzyme Microb. Technol. 27:100-107. [DOI] [PubMed] [Google Scholar]
- 43.Schwedt, G. 1996. Toxikologisches Lexikon, p. 72. Vogel Verlag, Würzburg, Germany.
- 44.Shaul, G. M., T. J. Holdsworth, C. R. Dempsey, and K. A. Dostal. 1991. Fate of water soluble azo dyes in activated sludge process. Chemosphere 22:107-119. [Google Scholar]
- 45.Smyth, W. F., S. McClean, E. O′Kane, I. Banat, and G. McMullan. 1999. Application of electrospray mass spectrometry in the detection and determination of Remazol textile dyes. J. Chromatogr. A 854:259-274. [DOI] [PubMed] [Google Scholar]
- 46.Sterner, O. 1999. Chemistry. Health and Environment. Wiley-VCH, Weinheim, Germany.
- 47.Stock, N., J. Peller, K. Vinodgopal, and P. V. Kamat. 2000. Combinative sonolysis and photocatalysis for textile dye degradation. Environ. Sci. Technol. 34:1747-1750. [Google Scholar]
- 48.Suslick, K. S. (ed.). 1988. Ultrasound, p. 123-163. VCH, Weinheim, Germany.
- 49.Vinodgopal, K., J. Peller, O. Makogon, P. V. Kamat, and K. Vinodgopal. 1998. Ultrasonic mineralization of a reactive textile azo dye, Remazol Black B. Water Res. 32:3646-3650. [Google Scholar]
- 50.Wahleithner, J. A., F. Xu, K. M. Brown, St. H. Brown, E. J. Golightly, T. Halkier, S. Kauppinen, A. Pedrson, and P. Schneider. 1996. The identification and characterization of four laccases from the plant pathogenic fungus Rhizoctonia solani. Curr. Genet. 29:395-403. [DOI] [PubMed] [Google Scholar]
- 51.Walker, R. 1970. The metabolism of azo compounds: a review of the literature. Food Cosmet. Toxicol. 8:659-676. [DOI] [PubMed] [Google Scholar]
- 52.Wong, Y., and J. Yu. 1999. Laccase-catalyzed decolorization of synthetic dyes. Water Res. 33:3512-3520. [Google Scholar]
- 53.Xu, F. 1996. Oxidation of phenols, anilines, and benzenethiols by fungal laccases: correlation between activity and redox potential as well as halide inhibition. Biochemistry 35:7608-7614. [DOI] [PubMed] [Google Scholar]
- 54.Xu, F., W. Shin, S. H. Brown, J. A. Wahleithner, U. M. Sundaram, and E. I. Solomon. 1996. A study of a series of recombinant fungal laccases and bilirubin oxidase that exhibit significant differences in redox potential, substrate specificity, and stability. Biochim. Biophys. Acta 1292:303-311. [DOI] [PubMed] [Google Scholar]
- 55.Xu, F. 1999. Laccase, p. 1545-1554. Encyclopedia of bioprocess technology: fermentation, biocatalysis and bioseparation. Wiley, New York, N.Y.
- 56.Yaropolov, A. I., O. V. Skorobogat′ko, S. S. Vartanov, and S. D. Varfolomeyev. 1994. Laccase—properties, catalytic mechanism and application. Appl. Biochem. Biotechnol. 49:257-280. [Google Scholar]
- 57.Zollinger, H. 1998. Color chemistry, 2nd revision. VCH, Weinheim, Germany.