Abstract
Soft ticks (Ixodida: Argasidae) are ectoparasites of terrestrial vertebrates with worldwide distributions. As one representative group of Argasidae, the genus Argas has an important vectorial role in transmitting zoonotic diseases. However, our knowledge of the subgenus Argas in China is still limited, as most literature only lists occurrence records or describes specific case reports without providing detailed morphological characteristics and further molecular data. This study aims to characterize Argas vulgaris through complete mitochondrial sequencing and morphological diagnostic techniques based on a batch of adult specimens collected from Ningxia Hui Autonomous Regions (NXHAR), North China. The morphology and microstructures of Ar. vulgaris and other lectotypes of argasid ticks in the subgenus Argas were also observed using a stereomicroscope. Following DNA extraction and sequencing, a complete mitochondrial sequence of Ar. vulgaris was assembled and analyzed within a phylogenetic context. The 14,479 bp mitogenome of Ar. vulgaris consists of 37 genes, including 13 genes for protein coding, two for ribosomal RNA, 22 for transfer RNA, and one for control region (D-loops). Phylogenetic analysis of Ar. vulgaris showed 98.27%–100% nucleotide identity with Ar. japonicus, indicating a close relationship between the two tick species. The morphological diagnostic features to differentiate Ar. vulgaris from other ticks within the subgenus Argas included the location of the anus and setae on the anterior lip of the female genital aperture. This study provided high-resolution scanning electron microscope images of female Ar. vulgaris and corresponding molecular data, representing valuable resources for future accurate species identification.
Keywords: Argas, Morphological, Mitochondrial genome, Phylogenetic
Graphical abstract
Highlights
-
•
The first complete mitochondrial sequence of Ar. vulgaris were assembled.
-
•
High-resolution SEM images of Ar. vulgaris were presented.
-
•
Morphological diagnostic features of Ar. vulgaris were compared with other argasid ticks within the subgenus Argas.
1. Introduction
Ticks (Acari: Ixodidae) are obligatory hematophagous arthropods with a global distribution, known for their ability to transmit a diverse array of pathogens (Rochlin and Toledo, 2020; Sarwar, 2017). They are believed to surpass all other arthropods in the variety of infectious agents that they transmit (Sonenshine and Roe, 2013). Modern tick species are divided into five families, Argasidae (argasid ticks), Ixodidae (ixodid ticks), Nuttalliellidae (monotypic), and two newly erected families, Khimairidae (Chitimia-Dobler et al., 2022) and Deinocrotonidae (Peñalver et al., 2017). More than 221 argasid ticks have been described to date worldwide. In China, approximately 15 species from two genera were identified, including 10 species of the genus Argas and 5 species of the genus Ornithodoros (Chen and Liu, 2022).
Ticks of the genus Argas primarily parasitize domestic or feral pigeons, sparrows, swallows, and various other birds inhabiting arboreal nests (Estrada-Peña et al., 2010). There are two subgenera under the genus Argas in China (Sun et al., 2019). One is the subgenus Argas, which comprises Argas vulgaris, Ar. japonicus, Ar. assimilis, and Ar. beijingensis. Historically, Ar. vulgaris was scarcely collected, and misidentification was reported (Dusbábek, 1976; Teng, 1983) because this species was morphologically closely related to the other argasid ticks. For instance, the geographical distribution and host association of Ar. vulgaris coincided with that of the species in the genus Argas, which might lead to misidentification (Chen and Liu, 2022; Sun et al., 2019). The other subgenus Persicargas contains Ar. persicus and Ar. robertsi, the former of which is the most common species in China (Chen and Liu, 2022) may transmit a variety of pathogenic viruses (Zahid et al., 2021).
It is well known that numerous argasid ticks share such a high degree of external morphological resemblance, and discerning them from one another remains difficult (Muñoz-Leal et al., 2018). Currently, the higher hierarchical system of Argasidae is still argued by different classification schools and nearly two-thirds of argasid taxon species are controversial (Chen and Liu, 2022). To date, the argasid ticks mentioned above have not been thoroughly studied except Ar. persicus, which spreads widely along with its hosts, such as birds. The molecular species identification of argasid ticks relies on mitochondrial genes (Guzmán-Cornejo et al., 2017; Muñoz-Leal et al., 2016; Labruna et al., 2016; Mans et al., 2021), but the complete mitochondrial sequence of these species remains scarce. For instance, there is only a partial 16 S rRNA sequence of Ar. vulgaris in GenBank, but the COI sequence is unavailable. In addition, the morphological characteristics of the sequenced sample of Ar. vulgaris were not provided. Herein, a complete mitochondrial sequence of Ar. vulgaris may apply to distinguishing morphologically similar species. Phylogenetic analysis revealed multiple closely related species of Ar. vulgaris based on the mitochondrial genetic marker, 16 S rRNA gene. Therefore, we provided scanning electron microscope images of Ar. vulgaris based on specimens collected in Ningxia province, China. A detailed morphology comparison among Ar. vulgaris with other argasid ticks in the subgenus Argas were conducted to facilitate future tick species identification. The sequence data and images provided in this study are valuable resources for the accurate identification of these tick species, which are vital for vector surveillance and tick-borne disease control.
2. Materials and methods
2.1. Sample collection and morphological identification
The samples of Ar. vulgaris were collected in Ningxia Province (105°41′55″E, 37°29′21″N), China. We searched areas frequented by domestic pigeons and inspected cracks in the walls and fowl nests in April 2022. The collected ticks were preserved in 70% ethanol and the dorsal and ventral views of ticks were examined by a stereo microscope. Adults of Ar. vulgaris were coated with carbon-gold and examined with a scanning electron microscope (SEM). Under SEM, tick species were identified according to the keys described by Walker (2003) and Hoogstraal (Kaiser et al., 1964). Samples for SEM were prepared according to the methods presented by Keirans et al. (1976).
2.2. Specimens examination
Argas beijingensis: 3♀, 2♂ from home pigeon, Columba livia, in Dis. Shijingshan Beijing, Deposited in Institute of Zoology, Chinese Academy of Sciences.
Argas assimilis: 6♀, 3 ♂ from swallow, Hirundo daurica japonica, in Tonggu County, Jiangxi Province, collected by Jieyi Song on Nov. 9, 1980. Deposited in Institute of Zoology, Chinese Academy of Sciences.
Argas japonicus: 8 N, from swallow, Hirundo daurica japonica, in Changchun City, Jilin Province, on Jun. 11, 1959. Deposited in Institute of Zoology, Chinese Academy of Sciences.
Argas vulgaris: 10♀, 7♂, from home pigeon, Columba livia, in Maigaiti County, Xinjiang Uygur Autonomous Regions, on March 5, 1992. Deposited in Center for Diseases Prevention and Control of Xinjiang Uygur Autonomous Regions.
2.3. DNA extraction and sequencing
DNA was extracted from a single female of Ar. vulgaris and the genomic DNA was selected by the Agencourt AMPure XP-Medium kit to an average size of 200–400bp. The integrity of the extracted DNA was assessed using Femto Pulse (https://www.agilent.com.cn/en/product/automated-electrophoresis/femto-pulse-systems/femto-pulse-system-software). The qualified libraries were sequenced on a MGISEQ2000 platform, and paired-end reads of 150 bp were generated.
2.4. Mitochondrial genome assembly and phylogenetic analyses
Based upon ∼50 Gb of data, the mitogenomes were de novo assembled using the “all” module from MitoZ (v2.3) (Meng et al., 2019). The GenBank files generated by MitoZ were visualized and manually corrected in Geneious Prime (version 2023.2) (Kearse et al., 2012). Subsequently, the 16 S rRNA and COI sequences were extracted from the assembled mitochondrial sequence. Then, phylogenetic trees were constructed based on sequences from complete mitochondrial genome, 16 S rRNA and COI of other argasid tick species available in GenBank. The sequences were aligned using MAFFT (v7.487) (Katoh and Standley, 2013) and the phylogenetic trees were built using IQ-TREE (v2.1.4) (Nguyen et al., 2015) with the best-fit substitution model and ultrafast bootstrap of 1000 replications. The genetic distances of each sequence were calculated using MEGA11 (Tamura et al., 2021).
3. Results
3.1. Molecular-phylogenetic relationships of Ar. vulgaris
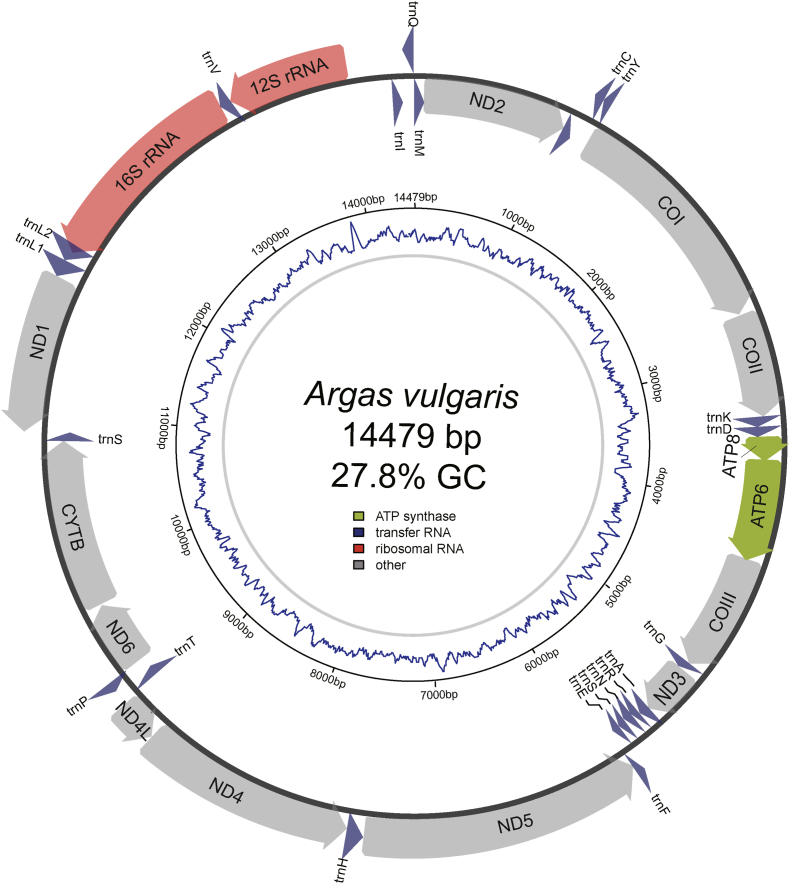
We obtained the first representative complete mitochondrial genome sequence of Ar. vulgaris through short-read sequencing and assembly (Fig. 1). The complete genome of Ar. vulgaris was 14,479 bp in length. Additionally, the circular and double-stranded DNA structure of the mitochondrial genomes of this species was successfully predicted. The gene arrangement of the mitochondrial genomes included 13 protein-coding genes (PCGs), 22 transfer RNA (tRNA) genes, and 2 ribosomal RNA (rRNA) genes, similar to other argasid ticks (Burger et al., 2014). Four of the 13 PCGs (ND5, ND1, ND4L, and ND4), nine tRNAs (trnF, trnC, trnQ, trnP, trnH, trnV, trnY, trnL2, and trnL1) and two rRNAs (16 S rRNA and 12 S rRNA) were coded on the light strand (L-strand), while the other 22 genes (trnI, trnT, trnS2, trnD, trnM, trnG, trnW, trnK, trnA, trnR, trnN, trnE, trnS1, ATP8, ATP6, ND6, CYTB, COIII, ND3, ND2, COI, and COII) were transcribed on the heavy strand (H-strand). The overall nucleotide composition of the complete mitochondrial genome was listed in Table 1. The nucleotide sequence of the Ar. vulgaris mitochondrial genome included approximately adenine = 5600 (38.67%), thymine = 4850 (33.50%), guanine = 1338 (9.24%), and cytosine = 2691 (18.59%). The proportion of adenine + thymine (72.17%) was substantially higher than that of guanine + cytosine (27.83%). GC skew ranged from −0.633 (for ND4L) to −0.15 (for COI), while AT skew ranged from −0.125 (for ND2) to 0.323 (for ND1). The whole AT skew and GC skew in the entire mitochondrial genome of Ar. vulgaris was 0.072 and −0.336, respectively.
Fig. 1.
The assembled mitogenome of Argas vulgaris. All the annotated genes are plotted in the outer circle and the inner tract shows the GC content.
Table 1.
Composition and skew values of the mitochondrial genome of Ar. vulgaris.
| Genes | Size (bp) | A (%) | C (%) | T (%) | G (%) | A + T (%) | G + C (%) | AT-Skew | GC-skew |
|---|---|---|---|---|---|---|---|---|---|
| Mitogenome | 14,479 | 38.67 | 18.59 | 33.5 | 9.24 | 72.17 | 27.83 | 0.072 | −0.336 |
| PCGs | 10,897 | 38.33 | 19.49 | 32.72 | 9.45 | 71.06 | 28.94 | 0.079 | −0.347 |
| tRNAs | 1365 | 39.85 | 14.80 | 35.09 | 10.26 | 74.95 | 25.05 | 0.064 | −0.181 |
| rRNAs | 2083 | 40.90 | 16.18 | 35.67 | 7.25 | 76.57 | 23.43 | 0.068 | −0.381 |
| D-loop | 238 | 31.09 | 18.07 | 39.50 | 11.34 | 70.59 | 29.41 | −0.119 | −0.229 |
| ND5 | 1663 | 46.54 | 19.24 | 25.32 | 8.90 | 71.86 | 28.14 | 0.295 | −0.368 |
| ND4 | 1326 | 47.96 | 18.93 | 24.81 | 8.30 | 72.78 | 27.22 | 0.318 | −0.391 |
| ND4l | 279 | 51.61 | 17.56 | 26.88 | 3.94 | 78.49 | 21.51 | 0.315 | −0.633 |
| ND6 | 476 | 38.24 | 15.34 | 40.13 | 6.30 | 78.36 | 21.64 | −0.024 | −0.417 |
| ND2 | 951 | 32.70 | 17.88 | 42.06 | 7.36 | 74.76 | 25.24 | −0.125 | −0.417 |
| COI | 1539 | 30.41 | 19.23 | 36.13 | 14.23 | 66.54 | 33.46 | −0.086 | −0.150 |
| COII | 676 | 33.28 | 21.45 | 33.58 | 11.69 | 66.86 | 33.14 | −0.004 | −0.295 |
| ATP8 | 156 | 37.18 | 17.31 | 41.03 | 4.49 | 78.21 | 21.79 | −0.049 | −0.588 |
| ATP6 | 669 | 34.53 | 20.78 | 35.87 | 8.82 | 70.40 | 29.60 | −0.019 | −0.404 |
| COIII | 779 | 31.45 | 20.15 | 37.48 | 10.91 | 68.93 | 31.07 | −0.088 | −0.298 |
| ND3 | 339 | 32.45 | 18.88 | 40.12 | 8.55 | 72.57 | 27.43 | −0.106 | −0.376 |
| ND1 | 939 | 47.07 | 21.09 | 24.07 | 7.77 | 71.14 | 28.86 | 0.323 | −0.461 |
| 16 S rRNA | 1277 | 41.43 | 16.13 | 35.71 | 6.73 | 77.13 | 22.87 | 0.074 | −0.411 |
| 12 S rRNA | 806 | 40.07 | 16.25 | 35.61 | 8.06 | 75.68 | 24.32 | 0.059 | −0.337 |
Abbreviations: PCG, protein-coding gene; rRNA, ribosomal RNA; tRNA, transport RNA.
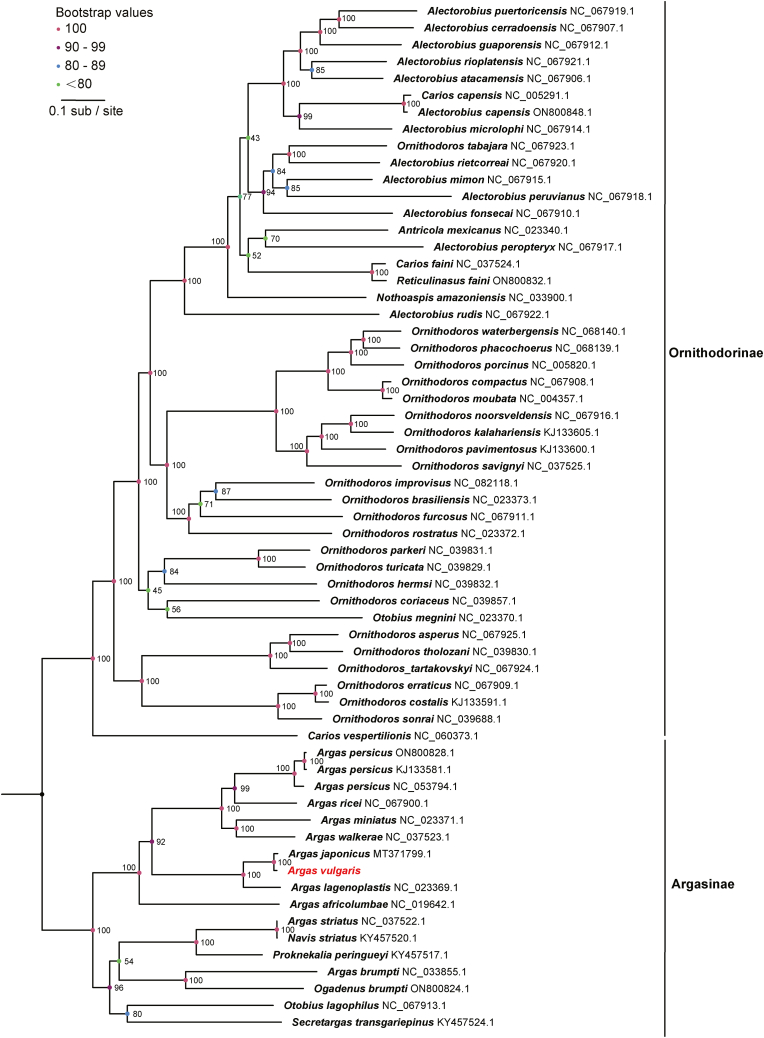
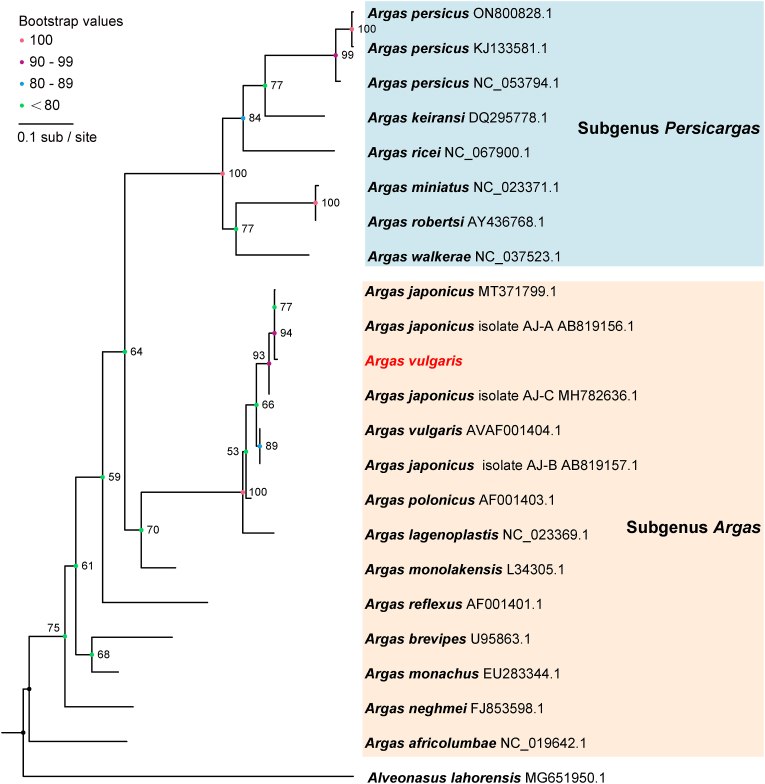
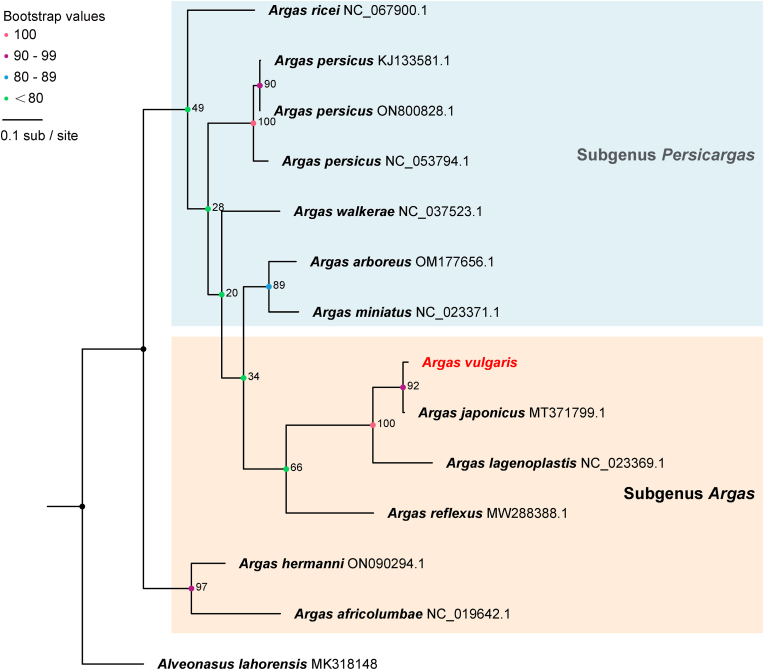
Phylogenetic analysis was then performed with available sequences of other argasid species deposited in GenBank. In terms of phylogenetic relationships within the genus Argas, the subgenus Persicargas was intricately nested within the subgenus Argas as reported in previous studies (Mans et al., 2019; Estrada-Peña et al., 2010), albeit with low bootstrap support value (Fig. 2, Fig. 3, Fig. 4). Among species from these two subgenera, a range of 69.96–70.83% nucleotide identity was observed in the complete mitogenomes, while the 16 S rRNA gene exhibited identities of 69.23–87.78%, and the COI gene showed identities of 75.92–79.67% (Supplementary Tables 1–3). Morphologically, the two subgenera were diagnosed by the combination of the absence or presence of postpalpal setae and the shape of marginal cells.
Fig. 2.
Evolutionary relationships among ticks of the Argasidae families. The phylogenetic tree of complete sequences of mitochondrial genomes. Numbers at the nodes are bootstrap values of the ML analysis. The GenBank accession numbers are listed after the species names.
Fig. 3.
Phylogenetic tree of Argas species based on the 16S rRNA gene contained in the mitochondrial genome. Numbers at the nodes are bootstrap values of the ML analysis. The GenBank accession numbers are listed after the species names.
Fig. 4.
Phylogenetic tree of Argas species based on the COI gene contained in the mitochondrial genome. Numbers at the nodes are bootstrap values of the ML analysis. The GenBank accession numbers are listed after the species names.
Notably, a closely related species of Ar. vulgaris was identified in the phylogenetic analysis. In the phylogenetic tree constructed from complete mitogenomes (Fig. 2), the sequence of Ar. vulgaris obtained in this study clustered with the sequence of Ar. japonicus (MT371799), showing a nucleotide identity of 98.56% (Supplementary Table 1). Based on the 16 S rRNA sequences (Fig. 3), Ar. vulgaris in this study clustered with sequences of both Ar. japonicus and Ar. vulgaris from GenBank. This cluster could be divided into two clades. In the first clade, the 16 S rRNA of Ar. vulgaris obtained in this study displayed 100% similarity with Ar. japonicus Aj-A from Japan (AB819156.1) and 99.51% with Ar. japonicus Aj-C (MH782636.1). In the second clade, the 16 S rRNA of Ar. vulgaris from the Kyrgyzstan (Dabert et al., 1999) (AVAF001404.1) in GenBank and Ar. japonicus Aj-B from Japan (AB819157.1) were grouped, showing 100% identity to each other (Supplementary Table 2). The phylogeny based on the COI gene was concordant with the topology based on 16 S rRNA. The COI gene sequence of Ar. vulgaris clustered with Ar. japonicus (Fig. 4), and the two sequences showed 98.69% nucleotide identity with each other (Supplementary Table 3). These results suggest that Ar. vulgaris and Ar. japonicus cannot be distinguished merely based on sequences of 16 S rRNA, COI and even the whole mitogenome.
3.2. Diagnostic characters of Ar. vulgaris and notes with Argas species relevant
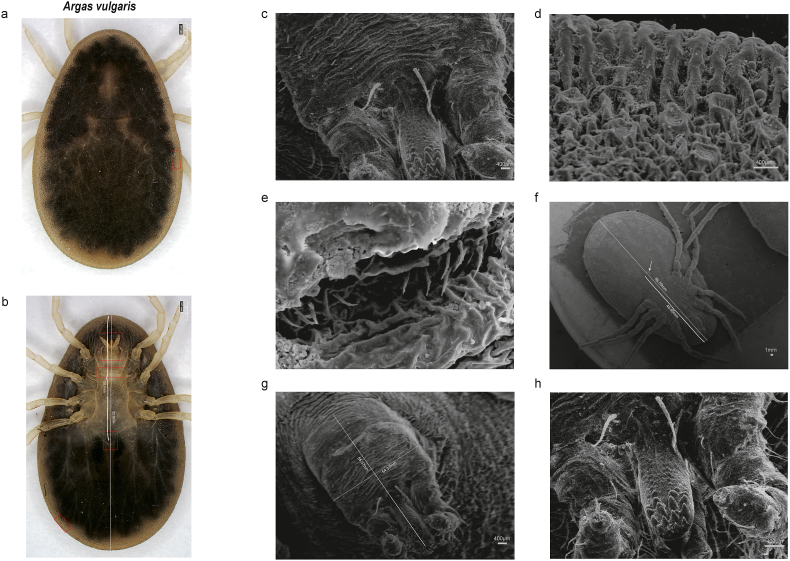
The ticks collected in this study were morphologically recognized as Ar. vulgaris (Fig. 5a and b) according to the following features, including the invisible postpalpal setae (Fig. 5c), marginal cell fenced with a narrow and long rugose ridge (Fig. 5d), and the anterior lip of genital aperture without setae (Fig. 5e) (Sun et al., 2019). In addition, the anal of Ar. vulgaris was situated posteriorly, far from the middle level of the body (Fig. 5f).
Fig. 5.
Key morphological characters of female Ar. vulgaris. (a) Dorsal view of female Ar. vulgaris. (b) Ventral view of female Ar. vulgaris. (c) Posthypostomal seta, ventral view. (d) Details structure of the marginal cells between the dorsal and ventral surfaces. (e) Genital aperture of female Ar. vulgaris. (f) Ventral view of anus location. (g) Ventral view of capitulum. (h) Ventral view of hypostome of the dentition formula.
We further compared the morphological traits of Ar. vulgaris to that of Ar. japonicus, which was a closely related species to Ar. vulgaris based on the phylogenetic analysis, revealing several distinctive differences between the two species. Ar. japonicus exhibited numerous short frayed setae on the anterior lip (Yamaguti et al., 1968), whereas the setae of Ar. vulgaris were located on the posterior lip (Fig. 5e). Meanwhile, the basic capitula wide/long ratio as well as the positions of the genital aperture and anus of these two species were also different. Additionally, Ar. japonicus was rectangular with a wide/long ratio of 1.7 (Hu et al., 2021), while Ar. vulgaris in this study had a partial round shape with a wide/long ratio of 1.5 (Fig. 5g). The posterolateral setae on the basis capitula of Ar. vulgaris exceeded 6 pairs, whereas 3–4 pairs were observed in Ar. japonicus (Yamaguti et al., 1968). The genital aperture and anus of Ar. vulgaris situated closer to the anterior margin of the body compared to Ar. japonicus (Hu et al., 2021). In terms of the palpal segment, Ar. vulgaris exhibited a decreased length from segments I to IV, with segments III and IV being nearly equal in length (Fig. 5f). The ossification ring at the base of the marginal sensilla was either smaller than or equal to that of the bell-shaped sensilla (Teng, 1983). Furthermore, in Ar. japonicus, segments I and II were of equal length, shorter than segments III and IV, which were also equal in length. The ossification ring at the base of the body-marginal sensilla of Ar. japonicus was larger than that of the bell-shaped sensilla (Sun et al., 2019). The terminal portion of Tarsus I in Ar. vulgaris exhibited a sloping gradient, whereas in Ar. japonicus, it was curved apically on a fleshy projection at its end (Yamaguti et al., 1968). However, other distinguishing features such as the shortness of eyes and arrangement of anal setae were same in both species. Besides, the postpalpal setae in both Ar. japonicus and Ar. vulgaris were invisible on ventral view of basic capitula (Yamaguti et al., 1968) (Table 2).
Table 2.
Summary of most important diagnostic characteristics that differ or are similar between Ar. vulgaris and Ar. japonicus females.
| Argas vulgaris | Argas japonicus | |
|---|---|---|
| Ventral view | Basis capituli rectangular, two-thirds as long as wide, with a wide/long ratio of 1.5 (Fig. 5 g). | Basis capituli rectangular, half as long as wide, with a wide/long ratio of 1.7 (Hu et al., 2021). |
| A pair of posthypostomal seta, postpalpal setae absent (Fig. 5 c), exceeded 6 pairs of posterolateral setae on the basis capitula. | A pair of posthypostomal seta, postpalpal setae absent, 3–4 pairs of posterolateral setae on the basis capitula (Yamaguti et al., 1968). | |
| Dental formula 2/2 on middle of shaft, each row with 7∼9 denticles (Fig. 5 h). | Dental formula 2/2–3/3 on middle of shaft, 3/3 to 5/5 smaller denticles with blunt edges in more than 7 rows at base (Hu et al., 2021). | |
| Posterior lip of female genital aperture with setae numerous, but invisible in anterior lip (Fig. 5 e), transversely lobed, located at the level between coxae I and II (Fig. 5 f). | Anterior lip of female genital aperture with numerous short frayed setae (Yamaguti et al., 1968), transversely slit, located at the level of posterior border of the coxae I (Hu et al., 2021). | |
| Anus oval, far from the middle level of body (Fig. 5 f). | Anus elliptical, situated immediately posterior to body midlength (Hu et al., 2021). | |
| Dorsal view | Eyes absent. | Eyes absent (Yamaguti et al., 1968). |
| Long rugose ridge, discrete, groove broad and bent dramatically (Fig. 5 d). | Long rugose ridge, discrete, groove broad and bent dramatically (Sun et al., 2019). | |
| Disc varied in size, round or ovoid, arranged radially toward the margin, slightly larger near the central disc, smaller and irregularly arranged near the margin. | Discs radially arranged, radial elevation of integument among rows of radial discs more prominent in posterior 1/2 of body (Yamaguti et al., 1968). Anteromedian two discs comparatively larger, oval, or spindle shape, others towards margins mostly relatively small, subcircular (Hu et al., 2021). | |
| Lateral view | Marginal cells striated or fenced with slightly continued (Fig. 5 d). | Marginal cells striated or fenced with slightly continued (Hu et al., 2021). |
| A decreased length from segments I to IV, with segments III and IV nearly equal in length (Fig. 5 f). | Segments globose, I widest, IV narrowest, segments II and III subequal in width, IV about equal in length to III (Hu et al., 2021). | |
| The ossification ring at the base of the marginal sensilla smaller than or equal to the bell-shaped sensilla (Teng, 1983). | The ossification ring at the base of the marginal sensilla larger than the bell-shaped sensilla (Sun et al., 2019). | |
| The tarsus I slope gradient terminally. | The tarsus I curved apically on fleshy projection in its end (Yamaguti et al., 1968) |
Ar. assimilis was similar to Ar. japonicus, in that the integumental ridges were relatively narrower and markedly raised (integumental ridges were thick and not markedly raised in Ar. japonicus); peripheral integumental ridges were narrower and elongated and regularly arranged (peripheral ridges are thick and short, irregularly arranged in Ar. japonicus); the hypostome of females extending to the mid-length of palpal article 3rd (extended to mid-length of palpal article 2nd in Ar. japonicus); article 3rd was shorter than article 4th (article 3rd is equal to article 4th in Ar. japonicus); the basic capitula of Ar. assimilis was rectangular with a wide/long ratio of 1.8, and the anterior lip of the genital aperture with setae (Teng, 1983; Teng and Song, 1983). Ar. beijingensis also closely resembled Ar. vulgaris, but could be distinguished by the combination of the following characteristics. The anus of adults was slightly posterior to the center of the venter (much more separated from the middle of the ventral body surface in Ar. vulgaris), peripheral integumental ridges were short and sinuous (relatively narrower and longer in Ar. vulgaris Fig. 5d) (Chen and Liu, 2022), the dental formula was 2|2 for the first three rows and approximately 3|3 backward (2|2 type of hypostome dental formula, and each row has 7 to 9 teeth in Ar. vulgaris Fig. 5h). The palpal segments were cylindrical, widest in segment I, narrowed markedly in segment II, and progressively narrower in segments III and IV. The basic capitula of Ar. beijingensis was rectangular with a wide/long ratio of 2, and the anterior lip of the genital aperture had setae (Teng, 1983).
4. Discussion
To date, reliable morphological characteristics for systematic taxonomy of argasid ticks are still inadequate due to the unstable higher hierarchy of Argasidae designed by different classification schools (Mans et al., 2019). Currently, Argasidae comprises two subfamilies, Argasinae and Ornithodorinae, both with different numbers of genera and subgenera. The long-lasting arguments on the systematics of Argasidae are prone to cause misidentification or confusion in the correct interpretation of the systematic relationship between different taxa (Estrada-Peña et al., 2010). Traditionally, Argasidae species were usually identified with diagnostic characters derived by skilled taxonomists. The conclusive results depended greatly on the weights of external morphological characters with progeny and ancestor significance (Queiroz, 1985). However, the valuable diagnostic characteristics are not easy to stand due to the scarcity of specimens. It would make much sense to explore reliable, optional protocols to assist our accurate identification of argasid ticks with immense medical relevance. In the current study, the first complete mitochondrial sequence of Ar. vulgaris was provided in the combination of diagnostic characters investigated under high-resolution SEM techniques. The detailed morphological traits and molecular evidence yield valuable insights into the systematics of Ar. vulgaris and reliable differential protocols to distinguish the species from other related species in subgenus Argas.
The primary morphology characteristic distinguishing Ar. vulgaris from other argasid ticks in subgenus Argas was the absence of setae on the anterior lip of the genital aperture, which was frequently ignored in taxonomy identification. We provided a clear visualization of the genital aperture morphology of Ar. vulgaris for future reference. In addition, other morphological traits were also informative for differentiating this species from other argasid ticks, including body size, coloration, index of base capitula, size of palpal segments, and anal location. These morphological differences were essential for rapid identification, especially in regions where these argasid tick species coexist.
Molecular biology identification approaches offer unique advantages over morphological identification, particularly in accurately identifying incomplete or fragmentary specimens and differentiating morphological similar species (Ball and Armstrong, 2006). Reliance on morphological characters that vary within a species may lead to inflation of species. To overcome the limitation of morphological identification, various molecular identifications were explored. The phylogenetic analysis on the 16 S rRNA and COI sequences helps much in tick species identification. However, DNA barcoding might also overestimate the number of species (Song et al., 2008) and 16 S rRNA misidentification has been observed (Dash et al., 2012). A practicable classification system should integrate diagnostic morphological characters with molecular phylogenetic studies, whenever possible, as well as other related characters (e.g. anatomy, ecology, geography, palynology, phytochemistry). In our study, despite distinct morphological traits observed in Ar. vulgaris and Ar. japonicus, molecular biology identification unveiled a nucleotide identity of 98.56% between the mitogenome sequences of the two species, suggesting a close relationship between them. Since mitochondrial genes provide only partial phylogenetic information, the inconsistency between morphological traits and molecular evidence might be addressed through a comprehensive approach based on more accurate molecular markers and a broader range of specimens in the future.
5. Conclusions
We compared the morphological traits of Ar. vulgaris with those of other species in the subgenus Argas with high-resolution SEM images. In addition, the first mitochondrial sequence and phylogenetic analysis of Ar. vulgaris was provided. Interestingly, molecular analysis using mitogenome sequences suggested a very close relationship between Ar. japonicus and Ar. vulgaris, but morphological traits supported that they are two distinct species. Future investigations may necessitate the utilization of whole-genome data and a broader range of specimens to achieve a more precise differentiation between these two tick species.
Author contributions
W.-C.C., W.-Q.S., Y.S., and Y.-F.W. conceptualized the project; X.-M.C., X.-Y.S., D.T., N.W., W.-Y.G., and B.-H.W. collected samples and provided resources; Y.S. identified the tick species; M.-Z.Z. was in charge of DNA extraction and digital photography; Y.-F.W. and J.-J.Z. analyzed the data; Y.-F.W., L.-F.D., X.-Y.H., and N.C. created visualizations; Y.-F.W. wrote the original draft; W.-C.C., Y.S., and W.S. reviewed and edited the article; W.-C.C. and J.-F.J. supervised the project. All the authors have read and approved the final manuscript.
Funding
This work was supported by research grants from the Natural Science Foundation of China (81621005, W.-C.C.).
Availability of data and materials
The mitochondrial genome assemblies and annotations generated in this study are deposited in GenBank under the following accession numbers: OR892711 for Ar. vulgaris. All other relevant data are included in the manuscript and the references are available upon request by the corresponding author.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
The authors are grateful for the help from the State Key Laboratory of Pathogen and Biosecurity, Beijing Institute of Microbiology and Epidemiology for technical support of SEM, and Ningxia Medical University for obtaining the samples of Ar. vulgaris.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2024.100912.
Contributor Information
Yi Sun, Email: sunyi7310@sina.com.
Wenqiang Shi, Email: wqshi_mail@163.com.
Wu-Chun Cao, Email: caowuchun@126.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Ball S.L., Armstrong K.F. DNA barcodes for insect pest identification: a test case with tussock moths (Lepidoptera: lymantriidae) Can. J. For. Res. 2006;36(2):337–350. [Google Scholar]
- Burger T.D., Shao R., Labruna M.B., Barker S.C. Molecular phylogeny of soft ticks (Ixodida: Argasidae) inferred from mitochondrial genome and nuclear rRNA sequences. Ticks Tick Borne Dis. 2014;5:195–207. doi: 10.1016/j.ttbdis.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Chen Z., Liu J. A review of argasid ticks and associated pathogens of China. Front. Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.865664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitimia-Dobler L., Mans B.J., Handschuh S., Dunlop J.A. A remarkable assemblage of ticks from mid-Cretaceous Burmese amber. Parasitology. 2022;149(6):1–36. doi: 10.1017/S0031182022000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabert M., Dabert J., Siuda K. Ecology and Evolution of the Acari: Proceedings of the 3rd Symposium of the European Association of Acarologists 1–5 July 1996. Springer; Amsterdam, The Netherlands: 1999. Species validity of the soft-tick Argas polonicus (Acari: Argasidae) based on 16S rDNA sequence analysis; pp. 667–671. [Google Scholar]
- Dash N., Panigrahi D., Al-Zarouni M., Mishra S. 16S rRNA gene sequence analysis of a Brucella melitensis infection misidentified as Bergeyella zoohelcum. J Infect Dev Ctries. 2012;6(3):283–286. doi: 10.3855/jidc.2252. [DOI] [PubMed] [Google Scholar]
- Dusbábek F. Argas (Argas) vulgaris Filippova, 1961, a new member of Czechoslovak tick fauna. Folia Parasitol (Praha) 1976;23(3):281–283. [PubMed] [Google Scholar]
- Estrada-Peña A., Mangold A.J., Nava S., Venzal J.M., Labruna M., Guglielmone A.A. A review of the systematics of the tick family Argasidae (Ixodida) Acarologia. 2010;50(3):317–333. [Google Scholar]
- Guzmán-Cornejo C., García-Prieto L., Rebollo-Hernández A., Venzal J.M., Nava S., Sánchez-Montes S. Molecular evidence and additional morphological characters to distinguish Ornithodoros brodyi and Ornithodoros yumatensis (Ixodida: Argasidae) in their different developmental stages. Acta Parasitol. 2017;62(2):432–448. doi: 10.1515/ap-2017-0051. [DOI] [PubMed] [Google Scholar]
- Hu X., Liu J., Bao R. Redescription and molecular characterization of the tick Argas japonicus yamaguti, clifford & tipton, 1968 (ixodida: Argasidae) Parasitol. Res. 2021;120(11):3645–3651. doi: 10.1007/s00436-021-07320-7. [DOI] [PubMed] [Google Scholar]
- Kaiser M.N., Hoogstraal H., Kohls G.M. The subgenus Persicargas new subgenus (Ixodoidea, Argasidae, Argas). 1. A.(P.) arboreus, new species, an Egyptian persicus-like parasite of wild birds, with a redefinition of the subgenus Argas. Ann. Entomol. 1964;57(1):60–69. [Google Scholar]
- Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30(4):772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., Buxton S., Cooper A., Markowitz S., Duran C., Thierer T., Ashton B., Meintjes P., Drummond A. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28(12):1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirans J.E., Clifford C.M., Corwin D. Ixodes sigelos, n. sp. (Acarina : Ixodidae), a parasite of rodents in Chile, with a method for preparing ticks for examination by scanning electron microscopy. Acarologia. 1976;18(2):217–225. [PubMed] [Google Scholar]
- Labruna M.B., Nava S., Marcili A., Barbieri A.R., Nunes P.H., Horta M.C., Venzal J.M. A new argasid tick species (Acari: Argasidae) associated with the rock cavy, Kerodon rupestris Wied-Neuwied (Rodentia: caviidae), in a semiarid region of Brazil. Parasites Vectors. 2016;9(1):511. doi: 10.1186/s13071-016-1796-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mans B.J., Featherston J., Kvas M., Pillay K.-A., de Klerk D.G., Pienaar R., de Castro M.H., Schwan T.G., Lopez J.E., Teel P.J.T., diseases t.-b. Argasid and ixodid systematics: implications for soft tick evolution and systematics, with a new argasid species list. Ticks Tick Borne Dis. 2019;10:219–240. doi: 10.1016/j.ttbdis.2018.09.010. [DOI] [PubMed] [Google Scholar]
- Mans B.J., Kelava S., Pienaar R., Featherston J., de Castro M.H., Quetglas J., Reeves W.K., Durden L.A., Miller M.M., Laverty T.M., Shao R., Takano A., Kawabata H., Moustafa M.A.M., Nakao R., Matsuno K., Greay T.L., Evasco K.L., Barker D., Barker S.C. Nuclear (18S-28S rRNA) and mitochondrial genome markers of Carios (Carios) vespertilionis (Argasidae) support Carios Latreille, 1796 as a lineage embedded in the Ornithodorinae: re-classification of the Carios sensu Klompen and Oliver (1993) clade into its respective subgenera. Ticks Tick Borne Dis. 2021;12(4) doi: 10.1016/j.ttbdis.2021.101688. [DOI] [PubMed] [Google Scholar]
- Meng G., Li Y., Yang C., Liu S. MitoZ: a toolkit for animal mitochondrial genome assembly, annotation and visualization. Nucleic Acids Res. 2019;47(11):e63. doi: 10.1093/nar/gkz173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Leal S., Venzal J.M., González-Acuña D., Nava S., Lopes M.G., Martins T.F., Figueroa C., Fernández N., Labruna M.B. A new species of Ornithodoros (Acari: Argasidae) from desert areas of northern Chile. Ticks Tick Borne Dis. 2016;7(5):901–910. doi: 10.1016/j.ttbdis.2016.04.008. [DOI] [PubMed] [Google Scholar]
- Muñoz-Leal S., Venzal J.M., Nava S., Reyes M., Martins T.F., Leite R.C., Vilela V.L.R., Benatti H.R., Ríos-Rosas D., Barros-Battesti D.M., González-Acuña D., Labruna M.B. The geographic distribution of Argas (Persicargas) miniatus and Argas (Persicargas) persicus (Acari: Argasidae) in America, with morphological and molecular diagnoses from Brazil, Chile and Cuba. Ticks Tick Borne Dis. 2018;9(1):44–56. doi: 10.1016/j.ttbdis.2017.10.009. [DOI] [PubMed] [Google Scholar]
- Nguyen L.T., Schmidt H.A., von Haeseler A., Minh B.Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015;32(1):268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñalver E., Arillo A., Delclòs X., Peris D., Grimaldi D.A., Anderson S.R., Nascimbene P.C., Pérez-de la Fuente R. Parasitised feathered dinosaurs as revealed by Cretaceous amber assemblages. Nat. Commun. 2017;8(1):1924. doi: 10.1038/s41467-017-01550-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queiroz K.D. The ontogenetic method for determining characterpolarity and its relevance to phylogenetic systematics. Syst. Zool. 1985;34:280–299. [Google Scholar]
- Rochlin I., Toledo A. Emerging tick-borne pathogens of public health importance: a mini-review. J. Med. Microbiol. 2020;69(6):781–791. doi: 10.1099/jmm.0.001206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwar M. Status of argasid (soft) ticks (Acari: parasitiformes: Argasidae) in relation to transmission of human pathogens. Int J Vaccines Vaccin. 2017;4(4) [Google Scholar]
- Sonenshine D.E., Roe R.M. vol. 1. University of Oxford Press; New York: 2013. Ticks, people, and animals. (Biology of Ticks). [Google Scholar]
- Song H., Buhay J.E., Whiting M.F., Crandall K.A. Many species in one: DNA barcoding overestimates the number of species when nuclear mitochondrial pseudogenes are coamplified. Proc Natl Acad Sci U S A. 2008;105(36):13486–13491. doi: 10.1073/pnas.0803076105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Xu R., Wu M.J. Systematics of argasid ticks (Ixodida: Argasidae) in China with a pictorial key to species. Acta Parasetol. Med. Entomol. Sinica. 2019;26:231–250. [Google Scholar]
- Tamura K., Stecher G., Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021;38(7):3022–3027. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng K.F. Notes on Chinese ticks of the subgenus Argas (Acarina: Argasidae: Argas) Acta Zootaxonomica Sin. 1983;8:255–261. [Google Scholar]
- Teng K.F., Song J. A new species of Argas from Jiangxi, China (Acarina:Argasidae) Acta Zootaxonomica Sin. 1983;8:153–156. [Google Scholar]
- Walker A.R. Ticks of domestic animals in Africa: a guide to identification of species. Bioscience Reports Edinburgh. 2003 [Google Scholar]
- Yamaguti N., Clifford C.M., Tipton V.J. Argas (Argas) japonicus, new species, associated with swallows in Japan and Korea. (Ixodoidea Argasidae) J. Med. Entomol. 1968;5:453–459. doi: 10.1093/jmedent/5.4.453. [DOI] [PubMed] [Google Scholar]
- Zahid H., Muñoz-Leal S., Khan M.Q., Alouffi A.S., Labruna M.B., Ali A. Life cycle and genetic identification of Argas persicus infesting domestic fowl in Khyber Pakhtunkhwa, Pakistan. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.664731. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The mitochondrial genome assemblies and annotations generated in this study are deposited in GenBank under the following accession numbers: OR892711 for Ar. vulgaris. All other relevant data are included in the manuscript and the references are available upon request by the corresponding author.