Abstract
The molecular basis for the resistance of serogroup B Neisseria meningitidis to the bactericidal activity of normal human sera (NHS) was examined with a NHS-resistant, invasive serogroup B meningococcal isolate and genetically and structurally defined capsule-, lipooligosaccharide (LOS)-, and sialylation-altered mutants of the wild-type strain. Expression of the (α2→8)-linked polysialic acid serogroup B capsule was essential for meningococcal resistance to NHS. The very NHS-sensitive phenotype of acapsular mutants (99.9 to 100% killed in 10, 25, and 50% NHS) was not rescued by complete LOS sialylation or changes in LOS structure. However, expression of the capsule was necessary but not sufficient for a fully NHS-resistant phenotype. In an encapsulated background, loss of LOS sialylation by interrupting the α2,3 sialyltransferase gene, lst, increased sensitivity to 50% NHS. In contrast, replacement of the lacto-N-neotetraose α-chain (Galβ1-4GlcNAcβ1-3Galβ1-4Glc) with glucose extensions (GlcN) in a galE mutant resulted in a strain resistant to killing by 50% NHS at all time points. Encapsulated meningococci expressing a Hep2(GlcNAc)→KDO2→lipid A LOS without an α-chain demonstrated enhanced sensitivity to 50% NHS (98% killed at 30 min) mediated through the antibody-dependent classical complement pathway. Encapsulated LOS mutants expressing truncated Hep2→KDO2→lipid A and KDO2→lipid A structures were also sensitive to 50% NHS (98 to 100% killed at 30 min) but, unlike the wild-type strain and mutants with larger oligosaccharide structures, they were killed by hypogammaglobulinemic sera. These data indicate that encapsulation is essential but that the LOS structure contributes to the ability of serogroup B N. meningitidis to resist the bactericidal activity of NHS.
Serogroup B Neisseria meningitidis (the meningococcus) is an obligate human pathogen and remains a leading cause of fulminant septicemia and meningitis. In addition to sporadic outbreaks, large epidemics of serogroup B meningococcal disease continue to occur in many parts of the world, including South America, the United States Pacific Northwest, Western Europe, and New Zealand (4, 22). After penetrating upper respiratory tract mucosal surfaces, N. meningitidis must survive and multiply in the bloodstream to cause sepsis, meningitis, and other manifestations of invasive meningococcal disease. A major mechanism inhibiting or preventing the multiplication of meningococci in the blood is the complement-mediated bactericidal activity of human sera (17, 39). The importance of this activity in the prevention of systemic meningococcal disease is reinforced by host factors that alter bactericidal activity and increase the risk for development of invasive disease. These factors include the absence of bactericidal antibodies against meningococci (17, 18, 45), deficiencies in the complement cascade (13), and the presence of blocking immunoglobulin A antibodies that inhibit the bactericidal activity of human sera (19). The bactericidal activity of human sera against meningococci is also used as a surrogate marker for assessing meningococcal vaccine efficacy.
Meningococci have evolved mechanisms that protect them from the bactericidal activity of human sera. Invasive serogroup B meningococcal strains recovered from blood and cerebrospinal fluid often resist being killed by human sera (48). The molecular basis for resistance has been attributed to the expression by this organism of an (α2→8)-linked polysialic acid capsule and a short-chained lipooligosaccharide (LOS) with terminal sialic acid residues (23, 34, 35). Meningococci isolated from the bloodstream in invasive disease, in contrast to nasopharyngeal isolates, are heavily encapsulated (9) and express the L3,7,9 LOS immunotypes (28). These immunotypes have a lacto-N-neotetraose originating from HepI of the inner core, which may be terminally sialylated (34, 62). However, the experimental data defining the precise contributions of the capsule, LOS sialylation, and LOS structure to the ability of serogroup B meningococci to resist the bactericidal activity of human sera is conflicting (11, 15, 20, 21, 27, 37, 63–65).
LOS epitopes are immunogenic in infants and children and induce protective bactericidal antibodies in convalescent sera (10, 12). These bactericidal LOS antibodies appear to be directed at conserved low-molecular-weight LOS epitopes (10, 12). LOS is also a component of new serogroup B outer membrane vesicle (OMV) vaccines and is proposed as a basis for other new meningococcal vaccines (1–3, 50). Although changes in the structure of LOS are known to influence the amount and epitopes of bactericidal and other functional antibodies elicited by OMV vaccines (2), the precise LOS structure(s) to include in these and other LOS-containing meningococcal vaccines is uncertain.
To help understand the basis for meningococcal survival following mucosal invasion and to facilitate development of meningococcal vaccines which may contain LOS, we created a series of genetically and structurally defined capsule-, sialylation-, and LOS-altered mutants of the serogroup B meningococcal strain NMB. We used these mutants to study the contributions of the capsule, LOS sialylation, and changes in LOS structure to meningococcal resistance to the bactericidal activity of normal human sera (NHS).
MATERIALS AND METHODS
Strains and media.
The serogroup B meningococcal strain NMB and a series of genetically and structurally defined mutants of this strain were used. These mutants were defective in capsule formation, LOS sialylation, or altered in LOS structure or had a combination of these phenotypes. The characteristics of strain NMB are summarized in Table 1, and the creation and selection of the mutants have been described previously (29, 30, 32, 56, 57, 60, 69) or are described below. The serum-resistant gonococcal strain FA19 was also used (55). The strains were grown on gonococcal (GC) agar (Difco) with supplements according to the recommendations of Morse and Bartenstein (38). The following antibiotics were used where appropriate: 5 μg of tetracycline/ml and 60 μg of spectinomycin/ml. Mutants containing the aphA::3 cassette were grown on brain heart infusion agar supplemented with 2.5% fetal bovine serum and containing 80 μg of kanamycin/ml.
TABLE 1.
Characteristics of strains used in this study
| Geno-typed | Phenotype
|
Log10 survivala ± SEM
|
||||||
|---|---|---|---|---|---|---|---|---|
| Encapsu-lation | LOS sialylation | LOS structureb | NHS
|
HGS | C2DS | |||
| 10% | 25% | 50% | ||||||
| galE | Cap+ | LOSsial− | GlcNβ1→4Glcβ1→4Hep2(GlcNAc,Glc)PEA→KDO2→lipid A | 4.3 ± 0.031 | 4.2 ± 0.37 | 4.06 ± 0.75 | ND | ND |
| Wild type | Cap+ | LOSsial− | NANAα2→3Galβ1→4GlcNAcβ1→3Galβ1→4Glcβ1→4Hep2(GlcNAc,Glc)PEA→KDO2→lipid A | 4.26 ± 0.046 | 4.07 ± 0.073 | 3.63 ± 0.071 | 4.22 ± 0.082 | 4.24 ± 0.015 |
| lst | Cap+ | LOSsial− | Galβ1→4GlcNAcβ1→3Galβ1→4Glcβ1→4Hep2(GlcNAc,Glc)PEA→KDO2→lipid A | 4.37 ± 0.27 | 4.28 ± 0.057 | 3.1 ± 0.2 | ND | 4.48 ± 0.016 |
| pgmc | Cap+ | LOSsial− | Hep2(GlcNAc)PEA→KDO2→lipid A | 3.98 ± 0.077 | 3.15 ± 0.20 | 2.3 ± 0.11 | 4.19 ± 0.005 | 4.25 ± 0.029 |
| orfA | Cap+ | LOSsial− | KDO2→lipid A | 4.06 ± 0.038 | 3.80 ± 0.098 | 2.47 ± 0.15 | 0 ± 0 | 4.48 ± 0.01 |
| rfaK | Cap+ | LOSsial− | Hep2PEA→KDO2→lipid A | 3.97 ± 0.065 | 3.15 ± 0.71 | 0 ± 0 | 0 ± 0 | 4.50 ± 0.033 |
| synD | Cap− | LOSsial+ | NANAα2→3Galβ1→4GlcNAcβ1→3Galβ1→4Glcβ1→4Hep2(GlcNAc,Glc)PEA→KDO2→lipid A | 0 ± 0 | 0 ± 0 | 0 ± 0 | ND | ND |
| synA | Cap− | LOSsial− | Galβ1→4GlcNAcβ1→3Galβ1→4Glcβ1→4Hep2(GlcNAc,Glc)PEA→KDO2→lipid A | 0 ± 0 | 0 ± 0 | 0 ± 0 | 3.92 ± 0.037 | 3.11 ± 0.22 |
Resistance to killing by NHS (10, 25, and 50% [vol/vol] concentrations), hypogammaglobulinemic sera (HGS) (50% [vol/vol] concentration), or C2-deficient sera (C2DS) (50% [vol/vol] concentration) over 30 min compared to time 0 value (∼4.0 log units). The data represent 2 to 15 separate experiments per variable, with quadruplicate determinations of each variable per experiment. ND, not done.
For phosphorylation patterns of lipid A of LOS expressed in each strain, see Fig. 1.
CMK2, the lgtF mutant, expresses the same phenotype as R6 and behaves similarly in NHS. CMK2 was not tested in hypogammaglobulinemic sera or C2-deficient sera.
The indicated genes have been inactivated (see the text for details).
Construction of synA::Ω mutants.
An internal section of synA (siaA) (57, 59) was amplified by PCR with the primers JS51 (5′-GCAATACCATTACGTTTATCTCTC-3′) and JS40 (5′-GTTTCAGGATTGTTGATTACTTCAGC-3′). The TA cloning kit (Invitrogen) was used to clone this PCR product into the polylinker of the plasmid pCR2 to form pCK3. A MscI partial digest of pCK3 was ligated with a SmaI-digested pHP45 (41) and transformed into Escherichia coli JM109. Transformants were selected for resistance to kanamycin and spectinomycin. Restriction mapping of the recombinant plasmids confirmed the insertion of the spectinomycin cassette (Ω) into the internal site of the cloned synA fragment in plasmid pCK4. The synA::Ω cassette was cloned from pCK4 into the vector pHSG298 to form pCK5.
The plasmid pCK5 was transformed into strains NMB, SS3, R6, 559, and 469 by the plate transformation method (60), and transformants were identified by their acquisition of spectinomycin resistance. The site of insertion of synA::Ω was confirmed by PCR of the chromosomal synA locus.
Construction of lst::Ω mutant.
A unique HincII site was introduced into an internal region of the LOS sialyltransferase gene, lst (16), using PCR. The primer pairs lst4Hc (5′-GAAGGTAAAGTCGAGCTGCTGC-3′) with cycl (5′-GCAAATCCTGCCACGACAGTTTCC-3′) and lst5Hc (5′-CAGCAGCGTCGACTTTACCTTCAGC-3′) with icdl (5′-CAAAAGCCTGCACAATCGGCAGC-3′) were used to amplify the 3′ and 5′ ends of the lst gene. Equimolar amounts of these two PCR products were used as a template in a standard PCR with the nested primer pair lst2 (5′-GAATGCGGTTTCCCTGCTGAAGG-3′) and lst3 (5′-CAGCGGCAGGTAAGTCATCTTGC-3′). The resultant PCR product, which represents an internal region of lst containing a unique HincII site, was cloned into the polylinker HincII site of the low-copy-number vector pHSG576 to create pCK90. The polar spectinomycin cassette, Ω, was released from pHP45 (41) by using SmaI and ligated with HincII-digested pCK90. A combination of recipient vector and cassette antibiotic resistances was used to select transformants harboring the correct recombinant plasmid. The resultant plasmid was called pCK91. The lst::Ω cassette was released from pCK91 with EcoRI and PstI and cloned into corresponding sites in the high-copy-number vector pHSG298 to form pCK92. The wild-type parent, NMB, was transformed with pCK92 by the plate transformation method (60), and transformants were identified by their acquisition of spectinomycin resistance. The site of insertion of lst::Ω was confirmed by PCR of the chromosomal lst locus with the icd1 and cyc1 primer pair.
Construction of FA19 LOS mutants.
Piliated FA19 was transformed with chromosomal DNA from the meningococcal LOS mutants R6 and 469. Transformants were selected for acquisition of the tetracycline marker, and the LOS profiles of these isolates were further characterized by Tricine sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (29). Those transformants which were tetracycline resistant and expressed truncated LOS structures characteristic of the corresponding meningococcal mutants were used.
Assessment of outer membrane structures.
The outer membrane structures of meningococcal strain NMB and of each mutant were extensively studied. Piliation of the wild-type parent was assessed by electron microscopy of negatively stained preparations and by monoclonal antibody reactivity (54) and, in the case of the mutants, by competence for transformation. Outer membrane proteins (OMP) were assessed by serotyping and subtyping (44) (kindly performed by George Carlone, Centers for Disease Control and Prevention) and by Coomassie-stained Laemmli SDS-PAGE of isolated outer membranes (5). LOS immunotyping was performed as previously described (44). Structural analysis of LOS expressed by strain NMB and the mutants was performed by Tricine SDS-PAGE, compositional studies, nuclear magnetic resonance (NMR), and mass spectrometry (29, 30, 32, 42). Immunoblots and whole-cell enzyme-linked immunosorbent assays (57, 59, 60) were used to quantitate amounts of (α2→8)-linked polysialic acid capsule. Phospholipid analysis of strain NMB and its mutants was performed by mass spectrometry and NMR (51).
SBA.
A microdilution serum bactericidal assay (SBA) was performed (55), modified as described below. Briefly, blood from five healthy adult subjects without a history of gonococcal or meningococcal disease and not under antibiotic therapy was collected under sterile conditions, allowed to clot for 3 min at room temperature (23°C), and then centrifuged at 500 × g at 4°C for 15 min. The serum was removed, pooled, divided into subsamples, and stored at −70°C until used in the experiments. Total hemolytic activity in the serum (measured by the 50% hemolytic complement value [the amount of complement required to lyse 50% of sensitized sheep erythrocytes]) was at normal levels. Isolates to be tested were grown on GC agar plates for 14 to 16 h at 37°C in 3% (vol/vol) CO2. The colonies were inoculated into 10 ml of GC broth containing supplements and grown to an optical density of 0.5 at 550 nm. The inocula were diluted in 1× minimal essential medium (MEM; Gibco) containing 50 mM HEPES, pH 7.3 (HEPES-MEM) to the desired concentration (105 cells/ml) and used immediately in the SBA. In some experiments heat-inactivated sera (56°C for 30 min), hypogammaglobulinemic serum from a patient with acquired common variable immunodeficiency, or C2-deficient human serum (Calbiochem, La Jolla, Calif., and Quidel, San Diego, Calif.) were used.
HEPES-MEM, serum, and bacterial inoculum in HEPES-MEM in that order were mixed in a microtiter plate well (Falcon tissue culture plate no. 3072) so that the final suspension contained 100 μl with 10, 25, or 50% (vol/vol) serum and 104 CFU/ml. The reaction mixture was incubated at 37°C in 3% (vol/vol) CO2. Controls included an assay without serum. Quadruplicate samples (0.01 ml) were withdrawn from the reaction mixture immediately after preparation and after 5, 15, and 30 min of incubation. Colony counts were performed as previously described (55). After overnight incubation of the plates, the number of CFU per sample was determined and the number of CFU per milliliter in the original mixture at each sampling time was calculated. Data were expressed as percentage survival or as log10 survival. The significance of differences between the means of two variables was determined by using Student’s t test with unpaired values and a two-tailed hypothesis (StatWorks version 1).
RESULTS
Outer membrane composition of meningococcal strain NMB and mutants.
The wild-type parent strain, NMB, is an encapsulated B:2b;P1.2,5:L3,7,9 (serogroup:serotype [class II or III OMP];serosubtype [class I OMP]:LOS immunotype) originally isolated from the cerebrospinal fluid of a patient with meningococcal meningitis in Pennsylvania in 1982. Strain NMB was competent and expressed class II pili, which can be glycosylated (Galβ1-4-Gal) (62a), and zero to two class V (Opa) proteins.
The encapsulation and LOS structure of strain NMB and each mutant are described below. Because the susceptibility of meningococci to the bactericidal activity of NHS may be influenced by phase-variable changes in the outer membrane, we also assessed the OMP expression and membrane phospholipid composition of the mutants. All mutants were competent and retained the OMP serotype and serosubtype of the parent. Except as noted, the OMP profiles of the mutants were identical by SDS-PAGE to that of the parent (data not shown). Strain NMB and the mutants all expressed similar membrane phospholipid profiles.
Wild-type parent strain NMB [encapsulated; NANAα2→ 3Galβ1→4GlcNAcβ1→3Galβ1→4Glcβ1→4Hep2(GlcNAc, Glc)PEA→KDO2→lipid A LOS].
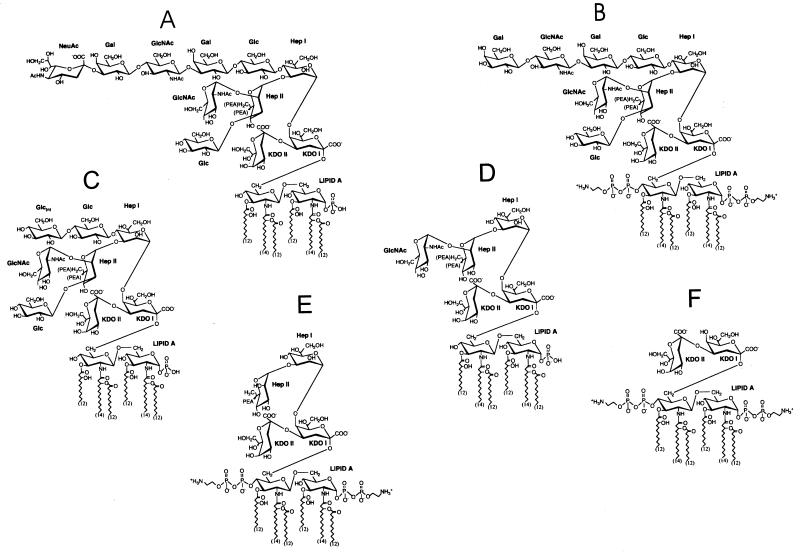
The predominant structure of the LOS of strain NMB, based on biochemical and physical studies, is shown in Fig. 1A. The LOS species contains a terminal lacto-N-neotetraose (Galβ1→4GlcNAcβ1→3Galβ1→4Glc) α-chain attached to the Hep2-KDO2-lipid A inner core. By routine immunotyping, NMB is an L3. However, physical studies indicate that strain NMB expresses both L3 and L2 LOS, with L2 as the predominant LOS structure (42). Both L2 and L3 structures have identical α-chains of lacto-N-neotetraose attached to HepI of the inner core. They differ primarily by the attachment of glucose to the 0-3 of HepII in L2 versus attachment of a phosphoethanolamine (PEA) group at the same position in L3. Antibodies that readily distinguish the 0-3 HepII region are not usually employed in standard immunologic typing techniques. Therefore, L2 and L3 structures appear the same in standard immunotyping.
FIG. 1.
Predominant LOS structure of the serogroup B N. meningitidis strain NMB and LOS structures expressed by a series of genetically defined NMB mutants. (A) The predominant LOS of parental strain NMB, (NANAα2→3)Galβ1→4GlcNAcβ1→3Galβ1→4Glc→Hep2(GlcNAc,Glc)PEA→KDO2, contains a terminal lacto-N-neotetraose (Galβ1→4GlcNAcβ1→3Galβ1→4Glc) in the α-chain, with a portion of the molecules containing a terminally linked sialic acid attached to the 0-3 lacto-N-neotetraose galactosyl residue and two heptoses attached through two KDOs to lipid A (41). (B) In contrast to the LOS of the wild-type strain, NMB, the LOS lacto-N-neotetraose of the sialic acid biosynthesis pathway mutant, M7 (synA− [siaA−]), and the α2→3 sialyltransferase mutant, Lst (lst−), lacks the terminally linked sialic acid. (C) In the UDP-Glc4-epimerase galE mutant, SS3, mass spectrometric analysis of O-deacylated LOS revealed the presence of multiple species, with the predominant LOS species in this mutant strain formed by the GlcN→Glcβ1→4Hep2(GlcNAc,Glc)PEA→KDO2 (32). (D) The LOS from HepIβ1-4 glucosyl transferase mutant NMBlgtF::aphA-3 (nonpolar lgtF mutant) had a GlcNAc/Hep ratio of 1:2.125 with no other hexoses detected, indicating that the predominant structure was Hep2(GlcNAc)KDO2→lipid A (29). The phosphoglucomutase pgm mutant, R6, has an identical structure (69). (E) Glycosyl composition and linkage analysis of rfaK− (α1,2 N-acetylglucosamine transferase mutant) LOS before and after de-O-acetylation showed that the oligosaccharide of this LOS consists of only Hep and KDO and that a PEA group is attached to position 3 of HepII. In addition, no glucose is present in the rfaK− LOS structure. Fast atom bombardment-mass spectrometry of the de-O-acylated LOS and one-dimensional proton NMR analyses of the oligosaccharide gave results that were consistent with the structure (29). (F) Electrospray-mass spectrometry of the de-O-acylated mutant 469 (orfA−) LOS preparation indicated that it contained one major molecular species, KDO2→lipid A.
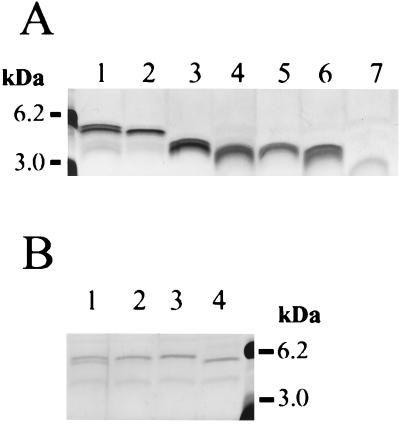
Meningococcal LOS structures with terminal galactose residues, like that found in strain NMB, may be terminated with N-acetylneuraminic acid (NANA) added by a LOS-specific α2,3 sialyltransferase (68). The cytidine monophosphate (CMP)-NANA substrate for this reaction (and also for capsule polymerization) is endogenously synthesized by serogroup B N. meningitidis (57). Under laboratory growth conditions, the α-chain of strain NMB was 20 to 50% sialylated (NANAα2→3) at the α-chain terminal galactose (42), resulting in two major LOS structures of different mobilities (4.5 and 4.8 kDa) when separated by Tricine SDS-PAGE (Fig. 2A and B).
FIG. 2.
LOS structures of N. meningitidis NMB and genetically defined LOS mutants separated by Tricine SDS-PAGE and visualized by silver staining (30). (A) The NMB parental LOS structure (lane 1) is compared to the truncated LOS from NMBlst::Ω (lane 2), SS3 (galE−) (lane 3), R6 (pgm−) (lane 4), CMK2 (lgtF−) (lane 5), CMK1 (rfaK−) (lane 6), and 469 (orfA−) (lane 7). (B) The parent strain, NMB (lane 1), expresses LOS which is partially sialylated under normal growth conditions and which separates as two bands in Tricine SDS-PAGE. Mutant 43 (synD−) (lane 2) expresses LOS which is almost completely sialylated, since little further sialylation can be achieved when the strain is grown in the presence of 50 μg of CMP-NANA/ml (lane 3). In comparison, mutant M7 (synA−) cannot synthesize sialic acid and expresses only nonsialylated LOS (lane 4). Molecular mass standards (Boehringer Mannheim) are also shown.
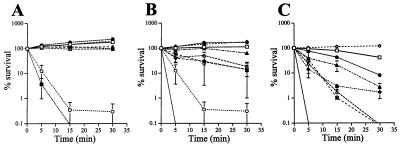
Strain NMB was completely resistant to killing by 10 and 25% NHS at all time points (Fig. 3). In 50% NHS, NMB was resistant at 5 min and demonstrated 82 and 43% survival (<1 log unit decrease in CFU) at 15 and 30 min, respectively (Fig. 3 and Table 1). Complement-inactivated NHS (50% [vol/vol] heated at 56°C for 30 min; designated ΔNHS) did not kill strain NMB at any time point. Increasing the sialylation of the LOS of strain NMB to 100% by growth in CMP-NANA did not change the survival of NMB in 50% NHS (data not shown). In C2-deficient and hypogammaglobulinemic sera, strain NMB was completely resistant after 30 min of incubation.
FIG. 3.
Survival in 10 (A), 25 (B), and 50% (C) NHS of the wild-type parental meningococcal strain, NMB (□); sialic acid or capsule pathway mutants expressing different sialylation phenotypes (synA− [■], synD− [○], and NMBlst::Ω [•]; or mutants expressing truncated LOS structures (SS3 [galE−] [◊], R6 [pgm−], [⧫], CMK2 [lgtF−], [∗], CMK1 [rfaK−] [▵], and 469 [orfA−] [▴]). Error bars indicate standard error of the means.
SynA mutation [nonencapsulated; Galβ1→4GlcNAcβ1→ 3Galβ1→4Glcβ→4Hep2(GlcNAc,Glc)PEA→KDO2→lipid A LOS].
A mutant of strain NMB designated M7 contains a single truncated Tn916 inserted in synA, the first gene of the capsule biosynthesis operon (synABCD) of serogroup B N. meningitidis (57, 60). SynA is responsible for conversion of N-acetyl-d-glucosamine-6-phosphate to N-acetyl-d-mannosamine-6-phosphate in the CMP-NANA biosynthesis pathway. Insertional activation of any of the CMP-NANA biosynthetic genes, synA, -B, or -C, prevents endogenous LOS sialylation as well as encapsulation. The SynA mutant is completely defective in both sialic acid biosynthesis (Fig. 2B) and capsule expression (57). The predominant LOS of the SynA mutant (Fig. 1B) was otherwise identical to the lacto-N-neotetraose-containing L2 structure of the parent, except that the LOS was not sialylated (Fig. 2) and phosphorylation of the lipid A was increased (42).
In contrast to the encapsulated, LOS-sialylated parent strain, NMB, the nonencapsulated, LOS-nonsialylated SynA mutant was rapidly and completely killed in 5 min by 10, 25, and 50% NHS (P < 3.5 × 10−5) (Fig. 3). Thus, in an unencapsulated background the bactericidal activity of NHS was not inhibited by expression of an unsialylated, lacto-N-neotetraose-containing LOS.
The rapid bactericidal activity of NHS against the SynA mutant was mediated through the antibody-dependent, classical complement pathway. Fifty percent ΔNHS did not kill the SynA mutant at any time point. The rapid bactericidal activity observed in 10% NHS was probably below a critical concentration for alternative-pathway-mediated complement activation (7, 61), and no killing of the SynA mutant was observed with 10% C2-deficient sera. In 50% hypogammaglobulinemic sera, minimal killing was observed over 30 min (3.92 log10 [83%] survival [Table 1]). In 50% C2-deficient sera, ∼1 log unit of killing was observed over 30 min (Table 1). Restoration of C2-deficient sera with exogenous C2 restored rapid and complete killing (0% survival at 5 min). These data indicate that bactericidal antibodies in NHS, recognizing exposed outer membrane structures in an unencapsulated background, produce rapid and complete killing of the SynA mutant. The data predict that unencapsulated, nonsialylated meningococci which reach the bloodstream would be rapidly killed.
SynD mutation [nonencapsulated; NANAα2→3Galβ1→ 4GlcNAcβ1→3Galβ1→4Glcβ1→4Hep2(GlcNAc,Glc)PEA→ KDO2→lipid A LOS].
The SynD mutant, designated mutant 43, is defective in capsule expression due to a Tn916 insertion in the fourth gene, synD, of the serogroup B capsule biosynthesis operon (57, 58). Inactivation of synD, which encodes the serogroup B capsule α2→8 polysialyltransferase for capsule formation from CMP-NANA, results in an unencapsulated phenotype while retaining LOS sialylation. In contrast to the 20- to 50%-sialylated parent, the SynD mutant, under standard laboratory growth conditions, produced an almost completely sialylated LOS (Fig. 2B), presumably because the block in capsule production shunts CMP-NANA into the LOS sialylation pathway.
Like the unencapsulated, LOS-nonsialylated SynA mutant, the unencapsulated LOS-sialylated SynD mutant was very sensitive to killing by NHS. In 10, 25, and 50% NHS, the SynD mutant was more rapidly and completely killed than the encapsulated wild-type parent (Fig. 3). Growth of the SynD mutant in CMP-NANA, to further ensure complete sialylation of LOS (Fig. 2B), did not enhance the survival of this mutant in NHS (data not shown). Thus, complete sialylation of the lacto-N-neotetraose of LOS in the absence of a capsule did not rescue the acapsular, serum-sensitive phenotype. These data confirm the necessity of the (α2→8)-linked polysialic acid capsule of group B meningococci for conferring resistance to the bactericidal activity of NHS.
Lst mutation [encapsulated; Galβ1→4GlcNAcβ1→3Galβ1→ 4Glcβ1→4Hep2(GlcNAc,Glc)PEA→KDO2→lipid A LOS].
We next asked whether LOS structure influenced serum bactericidal activity in an encapsulated meningococcal background. A mutation in the α2,3 sialyltransferase gene, lst, was created, resulting in the inability to add NANA to the 0-3 lacto-N-neotetraose galactosyl residue (Fig. 1B and 2B). The lst mutant was resistant to 10 and 25% NHS at all time points (Fig. 3A and B). In 50% NHS, the lst mutant was more sensitive than the parent at 15 min (P = 0.0192) and at 30 min (P = 0.00125) (Fig. 3C and Table 1).
GalE mutation [encapsulated; GlcN-Hep2(GlcNAc,Glc)PEA→KDO2→lipid A LOS].
A galE mutant, designated SS3, was fully encapsulated but produced a truncated LOS which migrated at 3.4 kDa (Fig. 2A). The mutation was due to a Tn916 insertion in the amino terminus of the functional copy of the UDP-Glc4-epimerase, galE (32). UDP-Glc 4-epimerase activity was present in N. meningitidis NMB but not in SS3, indicating that the Tn916 insertion had abolished this activity. Structural analysis of LOS from mutant SS3 revealed that there was no galactose present in the structure (Fig. 1C), but multiple terminal glucoses were present. The original galE transformant expressed one class V (Opa) protein rather than the two expressed by the wild-type parent. However, class V expression was not linked to the galE mutation, and only galE mutants which matched the class V expression profile of the parent were used in the bactericidal assays.
The galE mutant was completely resistant to NHS and 50% ΔNHS at all serum concentrations and at all time points (Fig. 3 and Table 1). The galE mutant was more resistant than the wild-type parent in 50% NHS (P < 0.02 at 15 min and P < 0.0005 at 30 min). Thus, an encapsulated mutant lacking lacto-N-neotetraose and LOS sialylation, but expressing a GlcN→HepI α-chain, demonstrated enhanced resistance to NHS.
Pgm mutation [encapsulated; Hep2(GlcNAc)PEA→KDO2→lipid A LOS].
The LOS mutant designated R6 produced a truncated LOS molecule of 3.1 to 3.2 kDa (Fig. 2) and was shown to contain a Tn916 inserted in the 3′ end of pgm, the gene encoding the meningococcal and gonococcal phosphoglucomutase (69). The inability of mutant R6 to convert glucose-6-phosphate to glucose-1-phosphate, which is required for UDP-glucose and UDP-galactose synthesis, resulted in the truncated LOS phenotype expressed by this mutant. Analysis of R6 LOS by mass spectrometry (69) showed that the predominant LOS structure was Hep2(GlcNAc)PEA→KDO2→lipid A (Fig. 1D).
The Pgm mutant was resistant to 10% NHS but, compared to the parent strain NMB, demonstrated increased sensitivity at 5, 15, and 30 min to 25 (P < 0.003) and 50% (P < 0.002) NHS (Fig. 3 and Table 1). By 30 min, 98% killing was observed for the Pgm mutant in 25 and 50% NHS (Fig. 3 and Table 1). Killing of the Pgm mutant in 50% NHS was not seen with C2-depleted or hypogammaglobulinemic sera, indicating that killing of this mutant was largely due to the antibody-dependent classical complement pathway.
LgtF mutation (CMK2) [encapsulated; Hep2(GlcNAc)→PEA→KDO2→lipid A LOS].
LgtF is the β1-4 glucosyl transferase initiating synthesis of the α-chain from HepI and is found in the inner core extension (ice1) operon with rfaK, the α1,2 N-acetylglucosamine transferase (see below) (30). When lgtF was inactivated by a nonpolar cassette, the LOS α-chain was missing but the inner core GlcNAc attached to HepII was present. No glucose was present in the LgtF mutant LOS structure. Although produced by distinct mutations, the predominant LOS structures expressed by the LgtF and Pgm mutants were identical (Fig. 1D).
The susceptibility of the LgtF mutant to killing by NHS did not differ from the susceptibility of the Pgm mutant to NHS at any time point or serum concentration. Like the Pgm mutant, the LgtF mutant was resistant to killing by 10% NHS but showed increased susceptibility at 15 and 30 min compared to the parent strain in 25 (P < 0.01) and 50% (P < 0.0009) NHS (Fig. 3). Thus, mutants expressing Hep2(GlcNAc)→KDO2→lipid A, created by inactivation of two different genes (pgm and lgtF), demonstrated identical profiles of increased sensitivity to NHS.
RfaK mutation (encapsulated; Hep2PEA→KDO2→lipid A LOS).
Mutant CMK1 is created by an Ω insertion in the α1,2 N-acetylglucosamine transferase gene, rfaK, encoding the enzyme responsible for the addition of N-acetylglucosamine to HepII (29). The CMK1 mutant expressed a truncated LOS of 3.0 kDa (Fig. 2A), that is, a Hep2PEA→KDO2→lipid A structure (Fig. 1E). The mutant was totally deficient in the addition of GlcNAc to the inner core LOS and also completely lacked an α-chain on HepI. The lack of an α-chain extension from HepI in mutant CMK1 is likely due to structural constraints imposed by the incomplete biosynthesis of the LOS inner core (29).
The susceptibility of the RfaK mutant to NHS resembled those of the Pgm and LgtF mutants (Fig. 3). Compared to the parent strain, NMB, the RfaK mutant was resistant in 10% NHS but demonstrated increased susceptibility at 15 and 30 min to 25% NHS (P < 0.02) and was completely killed in 50% NHS (P < .0009). However, unlike the Pgm mutant, the RfaK mutant was completely killed by 50% hypogammaglobulinemic sera (0% survival at 30 min) but not by 50% C2-deficient sera (>100% survival at 30 min) (Table 1).
OrfA mutation (encapsulated; KDO2→lipid A LOS).
The LOS mutant designated 469 exhibited a markedly truncated LOS structure of 2.9 kDa (Fig. 2A). The mutant contained a single truncated Tn916 insertion located in a second LOS inner core extension operon, ice2. Inactivation of the gene orfA in the ice2 operon was responsible for the truncated LOS phenotype. Glycosyl composition and linkage analysis of the mutant OrfA LOS showed that it consisted of KDO2→lipid A (52) (Fig. 1F).
In contrast to the LOS mutants LgtF, Pgm, and RfaK, the OrfA mutant was more resistant to killing in 25% NHS at all time points (Fig. 3). However, compared to the parent strain, NMB, the OrfA mutant was more sensitive to killing by 50% NHS (P < 0.002). Fifty percent ΔNHS and C2-deficient sera did not kill the OrfA mutant. However, as with the RfaK mutant, hypogammaglobulinemic serum completely killed the OrfA mutant (e.g., 0% survival at 30 min).
Effect of combined LOS and capsule mutations.
The plasmid pCK5, containing the synA::Ω cassette, was used to transform the pgm, galE, rfaK, and orfA mutants. The lack of capsule expression of the transformants was confirmed by colony immunoblotting. Each of the acapsular, LOS-truncated mutants was rapidly and completely killed at 5 min by 10% NHS (data not shown). This profile was not significantly different from the killing of the synA or synD mutants that expressed the LOS α-chain composed of sialylated or nonsialylated lacto-N-neotetraose.
Effects of LOS mutations in a Neisseria gonorrhoeae background.
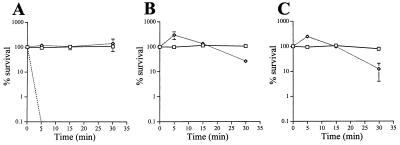
To assess whether the influence of LOS structure on meningococcal sensitivity to NHS was dependent on an encapsulated meningococcal background, we transformed the pgm and 469 (orfA) mutations into the serum-resistant gonococcal strain FA19. Compared to the serum-resistant parent, the pgm mutation enhanced serum sensitivity (90% killing) in the gonococcal background (Fig. 4), similar to that observed in the meningococcal background. However, the orfA mutation resulted in a very NHS-sensitive gonococcal phenotype that was completely killed in 10% NHS at 5 min (Fig. 4) compared to the same mutation in meningococci (Fig. 3). Thus, examination of LOS mutations in the appropriate meningococcal or gonococcal background was important for the assessment of the contributions of LOS to killing by NHS.
FIG. 4.
Resistances to NHS of the parental gonococcal strain, FA19 (□), and the LOS mutants containing the pgm mutation (FA19/R6) (◊) and the orfA mutation (FA19/469) (–––) are shown at 10 (A), 25 (B), and 50% (C) NHS. Error bars indicate standard error of the means.
DISCUSSION
The wild-type meningococcal strain used in this study resembled other invasive serogroup B meningococcal strains (11, 65) in that it was encapsulated, expressed a sialylated lacto-N-neotetraose LOS, and resisted the bactericidal activity of up to 50% NHS. The (α2→8)-linked polysialic acid capsule was the major determinant that allowed this strain to resist the bactericidal activity of NHS. The serogroup B capsule is poorly immunogenic due to its identity with host cell molecules, such as the neural cell adhesion molecule, N-CAM (14). Through steric or electrostatic hindrance, the polysialic acid capsule may decrease the binding of antibodies directed toward other meningococcal surface structures. In addition, sialic acid capsules have been shown to redirect overall C3 deposition on the bacterial surface (24, 27, 65), to increase the quantity of amide-linked C3 (25), and, although not clearly shown for sialic acid polymers, perhaps to render surface-bound C3 more accessible to the action of factors H and I with subsequent blocking of factor B binding and enhanced inactivation of C3b to iC3b (36, 40). Vogel et al. (65) recently showed that deposition of C3b and its derivatives (e.g., iC3b) on encapsulated group B meningococci was initially dependent on the classical pathway and that the location of C3b differed in the encapsulated parent versus unencapsulated mutants.
Unencapsulated variants of our serogroup B meningococcal strain, regardless of LOS structure, were rapidly killed by NHS. In addition, expression of a fully sialylated lacto-N-neotetraose through endogenous or exogenous CMP-NANA did not protect the unencapsulated phenotype. These data are consistent with those of Klein et al. (31), who found that the blood-sensitive phenotype of acapsular N. meningitidis could not be rescued by the presence of sialylated LOS, and with those of Fox et al. (15), who were unable to increase serum resistance by growing meningococci in the presence of exogenous CMP-NANA. In N. gonorrhoeae, however, exogenous sialylation of LOS inhibits complement-mediated killing by serum (8, 43, 53, 67). These data emphasize the critical role of the meningococcal serogroup B polysialic acid capsule in inhibiting the bactericidal activity of NHS and the differences in the mechanisms of resistance of meningococci and gonococci to NHS.
Capsule expression, while necessary, was not sufficient to confer a fully NHS-resistant phenotype on group B meningococci. In an encapsulated background, expression of LOS α-chain structures from HepI was needed for high-level resistance to NHS. These α-chain structures included, but did not require, a sialylated lacto-N-neotetraose. Moran et al. (37) showed that meningococci expressing the sialylatable lacto-N-neotetraose of the L3 LOS immunotype were more resistant to the bactericidal activity of serum than meningococci expressing the nonsialylatable Galβ1→4Glcβ1→4HepI of the L8 immunotype. Vogel et al. (64, 65) and Hammerschmidt et al. (21) initially reported that sialylation of LOS and encapsulation were both indispensible for serogroup B meningococcal resistance to complement-mediated killing by NHS and for bacteremia in the infant rat model. However, these conclusions were based on the increased serum sensitivity of a LOS-truncated galE mutant. As shown, our galE mutant was completely resistant at all concentrations of NHS and was even more resistant than the wild-type parent. Our mutant contains GlcN extensions from Hep (32) in contrast to the truncated α-chain containing one or two glucose moieties present on other meningococcal galE mutants (66). Interestingly, Robertson et al. (47) reported that galE mutants of the gonococcal strain MS11 also exhibit increased resistance to NHS compared to the sialylated, lacto-N-neotetraose-expressing parent. Recently, Estabrook et al. (11) reported that bactericidal activity of NHS against serogroup C meningococcal strains correlated with the amount of free lacto-N-neotetraose exposed, not with LOS sialylation per se. While antibodies in NHS directed at lacto-N-neotetraose could explain these observations, this molecule is poorly immunogenic, since the lacto-N-neotetraose structure is common to the human Ii antigens (33) and NHS does not contain significant amounts of antibodies directed against lacto-N-neotetraose (11). Estabrook et al. (11) suggest that bactericidal activity may be due to binding of C3 to the terminal galactose of unsialylated lacto-N-neotetraose on LOS. In contrast, Vogel et al. (63) recently reported that a serogroup B encapsulated lst (α2,3 sialyltransferase) mutant, which was unable to terminally sialylate LOS, was as resistant to NHS as the wild-type parent, despite enhanced C3b binding. In our studies in a serogroup B encapsulated background, loss of sialylation enhanced susceptibility to NHS. This may be due to the increased exposure of terminal galactose of the α-chain lacto-N-neotetraose or loss of the effect of LOS sialylation in blocking complement pathway activation (43). Certainly, the length and nature of meningococcal LOS α-chain extending beyond Glcβ1→4HepI was an important determinant for facilitating meningococcal resistance to NHS.
In an encapsulated background, loss of the α-chain and exposure of inner core LOS structures [Hep2(GlcNAc)→KDO2→lipid A or Hep2→KDO2→lipid A] enhanced meningococcal susceptibility to killing by NHS. Though the kinetics and mechanisms of killing were different, truncation of LOS to Hep2 inner core structures made these encapsulated mutants as sensitive to NHS as the capsule-deficient mutants (i.e., up to 100% killing). For the Hep2(GlcNAc)→KDO2→lipid A mutants, killing was largely mediated by an antibody-initiated classical complement pathway, since killing was not observed in hypogammaglobulinemic sera. Bactericidal antibodies to conserved inner core LOS structures are found in sera of meningococcal carriers, in convalescent sera of patients following meningococcal disease, and in NHS (10, 12). The NHS used in this study contained antibodies which recognized the minimal LOS structure, Hep2(GlcNAc)→KDO2→lipid A (data not shown). Enhanced immunoglobulin M antibody binding to the truncated galE mutant described by Vogel et al. (65) was seen.
Alternatively, truncated LOS structures may alter the topology of the outer membrane (including sialic acid capsule polymers) and enhance exposure of other outer membrane components. This may enhance susceptibility to complement-mediated killing by NHS through direct activation of C1q (6, 26), activation of the alternative complement pathway, enhanced binding of bactericidal antibodies to outer membrane structures other than LOS, or allowing the binding of blocking antibodies. The rapid killing of Hep2→KDO2→lipid A and KDO2→lipid A mutants in hypogammaglobulinemic sera but not C2-deficient sera suggests that these strains are not killed by bactericidal antibody or the alternative complement pathway but may directly activate C1q. Surprisingly, despite their marked sensitivity to hypogammaglobulinemic sera, the Hep2→KDO2→lipid A and KDO2→lipid A mutants were partially resistant to NHS, suggesting that extensive truncation of meningococcal LOS may expose epitopes (e.g., class IV proteins) recognized by nonbactericidal or blocking antibodies. Exposure of certain inner core LOS structures in a gonococcal background resulted in serum killing profiles different from those seen in the corresponding meningococcal mutants. Although the expression of Hep2(GlcNAc)PEA→KDO2→lipid A in gonococci also produced a serum-sensitive phenotype similar to that in meningococci, the deep rough mutant, FA19/469, in contrast to the meningococcal 469 mutant, was extremely sensitive to serum. These findings reinforce the concept that truncation of LOS structures in gonococcal and meningococcal strains may have different effects on the topology of the outer membrane.
In addition to heterogeneity in oligosaccharide structure, meningococcal LOS displays heterogeneity in the phosphorylation substitution of lipid A. We have previously noted that the lipid A of meningococcal LOS from a capsule-defective mutant is more heavily substituted with phosphate than lipid A of the parent (42). However, the biological role of this modification of lipid A in capsule-defective and truncated LOS mutants remains unclear. We found no correlation of meningococcal lipid A phosphorylation with sensitivity to NHS. However, monophosphoryl enteric lipid A is less bioactive than diphosphoryl lipid A, as shown by the rabbit pyrogenicity test, the chicken embryo lethal dose test, and the Schwartzmann reaction, as well as by a reduced ability to stimulate monokine induction in murine macrophage cell lines (46). Indeed, a brief study of the ability of meningococcal and gonococcal LOS to clot Limulus amebocyte lysate revealed that this activity was strain dependent and possibly related to the amount of phosphorylated lipid A expressed by each isolate (49). The biological importance of phosphorylation of meningococcal lipid A remains to be elucidated.
Defining the role of the capsule, LOS sialylation, and LOS structure in serum killing of meningococci has implications for the development of new serogroup B meningococcal vaccines containing OMVs or LOS. Since the (α2→8)-linked serogroup B capsule and the LOS lacto-N-neotetraose and digalactose structures of the α-chain molecule are weakly immunogenic, the use of truncated LOS molecules with Hep2(GlcNAc)→KDO2→lipid A structures in OMV vaccines may boost LOS antibodies produced by these vaccines. Andersen et al. have recently shown that this may be true by using a Glc→Hep2(GlcNAc)→KDO2→lipid A truncated LOS in an OMV vaccine given to mice (2). OMV vaccines containing Hep2(GlcNAc)→KDO2→lipid A in addition to higher-molecular-weight LOS components may also be useful as a means of avoiding immune escape (3). Further truncation of the LOS molecule appears less likely to induce bactericidal LOS antibodies.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AI-33517 and AI-40247 from the National Institutes of Health and by the Research Service of the VA Medical Center.
We thank Lane Pucko for manuscript preparation.
REFERENCES
- 1.Alving C R. Lipopolysaccharide, lipid A, and liposomes containing lipid A as immunologic adjuvants. Immunobiology. 1993;187:430–446. doi: 10.1016/S0171-2985(11)80355-4. [DOI] [PubMed] [Google Scholar]
- 2.Andersen S R, Bjune G, Høiby E A, Michaelsen T E, Aase A, Rye U, Jantzen E. Outer membrane vesicle vaccines made from short-chain lipopolysaccharide mutants of serogroup B Neisseria meningitidis: effect of the carbohydrate chain length on the immune response. Microb Pathog. 1997;23:139–155. doi: 10.1016/s0264-410x(97)00030-3. [DOI] [PubMed] [Google Scholar]
- 3.Andersen S R, Kolberg J, Høiby E A, Namork E, Caugant D A, Frøholm L O, Jantzen E, Bjune G. Lipopolysaccharide heterogeneity and escape mechanisms of Neisseria meningitidis: possible consequences for vaccine development. Microb Pathog. 1997;23:139–155. doi: 10.1006/mpat.1997.0143. [DOI] [PubMed] [Google Scholar]
- 4.Caugant D A, Froholm L O, Bovre K, Holten E, Frasch C E, Mocca L F, Zollinger W D, Selander R K. Intercontinental spread of a genetically distinctive complex of clones of Neisseria meningitidis causing epidemic disease. Proc Natl Acad Sci USA. 1986;83:4927–4931. doi: 10.1073/pnas.83.13.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark V L, Campbell L A, Palermo D A, Evans T M, Klimpel K W. Induction and repression of outer membrane proteins by anaerobic growth of Neisseria gonorrhoeae. Infect Immun. 1987;55:1359–1364. doi: 10.1128/iai.55.6.1359-1364.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper N R, Morrison D C. Binding and activation of the first component of human complement by the lipid A region of lipopolysaccharides. J Immunol. 1978;120:1862–1868. [PubMed] [Google Scholar]
- 7.Croize J, Arvieux J, Berche P, Colomb M G. Activation of the human complement alternative pathway by Listeria monocytogenes: evidence for direct binding and proteolysis of the C3 component on bacteria. Infect Immun. 1993;61:5134–5139. doi: 10.1128/iai.61.12.5134-5139.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de la Paz J, Cook S J, Heckels J E. Effect of sialylation of lipopolysaccharide of Neisseria gonorrhoeae on recognition and complement-mediated killing by monoclonal antibodies directed against different outer membrane antigens. Microbiology. 1995;141:913–920. doi: 10.1099/13500872-141-4-913. [DOI] [PubMed] [Google Scholar]
- 9.DeVoe I W. The meningococcus and mechanisms of pathogenicity. Microbiol Rev. 1982;46:162–190. doi: 10.1128/mr.46.2.162-190.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estabrook M M, Baker C J, Griffiss J M. The immune response of children to meningococcal lipooligosaccharides during disseminated disease is directed primarily against two monoclonal antibody-defined epitopes. J Infect Dis. 1993;167:966–970. doi: 10.1093/infdis/167.4.966. [DOI] [PubMed] [Google Scholar]
- 11.Estabrook M M, Griffiss J M, Jarvis G A. Sialylation of Neisseria meningitidis lipooligosaccharide inhibits serum bactericidal activity by masking lacto-N-neotetraose. Infect Immun. 1997;65:4436–4444. doi: 10.1128/iai.65.11.4436-4444.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estabrook M M, Mandrell R E, Apicella M A, Griffiss J M. Measurement of the human immune response to meningococcal lipooligosaccharide antigens by using serum to inhibit monoclonal antibody binding to purified lipooligosaccharide. Infect Immun. 1990;58:2204–2213. doi: 10.1128/iai.58.7.2204-2213.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Figueroa J, Andreoni J, Densen P. Complement deficiency states and meningococcal disease. Immunol Res. 1993;12:295–311. doi: 10.1007/BF02918259. [DOI] [PubMed] [Google Scholar]
- 14.Finne J, Leinonen M, Makela P H. Antigenic similarities between brain components and bacteria causing meningitis. Lancet. 1983;ii:355–356. doi: 10.1016/s0140-6736(83)90340-9. [DOI] [PubMed] [Google Scholar]
- 15.Fox A J, Jones D M, Scotland S M, et al. Serum killing of meningococci and several other gram-negative bacterial species is not decreased by incubating them with cytidine-5′-monophospho-N-acetyl acid. Microb Pathog. 1989;7:317–318. doi: 10.1016/0882-4010(89)90050-8. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert M, Watson D C, Cunningham A, Jennings M P, Young N M, Wakarchuk W W. Cloning of the lipooligosaccharide α2,3 sialyltransferase from the bacterial pathogens Neisseria meningitidis and Neisseria gonorrhoeae. J Biol Chem. 1996;271:28271–28276. doi: 10.1074/jbc.271.45.28271. [DOI] [PubMed] [Google Scholar]
- 17.Goldschneider I, Gotschlich E C, Artenstein M. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldschneider I, Gotschlich E C, Artenstein M S. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969;129:1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffiss J M, Broud D D, Bertram M A. Bactericidal activity of meningococcal antisera. Blocking by IgA of lytic antibody in human convalescent sera. J Immunol. 1975;114:1779–1784. [PubMed] [Google Scholar]
- 20.Hamadeh R M, Estabrook M M, Zhou P, Jarvis G A, Griffiss J M. Anti-Gal binds to pili of Neisseria meningitidis: the immunoglobulin A isotype blocks complement-mediated killing. Infect Immun. 1995;63:4900–4906. doi: 10.1128/iai.63.12.4900-4906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammerschmidt S, Birkholz C, Zähringer U, Robertson B D, van Putten J P M, Ebeling O, Frosch M. Contribution of genes from the capsule gene complex (cps) to lipooligosaccharide biosynthesis and serum resistance in Neisseria meningitidis. Mol Microbiol. 1994;11:885–896. doi: 10.1111/j.1365-2958.1994.tb00367.x. [DOI] [PubMed] [Google Scholar]
- 22.Hedberg K, Hoesly F, Fleming D, Steingart K, Goldoft M, Stehr-Green P. Serogroup B meningococcal disease—Oregon, 1994. Morbid Mortal Weekly Rep. 1995;44:121–124. [PubMed] [Google Scholar]
- 23.Horstmann R D. Target recognition failure by the nonspecific defense system: surface constituents of pathogens interfere with the alternative pathway of complement activation. Infect Immun. 1992;60:721–727. doi: 10.1128/iai.60.3.721-727.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarvis G A. Analysis of C3 deposition and degradation on Neisseria meningitidis and Neisseria gonorrhoeae. Infect Immun. 1994;62:1755–1760. doi: 10.1128/iai.62.5.1755-1760.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarvis G A, Griffiss J M. Human IgA initiates complement-mediated killing of Neisseria meningitidis. J Immunol. 1989;143:1703–1709. [PubMed] [Google Scholar]
- 26.Jarvis G A, Li J. Complement component C1q is required for IgA1-initiated killing of Neisseria meningitidis. In: Zollinger W D, Freasch C E, Deal C D, editors. Abstracts of the Tenth International Pathogenic Neisseria Conference. 1996. p. 287. [Google Scholar]
- 27.Jarvis G A, Vedros N A. Sialic acid of group B Neisseria meningitidis regulates alternative complement pathway activation. Infect Immun. 1987;55:174–180. doi: 10.1128/iai.55.1.174-180.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones D M, Borrow R, Fox A J, Gray S, Cartwright K A, Poolman J T. The lipooligosaccharide immunotype as a virulence determinant in Neisseria meningitidis. Microb Pathog. 1992;13:219–224. doi: 10.1016/0882-4010(92)90022-g. [DOI] [PubMed] [Google Scholar]
- 29.Kahler C M, Carlson R W, Rahman M M, Martin L E, Stephens D S. Inner core biosynthesis of lipooligosaccharide (LOS) in Neisseria meningitidis serogroup B: identification and role in LOS assembly of the α1,2 N-acetylglucosamine transferase (RfaK) J Bacteriol. 1996;178:1265–1273. doi: 10.1128/jb.178.5.1265-1273.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kahler C M, Carlson R W, Rahman M M, Martin L E, Stephens D S. Two glycosyltransferase genes, lgtF and rfaK, constitute the lipooligosaccharide ice (inner core extension) biosynthesis operon of Neisseria meningitidis. J Bacteriol. 1996;178:6677–6684. doi: 10.1128/jb.178.23.6677-6684.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein N J, Ison C A, Peak M, Levin M, Hammerschmidt S, Frosch M, Heyderman R S. The influence of capsulation and lipooligosaccharide structure on neutrophil adhesion molecule expression and endothelial injury by Neisseria meningitidis. J Infect Dis. 1996;173:172–179. doi: 10.1093/infdis/173.1.172. [DOI] [PubMed] [Google Scholar]
- 32.Lee F K N, Stephens D S, Gibson B W, Engstrom J J, Zhou D, Apicella M A. Microheterogeneity of Neisseria lipooligosaccharide: analysis of a UDP-glucose 4-epimerase mutant of Neisseria meningitidis NMB. Infect Immun. 1995;63:2508–2515. doi: 10.1128/iai.63.7.2508-2515.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mandrell R E, Griffiss J M, Macher B A. Lipooligosaccharides (LOS) of Neisseria gonorrhoeae and Neisseria meningitidis have components that are immunochemically similar to precursors of human blood group antigens. Carbohydrate sequence specificity of the mouse monoclonal antibodies that recognize crossreacting antigens on LOS and human erythrocytes. J Exp Med. 1988;168:107–126. doi: 10.1084/jem.168.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mandrell R E, Kim J J, John C M, Gibson B W, Sugai J V, Apicella M A, Griffiss J M, Yaurasaki R. Endogenous sialylation of the lipooligosaccharides of Neisseria meningitidis. J Bacteriol. 1991;173:2823–2832. doi: 10.1128/jb.173.9.2823-2832.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masson L, Holbein B E, Ashton F E. Virulence linked to polysaccharide production in serogroup B Neisseria meningitidis. FEMS Microbiol Lett. 1982;13:187–190. [Google Scholar]
- 36.Meri S, Pangburn M K. Discrimination between activators and nonactivators of the alternative pathway of complement: regulation via a sialic acid/polyanion binding site on factor H. Proc Natl Acad Sci USA. 1990;87:3982–3986. doi: 10.1073/pnas.87.10.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moran E E, Brandt B L, Zollinger W D. Expression of the L8 lipopolysaccharide determinant increases the sensitivity of Neisseria meningitidis to serum bactericidal activity. Infect Immun. 1994;62:5290–5295. doi: 10.1128/iai.62.12.5290-5295.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morse S A, Bartenstein L. Factors affecting autolysis of Neisseria gonorrhoeae. Proc Soc Exp Biol Med. 1974;145:1418–1421. doi: 10.3181/00379727-145-38025. [DOI] [PubMed] [Google Scholar]
- 39.Nicholson A, Lepow I H. Host defense against Neisseria meningitidis requires a complement-dependent bactericidal activity. Science. 1979;205:298–299. doi: 10.1126/science.451601. [DOI] [PubMed] [Google Scholar]
- 40.Pangburn M K, Muller-Eberhard H J. Complement C3 convertase: cell surface restriction of B1H control and generation of restriction on neuraminidase-treated cells. Proc Natl Acad Sci USA. 1978;75:2416–2420. doi: 10.1073/pnas.75.5.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prentki P, Krisch H M. In vivo insertional mutagenesis with a selectable DNA fragment. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 42.Rahman M M, Stephens D S, Kahler C M, Gluska J, Carlson R W. The lipooligosaccharide (LOS) of Neisseria meningitidis serogroup B strain NMB contains L2, L3 and novel oligosaccharides and lacks the lipid-A 4′ phosphate substituent. Carbohydrate Res. 1998;307:311–324. doi: 10.1016/s0008-6215(98)00012-3. [DOI] [PubMed] [Google Scholar]
- 43.Ram S, Sharma A K, Simpson S D, Gulati S, McQuillen D P, Pangburn M K, Rice P A. A novel sialic acid binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J Exp Med. 1998;187:743–752. doi: 10.1084/jem.187.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raymond N J, Reeves M, Ajello G, Baughman W, Gheesling L, Carlone G, Wenger J D, Stephens D S. Molecular epidemiology of sporadic (endemic) serogroup C meningococcal disease. J Infect Dis. 1997;176:1277–1284. doi: 10.1086/514123. [DOI] [PubMed] [Google Scholar]
- 45.Reller L B, MacGregor R R, Beaty H N. Bactericidal antibody after colonization with Neisseria meningitidis. J Infect Dis. 1978;127:56–62. doi: 10.1093/infdis/127.1.56. [DOI] [PubMed] [Google Scholar]
- 46.Rietschel E T, Kirikae T, Schade F U, Mamat U, Schmidt G, Loppnow H, Ulmer A J, Zahringer U, Seydel U, di Padova F, Schreier M, Brade H. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994;8:217–225. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 47.Robertson B D, Frosch M, van Putten J P M. The role of galE in the biosynthesis and function of gonococcal lipopolysaccharide. Mol Microbiol. 1993;8:891–901. doi: 10.1111/j.1365-2958.1993.tb01635.x. [DOI] [PubMed] [Google Scholar]
- 48.Ross S C, Rosenthal P J, Berberich H M, Densen P. Killing of Neisseria meningitidis by human neutrophils: implication for normal and complement deficient individuals. J Infect Dis. 1987;155:1266–1275. doi: 10.1093/infdis/155.6.1266. [DOI] [PubMed] [Google Scholar]
- 49.Roth R I, Yamasaki R, Mandrell R E, Griffiss J M. Ability of gonococcal and meningococcal lipooligosaccharides to clot Limulus amebocyte lysate. Infect Immun. 1992;60:762–767. doi: 10.1128/iai.60.3.762-767.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saukkonen K, Leinonen M, Abdillahi H, Pooman J T. Comparative evaluation of potential components for group B meningococcal vaccine by passive protection in the infant rat and in vitro bactericidal assay. Vaccine. 1989;7:325–328. doi: 10.1016/0264-410x(89)90194-1. [DOI] [PubMed] [Google Scholar]
- 51.Shih, G. C., C. M. Kahler, J. S. Swartley, M. M. Rahman, J. Coleman, R. W. Carlson, and D. S. Stephens. Multiple lysophosphatidic acyltransferases in Neisseria meningitidis. Submitted for publication. [DOI] [PubMed]
- 52.Shih, G. C., C. M. Kahler, R. W. Carlson, M. M. Rahman, and D. S. Stephens. Unpublished data.
- 53.Smith H, Parsons N J, Cole J A. Sialylation of neisserial lipopolysaccharide: a major influence on pathogenicity. Microb Pathog. 1995;19:365–377. doi: 10.1006/mpat.1995.0071. [DOI] [PubMed] [Google Scholar]
- 54.Stephens D S, Whitney A M, Schoolnik G K, Zollinger W D. Common epitopes of pilin of Neisseria meningitidis. J Infect Dis. 1988;158:332–342. doi: 10.1093/infdis/158.2.332. [DOI] [PubMed] [Google Scholar]
- 55.Stephens D S, Shafer W M. Evidence that serum resistance genetic locus sac-3 of Neisseria gonorrhoeae is involved in lipopolysaccharide structure. J Gen Microbiol. 1987;133:2671–2679. doi: 10.1099/00221287-133-9-2671. [DOI] [PubMed] [Google Scholar]
- 56.Stephens D S, McAllister C F, Zhou D, Lee F K, Apicella M A. Tn916-generated, lipooligosaccharide mutants of Neisseria meningitidis and Neisseria gonorrhoeae. Infect Immun. 1994;62:2947–2952. doi: 10.1128/iai.62.7.2947-2952.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Swartley J, Ahn J, Stephens D S. Expression of sialic acid and polysialic acid in serogroup B Neisseria meningitidis: divergent transcription of biosynthesis and transport operons through a common reporter region. J Bacteriol. 1996;178:4052–4059. doi: 10.1128/jb.178.14.4052-4059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swartley J S, Marfin A A, Edupuganti S, Liu L-J, Cieslak P, Perkins B, Wenger J D, Stephens D S. Capsule switching of Neisseria meningitidis. Proc Natl Acad Sci USA. 1997;94:271–276. doi: 10.1073/pnas.94.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swartley J S, Liu L-J, Miller Y K, Martin L E, Edupuganti S, Stephens D S. Characterization of the gene cassette required for biosynthesis of the (α1→6)-linked N-acetyl-d-mannosamine-1-phosphate capsule of serogroup A Neisseria meningitidis. J Bacteriol. 1998;180:1533–1539. doi: 10.1128/jb.180.6.1533-1539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Swartley J S, Stephens D S. Identification of a genetic locus involved in the biosynthesis of N-acetyl-d-mannosamine, a precursor of the (α2→8)-linked polysialic acid capsule of serogroup B Neisseria meningitidis. J Bacteriol. 1994;176:1530–1534. doi: 10.1128/jb.176.5.1530-1534.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taylor P W. Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol Rev. 1983;47:46–83. doi: 10.1128/mr.47.1.46-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsai C-M, Civin C I. Eight lipooligosaccharides of Neisseria meningitidis react with a monoclonal antibody which binds lacto-N-neotetraose (Galβ1-4GlcNAcβ1-3Galβ1-4Glc) Infect Immun. 1991;59:3604–3609. doi: 10.1128/iai.59.10.3604-3609.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62a.Virji, M., and D. S. Stephens. Unpublished data.
- 63.Vogel U, Claus H, Heinze G, Frosch M. Functional characterization of an isogenic meningococcal alpha-2,3-sialyltransferase mutant: the role of lipooligosaccharide sialylation for serum resistance in serogroup B meningococci. Med Microbiol Immunol. 1997;186:159–166. doi: 10.1007/s004300050059. [DOI] [PubMed] [Google Scholar]
- 64.Vogel U, Hammerschmidt S, Frosch M. Sialic acids of both the capsule and the sialylated lipooligosaccharide of Neisseria meningitidis serogroup B are prerequisites for virulence of meningococci in the infant rat. Med Microbiol Immunol. 1996;185:81–87. doi: 10.1007/s004300050018. [DOI] [PubMed] [Google Scholar]
- 65.Vogel U, Weinberger A, Frank R, Müller A, Köhl J, Atkinson J P, Frosch M. Complement factor C3 deposition and serum resistance in isogenic capsule and lipooligosaccharide sialic acid mutants of serogroup B Neisseria meningitidis. Infect Immun. 1997;65:4022–4029. doi: 10.1128/iai.65.10.4022-4029.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wakarchuk W, Martin A, Jennings M P, Moxon E R, Richards J C. Functional relationships of the genetic locus encoding the glycosyltransferase enzymes involved in expression of the lacto-N-neotetraose terminal lipopolysaccharide structure in Neisseria meningitidis. J Biol Chem. 1996;271:19166–19173. doi: 10.1074/jbc.271.32.19166. [DOI] [PubMed] [Google Scholar]
- 67.Wetzler L M, Barry K, Blake M S, Gotschlich E C. Gonococcal lipooligosaccharide sialylation prevents complement-dependent killing by immune sera. Infect Immun. 1992;60:39–43. doi: 10.1128/iai.60.1.39-43.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamasaki R, Griffiss J M, Quinn K P, Mandrell R E. Neuraminic acid is α2→3 linked in the lipooligosaccharide of Neisseria meningitidis serogroup B strain 6275. J Bacteriol. 1993;175:4565–4568. doi: 10.1128/jb.175.14.4565-4568.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou D, Stephens D S, Gibson B W, Engstrom J, McAllister C F, Lee F K N, Apicella M A. Lipooligosaccharide biosynthesis in pathogenic Neisseria: cloning, identification and characterization of the phosphoglucomutase gene. J Biol Chem. 1994;269:11162–11169. [PubMed] [Google Scholar]