Abstract
Clostridium acetobutylicum ATCC 824 was selected for the homologous overexpression of its Fe-only hydrogenase and for the heterologous expressions of the Chlamydomonas reinhardtii and Scenedesmus obliquus HydA1 Fe-only hydrogenases. The three Strep tag II-tagged Fe-only hydrogenases were isolated with high specific activities by two-step column chromatography. The purified algal hydrogenases evolve hydrogen with rates of around 700 μmol H2 min−1 mg−1, while HydA from C. acetobutylicum (HydACa) shows the highest activity (5,522 μmol H2 min−1 mg−1) in the direction of hydrogen uptake. Further, kinetic parameters and substrate specificity were reported. An electron paramagnetic resonance (EPR) analysis of the thionin-oxidized HydACa protein indicates a characteristic rhombic EPR signal that is typical for the oxidized H cluster of Fe-only hydrogenases.
The great biotechnological interest of hydrogen is its use as a fuel, an alternative “clean” energy carrier. Hydrogenases catalyze the interconversion of hydrogen gas (H2) and its elementary particle constituents, protons and electrons. They are a diverse group of enzymes, often classified according to the transition metal cofactors associated with the protein (25). Among the hydrogenases, the Fe-only hydrogenases often catalyze the reduction of protons to yield hydrogen at high turnover levels (2, 17, 19).
To our knowledge, the fastest reported microorganism for hydrogen production from hexose is the anaerobic bacterium Clostridium acetobutylicum, with a productivity in a chemostat of 2.4 liters H2 liter−1 h−1 (22). C. acetobutylicum ATCC 824 contains an Fe-only hydrogenase (12) and a putative NiFe-hydrogenase revealed by genome sequencing (18). The Fe-only hydrogenase gene is located on the chromosome, whereas the NiFe-hydrogenase is located on a separate megaplasmid. Only one report described the purification of the C. acetobutylicum Fe-hydrogenase related to its ability to reduce 2,4,6-trinitrotoluene (27), but nothing yet is known on the respective involvement of NiFe- and Fe-hydrogenases in the hydrogen metabolism of C. acetobutylicum.
Hydrogen evolution from light and water by green algal Fe-hydrogenases is an active field of basic and applied research. Chlamydomonas reinhardtii contains two Fe-hydrogenases (HydA1 and HydA2). HydA1 is located in the chloroplast and linked to the photosynthetic electron transport chain via the ferredoxin PetF (9, 13, 14). Two copies of Fe-hydrogenase genes were also identified in the green alga Scenedesmus obliquus (7, 29). All these algal Fe-hydrogenases have transcripts expressed upon anaerobic induction (7, 9, 13). The “classical” isolation of native C. reinhardtii and S. obliquus Fe-only hydrogenases revealed highly active hydrogenases showing hydrogen evolution activities above 700 μmol H2 min−1 mg−1 (7, 13, 14). Those Fe-hydrogenases from algae and clostridia reveal common features, e.g., monocistronic gene organization, a monomeric structure (between 44.5 and 64 kDa), and high homology at the amino acid sequence level in the C-terminal region including the H catalytic center (7, 25). However, algal hydrogenases lack an additional Fe-S cluster in the N-terminal domain (25).
To develop an efficient hydrogen production system, the study of structure-function relationships of Fe-hydrogenases is needed, requiring the characterization of overexpressed purified native and modified Fe-hydrogenases. Since the heterologous expression of Fe-only hydrogenases in Escherichia coli or in the cyanobacterium Synechococcus sp. led to enzymes in an inactive form (5, 12, 26) or with low hydrogen evolution activity (4, 20), we developed a homologous and heterologous overexpression and purification system functional in C. acetobutylicum, an essential tool for the characterization of Fe-hydrogenases.
Development of an overexpression and purification system functional in C. acetobutylicum.
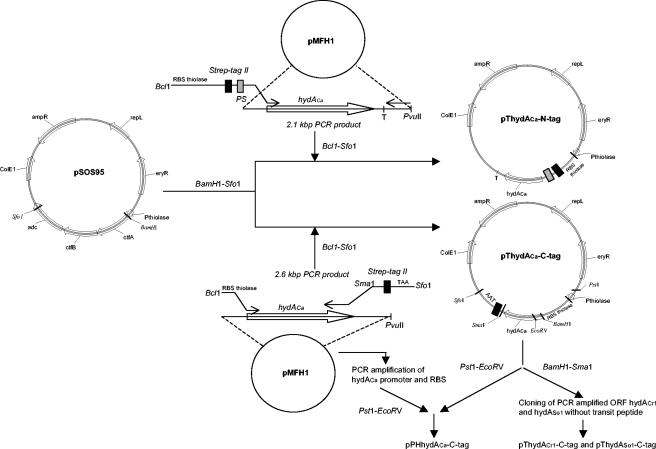
Our system is based on (i) E. coli-C. acetobutylicum shuttle vectors for overexpression in C. acetobutylicum and (ii) a fusion protein technique for a rapid and efficient purification procedure. The development of this system was performed with the C. acetobutylicum Fe-hydrogenase (HydACa). Since first attempts using glutathione S-transferase protein led mainly to an insoluble fusion protein, the small tag Strep tag II, consisting of only eight amino acids, was evaluated. This tag was reported to be efficient in metalloenzyme purification (15). Strep tag II was positioned in the N-terminal (pThydACa-N-tag vector) or C-terminal (pThydACa-C-tag vector) position of HydACa, under control of the ribosome binding site (RBS) and promoter (PthlA) from clostridial thiolase (Table 1 and Fig. 1). All plasmids were constructed in E. coli. The hydACa gene was sequenced in pThydACa-N-tag and pThydACa-C-tag before transfer into C. acetobutylicum (16). C. acetobutylicum recombinant strains were stored in spore form at −20°C, being stable for months. Strep tag II-HydACa (64 kDa) was overexpressed in C. acetobutylicum. The tagged protein's solubility was greatly influenced by the tag position. After sonication, N-terminal Strep tag II-HydACa was mostly found in the postsonication pellet while C-terminal Strep tag II-HydACa was exclusively detected in the cell extract (Fig. 2). The C-terminal Strep tag II-HydACa construct was selected for the following optimization experiments.
TABLE 1.
Bacterial strain and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| C. acetobutylicum ATCC 824 | Wild type | ATCCb |
| Plasmids | ||
| pMFH1 | hydACa gene, ORF2, ORF3, Ampr, ColE1 origin | 12 |
| pPHhydACa-C-tag | hydACa promoter, hydACa gene, Strep tag II, Apr, Emr, repL gene, ColE1 origin | This study |
| pSOS95c | thlA promoter, acetone operon, Ampr, Eryr, repL gene, ColE1 origin | 8 |
| pThydACa-C-tag | thlA promoter, hydACa gene, Strep tag II, Ampr, Eryr, repL gene, ColE1 origin | This study |
| pThydACa-N-tag | thlA promoter, Strep tag II, PS sequence, hydACa gene, Ampr, Eryr, repL gene, ColE1 origin | This study |
| pThydACrl-C-tag | thlA promoter, Strep tag II, PS sequence, hydACrl gene, Ampr, Eryr, repL gene, ColE1 origin | This study |
| pThydASol-C-tag | thlA promoter, Strep tag II, PS sequence, hydASol gene, Ampr, Eryr, repL gene, ColE1 origin | This study |
Abbreviations: Ampr, ampicillin resistance; Eryr, erythromycin resistance; repL gene, gram-positive origin of replication from pIM13; thlA promoter, promoter of the C. acetobutylicum ATCC 824 thiolase gene (23); hydACa gene, C. acetobutylicum ATCC 824 [Fe]-hydrogenase gene; hydACrl gene, C. reinhardtii HydA1 [Fe]-hydrogenase gene; hydASol gene, S. obliquus HydA1 [Fe]-hydrogenase gene; PS sequence, cleavage recognition site of the prescission protease (Amersham).
ATCC, American Type Culture Collection Manassas, Va.
GenBank accession number AY187686.
FIG. 1.
Construction of the shuttle expression vectors. For each plasmid, the locations and directions of relevant genes and restriction sites are shown. adc and ctfAB are genes encoding C. acetobutylicum ATCC 824 acetoacetate decarboxylase and coenzyme A transferase in the acetone operon. T, hydACa terminator. For definitions of other abbreviations, see the footnotes to Table 1.
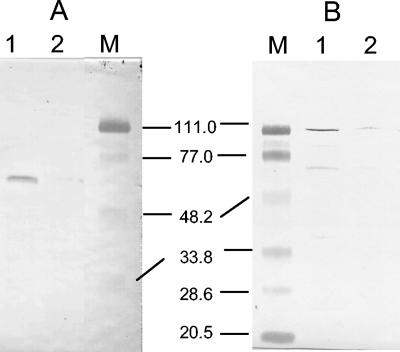
FIG. 2.
Immunoblot detection with Strep Tactin HPR conjugate (IBA GmbH, Göttingen, Germany) of Strep tag II-HydACa in sonicated C. acetobutylicum cells with Strep tag II in the N-terminal position (A) (lane 1, postsonication pellet; lane 2, cell extract) and Strep tag II in the C-terminal position (B) (lane 1, cell extract; lane 2, postsonication pellet). M, prestained low-range standard proteins (sizes are in kDa).
The expression level of recombinant HydACa in C. acetobutylicum was increased after exchanging in the expression vector the PthlA promoter for the 14 times stronger hydACa promoter (PhydA) as previously demonstrated in cultures maintained at pH 6.5 (10). Consequently, PhydA, together with the Fe-only hydrogenase RBS, was cloned in place of the thiolase promoter and RBS into the pThydACa-C-tag vector to yield pPHhydACa-C-tag. The level of Strep tag II-HydACa expression was compared between cell extracts of C. acetobutylicum strains pThydACa-C-tag and pPHhydACa-C-tag grown in batch cultures at a pH regulated at 6.5. For both recombinant strains, Strep tag II-HydACa expression was constant throughout the exponential phase. However, for the same culture volume, 10 times more Strep tag II-HydACa could be purified with the same level of specific activity using C. acetobutylicum pPHhydACa-C-tag compared with C. acetobutylicum pThydACa-C-tag (data not shown).
Homologous overexpression, purification, and characterization of the C. acetobutylicum Fe-only hydrogenase.
Anaerobic conditions were maintained throughout the entire expression-purification procedure in the presence of 2 mM Na-dithionite. C. acetobutylicum pPHhydACa-C-tag was grown on a minimum medium (24) supplemented with erythromycin (40 μg ml−1), calcium carbonate (2 g liter−1), and a mixed solution of iron sulfate (100 mg liter−1), nickel II chloride (6 mg liter−1), zinc sulfate (120 mg liter−1), and nitrilotriacetic acid (400 mg liter−1) in a 1.3-liter batch culture maintained at 37°C and pH 6.5, the optimal pH of PhydA expression (10). While cells were still in the exponential growth phase (optical density at 620 nm in the range of 5 to 6), they were anaerobically harvested (15 min at 9,000 rpm and 4°C), transferred into the anaerobic chamber, washed in 100 mM Tris/HCl (pH 7.6)-2 mM Na-dithionite-10% glycerol, concentrated 30 times, and frozen at −20°C. The cell yield was around 1.5 to 2 g liter−1. Later, again in the anaerobic chamber, the frozen cells were thawed and broken by sonication and debris was removed by centrifugation (10 min at 6,000 rpm). Nucleic acids were precipitated by addition of streptomycin sulfate (2 g liter−1) in the supernatant and removed by centrifugation. A first rough protein separation was performed in batch by anionic chromatography on 2 ml Q-Sepharose (Amersham) in 25 mM Tris/HCl-2 mM Na-dithionite at pH 8.3. Elution occurred stepwise, eluting Strep tag II-HydACa in the 0.3 M NaCl fraction. The second purification step was affinity chromatography on a 1-ml Strep-Tactin Superflow column performed as described by the manufacturer (IBA GmbH, Göttingen, Germany). Strep tag II-HydACa was eluted by gravity in pure form in a single step by competition with 2.5 mM desthiobiotin (Fig. 3). An accurate purification factor could not be calculated since the native hydrogenases separated from the recombinant hydrogenase only on the second column. From a 1-liter culture we could routinely obtain 0.4 mg of recombinant Strep tag II-HydACa protein, a yield sufficiently high to get enough protein for biophysical characterizations.
FIG. 3.
Purification of Strep tag II-HydACa. (A) Coomassie staining. Lane 1, crude C. acetobutylicum cell extract; lane 2, purified Strep tag II-HydACa protein (∼2 μg). (B) Immunoblot detection with Strep Tactin HPR conjugate. Lane 1, crude C. acetobutylicum cell extract; lane 2, purified Strep tag II-HydACa protein (∼2 μg). M, low-range standard proteins (sizes are in kDa).
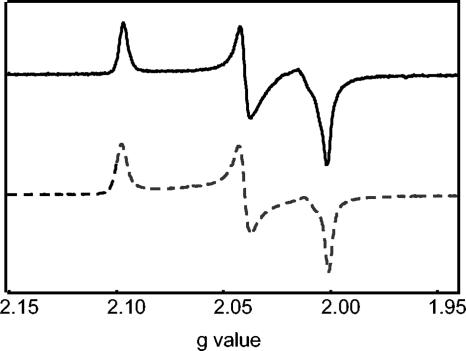
For electron paramagnetic resonance (EPR) analysis, a 100-μl sample of the isolated Strep tag II-HydACa protein (10 mg ml−1) was oxidized by anaerobic direct addition of 3,7-diamino-5-phenothiazinium (thionin, >85%; Sigma) to a final concentration of 20 μM. C. pasteurianum Cp1 Fe-hydrogenase was prepared in exactly the same manner as the Strep tag II-HydACa protein. The samples were then anaerobically transferred to a quartz EPR tube (Wilmad, Buena, NJ) and immediately frozen in liquid nitrogen. X-band EPR spectroscopy was performed using a Bruker EMX spectrometer equipped with an ER 4119HS high-sensitivity cavity and an Oxford Instruments ESR-900 helium flow cryostat. The EPR spectrum was recorded at 15 K, a microwave power of 2 mW, a modulation amplitude of 10.0 G, a modulation frequency of 100 kHz, a sweep rate of 100 Gs−1, and a microwave frequency of 9.84 GHz. An independently recorded background spectrum of the cavity and a thionin contaminant was aligned with and subtracted from the experimental spectrum to ensure that the final generated spectrum was solely derived from the paramagnetic component of the Strep tag II-HydACa protein. The X-band EPR spectrum of thionin-oxidized Strep tag II-HydACa protein is presented in Fig. 4. The thionin-oxidized Strep tag II-HydACa protein exhibited a characteristic rhombic EPR signal due to a single paramagnetic species. The signal is due to the oxidized H cluster of the protein, which exhibits an S = 1/2 paramagnetic ground state with the line shape and g values (2.096, 2.039, and 2.00) of the spectrum being in close agreement with those previously reported for other oxidized Fe-only hydrogenases (Fig. 4 and references 1, 3, and 6). The lack of any significant perturbations to the typical oxidized EPR signal of the Strep tag II-HydACa protein suggests the electronic properties of the H cluster within the protein have not been affected by the addition of the Strep tag II tag.
FIG. 4.
X-band EPR spectrum of thionin-oxidized Strep tag II-HydACa protein (solid line). For comparison, X-band EPR spectrum of thionin-oxidized Cp1 protein is shown (dashed line). Spectroscopic parameters are detailed in the text.
To measure purified HydACa activities, the in vitro hydrogen uptake assay was adopted from Vasconcelos et al. (24) while the in vitro hydrogen evolution activity was measured as previously described (11). The hydrogen uptake and evolution activities were 5,522 and 10 μmol H2 min−1 mg−1, respectively, using methylviologen. These activities are significantly lower than those of C. pasteurianum Fe-only hydrogenase Cp1—4.3 and 340 times lower for the hydrogen uptake and evolution activities, respectively (2, 19)—and also lower than activities of the native and recombinant green algal hydrogenases (7, 14, and next section).
For hydrogen uptake activity, the turnover (kcat) was 60 times higher with methylviologen while an almost 100-fold higher catalytic efficiency (kcat/Km) was measured for ferredoxin compared to methylviologen as electron acceptor (Table 2). For hydrogen evolution activity, both kcat and kcat/Km were higher for reduced ferredoxin than for reduced methylviologen, 5 and 250 times, respectively (Table 2). Obviously, the natural substrate ferredoxin was the more suitable substrate for efficient hydrogen uptake and hydrogen evolution activities of C. acetobutylicum Fe-only hydrogenase. For comparison, Quinkal and coworkers (21) have reported a slightly higher affinity of Cp1 for reduced ferredoxin associated with a 50 times higher Vmax.
TABLE 2.
Kinetic parameters of HydACa for methylviologen and ferredoxin as electron acceptor or donor
| Electron acceptor or donor | Km (M) | Vmax (μmol min−1 mg−1) | kcat (s−1) | kcat/Km (M−1 s−1) |
|---|---|---|---|---|
| Oxidized methylviologen | 128 × 10−3 | 10,057 | 10,793 | 8.4 × 104 |
| Oxidized ferredoxin | 23 × 10−6 | 160 | 172 | 7.5 × 106 |
| Reduced methylviologen | 0.3 × 10−3 | 10 | 11 | 3.6 × 104 |
| Reduced ferredoxin | 6 × 10−6 | 51 | 55 | 9.1 × 106 |
Heterologous expression and characterization of recombinant HydA from green algae.
Fe-only hydrogenases from the green algae C. reinhardtii and S. obliquus were expressed in C. acetobutylicum using the expression system described above. The cDNA coding region was cloned without the 5′ region encoding the N-terminal transit peptide of the hydA1 genes from C. reinhardtii and S. obliquus (Fig. 1). The inserts of pThydACr1-C-tag and pThydASo1-C-tag were sequenced, confirming that the fragments contain the exact full coding region of the algal hydrogenase without transit peptide.
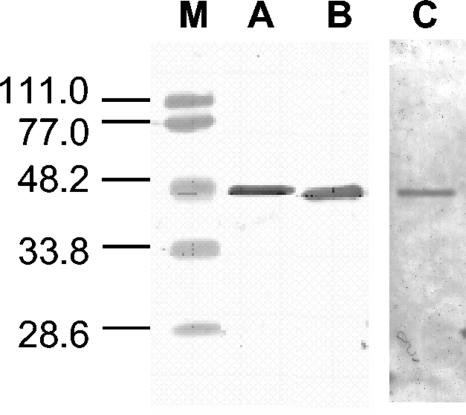
Expression and purification occurred from C. acetobutylicum transformants containing the plasmids pThydACr1-C-tag and pThydASo1-C-tag. C. acetobutylicum cells were grown in complex CGM medium (28) in 1.3-liter batch cultures at a constant pH of 6.5. Similar expression and purification steps as described for homologous hydrogenase from C. acetobutylicum were performed for the algal hydrogenases. Due to the high oxygen sensitivity of the enzymes, high concentrations of Na-dithionite (10 mM) had to be maintained throughout the whole procedure. During the stepwise elution from Q-Sepharose, the algal hydrogenases were eluted at 0.5 M NaCl. After purification using the StrepTactin Superflow column, about 0.1 mg algal hydrogenase was isolated from a 1-liter culture (Fig. 5). Although the amount of HydA is not high, similar values were obtained for HydA isolated from algal cultures of C. reinhardtii and S. obliquus (7, 13, 14). It should be noted that the thiolase promoter (PthlA) was used in this study and that by using the hydrogenase promoter (PhydA) the expression level could be increased significantly.
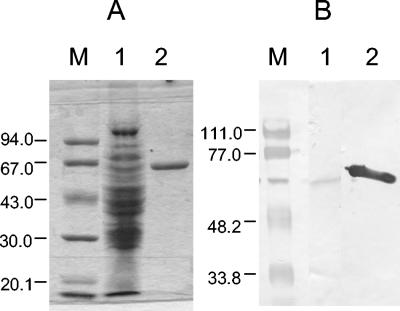
FIG. 5.
Purification of HydACr1. Immunoblot detection probed with Strep Tactin HPR conjugate (A), HydACr1 antibody (B), or Ponceau red staining (C). M, low-range standard proteins (sizes are in kDa). Protein concentration, ∼2 μg.
The isolated Fe-only hydrogenases were highly stable under anaerobic conditions. At 30 μg/ml, recombinant algal hydrogenase and Strep tag II-HydACa had lost 25 and 40% of their activity, respectively, when stored for 7 days in the absence of oxygen at room temperature kept in 0.1 M Tris/HCl (pH 8.0)-10 mM Na-dithionite.
Hydrogen evolution rates of 760 and 633 μmol H2 min−1 mg−1 and Km values of 508 and 386 μM for HydACr1 and HydASo1, respectively, were measured with methylviologen as electron donor. These kinetic parameters were in the same range as described earlier in the literature (7, 14) for the native algal and clostridial Fe-only hydrogenases. The hydrogen evolution rate of HydACr1 expressed in C. acetobutylicum was significantly higher than the 0.4 μmol H2 min−1 mg−1 reported when the enzyme was expressed in E. coli (20). Due to high Na-dithionite concentrations in the buffer of purified protein, hydrogen consumption activity tests could not be carried out in a quantitative manner.
Interestingly, the same H2 production rates were determined using clostridial ferredoxin as electron donor (data not shown). This indicates that the algal hydrogenases accept not only plant-type ferredoxin with a [2Fe-2S] cluster but also [4Fe-4S] bacterial-type ferredoxin. It is known that [2Fe-2S] plant-type ferredoxins are efficient electron mediators to clostridial hydrogenases (25).
In summary, the Strep tag II-Fe-only hydrogenase of C. acetobutylicum has been purified. In comparison with Cp1, the electronic properties of its H cluster were similar but its hydrogen evolution activity was strongly reduced. The heterologously expressed C. reinhardtii and S. obliquus Fe-only hydrogenases were fully active with high specific activities. This overexpression and purification system represents a major breakthrough for the study of the structure-function relationships of Fe-only hydrogenases.
Acknowledgments
This research was supported by grant NSF MCB-0110269 to J.W.P. and by Deutsche Forschungsgemeinschaft award SFB 480 to T.H.
REFERENCES
- 1.Adams, M. W. 1987. The mechanisms of H2 activation and CO binding by hydrogenase I and hydrogenase II of Clostridium pasteurianum. J. Biol. Chem. 262:15054-15061. [PubMed] [Google Scholar]
- 2.Adams, M. W. 1990. The structure and mechanism of iron-hydrogenase. Biochim. Biophys. Acta 1020:115-145. [DOI] [PubMed] [Google Scholar]
- 3.Adams, M. W., M. K. Johnson, I. C. Zambrano, and L. E. Mortenson. 1986. On the novel H2-activating iron-sulfur center of the “Fe-only” hydrogenases. Biochimie 68:35-42. [DOI] [PubMed] [Google Scholar]
- 4.Asada, Y., Y. Koike, J. Schnackenberg, M. Miyake, I. Uemura, and J. Miyake. 2000. Heterologous expression of clostridial hydrogenase in the cyanobacterium Synechococcus PCC7942. Biochim. Biophys. Acta 1490:269-278. [DOI] [PubMed] [Google Scholar]
- 5.Atta, M., and J. Meyer. 2000. Characterization of the gene encoding the [Fe]-hydrogenase from Megasphaera elsdenii. Biochim. Biophys. Acta 1476:368-371. [DOI] [PubMed] [Google Scholar]
- 6.Bennett, B., B. J. Lemon, and J. W. Peters. 2000. Reversible carbon monoxide binding and inhibition at the active site of the Fe-only hydrogenase. Biochemistry 39:7455-7460. [DOI] [PubMed] [Google Scholar]
- 7.Florin, L., A. Tsokoglou, and T. Happe. 2001. A novel type of iron hydrogenase in the green alga Scenedesmus obliquus is linked to the photosynthetic electron transport chain. J. Biol. Chem. 276:6125-6132. [DOI] [PubMed] [Google Scholar]
- 8.Fontaine, L., I. Meynial-Salles, L. Girbal, X. Yang, C. Croux, and P. Soucaille. 2002. Molecular characterization and transcriptional analysis of adhE2, the gene encoding the NADH-dependent aldehyde/alcohol dehydrogenase responsible for butanol production in alcohologenic cultures of Clostridium acetobutylicum ATCC 824. J. Bacteriol. 154:821-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forestier, M., P. King, L. Zhang, M. Posewitz, S. Schwarzer, T. Happe, M. L. Ghirardi, and M. Seibert. 2003. Expression of two [Fe]-hydrogenases in Chlamydomonas reinhardtii under anaerobic conditions. Eur. J. Biochem. 270:2750-2758. [DOI] [PubMed] [Google Scholar]
- 10.Girbal, L., I. Mortier-Barrière, F. Raynaud, C. Rouanet, C. Croux, and P. Soucaille. 2003. Development of a sensitive gene expression reporter system and an inducible promoter-repressor system for Clostridium acetobutylicum. Appl. Environ. Microbiol. 69:4985-4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girbal, L., I. Vasconcelos, S. Saint-Amans, and P. Soucaille. 1995. How neutral red modified carbon and electron flow in Clostridium acetobutylicum grown in chemostat culture at neutral pH. FEMS Microbiol. Rev. 16:151-162. [Google Scholar]
- 12.Gorwa, M. F., C. Croux, and P. Soucaille. 1996. Molecular characterization and transcriptional analysis of the putative hydrogenase gene of Clostridium acetobutylicum ATCC 824. J. Bacteriol. 178:2668-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Happe, T., and A. Kaminski. 2002. Differential regulation of the Fe-hydrogenase during anaerobic adaptation in the green alga Chlamydomonas reinhardtii. Eur. J. Biochem. 269:1022-1032. [DOI] [PubMed] [Google Scholar]
- 14.Happe, T., and J. D. Naber. 1993. Isolation, characterization and N-terminal amino acid sequence of hydrogenase from the green alga Chlamydomonas reinhardtii. Eur. J. Biochem. 214:475-481. [DOI] [PubMed] [Google Scholar]
- 15.Maier, T., N. Drapal, M. Thanbichler, and A. Böck. 1998. Strep-Tag II affinity purification: an approach to study intermediates of metalloenzyme biosynthesis. Anal. Biochem. 259:68-73. [DOI] [PubMed] [Google Scholar]
- 16.Melmerstein, L. D., and E. T. Papoutsakis. 1993. In vivo methylation in Escherichia coli by the Bacillus subtilis phage φ3T I methyltransferase to protect plasmids from restriction upon transformation of Clostridium acetobutylicum ATCC 824. Appl. Environ. Microbiol. 59:1077-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicolet, Y., C. Piras, P. Legrand, C. E. Hatchikian, and J. C. Fontecilla-Camps. 1999. Desulfovibrio desulfuricans iron hydrogenase: the structure shows unusual coordination to an active Fe binuclear center. Structure 7:13-23. [DOI] [PubMed] [Google Scholar]
- 18.Nölling, J., G. Breton, M. V. Omelchenko, K. S. Makarova, Q. Zeng, R. Gibson, H. M. Lee, J. Dubois, D. Qiu, J. Hitti, Y. I. Wolf, R. L. Tatusov, F. Sabathe, L. Doucette-Stamm, P. Soucaille, M. J. Daly, G. N. Bennett, E. V. Koonin, and D. R. Smith. 2001. Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum. J. Bacteriol. 183:4823-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters, J. W., W. N. Lanzilotta, B. J. Lemon, and L. C. Seefeldt. 1998. X-ray crystal structure of the Fe-only hydrogenase (Cp1) from Clostridium pasteurianum. Science 282:1853-1858. [DOI] [PubMed] [Google Scholar]
- 20.Posewitz, M. C., P. W. King, S. L. Smolinsky, L. Zhang, M. Seibert, and M. L. Ghirardi. 2004. Discovery of two novel radical SAM proteins required for the assembly of an active [Fe]-hydrogenase. J. Biol. Chem. 279:25711-25720. [DOI] [PubMed] [Google Scholar]
- 21.Quinkal, I., V. Davasse, J. Gaillard, and J.-M. Moulis. 1994. On the role of conserved proline residues in the structure and function of Clostridium pasteurianum 2[4Fe-4S] ferredoxin. Protein Eng. 7:681-687. [DOI] [PubMed] [Google Scholar]
- 22.Soni, B. K., P. Soucaille, and G. Goma. 1987. Continuous acetone butanol fermentation: influence of vitamins on the metabolic activity of Clostridium acetobutylicum. Appl. Microbiol. Biotechnol. 2:1-5. [Google Scholar]
- 23.Stim-Herndon, K. P., D. J. Petersen, and G. N. Bennett. 1995. Characterization of an acetyl-CoA C-acetyltransferase (thiolase) gene from Clostridium acetobutylicum ATCC 824. Gene 154:81-85. [DOI] [PubMed] [Google Scholar]
- 24.Vasconcelos, I., L. Girbal, and P. Soucaille. 1994. Regulation of carbon and electron flow in Clostridium acetobutylicum grown in chemostat culture at neutral pH on mixtures of glucose and glycerol. J. Bacteriol. 176:1443-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vignais, P. M., B. Billoud, and J. Meyer. 2001. Classification and phylogeny of hydrogenases. FEMS Microbiol. Rev. 25:455-501. [DOI] [PubMed] [Google Scholar]
- 26.Voordouw, G., W. R. Hagen, K. M. Krüse-Wolters, A. van Berkel-Arts, and C. Veeger. 1987. Purification and characterization of Desulfovibrio vulgaris (Hildenborough) hydrogenase expressed in Escherichia coli. Eur. J. Biochem. 162:31-36. [DOI] [PubMed] [Google Scholar]
- 27.Watrous, M. M., S. Clark, R. Kutty, S. Huang, F. B. Rudolph, J. B. Hughes, and G. N. Bennett. 2003. 2,4,6-Trinitrotoluene reduction by an Fe-only hydrogenase in Clostridium acetobutylicum. Appl. Environ. Microbiol. 69:1542-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiesenborn, D. P., F. B. Rudolph, and E. T. Papoutsakis. 1988. Thiolase from Clostridium acetobutylicum ATCC 824 and its role in the synthesis of acids and solvents. Appl. Environ. Microbiol. 54:2717-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wünschiers, R., K. Stangier, H. Senger, and R. Schulz. 2001. Molecular evidence for a Fe-hydrogenase in the green alga Scenedesmus obliquus. Curr. Microbiol. 42:353-360. [DOI] [PubMed] [Google Scholar]